Prolonged Humid Heat Triggers Systemic Inflammation and Stress Signaling: Fluid Intake Modulates NF-κB, p38, JNK2, and STAT3α Pathways
Abstract
1. Introduction
2. Results
2.1. Hydration Status
2.2. Body Temperatures
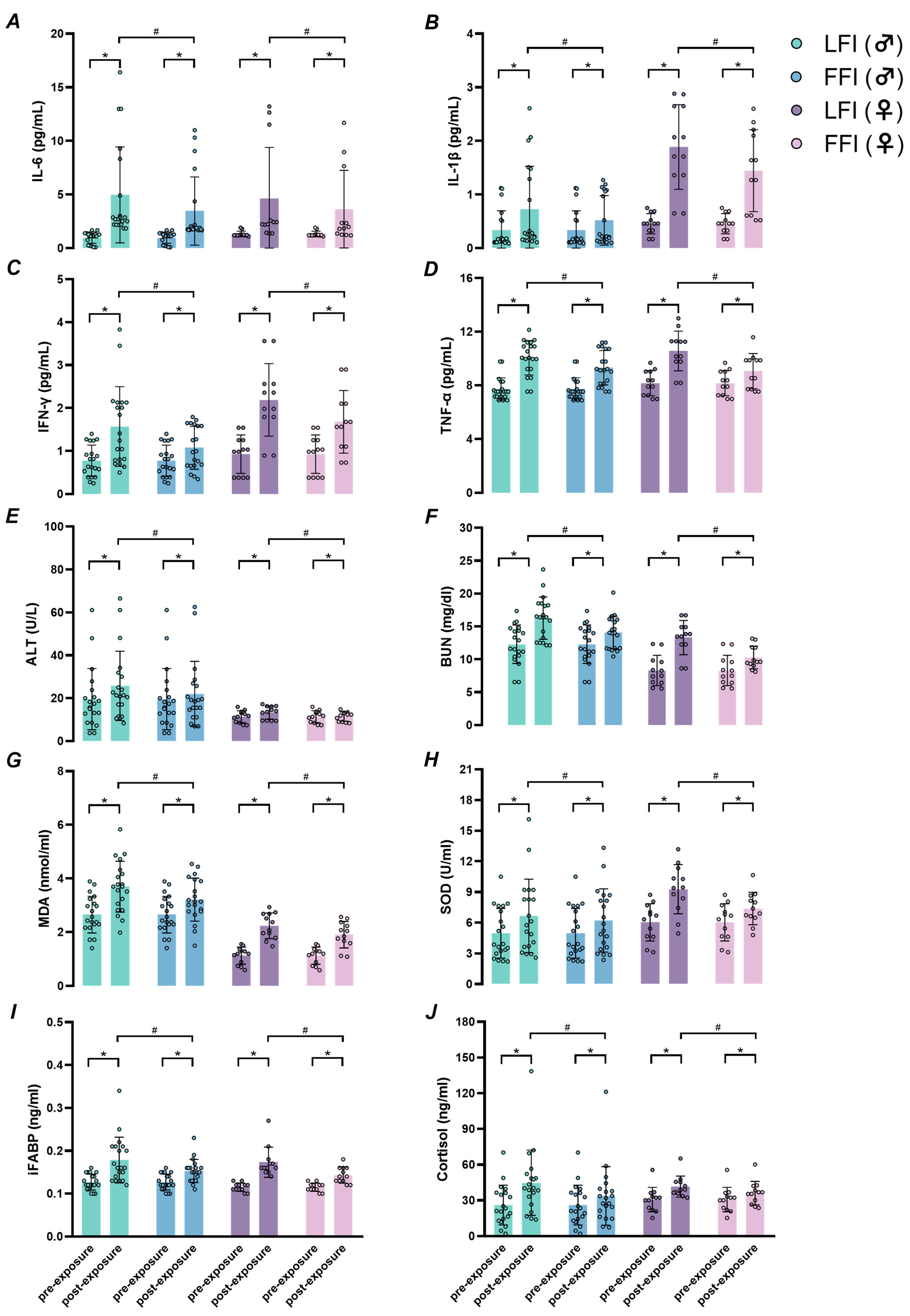
2.3. Systemic Inflammation and Organ Function
2.4. Oxidative Stress, Intestinal Cell Injury, and Cortisol Levels
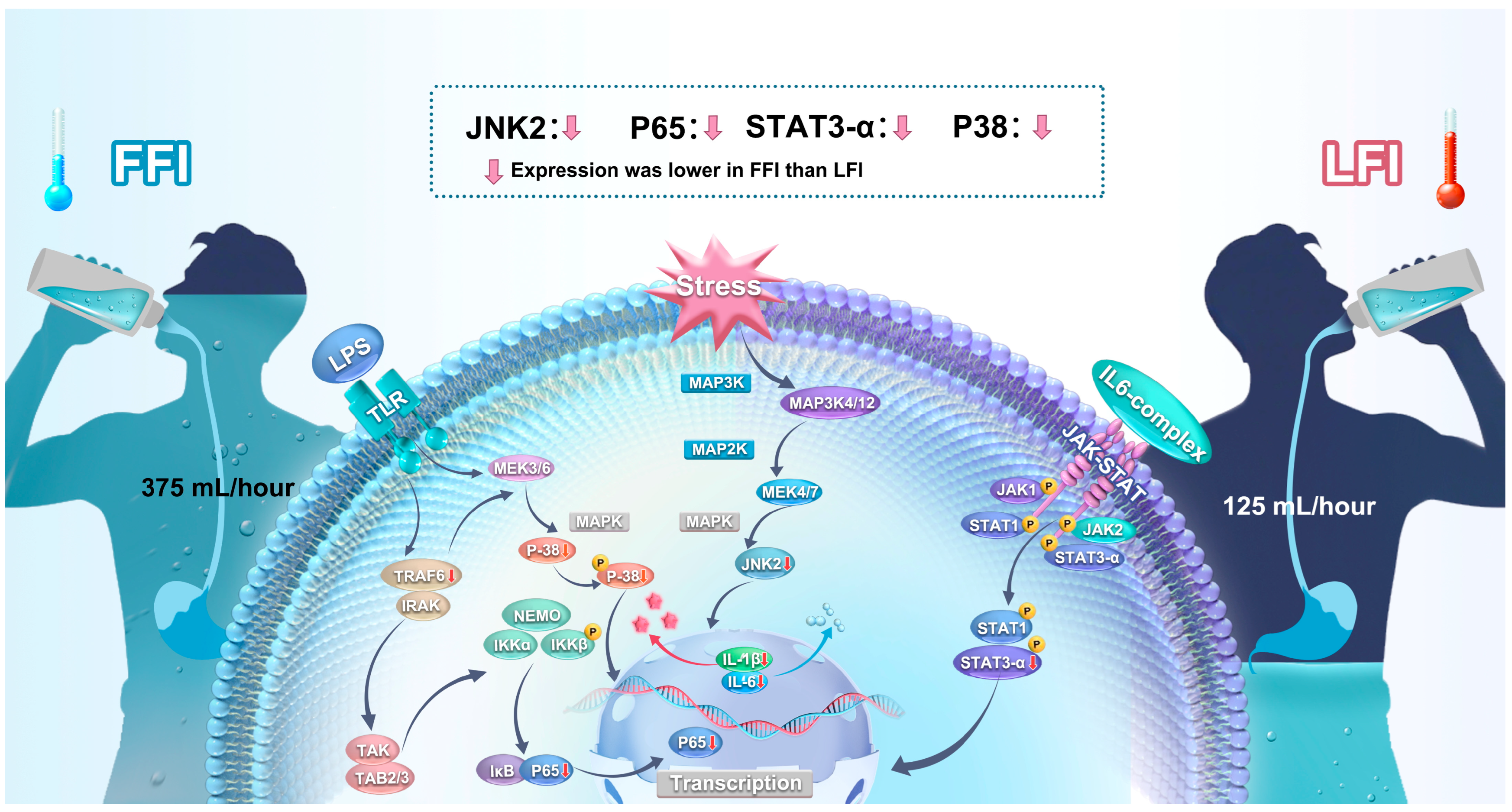
2.5. Inflammatory Signaling Pathways
3. Discussion
3.1. Limitations
3.2. Physiological Relevance and Practical Significance
4. Materials and Methods
4.1. Ethical Approval and Participants
4.2. Experimental Protocol
4.3. Measurements
4.3.1. Blood Preparation
4.3.2. Inflammatory Cytokines
4.3.3. Oxidative Stress, Intestinal Cell Injury, and Cortisol
4.3.4. Organ Function Markers
4.3.5. Whole Blood Cell Counts
4.4. Western Blotting
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LFI | Limited fluid intake |
| FFI | Full fluid intake |
| NF-κB | Nuclear factor kappa B |
| MAPKs | Mitogen-activated protein kinases |
| JAK/STAT | Janus kinase/signal transducer and activator of transcription |
| JNK | C-June N-terminal kinase |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| USG | Urine specific gravity |
| Tcore | Core temperature |
| Tsk | Mean skin temperature |
| IFN-γ | Interferon-γ |
| TNF-α | Tumor necrosis factor-α |
| ALT | Alanine aminotransferase |
| BUN | Blood urea nitrogen |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| iFABP | Intestinal fatty acid-binding protein |
| p-NF-κB p65 | Phosphorylated NF-κB p65 |
| p-JNK1 | Phosphorylated JNK1 |
| p-JNK2 | Phosphorylated JNK2 |
| p-p38 | Phosphorylated p38 |
| p-STAT3α | Phosphorylated STAT3α |
| p-STAT3β | Phosphorylated STAT3β |
| LPS | Lipopolysaccharide |
| TLR4 | Toll-like receptor 4 |
| HPA | Hypothalamic–pituitary–adrenal axis |
| PBMCs | Peripheral blood mononuclear cells |
| SIRS | Systemic inflammatory response syndrome |
| BP | Blood pressure |
| DBP | Diastolic blood pressure |
| SBP | Systolic blood pressure |
| BCA | Bicinchoninic acid |
| PVDF | Polyvinylidene fluoride |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
| CV | Coefficient of variation |
References
- Walsh, N.P.; Whitham, M. Exercising in environmental extremes: A greater threat to immune function? Sports Med. 2006, 36, 941–976. [Google Scholar] [CrossRef] [PubMed]
- Barberio, M.D.; Elmer, D.J.; Laird, R.H.; Lee, K.A.; Gladden, B.; Pascoe, D.D. Systemic LPS and inflammatory response during consecutive days of exercise in heat. Int. J. Sports Med. 2015, 36, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Helms, J.; Levi, M.; Levy, J.H. Inflammation, coagulation, and cellular injury in heat-induced shock. Inflamm. Res. 2023, 72, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Russell, S.L.; Meade, R.D.; McCormick, J.J.; King, K.E.; Kenny, G.P. Markers of enterocyte damage, microbial translocation, and systemic inflammation following 9 h of heat exposure in young and older adults. Appl. Physiol. Nutr. Metab. 2024, 49, 1241–1251. [Google Scholar] [CrossRef]
- Lei, T.H.; Wang, F. Looking ahead of 2021 Tokyo Summer Olympic Games: How Does Humid Heat Affect Endurance Performance? Insight into physiological mechanism and heat-related illness prevention strategies. J. Therm. Biol. 2021, 99, 102975. [Google Scholar] [CrossRef]
- Frey, B.; Weiss, E.M.; Rubner, Y.; Wunderlich, R.; Ott, O.J.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Old and new facts about hyperthermia-induced modulations of the immune system. Int. J. Hyperth. 2012, 28, 528–542. [Google Scholar] [CrossRef]
- Enomoto, A.; Fukasawa, T. JNK signaling dominance in hyperthermia. Cell Stress Chaperones 2025, 30, 100080. [Google Scholar] [CrossRef]
- Enomoto, A.; Fukasawa, T.; Terunuma, H.; Nakagawa, K.; Yoshizaki, A.; Sato, S.; Hosoya, N.; Miyagawa, K. Deregulated JNK signaling enhances apoptosis during hyperthermia. Int. J. Hyperth. 2024, 41, 2335199. [Google Scholar] [CrossRef]
- Chapman, C.L.; Johnson, B.D.; Parker, M.D.; Hostler, D.; Pryor, R.R.; Schlader, Z.J. Kidney physiology and pathophysiology during heat stress and the modification by exercise, dehydration, heat acclimation and aging. Temperature 2021, 8, 108–159. [Google Scholar] [CrossRef]
- Welc, S.S.; Phillips, N.A.; Oca-Cossio, J.; Wallet, S.M.; Chen, D.L.; Clanton, T.L. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am. J. Physiol. Cell. Physiol. 2012, 303, C455–C466. [Google Scholar] [CrossRef]
- Welc, S.S.; Clanton, T.L.; Dineen, S.M.; Leon, L.R. Heat stroke activates a stress-induced cytokine response in skeletal muscle. J. Appl. Physiol. 2013, 115, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Paszek, A.; Kardyńska, M.; Bagnall, J.; Śmieja, J.; Spiller, D.G.; Widłak, P.; Kimmel, M.; Widlak, W.; Paszek, P. Heat shock response regulates stimulus-specificity and sensitivity of the pro-inflammatory NF-κB signalling. Cell. Commun. Signal. 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Winter-Vann, A.M.; Johnson, G.L. Integrated activation of MAP3Ks balances cell fate in response to stress. J. Cell. Biochem. 2007, 102, 848–858. [Google Scholar] [CrossRef]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Dmitrieva, N.I.; Boehm, M.; Yancey, P.H.; Enhörning, S. Long-term health outcomes associated with hydration status. Nat. Rev. Nephrol. 2024, 20, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Montain, S.J.; Latzka, W.A. Hydration effects on thermoregulation and performance in the heat. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001, 128, 679–690. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Dugas, J.P.; McFarlin, B.K.; Nelson, M.J. Effect of exercise, heat stress, and hydration on immune cell number and function. Med. Sci. Sports Exerc. 2002, 34, 1941–1950. [Google Scholar] [CrossRef]
- Xiang, N.L.; Liu, J.; Liao, Y.J.; Huang, Y.W.; Wu, Z.; Bai, Z.Q.; Lin, X.; Zhang, J.H. Abrogating ClC-3 inhibits LPS-induced inflammation via blocking the TLR4/NF-κB pathway. Sci. Rep. 2016, 6, 27583. [Google Scholar] [CrossRef]
- Sawka, M.N.; Montain, S.J. Fluid and electrolyte supplementation for exercise heat stress. Am. J. Clin. Nutr. 2000, 72, 564S–572S. [Google Scholar] [CrossRef]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. Exercise and fluid replacement. Med. Sci. Sports. Exerc. 2007, 39, 377–390. [Google Scholar] [PubMed]
- Costello, J.T.; Rendell, R.A.; Furber, M.; Massey, H.C.; Tipton, M.J.; Young, J.S.; Corbett, J. Effects of acute or chronic heat exposure, exercise and dehydration on plasma cortisol, IL-6 and CRP levels in trained males. Cytokine 2018, 110, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cottle, R.M.; Lichter, Z.S.; Vecellio, D.J.; Wolf, S.T.; Kenney, W.L. Core temperature responses to compensable versus uncompensable heat stress in young adults (PSU HEAT Project). J. Appl. Physiol. 2022, 133, 1011–1018. [Google Scholar] [CrossRef]
- McCormick, J.J.; Meade, R.D.; King, K.E.; Notley, S.R.; Akerman, A.P.; McGarr, G.W.; Richards, B.J.; McCourt, E.R.; Boulay, P.; Sigal, R.J.; et al. Physiological responses to 9 hours of heat exposure in young and older adults. Part II: Autophagy and the acute cellular stress response. J. Appl. Physiol. 2023, 135, 688–695. [Google Scholar] [CrossRef]
- Leon, L.R.; Helwig, B.G. Heat stroke: Role of the systemic inflammatory response. J. Appl. Physiol. 2010, 109, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Lambert, G.P. Role of gastrointestinal permeability in exertional heatstroke. Exerc. Sport. Sci. Rev. 2004, 32, 185–190. [Google Scholar] [CrossRef]
- Lang, C.H.; Silvis, C.; Deshpande, N.; Nystrom, G.; Frost, R.A. Endotoxin stimulates in vivo expression of inflammatory cytokines tumor necrosis factor alpha, interleukin-1β, -6, and high-mobility-group protein-1 in skeletal muscle. Shock 2003, 19, 538–546. [Google Scholar] [CrossRef]
- March, D.S.; Marchbank, T.; Playford, R.J.; Jones, A.W.; Thatcher, R.; Davison, G. Intestinal fatty acid-binding protein and gut permeability responses to exercise. J. Appl. Physiol. 2017, 117, 931–941. [Google Scholar] [CrossRef]
- Ogden, H.B.; Child, R.B.; Fallowfield, J.L.; Delves, S.K.; Westwood, C.S.; Layden, J.D. The gastrointestinal exertional heat stroke paradigm: Pathophysiology, assessment, severity, aetiology and nutritional countermeasures. Nutrients 2020, 12, 537. [Google Scholar] [CrossRef]
- Pires, W.; Veneroso, C.E.; Wanner, S.P.; Pacheco, D.A.; Vaz, G.C.; Amorim, F.T.; Tonoli, C.; Soares, D.D.; Coimbra, C.C. Association between exercise-induced hyperthermia and intestinal permeability: A systematic review. Sports. Med. 2017, 47, 1389–1403. [Google Scholar] [CrossRef]
- Yamakawa, K.; Matsunaga, M.; Isowa, T.; Kimura, K.; Kasugai, K.; Yoneda, M.; Kaneko, H.; Ohira, H. Transient responses of inflammatory cytokines in acute stress. Biol. Psychol. 2009, 82, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.L.; Johnson, B.D.; Vargas, N.T.; Hostler, D.; Parker, M.D.; Schlader, Z.J. Both hyperthermia and dehydration during physical work in the heat contribute to the risk of acute kidney injury. J. Appl. Physiol. 2020, 128, 715–728. [Google Scholar] [CrossRef]
- Giercksky, T.; Boberg, K.; Farstad, I.N.; Halvorsen, S.; Schrumpf, E. Severe liver failure in exertional heat stroke. Scand. J. Gastroenterol. 1999, 34, 824–827. [Google Scholar] [PubMed]
- Geng, Y.; Ma, Q.; Liu, Y.N.; Peng, N.; Yuan, F.F.; Li, X.G.; Li, M.; Wu, Y.S.; Li, B.L.; Song, W.B.; et al. Heatstroke induces liver injury via IL-1β and HMGB1-induced pyroptosis. J. Hepatol. 2015, 63, 622–633. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Li, J.; Xia, H.; Zhang, D.; Yao, S. The pathogenesis and therapeutic strategies of heat stroke-induced liver injury. Crit. Care 2022, 26, 391. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Song, C.; Lin, A.; De Jongh, R.; Van Gastel, A.; Kenis, G.; Bosmans, E.; Meester, I.D.; Benoy, I.; Neels, H.; et al. The effects of psychological stress on humans: Increased production of pro-inflammatory cytokines and Th1-like response in stress-induced anxiety. Cytokine 1998, 10, 313–318. [Google Scholar] [CrossRef]
- Ip, Y.T.; Davis, R.J. Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr. Opin. Cell. Biol. 1998, 10, 205–219. [Google Scholar] [CrossRef]
- Maritano, D.; Sugrue, M.L.; Tininini, S.; Dewilde, S.; Strobl, B.; Fu, X.; Murray-Tait, V.; Chiarle, R.; Poli, V. The STAT3 isoforms alpha and beta have unique and specific functions. Nat. Immunol. 2004, 5, 401–409. [Google Scholar] [CrossRef]
- Borra, V.; De Brier, N.; Berry, D.C.; Zideman, D.; Singletary, E.; De Buck, E.; International Liaison Committee on Resuscitation First Aid Task Force. Oral rehydration beverages for treating exercise-associated dehydration: A systematic review, Part I. Carbohydrate-electrolyte solutions. J. Athl. Train 2025, 60, 34–54. [Google Scholar] [CrossRef]
- De Brier, N.; Borra, V.; Berry, D.C.; Zideman, D.; Singletary, E.; De Buck, E.; International Liaison Committee on Resuscitation First Aid Task Force. Oral rehydration beverages for treating exercise-associated dehydration: A systematic review, Part II. The effectiveness of alternatives to carbohydrate-electrolyte drinks. J. Athl. Train 2025, 60, 55–69. [Google Scholar] [CrossRef]
- Speakman, J.R.; Hall, K.D. Carbohydrates, insulin, and obesity. Science 2021, 372, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Nieman, D.C. Influence of carbohydrate on the immune response to intensive, prolonged exercise. Exerc. Immunol. Rev. 1998, 4, 64–76. [Google Scholar] [PubMed]
- Davies, M.G.; Hagen, P.O. Systemic inflammatory response syndrome. Br. J. Surg. 1997, 84, 920–935. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Frausto, M.S.; Pittet, D.; Costigan, M.; Hwang, T.; Davis, C.S.; Wenzel, R.P. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA 1995, 273, 117–123. [Google Scholar] [CrossRef]
- Oppliger, R.A.; Magnes, S.A.; Popowski, L.A.; Gisolfi, C.V. Accuracy of urine specific gravity and osmolality as indicators of hydration status. Int. J. Sport. Nutr. Exerc. Metab. 2005, 15, 236–251. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Q.; Liu, A.; Zuo, J.; Zhang, W.; Zou, S.; Li, X.; Lu, L.; Pan, H.; Hu, X. Fluid intake of adults in four Chinese cities. Nutr. Rev. 2012, 70, 105–110. [Google Scholar] [CrossRef]
- Popkin, B.M.; D’Anci, K.E.; Rosenberg, I.H. Water, hydration and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef]
- Nes, B.M.; Janszky, I.; Wisløff, U.; Støylen, A.; Karlsen, T. Age-predicted maximal heart rate in healthy subjects: The HUNT fitness study. Scand. J. Med. Sci. Sports 2013, 23, 697–704. [Google Scholar] [CrossRef]
- Taylor, B.C.; Wilt, T.J.; Welch, H.G. Impact of diastolic and systolic blood pressure on mortality: Implications for the definition of “normal”. J. Gen. Intern. Med. 2011, 26, 685–690. [Google Scholar] [CrossRef]
- Dill, D.B.; Costill, D.L. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J. Appl. Physiol. 1974, 37, 247–248. [Google Scholar] [CrossRef]
- Adams, J.D.; Myatich, A.I.; McCullough, A.S. Thirst as an ingestive behavior: A brief review on physiology and assessment. Nutr. Health 2020, 26, 271–274. [Google Scholar] [CrossRef] [PubMed]
- ASHRAE Standard 55-2023; Thermal Environmental Conditions for Human Occupancy. American Society of Heating, Refrigeration and Air Conditioning Engineers (ASHRAE): Atlanta, GA, USA, 2023.
- Filingeri, D.; Redortier, B.; Hodder, S.; Havenith, G. Warm temperature stimulus suppresses the perception of skin wetness during initial contact with a wet surface. Skin Res. Technol. 2015, 21, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, N.L. A new weighting system for mean surface temperature of the human body. J. Appl. Physiol. 1964, 19, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Williams, V.S.; Morlock, R.J.; Feltner, D. Psychometric evaluation of a visual analog scale for the assessment of anxiety. Health Qual. Life Outcomes 2010, 8, 57. [Google Scholar] [CrossRef]

| LFI (Limited Fluid Intake) | FFI (Full Fluid Intake) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| Body temperatures | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Tcore (°C) | 36.9 ± 0.2 | 38.1 ± 0.3 * | 37.0 ± 0.1 | 38.0 ± 0.3 * | 36.9 ± 0.2 | 37.9 ± 0.4 *# | 37.1 ± 0.1 † | 37.6 ± 0.2 *#† |
| Tsk (°C) | 33.8 ± 0.3 | 37.6 ± 0.4 * | 33.0 ± 0.6 | 37.7 ± 0.4 * | 33.9 ± 0.4 | 37.5 ± 0.5 * | 33.5 ± 0.8 † | 37.4 ± 0.2 * |
| Cardiovascular | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| SBP (mmHg) | 118 ± 7 | 112 ± 8 * | 113 ± 10 | 105 ± 8 * | 119 ± 9 | 111 ± 7 * | 112 ± 5 † | 103 ± 5 *† |
| DBP (mmHg) | 72 ± 6 | 63 ± 7 * | 68 ± 8 | 58 ± 4 * | 70 ± 5 | 63 ± 5 * | 65 ± 5 † | 57 ± 5 *† |
| MAP (mmHg) | 87 ± 6 | 79 ± 6 * | 83 ± 7 | 74 ± 4 * | 86 ± 6 | 79 ± 6 * | 81 ± 4 † | 73 ± 5 *† |
| HR (bpm) | 65 ± 8 | 118 ± 8 * | 64 ± 7 | 112 ± 13 * | 59 ± 5 | 110 ± 9 * | 63 ± 4 | 113 ± 12 * |
| Blood biomarkers | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Hematocrit (%) | 43.4 ± 3.3 | 46.9 ± 2.0 * | 30.8 ± 3.6 | 37.0 ± 3.7 * | 43.9 ± 3.8 | 46.4 ± 2.0 * | 34.1 ± 2.2 † | 37.0 ± 3.3 *† |
| Leukocytes (109/L) | 6.8 ± 1.2 | 13.6 ± 2.0 * | 5.5 ± 0.7 | 10.8 ± 2.4 * | 6.3 ± 1.2 | 12.5 ± 2.0 * | 6.5 ± 0.7 | 10.2 ± 2.0 *† |
| Neutrophils (109/L) | 3.5 ± 0.7 | 8.7 ± 1.8 * | 3.1 ± 0.6 | 6.6 ± 0.8 * | 3.3 ± 0.7 | 8.1 ± 1.6 * | 2.9 ± 0.6 | 5.7 ± 1.2 *† |
| K+ (mmol/L) | 3.9 ± 0.1 | 4.0 ± 0.2 * | 4.0 ± 0.2 | 4.1 ± 0.3 * | 4.0 ± 0.2 | 3.8 ± 0.3 * | 4.0 ± 0.2 | 3.8 ± 0.5 * |
| Na+ (mmol/L) | 144.7 ± 3.4 | 146.1 ± 1.9 * | 142.2 ± 5.3 | 142.7 ± 3.5 * | 142.4 ± 3.0 | 141.1 ± 2.8 * | 142.2 ± 5.3 | 130.0 ± 13.8 *† |
| Cl− (mmol/L) | 103.6 ± 3.6 | 110.1 ± 6.2 * | 104.9 ± 2.2 | 105.4 ± 1.5 * | 101.5 ± 3.9 | 99.5 ± 2.3 * | 103.3 ± 4.6 | 97.1 ± 9.6 *† |
| PV change (%) | - | −6.7% | - | −7.4% | - | 6.4% | - | 8.7% |
| Hydration | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| USG (g/mL) | 1.013 ± 0.005 | 1.030 ± 0.004 * | 1.012 ± 0.003 | 1.030 ± 0.002 * | 1.016 ± 0.007 | 1.014 ± 0.010 *# | 1.011 ± 0.001 | 1.013 ± 0.003 * |
| Dehydration rate (%) | - | 3.0 ± 0.5 * | - | 2.8 ± 0.4 * | - | - | - | - |
| Sweat rate (g/h) | - | 237 ± 48 * | - | 165 ± 58 * | - | 276 ± 80 * | - | 214 ± 47 *† |
| Perceptions | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Thermal sensation | 0.8 ± 0.5 | 2.6 ± 0.4 * | 0.7 ± 0.5 | 3.3 ± 0.5 * | 0.8 ± 0.5 | 2.5 ± 0.8 * | 0.7 ± 0.5 | 2.7 ± 0.5 *† |
| Thermal comfort | −0.5 ± 0.6 | −2.5 ± 0.5 * | 0.5 ± 0.8 | −3.2 ± 0.9 * | −0.7 ± 0.6 | −2.0 ± 0.7 * | 0.3 ± 0.5 † | −2.7 ± 0.5 *† |
| Wetness perception | −0.8 ± 0.6 | −2.4 ± 0.5 * | −0.3 ± 0.5 | −3.3 ± 0.5 * | −0.9 ± 0.6 | −2.2 ± 0.5 * | −0.5 ± 0.5 † | −2.7 ± 0.5 *† |
| Thirst sensation | 2.1 ± 0.7 | 5.1 ± 0.8 * | 1.2 ± 0.4 | 4.8 ± 0.8 * | 1.6 ± 0.7 | 2.5 ± 1.0 * | 1.8 ± 0.7 | 2.2 ± 0.4 * |
| Psychological stress | 0.8 ± 0.7 | 6.5 ± 1.4 * | 1.1 ± 1.0 | 6.3 ± 1.6 * | 0.8 ± 0.8 | 5.0 ± 1.3 * | 0.8 ± 0.9 | 5.5 ± 1.3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Lu, C.; Lei, Y.; Lei, T.-H. Prolonged Humid Heat Triggers Systemic Inflammation and Stress Signaling: Fluid Intake Modulates NF-κB, p38, JNK2, and STAT3α Pathways. Int. J. Mol. Sci. 2025, 26, 5114. https://doi.org/10.3390/ijms26115114
Wang F, Lu C, Lei Y, Lei T-H. Prolonged Humid Heat Triggers Systemic Inflammation and Stress Signaling: Fluid Intake Modulates NF-κB, p38, JNK2, and STAT3α Pathways. International Journal of Molecular Sciences. 2025; 26(11):5114. https://doi.org/10.3390/ijms26115114
Chicago/Turabian StyleWang, Faming, Caiping Lu, Ying Lei, and Tze-Huan Lei. 2025. "Prolonged Humid Heat Triggers Systemic Inflammation and Stress Signaling: Fluid Intake Modulates NF-κB, p38, JNK2, and STAT3α Pathways" International Journal of Molecular Sciences 26, no. 11: 5114. https://doi.org/10.3390/ijms26115114
APA StyleWang, F., Lu, C., Lei, Y., & Lei, T.-H. (2025). Prolonged Humid Heat Triggers Systemic Inflammation and Stress Signaling: Fluid Intake Modulates NF-κB, p38, JNK2, and STAT3α Pathways. International Journal of Molecular Sciences, 26(11), 5114. https://doi.org/10.3390/ijms26115114







