Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei
Abstract
1. Introduction
2. Results
2.1. Identification and Tissue Distribution of Hemocyanin Linear Ubiquitination
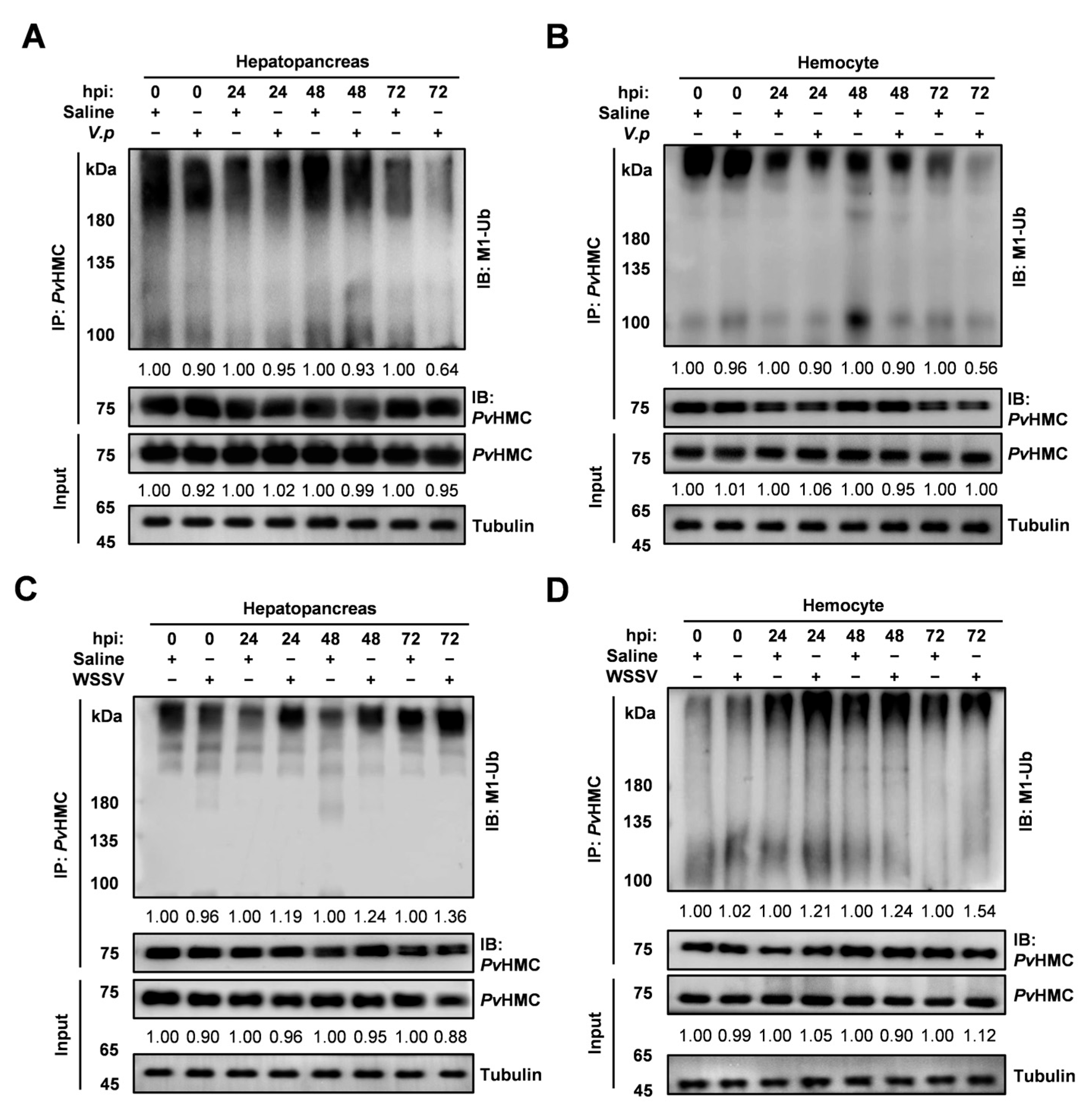
2.2. Pathogen-Induced Changes in Linear Ubiquitination of Hemocyanin
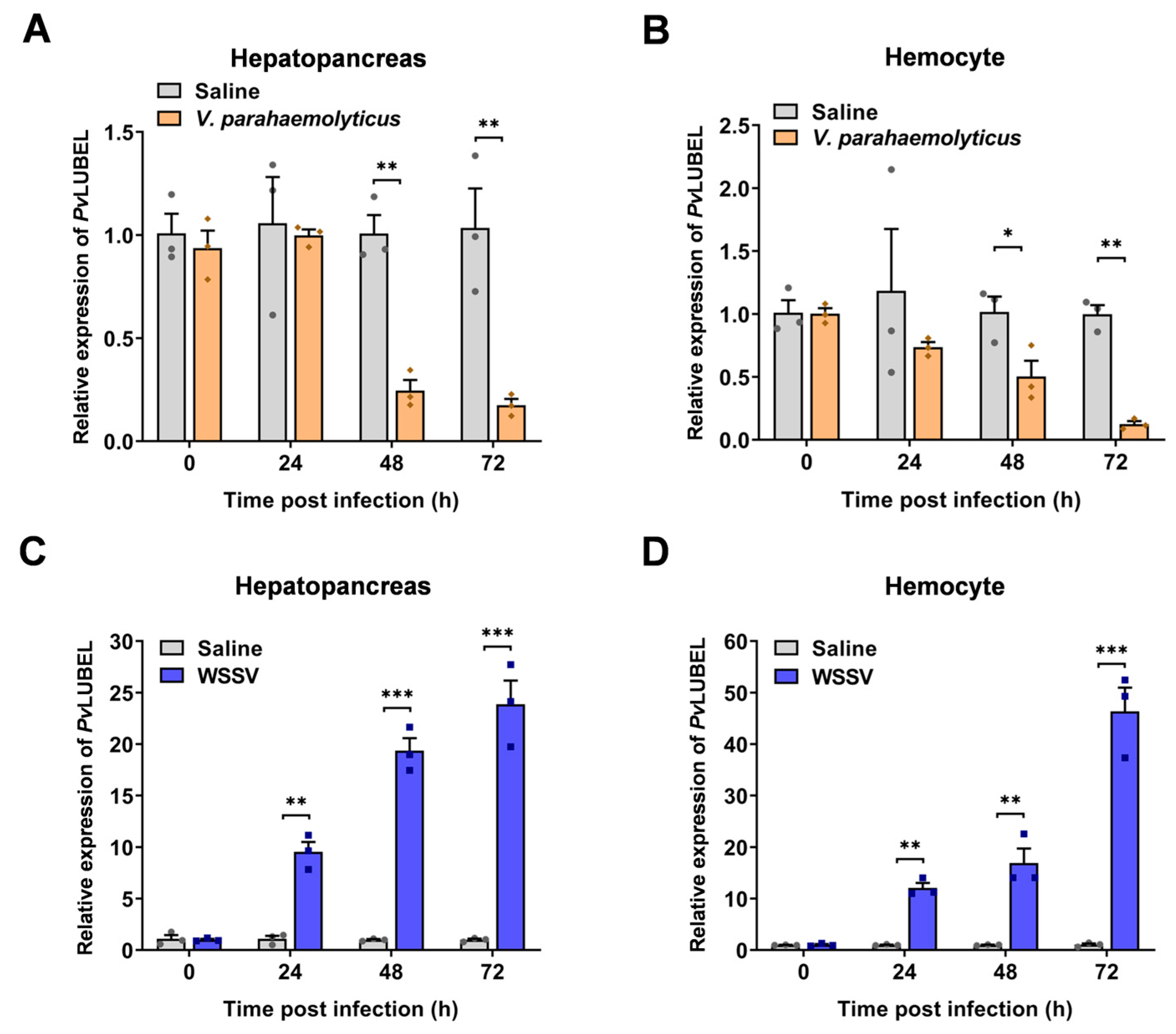
2.3. Identification and Functional Analysis of E3 Ubiquitin–Protein Ligase PvLUBEL
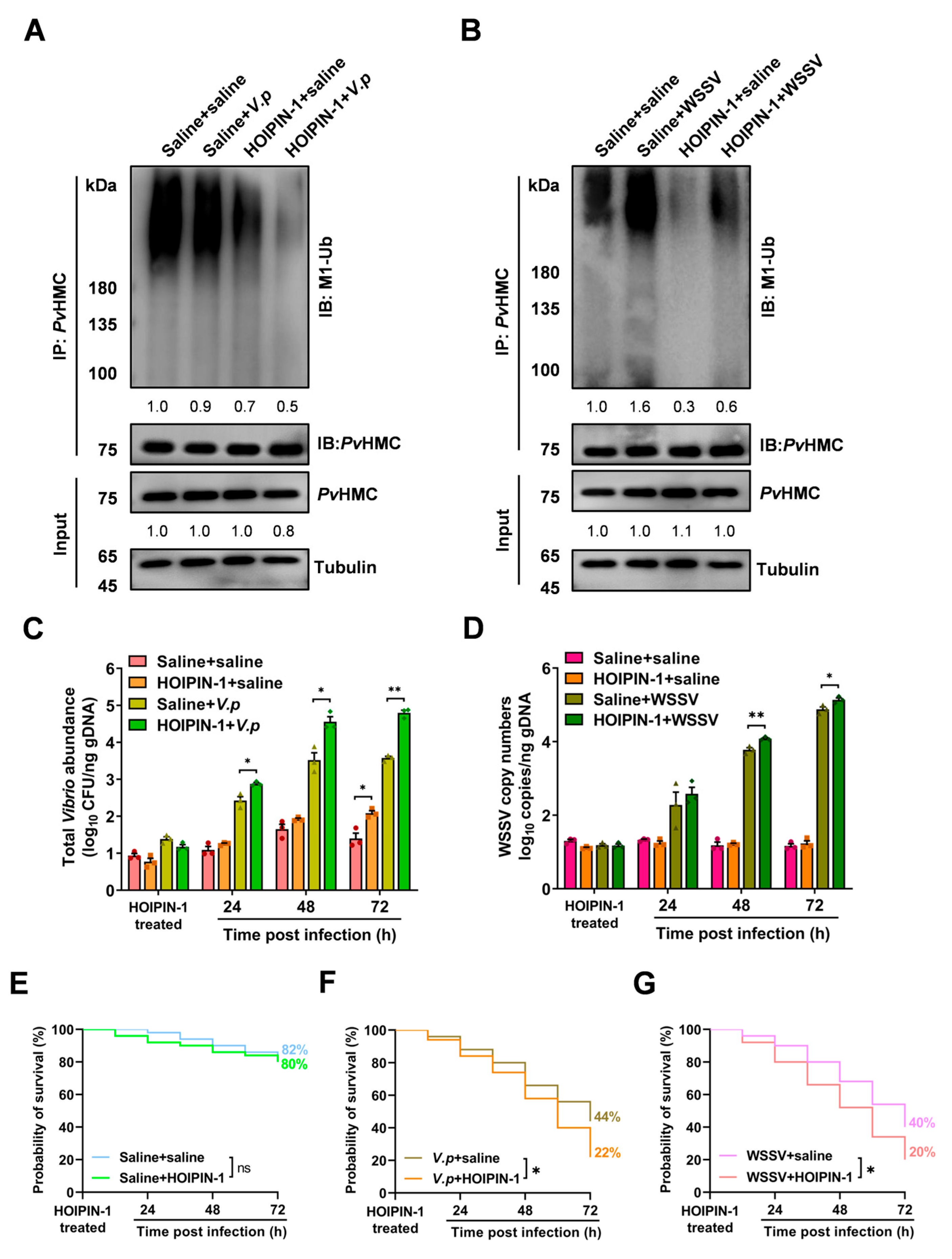
2.4. HOIPIN-1 Suppresses PvHMC Linear Ubiquitination In Vivo
2.5. Inhibition of PvHMC Linear Ubiquitination Enhances Pathogen Proliferation
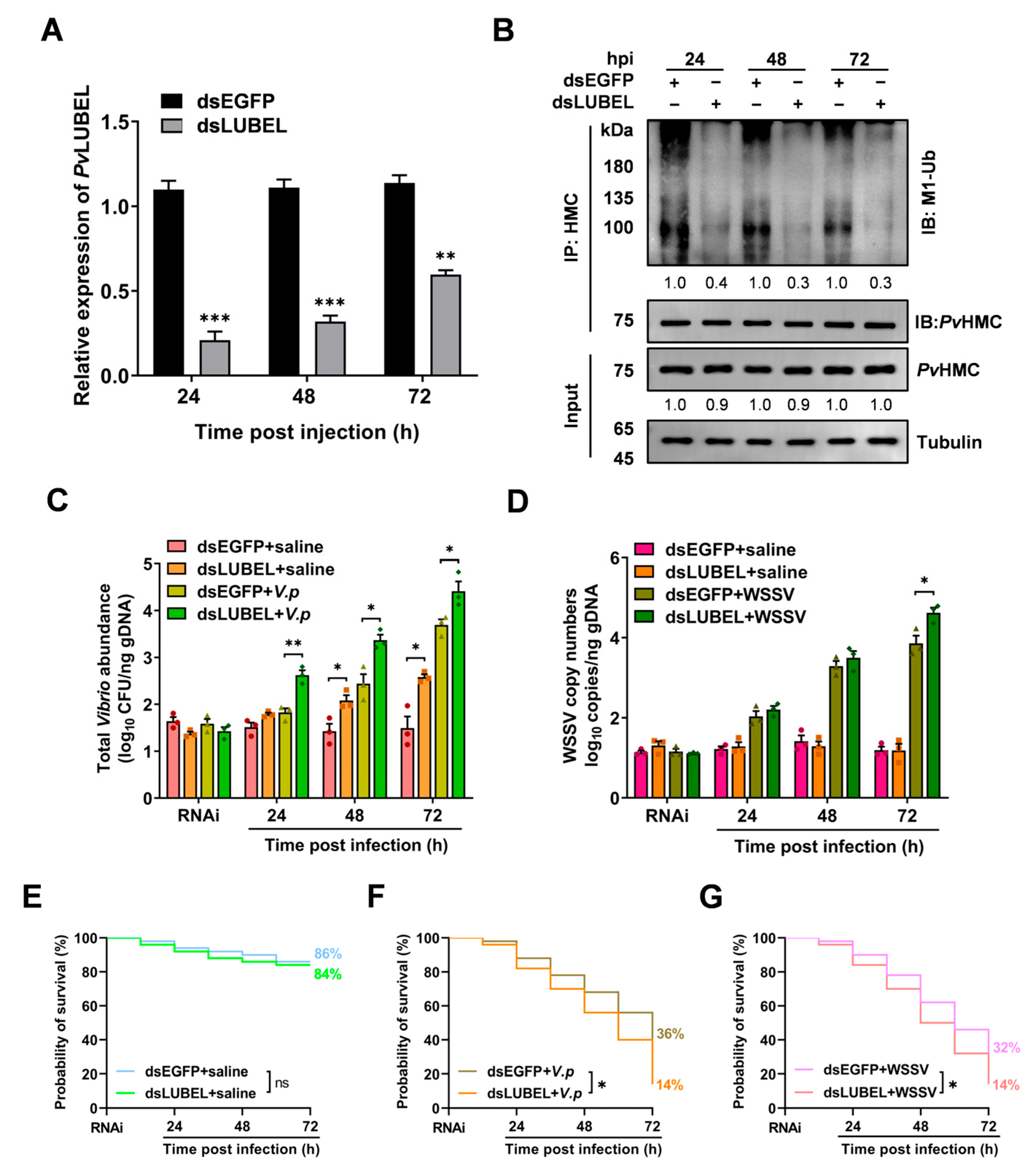
2.6. Impact of PvLUBEL Knockdown on Pathogen Proliferation and Shrimp Survival
3. Discussion
4. Materials and Methods
4.1. Penaeid Shrimp Sample Collection and Preparation
4.2. Protein Extraction and Immunoprecipitation
4.3. RNA Interference and Pathogen Challenge
4.4. Molecular Docking Simulation and Inhibitor Treatment
4.5. Total RNA Extraction, cDNA Synthesis, and qRT-PCR
4.6. Western Blotting Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swatek, K.N.; Komander, D. Ubiquitin modifications. Cell Res. 2016, 26, 399–422. [Google Scholar] [CrossRef] [PubMed]
- Buetow, L.; Huang, D.T. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 2016, 17, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Pao, K.-C.; Wood, N.T.; Knebel, A.; Rafie, K.; Stanley, M.; Mabbitt, P.D.; Sundaramoorthy, R.; Hofmann, K.; van Aalten, D.M.F.; Virdee, S. Activity-based E3 ligase profiling uncovers an E3 ligase with esterification activity. Nature 2018, 556, 381–385. [Google Scholar] [CrossRef]
- Ikeda, F. Protein and nonprotein targets of ubiquitin modification. Am. J. Physiol. Cell Physiol. 2023, 324, C1053–C1060. [Google Scholar] [CrossRef]
- Ciechanover, A. The unravelling of the ubiquitin system. Nat. Rev. Mol. Cell Biol. 2015, 16, 322–324. [Google Scholar] [CrossRef]
- Meyer, H.-J.; Rape, M. Enhanced Protein Degradation by Branched Ubiquitin Chains. Cell 2014, 157, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Fuseya, Y.; Fujita, H.; Kim, M.; Ohtake, F.; Nishide, A.; Sasaki, K.; Saeki, Y.; Tanaka, K.; Takahashi, R.; Iwai, K. The HOIL-1L ligase modulates immune signalling and cell death via monoubiquitination of LUBAC. Nat. Cell Biol. 2020, 22, 663–673. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Gong, Y.; Fu, T.; Hu, S.; Zhou, Z.; Pan, L. Structural Insights into SHARPIN-Mediated Activation of HOIP for the Linear Ubiquitin Chain Assembly. Cell Rep. 2017, 21, 27–36. [Google Scholar] [CrossRef]
- Fuseya, Y.; Kadoba, K.; Liu, X.; Suetsugu, H.; Iwasaki, T.; Ohmura, K.; Sumida, T.; Kochi, Y.; Morinobu, A.; Terao, C.; et al. Attenuation of HOIL-1L ligase activity promotes systemic autoimmune disorders by augmenting linear ubiquitin signaling. JCI Insight 2024, 9, e171108. [Google Scholar] [CrossRef]
- Wang, C.; Gu, C.; Lv, Y.; Liu, H.; Wang, Y.; Zuo, Y.; Jiang, G.; Liu, L.; Liu, J. AlphaFold2 assists in providing novel mechanistic insights into the interactions among the LUBAC subunits. Acta Biochim. Biophys. Sin. 2024, 56, 1034–1043. [Google Scholar] [CrossRef]
- Wu, Z.; Berlemann, L.A.; Bader, V.; Sehr, D.A.; Dawin, E.; Covallero, A.; Meschede, J.; Angersbach, L.; Showkat, C.; Michaelis, J.B.; et al. LUBAC assembles a ubiquitin signaling platform at mitochondria for signal amplification and transport of NF-κB to the nucleus. EMBO J. 2022, 41, e112006. [Google Scholar] [CrossRef] [PubMed]
- Klein, T.; Fung, S.Y.; Renner, F.; Blank, M.; Dufour, A.; Kang, S.; Bolger-Munro, M.; Scurll, J.M.; Priatel, J.J.; Schweigler, P.; et al. The paracaspase MALT1 cleaves HOIL1 reducing linear ubiquitination by LUBAC to dampen lymphocyte NF-κB signalling. Nat. Commun. 2015, 6, 8777. [Google Scholar] [CrossRef]
- Belgnaoui, S.M.; Paz, S.; Samuel, S.; Goulet, M.-L.; Sun, Q.; Kikkert, M.; Iwai, K.; Dikic, I.; Hiscott, J.; Lin, R. Linear Ubiquitination of NEMO Negatively Regulates the Interferon Antiviral Response through Disruption of the MAVS-TRAF3 Complex. Cell Host Microbe 2012, 12, 211–222. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Jiang, K.; Wen, Z.; Cao, X.; Wu, S. Linear ubiquitination of LKB1 activates AMPK pathway to inhibit NLRP3 inflammasome response and reduce chondrocyte pyroptosis in osteoarthritis. J. Orthop. Transl. 2023, 39, 1–11. [Google Scholar] [CrossRef]
- Asaoka, T.; Almagro, J.; Ehrhardt, C.; Tsai, I.; Schleiffer, A.; Deszcz, L.; Junttila, S.; Ringrose, L.; Mechtler, K.; Kavirayani, A.; et al. Linear ubiquitination by LUBEL has a role in Drosophila heat stress response. EMBO Rep. 2016, 17, 1624–1640. [Google Scholar] [CrossRef]
- Aalto, A.; Mohan, A.K.; Schwintzer, L.; Kupka, S.; Kietz, C.; Walczak, H.; Broemer, M.; Meinander, A. M1-linked ubiquitination by LUBEL is required for inflammatory responses to oral infection in Drosophila. Cell Death Differ. 2019, 26, 860–876. [Google Scholar] [CrossRef]
- Aalto, A.; Martínez-Chacón, G.; Kietz, C.; Tsyganova, N.; Kreutzer, J.; Kallio, P.; Broemer, M.; Meinander, A. M1-linked ubiquitination facilitates NF-κB activation and survival during sterile inflammation. FEBS J. 2022, 289, 5180–5197. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.; Guan, L.; Hu, Z.; Cheng, Y.; Cai, M.; Zhao, G.; Zang, J. A comprehensive review on hemocyanin from marine products: Structure, functions, its implications for the food industry and beyond. Int. J. Biol. Macromol. 2024, 269, 132041. [Google Scholar] [CrossRef]
- Li, J.; Zhao, M.; Zhang, X.; Zheng, Z.; Yao, D.; Yang, S.; Chen, T.; Zhang, Y.; Aweya, J.J. The evolutionary adaptation of shrimp hemocyanin subtypes and the consequences on their structure and functions. Fish Shellfish Immunol. 2024, 145, 109347. [Google Scholar] [CrossRef]
- Coates, C.J.; Decker, H. Immunological properties of oxygen-transport proteins: Hemoglobin, hemocyanin and hemerythrin. Cell. Mol. Life Sci. 2017, 74, 293–317. [Google Scholar] [CrossRef]
- Sairi, F.; Gomes, V.G.; Dehghani, F.; Valtchev, P. Lipoprotein-induced cell growth and hemocyanin biosynthesis in rhogocytes. Cell Tissue Res. 2022, 388, 359–371. [Google Scholar] [CrossRef]
- Li, Z.S.; Ma, S.; Shan, H.W.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotoxicol. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, F.; Aweya, J.J.; Yao, D.; Zheng, Z.; Huang, H.; Li, S.; Zhang, Y. Trypsin of Litopenaeus vannamei is required for the generation of hemocyanin-derived peptides. Dev. Comp. Immunol. 2018, 79, 95–104. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, J.; Zhang, P.; Zhang, Z.; Aweya, J.J.; Chen, X.; Zhao, Y.; Zhang, Y. Protein Diversity and Immune Specificity of Hemocyanin From Shrimp Litopenaeus vannamei. Front. Immunol. 2021, 12, 772091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, R.; Aweya, J.J.; Wang, F.; Zhong, M.; Zhang, Y. Identification and characterization of glycosylation sites on Litopenaeus vannamei hemocyanin. FEBS Lett. 2019, 593, 820–830. [Google Scholar] [CrossRef]
- Feng, Q.; Aweya, J.J.; Huang, Y.-Q.; Zhang, P.; Wang, F.; Yao, D.-F.; Zheng, Z.-H.; Li, E.-M.; Zhang, Y.-L. Dephosphorylation of T517 on Hemocyanin Is Required for Antibacterial Activity in Penaeus vannamei. J. Immunol. 2023, 210, 1396–1407. [Google Scholar] [CrossRef]
- Nie, J.; Aweya, J.J.; Yu, Z.; Zhou, H.; Wang, F.; Yao, D.; Zheng, Z.; Li, S.; Ma, H.; Zhang, Y. Deacetylation of K481 and K484 on Penaeid Shrimp Hemocyanin Is Critical for Antibacterial Activity. J. Immunol. 2022, 209, 476–487. [Google Scholar] [CrossRef]
- Kumar, M.; Chadha, N.K.; Prakash, S.; Pavan-Kumar, A.; Harikrishna, V.; Gireesh-Babu, P.; Krishna, G. Salinity, stocking density, and their interactive effects on growth performance and physiological parameters of white-leg shrimp, Penaeus vannamei (Boone, 1931), reared in inland ground saline water. Aquac. Int. 2024, 32, 675–690. [Google Scholar] [CrossRef]
- Joffre, O.M.; Poortvliet, P.M.; Klerkx, L. Are shrimp farmers actual gamblers? An analysis of risk perception and risk management behaviors among shrimp farmers in the Mekong Delta. Aquaculture 2018, 495, 528–537. [Google Scholar] [CrossRef]
- Andrade, T.P.D.; Cruz-Flores, R.; Mai, H.N.; Dhar, A.K. Novel infectious myonecrosis virus (IMNV) variant is associated with recent disease outbreaks in Penaeus vannamei shrimp in Brazil. Aquaculture 2022, 554, 738159. [Google Scholar] [CrossRef]
- Phanse, Y.; Puttamreddy, S.; Loy, D.; Ramirez, J.V.; Ross, K.A.; Alvarez-Castro, I.; Mogler, M.; Broderick, S.; Rajan, K.; Narasimhan, B.; et al. RNA Nanovaccine Protects against White Spot Syndrome Virus in Shrimp. Vaccines 2022, 10, 1428. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.N.; Aranguren Caro, L.F.; Cruz-Flores, R.; Dhar, A.K. Development of a Recombinase Polymerase Amplification (RPA) assay for acute hepatopancreatic necrosis disease (AHPND) detection in Pacific white shrimp (Penaeus vannamei). Mol. Cell. Probes 2021, 57, 101710. [Google Scholar] [CrossRef] [PubMed]
- Reyes, G.; Betancourt, I.; Andrade, B.; Panchana, F.; Román, R.; Sorroza, L.; Trujillo, L.E.; Bayot, B. Microbiome of Penaeus vannamei Larvae and Potential Biomarkers Associated with High and Low Survival in Shrimp Hatchery Tanks Affected by Acute Hepatopancreatic Necrosis Disease. Front. Microbiol. 2022, 13, 838640. [Google Scholar] [CrossRef]
- Keusekotten, K.; Elliott, P.R.; Glockner, L.; Fiil, B.K.; Damgaard, R.B.; Kulathu, Y.; Wauer, T.; Hospenthal, M.K.; Gyrd-Hansen, M.; Krappmann, D.; et al. OTULIN Antagonizes LUBAC Signaling by Specifically Hydrolyzing Met1-Linked Polyubiquitin. Cell 2013, 153, 1312–1326. [Google Scholar] [CrossRef]
- Stieglitz, B.; Rana, R.R.; Koliopoulos, M.G.; Morris-Davies, A.C.; Schaeffer, V.; Christodoulou, E.; Howell, S.; Brown, N.R.; Đikić, I.; Rittinger, K. Structural basis for ligase-specific conjugation of linear ubiquitin chains by HOIP. Nature 2013, 503, 422–426. [Google Scholar] [CrossRef]
- Katsuya, K.; Hori, Y.; Oikawa, D.; Yamamoto, T.; Umetani, K.; Urashima, T.; Kinoshita, T.; Ayukawa, K.; Tokunaga, F.; Tamaru, M. High-Throughput Screening for Linear Ubiquitin Chain Assembly Complex (LUBAC) Selective Inhibitors Using Homogenous Time-Resolved Fluorescence (HTRF)-Based Assay System. SLAS Discov. 2018, 23, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, K.; Oikawa, D.; Iio, K.; Obika, S.; Hori, Y.; Urashima, T.; Ayukawa, K.; Tokunaga, F. Small-molecule inhibitors of linear ubiquitin chain assembly complex (LUBAC), HOIPINs, suppress NF-κB signaling. Biochem. Biophys. Res. Commun. 2019, 509, 700–706. [Google Scholar] [CrossRef]
- Yang, P.; Aweya, J.J.; Yao, D.; Wang, F.; Lun, J.; Hong, Y.; Sun, K.; Zhang, Y. The krüppel-like factor of Penaeus vannamei negatively regulates transcription of the small subunit hemocyanin gene as part of shrimp immune response. Fish Shellfish Immunol. 2020, 100, 397–406. [Google Scholar] [CrossRef]
- Zhao, M.; Aweya, J.J.; Feng, Q.; Zheng, Z.; Yao, D.; Zhao, Y.; Chen, X.; Zhang, Y. Ammonia stress affects the structure and function of hemocyanin in Penaeus vannamei. Ecotoxicol. Environ. Saf. 2022, 241, 113827. [Google Scholar] [CrossRef]
- Wang, Z.; Aweya, J.J.; Yao, D.; Zheng, Z.; Wang, C.; Zhao, Y.; Li, S.; Zhang, Y. Taurine metabolism is modulated in Vibrio-infected Penaeus vannamei to shape shrimp antibacterial response and survival. Microbiome 2022, 10, 213. [Google Scholar] [CrossRef]
- Rubio, T.; Oyanedel, D.; Labreuche, Y.; Toulza, E.; Luo, X.; Bruto, M.; Chaparro, C.; Torres, M.; de Lorgeril, J.; Haffner, P.; et al. Species-specific mechanisms of cytotoxicity toward immune cells determine the successful outcome of Vibrio infections. Proc. Natl. Acad. Sci. USA 2019, 116, 14238–14247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Aweya, J.J.; Yao, D.; Zheng, Z.; Tran, N.T.; Li, S.; Zhang, Y. Ubiquitination as an Important Host-Immune Response Strategy in Penaeid Shrimp: Inferences From Other Species. Front. Immunol. 2021, 12, 697397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Zheng, Z.; Wang, C.; Yao, D.; Lin, Z.; Zhao, Y.; Chen, X.; Li, S.; Aweya, J.J.; Zhang, Y. Penaeid shrimp counteract high ammonia stress by generating and using functional peptides from hemocyanin, such as HMCs27. Sci. Total Environ. 2023, 905, 167073. [Google Scholar] [CrossRef]
- Chen, D.-D.; Jiang, J.-Y.; Lu, L.-F.; Zhang, C.; Zhou, X.-Y.; Li, Z.-C.; Zhou, Y.; Li, S. Zebrafish Uba1 Degrades IRF3 through K48-Linked Ubiquitination to Inhibit IFN Production. J. Immunol. 2021, 207, 512–522. [Google Scholar] [CrossRef]
- Samant, R.S.; Livingston, C.M.; Sontag, E.M.; Frydman, J. Distinct proteostasis circuits cooperate in nuclear and cytoplasmic protein quality control. Nature 2018, 563, 407–411. [Google Scholar] [CrossRef]
- Sheng, X.; Xia, Z.; Yang, H.; Hu, R. The ubiquitin codes in cellular stress responses. Protein Cell 2023, 15, 157–190. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, L.; Ye, C.; Zha, Z.; Li, C.; Feng, C.; Zhang, Y.; Jin, Q.; Pan, J. Destruction of self-derived PAMP via T3SS2 effector VopY to subvert PAMP-triggered immunity mediates Vibrio parahaemolyticus pathogenicity. Cell Rep. 2023, 42, 113261. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Liang, H.; Li, J.; Deng, T.; Bakky, M.A.H.; Zhang, M.; Li, S. Cellular responses in crustaceans under white spot syndrome virus infection. Fish Shellfish Immunol. 2023, 140, 108984. [Google Scholar] [CrossRef]
- Shariq, M.; Quadir, N.; Alam, A.; Zarin, S.; Sheikh, J.A.; Sharma, N.; Samal, J.; Ahmad, U.; Kumari, I.; Hasnain, S.E.; et al. The exploitation of host autophagy and ubiquitin machinery by Mycobacterium tuberculosis in shaping immune responses and host defense during infection. Autophagy 2022, 19, 3–23. [Google Scholar] [CrossRef]
- Noad, J.; von der Malsburg, A.; Pathe, C.; Michel, M.A.; Komander, D.; Randow, F. LUBAC-synthesized linear ubiquitin chains restrict cytosol-invading bacteria by activating autophagy and NF-κB. Nat. Microbiol. 2017, 2, 17063. [Google Scholar] [CrossRef]
- Lechtenberg, B.C.; Rajput, A.; Sanishvili, R.; Dobaczewska, M.K.; Ware, C.F.; Mace, P.D.; Riedl, S.J. Structure of a HOIP/E2~ubiquitin complex reveals RBR E3 ligase mechanism and regulation. Nature 2016, 529, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Mengal, K.; Kor, G.; Siino, V.; Buřič, M.; Kozák, P.; Levander, F.; Niksirat, H. Quantification of proteomic profile changes in the hemolymph of crayfish during in vitro coagulation. Dev. Comp. Immunol. 2023, 147, 104760. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Hillyer, J.F. The immune and circulatory systems are functionally integrated across insect evolution. Sci. Adv. 2020, 6, eabb3164. [Google Scholar] [CrossRef]
- Oikawa, D.; Sato, Y.; Ohtake, F.; Komakura, K.; Hanada, K.; Sugawara, K.; Terawaki, S.; Mizukami, Y.; Phuong, H.T.; Iio, K.; et al. Molecular bases for HOIPINs-mediated inhibition of LUBAC and innate immune responses. Commun. Biol. 2020, 3, 163. [Google Scholar] [CrossRef]
- Fennell, L.M.; Diaz, C.G.; Deszcz, L.; Kavirayani, A.; Hoffmann, D.; Yanagitani, K.; Schleiffer, A.; Mechtler, K.; Hagelkruys, A.; Penninger, J.; et al. Site-specific ubiquitination of the E3 ligase HOIP regulates apoptosis and immune signaling. EMBO J. 2020, 39, e103303. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Xiao, T.; Qi, F.; Fan, L.; Zhang, S.; Zhou, J.; He, Y.; Gao, X.; Zeng, H.; et al. Multiomics approach reveals the ubiquitination-specific processes hijacked by SARS-CoV-2. Signal Transduct. Target. Ther. 2022, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Wang, Z.; Wei, M.; Zhao, X.; Aweya, J.J.; Zhong, M.; Li, S.; Zhang, Y. Analysis of Litopenaeus vannamei hemocyanin interacting proteins reveals its role in hemolymph clotting. J. Proteom. 2019, 201, 57–64. [Google Scholar] [CrossRef]
- Varadi, M.; Bertoni, D.; Magana, P.; Paramval, U.; Pidruchna, I.; Radhakrishnan, M.; Tsenkov, M.; Nair, S.; Mirdita, M.; Yeo, J.; et al. AlphaFold Protein Structure Database in 2024: Providing structure coverage for over 214 million protein sequences. Nucleic Acids Res. 2023, 52, D368–D375. [Google Scholar] [CrossRef]
- E3 Ubiquitin-Protein Ligase Lubel [Penaeus vannamei]. Available online: https://www.ncbi.nlm.nih.gov/protein/XP_069991990.1/ (accessed on 1 September 2024).
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2020, 49, D458–D460. [Google Scholar] [CrossRef]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational protein–ligand docking and virtual drug screening with the AutoDock suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; Version 1.8; Schrödinger, LLC: Palo Alto, CA, USA, 2015. [Google Scholar]
- Schroeder, A.B.; Dobson, E.T.A.; Rueden, C.T.; Tomancak, P.; Jug, F.; Eliceiri, K.W. The ImageJ ecosystem: Open-source software for image visualization, processing, and analysis. Protein Sci. 2021, 30, 234–249. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, H.; Zhang, Y.; Lin, Z. Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei. Int. J. Mol. Sci. 2025, 26, 5110. https://doi.org/10.3390/ijms26115110
Zhang X, Zhang H, Zhang Y, Lin Z. Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei. International Journal of Molecular Sciences. 2025; 26(11):5110. https://doi.org/10.3390/ijms26115110
Chicago/Turabian StyleZhang, Xiaojun, Hanfeng Zhang, Yueling Zhang, and Zhongyang Lin. 2025. "Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei" International Journal of Molecular Sciences 26, no. 11: 5110. https://doi.org/10.3390/ijms26115110
APA StyleZhang, X., Zhang, H., Zhang, Y., & Lin, Z. (2025). Linear Ubiquitination of Hemocyanin Mediated by LUBEL Regulates Innate Immunity in Penaeus vannamei. International Journal of Molecular Sciences, 26(11), 5110. https://doi.org/10.3390/ijms26115110




