Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction
Abstract
1. Introduction
2. Age-Related Changes in Mitochondrial Function
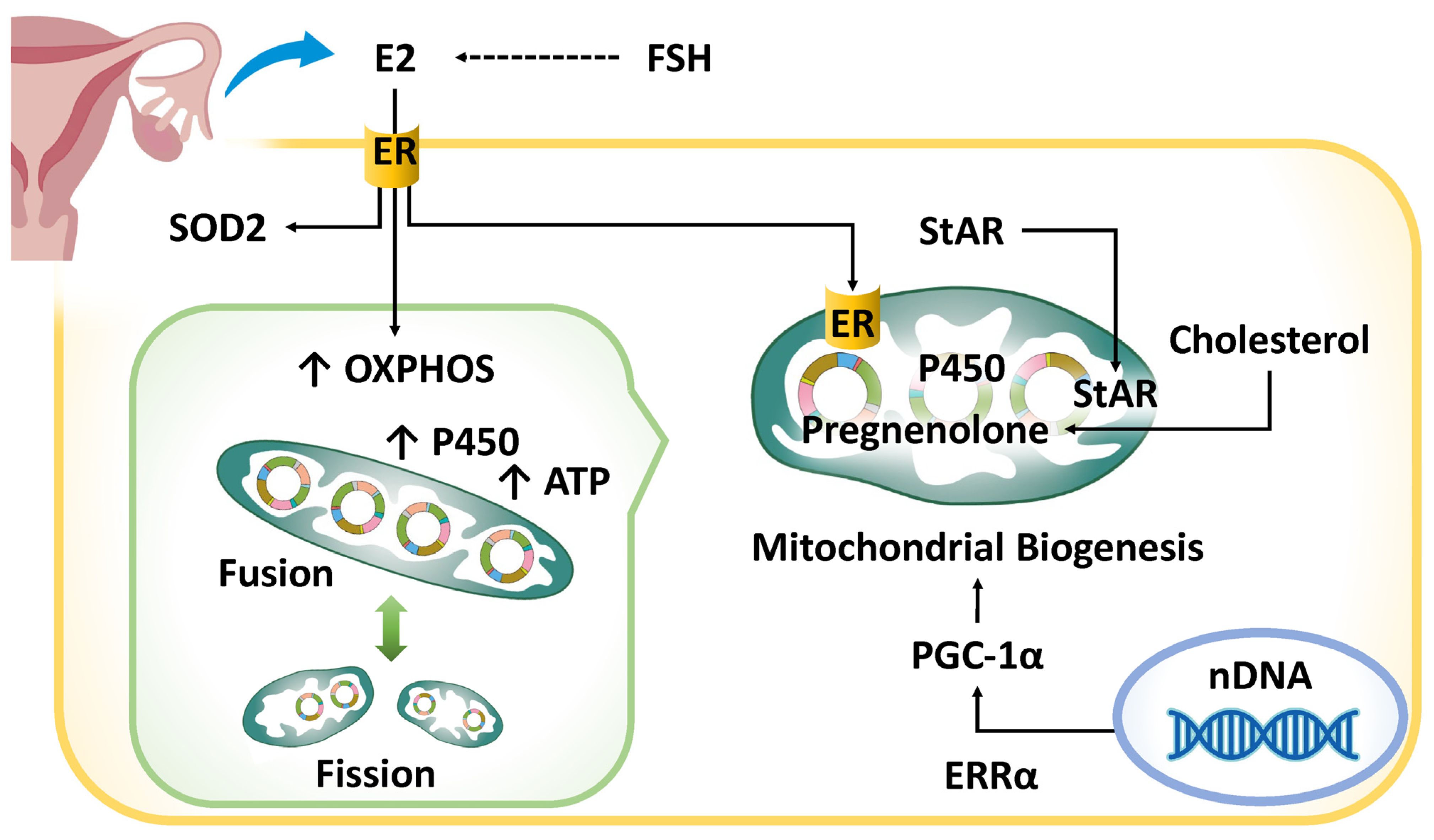
2.1. Mitochondrial Structure and Function
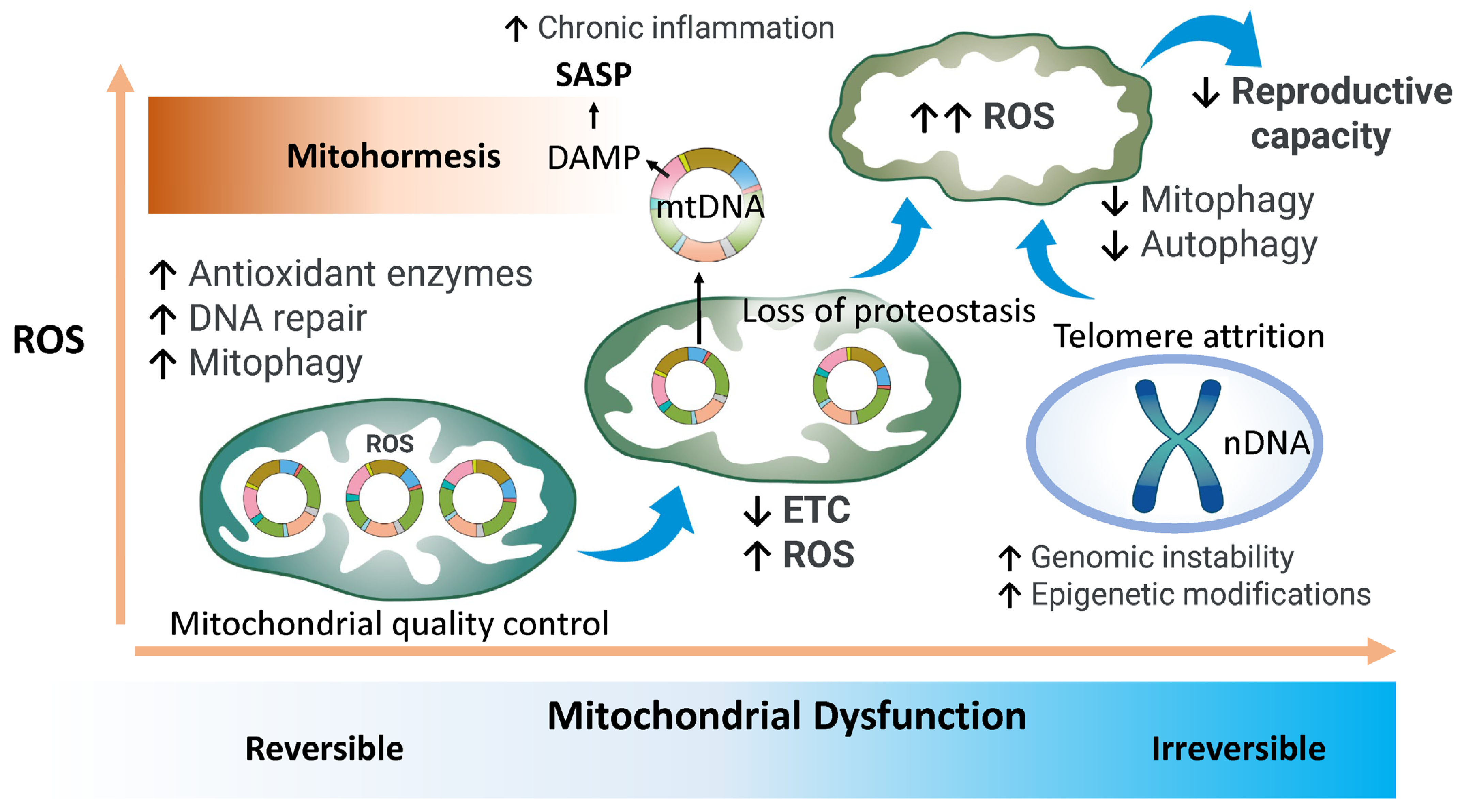
2.2. Mitochondrial Quality Control
2.3. Autophagy and Mitophagy
2.3.1. Autophagy
2.3.2. Mitophagy
3. Age-Related Regulation of Endometrial Receptivity
4. Characteristics of Endometrial Aging
4.1. Hormonal Abnormalities
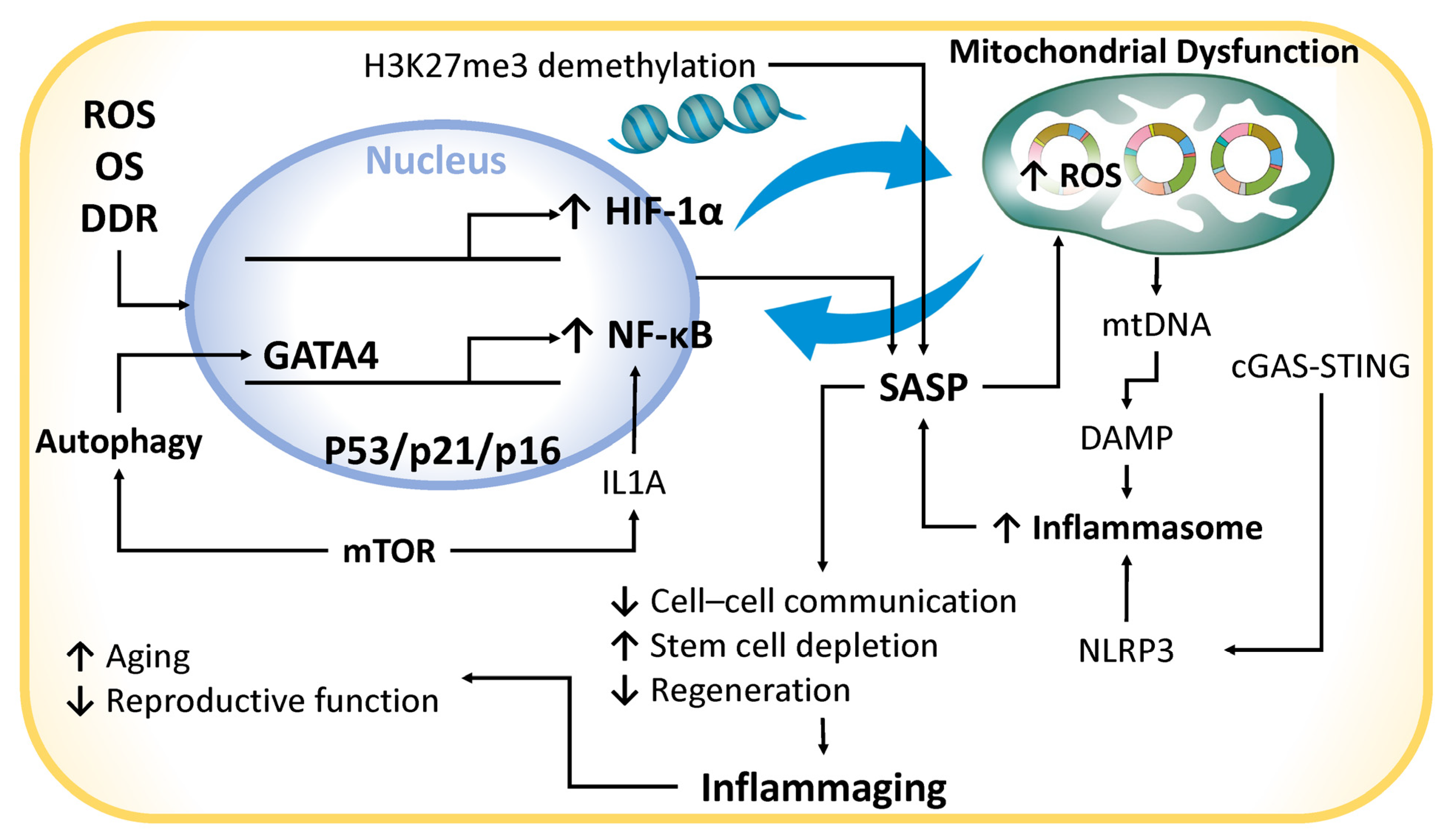
4.2. Chronic Inflammation
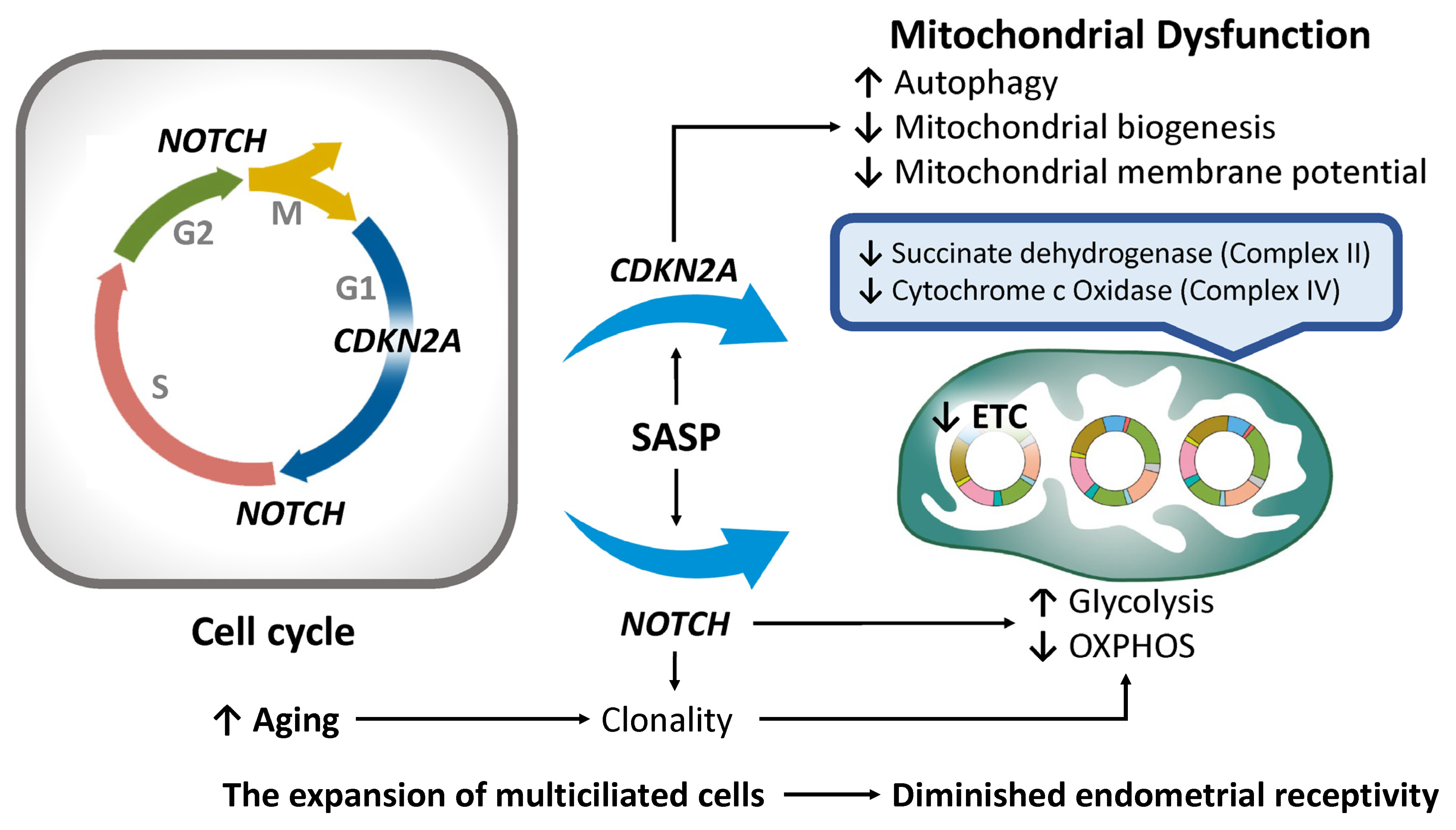
4.3. Cell Cycle Arrest
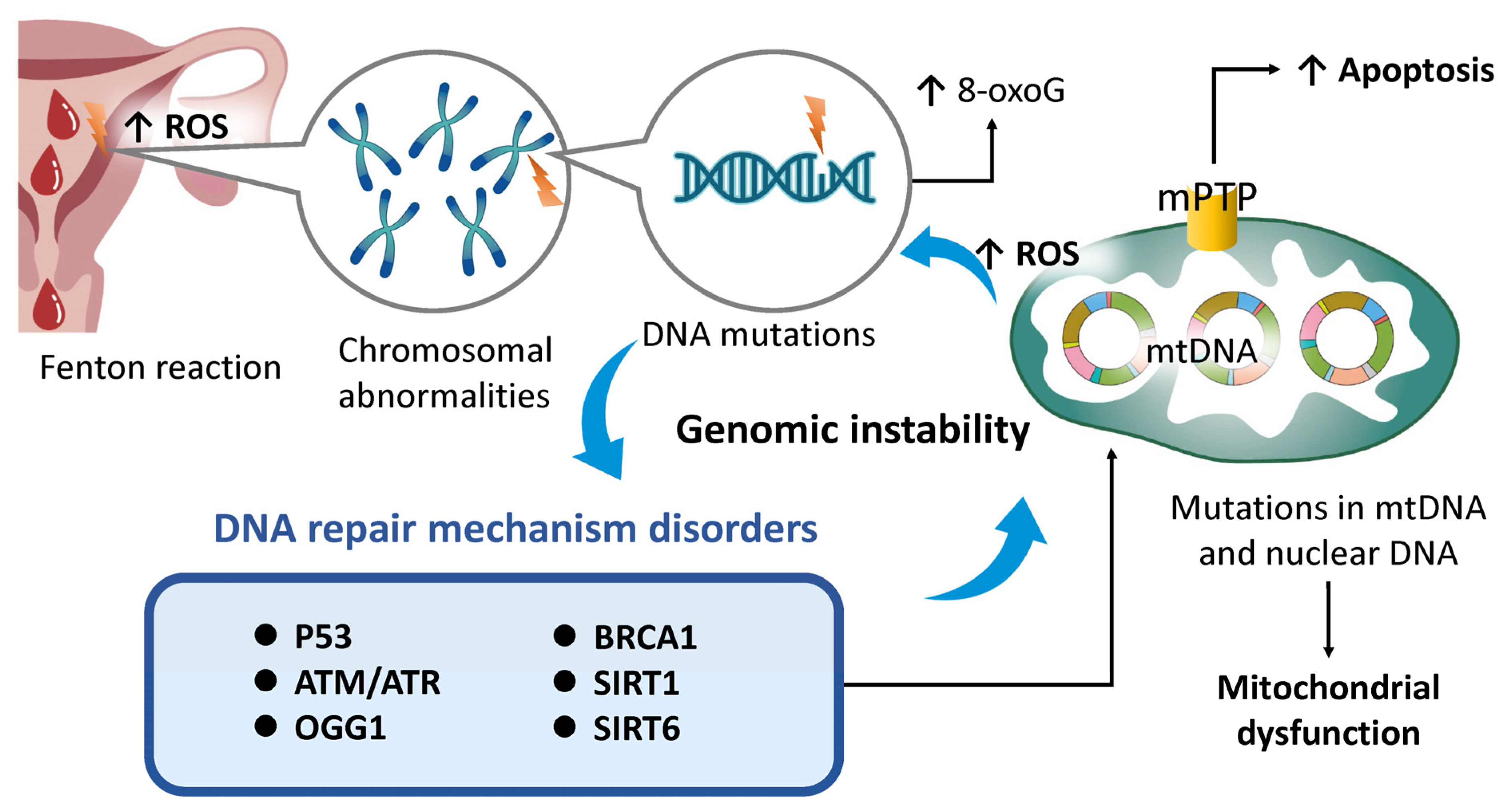
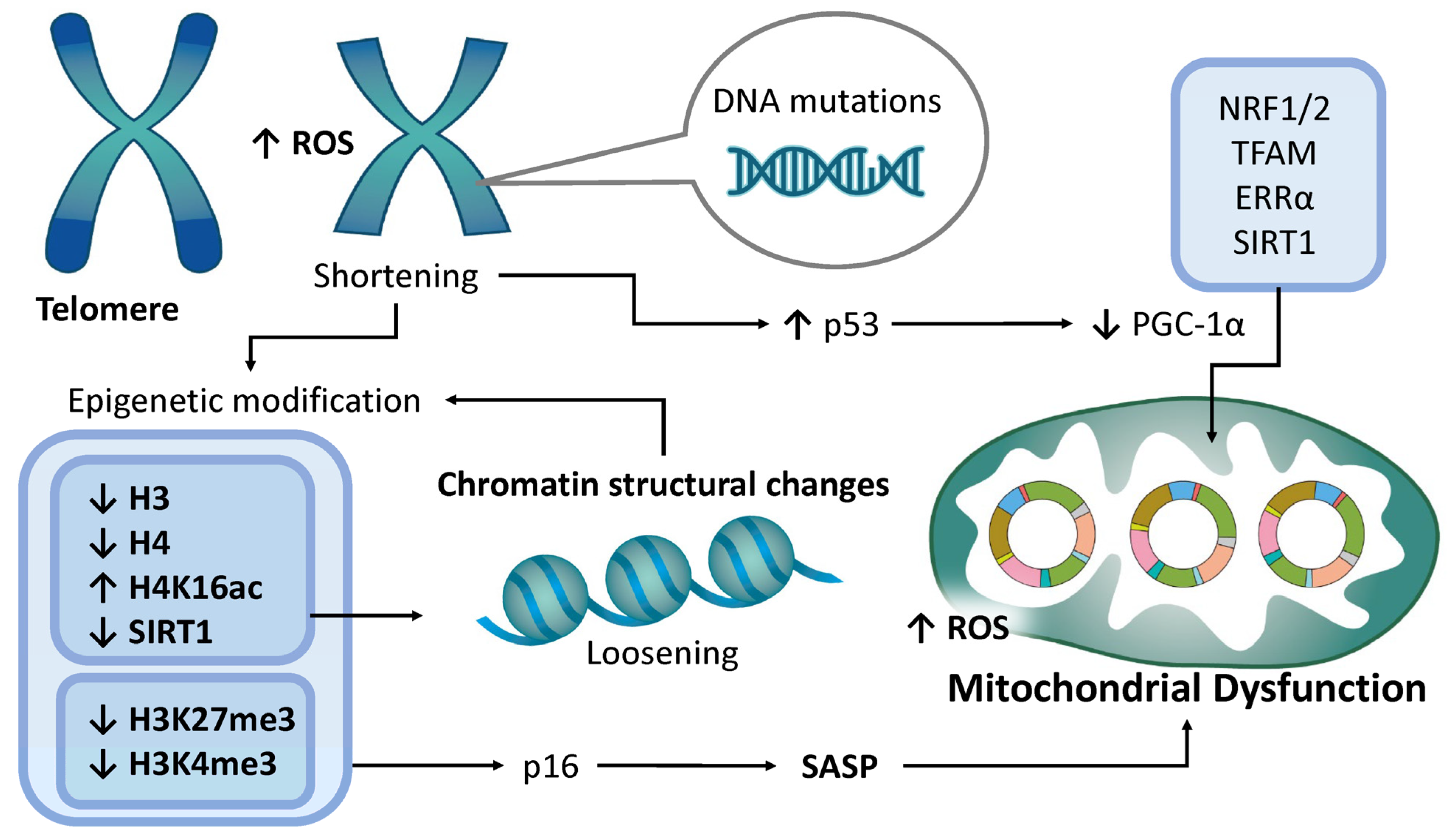
4.4. Genomic Instability
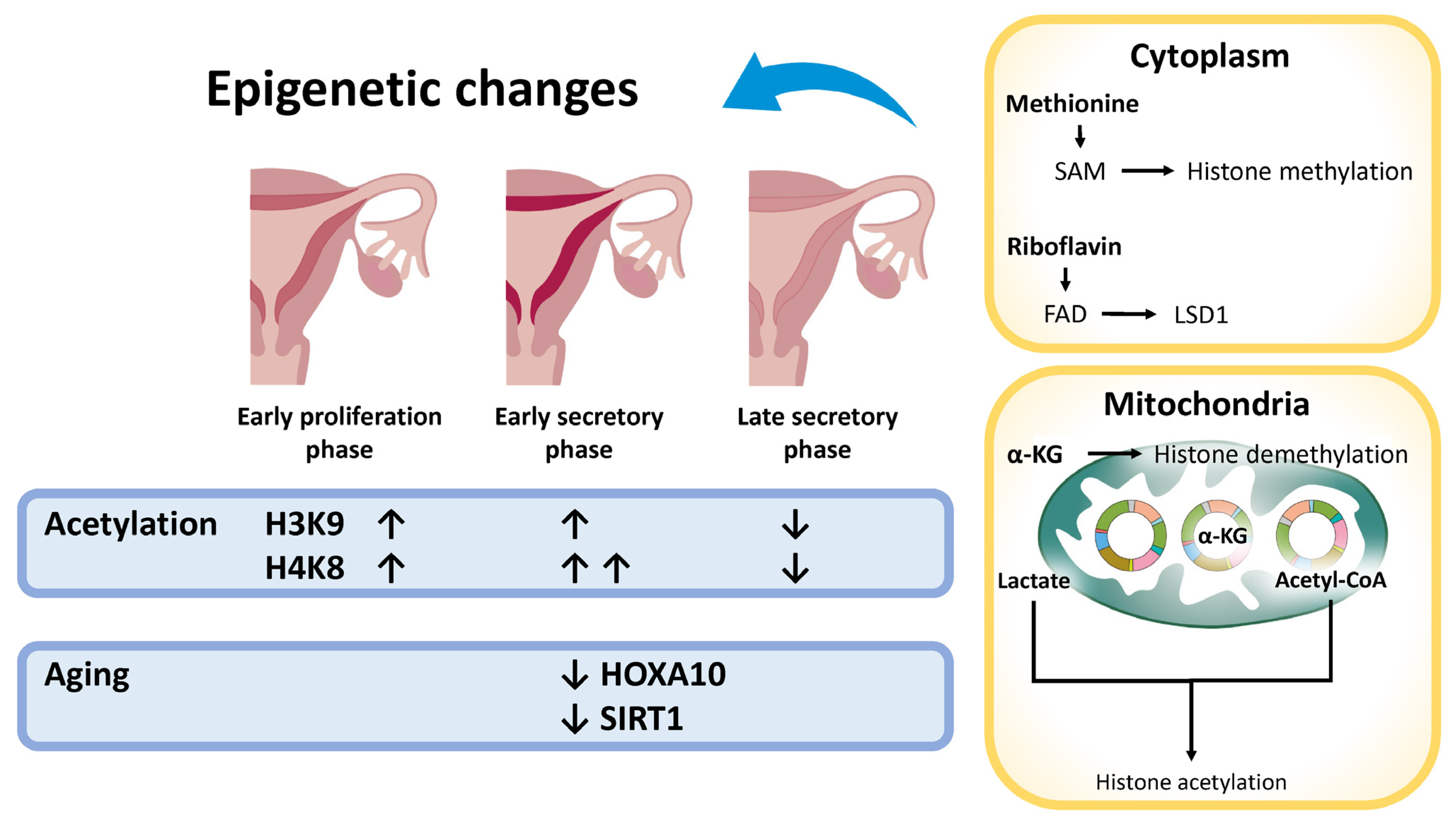
4.5. Epigenetic Modifications
4.6. Telomere Attrition
4.7. MicroRNA Changes
4.8. Loss of Protein Homeostasis
4.9. Stem Cell Depletion
4.10. Altered Intercellular Communication
4.11. Immune Regulation
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, M.; Rindfuss, R.R.; McDonald, P.; Velde, E.T.; ESHRE Reproduction and Society Task Force. Why do people postpone parenthood? Reasons and social policy incentives. Hum. Reprod. Updat. 2011, 17, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H. When is the right time to stop autologous in vitro fertilization treatment in poor responders? Fertil. Steril. 2022, 117, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Osaikhuwuomwan, J.A.; Aziken, M.E. Pregnancy in Older Women: Analysis of Outcomes in Pregnancies from Do-nor oocyte In-vitro Fertilization. J. Hum. Reprod. Sci. 2021, 14, 300–306. [Google Scholar] [CrossRef]
- Chen, T.-S.; Kuo, P.-L.; Yu, T.; Wu, M.-H. IVF and obstetric outcomes among women of advanced maternal age (≥45 years) using donor eggs. Reprod. Biomed. Online 2024, 49, 104291. [Google Scholar] [CrossRef]
- Pantos, K.; Meimeti-Damianaki, T.; Vaxevanoglou, T.; Kapetanakis, E. Oocyte donation in menopausal women aged over 40 years. Hum. Reprod. 1993, 8, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, A.; García, D.; Rodríguez, A.; Vassena, R.; Figueras, F.; Vernaeve, V. Is oocyte donation a risk factor for preeclampsia? A systematic review and meta-analysis. J. Assist. Reprod. Genet. 2016, 33, 855–863. [Google Scholar] [CrossRef]
- Schwarze, J.E.; Borda, P.; Vásquez, P.; Ortega, C.; Villa, S.; Crosby, J.A.; Pommer, R. Is the risk of preeclampsia higher in donor oocyte pregnancies? A systematic review and meta-analysis. JBRA Assist. Reprod. 2018, 22, 15–19. [Google Scholar] [CrossRef]
- Sanders, K.D.; Silvestri, G.; Gordon, T.; Griffin, D.K. Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK Human Fertilisation and Embryology Authority data collection 2016–2018. J. Assist. Reprod. Genet. 2021, 38, 3277–3285. [Google Scholar] [CrossRef]
- Vitagliano, A.; Paffoni, A.; Viganò, P. Does maternal age affect assisted reproduction technology success rates after euploid embryo transfer? A systematic review and meta-analysis. Fertil. Steril. 2023, 120, 251–265. [Google Scholar] [CrossRef]
- Pethő, B.; Váncsa, S.; Váradi, A.; Agócs, G.; Mátrai, Á.; Zászkaliczky-Iker, F.; Balogh, Z.; Bánhidy, F.; Hegyi, P.; Ács, N. Very young and advanced maternal age strongly elevates the occurrence of nonchromosomal congenital anomalies: A systematic review and meta-analysis of population-based studies. Am. J. Obstet. Gynecol. 2024, 231, 490–500.e73. [Google Scholar] [CrossRef] [PubMed]
- Sugai, S.; Nishijima, K.; Haino, K.; Yoshihara, K. Pregnancy outcomes at maternal age over 45 years: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2023, 5, 100885. [Google Scholar] [CrossRef] [PubMed]
- Machado-Gédéon, A.; Badeghiesh, A.; Baghlaf, H.; Dahan, M.H. Adverse pregnancy, delivery and neonatal outcomes across different advanced maternal ages: A population-based retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. X 2023, 17, 100180. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Katsukura, Y.; Henmi, Y.; Ozawa, R.; Shimazaki, S.; Kurosawa, A.; Torii, Y.; Takahashi, H.; Iwata, H.; Kuwayama, T.; et al. Advanced maternal age induces fetal growth restriction through decreased placental inflammatory cytokine expression and immune cell accumulation in mice. J. Reprod. Dev. 2021, 67, 257–264. [Google Scholar] [CrossRef]
- Lean, S.C.; Heazell, A.E.P.; Dilworth, M.R.; Mills, T.A.; Jones, R.L. Placental Dysfunction Underlies Increased Risk of Fetal Growth Restriction and Stillbirth in Advanced Maternal Age Women. Sci. Rep. 2017, 7, 9677. [Google Scholar] [CrossRef]
- Napso, T.; Hung, Y.-P.; Davidge, S.T.; Care, A.S.; Sferruzzi-Perri, A.N. Advanced maternal age compromises fetal growth and induces sex-specific changes in placental phenotype in rats. Sci. Rep. 2019, 9, 16916. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Wu, C.; Chen, D.; Stout, M.B.; Wu, M.; Wang, S. Hallmarks of ovarian aging. Trends Endocrinol. Metab. 2025, 36, 418–439. [Google Scholar] [CrossRef]
- Tinelli, A.; Andjić, M.; Morciano, A.; Pecorella, G.; Malvasi, A.; D’amato, A.; Sparić, R. Uterine Aging and Reproduction: Dealing with a Puzzle Biologic Topic. Int. J. Mol. Sci. 2023, 25, 322. [Google Scholar] [CrossRef]
- Kobayashi, H.; Imanaka, S. Mitochondrial DNA Damage and Its Repair Mechanisms in Aging Oocytes. Int. J. Mol. Sci. 2024, 25, 13144. [Google Scholar] [CrossRef]
- Ju, W.; Zhao, Y.; Yu, Y.; Zhao, S.; Xiang, S.; Lian, F. Mechanisms of mitochondrial dysfunction in ovarian aging and potential interventions. Front. Endocrinol. 2024, 15, 1361289. [Google Scholar] [CrossRef] [PubMed]
- Park, S.U.; Walsh, L.; Berkowitz, K.M. Mechanisms of ovarian aging. Reproduction 2021, 162, R19–R33. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Matsubara, S.; Yoshimoto, C.; Shigetomi, H.; Imanaka, S. The role of mitochondrial dynamics in the pathophysiology of endometriosis. J. Obstet. Gynaecol. Res. 2023, 49, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Marino, Y.; Inferrera, F.; Genovese, T.; Cuzzocrea, S.; Fusco, R.; Di Paola, R. Mitochondrial dynamics: Molecular mechanism and implications in endometriosis. Biochimie 2025, 231, 163–175. [Google Scholar] [CrossRef]
- Siemers, K.M.; Klein, A.K.; Baack, M.L. Mitochondrial Dysfunction in PCOS: Insights into Reproductive Organ Pathophysiology. Int. J. Mol. Sci. 2023, 24, 13123. [Google Scholar] [CrossRef]
- Kobayashi, H.; Matsubara, S.; Yoshimoto, C.; Shigetomi, H.; Imanaka, S. A Comprehensive Review of the Contribution of Mitochondrial DNA Mutations and Dysfunction in Polycystic Ovary Syndrome, Supported by Secondary Database Analysis. Int. J. Mol. Sci. 2025, 26, 1172. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Zhang, L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants 2021, 10, 405. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. An integral role of mitochondrial function in the pathophysiology of preeclampsia. Mol. Biol. Rep. 2024, 51, 330. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Ergun, Y.; Basar, M. Mitochondrial Dysfunction, Mitophagy and Their Correlation with Perinatal Complications: Preeclampsia and Low Birth Weight. Biomedicines 2022, 10, 2539. [Google Scholar] [CrossRef]
- Say, R.E.; Whittaker, R.G.; Turnbull, H.E.; McFarland, R.; Taylor, R.W.; Turnbull, D.M. Mitochondrial disease in pregnancy: A systematic review. Obstet. Med. 2011, 4, 90–94. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Sobenin, I.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. BioMed. Res. Int. 2014, 2014, 238463. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial dysfunction in aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S. The Mitochondrial Basis of Aging and Age-Related Disorders. Genes 2017, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Yavorska, T.; Esfandiari, N.; Jurisicova, A.; Casper, R.F. The contribution of mitochondrial function to reproductive aging. J. Assist. Reprod. Genet. 2011, 28, 773–783. [Google Scholar] [CrossRef]
- Babayev, E.; Wang, T.; Szigeti-Buck, K.; Lowther, K.; Taylor, H.S.; Horvath, T.; Seli, E. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas 2016, 93, 121–130. [Google Scholar] [CrossRef]
- Song, J.; Xiao, L.; Zhang, Z.; Wang, Y.; Kouis, P.; Rasmussen, L.J.; Dai, F. Effects of reactive oxygen species and mitochondrial dysfunction on reproductive aging. Front. Cell Dev. Biol. 2024, 12, 1347286. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, D.; Zhao, S.; Zhang, H.; Sun, Y.; Yan, L. A Preliminary Study on the Correlation Between Age and Endometrial Receptivity. Pharmacogenomics Pers. Med. 2023, 16, 425–432. [Google Scholar] [CrossRef]
- Anderson, S.; Bankier, A.T.; Barrell, B.G.; De Bruijn, M.H.L.; Coulson, A.R.; Drouin, J.; Eperon, I.C.; Nierlich, D.P.; Roe, B.A.; Sanger, F.; et al. Sequence and organization of the human mitochondrial genome. Nature 1981, 290, 457–465. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Casanova, A.; Wevers, A.; Navarro-Ledesma, S.; Pruimboom, L. Mitochondria: It is all about energy. Front. Physiol. 2023, 14, 1114231. [Google Scholar] [CrossRef]
- Yoshikawa, T.; You, F. Oxidative Stress and Bio-Regulation. Int. J. Mol. Sci. 2024, 25, 3360. [Google Scholar] [CrossRef]
- Choksi, K.B.; Papaconstantinou, J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free. Radic. Biol. Med. 2008, 44, 1795–1805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Trifunovic, A.; Hansson, A.; Wredenberg, A.; Rovio, A.T.; Dufour, E.; Khvorostov, I.; Spelbrink, J.N.; Wibom, R.; Jacobs, H.T.; Larsson, N.-G. Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Proc. Natl. Acad. Sci. USA 2005, 102, 17993–17998. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Zhang, J.; Hancock, S.; Derbeneva, O.; Golhar, R.; Golik, P.; O’Hearn, S.; Levy, S.; Potluri, P.; Lvova, M.; et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc. Natl. Acad. Sci. USA 2014, 111, E4033–E4042. [Google Scholar] [CrossRef]
- Long, S.; Zheng, Y.; Deng, X.; Guo, J.; Xu, Z.; Scharffetter-Kochanek, K.; Dou, Y.; Jiang, M. Maintaining mitochondrial DNA copy number mitigates ROS-induced oocyte decline and female reproductive aging. Commun. Biol. 2024, 7, 1229. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Contreras, M.; Sweetwyne, M.T.; Tsantilas, K.A.; Whitson, J.A.; Campbell, M.D.; Kohrn, B.F.; Kim, H.J.; Hipp, M.J.; Fredrickson, J.; Nguyen, M.M.; et al. The multi-tissue landscape of somatic mtDNA mutations indicates tissue-specific accumulation and removal in aging. eLife 2023, 12, e83395. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef]
- Harman, D. Aging: A Theory Based on Free Radical and Radiation Chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Cozzolino, M.; Marin, D.; Sisti, G. New Frontiers in IVF: mtDNA and autologous germline mitochondrial energy transfer. Reprod. Biol. Endocrinol. 2019, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Shadel, G.S. Live longer on MARS: A yeast paradigm of mitochondrial adaptive ROS signaling in aging. Microb. Cell 2014, 1, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Scialò, F.; Sriram, A.; Fernández-Ayala, D.J.M.; Gubina, N.; Lõhmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enríquez, J.A.; et al. Mitochondrial ROS Produced via Reverse Electron Transport Extend Animal Lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef]
- Da, W.; Chen, Q.; Shen, B. The current insights of mitochondrial hormesis in the occurrence and treatment of bone and cartilage degeneration. Biol. Res. 2024, 57, 37. [Google Scholar] [CrossRef] [PubMed]
- Guachalla, L.M.; Ju, Z.; Koziel, R.; von Figura, G.; Song, Z.; Fusser, M.; Epe, B.; Jansen-Dűrr, P.; Rudolph, K.L. Sod2 haploinsufficiency does not accelerate aging of telomere dysfunctional mice. Aging 2009, 1, 303–315. [Google Scholar] [CrossRef]
- da Cunha, F.M.; Torelli, N.Q.; Kowaltowski, A.J. Mitochondrial Retrograde Signaling: Triggers, Pathways, and Outcomes. Oxidative Med. Cell. Longev. 2015, 2015, 482582. [Google Scholar] [CrossRef]
- Walker, B.R.; Moraes, C.T. Nuclear-Mitochondrial Interactions. Biomolecules 2022, 12, 427. [Google Scholar] [CrossRef]
- Saki, M.; Prakash, A. DNA damage related crosstalk between the nucleus and mitochondria. Free. Radic. Biol. Med. 2017, 107, 216–227. [Google Scholar] [CrossRef]
- Yapa, N.M.; Lisnyak, V.; Reljic, B.; Ryan, M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021, 595, 1184–1204. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Um, J.; Yoon, J.; Lee, D.; Lee, Y.J.; Kim, D.H.; Park, J.; Yun, J. p53 regulates mitochondrial dynamics by inhibiting Drp1 translocation into mitochondria during cellular senescence. FASEB J. 2020, 34, 2451–2464. [Google Scholar] [CrossRef]
- Lin, J.-R.; Shen, W.-L.; Yan, C.; Gao, P.-J. Downregulation of Dynamin-Related Protein 1 Contributes to Impaired Autophagic Flux and Angiogenic Function in Senescent Endothelial Cells. Arter. Thromb. Vasc. Biol. 2015, 35, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, A.; Shimauchi, T.; Tanaka, T.; Shimoda, K.; Toyama, T.; Kitajima, N.; Ishikawa, T.; Shindo, N.; Numaga-Tomita, T.; Yasuda, S.; et al. Hypoxia-induced interaction of filamin with Drp1 causes mitochondrial hyperfission–associated myocardial senescence. Sci. Signal. 2018, 11, eaat5185. [Google Scholar] [CrossRef] [PubMed]
- Seo, A.Y.; Joseph, A.-M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: Mitochondrial dynamics and more. J. Cell Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Zhang, J. Autophagy and mitophagy in cellular damage control. Redox Biol. 2013, 1, 19–23. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [CrossRef]
- Popli, P.; Sun, A.J.; Kommagani, R. The Multifaceted Role of Autophagy in Endometrium Homeostasis and Disease. Reprod. Sci. 2022, 29, 1054–1067. [Google Scholar] [CrossRef]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Li, J.; Zhang, S.; Zhang, Y.; Dai, Y.; Zhang, Y.; Yang, A.; Hong, F.; Pan, Y. Atg9A-mediated mitophagy is required for decidual differentiation of endometrial stromal cells. Reprod. Biol. 2022, 22, 100707. [Google Scholar] [CrossRef]
- Shen, H.-H.; Zhang, T.; Yang, H.-L.; Lai, Z.-Z.; Zhou, W.-J.; Mei, J.; Shi, J.-W.; Zhu, R.; Xu, F.-Y.; Li, D.-J.; et al. Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis. Theranostics 2021, 11, 3512–3526. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shin, H.; Song, H.; Lim, H.J. Suppression of autophagic activation in the mouse uterus by estrogen and progesterone. J. Endocrinol. 2014, 221, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Rapamycin extends life- and health span because it slows aging. Aging 2013, 5, 592–598. [Google Scholar] [CrossRef]
- Templeman, N.M.; Murphy, C.T. Regulation of reproduction and longevity by nutrient-sensing pathways. J. Cell Biol. 2018, 217, 93–106. [Google Scholar] [CrossRef]
- McCallum, M.L.; Pru, C.A.; Smith, A.R.; Kelp, N.C.; Foretz, M.; Viollet, B.; Du, M.; Pru, J.K. A functional role for AMPK in female fertility and endometrial regeneration. Reproduction 2018, 156, 501–513. [Google Scholar] [CrossRef]
- Cummings, M.J.; Yu, H.; Paudel, S.; Hu, G.; Li, X.; Hemberger, M.; Wang, X. Uterine-specific SIRT1 deficiency confers premature uterine aging and impairs invasion and spacing of blastocyst, and stromal cell decidualization, in mice. Mol. Hum. Reprod. 2022, 28, gaac016. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Yu, Q. The PI3K/Akt signaling pathway exerts effects on the implantation of mouse embryos by regulating the expression of RhoA. Int. J. Mol. Med. 2014, 33, 1089–1096. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, J.-J.; He, J.-L.; Liu, X.-Q.; Chen, X.-M.; Ding, Y.-B.; Tong, C.; Peng, C.; Geng, Y.-Q.; Wang, Y.-X.; et al. Endometrial autophagy is essential for embryo implantation during early pregnancy. J. Mol. Med. 2020, 98, 555–567. [Google Scholar] [CrossRef]
- Park, H.; Cho, M.; Do, Y.; Park, J.-K.; Bae, S.-J.; Joo, J.; Ha, K.-T. Autophagy as a Therapeutic Target of Natural Products Enhancing Embryo Implantation. Pharmaceuticals 2021, 15, 53. [Google Scholar] [CrossRef]
- Ruiz, A.; Rockfield, S.; Taran, N.; Haller, E.; Engelman, R.W.; Flores, I.; Panina-Bordignon, P.; Nanjundan, M. Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis. 2016, 7, e2059. [Google Scholar] [CrossRef] [PubMed]
- Oestreich, A.K.; Chadchan, S.B.; Medvedeva, A.; Lydon, J.P.; Jungheim, E.S.; Moley, K.H.; Kommagani, R. The autophagy protein, FIP200 (RB1CC1) mediates progesterone responses governing uterine receptivity and decidualization. Biol. Reprod. 2020, 102, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Vervier, J.; Squatrito, M.; Nisolle, M.; Henry, L.; Munaut, C. Controversial Roles of Autophagy in Adenomyosis and Its Implications for Fertility Outcomes—A Systematic Review. J. Clin. Med. 2024, 13, 7501. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Furuta, A.; Yamada, K.; Yoshida-Kawaguchi, M.; Yamaki-Ushijima, A.; Yasuda, I.; Ito, M.; Yamashita, S.; Tsuda, S.; Yoneda, S.; et al. The Role of Autophagy in the Female Reproduction System: For Beginners to Experts in This Field. Biology 2023, 12, 373. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Li, D.; Li, M. Role of Endometrial Autophagy in Physiological and Pathophysiological Processes. J. Cancer 2019, 10, 3459–3471. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, J.; Wang, J.; Liu, X. When autophagy meets placenta development and pregnancy complications. Front. Cell Dev. Biol. 2024, 12, 1327167. [Google Scholar] [CrossRef]
- Vasileiou, P.V.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.-G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef]
- Eiyama, A.; Okamoto, K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr. Opin. Cell Biol. 2015, 33, 95–101. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Wang, Z.-J.; Liu, H.-C.; Wang, C.-Y.; Wang, Y.-Q.; Yue, Y.; Zhao, C.; Wang, G.; Wan, J.-P. Targeting mitochondria for ovarian aging: New insights into mechanisms and therapeutic potential. Front. Endocrinol. 2024, 15, 1417007. [Google Scholar] [CrossRef]
- Zhao, Q.; Ye, M.; Yang, W.; Wang, M.; Li, M.; Gu, C.; Zhao, L.; Zhang, Z.; Han, W.; Fan, W.; et al. Effect of Mst1 on Endometriosis Apoptosis and Migration: Role of Drp1-Related Mitochondrial Fission and Parkin-Required Mitophagy. Cell. Physiol. Biochem. 2018, 45, 1172–1190. [Google Scholar] [CrossRef] [PubMed]
- Chemerinski, A.; de Paredes, J.G.; Blackledge, K.; Douglas, N.C.; Morelli, S.S. Mechanisms of endometrial aging: Lessons from natural conceptions and assisted reproductive technology cycles. Front. Physiol. 2024, 15, 1332946. [Google Scholar] [CrossRef] [PubMed]
- Bolumar, D.; Moncayo-Arlandi, J.; Gonzalez-Fernandez, J.; Ochando, A.; Moreno, I.; Monteagudo-Sanchez, A.; Marin, C.; Diez, A.; Fabra, P.; Checa, M.A.; et al. Vertical transmission of maternal DNA through extracellular vesicles associates with altered embryo bioenergetics during the periconception period. eLife 2023, 12, RP88008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zeng, C.; Sun, X.; Zhang, Q.; Wang, Y.; Xia, R.; Mai, Q.; Xue, G.; Huang, H.; Wang, F. Zearalenone modulates the function of goat endometrial cells via the mitochondrial quality control system. FASEB J. 2024, 38, e23701. [Google Scholar] [CrossRef]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An Emerging Role in Aging and Age-Associated Diseases. Front. Cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef]
- Atkins, H.M.; Bharadwaj, M.S.; Cox, A.O.; Furdui, C.M.; Appt, S.E.; Caudell, D.L. Endometrium and endometriosis tissue mitochondrial energy metabolism in a nonhuman primate model. Reprod. Biol. Endocrinol. 2019, 17, 70. [Google Scholar] [CrossRef]
- Guo, J.; Chiang, W. Mitophagy in aging and longevity. IUBMB Life 2022, 74, 296–316. [Google Scholar] [CrossRef]
- Cota, V.; Sohrabi, S.; Kaletsky, R.; Murphy, C.T. Oocyte mitophagy is critical for extended reproductive longevity. PLoS Genet. 2022, 18, e1010400. [Google Scholar] [CrossRef]
- Moshkalova, G.; Karibayeva, I.; Kurmanova, A.; Mamedalieva, N.; Aimbetova, A.; Terlikbayeva, A.; Mamutova, A.; Yerzhan, Z.; Yerkenova, S.; Zheksembay, B. Endometrial thickness and live birth rates after IVF: A systematic review. Acta Biomed. 2023, 94, e2023152. [Google Scholar] [CrossRef]
- Marti-Garcia, D.; Martinez-Martinez, A.; Sanz, F.J.; Devesa-Peiro, A.; Sebastian-Leon, P.; del Aguila, N.; Pellicer, A.; Diaz-Gimeno, P. Age-related uterine changes and its association with poor reproductive outcomes: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2024, 22, hoae048. [Google Scholar] [CrossRef]
- Crawford, B.S.; Davis, J.; Harrigill, K. Uterine artery atherosclerotic disease: Histologic features and clinical correlation. Obstet. Gynecol. 1997, 90, 210–215. [Google Scholar] [CrossRef]
- Loid, M.; Obukhova, D.; Kask, K.; Apostolov, A.; Meltsov, A.; Tserpelis, D.; Wijngaard, A.v.D.; Altmäe, S.; Yahubyan, G.; Baev, V.; et al. Aging promotes accumulation of senescent and multiciliated cells in human endometrial epithelium. Hum. Reprod. Open 2024, 2024, hoae048. [Google Scholar] [CrossRef]
- Makabe, S.; Motta, P.M.; Naguro, T.; Vizza, E.; Perrone, G.; Zichella, L. Microanatomy of the female reproductive organs in postmenopause by scanning electron microscopy. Climacteric 1998, 1, 63–71. [Google Scholar] [CrossRef]
- Devesa-Peiro, A.; Sebastian-Leon, P.; Parraga-Leo, A.; Pellicer, A.; Diaz-Gimeno, P. Breaking the ageing paradigm in endometrium: Endometrial gene expression related to cilia and ageing hallmarks in women over 35 years. Hum. Reprod. 2022, 37, 762–776. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.; Ma, J.; Diao, L.; Chen, J.; Cheng, Y.; Li, L. Endometrial proteomic profile of patients with repeated implantation failure. Front. Endocrinol. 2023, 14, 1144393. [Google Scholar] [CrossRef]
- Yang, S.-H.; Liu, R.; Perez, E.J.; Wen, Y.; Stevens, S.M.; Valencia, T.; Brun-Zinkernagel, A.-M.; Prokai, L.; Will, Y.; Dykens, J.; et al. Mitochondrial localization of estrogen receptor β. Proc. Natl. Acad. Sci. USA 2004, 101, 4130–4135. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, C.; Du, S.; Fan, L.; Zhang, D.; Wang, X.; He, M. Estrogen Regulates Endoplasmic Reticulum Stress–Mediated Apoptosis by ERK-p65 Pathway to Promote Endometrial Angiogenesis. Reprod. Sci. 2021, 28, 1216–1226. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, J.-S. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Torre, M.D.; Pittari, D.; Boletta, A.; Cassina, L.; Sitia, R.; Anelli, T. Mitochondria remodeling during endometrial stromal cell decidualization. Life Sci. Alliance 2024, 7, e202402627. [Google Scholar] [CrossRef]
- Gong, D.; Zhu, H.; Zeng, L.; Hu, R.; Hu, J.; Ding, J. Overexpression of HOXA10 promotes the growth and metastasis of nasopharyngeal carcinoma. Exp. Biol. Med. 2021, 246, 2454–2462. [Google Scholar] [CrossRef]
- Arici, A.; Engin, O.; Attar, E.; Olive, D.L. Modulation of leukemia inhibitory factor gene expression and protein biosynthesis in human endometrium. J. Clin. Endocrinol. Metab. 1995, 80, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Li, C.; Zhang, H.; Li, R.; Li, M. Letrozole promotes the expression of integrin alphavbeta3 and HOXA10 in endometrium of endometriosis. Syst. Biol. Reprod. Med. 2022, 68, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Hautala, L.C.; Pang, P.-C.; Antonopoulos, A.; Pasanen, A.; Lee, C.-L.; Chiu, P.C.N.; Yeung, W.S.B.; Loukovaara, M.; Bützow, R.; Haslam, S.M.; et al. Altered glycosylation of glycodelin in endometrial carcinoma. Mod. Pathol. 2020, 100, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Boicea, A.; Barrett, K.L.; James, C.O.; Bagchi, I.C.; Bagchi, M.K.; Nezhat, C.; Sidell, N.; Taylor, R.N. Reduced connexin 43 in eutopic endometrium and cultured endometrial stromal cells from subjects with endometriosis. Mol. Hum. Reprod. 2014, 20, 260–270. [Google Scholar] [CrossRef]
- Pathare, A.D.S.; Loid, M.; Saare, M.; Gidlöf, S.B.; Esteki, M.Z.; Acharya, G.; Peters, M.; Salumets, A. Endometrial receptivity in women of advanced age: An underrated factor in infertility. Hum. Reprod. Updat. 2023, 29, 773–793. [Google Scholar] [CrossRef]
- Cano, F.; Simón, C.; Remohí, J.; Pellicer, A. Effect of aging on the female reproductive system: Evidence for a role of uterine senescence in the decline in female fecundity. Fertil. Steril. 1995, 64, 584–589. [Google Scholar] [CrossRef]
- Marino, A.A.; Volpes, A.; Sammartano, F.; Modica, M.; Scaglione, P.; Gullo, S.; Quintero, L.; Allegra, A. Recipients’ age, fresh embryo and blastocyst-stage embryo transfer as favorable factors in a transnational oocyte donation program. Minerva Obstet. Gynecol. 2024. [Google Scholar] [CrossRef]
- Yeh, J.S.; Steward, R.G.; Dude, A.M.; Shah, A.A.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline in recipients over age 44: An analysis of 27,959 fresh donor oocyte in vitro fertilization cycles from the Society for Assisted Reproductive Technology. Fertil. Steril. 2014, 101, 1331–1336.e1. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Idda, M.L.; McClusky, W.G.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Rossi, M.; Gorospe, M. Survey of senescent cell markers with age in human tissues. Aging 2020, 12, 4052–4066. [Google Scholar] [CrossRef]
- Kim, J.J.; Fazleabas, A.T. Uterine receptivity and implantation: The regulation and action of insulin-like growth factor binding protein-1 (IGFBP-1), HOXA10 and forkhead transcription factor-1 (FOXO-1) in the baboon endometrium. Reprod. Biol. Endocrinol. 2004, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Gesell, M.S.; Roth, G.S. Decrease in Rat Uterine Estrogen Receptors during Aging: Physio- and Immunochemical Properties. Endocrinology 1981, 109, 1502–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, J.; Xu, G.; Zhang, R.; Zhou, C.; Qian, Y.; Liu, Y.; Chen, L.; Zhu, B.; Ye, X.; et al. Follicle-stimulating hormone promotes age-related endometrial atrophy through cross-talk with transforming growth factor beta signal transduction pathway. Aging Cell 2015, 14, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. StAR Protein and the Regulation of Steroid Hormone Biosynthesis. Annu. Rev. Physiol. 2001, 63, 193–213. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Duarte, A.; Poderoso, C.; Cooke, M.; Soria, G.; Maciel, F.C.; Gottifredi, V.; Podestá, E.J. Mitochondrial Fusion Is Essential for Steroid Biosynthesis. PLoS ONE 2012, 7, e45829. [Google Scholar] [CrossRef]
- Simpkins, J.W.; Yang, S.-H.; Sarkar, S.N.; Pearce, V. Estrogen actions on mitochondria—Physiological and pathological implications. Mol. Cell. Endocrinol. 2008, 290, 51–59. [Google Scholar] [CrossRef]
- Guajardo-Correa, E.; Silva-Agüero, J.F.; Calle, X.; Chiong, M.; Henríquez, M.; García-Rivas, G.; Latorre, M.; Parra, V. Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front. Cell Dev. Biol. 2022, 10, 968373. [Google Scholar] [CrossRef]
- Velarde, M.C. Pleiotropic actions of estrogen: A mitochondrial matter. Physiol. Genom. 2013, 45, 106–109. [Google Scholar] [CrossRef]
- Taylor, R.N.; Berga, S.L.; Zou, E.; Washington, J.; Song, S.; Marzullo, B.J.; Bagchi, I.C.; Bagchi, M.K.; Yu, J. Interleukin-1β induces and accelerates human endometrial stromal cell senescence and impairs decidualization via the c-Jun N-terminal kinase pathway. Cell Death Discov. 2024, 10, 288. [Google Scholar] [CrossRef]
- Ivanov, D.; Drobintseva, A.; Rodichkina, V.; Mironova, E.; Zubareva, T.; Krylova, Y.; Morozkina, S.; Marasco, M.G.P.; Mazzoccoli, G.; Nasyrov, R.; et al. Inflammaging: Expansion of Molecular Phenotype and Role in Age-Associated Female Infertility. Biomedicines 2024, 12, 1987. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Sánchez-López, E.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. Hypoxia-Inducible Factor-1α: The Master Regulator of Endothelial Cell Senescence in Vascular Aging. Cells 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Xu, Q.; Martin, T.D.; Li, M.Z.; DeMaria, M.; Aron, L.; Lu, T.; Yankner, B.A.; Campisi, J.; Elledge, S.J. The DNA damage response induces inflammation and senescence by inhibiting autophagy of GATA4. Science 2015, 349, aaa5612. [Google Scholar] [CrossRef]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061. [Google Scholar] [CrossRef]
- Dasgupta, N.; Arnold, R.; Equey, A.; Gandhi, A.; Adams, P.D. The role of the dynamic epigenetic landscape in senescence: Orchestrating SASP expression. NPJ Aging 2024, 10, 48. [Google Scholar] [CrossRef]
- Martini, H.; Passos, J.F. Cellular senescence: All roads lead to mitochondria. FEBS J. 2023, 290, 1186–1202. [Google Scholar] [CrossRef]
- Gnainsky, Y.; Granot, I.; Aldo, P.; Barash, A.; Or, Y.; Mor, G.; Dekel, N. Biopsy-induced inflammatory conditions improve endometrial receptivity: The mechanism of action. Reproduction 2015, 149, 75–85. [Google Scholar] [CrossRef]
- Tanikawa, N.; Ohtsu, A.; Kawahara-Miki, R.; Kimura, K.; Matsuyama, S.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Age-associated mRNA expression changes in bovine endometrial cells in vitro. Reprod. Biol. Endocrinol. 2017, 15, 63. [Google Scholar] [CrossRef]
- Shirasuna, K.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef]
- Kwong, J.Q.; Henning, M.S.; Starkov, A.A.; Manfredi, G. The mitochondrial respiratory chain is a modulator of apoptosis. J. Cell Biol. 2007, 179, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, X.; Mittra, N.; Bhattacharjee, A.; Wang, H.; Song, S.; Zhao, S.; Liu, F.; Han, X. Microglial cGAS Deletion Preserves Intercellular Communication and Alleviates Amyloid-β-Induced Pathogenesis of Alzheimer’s Disease. Adv. Sci. 2025, 12, e2410910. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shi, W.; Ran, F.; Liu, C.; Luo, H.; Wu, L.; Wu, Y.; Zhang, T.; Yang, Z. The activation of cGAS-STING pathway causes abnormal uterine receptivity in aged mice. Aging Cell 2024, 23, e14303. [Google Scholar] [CrossRef]
- Spel, L.; Zaffalon, L.; Hou, C.; Nganko, N.; Chapuis, C.; Martinon, F. CDC42 regulates PYRIN inflammasome assembly. Cell Rep. 2022, 41, 111636. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, Y.; Cao, Z.; Wang, X.; Cai, X.; Tang, Y.; Zhou, J.; Wu, M.; Zhen, X.; Ding, L.; et al. CDC42 deficiency leads to endometrial stromal cell senescence in recurrent implantation failure. Hum. Reprod. 2024, 39, 2768–2784. [Google Scholar] [CrossRef]
- Griukova, A.; Deryabin, P.; Shatrova, A.; Burova, E.; Severino, V.; Farina, A.; Nikolsky, N.; Borodkina, A. Molecular basis of senescence transmitting in the population of human endometrial stromal cells. Aging 2019, 11, 9912–9931. [Google Scholar] [CrossRef]
- Buj, R.; Leon, K.E.; Anguelov, M.A.; Aird, K.M. Suppression of p16 alleviates the senescence-associated secretory phenotype. Aging 2021, 13, 3290–3312. [Google Scholar] [CrossRef]
- Budina-Kolomets, A.; Hontz, R.D.; Pimkina, J.; Murphy, M.E. A conserved domain in exon 2 coding for the human and murine ARF tumor suppressor protein is required for autophagy induction. Autophagy 2013, 9, 1553–1565. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, K.; Markby, G.R.; Parys, M.; Phadwal, K.; MacRae, V.E.; Corcoran, B.M. Autophagy regulates cellular senescence by mediating the degradation of CDKN1A/p21 and CDKN2A/p16 through SQSTM1/p62-mediated selective autophagy in myxomatous mitral valve degeneration. Autophagy 2025, 1–23. [Google Scholar] [CrossRef]
- Tyagi, E.; Liu, B.; Li, C.; Liu, T.; Rutter, J.; Grossman, D. Loss of p16INK4A stimulates aberrant mitochondrial biogenesis through a CDK4/Rb-independent pathway. Oncotarget 2017, 8, 55848–55862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia-Alonso, L.; Handfield, L.-F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Chi, F.; Guo, T.; Punj, V.; Lee, W.P.; French, S.W.; Tsukamoto, H. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J. Clin. Investig. 2015, 125, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, W.; Wang, X.; Zhan, Y.; Wang, W.; Yang, L.; Zhu, Y. Notch mediates the glycolytic switch via PI3K/Akt signaling to support embryonic development. Cell. Mol. Biol. Lett. 2023, 28, 50. [Google Scholar] [CrossRef]
- Perumalsamy, L.R.; Nagala, M.; Sarin, A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc. Natl. Acad. Sci. USA 2010, 107, 6882–6887. [Google Scholar] [CrossRef]
- Choi, H.Y.; Zhu, Y.; Zhao, X.; Mehta, S.; Hernandez, J.C.; Lee, J.-J.; Kou, Y.; Machida, R.; Giacca, M.; Del Sal, G.; et al. NOTCH localizes to mitochondria through the TBC1D15-FIS1 interaction and is stabilized via blockade of E3 ligase and CDK8 recruitment to reprogram tumor-initiating cells. Exp. Mol. Med. 2024, 56, 461–477. [Google Scholar] [CrossRef]
- Mori, H.; Funahashi, Y.; Yoshino, Y.; Kumon, H.; Ozaki, Y.; Yamazaki, K.; Ochi, S.; Tachibana, A.; Yoshida, T.; Shimizu, H.; et al. Blood CDKN2A Gene Expression in Aging and Neurodegenerative Diseases. J. Alzheimer’s Dis. 2021, 82, 1737–1744. [Google Scholar] [CrossRef]
- Lynch, M.D.; Lynch, C.N.S.; Craythorne, E.; Liakath-Ali, K.; Mallipeddi, R.; Barker, J.N.; Watt, F.M. Spatial constraints govern competition of mutant clones in human epidermis. Nat. Commun. 2017, 8, 1119. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Prattichizzo, F.; Olivieri, F.; Procopio, A.D.; Rippo, M.R.; Giuliani, A. Where Metabolism Meets Senescence: Focus on Endothelial Cells. Front. Physiol. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Hackl, H. Metabolism of Glucose in the Human Endometrium with Special Reference to Fertility and Contraception. Acta Obstet. Et Gynecol. Scand. 1973, 52, 135–140. [Google Scholar] [CrossRef]
- Vijg, J.; Suh, Y. Genome Instability and Aging. Annu. Rev. Physiol. 2013, 75, 645–668. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, P.I.; Borodkina, A.V. Epigenetic clocks provide clues to the mystery of uterine ageing. Hum. Reprod. Updat. 2023, 29, 259–271. [Google Scholar] [CrossRef]
- Wyatt, J.; Fernando, S.M.; Powell, S.G.; Hill, C.J.; Arshad, I.; Probert, C.; Ahmed, S.; Hapangama, D.K. The role of iron in the pathogenesis of endometriosis: A systematic review. Hum. Reprod. Open 2023, 2023, hoad033. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020, 37, 101726. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Dong, Z.; Wang, G. Identification of iron metabolism-related predictive markers of endometriosis and endometriosis-relevant ovarian cancer. Medicine 2023, 102, e33478. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Ježek, J.; Cooper, K.F.; Strich, R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants 2018, 7, 13. [Google Scholar] [CrossRef]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Clarke, T.L.; Mostoslavsky, R. DNA repair as a shared hallmark in cancer and ageing. Mol. Oncol. 2022, 16, 3352–3379. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Li, C.; Deng, S.; Chen, X.; Han, J.; Zheng, X.; Tian, M.; Hao, W.; Pan, L.; Boldogh, I.; et al. 8-Oxoguanine DNA glycosylase 1 selectively modulates ROS-responsive NF-κB targets through recruitment of MSK1 and phosphorylation of RelA/p65 at Ser276. J. Biol. Chem. 2023, 299, 105308. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Titus, S.; Moy, F.; Ginsburg, E.S.; Oktay, K. Ovarian Aging in Women With BRCA Germline Mutations. J. Clin. Endocrinol. Metab. 2017, 102, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.T.; Beattie, M.; Chen, L.; Oktay, K.; Crawford, S.L.; Gold, E.B.; Cedars, M.; Rosen, M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non–clinic-based sample of women in northern California. Cancer 2013, 119, 1652–1659. [Google Scholar] [CrossRef]
- Dzidek, A.; Czerwińska-Ledwig, O.; Żychowska, M.; Pilch, W.; Piotrowska, A. The Role of Increased Expression of Sirtuin 6 in the Prevention of Premature Aging Pathomechanisms. Int. J. Mol. Sci. 2023, 24, 9655. [Google Scholar] [CrossRef]
- Demain, L.A.M.; Conway, G.S.; Newman, W.G. Genetics of mitochondrial dysfunction and infertility. Clin. Genet. 2017, 91, 199–207. [Google Scholar] [CrossRef]
- Habermann, J.K.; Bündgen, N.K.; Gemoll, T.; Hautaniemi, S.; Lundgren, C.; Wangsa, D.; Doering, J.; Bruch, H.-P.; Nordstroem, B.; Roblick, U.J.; et al. Genomic instability influences the transcriptome and proteome in endometrial cancer subtypes. Mol. Cancer 2011, 10, 132. [Google Scholar] [CrossRef]
- Panier, S.; Wang, S.; Schumacher, B. Genome Instability and DNA Repair in Somatic and Reproductive Aging. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 261–290. [Google Scholar] [CrossRef]
- Lee, Y.; Shin, M.H.; Kim, M.-K.; Kim, Y.K.; Shin, H.S.; Lee, D.H.; Chung, J.H. Increased Histone Acetylation and Decreased Expression of Specific Histone Deacetylases in Ultraviolet-Irradiated and Intrinsically Aged Human Skin In Vivo. Int. J. Mol. Sci. 2021, 22, 2032. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, C.; Zhang, Y. Aging-related histone modification changes in brain function. Ibrain 2023, 9, 205–213. [Google Scholar] [CrossRef]
- Retis-Resendiz, A.M.; González-García, I.N.; León-Juárez, M.; Camacho-Arroyo, I.; Cerbón, M.; Vázquez-Martínez, E.R. The role of epigenetic mechanisms in the regulation of gene expression in the cyclical endometrium. Clin. Epigenetics 2021, 13, 116. [Google Scholar] [CrossRef]
- Satoh, A.; Brace, C.S.; Rensing, N.; Cliften, P.; Wozniak, D.F.; Herzog, E.D.; Yamada, K.A.; Imai, S.-I. Sirt1 Extends Life Span and Delays Aging in Mice through the Regulation of Nk2 Homeobox 1 in the DMH and LH. Cell Metab. 2013, 18, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xu, J.; Song, B.; Zhu, R.; Liu, J.; Liu, Y.F.; Ma, Y.J. The role of epigenetics in women’s reproductive health: The impact of environmental factors. Front. Endocrinol. 2024, 15, 1399757. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.; Morgan, N.; Zhao, X.; Dean, W.; Perez-Garcia, V.; Hemberger, M. Epigenetic changes occur at decidualisation genes as a function of reproductive ageing in mice. Development 2020, 147, dev185629. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Melkani, G.C. Mitochondrial epigenetic modifications and nuclear-mitochondrial communication: A new dimension towards understanding and attenuating the pathogenesis in women with PCOS. Rev. Endocr. Metab. Disord. 2023, 24, 317–326. [Google Scholar] [CrossRef]
- Chatterjee, D.; Das, P.; Chakrabarti, O. Mitochondrial Epigenetics Regulating Inflammation in Cancer and Aging. Front. Cell Dev. Biol. 2022, 10, 929708. [Google Scholar] [CrossRef]
- Yu, X.; Ma, R.; Wu, Y.; Zhai, Y.; Li, S. Reciprocal Regulation of Metabolic Reprogramming and Epigenetic Modifications in Cancer. Front. Genet. 2018, 9, 394. [Google Scholar] [CrossRef]
- Mani, S.; Srivastava, V.; Shandilya, C.; Kaushik, A.; Singh, K.K. Mitochondria: The epigenetic regulators of ovarian aging and longevity. Front. Endocrinol. 2024, 15, 1424826. [Google Scholar] [CrossRef]
- Mishra, A.; Modi, D. Role of HOXA10 in pathologies of the endometrium. Rev. Endocr. Metab. Disord. 2025, 26, 81–96. [Google Scholar] [CrossRef]
- Valentijn, A.J.; Saretzki, G.; Tempest, N.; Critchley, H.O.D.; Hapangama, D.K. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum. Reprod. 2015, 30, 2816–2828. [Google Scholar] [CrossRef]
- Crabbe, L.; Jauch, A.; Naeger, C.M.; Holtgreve-Grez, H.; Karlseder, J. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl. Acad. Sci. USA 2007, 104, 2205–2210. [Google Scholar] [CrossRef] [PubMed]
- Galati, A.; Micheli, E.; Cacchione, S. Chromatin Structure in Telomere Dynamics. Front. Oncol. 2013, 3, 46. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.K.; Dubey, R.; Kleinman, M.E. Unraveling Histone Loss in Aging and Senescence. Cells 2024, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Hayakawa, T.; Iwai, M.; Aoki, S.; Takimoto, K.; Maruyama, M.; Maruyama, W.; Motoyama, N. SIRT1 Suppresses the Senescence-Associated Secretory Phenotype through Epigenetic Gene Regulation. PLoS ONE 2015, 10, e0116480. [Google Scholar] [CrossRef]
- Bartosch, C.; Monteiro-Reis, S.; Almeida-Rios, D.; Vieira, R.; Castro, A.; Moutinho, M.; Rodrigues, M.; Graça, I.; Lopes, J.M.; Jerónimo, C. Assessing sirtuin expression in endometrial carcinoma and non-neoplastic endometrium. Oncotarget 2016, 7, 1144–1154. [Google Scholar] [CrossRef]
- Ito, T.; Teo, Y.V.; Evans, S.A.; Neretti, N.; Sedivy, J.M. Regulation of Cellular Senescence by Polycomb Chromatin Modifiers through Distinct DNA Damage- and Histone Methylation-Dependent Pathways. Cell Rep. 2018, 22, 3480–3492. [Google Scholar] [CrossRef]
- Jezek, M.; Green, E.M. Histone Modifications and the Maintenance of Telomere Integrity. Cells 2019, 8, 199. [Google Scholar] [CrossRef]
- Fukui, Y.; Hirota, Y.; Aikawa, S.; Sakashita, A.; Shimizu-Hirota, R.; Takeda, N.; Ishizawa, C.; Iida, R.; Kaku, T.; Hirata, T.; et al. The EZH2–PRC2–H3K27me3 axis governs the endometrial cell cycle and differentiation for blastocyst invasion. Cell Death Dis. 2023, 14, 320. [Google Scholar] [CrossRef]
- Liu, L.; Trimarchi, J.R.; Smith, P.J.S.; Keefe, D.L. Mitochondrial dysfunction leads to telomere attrition and genomic instability. Aging Cell 2002, 1, 40–46. [Google Scholar] [CrossRef]
- Gao, X.; Yu, X.; Zhang, C.; Wang, Y.; Sun, Y.; Sun, H.; Zhang, H.; Shi, Y.; He, X. Telomeres and Mitochondrial Metabolism: Implications for Cellular Senescence and Age-related Diseases. Stem Cell Rev. Rep. 2022, 18, 2315–2327. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Colla, S.; Liesa, M.; Moslehi, J.; Müller, F.L.; Guo, M.; Cooper, M.; Kotton, D.; Fabian, A.J.; Walkey, C.; et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 2011, 470, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhu, Y.; Deng, C.; Liang, Z.; Chen, J.; Chen, Y.; Wang, X.; Liu, Y.; Tian, Y.; Yang, Y. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases. Signal Transduct. Target. Ther. 2024, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Kamal, A.; Saretzki, G. Implications of telomeres and telomerase in endometrial pathology. Hum. Reprod. Update 2017, 23, 166–187. [Google Scholar] [CrossRef]
- Toupance, S.; Fattet, A.-J.; Thornton, S.N.; Benetos, A.; Guéant, J.-L.; Koscinski, I. Ovarian Telomerase and Female Fertility. Biomedicines 2021, 9, 842. [Google Scholar] [CrossRef]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G.; et al. The association of female and male infertility with telomere length (Review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef]
- Kalmbach, K.H.; Antunes, D.M.F.; Dracxler, R.C.; Knier, T.W.; Seth-Smith, M.L.; Wang, F.; Liu, L.; Keefe, D.L. Telomeres and human reproduction. Fertil. Steril. 2013, 99, 23–29. [Google Scholar] [CrossRef]
- Tan, Y.; Du, B.; Chen, X.; Chen, M. Correlation of MicroRNA-31 with Endometrial Receptivity in Patients with Repeated Implantation Failure of In Vitro Fertilization and Embryo Transfer. Organogenesis 2025, 21, 2460263. [Google Scholar] [CrossRef]
- Kolanska, K.; Bendifallah, S.; Canlorbe, G.; Mekinian, A.; Touboul, C.; Aractingi, S.; Chabbert-Buffet, N.; Daraï, E. Role of miRNAs in Normal Endometrium and in Endometrial Disorders: Comprehensive Review. J. Clin. Med. 2021, 10, 3457. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zheng, Y.-F.; Wu, D.; Li, Y.; Zhou, L.-N.; Chen, Y.-G. microRNA-1203 targets and silences cyclophilin D to protect human endometrial cells from oxygen and glucose deprivation-re-oxygenation. Aging 2020, 12, 3010–3024. [Google Scholar] [CrossRef]
- Altmäe, S.; Martinez-Conejero, J.A.; Esteban, F.J.; Ruiz-Alonso, M.; Stavreus-Evers, A.; Horcajadas, J.A.; Salumets, A. MicroRNAs miR-30b, miR-30d, and miR-494 Regulate Human Endometrial Receptivity. Reprod. Sci. 2013, 20, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martínez, S.; Marcilla, A.; Simón, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221. [Google Scholar] [CrossRef] [PubMed]
- Kresowik, J.D.; Devor, E.J.; Van Voorhis, B.J.; Leslie, K.K. MicroRNA-31 is significantly elevated in both human endometrium and serum during the window of implantation: A potential biomarker for optimum receptivity. Biol. Reprod. 2014, 91, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Van Sinderen, M.; Rainczuk, K.; Menkhorst, E.; Sorby, K.; Osianlis, T.; Pangestu, M.; Santos, L.; Rombauts, L.; Rosello-Diez, A.; et al. Dysregulated miR-124-3p in endometrial epithelial cells reduces endometrial receptivity by altering polarity and adhesion. Proc. Natl. Acad. Sci. USA 2024, 121, e2401071121. [Google Scholar] [CrossRef]
- Shekibi, M.; Heng, S.; Nie, G. MicroRNAs in the Regulation of Endometrial Receptivity for Embryo Implantation. Int. J. Mol. Sci. 2022, 23, 6210. [Google Scholar] [CrossRef]
- Zang, X.; Zhou, C.; Wang, W.; Gan, J.; Li, Y.; Liu, D.; Liu, G.; Hong, L. Differential MicroRNA Expression Involved in Endometrial Receptivity of Goats. Biomolecules 2021, 11, 472. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. MicroRNA biogenesis pathway alterations in aging. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Rivera, J.; Gangwani, L.; Kumar, S. Mitochondria Localized microRNAs: An Unexplored miRNA Niche in Alzheimer’s Disease and Aging. Cells 2023, 12, 742. [Google Scholar] [CrossRef]
- John, A.; Kubosumi, A.; Reddy, P.H. Mitochondrial MicroRNAs in Aging and Neurodegenerative Diseases. Cells 2020, 9, 1345. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, Å.B. Mitochondrial Quality Control and Cellular Proteostasis: Two Sides of the Same Coin. Front. Physiol. 2020, 11, 515. [Google Scholar] [CrossRef]
- Labbadia, J.; Morimoto, R.I. The Biology of Proteostasis in Aging and Disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S.; Kong, Q.; Satyaswaroop, P.; Babaknia, A. Heat shock proteins in human endometrium throughout the menstrual cycle. Hum. Reprod. 1996, 11, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Jee, B.; Dhar, R.; Singh, S.; Karmakar, S. Heat Shock Proteins and Their Role in Pregnancy: Redefining the Function of “Old Rum in a New Bottle”. Front. Cell Dev. Biol. 2021, 9, 648463. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, I.V.; Shashova, E.E.; Sidenko, E.A.; Astakhova, T.M.; Zakharova, L.; Sharova, N.P. Estrogen Receptors and Ubiquitin Proteasome System: Mutual Regulation. Biomolecules 2020, 10, 500. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, H. Female fertility: The role of mitochondrial protease LONP1 in oocyte development and survival. EBioMedicine 2022, 77, 103881. [Google Scholar] [CrossRef]
- Lu, B.; Guo, S. Mechanisms Linking Mitochondrial Dysfunction and Proteostasis Failure. Trends Cell Biol. 2020, 30, 317–328. [Google Scholar] [CrossRef]
- Bartman, S.; Coppotelli, G.; Ross, J.M. Mitochondrial Dysfunction: A Key Player in Brain Aging and Diseases. Curr. Issues Mol. Biol. 2024, 46, 1987–2026. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Zhu, Y.; Chen, Q. Mitochondria organize the cellular proteostatic response and promote cellular senescence. Cell Stress 2019, 3, 110–114. [Google Scholar] [CrossRef]
- Ross, J.M.; Olson, L.; Coppotelli, G. Mitochondrial Dysfunction and Protein Homeostasis in Aging: Insights from a Premature-Aging Mouse Model. Biomolecules 2024, 14, 162. [Google Scholar] [CrossRef]
- Takenaka, Y.; Inoue, I.; Nakano, T.; Ikeda, M.; Kakinuma, Y. Prolonged disturbance of proteostasis induces cellular senescence via temporal mitochondrial dysfunction and subsequent mitochondrial accumulation in human fibroblasts. FEBS J. 2022, 289, 1650–1667. [Google Scholar] [CrossRef]
- Cho, A.; Park, S.-R.; Kim, S.-R.; Nam, S.; Lim, S.; Park, C.H.; Lee, H.-Y.; Hong, I.-S. An Endogenous Anti-aging Factor, Sonic Hedgehog, Suppresses Endometrial Stem Cell Aging through SERPINB2. Mol. Ther. 2019, 27, 1286–1298. [Google Scholar] [CrossRef]
- Wei, C.; Pan, Y.; Zhang, Y.; Dai, Y.; Jiang, L.; Shi, L.; Yang, W.; Xu, S.; Zhang, Y.; Xu, W.; et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell Death Dis. 2020, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, H.-Y. Exploring distinct properties of endometrial stem cells through advanced single-cell analysis platforms. Stem Cell Res. Ther. 2023, 14, 379. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.J.; Yoon, S.H.; Do, J.T. Mitochondrial Dynamics in Stem Cells and Differentiation. Int. J. Mol. Sci. 2018, 19, 3893. [Google Scholar] [CrossRef]
- Oh, J.; Lee, Y.D.; Wagers, A.J. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat. Med. 2014, 20, 870–880. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Hong, I.-S. Endometrial Stem Cells: Orchestrating Dynamic Regeneration of Endometrium and Their Implications in Diverse Endometrial Disorders. Int. J. Biol. Sci. 2024, 20, 864–879. [Google Scholar] [CrossRef]
- Velarde, M.C.; Demaria, M.; Melov, S.; Campisi, J. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc. Natl. Acad. Sci. USA 2015, 112, 10407–10412. [Google Scholar] [CrossRef]
- Khacho, M.; Clark, A.; Svoboda, D.S.; MacLaurin, J.G.; Lagace, D.C.; Park, D.S.; Slack, R.S. Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum. Mol. Genet. 2017, 26, 3327–3341. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef]
- Beal, J.R.; Ma, Q.; Bagchi, I.C.; Bagchi, M.K. Role of Endometrial Extracellular Vesicles in Mediating Cell-to-Cell Communication in the Uterus: A Review. Cells 2023, 12, 2584. [Google Scholar] [CrossRef] [PubMed]
- Mariadas, H.; Chen, J.-H.; Chen, K.-H. The Molecular and Cellular Mechanisms of Endometriosis: From Basic Pathophysiology to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 2458. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, S.; Maruyama, S.; Kimura, M.; Nagayasu, M.; Kobayashi, H. Towards an understanding of the molecular mechanisms of endometriosis-associated symptoms (Review). World Acad. Sci. J. 2020, 2, 12. [Google Scholar] [CrossRef]
- Salmasi, S.; Heidar, M.S.; Mahabady, M.K.; Rashidi, B.; Mirzaei, H. MicroRNAs, endometrial receptivity and molecular pathways. Reprod. Biol. Endocrinol. 2024, 22, 139. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Kurdys, J.G.; Muntean, D.M.; Rosca, M.G. Mitochondrial NAD+/NADH Redox State and Diabetic Cardiomyopathy. Antioxid. Redox Signal. 2019, 30, 375–398. [Google Scholar] [CrossRef]
- Li, Y.; Berliocchi, L.; Li, Z.; Rasmussen, L.J. Interactions between mitochondrial dysfunction and other hallmarks of aging: Paving a path toward interventions that promote healthy old age. Aging Cell 2024, 23, e13942. [Google Scholar] [CrossRef]
- Wu, H.-M.; Chen, L.-H.; Hsu, L.-T.; Lai, C.-H. Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 13382. [Google Scholar] [CrossRef]
- Patel, M.V.; Shen, Z.; Wira, C.R. Do endometrial immune changes with age prior to menopause compromise fertility in women? Explor. Immunol. 2022, 2, 677–692. [Google Scholar] [CrossRef]
- Balci, C.N.; Acar, N. NLRP3 inflammasome pathway, the hidden balance in pregnancy: A comprehensive review. J. Reprod. Immunol. 2024, 161, 104173. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Omidvar-Mehrabadi, A.; Shahbazi, M.; Mohammadnia-Afrouzi, M. Innate and Adaptive Immune Dysregulation in Women with Recurrent Implantation Failure. J. Reprod. Immunol. 2024, 164, 104262. [Google Scholar] [CrossRef]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Vexø, L.E.; Stormlund, S.; Landersoe, S.K.; Jørgensen, H.L.; Humaidan, P.; Bergh, C.; Englund, A.L.M.; Klajnbard, A.; Bogstad, J.W.; Freiesleben, N.l.C.; et al. Low-grade inflammation is negatively associated with live birth in women undergoing IVF. Reprod. Biomed. Online 2023, 46, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, S.; Shen, Z.; Carrillo-Salinas, F.J.; Iyer, V.; Vogell, A.; Illanes, D.; Wira, C.R.; Rodriguez-Garcia, M. Aging modifies endometrial dendritic cell function and unconventional double negative T cells in the human genital mucosa. Immun. Ageing 2023, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Tomari, H.; Onoyama, I.; Araki, H.; Yasunaga, M.; Lin, C.; Kawamura, K.; Yokota, N.; Yoshida, S.; Yagi, H.; et al. Identification of genes associated with endometrial cell ageing. Mol. Hum. Reprod. 2021, 27, gaaa078. [Google Scholar] [CrossRef]
- Kawamura, K.; Matsumura, Y.; Kawamura, T.; Araki, H.; Hamada, N.; Kuramoto, K.; Yagi, H.; Onoyama, I.; Asanoma, K.; Kato, K. Endometrial senescence is mediated by interleukin 17 receptor B signaling. Cell Commun. Signal. 2024, 22, 363. [Google Scholar] [CrossRef]
- Küchenhoff, A.; Seliger, G.; Klonisch, T.; Tscheudschilsuren, G.; Kaltwaßer, P.; Seliger, E.; Buchmann, J.; Fischer, B. Arylhydrocarbon receptor expression in the human endometrium. Fertil. Steril. 1999, 71, 354–360. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef]
- Grazioli, S.; Pugin, J. Mitochondrial Damage-Associated Molecular Patterns: From Inflammatory Signaling to Human Diseases. Front. Immunol. 2018, 9, 832. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Zorzano, A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef]
- Garg, M.; Johri, S.; Chakraborty, K. Immunomodulatory role of mitochondrial DAMPs: A missing link in pathology? FEBS J. 2023, 290, 4395–4418. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, F. Effects of adverse fertility-related factors on mitochondrial DNA in the oocyte: A comprehensive review. Reprod. Biol. Endocrinol. 2023, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Salmonowicz, H.; Chapman, J.; Martini, H.; Vizioli, M.G.; Riley, J.S.; Cloix, C.; Hall-Younger, E.; Espindola-Netto, J.M.; Jurk, D.; et al. Apoptotic stress causes mtDNA release during senescence and drives the SASP. Nature 2023, 622, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Korolchuk, V.I.; Miwa, S.; Carroll, B.; von Zglinicki, T. Mitochondria in Cell Senescence: Is Mitophagy the Weakest Link? EBioMedicine 2017, 21, 7–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Nishio, M.; Umetani, M.; Shigetomi, H.; Imanaka, S.; Hashimoto, H. Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 5060. https://doi.org/10.3390/ijms26115060
Kobayashi H, Nishio M, Umetani M, Shigetomi H, Imanaka S, Hashimoto H. Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction. International Journal of Molecular Sciences. 2025; 26(11):5060. https://doi.org/10.3390/ijms26115060
Chicago/Turabian StyleKobayashi, Hiroshi, Miki Nishio, Mai Umetani, Hiroshi Shigetomi, Shogo Imanaka, and Hiratsugu Hashimoto. 2025. "Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction" International Journal of Molecular Sciences 26, no. 11: 5060. https://doi.org/10.3390/ijms26115060
APA StyleKobayashi, H., Nishio, M., Umetani, M., Shigetomi, H., Imanaka, S., & Hashimoto, H. (2025). Endometrial Aging and Reproductive Decline: The Central Role of Mitochondrial Dysfunction. International Journal of Molecular Sciences, 26(11), 5060. https://doi.org/10.3390/ijms26115060







