Rewiring the Spine—Cutting-Edge Stem Cell Therapies for Spinal Cord Repair
Abstract
1. Introduction
2. Harnessing the Therapeutic Potential of Stem Cells for Spinal Cord Injury Recovery
3. Strategies for Stem Cell Therapy in Spinal Cord Injury
4. Mechanisms of Stem Cell Treatment for Spinal Cord Injury
4.1. Inflammation Modulation
4.2. Enhancement of Angiogenesis
4.3. Restoration and Repair
4.4. Inhibition of Cell Death
4.5. Nerve Growth Promotion and Regeneration
5. Clinical Trials and Applications of Stem Cell Therapy for Spinal Cord Injury
6. Safety and Efficacy Considerations in Stem Cell Therapy for Spinal Cord Injury
7. The Therapeutic Role of Stem Cell-Derived Exosomes in Spinal Cord Injury Recovery
Mechanisms of Action
- I.
- Neuroinflammation Control: Exosomes reduce pro-inflammatory responses by shifting macrophages towards the anti-inflammatory M2 phenotype, characterized by increased CD206 expression and reduced Iba-1 levels. Exosomes also carry miRNAs (e.g., miRNA-125a, miRNA-216a, and miRNA-23b) and siRNAs that inhibit inflammasome activation and suppress the NF-κB signaling pathway, thereby decreasing pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 [154,155,156,157,158,159,160,161,162].
- II.
- Apoptosis Inhibition: Exosome treatment upregulates anti-apoptotic proteins (e.g., Bcl-2) while downregulating pro-apoptotic factors (e.g., Bax and caspase-3). miRNAs such as miRNA-21 and miRNA-19 play a key role in regulating apoptosis-related genes, contributing to reduced neuronal death and improved recovery [163,164,165,166,167,168].
- III.
- IV.
8. Cell-Based Treatments for Spinal Cord Injury (SCI)
8.1. Neural Progenitor Cells
8.2. Oligodendrocyte Progenitor Cells (OPCs)
8.3. Astrocytes
8.4. Microglia
8.5. Multi-Modal Therapeutic Approaches
8.5.1. Biomaterial Scaffolds
8.5.2. Growth Factors
8.5.3. Electrical Stimulation
8.5.4. Decellularized Extracellular Matrix (dECM) Scaffolds
9. Management of Chronic Spinal Cord Injury
10. Stem Cell Therapy: Timing and Dosage Considerations
11. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- van den Berg, M.E.L.; Castellote, J.M.; Mahillo-Fernandez, I.; de Pedro-Cuesta, J. Incidence of Spinal Cord Injury Worldwide: A Systematic Review. Neuroepidemiology 2010, 34, 184–192; discussion 192. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.J.; Martin, M.J. Trauma: Spinal Cord Injury. Surg. Clin. N. Am. 2017, 97, 1031–1045. [Google Scholar] [CrossRef] [PubMed]
- Frontera, J.E.; Mollett, P. Aging with Spinal Cord Injury: An Update. Phys. Med. Rehabil. Clin. N. Am. 2017, 28, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Sabre, L.; Harro, J.; Eensoo, D.; Vaht, M.; Kabel, V.; Pakkanen, M.; Asser, T.; Kõrv, J. A New Risk Factor for Traumatic Spinal Cord Injury. J. Neurotrauma 2016, 33, 1946–1949. [Google Scholar] [CrossRef]
- Krause, J.S. Risk for Subsequent Injuries after Spinal Cord Injury: A 10-Year Longitudinal Analysis. Arch. Phys. Med. Rehabil. 2010, 91, 1741–1746. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From Basics to Clinical: A Comprehensive Review on Spinal Cord Injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Stenudd, M.; Sabelström, H.; Frisén, J. Role of Endogenous Neural Stem Cells in Spinal Cord Injury and Repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Tetreault, L.A.; Wilson, J.R.; Kwon, B.K.; Burns, A.S.; Martin, A.R.; Hawryluk, G.; Harrop, J.S. A Clinical Practice Guideline for the Management of Acute Spinal Cord Injury: Introduction, Rationale, and Scope. Glob. Spine J. 2017, 7, 84S–94S. [Google Scholar] [CrossRef]
- Weissman, I.L.; Anderson, D.J.; Gage, F. Stem and Progenitor Cells: Origins, Phenotypes, Lineage Commitments, and Transdifferentiations. Annu. Rev. Cell Dev. Biol. 2001, 17, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Blommestein, H.M.; Verelst, S.G.R.; Huijgens, P.C.; Blijlevens, N.M.A.; Cornelissen, J.J.; Uyl-de Groot, C.A. Real-World Costs of Autologous and Allogeneic Stem Cell Transplantations for Haematological Diseases: A Multicentre Study. Ann. Hematol. 2012, 91, 1945–1952. [Google Scholar] [CrossRef]
- Gao, L.; Xu, W.; Li, T.; Chen, J.; Shao, A.; Yan, F.; Chen, G. Stem Cell Therapy: A Promising Therapeutic Method for Intracerebral Hemorrhage. Cell Transplant. 2018, 27, 1809–1824. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Wilkinson, M.; Reis, H.; Akyol, O.; Gospodarev, V.; Araujo, C.; Chen, S.; Zhang, J.H. A Look into Stem Cell Therapy: Exploring the Options for Treatment of Ischemic Stroke. Stem Cells Int. 2017, 2017, 3267352. [Google Scholar] [CrossRef] [PubMed]
- Muheremu, A.; Peng, J.; Ao, Q. Stem Cell Based Therapies for Spinal Cord Injury. Tissue Cell 2016, 48, 328–333. [Google Scholar] [CrossRef] [PubMed]
- De Feo, D.; Merlini, A.; Laterza, C.; Martino, G. Neural Stem Cell Transplantation in Central Nervous System Disorders: From Cell Replacement to Neuroprotection. Curr. Opin. Neurol. 2012, 25, 322–333. [Google Scholar] [CrossRef]
- Oliveri, R.S.; Bello, S.; Biering-Sørensen, F. Mesenchymal Stem Cells Improve Locomotor Recovery in Traumatic Spinal Cord Injury: Systematic Review with Meta-Analyses of Rat Models. Neurobiol. Dis. 2014, 62, 338–353. [Google Scholar] [CrossRef]
- Cusimano, M.; Biziato, D.; Brambilla, E.; Donegà, M.; Alfaro-Cervello, C.; Snider, S.; Salani, G.; Pucci, F.; Comi, G.; Garcia-Verdugo, J.M.; et al. Transplanted Neural Stem/Precursor Cells Instruct Phagocytes and Reduce Secondary Tissue Damage in the Injured Spinal Cord. Brain 2012, 135, 447–460. [Google Scholar] [CrossRef]
- Gao, L.; Peng, Y.; Xu, W.; He, P.; Li, T.; Lu, X.; Chen, G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020, 2020, 2853650. [Google Scholar] [CrossRef]
- Bain, G.; Kitchens, D.; Yao, M.; Huettner, J.E.; Gottlieb, D.I. Embryonic Stem Cells Express Neuronal Properties in Vitro. Dev. Biol. 1995, 168, 342–357. [Google Scholar] [CrossRef]
- Martello, G.; Smith, A. The Nature of Embryonic Stem Cells. Annu. Rev. Cell Dev. Biol. 2014, 30, 647–675. [Google Scholar] [CrossRef]
- Yang, J.-R.; Liao, C.-H.; Pang, C.-Y.; Huang, L.L.-H.; Chen, Y.-L.; Shiue, Y.-L.; Chen, L.-R. Transplantation of Porcine Embryonic Stem Cells and Their Derived Neuronal Progenitors in a Spinal Cord Injury Rat Model. Cytotherapy 2013, 15, 201–208. [Google Scholar] [CrossRef]
- Kim, Y.; Jo, S.-H.; Kim, W.H.; Kweon, O.-K. Antioxidant and Anti-Inflammatory Effects of Intravenously Injected Adipose Derived Mesenchymal Stem Cells in Dogs with Acute Spinal Cord Injury. Stem Cell Res. Ther. 2015, 6, 229. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Li, H.; Wang, C.; Song, X.; Ding, Y.; Zheng, M.; Liu, W.; Chen, Y.; Zhang, X.; Wang, L. Bone Marrow Mesenchymal Stem Cells Decrease CHOP Expression and Neuronal Apoptosis after Spinal Cord Injury. Neurosci. Lett. 2017, 636, 282–289. [Google Scholar] [CrossRef]
- Hur, J.W.; Cho, T.-H.; Park, D.-H.; Lee, J.-B.; Park, J.-Y.; Chung, Y.-G. Intrathecal Transplantation of Autologous Adipose-Derived Mesenchymal Stem Cells for Treating Spinal Cord Injury: A Human Trial. J. Spinal Cord. Med. 2016, 39, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-Inflammatory Mechanism of Bone Marrow Mesenchymal Stem Cell Transplantation in Rat Model of Spinal Cord Injury. Cell Biochem. Biophys. 2015, 71, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Bydon, M.; Qu, W.; Moinuddin, F.M.; Hunt, C.L.; Garlanger, K.L.; Reeves, R.K.; Windebank, A.J.; Zhao, K.D.; Jarrah, R.; Trammell, B.C.; et al. Intrathecal Delivery of Adipose-Derived Mesenchymal Stem Cells in Traumatic Spinal Cord Injury: Phase I Trial. Nat. Commun. 2024, 15, 2201. [Google Scholar] [CrossRef]
- Arias, J.; Yu, J.; Varshney, M.; Inzunza, J.; Nalvarte, I. Hematopoietic Stem Cell- and Induced Pluripotent Stem Cell-Derived CAR-NK Cells as Reliable Cell-Based Therapy Solutions. Stem Cells Transl. Med. 2021, 10, 987–995. [Google Scholar] [CrossRef]
- Frolov, A.A.; Bryukhovetskiy, A.S. Effects of Hematopoietic Autologous Stem Cell Transplantation to the Chronically Injured Human Spinal Cord Evaluated by Motor and Somatosensory Evoked Potentials Methods. Cell Transplant. 2012, 21 (Suppl. S1), 49–55. [Google Scholar] [CrossRef]
- Koshizuka, S.; Okada, S.; Okawa, A.; Koda, M.; Murasawa, M.; Hashimoto, M.; Kamada, T.; Yoshinaga, K.; Murakami, M.; Moriya, H.; et al. Transplanted Hematopoietic Stem Cells from Bone Marrow Differentiate into Neural Lineage Cells and Promote Functional Recovery after Spinal Cord Injury in Mice. J. Neuropathol. Exp. Neurol. 2004, 63, 64–72. [Google Scholar] [CrossRef]
- Kumar, R.S.; Goyal, N. Estrogens as Regulator of Hematopoietic Stem Cell, Immune Cells and Bone Biology. Life Sci. 2021, 269, 119091. [Google Scholar] [CrossRef]
- You, Y.; Che, L.; Lee, H.Y.; Lee, H.-L.; Yun, Y.; Lee, M.; Oh, J.; Ha, Y. Antiapoptotic Effect of Highly Secreted GMCSF From Neuronal Cell-Specific GMCSF Overexpressing Neural Stem Cells in Spinal Cord Injury Model. Spine (Phila Pa 1976) 2015, 40, E1284–E1291. [Google Scholar] [CrossRef]
- Führmann, T.; Tam, R.Y.; Ballarin, B.; Coles, B.; Elliott Donaghue, I.; van der Kooy, D.; Nagy, A.; Tator, C.H.; Morshead, C.M.; Shoichet, M.S. Injectable Hydrogel Promotes Early Survival of Induced Pluripotent Stem Cell-Derived Oligodendrocytes and Attenuates Longterm Teratoma Formation in a Spinal Cord Injury Model. Biomaterials 2016, 83, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Devito, L.; Miere, C.; Codognotto, S. Human Embryonic and Induced Pluripotent Stem Cells in Clinical Trials. Br. Med. Bull. 2015, 116, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Okano, H. Applications of Induced Pluripotent Stem Cell Technologies in Spinal Cord Injury. J. Neurochem. 2017, 141, 848–860. [Google Scholar] [CrossRef]
- Oh, J.; Lee, K.-I.; Kim, H.-T.; You, Y.; Yoon, D.H.; Song, K.Y.; Cheong, E.; Ha, Y.; Hwang, D.-Y. Human-Induced Pluripotent Stem Cells Generated from Intervertebral Disc Cells Improve Neurologic Functions in Spinal Cord Injury. Stem Cell Res. Ther. 2015, 6, 125. [Google Scholar] [CrossRef]
- Olushanu, M.; Collins, A.; Li, Y.; Li, D. The Impact of Tissue Storage Conditions on Rat Olfactory Ensheathing Cell Yield and the Future Clinical Implications. Cell Transplant. 2018, 27, 1320–1327. [Google Scholar] [CrossRef]
- Taghipour, Z.; Karbalaie, K.; Kiani, A.; Niapour, A.; Bahramian, H.; Nasr-Esfahani, M.H.; Baharvand, H. Transplantation of Undifferentiated and Induced Human Exfoliated Deciduous Teeth-Derived Stem Cells Promote Functional Recovery of Rat Spinal Cord Contusion Injury Model. Stem Cells Dev. 2012, 21, 1794–1802. [Google Scholar] [CrossRef]
- Martens, W.; Sanen, K.; Georgiou, M.; Struys, T.; Bronckaers, A.; Ameloot, M.; Phillips, J.; Lambrichts, I. Human Dental Pulp Stem Cells Can Differentiate into Schwann Cells and Promote and Guide Neurite Outgrowth in an Aligned Tissue-Engineered Collagen Construct in Vitro. FASEB J. 2014, 28, 1634–1643. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Concise Review: Dental Pulp Stem Cells: A Novel Cell Therapy for Retinal and Central Nervous System Repair. Stem Cells 2017, 35, 61–67. [Google Scholar] [CrossRef]
- Czyz, M.; Tabakow, P.; Hernandez-Sanchez, I.; Jarmundowicz, W.; Raisman, G. Obtaining the Olfactory Bulb as a Source of Olfactory Ensheathing Cells with the Use of Minimally Invasive Neuroendoscopy-Assisted Supraorbital Keyhole Approach--Cadaveric Feasibility Study. Br. J. Neurosurg. 2015, 29, 362–370. [Google Scholar] [CrossRef]
- Gómez, R.M.; Sánchez, M.Y.; Portela-Lomba, M.; Ghotme, K.; Barreto, G.E.; Sierra, J.; Moreno-Flores, M.T. Cell Therapy for Spinal Cord Injury with Olfactory Ensheathing Glia Cells (OECs). Glia 2018, 66, 1267–1301. [Google Scholar] [CrossRef] [PubMed]
- Roet, K.C.D.; Verhaagen, J. Understanding the Neural Repair-Promoting Properties of Olfactory Ensheathing Cells. Exp. Neurol. 2014, 261, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Tabakow, P.; Raisman, G.; Fortuna, W.; Czyz, M.; Huber, J.; Li, D.; Szewczyk, P.; Okurowski, S.; Miedzybrodzki, R.; Czapiga, B.; et al. Functional Regeneration of Supraspinal Connections in a Patient with Transected Spinal Cord Following Transplantation of Bulbar Olfactory Ensheathing Cells with Peripheral Nerve Bridging. Cell Transplant. 2014, 23, 1631–1655. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.A.; Todorovic, M.; Tello-Velasquez, J.; Rayfield, A.J.; St John, J.A.; Ekberg, J.A. Enhancing the Therapeutic Potential of Olfactory Ensheathing Cells in Spinal Cord Repair Using Neurotrophins. Cell Transplant. 2018, 27, 867–878. [Google Scholar] [CrossRef]
- Yu, A.; Mao, L.; Zhao, F.; Sun, B. Olfactory Ensheathing Cells Transplantation Attenuates Chronic Cerebral Hypoperfusion Induced Cognitive Dysfunction and Brain Damages by Activating Nrf2/HO-1 Signaling Pathway. Am. J. Transl. Res. 2018, 10, 3111–3121. [Google Scholar]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of Autologous Bone Marrow Stem Cells into Spinal Cord Injury Patients via Multiple Routes Is Safe and Improves Their Quality of Life: Comprehensive Case Studies. Cell Transplant. 2008, 17, 1277–1293. [Google Scholar] [CrossRef]
- Oh, S.K.; Jeon, S.R. Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review. Korean J. Neurotrauma 2016, 12, 40–46. [Google Scholar] [CrossRef]
- Ninomiya, K.; Iwatsuki, K.; Ohnishi, Y.; Ohkawa, T.; Yoshimine, T. Intranasal Delivery of Bone Marrow Stromal Cells to Spinal Cord Lesions. J. Neurosurg. Spine 2015, 23, 111–119. [Google Scholar] [CrossRef]
- Takeuchi, H.; Natsume, A.; Wakabayashi, T.; Aoshima, C.; Shimato, S.; Ito, M.; Ishii, J.; Maeda, Y.; Hara, M.; Kim, S.U.; et al. Intravenously Transplanted Human Neural Stem Cells Migrate to the Injured Spinal Cord in Adult Mice in an SDF-1- and HGF-Dependent Manner. Neurosci. Lett. 2007, 426, 69–74. [Google Scholar] [CrossRef]
- Ohta, Y.; Hamaguchi, A.; Ootaki, M.; Watanabe, M.; Takeba, Y.; Iiri, T.; Matsumoto, N.; Takenaga, M. Intravenous Infusion of Adipose-Derived Stem/Stromal Cells Improves Functional Recovery of Rats with Spinal Cord Injury. Cytotherapy 2017, 19, 839–848. [Google Scholar] [CrossRef]
- Bakshi, A.; Hunter, C.; Swanger, S.; Lepore, A.; Fischer, I. Minimally Invasive Delivery of Stem Cells for Spinal Cord Injury: Advantages of the Lumbar Puncture Technique. J. Neurosurg. Spine 2004, 1, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Paul, C.; Samdani, A.F.; Betz, R.R.; Fischer, I.; Neuhuber, B. Grafting of Human Bone Marrow Stromal Cells into Spinal Cord Injury: A Comparison of Delivery Methods. Spine (Phila Pa 1976) 2009, 34, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.D.; Okonkwo, D.O.; Park, P.; Jenkins, A.L.; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging Safety of Intramedullary Transplantation of Human Neural Stem Cells in Chronic Cervical and Thoracic Spinal Cord Injury. Neurosurgery 2018, 82, 562–575. [Google Scholar] [CrossRef]
- Amemori, T.; Ruzicka, J.; Romanyuk, N.; Jhanwar-Uniyal, M.; Sykova, E.; Jendelova, P. Comparison of Intraspinal and Intrathecal Implantation of Induced Pluripotent Stem Cell-Derived Neural Precursors for the Treatment of Spinal Cord Injury in Rats. Stem Cell Res. Ther. 2015, 6, 257. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, B.D.S.; Almeida, F.M.; de Sales, C.M.; de Lima, S.; Martinez, A.M.B. Injection of Bone Marrow Mesenchymal Stem Cells by Intravenous or Intraperitoneal Routes Is a Viable Alternative to Spinal Cord Injury Treatment in Mice. Neural Regen. Res. 2018, 13, 1046–1053. [Google Scholar] [CrossRef]
- Schwarz, Q.; Ruhrberg, C. Neuropilin, You Gotta Let Me Know: Should I Stay or Should I Go? Cell Adhes. Migr. 2010, 4, 61–66. [Google Scholar] [CrossRef]
- Baloh, R.H.; Tansey, M.G.; Lampe, P.A.; Fahrner, T.J.; Enomoto, H.; Simburger, K.S.; Leitner, M.L.; Araki, T.; Johnson, E.M.; Milbrandt, J. Artemin, a Novel Member of the GDNF Ligand Family, Supports Peripheral and Central Neurons and Signals through the GFRalpha3-RET Receptor Complex. Neuron 1998, 21, 1291–1302. [Google Scholar] [CrossRef]
- Iwai, H.; Nori, S.; Nishimura, S.; Yasuda, A.; Takano, M.; Tsuji, O.; Fujiyoshi, K.; Toyama, Y.; Okano, H.; Nakamura, M. Transplantation of Neural Stem/Progenitor Cells at Different Locations in Mice with Spinal Cord Injury. Cell Transplant. 2014, 23, 1451–1464. [Google Scholar] [CrossRef]
- Piltti, K.M.; Funes, G.M.; Avakian, S.N.; Salibian, A.A.; Huang, K.I.; Carta, K.; Kamei, N.; Flanagan, L.A.; Monuki, E.S.; Uchida, N.; et al. Increasing Human Neural Stem Cell Transplantation Dose Alters Oligodendroglial and Neuronal Differentiation after Spinal Cord Injury. Stem Cell Rep. 2017, 8, 1534–1548. [Google Scholar] [CrossRef]
- García-Alías, G.; Barkhuysen, S.; Buckle, M.; Fawcett, J.W. Chondroitinase ABC Treatment Opens a Window of Opportunity for Task-Specific Rehabilitation. Nat. Neurosci. 2009, 12, 1145–1151. [Google Scholar] [CrossRef]
- García-Alías, G.; Fawcett, J.W. Training and Anti-CSPG Combination Therapy for Spinal Cord Injury. Exp. Neurol. 2012, 235, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Uchida, K.; Nakajima, H.; Matsuo, H.; Sugita, D.; Yoshida, A.; Honjoh, K.; Johnson, W.E.B.; Baba, H. Early Transplantation of Mesenchymal Stem Cells after Spinal Cord Injury Relieves Pain Hypersensitivity through Suppression of Pain-Related Signaling Cascades and Reduced Inflammatory Cell Recruitment. Stem Cells 2015, 33, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, C.; Reges, D.; Rocha, E.; Gagliardi, V.; Silva, G.S. Traumatic Spinal Cord Injury: Current Concepts and Treatment Update. Arq. Neuropsiquiatr. 2017, 75, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.B.; et al. Transplantation of Mesenchymal Stem Cells Promotes an Alternative Pathway of Macrophage Activation and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef]
- All, A.H.; Gharibani, P.; Gupta, S.; Bazley, F.A.; Pashai, N.; Chou, B.-K.; Shah, S.; Resar, L.M.; Cheng, L.; Gearhart, J.D.; et al. Early Intervention for Spinal Cord Injury with Human Induced Pluripotent Stem Cells Oligodendrocyte Progenitors. PLoS ONE 2015, 10, e0116933. [Google Scholar] [CrossRef]
- Antonic, A.; Sena, E.S.; Lees, J.S.; Wills, T.E.; Skeers, P.; Batchelor, P.E.; Macleod, M.R.; Howells, D.W. Stem Cell Transplantation in Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis of Animal Studies. PLoS Biol. 2013, 11, e1001738. [Google Scholar] [CrossRef]
- Moon, S.M.; Kim, W.; Chung, J.Y.; Im, W.; Yoo, D.Y.; Jung, H.Y.; Won, M.-H.; Choi, J.H.; Hwang, I.K. Neuroprotective Effects of Adipose-Derived Stem Cells Are Maintained for 3 Weeks against Ischemic Damage in the Rabbit Spinal Cord. Biomed. Res. Int. 2014, 2014, 539051. [Google Scholar] [CrossRef]
- Xu, J.; Fan, G.; Chen, S.; Wu, Y.; Xu, X.M.; Hsu, C.Y. Methylprednisolone Inhibition of TNF-Alpha Expression and NF-kB Activation after Spinal Cord Injury in Rats. Brain Res. Mol. Brain Res. 1998, 59, 135–142. [Google Scholar] [CrossRef]
- Ensor, C.R.; Trofe-Clark, J.; Gabardi, S.; McDevitt-Potter, L.M.; Shullo, M.A. Generic Maintenance Immunosuppression in Solid Organ Transplant Recipients. Pharmacotherapy 2011, 31, 1111–1129. [Google Scholar] [CrossRef]
- Swanger, S.A.; Neuhuber, B.; Himes, B.T.; Bakshi, A.; Fischer, I. Analysis of Allogeneic and Syngeneic Bone Marrow Stromal Cell Graft Survival in the Spinal Cord. Cell Transplant. 2005, 14, 775–786. [Google Scholar] [CrossRef]
- Torres-Espín, A.; Hernández, J.; Navarro, X. Gene Expression Changes in the Injured Spinal Cord Following Transplantation of Mesenchymal Stem Cells or Olfactory Ensheathing Cells. PLoS ONE 2013, 8, e76141. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct Isolation of Human Central Nervous System Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Uchida, N.; Tamaki, S.J.; Salazar, D.L.; Hooshmand, M.; Summers, R.; Gage, F.H.; Anderson, A.J. Human Neural Stem Cells Differentiate and Promote Locomotor Recovery in Spinal Cord-Injured Mice. Proc. Natl. Acad. Sci. USA 2005, 102, 14069–14074. [Google Scholar] [CrossRef] [PubMed]
- Salazar, D.L.; Uchida, N.; Hamers, F.P.T.; Cummings, B.J.; Anderson, A.J. Human Neural Stem Cells Differentiate and Promote Locomotor Recovery in an Early Chronic Spinal Cord Injury NOD-Scid Mouse Model. PLoS ONE 2010, 5, e12272. [Google Scholar] [CrossRef]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Moghadas Jafari, A.; Asady, H.; Razavi Tousi, S.M.T.; Hosseini, M. Neural Stem/Progenitor Cell Transplantation for Spinal Cord Injury Treatment; A Systematic Review and Meta-Analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef]
- Madsen, J.R.; MacDonald, P.; Irwin, N.; Goldberg, D.E.; Yao, G.L.; Meiri, K.F.; Rimm, I.J.; Stieg, P.E.; Benowitz, L.I. Tacrolimus (FK506) Increases Neuronal Expression of GAP-43 and Improves Functional Recovery after Spinal Cord Injury in Rats. Exp. Neurol. 1998, 154, 673–683. [Google Scholar] [CrossRef]
- Park, D.Y.; Mayle, R.E.; Smith, R.L.; Corcoran-Schwartz, I.; Kharazi, A.I.; Cheng, I. Combined Transplantation of Human Neuronal and Mesenchymal Stem Cells Following Spinal Cord Injury. Global Spine J. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Hunt, J.; Cheng, A.; Hoyles, A.; Jervis, E.; Morshead, C.M. Cyclosporin A Has Direct Effects on Adult Neural Precursor Cells. J. Neurosci. 2010, 30, 2888–2896. [Google Scholar] [CrossRef]
- Song, L.H.; Pan, W.; Yu, Y.H.; Quarles, L.D.; Zhou, H.H.; Xiao, Z.S. Resveratrol Prevents CsA Inhibition of Proliferation and Osteoblastic Differentiation of Mouse Bone Marrow-Derived Mesenchymal Stem Cells through an ER/NO/cGMP Pathway. Toxicol 2006, 20, 915–922. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Yamamoto, A.; Sakai, K.; Matsubara, K.; Kano, F.; Ueda, M. Multifaceted Neuro-Regenerative Activities of Human Dental Pulp Stem Cells for Functional Recovery after Spinal Cord Injury. Neurosci. Res. 2014, 78, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhu, W.; Cao, K.; Wu, F.; Li, J.; Wang, G.; Li, H.; Lu, M.; Ren, Y.; He, X. Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. Int. J. Mol. Sci. 2016, 17, 1380. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Bosco, D.B.; Sun, L.; Chen, X.; Xu, Y.; Tai, W.; Didier, R.; Li, J.; Fan, J.; He, X.; et al. Neural Stem Cell-Conditioned Medium Suppresses Inflammation and Promotes Spinal Cord Injury Recovery. Cell Transplant. 2017, 26, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Chen, J.; Zhou, J.; Nong, F.; Lv, J.-H.; Liu, J. Reduced Inflammatory Cell Recruitment and Tissue Damage in Spinal Cord Injury by Acellular Spinal Cord Scaffold Seeded with Mesenchymal Stem Cells. Exp. Ther. Med. 2017, 13, 203–207. [Google Scholar] [CrossRef]
- Yahata, K.; Kanno, H.; Ozawa, H.; Yamaya, S.; Tateda, S.; Ito, K.; Shimokawa, H.; Itoi, E. Low-energy extracorporeal shock wave therapy for promotion of vascular endothelial growth factor expression and angiogenesis and improvement of locomotor and sensory functions after spinal cord injury. J. Neurosurg. Spine 2016, 25, 745–755. [Google Scholar] [CrossRef]
- Li, Z.; Guo, G.-H.; Wang, G.-S.; Guan, C.-X.; Yue, L. Influence of Neural Stem Cell Transplantation on Angiogenesis in Rats with Spinal Cord Injury. Genet. Mol. Res. 2014, 13, 6083–6092. [Google Scholar] [CrossRef]
- Yu, S.; Yao, S.; Wen, Y.; Wang, Y.; Wang, H.; Xu, Q. Angiogenic Microspheres Promote Neural Regeneration and Motor Function Recovery after Spinal Cord Injury in Rats. Sci. Rep. 2016, 6, 33428. [Google Scholar] [CrossRef]
- Seo, J.H.; Cho, S.-R. Neurorestoration Induced by Mesenchymal Stem Cells: Potential Therapeutic Mechanisms for Clinical Trials. Yonsei Med. J. 2012, 53, 1059–1067. [Google Scholar] [CrossRef]
- Lu, P. Stem Cell Transplantation for Spinal Cord Injury Repair. Prog. Brain Res. 2017, 231, 1–32. [Google Scholar] [CrossRef]
- Iwanami, A.; Kaneko, S.; Nakamura, M.; Kanemura, Y.; Mori, H.; Kobayashi, S.; Yamasaki, M.; Momoshima, S.; Ishii, H.; Ando, K.; et al. Transplantation of Human Neural Stem Cells for Spinal Cord Injury in Primates. J. Neurosci. Res. 2005, 80, 182–190. [Google Scholar] [CrossRef]
- Zhao, J.; Sun, W.; Cho, H.M.; Ouyang, H.; Li, W.; Lin, Y.; Do, J.; Zhang, L.; Ding, S.; Liu, Y.; et al. Integration and Long Distance Axonal Regeneration in the Central Nervous System from Transplanted Primitive Neural Stem Cells. J. Biol. Chem. 2013, 288, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Chen, S.; Fang, S.; Liu, S.; Jin, M.; Guo, Z.; Yuan, Y.; Wang, Y.; Liu, C.; Mei, X. The Neuroprotective Effects of Muscle-Derived Stem Cells via Brain-Derived Neurotrophic Factor in Spinal Cord Injury Model. Biomed. Res. Int. 2017, 2017, 1972608. [Google Scholar] [CrossRef] [PubMed]
- Sabelström, H.; Stenudd, M.; Réu, P.; Dias, D.O.; Elfineh, M.; Zdunek, S.; Damberg, P.; Göritz, C.; Frisén, J. Resident Neural Stem Cells Restrict Tissue Damage and Neuronal Loss after Spinal Cord Injury in Mice. Science 2013, 342, 637–640. [Google Scholar] [CrossRef]
- do Couto Nicola, F.; Marques, M.R.; Odorcyk, F.; Arcego, D.M.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; et al. Neuroprotector Effect of Stem Cells from Human Exfoliated Deciduous Teeth Transplanted after Traumatic Spinal Cord Injury Involves Inhibition of Early Neuronal Apoptosis. Brain Res 2017, 1663, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Song, W.; Zhao, W.; Gao, Y.; Yang, Z.; Li, X. Endogenous Neurogenesis in Adult Mammals after Spinal Cord Injury. Sci. China Life Sci. 2016, 59, 1313–1318. [Google Scholar] [CrossRef]
- Hodgetts, S.I.; Harvey, A.R. Neurotrophic Factors Used to Treat Spinal Cord Injury. Vitam. Horm. 2017, 104, 405–457. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, H.; Liu, X.; Chen, J.-T.; Xiang, L.-B.; Zhou, D.-P. Neurogenic Differentiation from Adipose-Derived Stem Cells and Application for Autologous Transplantation in Spinal Cord Injury. Cell Tissue Bank. 2015, 16, 335–342. [Google Scholar] [CrossRef]
- Pandamooz, S.; Salehi, M.S.; Zibaii, M.I.; Ahmadiani, A.; Nabiuni, M.; Dargahi, L. Epidermal Neural Crest Stem Cell-Derived Glia Enhance Neurotrophic Elements in an Ex Vivo Model of Spinal Cord Injury. J. Cell Biochem. 2018, 119, 3486–3496. [Google Scholar] [CrossRef]
- Liao, Y.; Zhong, D.; Kang, M.; Yao, S.; Zhang, Y.; Yu, Y. Transplantation of Neural Stem Cells Induced by All-Trans- Retinoic Acid Combined with Glial Cell Line Derived Neurotrophic Factor and Chondroitinase Abc for Repairing Spinal Cord Injury of Rats. Chin. J. Reparative Reconstr. Surg. 2015, 29, 1009–1015. [Google Scholar]
- Ra, J.C.; Shin, I.S.; Kim, S.H.; Kang, S.K.; Kang, B.C.; Lee, H.Y.; Kim, Y.J.; Jo, J.Y.; Yoon, E.J.; Choi, H.J.; et al. Safety of Intravenous Infusion of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Animals and Humans. Stem Cells Dev. 2011, 20, 1297–1308. [Google Scholar] [CrossRef]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, M.V.P.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and Neurological Assessments after Autologous Transplantation of Bone Marrow Mesenchymal Stem Cells in Subjects with Chronic Spinal Cord Injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef] [PubMed]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous Mesenchymal Stromal Cell Transplantation for Spinal Cord Injury: A Phase I Pilot Study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.-S.; Yun, S.; Lee, H.; Jung, K.; Hwang, K.; Kim, M.; Lee, I.-S.; et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015, 2015, 630932. [Google Scholar] [CrossRef]
- Ghobrial, G.M.; Anderson, K.D.; Dididze, M.; Martinez-Barrizonte, J.; Sunn, G.H.; Gant, K.L.; Levi, A.D. Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury: Functional Outcomes at 12 Months in a Phase II Clinical Trial. Neurosurgery 2017, 64, 87–91. [Google Scholar] [CrossRef]
- El-Kheir, W.A.; Gabr, H.; Awad, M.R.; Ghannam, O.; Barakat, Y.; Farghali, H.A.M.A.; El Maadawi, Z.M.; Ewes, I.; Sabaawy, H.E. Autologous Bone Marrow-Derived Cell Therapy Combined with Physical Therapy Induces Functional Improvement in Chronic Spinal Cord Injury Patients. Cell Transplant. 2014, 23, 729–745. [Google Scholar] [CrossRef]
- Oh, S.K.; Choi, K.H.; Yoo, J.Y.; Kim, D.Y.; Kim, S.J.; Jeon, S.R. A Phase III Clinical Trial Showing Limited Efficacy of Autologous Mesenchymal Stem Cell Therapy for Spinal Cord Injury. Neurosurgery 2016, 78, 436–447; discussion 447. [Google Scholar] [CrossRef]
- NCT02152657, 2017 Study Details Evaluation of Autologous Mesenchymal Stem Cell Transplantation in Chronic Spinal Cord Injury: A Pilot Study ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02152657 (accessed on 22 April 2025).
- dos Santos, R.R. Phase 1 Study of Autologous Bone Marrow Stem Cell Transplantation in Patients with Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2017.
- National Institute of Blood and Marrow Transplant (NIBMT). Autologous Transplantation of Bone Marrow Mesenchymal Stem Cells in Patients of Spinal Cord Injury-Phase I Clinical Trial; clinicaltrials.gov: Bethesda, MD, USA, 2016.
- Crespo, J.V. Phase I Pilot Study to Evaluate the Security of Local Administration of Autologous Stem Cells Obtained From the Bone Marrow Stroma, in Traumatic Injuries of the Spinal Cord; clinicaltrials.gov: Bethesda, MD, USA, 2019.
- Knight, A. Safety of Autologous Stem Cell Treatment for Spinal Cord Injury in Children; clinicaltrials.gov: Bethesda, MD, USA, 2024.
- Dr.SGA.Rao. Surgical Transplantation of Autologous Bone Marrow Stem Cells with Glial Scar Resection for Patients of Chronic Spinal Cord Injury and Intra-Thecal Injection for Acute and Subacute Injury—A Preliminary Study; clinicaltrials.gov: Bethesda, MD, USA, 2010.
- Sabaawy, H.E. Autologous Bone Marrow Derived Cell Transplant in Spinal Cord Injury Patients; clinicaltrials.gov: Bethesda, MD, USA, 2009.
- Crespo, J.V. Intrathecal Administration (Pattern 100/3) of Expanded Autologous Adult Bone Marrow Mesenchymal Stem Cells in Established Chronic Spinal Cord Injuries; clinicaltrials.gov: Bethesda, MD, USA, 2018.
- Jamali, F. Comparative Evaluation of Safety and Effectiveness of Autologous Bone Marrow Derived Mesenchymal Stem Cells (BM-MSC) vs. Adipose Tissue Derived Mesenchymal Stem Cells (AT-MSC) in the Treatment of Spinal Cord Injury (SCI) Patient; clinicaltrials.gov: Bethesda, MD, USA, 2019.
- R-Bio. Safety of Autologous Adipose Derived Mesenchymal Stem Cells in Patients with Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2014.
- Bukwang Pharmaceutical. The Effect of Intrathecal Transplantation of Autologous Adipose Tissue Derived Mesenchymal Stem Cells in the Patients with Spinal Cord Injury, Phase I Clinical Study; clinicaltrials.gov: Bethesda, MD, USA, 2015.
- R-Bio. Safety and Effect of Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Patients with Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2016.
- General Hospital of Chinese Armed Police Forces. Efficacy Difference Between Rehabilitation Therapy and Umbilical Cord Derived Mesenchymal Stem Cells Transplantation in Patients with Acute or Chronic Spinal Cord Injury in China; clinicaltrials.gov: Bethesda, MD, USA, 2011.
- General Hospital of Chinese Armed Police Forces. Different Efficacy Between Rehabilitation Therapy and Umbilical Cord Derived Mesenchymal Stem Cells Transplantation in Patients with Chronic Spinal Cord Injury in China; clinicaltrials.gov: Bethesda, MD, USA, 2018.
- StemCells, Inc. A Phase I;II Study of the Safety and Preliminary Efficacy of Intramedullary Spinal Cord Transplantation of Human Central Nervous System (CNS) Stem Cells (HuCNS-SC®) in Subjects with Thoracic (T2–T11) Spinal Cord Trauma; clinicaltrials.gov: Bethesda, MD, USA, 2015.
- StemCells, Inc. Long-Term Follow-up (LTFU) Study of the Phase I;II Safety and Preliminary Efficacy Investigation of Intramedullary Spinal Cord Transplantation of HuCNS-SC® in Subjects with Thoracic (T2–T11) Spinal Cord Trauma; clinicaltrials.gov: Bethesda, MD, USA, 2016.
- StemCells, Inc. A Single-Blind, Randomized, Parallel Arm, Phase II Proof-of-Concept Study of the Safety and Efficacy of Human Central Nervous System Stem Cells (HuCNS-SC) Transplantation in Cervical Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2016.
- Lineage Cell Therapeutics, Inc. A Phase 1/2a Dose Escalation Study of AST-OPC1 in Subjects with Subacute Cervical Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2021.
- Rong, L. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients with Late Stage of Chronic Spinal Cord Injury: A Multicenter, Prospective, Cohort Study; clinicaltrials.gov: Bethesda, MD, USA, 2019.
- Rong, L. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients with Sub-Acute Spinal Cord Injury: A Multicenter, Randomized, Controlled Trial; clinicaltrials.gov: Bethesda, MD, USA, 2019.
- Rong, L. The Effect of Intrathecal Transplantation of Umbilical Cord Mesenchymal Stem Cells in Patients with Early Stage of Chronic Spinal Cord Injury: A Multicenter, Randomized, Controlled Trial; clinicaltrials.gov: Bethesda, MD, USA, 2019.
- Banc de Sang i Teixits. A Phase I/IIa, Randomized, Double-Blind, Single-Dose, Placebo Controlled, Two-Way Crossover Clinical Trial to Assess the Safety and to Obtain Efficacy Data in Intrathecal Administration of Expanded Wharton’s Jelly Mesenchymal Stem Cells in Chronic Traumatic Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2020.
- Bydon, M. Phase I Clinical Trial of Autologous Adipose Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2022.
- Santos, R.R. Evaluation of the Safety and Potential Effectiveness of Autologous Mesenchymal Stem Cells Transplantation in Subjects with Cervical Chronic and Complete Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2017.
- Santos, R.R. Randomized Clinical Trial for the Evaluation of Autologous Mesenchymal Stem Cells Transplantation in Thoracolumbar Chronic and Complete Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2017.
- Pharmicell Co., Ltd. A Phase II/III Clinical Trial to Evaluate the Safety and Efficacy of Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Patients with Chronic Spinal Cord Injury; clinicaltrials.gov: Bethesda, MD, USA, 2021.
- Stem Cells Arabia. Transplantation of Purified Autologous Bone Marrow- or Leukapheresis-Derived CD34+ and CD133+ for Patients with Spinal Cord Injuries: A Long-Term Comparative Evaluation of Safety and Efficacy Study; clinicaltrials.gov: Bethesda, MD, USA, 2020.
- Neuralstem Inc. A Phase 1, Open-Label, Single-Site, Safety Study of Human Spinal Cord-Derived Neural Stem Cell Transplantation for the Treatment of Chronic SCI; clinicaltrials.gov: Bethesda, MD, USA, 2017.
- Neuroplast. A 3 Months Open Phase I Study to Assess the Safety of the Intrathecal Application of Neuro-Cells in End Stage (Chronic) Traumatic Spinal Cord Injury Patients; clinicaltrials.gov: Bethesda, MD, USA, 2023.
- Neuroplast. A Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Delayed Start Phase II/III Study to Assess the Efficacy and Safety of Neuro-Cells in (Sub)Acute Spinal Cord Injury Patients; clinicaltrials.gov: Bethesda, MD, USA, 2023.
- Mortazavi, M.M.; Harmon, O.A.; Adeeb, N.; Deep, A.; Tubbs, R.S. Treatment of Spinal Cord Injury: A Review of Engineering Using Neural and Mesenchymal Stem Cells. Clin. Anat. 2015, 28, 37–44. [Google Scholar] [CrossRef]
- Iyer, N.R.; Wilems, T.S.; Sakiyama-Elbert, S.E. Stem Cells for Spinal Cord Injury: Strategies to Inform Differentiation and Transplantation. Biotechnol. Bioeng. 2017, 114, 245–259. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.; Zhang, X.-M. Progress on bone marrow mesenchymal stem cells transplantation for spinal cord injury. China J. Orthop. Traumatol. 2014, 27, 437–440. [Google Scholar]
- Iida, T.; Iwanami, A.; Sanosaka, T.; Kohyama, J.; Miyoshi, H.; Nagoshi, N.; Kashiwagi, R.; Toyama, Y.; Matsumoto, M.; Nakamura, M.; et al. Whole-Genome DNA Methylation Analyses Revealed Epigenetic Instability in Tumorigenic Human iPS Cell-Derived Neural Stem/Progenitor Cells. Stem Cells 2017, 35, 1316–1327. [Google Scholar] [CrossRef]
- Miura, M.; Miura, Y.; Padilla-Nash, H.M.; Molinolo, A.A.; Fu, B.; Patel, V.; Seo, B.-M.; Sonoyama, W.; Zheng, J.J.; Baker, C.C.; et al. Accumulated Chromosomal Instability in Murine Bone Marrow Mesenchymal Stem Cells Leads to Malignant Transformation. Stem Cells 2006, 24, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, X. The Efficacy and Safety of Mesenchymal Stem Cell Transplantation for Spinal Cord Injury Patients: A Meta-Analysis and Systematic Review. Cell Transplant. 2019, 28, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Piltti, K.M.; Salazar, D.L.; Uchida, N.; Cummings, B.J.; Anderson, A.J. Safety of Human Neural Stem Cell Transplantation in Chronic Spinal Cord Injury. Stem Cells Transl. Med. 2013, 2, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Huang, J.-H.; Yin, X.-M.; Xu, Y.; Xu, C.-C.; Lin, X.; Ye, F.-B.; Cao, Y.; Lin, F.-Y. Systemic Administration of Exosomes Released from Mesenchymal Stromal Cells Attenuates Apoptosis, Inflammation, and Promotes Angiogenesis after Spinal Cord Injury in Rats. J. Neurotrauma 2017, 34, 3388–3396. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Meng, Y.; Katakowski, M.; Xin, H.; Mahmood, A.; Xiong, Y. Effect of Exosomes Derived from Multipluripotent Mesenchymal Stromal Cells on Functional Recovery and Neurovascular Plasticity in Rats after Traumatic Brain Injury. J. Neurosurg. 2015, 122, 856–867. [Google Scholar] [CrossRef]
- Fauré, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes Are Released by Cultured Cortical Neurones. Mol. Cell Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W.E. Proteomic Profiling of Exosomes: Current Perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk Factors in the Development of Stem Cell Therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef]
- Poongodi, R.; Hsu, Y.-W.; Yang, T.-H.; Huang, Y.-H.; Yang, K.D.; Lin, H.-C.; Cheng, J.-K. Stem Cell-Derived Extracellular Vesicle-Mediated Therapeutic Signaling in Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 723. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Gao, L.; Liu, R.-H.; Liu, Y.; Zhang, N.-S.; Chen, Z.-Y. microRNAs in Spinal Cord Injury: Potential Roles and Therapeutic Implications. Int. J. Biol. Sci. 2014, 10, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Esmailzadeh, S.; Mansoori, B.; Mohammadi, A.; Baradaran, B. Regulatory Roles of Micro-RNAs in T Cell Autoimmunity. Immunol. Investig. 2017, 46, 864–879. [Google Scholar] [CrossRef]
- Chang, Q.; Hao, Y.; Wang, Y.; Zhou, Y.; Zhuo, H.; Zhao, G. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal microRNA-125a Promotes M2 Macrophage Polarization in Spinal Cord Injury by Downregulating IRF5. Brain Res. Bull. 2021, 170, 199–210. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-Shuttled miR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Brand, F.; Adamczak, S.; Lee, S.W.; Perez-Barcena, J.; Wang, M.Y.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Exosome-Mediated Inflammasome Signaling after Central Nervous System Injury. J. Neurochem. 2016, 136 (Suppl. S1), 39–48. [Google Scholar] [CrossRef]
- Huang, J.-H.; Fu, C.-H.; Xu, Y.; Yin, X.-M.; Cao, Y.; Lin, F.-Y. Extracellular Vesicles Derived from Epidural Fat-Mesenchymal Stem Cells Attenuate NLRP3 Inflammasome Activation and Improve Functional Recovery After Spinal Cord Injury. Neurochem. Res. 2020, 45, 760–771. [Google Scholar] [CrossRef]
- Noori, L.; Arabzadeh, S.; Mohamadi, Y.; Mojaverrostami, S.; Mokhtari, T.; Akbari, M.; Hassanzadeh, G. Intrathecal Administration of the Extracellular Vesicles Derived from Human Wharton’s Jelly Stem Cells Inhibit Inflammation and Attenuate the Activity of Inflammasome Complexes after Spinal Cord Injury in Rats. Neurosci. Res. 2021, 170, 87–98. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, J. Mesenchymal Stem Cell-Derived Exosomes Containing miR-145-5p Reduce Inflammation in Spinal Cord Injury by Regulating the TLR4/NF-κB Signaling Pathway. Cell Cycle 2021, 20, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, X.; Qiu, J.; Xin, D.; Li, T.; Chu, X.; Yuan, H.; Wang, H.; Wang, Z.; Wang, D. Exosomes Derived From Bone Marrow Mesenchymal Stem Cells Inhibit Complement Activation In Rats With Spinal Cord Injury. Drug Des. Dev. Ther. 2019, 13, 3693–3704. [Google Scholar] [CrossRef] [PubMed]
- Strand, N.S.; Hoi, K.K.; Phan, T.M.T.; Ray, C.A.; Berndt, J.D.; Moon, R.T. Wnt/β-Catenin Signaling Promotes Regeneration after Adult Zebrafish Spinal Cord Injury. Biochem. Biophys. Res. Commun. 2016, 477, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Dong, J.; He, X.; Zhang, C.; Zhang, T. Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Reduce Apoptosis and Inflammatory Response during Spinal Cord Injury by Inhibiting the TLR4/MyD88/NF-κB Signaling Pathway. Hum. Exp. Toxicol. 2021, 40, 1612–1623. [Google Scholar] [CrossRef]
- Ji, W.; Jiang, W.; Li, M.; Li, J.; Li, Z. miR-21 Deficiency Contributes to the Impaired Protective Effects of Obese Rat Mesenchymal Stem Cell-Derived Exosomes against Spinal Cord Injury. Biochimie 2019, 167, 171–178. [Google Scholar] [CrossRef]
- Zhou, X.; Chu, X.; Yuan, H.; Qiu, J.; Zhao, C.; Xin, D.; Li, T.; Ma, W.; Wang, H.; Wang, Z.; et al. Mesenchymal Stem Cell Derived EVs Mediate Neuroprotection after Spinal Cord Injury in Rats via the microRNA-21-5p/FasL Gene Axis. Biomed. Pharmacother. 2019, 115, 108818. [Google Scholar] [CrossRef]
- Lambert, C.; Cisternas, P.; Inestrosa, N.C. Role of Wnt Signaling in Central Nervous System Injury. Mol. Neurobiol. 2016, 53, 2297–2311. [Google Scholar] [CrossRef]
- Li, C.; Jiao, G.; Wu, W.; Wang, H.; Ren, S.; Zhang, L.; Zhou, H.; Liu, H.; Chen, Y. Exosomes from Bone Marrow Mesenchymal Stem Cells Inhibit Neuronal Apoptosis and Promote Motor Function Recovery via the Wnt/β-Catenin Signaling Pathway. Cell Transplant. 2019, 28, 1373–1383. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Gong, F.; Rong, Y.; Luo, Y.; Tang, P.; Zhou, Z.; Zhou, Z.; Xu, T.; Jiang, T.; et al. Exosomes Derived from Bone Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Suppressing the Activation of A1 Neurotoxic Reactive Astrocytes. J. Neurotrauma 2019, 36, 469–484. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, C.; Xu, Y.; Li, C.; Cao, Y.; Li, P. Exosomes Derived from Human Placenta-Derived Mesenchymal Stem Cells Improve Neurologic Function by Promoting Angiogenesis after Spinal Cord Injury. Neurosci. Lett. 2020, 739, 135399. [Google Scholar] [CrossRef]
- Romanelli, P.; Bieler, L.; Scharler, C.; Pachler, K.; Kreutzer, C.; Zaunmair, P.; Jakubecova, D.; Mrowetz, H.; Benedetti, B.; Rivera, F.J.; et al. Extracellular Vesicles Can Deliver Anti-Inflammatory and Anti-Scarring Activities of Mesenchymal Stromal Cells After Spinal Cord Injury. Front. Neurol. 2019, 10, 1225. [Google Scholar] [CrossRef] [PubMed]
- Drommelschmidt, K.; Serdar, M.; Bendix, I.; Herz, J.; Bertling, F.; Prager, S.; Keller, M.; Ludwig, A.-K.; Duhan, V.; Radtke, S.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Ameliorate Inflammation-Induced Preterm Brain Injury. Brain Behav. Immun. 2017, 60, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Lv, B.; Zeng, H.; Shi, D.; Liu, Y.; Chen, F.; Li, F.; Liu, X.; Zhu, R.; Yu, L.; et al. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated With GFAP Downregulation After Ischemic Stroke via P38 MAPK and JNK. J. Cell Physiol. 2015, 230, 2461–2475. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, K.; Yang, H.; Li, B.; Zhou, T.; Wang, H.; Zhou, H.; Chen, S.; Zhou, X.; Wei, X.; et al. Extracellular Vesicles Derived from CD73 Modified Human Umbilical Cord Mesenchymal Stem Cells Ameliorate Inflammation after Spinal Cord Injury. J. Nanobiotechnol. 2021, 19, 274. [Google Scholar] [CrossRef]
- Yi, H.; Wang, Y. A Meta-Analysis of Exosome in the Treatment of Spinal Cord Injury. Open Med. 2021, 16, 1043–1060. [Google Scholar] [CrossRef]
- Zhang, C.; Deng, R.; Zhang, G.; He, X.; Chen, H.; Chen, B.; Wan, L.; Kang, X. Therapeutic Effect of Exosomes Derived From Stem Cells in Spinal Cord Injury: A Systematic Review Based on Animal Studies. Front. Neurol. 2022, 13, 847444. [Google Scholar] [CrossRef]
- Shang, Z.; Wang, M.; Zhang, B.; Wang, X.; Wanyan, P. Clinical Translation of Stem Cell Therapy for Spinal Cord Injury Still Premature: Results from a Single-Arm Meta-Analysis Based on 62 Clinical Trials. BMC Med. 2022, 20, 284. [Google Scholar] [CrossRef]
- Jabermoradi, S.; Paridari, P.; Ramawad, H.A.; Gharin, P.; Roshdi, S.; Toloui, A.; Yousefifard, M. Stem Cell-Derived Exosomes as a Therapeutic Option for Spinal Cord Injuries; a Systematic Review and Meta-Analysis. Arch. Acad. Emerg. Med. 2024, 13, e2. [Google Scholar]
- Csobonyeiova, M.; Polak, S.; Zamborsky, R.; Danisovic, L. Recent Progress in the Regeneration of Spinal Cord Injuries by Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2019, 20, 3838. [Google Scholar] [CrossRef]
- Barak, M.; Fedorova, V.; Pospisilova, V.; Raska, J.; Vochyanova, S.; Sedmik, J.; Hribkova, H.; Klimova, H.; Vanova, T.; Bohaciakova, D. Human iPSC-Derived Neural Models for Studying Alzheimer’s Disease: From Neural Stem Cells to Cerebral Organoids. Stem Cell Rev. Rep. 2022, 18, 792–820. [Google Scholar] [CrossRef]
- Cho, I.K.; Yang, B.; Forest, C.; Qian, L.; Chan, A.W.S. Amelioration of Huntington’s Disease Phenotype in Astrocytes Derived from iPSC-Derived Neural Progenitor Cells of Huntington’s Disease Monkeys. PLoS ONE 2019, 14, e0214156. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting Neural Progenitor Cells to Restore Connectivity after Spinal Cord Injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, N.; Okano, H. iPSC-Derived Neural Precursor Cells: Potential for Cell Transplantation Therapy in Spinal Cord Injury. Cell Mol. Life Sci. 2018, 75, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Ma, D.; Shen, H.; Zhao, Y.; Xu, B.; Fan, Y.; Sun, Z.; Chen, B.; Xue, W.; Shi, Y.; et al. Aligned Collagen Scaffold Combination with Human Spinal Cord-Derived Neural Stem Cells to Improve Spinal Cord Injury Repair. Biomater. Sci. 2020, 8, 5145–5156. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-Distance Growth and Connectivity of Neural Stem Cells after Severe Spinal Cord Injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Nori, S.; Okada, Y.; Yasuda, A.; Tsuji, O.; Takahashi, Y.; Kobayashi, Y.; Fujiyoshi, K.; Koike, M.; Uchiyama, Y.; Ikeda, E.; et al. Grafted Human-Induced Pluripotent Stem-Cell-Derived Neurospheres Promote Motor Functional Recovery after Spinal Cord Injury in Mice. Proc. Natl. Acad. Sci. USA 2011, 108, 16825–16830. [Google Scholar] [CrossRef]
- Ottoboni, L.; De Feo, D.; Merlini, A.; Martino, G. Commonalities in Immune Modulation between Mesenchymal Stem Cells (MSCs) and Neural Stem/Precursor Cells (NPCs). Immunol. Lett. 2015, 168, 228–239. [Google Scholar] [CrossRef]
- Wang, J.; Hu, W.-W.; Jiang, Z.; Feng, M.-J. Advances in Treatment of Neurodegenerative Diseases: Perspectives for Combination of Stem Cells with Neurotrophic Factors. World J. Stem Cells 2020, 12, 323–338. [Google Scholar] [CrossRef]
- McCrary, M.R.; Jiang, M.Q.; Jesson, K.; Gu, X.; Logun, M.T.; Wu, A.; Gonsalves, N.; Karumbaiah, L.; Yu, S.P.; Wei, L. Glycosaminoglycan Scaffolding and Neural Progenitor Cell Transplantation Promotes Regenerative Immunomodulation in the Mouse Ischemic Brain. Exp. Neurol. 2022, 357, 114177. [Google Scholar] [CrossRef]
- Bammidi, S.; Bali, P.; Kalra, J.; Anand, A. Transplantation Efficacy of Human Ciliary Epithelium Cells from Fetal Eye and Lin-ve Stem Cells from Umbilical Cord Blood in the Murine Retinal Degeneration Model of Laser Injury. Cell Transplant. 2020, 29, 963689720946031. [Google Scholar] [CrossRef]
- Czopka, T. Insights into Mechanisms of Central Nervous System Myelination Using Zebrafish. Glia 2016, 64, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Evonuk, K.S.; DeSilva, T.M. Microglia Phagocytose Oligodendrocyte Progenitor Cells and Synapses during Early Postnatal Development: Implications for White versus Gray Matter Maturation. FEBS J. 2022, 289, 2110–2127. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, S.; Takano, M.; Numasawa-Kuroiwa, Y.; Itakura, G.; Kobayashi, Y.; Nishiyama, Y.; Sugai, K.; Nishimura, S.; Iwai, H.; Isoda, M.; et al. Grafted Human iPS Cell-Derived Oligodendrocyte Precursor Cells Contribute to Robust Remyelination of Demyelinated Axons after Spinal Cord Injury. Stem Cell Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thiruvalluvan, A.; Czepiel, M.; Kap, Y.A.; Mantingh-Otter, I.; Vainchtein, I.; Kuipers, J.; Bijlard, M.; Baron, W.; Giepmans, B.; Brück, W.; et al. Survival and Functionality of Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes in a Nonhuman Primate Model for Multiple Sclerosis. Stem Cells Transl. Med. 2016, 5, 1550–1561. [Google Scholar] [CrossRef]
- Fessler, R.G.; Ehsanian, R.; Liu, C.Y.; Steinberg, G.K.; Jones, L.; Lebkowski, J.S.; Wirth, E.D.; McKenna, S.L. A Phase 1/2a Dose-Escalation Study of Oligodendrocyte Progenitor Cells in Individuals with Subacute Cervical Spinal Cord Injury. J. Neurosurg. Spine 2022, 37, 812–820. [Google Scholar] [CrossRef]
- Fan, B.; Wei, Z.; Feng, S. Progression in Translational Research on Spinal Cord Injury Based on Microenvironment Imbalance. Bone Res. 2022, 10, 35. [Google Scholar] [CrossRef]
- Kim, D.-S.; Jung, S.J.; Lee, J.S.; Lim, B.Y.; Kim, H.A.; Yoo, J.-E.; Kim, D.-W.; Leem, J.W. Rapid Generation of OPC-like Cells from Human Pluripotent Stem Cells for Treating Spinal Cord Injury. Exp. Mol. Med. 2017, 49, e361. [Google Scholar] [CrossRef]
- Yao, Z.-F.; Wang, Y.; Lin, Y.-H.; Wu, Y.; Zhu, A.-Y.; Wang, R.; Shen, L.; Xi, J.; Qi, Q.; Jiang, Z.-Q.; et al. Transplantation of PDGF-AA-Overexpressing Oligodendrocyte Precursor Cells Promotes Recovery in Rat Following Spinal Cord Injury. Front. Cell Neurosci. 2017, 11, 79. [Google Scholar] [CrossRef]
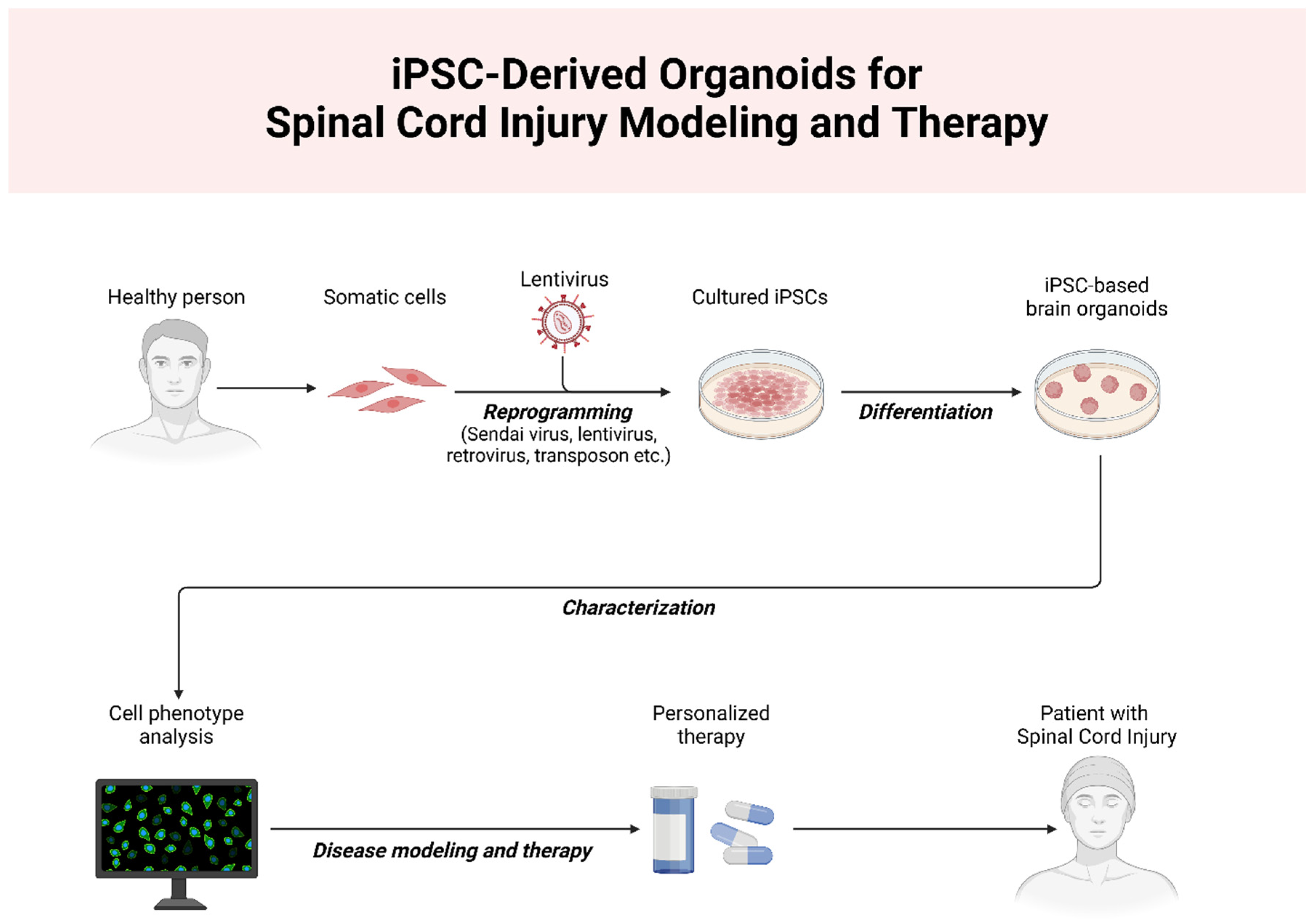
- Zeng, C.-W. Stem Cell-Based Approaches for Spinal Cord Injury: The Promise of iPSCs. Biology 2025, 14, 314. [Google Scholar] [CrossRef]
- Pestana, F.; Edwards-Faret, G.; Belgard, T.G.; Martirosyan, A.; Holt, M.G. No Longer Underappreciated: The Emerging Concept of Astrocyte Heterogeneity in Neuroscience. Brain Sci. 2020, 10, 168. [Google Scholar] [CrossRef]
- Hayashi, K.; Hashimoto, M.; Koda, M.; Naito, A.T.; Murata, A.; Okawa, A.; Takahashi, K.; Yamazaki, M. Increase of Sensitivity to Mechanical Stimulus after Transplantation of Murine Induced Pluripotent Stem Cell-Derived Astrocytes in a Rat Spinal Cord Injury Model. J. Neurosurg. Spine 2011, 15, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Arranz, A.M.; De Strooper, B. The Role of Astroglia in Alzheimer’s Disease: Pathophysiology and Clinical Implications. Lancet Neurol. 2019, 18, 406–414. [Google Scholar] [CrossRef]
- Strnadel, J.; Carromeu, C.; Bardy, C.; Navarro, M.; Platoshyn, O.; Glud, A.N.; Marsala, S.; Kafka, J.; Miyanohara, A.; Kato, T.; et al. Survival of Syngeneic and Allogeneic iPSC-Derived Neural Precursors after Spinal Grafting in Minipigs. Sci. Transl. Med. 2018, 10, eaam6651. [Google Scholar] [CrossRef]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef] [PubMed]
- Lukacova, N.; Kisucka, A.; Kiss Bimbova, K.; Bacova, M.; Ileninova, M.; Kuruc, T.; Galik, J. Glial-Neuronal Interactions in Pathogenesis and Treatment of Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 13577. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal Cord Injury: Pathophysiology, Treatment Strategies, Associated Challenges, and Future Implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Nicaise, A.M.; D’Angelo, A.; Ionescu, R.-B.; Krzak, G.; Willis, C.M.; Pluchino, S. The Role of Neural Stem Cells in Regulating Glial Scar Formation and Repair. Cell Tissue Res. 2022, 387, 399–414. [Google Scholar] [CrossRef]
- Pang, Q.M.; Chen, S.Y.; Xu, Q.J.; Fu, S.P.; Yang, Y.C.; Zou, W.H.; Zhang, M.; Liu, J.; Wan, W.H.; Peng, J.C.; et al. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front. Immunol. 2021, 12, 751021. [Google Scholar] [CrossRef]
- Raspa, A.; Carminati, L.; Pugliese, R.; Fontana, F.; Gelain, F. Self-Assembling Peptide Hydrogels for the Stabilization and Sustained Release of Active Chondroitinase ABC in Vitro and in Spinal Cord Injuries. J. Control Release 2021, 330, 1208–1219. [Google Scholar] [CrossRef]
- Thurgur, H.; Pinteaux, E. Microglia in the Neurovascular Unit: Blood-Brain Barrier-Microglia Interactions After Central Nervous System Disorders. Neuroscience 2019, 405, 55–67. [Google Scholar] [CrossRef]
- Zeng, C.-W. Immune Cell-NSPC Interactions: Friend or Foe in CNS Injury and Repair? Differentiation 2025, 143, 100855. [Google Scholar] [CrossRef] [PubMed]
- Francos-Quijorna, I.; Amo-Aparicio, J.; Martinez-Muriana, A.; López-Vales, R. IL-4 Drives Microglia and Macrophages toward a Phenotype Conducive for Tissue Repair and Functional Recovery after Spinal Cord Injury. Glia 2016, 64, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Caron, I.; Erba, E.; Panini, N.; De Paola, M.; Mariani, A.; Colombo, C.; Ferrari, R.; Pozzer, D.; Zanier, E.R.; et al. Early Modulation of Pro-Inflammatory Microglia by Minocycline Loaded Nanoparticles Confers Long Lasting Protection after Spinal Cord Injury. Biomaterials 2016, 75, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The Semantics of Microglia Activation: Neuroinflammation, Homeostasis, and Stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Badanjak, K.; Fixemer, S.; Smajić, S.; Skupin, A.; Grünewald, A. The Contribution of Microglia to Neuroinflammation in Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4676. [Google Scholar] [CrossRef]
- Reich, M.; Paris, I.; Ebeling, M.; Dahm, N.; Schweitzer, C.; Reinhardt, D.; Schmucki, R.; Prasad, M.; Köchl, F.; Leist, M.; et al. Alzheimer’s Risk Gene TREM2 Determines Functional Properties of New Type of Human iPSC-Derived Microglia. Front. Immunol. 2020, 11, 617860. [Google Scholar] [CrossRef]
- Marinelli, S.; Basilico, B.; Marrone, M.C.; Ragozzino, D. Microglia-Neuron Crosstalk: Signaling Mechanism and Control of Synaptic Transmission. Semin. Cell Dev. Biol. 2019, 94, 138–151. [Google Scholar] [CrossRef]
- Banerjee, A.; Lu, Y.; Do, K.; Mize, T.; Wu, X.; Chen, X.; Chen, J. Validation of Induced Microglia-Like Cells (iMG Cells) for Future Studies of Brain Diseases. Front. Cell Neurosci. 2021, 15, 629279. [Google Scholar] [CrossRef]
- Qu, W.; Li, L. Microglial TREM2 at the Intersection of Brain Aging and Alzheimer’s Disease. Neuroscientist 2023, 29, 302–316. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, Z.; Qie, S. The Implications of Microglial Regulation in Neuroplasticity-Dependent Stroke Recovery. Biomolecules 2023, 13, 571. [Google Scholar] [CrossRef]
- Teng, Y.D.; Lavik, E.B.; Qu, X.; Park, K.I.; Ourednik, J.; Zurakowski, D.; Langer, R.; Snyder, E.Y. Functional Recovery Following Traumatic Spinal Cord Injury Mediated by a Unique Polymer Scaffold Seeded with Neural Stem Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 3024–3029. [Google Scholar] [CrossRef] [PubMed]
- Beliën, H.; Evens, L.; Hendrikx, M.; Bito, V.; Bronckaers, A. Combining Stem Cells in Myocardial Infarction: The Road to Superior Repair? Med. Res. Rev. 2022, 42, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.G.; Park, S.A.; Lee, S.-H.; Choi, J.S.; Cho, H.; Lee, S.J.; Kwon, Y.-W.; Kwon, S.K. Transplantation of a 3D-Printed Tracheal Graft Combined with iPS Cell-Derived MSCs and Chondrocytes. Sci. Rep. 2020, 10, 4326. [Google Scholar] [CrossRef] [PubMed]
- Eugenis, I.; Wu, D.; Rando, T.A. Cells, Scaffolds, and Bioactive Factors: Engineering Strategies for Improving Regeneration Following Volumetric Muscle Loss. Biomaterials 2021, 278, 121173. [Google Scholar] [CrossRef]
- Larsson, L.; Decker, A.M.; Nibali, L.; Pilipchuk, S.P.; Berglundh, T.; Giannobile, W.V. Regenerative Medicine for Periodontal and Peri-Implant Diseases. J. Dent. Res. 2016, 95, 255–266. [Google Scholar] [CrossRef]
- Hosseini, M.; Shafiee, A. Engineering Bioactive Scaffolds for Skin Regeneration. Small 2021, 17, e2101384. [Google Scholar] [CrossRef]
- Assunção-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem Cells Int. 2015, 2015, 948040. [Google Scholar] [CrossRef]
- Friedman, J.A.; Windebank, A.J.; Moore, M.J.; Spinner, R.J.; Currier, B.L.; Yaszemski, M.J. Biodegradable Polymer Grafts for Surgical Repair of the Injured Spinal Cord. Neurosurgery 2002, 51, 742–751; discussion 751–752. [Google Scholar] [CrossRef]
- Percival, K.M.; Paul, V.; Husseini, G.A. Recent Advancements in Bone Tissue Engineering: Integrating Smart Scaffold Technologies and Bio-Responsive Systems for Enhanced Regeneration. Int. J. Mol. Sci. 2024, 25, 6012. [Google Scholar] [CrossRef]
- Liu, S.; Yu, J.-M.; Gan, Y.-C.; Qiu, X.-Z.; Gao, Z.-C.; Wang, H.; Chen, S.-X.; Xiong, Y.; Liu, G.-H.; Lin, S.-E.; et al. Biomimetic Natural Biomaterials for Tissue Engineering and Regenerative Medicine: New Biosynthesis Methods, Recent Advances, and Emerging Applications. Mil. Med. Res. 2023, 10, 16. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, K.; Luo, S.; Li, F.; Zuo, X.; Fan, C.; Li, Q. Programmable DNA Hydrogels as Artificial Extracellular Matrix. Small 2022, 18, e2107640. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Huang, W.-Y.; Chen, L.-H.; Liang, N.-W.; Wang, H.-C.; Lu, J.; Wang, X.; Wang, T.-W. Neural Tissue Engineering: The Influence of Scaffold Surface Topography and Extracellular Matrix Microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Idrisova, K.F.; Zeinalova, A.K.; Masgutova, G.A.; Bogov, A.A.; Allegrucci, C.; Syromiatnikova, V.Y.; Salafutdinov, I.I.; Garanina, E.E.; Andreeva, D.I.; Kadyrov, A.A.; et al. Application of Neurotrophic and Proangiogenic Factors as Therapy after Peripheral Nervous System Injury. Neural Regen. Res. 2022, 17, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, L.; Khakbiz, M.; Moosazadeh Moghaddam, M.; Bonakdar, S. A Biomaterials Approach to Schwann Cell Development in Neural Tissue Engineering. J. Biomed. Mater. Res. A 2019, 107, 2425–2446. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Ding, F.; Williams, D.F. Neural Tissue Engineering Options for Peripheral Nerve Regeneration. Biomaterials 2014, 35, 6143–6156. [Google Scholar] [CrossRef]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional Hydrogels as Therapeutic Tools for Spinal Cord Injury: New Perspectives on Immunopharmacological Interventions. Pharmacol. Ther. 2022, 234, 108043. [Google Scholar] [CrossRef]
- Tan, Z.; Xiao, L.; Ma, J.; Shi, K.; Liu, J.; Feng, F.; Xie, P.; Dai, Y.; Yuan, Q.; Wu, W.; et al. Integrating Hydrogels Manipulate ECM Deposition after Spinal Cord Injury for Specific Neural Reconnections via Neuronal Relays. Sci. Adv. 2024, 10, eado9120. [Google Scholar] [CrossRef]
- Han, C.; Jiao, J.; Gong, C.; Li, J.; Zhao, M.; Lu, X. Multidimensional Exploration of Hydrogels as Biological Scaffolds for Spinal Cord Regeneration: Mechanisms and Future Perspectives. Front. Bioeng. Biotechnol. 2025, 13, 1576524. [Google Scholar] [CrossRef]
- Liao, Z.; Bao, Q.; Saijilahu; Chimedtseren, C.; Tumurbaatar, K.; Saijilafu. Research Progress on Biomaterials for Spinal Cord Repair. Int. J. Nanomed. 2025, 20, 1773–1787. [Google Scholar] [CrossRef]
- Moswatsi, B.; Mahumane, G.D.; Kumar, P.; Choonara, Y.E. A Review of Bigels for Neurotrauma Therapeutics: Structural Insights for Tissue Microenvironment Alignment. Biomater. Adv. 2025, 174, 214315. [Google Scholar] [CrossRef]
- Yari-Ilkhchi, A.; Hamidi, N.; Mahkam, M.; Ebrahimi-Kalan, A. Graphene-Based Materials: An Innovative Approach for Neural Regeneration and Spinal Cord Injury Repair. RSC Adv. 2025, 15, 9829–9853. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Kudva, A.K.; Saxena, N.S.; Roy, K. Engineering Articular Cartilage with Spatially-Varying Matrix Composition and Mechanical Properties from a Single Stem Cell Population Using a Multi-Layered Hydrogel. Biomaterials 2011, 32, 6946–6952. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Chen, X.; Li, R.; Feng, Q.; Bian, L. Multivalent Host–Guest Hydrogels as Fatigue-Resistant 3D Matrix for Excessive Mechanical Stimulation of Encapsulated Cells. Chem. Mater. 2017, 29, 8604–8610. [Google Scholar] [CrossRef]
- Kerscher, P.; Turnbull, I.C.; Hodge, A.J.; Kim, J.; Seliktar, D.; Easley, C.J.; Costa, K.D.; Lipke, E.A. Direct Hydrogel Encapsulation of Pluripotent Stem Cells Enables Ontomimetic Differentiation and Growth of Engineered Human Heart Tissues. Biomaterials 2016, 83, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial Scaffolds for Stem Cell Proliferation and Differentiation in Tissue Engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef]
- Salehi, S.S.; Shamloo, A.; Hannani, S.K. Microfluidic Technologies to Engineer Mesenchymal Stem Cell Aggregates-Applications and Benefits. Biophys. Rev. 2020, 12, 123–133. [Google Scholar] [CrossRef]
- Zeng, C.-W. Advancing Spinal Cord Injury Treatment through Stem Cell Therapy: A Comprehensive Review of Cell Types, Challenges, and Emerging Technologies in Regenerative Medicine. Int. J. Mol. Sci. 2023, 24, 14349. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Recent Advances on the Role of Brain-Derived Neurotrophic Factor (BDNF) in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef]
- Li, J.; Xiang, X.; Xu, H.; Shi, Y. Cilostazol Promotes Angiogenesis and Increases Cell Proliferation After Myocardial Ischemia-Reperfusion Injury Through a cAMP-Dependent Mechanism. Cardiovasc. Eng. Technol. 2019, 10, 638–647. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Sun, J.-H.; Shi, L.-Y.; Huang, M.-Y.; Huang, L.-J.; Lin, Z.-J.; Lin, Q.-Y.; Lai, B.-Q.; Ma, Y.-H.; et al. An NT-3-Releasing Bioscaffold Supports the Formation of TrkC-Modified Neural Stem Cell-Derived Neural Network Tissue with Efficacy in Repairing Spinal Cord Injury. Bioact. Mater. 2021, 6, 3766–3781. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Howe, C.L.; Mobley, W.C. Nerve Growth Factor Signaling, Neuroprotection, and Neural Repair. Annu. Rev. Neurosci. 2001, 24, 1217–1281. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Grose, R. Fibroblast Growth Factor Signalling: From Development to Cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Halabian, R.; Imani Fooladi, A.A. A Revealing Review of Mesenchymal Stem Cells Therapy, Clinical Perspectives and Modification Strategies. Stem Cell Investig. 2019, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hsu, S.-H. Biomaterials and Neural Regeneration. Neural Regen. Res. 2020, 15, 1243–1244. [Google Scholar] [CrossRef]
- Nurkesh, A.; Jaguparov, A.; Jimi, S.; Saparov, A. Recent Advances in the Controlled Release of Growth Factors and Cytokines for Improving Cutaneous Wound Healing. Front. Cell Dev. Biol. 2020, 8, 638. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; van der Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The Leading Edge: Emerging Neuroprotective and Neuroregenerative Cell-Based Therapies for Spinal Cord Injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Ilic, D.; Ogilvie, C. Pluripotent Stem Cells in Clinical Setting-New Developments and Overview of Current Status. Stem Cells 2022, 40, 791–801. [Google Scholar] [CrossRef]
- Pizzolato, C.; Gunduz, M.A.; Palipana, D.; Wu, J.; Grant, G.; Hall, S.; Dennison, R.; Zafonte, R.D.; Lloyd, D.G.; Teng, Y.D. Non-Invasive Approaches to Functional Recovery after Spinal Cord Injury: Therapeutic Targets and Multimodal Device Interventions. Exp. Neurol. 2021, 339, 113612. [Google Scholar] [CrossRef]
- Fang, J.; Li, J.J.; Zhong, X.; Zhou, Y.; Lee, R.J.; Cheng, K.; Li, S. Engineering Stem Cell Therapeutics for Cardiac Repair. J. Mol. Cell Cardiol. 2022, 171, 56–68. [Google Scholar] [CrossRef]
- Tsuji, O.; Sugai, K.; Yamaguchi, R.; Tashiro, S.; Nagoshi, N.; Kohyama, J.; Iida, T.; Ohkubo, T.; Itakura, G.; Isoda, M.; et al. Concise Review: Laying the Groundwork for a First-In-Human Study of an Induced Pluripotent Stem Cell-Based Intervention for Spinal Cord Injury. Stem Cells 2019, 37, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ross, P.J.; Ellis, J.; Salter, M.W. Targeting NMDA Receptors in Neuropsychiatric Disorders by Drug Screening on Human Neurons Derived from Pluripotent Stem Cells. Transl. Psychiatry 2022, 12, 243. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.-C.; Khan, A.; Song, R.; Kai-Yu Tong, R. Rewiring the Lesioned Brain: Electrical Stimulation for Post-Stroke Motor Restoration. J. Stroke 2020, 22, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.-Q.; Zeng, X.; Han, W.-T.; Che, M.-T.; Ding, Y.; Li, G.; Zeng, Y.-S. Stem Cell-Derived Neuronal Relay Strategies and Functional Electrical Stimulation for Treatment of Spinal Cord Injury. Biomaterials 2021, 279, 121211. [Google Scholar] [CrossRef]
- Brown, M.; Li, J.; Moraes, C.; Tabrizian, M.; Li-Jessen, N.Y.K. Decellularized Extracellular Matrix: New Promising and Challenging Biomaterials for Regenerative Medicine. Biomaterials 2022, 289, 121786. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Yu, S.; Yan, F.; Chen, J.; Liu, J.; Dong, C. Decellularized Extracellular Matrix in the Treatment of Spinal Cord Injury. Exp. Neurol. 2023, 368, 114506. [Google Scholar] [CrossRef]
- Liu, S.; Xie, Y.-Y.; Wang, B. Role and Prospects of Regenerative Biomaterials in the Repair of Spinal Cord Injury. Neural Regen. Res. 2019, 14, 1352–1363. [Google Scholar] [CrossRef]
- Chen, K.; Yu, W.; Zheng, G.; Xu, Z.; Yang, C.; Wang, Y.; Yue, Z.; Yuan, W.; Hu, B.; Chen, H. Biomaterial-Based Regenerative Therapeutic Strategies for Spinal Cord Injury. NPG Asia Mater. 2024, 16, 1–29. [Google Scholar] [CrossRef]
- Liang, C.; Liao, L.; Tian, W. Advances Focusing on the Application of Decellularized Extracellular Matrix in Periodontal Regeneration. Biomolecules 2023, 13, 673. [Google Scholar] [CrossRef]
- Xing, H.; Ren, X.; Yin, H.; Sun, C.; Jiang, T. Construction of a NT-3 Sustained-Release System Cross-Linked with an Acellular Spinal Cord Scaffold and Its Effects on Differentiation of Cultured Bone Marrow Mesenchymal Stem Cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109902. [Google Scholar] [CrossRef]
- Yao, Q.; Zheng, Y.-W.; Lan, Q.-H.; Kou, L.; Xu, H.-L.; Zhao, Y.-Z. Recent Development and Biomedical Applications of Decellularized Extracellular Matrix Biomaterials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109942. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Teng, M.; Zhang, Y.; Ji, W. Decellularized Extracellular Matrix Scaffold Seeded with Adipose-Derived Stem Cells Promotes Neurorestoration and Functional Recovery after Spinal Cord Injury through Wnt/β-Catenin Signaling Pathway Regulation. Biomed. Mater. 2023, 19, 15007. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, S.K.; Bardia, A.; Lakkireddy, C.; Paspala, S.A.B.; Khan, A.A. Bioengineering Human Neurological Constructs Using Decellularized Meningeal Scaffolds for Application in Spinal Cord Injury. Front. Bioeng. Biotechnol. 2018, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.M.; Ganguly, K. Management of Chronic Spinal Cord Dysfunction. Continuum 2015, 21, 188–200. [Google Scholar] [CrossRef]

| Stem Cell Type | Key Features | Applications | References |
|---|---|---|---|
| ESCs | Differentiate into neurons and glial cells; Express neuron-specific antigens; Reduce neuropathic pain and improve motor function in animal models; Minimal adverse effects. | CNS disease treatment; SCI pain and function improvement. | [17,18,19,20] |
| MSCs | Includes BM-MSCs, HUC-MSCs, and AD-MSCs; Cross BBB; Reduce inflammation; Promote neuroprotection, axonal growth, and motor function recovery; Clinical and experimental evidence supports safety and efficacy. | Stroke, SCI; Reducing inflammation and apoptosis; Promoting axonal growth. | [15,21,22,23,24] |
| HSCs | Enhance neurotrophin expression; Promote oligodendrocyte and fiber formation; Improve sensory and motor functions; Safe in clinical studies. | SCI sensory and motor recovery; Safe for long-term outcomes. | [26,27,28,29] |
| NSCs | Endogenous and exogenous NSCs can repair neurons, reduce inflammation, and promote axonal regeneration; Induce anti-inflammatory responses; Improve motor recovery. | SCI repair; Reduce inflammation; Promote axonal growth and recovery. | [7,14,16,30] |
| iPSCs | Highly versatile; Differentiate into neurons and glial cells; Experimental studies show improved motor recovery; Challenges include low survival and tumorigenic risk. | Future potential in clinical application; Promising for motor recovery in SCI. | [26,31,32,33,34,35] |
| Other SCs (DPSCs, OECs) | DPSCs: Differentiate into neural-like cells; Enhance regeneration via neurotrophic factors; Supported by biomaterials like scaffolds. OECs: Modulate environment for remyelination; Reduce neuroinflammation; Clinically promising. | Promising for SCI; Enhance regeneration and reduce inflammation; Supported by biomaterials. | [36,37,38,39,40,41,42,43,44,45] |
| NCT ID | Study Title | Phase | Subjects | Cell Therapy | Route | Intervention | Efficacy | Safety | Reference | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT02152657 | Pilot Study: Autologous MSC Transplant in Chronic SCI | 1 | 5 (18–65 yrs) | BM-MSCs | Percutaneous | MSC Transplant | Not reported | Not reported | [108] | Completed |
| NCT01325103 | Autologous BMSC Transplant in SCI Patients | 1 | 14 (18–65 yrs) | BM-MSCs (5 × 106 cells/cm3, single) | Intralesional | Autologous BM-MSC Transplant | Improved sensitivity, motor function, AIS, SSEP, and pain in some | One CSF leak, no severe effects | [109] | Completed |
| NCT02482194 | Phase I: Autologous MSC Transplant for SCI | 1 | 9 (18–50 yrs) | BM-MSCs (1.2 × 106 cells/kg, 2–3 doses) | Intrathecal | Autologous BM-MSC Transplant | No MRI changes or ectopic tissue after 1 yr | Severe headache (1), tingling (2), no severe AE | [110] | Completed |
| NCT01909154 | Safety of BMSC in Chronic Paraplegia | 1 | 9 (18–50 yrs) | BM-MSCs (100 × 106 + 30 × 106 after 3 mo) | Intrathecal | Autologous BM-MSC Transplant | Sensitivity, pain, SSEP, urodynamic gains after 12 mo | AEs in all, no serious AEs | [111] | Completed |
| NCT01328860 | Autologous Stem Cells for Pediatric SCI | 1 | 10 (1–15 yrs) | BMPCs | Intravenous | Autologous BMPC Transplant | Not reported | Not reported | [112] | Completed |
| NCT01186679 | Safety/Efficacy of BMSC in SCI Treatment | 1/2 | 12 (20–55 yrs) | BM-MSCs | Intrathecal | Autologous BM-MSC Transplant | Not reported | Not reported | [113] | Completed |
| NCT00816803 | Cell Transplant for SCI Patients | 1/2 | 70 (10–36 yrs) | BM-MSCs | Intrathecal | Autologous BM-MSC Transplant | ASIA conversion (17/50), motor gains, tissue repair in some | Transient headache/pain, resolved | [114] | Completed |
| NCT02570932 | Expanded BM-MSC in Chronic SCI | 2 | 10 (18–70 yrs) | BM-MSCs (100 × 106, 3 doses) | Intrathecal | Autologous BM-MSC Transplant | Not reported | Not reported | [115] | Completed |
| NCT02981576 | BM-MSC vs. AT-MSC Safety/Efficacy in SCI | 1/2 | 14 (18–70 yrs) | BM-MSCs & AT-MSCs (3 doses) | Intrathecal | Autologous BM/AT-MSC Transplant | Not reported | Not reported | [116] | Completed |
| NCT01274975 | Autologous AT-MSC Transplant in SCI | 1 | 8 (19–60 yrs) | AT-MSCs (4 × 108, single) | Intravenous | Autologous AT-MSC Transplant | Reduced lesion size, ASIA A to C (1), motor/sensory gains | No severe AE, 19 AEs resolved/stabilized | [117] | Completed |
| NCT01624779 | Intrathecal AT-MSC Transplant in SCI | 1 | 15 (19–70 yrs) | AT-MSCs (9 × 107/3 mL, 3 doses) | Intrathecal | Autologous AT-MSC Transplant | Not reported | Not reported | [118] | Completed |
| NCT01769872 | Safety/Effect of AT-MSC in SCI | 1/2 | 15 (19–70 yrs) | AT-MSCs (variable doses) | IV, Intrathecal, Intralesional | Autologous AT-MSC Transplant | Not reported | Not reported | [119] | Completed |
| NCT01393977 | Stem Cells vs. Rehab in SCI (China) | 3 | 34 (20–50 yrs) | UC-MSCs (4 × 107) | Intrathecal | UC-MSC + Rehab Therapy | Motor, self-ability, tension gains (7/10) vs. minor rehab gains | No side effects | [120] | Completed |
| NCT01873547 | Stem Cells vs. Rehab Efficacy in SCI (China) | 3 | 300 (20–65 yrs) | UC-MSCs | Intrathecal | UC-MSC + Rehab Therapy | Not Applicable | Not Applicable | [121] | Ongoing |
| NCT01321333 | HuCNS-SC in Thoracic SCI | 1/2 | 12 (18–60 yrs) | Human CNS-SCs | Intramedullary | CNS-SC Transplant | Not reported | Not reported | [122] | Completed |
| NCT01725880 | Long-Term Follow-Up of HuCNS-SC in SCI | - | 12 (18–60 yrs) | Human CNS-SCs | Intramedullary | CNS-SC Transplant | Not reported | Not reported | [123] | Completed |
| NCT02163876 | HuCNS-SC Transplant in Cervical SCI | 2 | 31 (18–60 yrs) | Human CNS-SCs | Intramedullary | CNS-SC Transplant | Not reported | Not reported | [124] | Completed |
| NCT02302157 | AST-OPC1 Dose Escalation in SCI | 1/2 | 25 (18–69 yrs) | AST-OPC1 (10 M, 2 doses) | Not specified | AST-OPC1 Transplant | Not reported | Not reported | [125] | Completed |
| NCT03505034 | UC-MSC in Late-Stage Chronic SCI | 2 | 30 (18–65 yrs) | UC-MSCs (1 × 106/kg, 4 doses) | Intrathecal | Allogenic UC-MSC Transplant | Not Applicable | Not Applicable | [126] | Ongoing |
| NCT03521336 | UC-MSC in Sub-Acute SCI | 2 | 84 (18–65 yrs) | UC-MSCs (1 × 106/kg, 4 doses) | Intrathecal | Allogenic UC-MSC Transplant | Not Applicable | Not Applicable | [127] | Ongoing |
| NCT03521323 | UC-MSC in Early Chronic SCI | 2 | 66 (≥18 yrs) | UC-MSCs (1 × 106/kg, 4 doses) | Intrathecal | Allogenic UC-MSC Transplant | Not Applicable | Not Applicable | [128] | Ongoing |
| NCT03003364 | Wharton’s Jelly MSC in Chronic Traumatic SCI | 1/2a | 10 (18–65 yrs) | WJ-MSCs (2 doses) | Intrathecal | WJ-MSC Transplant | Not Applicable | Not Applicable | [129] | Ongoing |
| NCT03308565 | AT-MSC for Traumatic SCI | 1 | 10 (≥18 yrs) | AT-MSCs (100 M, single) | Intrathecal | Autologous AT-MSC Transplant | Not Applicable | Not Applicable | [130] | Ongoing |
| NCT02574572 | BM-MSC in Cervical Chronic Complete SCI | 1 | 10 (18–65 yrs) | BM-MSCs (2 doses) | Percutaneous | Autologous BM-MSC Transplant | Not Applicable | Not Applicable | [131] | Ongoing |
| NCT02574585 | BM-MSC in Thoracolumbar Chronic Complete SCI | 2 | 40 (18–65 yrs) | BM-MSCs (2 doses) | Percutaneous | Autologous BM-MSC Transplant | Not Applicable | Not Applicable | [132] | Ongoing |
| NCT01676441 | Safety/Efficacy of BM-MSC in Chronic SCI | 2/3 | 32 (16–65 yrs) | BM-MSCs (1 × 106 & 1 × 107, 2 doses) | Intrathecal | Autologous BM-MSC Transplant | Not Applicable | Not Applicable | [133] | Ongoing |
| NCT02687672 | BMSC Transplant for SCI Treatment | 1/2 | 50 (5–50 yrs) | BM-MSCs | Not specified | Autologous BM-MSC Transplant | Not Applicable | Not Applicable | [134] | Ongoing |
| NCT01772810 | Safety of Spinal Cord-Derived NSC in Chronic SCI | 1 | 8 (18–65 yrs) | Spinal Cord NSC | Not specified | NSC Transplant | Not Applicable | Not Applicable | [135] | Ongoing |
| NCT04205019 | Safety of Neuro-Cells in SCI | 1 | 10 (18–40 yrs) | Neuro-Cells | Intrathecal | Neuro-Cells Transplant | Not Applicable | No serious safety concerns or product-related adverse events | [136] | Completed |
| NCT03935724 | Autologous Stem Cell Product in (Sub)Acute SCI | 2/3 | 8 (18–65 yrs) | Neuro-Cells | Not specified | Neuro-Cells Transplant | Not Applicable | Not Applicable | [137] | Ongoing |
| Summary of Mechanisms of Action of Exosomes | |||
|---|---|---|---|
| Mechanism of Action | |||
| Mechanism of Action | Function | Key Molecules/Markers | References |
| Neuroinflammation Control | Reduces pro-inflammatory cytokines by shifting macrophages to the anti-inflammatory M2 phenotype. | CD206, miRNA-216a, NF-κB suppression | [154,155,156,157,158,159,160,161,162] |
| Apoptosis Inhibition | Prevents neuronal death by modulating pro- and anti-apoptotic factors. | Bcl-2 (upregulated), Bax, caspase-3 (downregulated) | [163,164,165,166,167,168] |
| Angiogenesis Promotion | Stimulates blood vessel formation to enhance oxygen and nutrient delivery at the injury site. | Angiogenic factors (VEGF, bFGF) | [148,169,170] |
| Glial Scarring Regulation | Decreases astrocyte activation and shifts towards the neuroprotective A2 astrocyte phenotype. | GFAP (reduced), neurotrophic factors | [169,171,172,173,174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riza, Y.M.; Alzahrani, F.A. Rewiring the Spine—Cutting-Edge Stem Cell Therapies for Spinal Cord Repair. Int. J. Mol. Sci. 2025, 26, 5048. https://doi.org/10.3390/ijms26115048
Riza YM, Alzahrani FA. Rewiring the Spine—Cutting-Edge Stem Cell Therapies for Spinal Cord Repair. International Journal of Molecular Sciences. 2025; 26(11):5048. https://doi.org/10.3390/ijms26115048
Chicago/Turabian StyleRiza, Yasir Mohamed, and Faisal A. Alzahrani. 2025. "Rewiring the Spine—Cutting-Edge Stem Cell Therapies for Spinal Cord Repair" International Journal of Molecular Sciences 26, no. 11: 5048. https://doi.org/10.3390/ijms26115048
APA StyleRiza, Y. M., & Alzahrani, F. A. (2025). Rewiring the Spine—Cutting-Edge Stem Cell Therapies for Spinal Cord Repair. International Journal of Molecular Sciences, 26(11), 5048. https://doi.org/10.3390/ijms26115048







