The Role of Long Non-Coding RNA in Anxiety Disorders: A Literature Review
Abstract
1. Introduction
2. Literature Screening Methods
3. Long Non-Coding RNA in the Pathogenesis of Anxiety Disorders
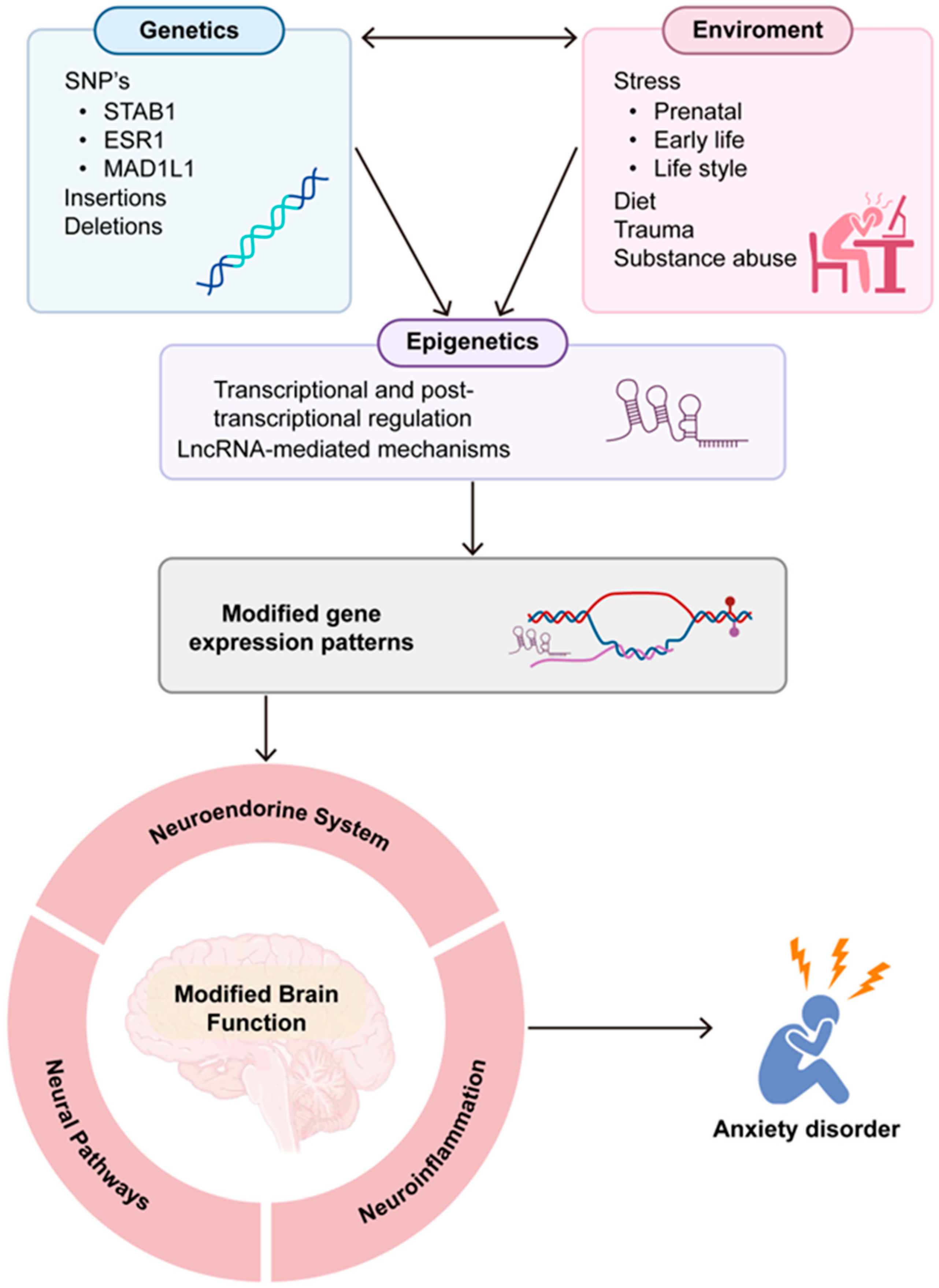
3.1. Anxiety Pathogenesis
3.2. Participation of lncRNAs in the Regulation of Neurodevelopment
3.3. Influence of lncRNAs on Neurotransmission
3.4. Neuroinflammation Related to Anxiety
4. Long Non-Coding RNA as a Potential Diagnostic Biomarker
5. Targeting Long Non-Coding RNA
5.1. Antisense Oligonucleotides (ASOs)
5.2. Small Interfering RNAs (siRNAs)
5.3. Natural Antisense Transcripts (NATs)
5.4. MiRNAs and LncRNAs
5.5. Other Molecular Approaches
6. Discussion
6.1. Contradictory Results
6.2. Clinical Translational Bottlenecks
6.3. Sex-Biased LncRNAs in the Brain
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADs | Anxiety disorders |
| AKT | Protein kinase B |
| AMI | Acute myocardial infarction |
| AML | Acute myeloid leukemia |
| ASOs | Antisense oligonucleotides |
| B200 | Brain cytoplasmic 200 lncRNA |
| BC1 | Brain cytoplasmic 200 lncRNA |
| BD | Bipolar disorder |
| BDNF | Brain-derived neurotrophic factor |
| BDNF-AS | Brain-derived neurotrophic factor antisense RNA |
| ceRNA | Competitive endogenous RNAs |
| circRNAs | Circular RNAs |
| CNS | Central nervous system |
| CRH | Corticotropin-releasing hormone |
| CRHBP | CRH binding protein |
| DISC1 | Disrupted in schizophrenia 1 |
| DLX | Distal-less homeobox |
| ERBB4 | Erb-B2 receptor tyrosine kinase 4 |
| ESR1 | Estrogen receptor 1 |
| Evf2 | Embryonic ventral forebrain 2 |
| GABA | Gamma-aminobutyric acid |
| GAD | Generalized anxiety disorder |
| H19 | H19 maternally imprinted expressed transcript |
| HOTAIR | HOX transcript antisense RNA |
| HPA | Hypothalamic–pituitary–adrenal |
| IL | Interleukin |
| JAK/STAT | Janus kinase/signal transducers and activators of transcription |
| JNK | c-Jun N-terminal kinases |
| lincRNA-p21 | Long intergenic ncRNA p21 |
| lncRNA(s) | Long non-coding RNA (s) |
| lncRNA-COX-2 | Cyclooxygenase 2 lncRNA |
| MAD1L1 | Mitotic arrest deficient 1 like 1 |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 |
| MECP2 | Methyl-CpG binding protein 2 |
| MEG3 | Maternally expressed gene 3 |
| MIAT | Myocardial infarction-associated transcript |
| miRNA(s) | MicroRNA (s) |
| MKK4 | Mitogen-activated protein kinase kinase 4 |
| mRNA | Messenger RNA |
| NATs | Natural antisense transcript (s) |
| ncRNA (s) | Non-coding RNA (s) |
| NEAT1 | Nuclear-enriched abundant transcript 1 |
| NF-κB | Nuclear factor kappa B |
| NKILA | NF-KappaB interacting lncRNA |
| NRLP3 | NLR family pyrin domain containing 3 |
| NSC | Neural stem cell |
| PI3K | Phosphoinositide-3 kinases |
| PRC2 | Polycomb repressive complex 2 |
| PTBP1 | Polypyrimidine tract binding protein 1 |
| PTSD | Post-traumatic stress disorder |
| RMRP | Mitochondrial RNA-processing endoribonuclease |
| RMST | Rhabdomyosarcoma 2-associated transcript |
| siRNAs | Small interfering RNAs |
| SNP | Single-nucleotide polymorphism |
| SNPs | Single-nucleotide polymorphisms |
| sRNA | Small RNA |
| STAB1 | Stabilin 1 protein coding gene |
| SZ | Schizophrenia |
| T2D | Type 2 diabetes |
| TAK1 | Transforming growth factor-β-activated kinase 1 |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| TUG1 | Taurine up-regulated 1 |
| WNT7B | Wingless-type family member 7B |
References
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [CrossRef] [PubMed]
- Domínguez, J.A.D.; Duque, V.E.; Tejera, E.T. Epidemiología de La Ansiedad y Su Contexto En Atención Primaria. Aten. Prim. Práct. 2024, 6, 100194. [Google Scholar] [CrossRef]
- Peedicayil, J. The Potential Role of Epigenetic Drugs in the Treatment of Anxiety Disorders. Neuropsychiatr. Dis. Treat. 2020, 16, 597. [Google Scholar] [CrossRef]
- Bie, F.; Yan, X.; Xing, J.; Wang, L.; Xu, Y.; Wang, G.; Wang, Q.; Guo, J.; Qiao, J.; Rao, Z. Rising Global Burden of Anxiety Disorders among Adolescents and Young Adults: Trends, Risk Factors, and the Impact of Socioeconomic Disparities and COVID-19 from 1990 to 2021. Front. Psychiatry 2024, 15, 1489427. [Google Scholar] [CrossRef]
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety Disorders. Lancet 2021, 397, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Fang, Y.; Chen, H.; Zhang, T.; Yin, X.; Man, J.; Yang, L.; Lu, M. Global, Regional and National Burden of Anxiety Disorders from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Epidemiol. Psychiatr. Sci. 2021, 30, e36. [Google Scholar] [CrossRef]
- Smoller, J.W. Anxiety Genetics Goes Genomic. Am. J. Psychiatry 2020, 177, 190–194. [Google Scholar] [CrossRef]
- Asociación Americana de Psiquiatría. Manual Diagnóstico y Estadístico de Los Trastornos Mentales (DSM-V), 5th ed.; Editorial Médica Panamericana: Madrid, Spain, 2014. [Google Scholar]
- Bosman, R.C.; van Balkom, A.J.L.M.; Rhebergen, D.; van Hemert, A.M.; Schoevers, R.A.; Penninx, B.W.J.H.; Batelaan, N.M. Predicting the Course of Anxiety Disorders: The Role of Biological Parameters. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 101, 109924. [Google Scholar] [CrossRef]
- Craske, M.G.; Stein, M.B. Anxiety. Lancet 2016, 388, 3048–3059. [Google Scholar] [CrossRef]
- González-Castro, T.B.; Pool-García, S.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Frésan, A.; Genis-Mendoza, A.D.; Pérez-Hernández, N.; Nicolini, H. Association between BDNF Val66Met Polymorphism and Generalized Anxiety Disorder and Clinical Characteristics in a Mexican Population. Medicine 2019, 98, e14838. [Google Scholar] [CrossRef]
- Shimada-Sugimoto, M.; Otowa, T.; Hettema, J.M. Genetics of Anxiety Disorders: Genetic Epidemiological and Molecular Studies in Humans. Psychiatry Clin. Neurosci. 2015, 69, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, M.-K.; Hovatta, I. Genetic Insights into the Neurobiology of Anxiety. Trends Neurosci. 2023, 46, 318–331. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.A.; Singh, R.; Hunter, R.G. Anxiety and Epigenetics. Adv. Exp. Med. Biol. 2017, 978, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Nechita, D.; Nechita, F.; Motorga, R. A Review of the Influence the Anxiety Exerts on Human Life. Rom. J. Morphol. Embryol. 2018, 59, 1045–1051. [Google Scholar]
- Zou, Y.; Guo, Q.; Chang, Y.; Zhong, Y.; Cheng, L.; Wei, W. Effects of Maternal High-Fructose Diet on Long Non-Coding RNAs and Anxiety-like Behaviors in Offspring. Int. J. Mol. Sci. 2023, 24, 4460. [Google Scholar] [CrossRef]
- Murphy, C.P.; Singewald, N. Role of MicroRNAs in Anxiety and Anxiety-Related Disorders. Curr. Top. Behav. Neurosci. 2019, 42, 185–219. [Google Scholar] [CrossRef]
- López-Jiménez, E.; Andrés-León, E. The Implications of NcRNAs in the Development of Human Diseases. Noncoding RNA 2021, 7, 17. [Google Scholar] [CrossRef]
- Chen, L.-L.; Kim, V.N. Small and Long Non-Coding RNAs: Past, Present, and Future. Cell 2024, 187, 6451–6485. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Aliperti, V.; Skonieczna, J.; Cerase, A. Long Non-Coding RNA (LncRNA) Roles in Cell Biology, Neurodevelopment and Neurological Disorders. Noncoding RNA 2021, 7, 36. [Google Scholar] [CrossRef]
- Ilieva, M.S. Non-Coding RNAs in Neurological and Neuropsychiatric Disorders: Unraveling the Hidden Players in Disease Pathogenesis. Cells 2024, 13, 1063. [Google Scholar] [CrossRef] [PubMed]
- Leija Montoya, G.; González Ramírez, J.; Sandoval Basilio, J.; Serafín Higuera, I.; Isiordia Espinoza, M.; González González, R.; Serafín Higuera, N. Long Non-Coding RNAs: Regulators of the Activity of Myeloid-Derived Suppressor Cells. Front. Immunol. 2019, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, I.A.; Mehler, M.F. Emerging Roles of Non-Coding RNAs in Brain Evolution, Development, Plasticity and Disease. Nat. Rev. Neurosci. 2012, 13, 528–541. [Google Scholar] [CrossRef]
- Dahariya, S.; Paddibhatla, I.; Kumar, S.; Raghuwanshi, S.; Pallepati, A.; Gutti, R.K. Long Non-Coding RNA: Classification, Biogenesis and Functions in Blood Cells. Mol. Immunol. 2019, 112, 82–92. [Google Scholar] [CrossRef]
- Munshi, S.; Loh, M.K.; Ferrara, N.; DeJoseph, M.R.; Ritger, A.; Padival, M.; Record, M.J.; Urban, J.H.; Rosenkranz, J.A. Repeated Stress Induces a Pro-Inflammatory State, Increases Amygdala Neuronal and Microglial Activation, and Causes Anxiety in Adult Male Rats. Brain Behav. Immun. 2020, 84, 180–199. [Google Scholar] [CrossRef]
- Tursich, M.; Neufeld, R.W.J.; Frewen, P.A.; Harricharan, S.; Kibler, J.L.; Rhind, S.G.; Lanius, R.A. Association of Trauma Exposure with Proinflammatory Activity: A Transdiagnostic Meta-Analysis. Transl. Psychiatry 2014, 4, e413. [Google Scholar] [CrossRef]
- Zheng, Z.-H.; Tu, J.-L.; Li, X.-H.; Hua, Q.; Liu, W.-Z.; Liu, Y.; Pan, B.-X.; Hu, P.; Zhang, W.-H. Neuroinflammation Induces Anxiety- and Depressive-like Behavior by Modulating Neuronal Plasticity in the Basolateral Amygdala. Brain Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Levey, D.F.; Gelernter, J.; Polimanti, R.; Zhou, H.; Cheng, Z.; Aslan, M.; Quaden, R.; Concato, J.; Radhakrishnan, K.; Bryois, J.; et al. Reproducible Genetic Risk Loci for Anxiety: Results From ∼200,000 Participants in the Million Veteran Program. Am. J. Psychiatry 2020, 177, 223–232. [Google Scholar] [CrossRef]
- Fox-Gaffney, K.A.; Singh, P.K. Genetic and Environmental Influences on Anxiety Disorders: A Systematic Review of Their Onset and Development. Cureus 2025, 17, e80157. [Google Scholar] [CrossRef]
- Jiang, S.; Postovit, L.; Cattaneo, A.; Binder, E.B.; Aitchison, K.J. Epigenetic Modifications in Stress Response Genes Associated with Childhood Trauma. Front. Psychiatry 2019, 10, 808. [Google Scholar] [CrossRef]
- Barry, G. Integrating the Roles of Long and Small Non-Coding RNA in Brain Function and Disease. Mol. Psychiatry 2014, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lu, Y.; Pan, B.-X.; Zhang, W.-H. New Insights into the Pivotal Role of the Amygdala in Inflammation-Related Depression and Anxiety Disorder. Int. J. Mol. Sci. 2022, 23, 11076. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Zhang, M.; Hao, W.; Wang, Y.; Zhang, T.; Liu, C. Neuroinflammation Mechanisms of Neuromodulation Therapies for Anxiety and Depression. Transl. Psychiatry 2023, 13, 5. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.L.; Kahn, U.N.; Grafe, L.A.; Hitti, F.L.; Fried, N.T.; Corbett, B.F. Stress Circuitry: Mechanisms behind Nervous and Immune System Communication That Influence Behavior. Front. Psychiatry 2023, 14, 1240783. [Google Scholar] [CrossRef]
- Kupcova, I.; Danisovic, L.; Grgac, I.; Harsanyi, S. Anxiety and Depression: What Do We Know of Neuropeptides? Behav. Sci. 2022, 12, 262. [Google Scholar] [CrossRef]
- Engel, S.; Laufer, S.; Knaevelsrud, C.; Schumacher, S. The Endogenous Oxytocin System in Depressive Disorders: A Systematic Review and Meta-Analysis. Psychoneuroendocrinology 2019, 101, 138–149. [Google Scholar] [CrossRef]
- Li, K.; Nakajima, M.; Ibañez-Tallon, I.; Heintz, N. A Cortical Circuit for Sexually Dimorphic Oxytocin-Dependent Anxiety Behaviors. Cell 2016, 167, 60–72.e11. [Google Scholar] [CrossRef]
- Policarpo, R.; Sierksma, A.; De Strooper, B.; d’Ydewalle, C. From Junk to Function: LncRNAs in CNS Health and Disease. Front. Mol. Neurosci. 2021, 14, 714768. [Google Scholar] [CrossRef]
- Accogli, A.; Addour-Boudrahem, N.; Srour, M. Neurogenesis, Neuronal Migration, and Axon Guidance. Handb. Clin. Neurol. 2020, 173, 25–42. [Google Scholar] [CrossRef]
- Briggs, J.A.; Wolvetang, E.J.; Mattick, J.S.; Rinn, J.L.; Barry, G. Mechanisms of Long Non-Coding RNAs in Mammalian Nervous System Development, Plasticity, Disease, and Evolution. Neuron 2015, 88, 861–877. [Google Scholar] [CrossRef] [PubMed]
- Zimmer-Bensch, G. Emerging Roles of Long Non-Coding RNAs as Drivers of Brain Evolution. Cells 2019, 8, 1399. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Vangompel, M.J.W.; Sametsky, E.A.; Clark, M.F.; Savage, J.C.; Disterhoft, J.F.; Kohtz, J.D. Balanced Gene Regulation by an Embryonic Brain NcRNA Is Critical for Adult Hippocampal GABA Circuitry. Nat. Neurosci. 2009, 12, 1020–1027. [Google Scholar] [CrossRef]
- Ramos, A.D.; Andersen, R.E.; Liu, S.J.; Nowakowski, T.J.; Hong, S.J.; Gertz, C.; Salinas, R.D.; Zarabi, H.; Kriegstein, A.R.; Lim, D.A. The Long Noncoding RNA Pnky Regulates Neuronal Differentiation of Embryonic and Postnatal Neural Stem Cells. Cell Stem Cell 2015, 16, 439–447. [Google Scholar] [CrossRef]
- Bernard, D.; Prasanth, K.V.; Tripathi, V.; Colasse, S.; Nakamura, T.; Xuan, Z.; Zhang, M.Q.; Sedel, F.; Jourdren, L.; Coulpier, F.; et al. A Long Nuclear-Retained Non-Coding RNA Regulates Synaptogenesis by Modulating Gene Expression. EMBO J. 2010, 29, 3082–3093. [Google Scholar] [CrossRef]
- Ming, Y.; Deng, Z.; Tian, X.; Jia, Y.; Ning, M.; Cheng, S. M6A Methyltransferase METTL3 Reduces Hippocampal Neuron Apoptosis in a Mouse Model of Autism Through the MALAT1/SFRP2/Wnt/β-Catenin Axis. Psychiatry Investig. 2022, 19, 771–787. [Google Scholar] [CrossRef]
- Zhang, B.; Arun, G.; Mao, Y.S.; Lazar, Z.; Hung, G.; Bhattacharjee, G.; Xiao, X.; Booth, C.J.; Wu, J.; Zhang, C.; et al. The LncRNA Malat1 Is Dispensable for Mouse Development but Its Transcription Plays a Cis-Regulatory Role in the Adult. Cell Rep. 2012, 2, 111–123. [Google Scholar] [CrossRef]
- Butler, A.A.; Johnston, D.R.; Kaur, S.; Lubin, F.D. Long Noncoding RNA NEAT1 Mediates Neuronal Histone Methylation and Age-Related Memory Impairment. Sci. Signal. 2019, 12, eaaw9277. [Google Scholar] [CrossRef]
- Kukharsky, M.S.; Ninkina, N.N.; An, H.; Telezhkin, V.; Wei, W.; De Meritens, C.R.; Cooper-Knock, J.; Nakagawa, S.; Hirose, T.; Buchman, V.L.; et al. Long Non-Coding RNA Neat1 Regulates Adaptive Behavioural Response to Stress in Mice. Transl. Psychiatry 2020, 10, 171. [Google Scholar] [CrossRef]
- Srinivas, T.; Mathias, C.; Oliveira-Mateos, C.; Guil, S. Roles of LncRNAs in Brain Development and Pathogenesis: Emerging Therapeutic Opportunities. Mol. Ther. 2023, 31, 1550. [Google Scholar] [CrossRef] [PubMed]
- Sas-Nowosielska, H.; Magalska, A. Long Noncoding RNAs—Crucial Players Organizing the Landscape of the Neuronal Nucleus. Int. J. Mol. Sci. 2021, 22, 3478. [Google Scholar] [CrossRef]
- Sone, M.; Hayashi, T.; Tarui, H.; Agata, K.; Takeichi, M.; Nakagawa, S. The MRNA-like Noncoding RNA Gomafu Constitutes a Novel Nuclear Domain in a Subset of Neurons. J. Cell Sci. 2007, 120, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.; Li, Y.; Ku, L.; Wang, F.; Goldsmith, D.R.; Wen, Z.; Yao, B.; Feng, Y. The Human LncRNA GOMAFU Suppresses Neuronal Interferon Response Pathways Affected in Neuropsychiatric Diseases. Brain Behav. Immun. 2023, 112, 175–187. [Google Scholar] [CrossRef]
- Wang, A.; Wang, J.; Liu, Y.; Zhou, Y. Mechanisms of Long Non-Coding RNAs in the Assembly and Plasticity of Neural Circuitry. Front. Neural Circuits 2017, 11, 76. [Google Scholar] [CrossRef]
- Briz, V.; Restivo, L.; Pasciuto, E.; Juczewski, K.; Mercaldo, V.; Lo, A.C.; Baatsen, P.; Gounko, N.V.; Borreca, A.; Girardi, T.; et al. The Non-Coding RNA BC1 Regulates Experience-Dependent Structural Plasticity and Learning. Nat. Commun. 2017, 8, 293. [Google Scholar] [CrossRef] [PubMed]
- Cogill, S.B.; Srivastava, A.K.; Yang, M.Q.; Wang, L. Co-Expression of Long Non-Coding RNAs and Autism Risk Genes in the Developing Human Brain. BMC Syst. Biol. 2018, 12, 91. [Google Scholar] [CrossRef]
- Rusconi, F.; Battaglioli, E.; Venturin, M. Psychiatric Disorders and LncRNAs: A Synaptic Match. Int. J. Mech. Sci. 2020, 21, 3030. [Google Scholar] [CrossRef]
- Shi, H.-J.; Wang, S.; Wang, X.-P.; Zhang, R.-X.; Zhu, L.-J. Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety. Neurosci. Bull. 2023, 39, 1009–1026. [Google Scholar] [CrossRef]
- Fink, G. Stress, Definitions, Mechanisms, and Effects Outlined: Lessons from Anxiety. Stress Concepts Cogn. Emot. Behav. 2016, 1, 3–11. [Google Scholar] [CrossRef]
- Maldonado, R.; Cabañero, D.; Martín-García, E. The Endocannabinoid System in Modulating Fear, Anxiety, and Stress. Dialogues Clin. Neurosci. 2020, 22, 229–239. [Google Scholar] [CrossRef]
- Ang, C.E.; Trevino, A.E.; Chang, H.Y. Diverse LncRNA Mechanisms in Brain Development and Disease. Curr. Opin. Genet. Dev. 2020, 65, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kumar, S. Association of LncRNA with Regulatory Molecular Factors in Brain and Their Role in the Pathophysiology of Schizophrenia. Metab. Brain Dis. 2021, 36, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Spadaro, P.A.; Flavell, C.R.; Widagdo, J.; Ratnu, V.S.; Troup, M.; Ragan, C.; Mattick, J.S.; Bredy, T.W. Long Noncoding RNA-Directed Epigenetic Regulation of Gene Expression Is Associated with Anxiety-like Behavior in Mice. Biol. Psychiatry 2015, 78, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, P.; Liu, L.; Goodwin, A.J.; Halushka, P.V.; Hirose, T.; Nakagawa, S.; Zhou, J.; Liu, M.; Fan, H. LncRNA Neat1 Regulates Neuronal Dysfunction Post-Sepsis via Stabilization of Hemoglobin Subunit Beta. Mol. Ther. 2022, 30, 2618. [Google Scholar] [CrossRef]
- Bohnsack, J.P.; Teppen, T.; Kyzar, E.J.; Dzitoyeva, S.; Pandey, S.C. The LncRNA BDNF-AS Is an Epigenetic Regulator in the Human Amygdala in Early Onset Alcohol Use Disorders. Transl. Psychiatry 2019, 9, 34. [Google Scholar] [CrossRef]
- Ni, Y.-Q.; Xu, H.; Liu, Y.-S. Roles of Long Non-Coding RNAs in the Development of Aging-Related Neurodegenerative Diseases. Front. Mol. Neurosci. 2022, 15, 844193. [Google Scholar] [CrossRef]
- Brigadski, T.; Leßmann, V. The physiology of regulated BDNF release. Cell Tissue Res. 2020, 382, 15–45. [Google Scholar] [CrossRef]
- Zhu, X.; Xie, X.; Das, H.; Tan, B.G.; Shi, Y.; Al-Behadili, A.; Peter, B.; Motori, E.; Valenzuela, S.; Posse, V.; et al. Non-Coding 7S RNA Inhibits Transcription via Mitochondrial RNA Polymerase Dimerization. Cell 2022, 185, 2309–2323.e24. [Google Scholar] [CrossRef]
- Reyes, A.; Rusecka, J.; Tońska, K.; Zeviani, M. RNase H1 Regulates Mitochondrial Transcription and Translation via the Degradation of 7S RNA. Front. Genet. 2020, 10, 1393. [Google Scholar] [CrossRef]
- Wang, X.; Memon, A.A.; Hedelius, A.; Grundberg, A.; Sundquist, J.; Sundquist, K. Circulating Mitochondrial Long Non-Coding 7S RNA in Primary Health Care Patients with Depression/Anxiety. J. Affect. Disord. 2024, 349, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Azman, K.F.; Yahaya, R.; Shafin, N.; Omar, N.; Ahmad, A.H.; Zakaria, R.; Wijaya, A.; Othman, Z. Brain-Derived Neurotrophic Factor (BDNF) in Schizophrenia Research: A Quantitative Review and Future Directions. AIMS Neurosci. 2023, 10, 5–32. [Google Scholar] [CrossRef] [PubMed]
- Hole, C.; Dhamsania, A.; Brown, C.; Ryznar, R. Immune Dysregulation in Depression and Anxiety: A Review of the Immune Response in Disease and Treatment. Cells 2025, 14, 607. [Google Scholar] [CrossRef]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2023, 24, 578. [Google Scholar] [CrossRef]
- Cunningham, A.J.; Murray, C.A.; O’Neill, L.A.J.; Lynch, M.A.; O’Connor, J.J. Interleukin-1β (IL-1β) and Tumour Necrosis Factor (TNF) Inhibit Long-Term Potentiation in the Rat Dentate Gyrus in Vitro. Neurosci. Lett. 1996, 203, 17–20. [Google Scholar] [CrossRef]
- Yue, N.; Huang, H.; Zhu, X.; Han, Q.; Wang, Y.; Li, B.; Liu, Q.; Wu, G.; Zhang, Y.; Yu, J. Activation of P2X7 Receptor and NLRP3 Inflammasome Assembly in Hippocampal Glial Cells Mediates Chronic Stress-Induced Depressive-like Behaviors. J. Neuroinflamm. 2017, 14, 102. [Google Scholar] [CrossRef]
- Green, H.F.; Nolan, Y.M. Inflammation and the Developing Brain: Consequences for Hippocampal Neurogenesis and Behavior. Neurosci. Biobehav. Rev. 2014, 40, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ohgidani, M.; Kato, T.A.; Sagata, N.; Hayakawa, K.; Shimokawa, N.; Sato-Kasai, M.; Kanba, S. TNF-α from Hippocampal Microglia Induces Working Memory Deficits by Acute Stress in Mice. Brain Behav. Immun. 2016, 55, 17–24. [Google Scholar] [CrossRef]
- Revest, J.-M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.-V.; Abrous, D.N. Adult Hippocampal Neurogenesis Is Involved in Anxiety-Related Behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Inagaki, T.K.; Muscatell, K.A.; Irwin, M.R.; Cole, S.W.; Eisenberger, N.I. Inflammation Selectively Enhances Amygdala Activity to Socially Threatening Images. Neuroimage 2012, 59, 3222–3226. [Google Scholar] [CrossRef]
- Lebow, M.A.; Chen, A. Overshadowed by the Amygdala: The Bed Nucleus of the Stria Terminalis Emerges as Key to Psychiatric Disorders. Mol. Psychiatry 2016, 21, 450–463. [Google Scholar] [CrossRef] [PubMed]
- Etkin, A.; Wager, T.D. Functional Neuroimaging of Anxiety: A Meta-Analysis of Emotional Processing in PTSD, Social Anxiety Disorder, and Specific Phobia. Am. J. Psychiatry 2007, 164, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Muscatell, K.A.; Dedovic, K.; Slavich, G.M.; Jarcho, M.R.; Breen, E.C.; Bower, J.E.; Irwin, M.R.; Eisenberger, N.I. Greater Amygdala Activity and Dorsomedial Prefrontal-Amygdala Coupling Are Associated with Enhanced Inflammatory Responses to Stress. Brain Behav. Immun. 2015, 43, 46–53. [Google Scholar] [CrossRef]
- Patel, R.R.; Wolfe, S.A.; Bajo, M.; Abeynaike, S.; Pahng, A.; Borgonetti, V.; D’Ambrosio, S.; Nikzad, R.; Edwards, S.; Paust, S.; et al. IL-10 Normalizes Aberrant Amygdala GABA Transmission and Reverses Anxiety-like Behavior and Dependence-Induced Escalation of Alcohol Intake. Prog. Neurobiol. 2021, 199, 101952. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How Neuroinflammation Contributes to Neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef]
- Han, C.-L.; Ge, M.; Liu, Y.-P.; Zhao, X.-M.; Wang, K.-L.; Chen, N.; Meng, W.-J.; Hu, W.; Zhang, J.-G.; Li, L.; et al. LncRNA H19 Contributes to Hippocampal Glial Cell Activation via JAK/STAT Signaling in a Rat Model of Temporal Lobe Epilepsy. J. Neuroinflamm. 2018, 15, 103. [Google Scholar] [CrossRef]
- Tripathi, R.K.P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020, 188, 111953. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, Y.; Fu, X. LncRNA Gm4419 Contributes to OGD/R Injury of Cerebral Microglial Cells via IκB Phosphorylation and NF-ΚB Activation. Biochem. Biophys. Res. Commun. 2017, 487, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Jiao, P.; Wang, J.; Li, Y.; Bao, B.; Luoreng, Z.; Wang, X. Role of Long Noncoding RNAs in the Regulation of Cellular Immune Response and Inflammatory Diseases. Cells 2022, 11, 3642. [Google Scholar] [CrossRef]
- Liu, R.; Li, F.; Zhao, W. Long Noncoding RNA NEAT1 Knockdown Inhibits MPP+-Induced Apoptosis, Inflammation and Cytotoxicity in SK-N-SH Cells by Regulating MiR-212-5p/RAB3IP Axis. Neurosci. Lett. 2020, 731, 135060. [Google Scholar] [CrossRef]
- Wang, H.; Liao, S.; Li, H.; Chen, Y.; Yu, J. Long Non-Coding RNA TUG1 Sponges Mir-145a-5p to Regulate Microglial Polarization After Oxygen-Glucose Deprivation. Front. Mol. Neurosci. 2019, 12, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.; Dai, Y.; Wang, B.; Li, L.; Jiang, B.; Wu, P.; Xu, J. The LncRNA H19/MiR-1-3p/CCL2 Axis Modulates Lipopolysaccharide (LPS) Stimulation-Induced Normal Human Astrocyte Proliferation and Activation. Cytokine 2020, 131, 155106. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cao, F.; Ran, Q.; Wang, F. Long Non-Coding RNA Gm4419 Promotes Trauma-Induced Astrocyte Apoptosis by Targeting Tumor Necrosis Factor α. Biochem. Biophys. Res. Commun. 2017, 491, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, Z.; Wu, Q.; Jin, S.; Yao, J.; Cheng, H. LncRNA RMST Activates TAK1-mediated NF-κB Signaling and Promotes Activation of Microglial Cells via Competitively Binding with HnRNPK. IUBMB Life 2019, 71, 1785–1793. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, Y.; Chen, S.; Zhou, Y. LncRNA HOTAIR Participates in Microglia Activation and Inflammatory Factor Release by Regulating the Ubiquitination of MYD88 in Traumatic Brain Injury. J. Mol. Neurosci. 2021, 71, 169–177. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, D.; Wang, L.; Gao, F.; Jin, B.; Lv, H.; Zhang, G.; Sun, X.; Liu, L.; Mo, D.; et al. Silencing of Long Noncoding RNA Nespas Aggravates Microglial Cell Death and Neuroinflammation in Ischemic Stroke. Stroke 2019, 50, 1850–1858. [Google Scholar] [CrossRef]
- Shih, R.-H.; Wang, C.-Y.; Yang, C.-M. NF-KappaB Signaling Pathways in Neurological Inflammation: A Mini Review. Front. Mol. Neurosci. 2015, 8, 77. [Google Scholar] [CrossRef]
- Kim, Y.-K. (Ed.) Anxiety Disorders: Rethinking and Understanding Recent Discoveries; Advances in Experimental Medicine and Biology; Springer: Singapore, 2020; Volume 1191. [Google Scholar] [CrossRef]
- Jackson, H.; Oler, E.; Torres-Calzada, C.; Kruger, R.; Hira, A.S.; López-Hernández, Y.; Pandit, D.; Wang, J.; Yang, K.; Fatokun, O.; et al. MarkerDB 2.0: A Comprehensive Molecular Biomarker Database for 2025. Nucleic Acids Res. 2025, 53, D1415–D1426. [Google Scholar] [CrossRef]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as Biomedical Bioindicators: Approaches and Techniques for the Detection, Analysis, and Validation of Novel Biomarkers of Diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef]
- Schmidt, U.; Keck, M.E.; Buell, D.R. MiRNAs and Other Non-Coding RNAs in Posttraumatic Stress Disorder: A Systematic Review of Clinical and Animal Studies. J. Psychiatr. Res. 2015, 65, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sheng, H.; Zhang, J.; Ma, Y.; Zhang, Y.; Dai, Y.; Jiang, R. LncRNA FBXL19-AS1 Is a Diagnosis Biomarker for Paediatric Patients with Acute Myeloid Leukemia. J. Gene Med. 2021, 23, e3317. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, J.-D.; Zhang, T.-J.; Ma, J.-C.; Xiao, G.-F.; Chen, Q.; Deng, Z.-Q.; Lin, J.; Qian, J.; Yao, D.-M. Overexpression of lncRNA PANDAR predicts adverse prognosis in acute myeloid leukemia. Cancer Manag. Res. 2018, 10, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jin, J.; Liu, E.; Zhang, J. A Novel Circulating Biomarker lnc-MALAT1 for Acute Myocardial Infarction: Its Relationship with Disease Risk, Features, Cytokines, and Major Adverse Cardiovascular Events. J. Clin. Lab. Anal. 2022, 36, e24771. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Wang, Z.; Su, Y.; Hou, Q.; Xu, Q.; Cai, R.; Wang, T.; Gong, X.; Yi, Q. Long Non-coding RNA Screening and Identification of Potential Biomarkers for Type 2 Diabetes. J. Clin. Lab. Anal. 2022, 36, e24280. [Google Scholar] [CrossRef]
- Rahni, Z.; Hosseini, S.M.; Shahrokh, S.; Saeedi Niasar, M.; Shoraka, S.; Mirjalali, H.; Nazemalhosseini-Mojarad, E.; Rostami-Nejad, M.; Malekpour, H.; Zali, M.R.; et al. Long Non-Coding RNAs ANRIL, THRIL, and NEAT1 as Potential Circulating Biomarkers of SARS-CoV-2 Infection and Disease Severity. Virus Res. 2023, 336, 199214. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zou, P.; Sun, W.; Fu, Y.; He, W.; Li, J. Identification of LncRNA NR_028138.1 as a Biomarker and Construction of a CeRNA Network for Bipolar Disorder. Sci. Rep. 2021, 11, 15653. [Google Scholar] [CrossRef]
- Chen, S.; Sun, X.; Niu, W.; Kong, L.; He, M.; Li, W.; Zhong, A.; Lu, J.; Zhang, L. Aberrant Expression of Long Non-Coding RNAs in Schizophrenia Patients. Med. Sci. Monit. 2016, 22, 3340–3351. [Google Scholar] [CrossRef]
- Liu, Y.; Rao, S.; Xu, Y.; Zhang, F.; Wang, Z.; Zhao, X. Changes in the Level of Long Non-Coding RNA Gomafu Gene Expression in Schizophrenia Patients before and after Antipsychotic Medication. Schizophr. Res. 2018, 195, 318–319. [Google Scholar] [CrossRef]
- Sim, D.; Brothers, M.C.; Slocik, J.M.; Islam, A.E.; Maruyama, B.; Grigsby, C.C.; Naik, R.R.; Kim, S.S. Biomarkers and Detection Platforms for Human Health and Performance Monitoring: A Review. Adv. Sci. 2022, 9, 2104426. [Google Scholar] [CrossRef]
- Garofalo, M.; Pandini, C.; Sproviero, D.; Pansarasa, O.; Cereda, C.; Gagliardi, S. Advances with Long Non-Coding RNAs in Alzheimer’s Disease as Peripheral Biomarker. Genes 2021, 12, 1124. [Google Scholar] [CrossRef]
- Cai, Z.; Li, S.; Yu, T.; Deng, J.; Li, X.; Jin, J. Non-Coding RNA Regulatory Network in Ischemic Stroke. Front. Neurol. 2022, 13, 820858. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, R.P.; Tepper, K.; Rönicke, R.; Soom, M.; Westermann, M.; Reymann, K.; Kaether, C.; Fändrich, M. Mechanism of Amyloid Plaque Formation Suggests an Intracellular Basis of Aβ Pathogenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 1942–1947. [Google Scholar] [CrossRef]
- Zhou, J.; Nagarkatti, P.; Zhong, Y.; Ginsberg, J.P.; Singh, N.P.; Zhang, J.; Nagarkatti, M. Dysregulation in MicroRNA Expression Is Associated with Alterations in Immune Functions in Combat Veterans with Post-Traumatic Stress Disorder. PLoS ONE 2014, 9, e94075. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Sun, X.Y.; Niu, W.; Kong, L.M.; He, M.J.; Fan, H.M.; Li, W.S.; Zhong, A.F.; Zhang, L.Y.; Lu, J. Correlation between the level of microRNA expression in peripheral blood mononuclear cells and symptomatology in patients with generalized anxiety disorder. Compr. Psychiatry 2016, 69, 216–224. [Google Scholar] [CrossRef]
- Vaisvaser, S.; Modai, S.; Farberov, L.; Lin, T.; Sharon, H.; Gilam, A.; Volk, N.; Admon, R.; Edry, L.; Fruchter, E.; et al. Neuro-Epigenetic Indications of Acute Stress Response in Humans: The Case of MicroRNA-29c. PLoS ONE 2016, 11, e0146236. [Google Scholar] [CrossRef] [PubMed]
- Katsuura, S.; Kuwano, Y.; Yamagishi, N.; Kurokawa, K.; Kajita, K.; Akaike, Y.; Nishida, K.; Masuda, K.; Tanahashi, T.; Rokutan, K. MicroRNAs MiR-144/144* and MiR-16 in Peripheral Blood Are Potential Biomarkers for Naturalistic Stress in Healthy Japanese Medical Students. Neurosci. Lett. 2012, 516, 79–84. [Google Scholar] [CrossRef]
- van Rensburg, D.J.; Womersley, J.S.; Martin, L.; Seedat, S.; Hemmings, S.M.J. Differential MicroRNA Expression in Adolescent Anxiety Proneness. Eur. J. Neurosci. 2024, 60, 5680–5693. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Qin, C. Long Non-Coding RNAs in Brain Development, Synaptic Biology, and Alzheimer’s Disease. Brain Res. Bull. 2017, 132, 160–169. [Google Scholar] [CrossRef]
- Khorkova, O.; Hsiao, J.; Wahlestedt, C. Basic biology and therapeutic implications of lncRNA. Adv. Drug Deliv. Rev. 2015, 87, 15–24. [Google Scholar] [CrossRef]
- Leucci, E.; Patella, F.; Waage, J.; Holmstrøm, K.; Lindow, M.; Porse, B.; Kauppinen, S.; Lund, A.H. MicroRNA-9 Targets the Long Non-Coding RNA MALAT1 for Degradation in the Nucleus. Sci. Rep. 2013, 3, 2535. [Google Scholar] [CrossRef] [PubMed]
- Almalki, W.H. LncRNAs and PTEN/PI3K signaling: A symphony of regulation in cancer biology. Pathol. Res. Pract. 2023, 249, 154764. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Zheng, X.; Jin, H.; Yu, F.; Zhao, W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics 2022, 14, 2252. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Li, J.; Chen, W.; Liu, C. CRISPRlnc: A machine learning method for lncRNA-specific single-guide RNA design of CRISPR/Cas9 system. Brief. Bioinform. 2024, 25, bbae066. [Google Scholar] [CrossRef]
- Roohi, E.; Jaafari, N.; Hashemian, F. On Inflammatory Hypothesis of Depression: What Is the Role of IL-6 in the Middle of the Chaos? J. Neuroinflamm. 2021, 18, 45. [Google Scholar] [CrossRef]
- Cătălina, G.R.; Gheorman, V.; Gheorman, V.; Forțofoiu, M.-C. The Role of Neuroinflammation in the Comorbidity of Psychiatric Disorders and Internal Diseases. Healthcare 2025, 13, 837. [Google Scholar] [CrossRef]
- Schett, G. Physiological Effects of Modulating the Interleukin-6 Axis. Rheumatology 2018, 57 (Suppl. S2), ii43–ii50. [Google Scholar] [CrossRef]
- Hossam Abdelmonem, B.; Kamal, L.T.; Wardy, L.W.; Ragheb, M.; Hanna, M.M.; Elsharkawy, M.; Abdelnaser, A. Non-Coding RNAs: Emerging Biomarkers and Therapeutic Targets in Cancer and Inflammatory Diseases. Front. Oncol. 2025, 15, 1534862. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.Y.; Gaddam, R.R.; Kravitz, M.; Gupta, A.; Vikram, A. The Challenges and Opportunities in the Development of MicroRNA Therapeutics: A Multidisciplinary Viewpoint. Cells 2021, 10, 3097. [Google Scholar] [CrossRef]
- Badowski, C.; He, B.; Garmire, L.X. Blood-Derived LncRNAs as Biomarkers for Cancer Diagnosis: The Good, the Bad and the Beauty. NPJ Precis. Oncol. 2022, 6, 40. [Google Scholar] [CrossRef]
- Chao, X.; Wang, P.; Ma, X.; Li, Z.; Xia, Y.; Guo, Y.; Ge, L.; Tian, L.; Zheng, H.; Du, Y.; et al. Comprehensive Analysis of LncRNAs as Biomarkers for Diagnosis, Prognosis, and Treatment Response in Clear Cell Renal Cell Carcinoma. Mol. Ther. Oncolytics 2021, 22, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, T.; Ryś, M.; Guglas, K.; Teresiak, A.; Bliźniak, R.; Mackiewicz, J.; Lamperska, K. Quantification of Long Non-Coding RNAs Using QRT-PCR: Comparison of Different CDNA Synthesis Methods and RNA Stability. Arch. Med. Sci. 2021, 17, 1006–1015. [Google Scholar] [CrossRef]
- Belmonte, T.; Rodríguez-Muñoz, C.; Ferruelo, A.; Exojo-Ramírez, S.M.; Amado-Rodríguez, L.; Barbé, F.; de Gonzalo-Calvo, D. Exploring the Translational Landscape of the Long Noncoding RNA Transcriptome in Acute Respiratory Distress Syndrome: It Is a Long Way to the Top. Eur. Respir. Rev. 2024, 33, 240013. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, X.; Zhu, H. Human-Specific Protein-Coding and LncRNA Genes Cast Sex-Biased Genes in the Brain and Their Relationships with Brain Diseases. Biol. Sex. Differ. 2024, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, C.; Di Palo, A.; Russo, A.; Potenza, N. The lncRNAs at X Chromosome Inactivation Center: Not Just a Matter of Sex Dosage Compensation. Int. J. Mol. Sci. 2022, 23, 611. [Google Scholar] [CrossRef]
- Rodríguez-Montes, L.; Ovchinnikova, S.; Yuan, X.; Studer, T.; Sarropoulos, I.; Anders, S.; Kaessmann, H.; Cardoso-Moreira, M. Sex-Biased Gene Expression across Mammalian Organ Development and Evolution. Science 2023, 382, eadf1046. [Google Scholar] [CrossRef]
- Herder, T.; Spoelstra, S.K.; Peters, A.W.M.; Knegtering, H. Sexual dysfunction related to psychiatric disorders: A systematic review. J. Sex. Med. 2023, 20, 965–976. [Google Scholar] [CrossRef]
- Marshall, E.A.; Stewart, G.L.; Sage, A.P.; Lam, W.L.; Brown, C.J. Beyond Sequence Homology: Cellular Biology Limits the Potential of XIST to Act as a MiRNA Sponge. PLoS ONE 2019, 14, e0221371. [Google Scholar] [CrossRef]
- Mitra, I.; Tsang, K.; Ladd-Acosta, C.; Croen, L.A.; Aldinger, K.A.; Hendren, R.L.; Traglia, M.; Lavillaureix, A.; Zaitlen, N.; Oldham, M.C.; et al. Pleiotropic Mechanisms Indicated for Sex Differences in Autism. PLoS Genet. 2016, 12, e1006425. [Google Scholar] [CrossRef]
- Vink, J.M.; Bartels, M.; van Beijsterveldt, T.C.E.M.; van Dongen, J.; van Beek, J.H.D.A.; Distel, M.A.; de Moor, M.H.M.; Smit, D.J.A.; Minica, C.C.; Ligthart, L.; et al. Sex Differences in Genetic Architecture of Complex Phenotypes? PLoS ONE 2012, 7, e47371. [Google Scholar] [CrossRef]
- Xia, Y.; Dai, R.; Wang, K.; Jiao, C.; Zhang, C.; Xu, Y.; Li, H.; Jing, X.; Chen, Y.; Jiang, Y.; et al. Sex-differential DNA methylation and associated regulation networks in human brain implicated in the sex-biased risks of psychiatric disorders. Mol. Psychiatry 2021, 26, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Barry, G.; Briggs, J.A.; Vanichkina, D.P.; Poth, E.M.; Beveridge, N.J.; Ratnu, V.S.; Nayler, S.P.; Nones, K.; Hu, J.; Bredy, T.W.; et al. The long non-coding RNA Gomafu is acutely regulated in response to neuronal activation and involved in schizophrenia-associated alternative splicing. Mol. Psychiatry 2014, 19, 486–494. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Williams, N.G.; Shelkovnikova, T.A. NEAT1 and Paraspeckles in Neurodegenerative Diseases: A Missing Lnc Found? Non-Coding RNA Res. 2018, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
| lncRNA | Regulatory Mechanism | Anxiety Connection | References |
|---|---|---|---|
| BDNF-AS | Represses the BDNF gene by recruiting PRC2; affects dendrite spine growth, neurogenesis. | Changes in spine density and neurogenesis contribute to anxiety-related behaviors in mouse models. | [43,44] |
| MALAT1 | Regulates synaptogenesis and neuroplasticity by modulating gene expression. | MALAT1 downregulation has been linked to reduced synaptic density and neuron apoptosis in the hippocampus in mouse models, which has been found to reinforce hyperactive fear responses. | [21,43,48] |
| NEAT1 | Maintains paraspeckle integrity. Regulates alternative splicing. Prevents neuron apoptosis. | NEAT1 downregulation alters alternative splicing of genes important to enabling adaptability in stress responses in mouse models. | [21,22,51] |
| GOMAFU | Binds DISC1, ERBB4, and WNT7B; regulates alternative splicing patterns, neurogenesis, glial cell differentiation, neuroplasticity, and neuron survival. | The downregulation of GOMAFU can alter brain excitability, and dysregulation is also associated with neuroinflammation, which promotes anxiety-like behaviors in mouse models. | [43,53,55,56] |
| Evf2 | Recruits DLX family genes and MECP2; controls the differentiation of GABAergic neurons in the hippocampus and dentate gyrus of mice. | GABAergic neurons play an important role in counterbalancing brain hyperactivity during and after stress responses and sustained excitatory states, especially on limbic system regions, contributing to anxiety-like behaviors. | [42,43] |
| PNKY | Interacts with PTBP1; regulates the expression and alternative splicing of gene transcripts that promote neurogenesis and migration in embryonic NSCs. | Studies in mice show that neurogenesis impairment enhances anxiety-like behaviors, especially in the hippocampus. | [42,43,46] |
| BC1/BC200 | Regulates local protein synthesis in synapses; modulates neuronal excitability and plasticity. | Studies in mouse models have shown that the absence of BC1/BC200 leads to altered glutamatergic transmission and maladaptive anxiety behaviors. | [21,43,57] |
| LncRNA | Mechanism(s) | References |
|---|---|---|
| BDNF-AS | Inhibits BDNF expression by recruiting repressive histone marks to its promoter. BDNF-AS upregulation promotes neurotoxicity as well as apoptosis and decreases cell viability. | [22,73,88] |
| GOMAFU | Regulates brain transmission through dopamine and glutamate pathways. Negatively modulates the IFN-γ pathway. | [55,64] |
| MALAT1 | Controls gene expression linked to synaptogenesis through SR protein interactions. Acts as a sponge for miR-125b, inhibiting neuron apoptosis and inflammation. Highly expressed in neurons. Dysregulation is associated with neuroinflammation. | [21,22,48] |
| NEAT1 | Acts as a sponge for miR-212-5p. Involved in paraspeckle body formation and regulates microglial activation. Part of the unfolded proteins response in cellular stress. Upregulates the expression of NLRP3 in macrophages, promoting occurrence of inflammatory responses. NEAT1 downregulation has been shown to reduce levels of IL-1β and TNF-α. | [21,88,90,91] |
| MEG3 | Acts as a miRNA sponge. Inactivates the PI3/AKT signaling pathway. Downregulates the NF-κB signaling pathway. Targets the miR-7a-5p/NLRP3 axis to regulate microglia activation and inflammatory response. | [22,88,90] |
| TUG1 | Acts as a sponge for miR-9 and miR-145a-5p, activating the NF-κB signaling pathway. | [22,88,92] |
| H19 | Promotes microglial and astrocyte activation by activating the JAK/STAT pathway. | [88,93] |
| Gm4419 | Serves as a decoy by binding and phosphorylating IkBα. | [88,94] |
| NKILA | Inhibits the NF-κB signaling pathway. | [88] |
| RMST | Activates the NF-κB signaling pathway. Favors microglial activation and neuronal apoptosis. | [88,95] |
| HOTAIR | Histone methylation and acetylation, functions as a scaffold for chromatin-remodeling complex PRC2. Glial activation in neuroinflammatory responses. | [22,96] |
| LncRNA Nespas | Inactivates the NF-κB signaling pathway via the suppression of TAK1. | [97] |
| GAS5 | Binds to PRC2, inhibiting M2 polarization. Acts as a sponge for miR-223-3p and positively regulates the NLRP3 inflammasome. | [88] |
| LincRNA-p21 | Competitively binds to the miR-181 family, inducing microglial activation. | [88] |
| LincRNA-Cox2 | Directly binds and promotes the NF-κB-p65 nuclear translocation and transcription of NLRP3. | [88] |
| Disease | Trial | LncRNA(s) | Sample | Reference |
|---|---|---|---|---|
| Acute myeloid leukemia (AML) | 137 patients 43 controls | ↑ FBXL19 antisense RNA 1 (FBXL19-AS1) in AML and overexpression associated with a bad prognosis. | Serum | [104] |
| Acute myeloid leukemia (AML) | 119 patients 26 controls | ↑ Promoter of CDKN1A antisense DNA damage-activated RNA (PANDAR) in AML, and a higher expression is associated with poor clinical outcomes. | Bone marrow | [105] |
| Acute myocardial infarction (AMI) | 160 patients (newly diagnosed with AMI) 50 controls (angina pectoris patients, no AMI) | ↑ MALAT1 in AMI and was positively correlated with CRP, troponin I, LDL, and infarct size, as well as TNF-alpha, IL-6 and IL-17A. | Peripheral blood | [106] |
| Type 2 diabetes (T2D) | 100 patients 100 controls | ↑ lncRNA XR_108954.2 and E2F2 mRNA in T2D | Peripheral blood | [107] |
| COVID-19 | 38 moderate and 25 severe COVID-19 patients 30 controls | ↑ ANRIL, THRIL and NEAT1 in COVID-19 patients. ANRIL and THRIL higher in severe vs. moderate. NEAT1 higher in both (moderate and severe) without significant difference. | Peripheral blood | [108] |
| Bipolar disorder (BD) | 130 patients 116 controls | ↑ lncRNA NR_028138.1. | Peripheral blood | [109] |
| Schizophrenia (SZ) | 106 patients 48 controls | ↑ NONHSAT089447 and NONHSAT041499 in SZ; both showed a significant reduction after treatment. | Peripheral blood | [110] |
| Schizophrenia (SZ) | 35 patients 49 controls | ↑ GOMAFU in SZ. | Peripheral blood | [111] |
| Disorder | Trial | LncRNA(s)/MiRNA(s) | Sample | Reference |
|---|---|---|---|---|
| Post-traumatic stress disorder | 30 patients 42 controls | ↑ miR-570, miR-219, miR-637, miR-668, miR-519a, miR-518f, and miR-615. ↓ miR-125a and miR-181c. | Peripheral blood | [116] |
| Generalized anxiety disorder | 76 patients 39 controls | ↑ miR-4484, miR-4674, miR-501, miR-663, and miR-4505. ↓ miR-1301 and miR-432. | Peripheral blood | [117] |
| Social stress | 49 healthy | ↑ miR-29c. | Peripheral blood | [118] |
| Anticipatory anxiety | 10 healthy medical students | ↑ miR-144 and miR-16, associated with an upcoming stressful exam. | Blood plasma | [119] |
| Anxiety proneness | 88 patients (adolescents with childhood trauma) | ↑ hsa-miR-28-5p. ↓ hsa-miR-502-3p and hsa-miR-500a-3p. | Peripheral blood | [120] |
| Depression/anxiety | 181 patients 59 controls | ↑ Mitochondiral 7S RNA. | Blood plasma | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Rocha, L.D.; Ruiz-Hernández, A.; Martínez-Coronilla, G.; Leija-Montoya, A.G.; Peña-Peña, M.; Sánchez-Muñoz, F.; Rieke-Campoy, U.; González-Ramírez, J. The Role of Long Non-Coding RNA in Anxiety Disorders: A Literature Review. Int. J. Mol. Sci. 2025, 26, 5042. https://doi.org/10.3390/ijms26115042
López-Rocha LD, Ruiz-Hernández A, Martínez-Coronilla G, Leija-Montoya AG, Peña-Peña M, Sánchez-Muñoz F, Rieke-Campoy U, González-Ramírez J. The Role of Long Non-Coding RNA in Anxiety Disorders: A Literature Review. International Journal of Molecular Sciences. 2025; 26(11):5042. https://doi.org/10.3390/ijms26115042
Chicago/Turabian StyleLópez-Rocha, Laura Dayanara, Armando Ruiz-Hernández, Gustavo Martínez-Coronilla, Ana Gabriela Leija-Montoya, Mario Peña-Peña, Fausto Sánchez-Muñoz, Ulises Rieke-Campoy, and Javier González-Ramírez. 2025. "The Role of Long Non-Coding RNA in Anxiety Disorders: A Literature Review" International Journal of Molecular Sciences 26, no. 11: 5042. https://doi.org/10.3390/ijms26115042
APA StyleLópez-Rocha, L. D., Ruiz-Hernández, A., Martínez-Coronilla, G., Leija-Montoya, A. G., Peña-Peña, M., Sánchez-Muñoz, F., Rieke-Campoy, U., & González-Ramírez, J. (2025). The Role of Long Non-Coding RNA in Anxiety Disorders: A Literature Review. International Journal of Molecular Sciences, 26(11), 5042. https://doi.org/10.3390/ijms26115042









