Tumor Microenvironment in Melanoma Brain Metastasis: A New Potential Target?
Abstract
1. Introduction
2. Current Melanoma Brain Metastases Therapies
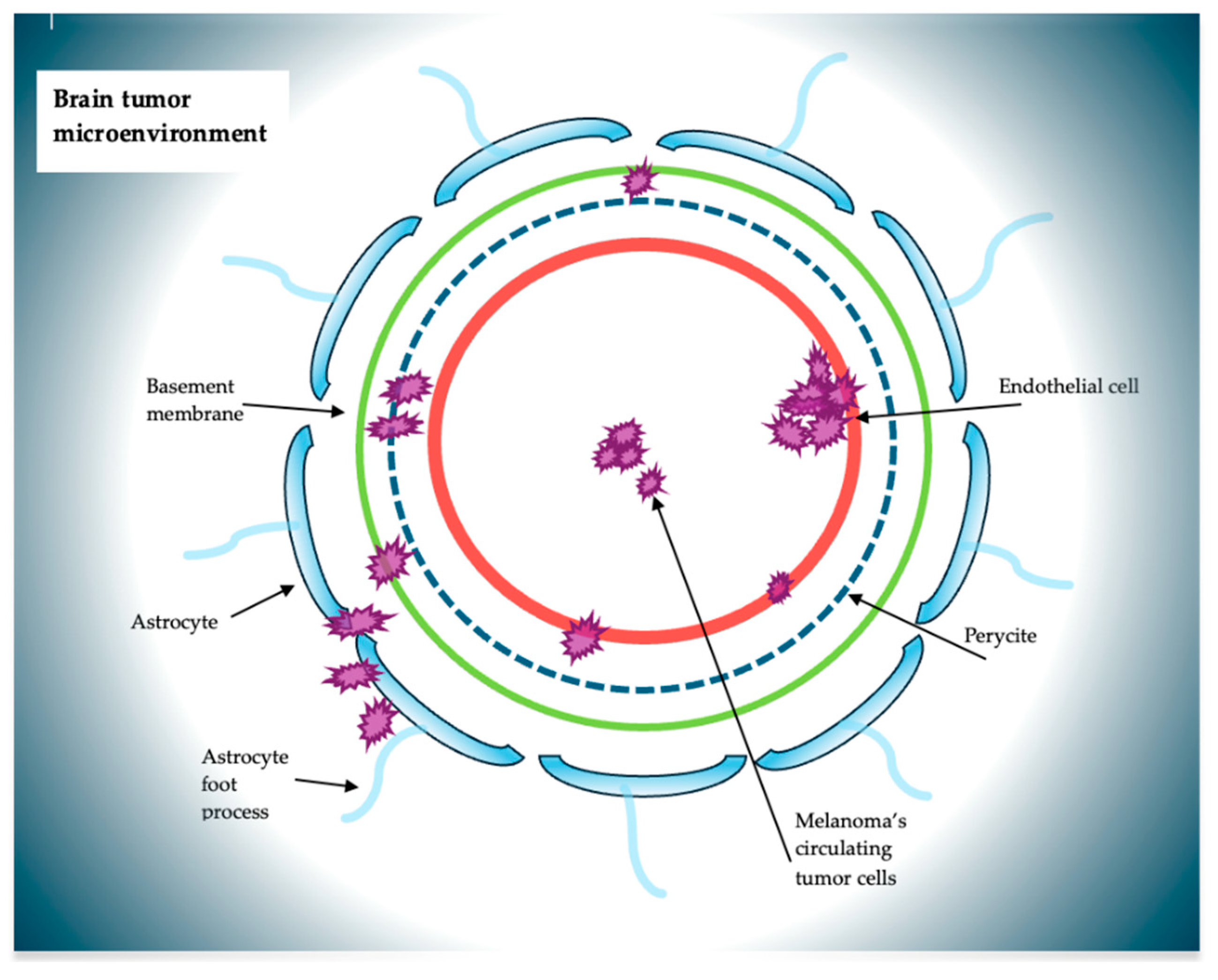
3. The Microenvironment in Melanoma Brain Metastases
4. CTCs and Cells Around the Microenvironment
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dreno, B.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur. J. Cancer 2022, 170, 236–255. [Google Scholar] [CrossRef]
- Whiteman, D.C.; Green, A.C.; Olsen, C.M. The Growing Burden of Invasive Melanoma: Projections of Incidence Rates and Numbers of New Cases in Six Susceptible Populations Through 2031. J. Investig. Dermatol. 2016, 136, 1161–1171. [Google Scholar] [CrossRef]
- Bander, E.D.; Yuan, M.; Carnevale, J.A.; Reiner, A.S.; Panageas, K.S.; Postow, M.A.; Tabar, V.; Moss, N.S. Melanoma Brain Metastasis Presentation, Treatment and Outcomes in the Age of Targeted- and Immuno-therapies. Cancer 2021, 127, 2062–2073. [Google Scholar] [CrossRef]
- Kleffman, K.; Levinson, G.; Rose, I.V.L.; Blumenberg, L.M.; Shadaloey, S.A.A.; Dhabaria, A.; Wong, E.; Galán-Echevarría, F.; Karz, A.; Argibay, D.; et al. Melanoma-Secreted Amyloid Beta Suppresses Neuroinflammation and Promotes Brain Metastasis. Cancer Discov. 2022, 12, 1314–1335. [Google Scholar] [CrossRef]
- Gutzmer, R.; Vordermark, D.; Hassel, J.C.; Krex, D.; Wendl, C.; Schadendorf, D.; Sickmann, T.; Rieken, S.; Pukrop, T.; Höller, C.; et al. Melanoma Brain Metastases—Interdisciplinary Management Recommendations 2020. Cancer Treat. Rev. 2020, 89, 102083. [Google Scholar] [CrossRef]
- Rishi, A.; Yu, H.-H.M. Current Treatment of Melanoma Brain Metastasis. Curr Treat. Options Oncol. 2020, 21, 45. [Google Scholar] [CrossRef]
- Caffo, M.; Barresi, V.; Caruso, G.; Cutugno, M.; La Fata, G.; Venza, M.; Alafaci, C.; Tomasello, F. Innovative Therapeutic Strategies in the Treatment of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 2135–2174. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Morris, S.L.; Low, S.H.; A’Hern, R.P.; Eisen, T.G.; Gore, M.E.; Nutting, C.M.; Harrington, K.J. A Prognostic Index that Predicts Outcome Following Palliative Whole Brain Radiotherapy for Patients with Metastatic Malignant Melanoma. Br. J. Cancer 2004, 91, 829–833. [Google Scholar] [CrossRef]
- de la Fuente, M.; Beal, K.; Carvajal, R.; Kaley, T.J. Whole Brain Radiotherapy in Patients with Brain Metastases from Melanoma. CNS Oncol. 2014, 3, 401–406. [Google Scholar] [CrossRef]
- Ewend, M.G.; Morris, D.E.; Carey, L.A.; Ladha, A.M.; Brem, S. Guidelines for the Initial Management of Metastatic Brain Tumors: Role of Surgery, Radiosurgery, and Radiation Therapy. J. Natl. Compr. Cancer Netw. 2008, 6, 505–513. [Google Scholar] [CrossRef]
- Middleton, M.R.; Grob, J.J.; Aaronson, N.; Fierlbeck, G.; Tilgen, W.; Seiter, S.; Gore, M.; Aamdal, S.; Cebon, J.; Coates, A.; et al. Randomized Phase III Study of Temozolomide Versus Dacarbazine in the Treatment of Patients with Advanced Metastatic Malignant Melanoma. J. Clin. Oncol. 2000, 18, 158–166. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Robert, C.; Hersey, P.; Nathan, P.; Garbe, C.; Milhem, M.; Demidov, L.V.; Hassel, J.C.; Rutkowski, P.; Mohr, P.; et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. N. Engl. J. Med. 2012, 367, 107–114. [Google Scholar] [CrossRef]
- Davies, M.A.; Saiag, P.; Robert, C.; Grob, J.J.; Flaherty, K.T.; Arance, A.; Chiarion-Sileni, V.; Thomas, L.; Lesimple, T.; Mortier, L.; et al. Dabrafenib plus Trametinib in Patients with BRAF(V600)-Mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-label, Phase 2 Trial. Lancet Oncol. 2017, 18, 863–873. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Dabrafenib and Trametinib Versus Dabrafenib and Placebo for Val600 BRAF-Mutant Melanoma: A Multicentre, Double-blind, Phase 3 Randomised Controlled Trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Wilson, E.H.; Weninger, W.; Hunter, C.A. Trafficking of Immune Cells in the Central Nervous System. J. Clin. Investig. 2010, 120, 1368–1379. [Google Scholar] [CrossRef]
- Sundararajan, S.; Thida, A.M.; Yadlapati, S.; Mukkamalla, S.K.R.; Koya, S. Metastatic Melanoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Srinivasan, E.S.; Deshpande, K.; Neman, J.; Winkler, F.; Khasraw, M. The Microenvironment of Brain Metastases from Solid Tumors. Neuro-Oncol. Adv. 2021, 3, v121–v132. [Google Scholar] [CrossRef]
- Caruso, G.; Caffo, M.; Raudino, G.; Alafaci, C.; Cafarella, D.; Lucerna, S.; Salpietro, F.M.; Tomasello, F. Could Nanoparticle Systems Have a Role in the Treatment of Cerebral Gliomas? Nanomedicine 2011, 7, 744–752. [Google Scholar] [CrossRef]
- Caruso, G.; Marino, D.; Caffo, M. Nanoparticles and CNS Delivery of Therapeutic Agents in the Treatment of Primary Brain Tumors. J. Anal. Oncol. 2014, 3, 105–112. [Google Scholar]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood-brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Sprowls, S.A.; Arsiwala, T.A.; Bumgarner, J.R.; Shah, N.; Lateef, S.S.; Kielkowski, B.N.; Lockman, P.R. Improving CNS Delivery to Brain Metastases by Blood-Tumor Barrier Disruption. Trends Cancer 2019, 5, 495–505. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood-Brain Barrier and Blood-Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.S.; Serres, S.; Anthony, D.C.; Sibson, N.R. Functional Role of Endothelial Adhesion Molecules in the Early Stages of Brain Metastasis. Neuro-Oncology 2014, 16, 540–551. [Google Scholar] [CrossRef]
- Zigler, M.; Villares, G.J.; Dobroff, A.S.; Wang, H.; Huang, L.; Braeuer, R.R.; Kamiya, T.; Melnikova, V.O.; Song, R.; Friedman, R.; et al. Expression of Id-1 is Regulated by MCAM/MUC18: A Missing Link in Melanoma Progression. Cancer Res. 2011, 71, 3494–3504. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Preusser, M. The Inflammatory Microenvironment in Brain Metastases: Potential Treatment Target? Chin. Clin. Oncol. 2015, 4, 21. [Google Scholar]
- Berghoff, A.S.; Liao, Y.; Karreman, M.A.; Ilhan-Mutlu, A.; Gunkel, K.; Sprick, M.R.; Eisen, C.; Kessler, T.; Osswald, M.; Wunsche, S.; et al. Identification and Characterization of Cancer Cells that Initiate Metastases to the Brain and Other Organs. Mol. Cancer Res. 2021, 19, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, Y.; Chen, W.; Zhang, M.; Qin, J. Malignant Melanoma-Derived Exosomes Induce Endothelial Damage and Glial Activation on a Human BBB Chip Model. Biosensors 2022, 12, 89. [Google Scholar] [CrossRef]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-santos, G.; Ghajar, C.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells Toward a Pro-metastatic Phenotype Through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef]
- Aliotta, J.M.; Pereira, M.; Johnson, K.W.; de Paz, N.; Dooner, M.S.; Puente, N.; Ayala, C.; Brilliant, K.; Berz, D.; Lee, D.; et al. Microvesicle Entry into Marrow Cells Mediates Tissue-Specific Changes in mRNA by Direct Delivery of mRNA and Induction of Transcription. Exp. Hematol. 2010, 38, 233–245. [Google Scholar] [CrossRef]
- Kuroda, H.; Tachikawa, M.; Yagi, Y.; Umetsu, M.; Nurdin, A.; Miyauchi, E.; Watanabe, M.; Uchida, Y.; Terasaki, T. Cluster of Differentiation 46 Is the Major Receptor in Human Blood-Brain Barrier Endothelial Cells for Uptake of Exosomes Derived from Brain-Metastatic Melanoma Cells (SK-Mel-28). Mol. Pharm. 2019, 16, 292–304. [Google Scholar] [CrossRef]
- Feng, S.; Cen, J.; Huang, Y.; Shen, H.; Yao, L.; Wang, Y.; Chen, Z. Matrix Metalloproteinase-2 and -9 Secreted by Leukemic Cells Increase the Permeability of Blood-Brain Barrier by Disrupting Tight Junction Proteins. PLoS ONE 2011, 6, e20599. [Google Scholar] [CrossRef]
- Kienast, Y.; Von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.F.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-Time Imaging Reveals the Single Steps of Brain Metastasis Formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef]
- Langley, R.R.; Fidler, I.J. The Seed and Soil Hypothesis Revisited—The Role of Tumor-Stroma Interactions in Metastasis to Different Organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Ramakrishna, R.; Rostomily, R. Seed, Soil, and beyond: The Basic Biology of Brain Metastasis. Surg. Neurol. Int. 2013, 4, S256–S264. [Google Scholar]
- Kushiro, K.; Chu, R.A.; Verma, A.; Núñez, N.P. Adipocytes Promote B16BL6 Melanoma Cell Invasion and the Epithelial-to-Mesenchymal Transition. Cancer Microenviron. 2011, 5, 73–82. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Y.; Jiang, X.; Peng, M.; Liu, Y.; Mo, Y.; Ren, D.; Hua, Y.; Yu, B.; Zhou, Y.; et al. Mechanisms of Vasculogenic Mimicry in Hypoxic Tumor Microenvironments. Mol. Cancer 2021, 20, 7. [Google Scholar] [CrossRef]
- García-Gómez, P.; Valiente, M. Vascular Co-Option in Brain Metastasis. Angiogenesis 2020, 23, 3–8. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, L.; Liang, N.; Xie, J.; Luo, H.; Deng, G.; Zhang, J. Vasculogenic Mimicry and Tumor Metastasis. J. BUON 2016, 21, 533–541. [Google Scholar]
- Comito, G.; Calvani, M.; Giannoni, E.; Bianchini, F.; Calorini, L.; Torre, E.; Migliore, C.; Giordano, S.; Chiarugi, P. HIF-1α Stabilization by Mitochondrial ROS Promotes Met-Dependent Invasive Growth and Vasculogenic Mimicry in Melanoma Cells. Free Radic. Biol. Med. 2011, 51, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.M.; Jalali, A.; Kircher, D.A.; Lee, W.C.; McQuade, J.L.; Haydu, L.E.; Yoon, A.Y.; Reuben, A.; de Macedo, M.P.; Carapeto, F.C.L.; et al. Molecular Profiling Reveals Unique Immune and Metabolic Features of Melanoma Brain Metastases. Cancer Discov. 2019, 9, 628–645. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Watters, A.; Cheng, N.; Perry, C.E.; Xu, K.; Alicea, G.M.; Parris, J.L.D.; Baraban, E.; Ray, P.; Nayak, A.; et al. Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 2019, 9, 1720–1735. [Google Scholar] [CrossRef] [PubMed]
- Placone, A.L.; Quiñones-Hinojosa, A.; Searson, P.C. The Role of Astrocytes in the Progression of Brain Cancer: Complicating the Picture of the Tumor Microenvironment. Tumor Biol. 2016, 37, 61–69. [Google Scholar] [CrossRef]
- Lin, Q.; Balasubramanian, K.; Fan, D.; Kim, S.J.; Guo, L.; Wang, H.; Bar-Eli, M.; Aldape, K.D.; Fidler, I.J. Reactive Astrocytes Protect Melanoma Cells from Chemotherapy by Sequestering Intracellular Calcium through Gap Junction Communication Channels. Neoplasia 2010, 12, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.; Schwartz, H.; Sagi-Assif, O.; Meshel, T.; Izraely, S.; Ben Menachem, S.; Bengaiev, R.; Ben-Shmuel, A.; Nahmias, C.; Couraud, P.; et al. Astrocytes Facilitate Melanoma Brain Metastasis Via Secretion of IL-23. J. Pathol. 2015, 236, 116–127. [Google Scholar] [CrossRef]
- Bieńkowski, M.; Preusser, M. Prognostic Role of Tumour-Infiltrating Inflammatory Cells in Brain Tumours: Literature Review. Curr. Opin. Neurol. 2015, 28, 647–658. [Google Scholar] [CrossRef]
- Lewis, C.E.; Pollard, J.W. Distinct Role of Macrophages in Different Tumor Microenvironments. Cancer Res. 2003, 66, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Fuchs, E.; Ricken, G.; Mlecnik, B.; Bindea, G.; Spanberger, T.; Hackl, M.; Widhalm, G.; Dieckmann, K.; Prayer, D.; et al. Density of Tumor-Infiltrating Lymphocytes Correlates with Extent of Brain Edema and Overall Survival Time in Patients with Brain Metastases. Oncoimmunology 2015, 5, e1057388. [Google Scholar] [CrossRef]
- Moran, A.; Laider-Trejo, L.; Shalom, V.; Shabtay-Orbach, A.; Krelin, Y.; Gil, Z. Characterization of the Melanoma Brain Metastatic Niche in Mice and Humans. Cancer Med. 2013, 2, 155–163. [Google Scholar]
- Holash, J.; Wiegand, S.J.; Yancopoulos, G.D. New Model of Tumor Angiogenesis: Dynamic Balance between Vessel Regression and Growth Mediated by Angiopoietins and VEGF. Oncogene 1999, 18, 5356–5362. [Google Scholar] [CrossRef]
- Jacobs, J.F.M.; Idema, A.J.; Bol, K.F.; Nierkens, S.; Grauer, O.M.; Wesseling, P.; Grothenuis, J.A.; Hoogerbrugge, P.M.; de Vries, I.J.M.; Adema, G.J. Regulatory T Cells and the PD-L1/PD-1 Pathway Mediate Immune Suppression in Malignant Human Brain Tumors. Neuro-Oncol. 2009, 11, 394–402. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Ricken, G.; Widhalm, G.; Rajky, O.; Dieckmann, K.; Birner, P.; Bartsch, R.; Holler, C.; Preusser, M. Tumour-Infiltrating Lymphocytes and Expression of Programmed Death Ligand 1 (PD-L1) in Melanoma Brain Metastases. Histopathology 2015, 66, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, J.; Goswami, S.; Jones, J.G.; Rohan, T.E.; Pieri, E.; Chen, X.; Adler, E.; Cox, D.; Maleki, S.; Bresnik, A.; et al. Invasive Breast Carcinoma Cells from Patients Exhibit MenaINV- and Macrophage-Dependent Transendothelial Migration. Sci. Signal. 2014, 7, ra112. [Google Scholar] [CrossRef]
- Mareel, M.; Madani, I. Tumour-Associated Host Cells Participating at Invasion and Metastasis: Targets for Therapy? Acta Chir. Belg. 2006, 106, 635–640. [Google Scholar] [CrossRef]
- Butovsky, O.; Weiner, H.L. Microglial Signatures and Their Role in Health and Disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Guldner, I.H.; Wang, Q.; Yang, L.; Golomb, S.M.; Zhao, Z.; Lopez, J.A.; Brunory, A.; Howe, E.N.; Zhang, Y.; Palakurthi, B.; et al. CNS-Native Myeloid Cells Drive Immune Suppression in the Brain Metastatic Niche through Cxcl10. Cell 2020, 183, 1234–1248. [Google Scholar] [CrossRef]
- Evans, K.T.; Blake, K.; Longworth, A.; Coburn, M.A.; Insua-Rodríguez, J.; McMullen, T.P.; Nguyen, Q.H.; Ma, D.; Lev, T.; Hernandez, G.A.; et al. Microglia Promote Anti-Tumour Immunity and Suppress Breast Cancer Brain Metastasis. Nat. Cell Biol. 2023, 25, 1848–1859. [Google Scholar] [CrossRef]
- Rodriguez-Baena, F.J.; Marquez-Galera, A.; Ballesteros-Martinez, P.; Castillo, A.; Diaz, E.; Moreno-Bueno, G.; Lopez-Atalaya, J.P.; sanchez-Laorden, B. Microglial Reprogramming Enhances Antitumor Immunity and Immunotherapy Response in Melanoma Brain Metastases. Cancer Cell 2025, 43, 413–427. [Google Scholar] [CrossRef]
- Fischer, G.M.; Guerrieri, R.A.; Hu, Q.; Joon, A.Y.; Kumar, S.; Haydu, L.E.; McQuade, J.L.; Vashisht Gopal, Y.N.; Knighton, B.; Deng, W.; et al. Clinical, Molecular, Metabolic, and Immune Features Associated with Oxidative Phosphorylation in Melanoma Brain Metastases. Neurooncol. Adv. 2021, 3, Vdaa177. [Google Scholar] [CrossRef]
- Lyle, L.T.; Lockman, P.R.; Adkins, C.E.; Mohammad, A.S.; Sechrest, E.; Hua, E.; Palmieri, D.; Liewher, D.J.; Steinberg, S.M.; Kloc, W.; et al. Alterations in Pericyte Subpopulations Are Associated with Elevated Blood-Tumor Barrier Permeability in Experimental Brain Metastasis of Breast Cancer. Clin. Cancer Res. 2016, 22, 5287–5299. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Q.; Li, X.Y.; Yang, Q.Y.; Xu, W.W.; Liu, G.L. Short-Term Anti-Vascular Endothelial Growth Factor Treatment Elicits Vasculogenic Mimicry Formation of Tumors to Accelerate Metastasis. J. Exp. Clin. Cancer Res. 2012, 31, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.J.L.; Zhou, Y.J.; Ding, R.L.; Xie, F.; Fu, S.Z.; Wu, J.B.; Yang, L.L.; Wen, Q.L. In Vitro and in Vivo Apatinib Inhibits Vasculogenic Mimicry in Melanoma MUM-2B cells. PLoS ONE 2018, 13, e0200845. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Ruma, I.M.W.; Putranto, E.W.; Kondo, E.; Murata, H.; Watanabe, M.; Huang, P.; Kinoshita, R.; Futami, J.; Inoue, Y.; Yamauchi, A.; et al. MCAM, as a Novel Receptor for S100A8/A9, Mediates Progression of Malignant Melanoma Through Prominent Activation of NF-κB and ROS Formation Upon Ligand Binding. Clin. Exp. Metastasis 2016, 33, 609–627. [Google Scholar] [CrossRef]
- Kinoshita, R.; Sato, H.; Yamauchi, A.; Takahashi, Y.; Inoue, Y.; Sumardika, I.W.; Chen, Y.; Tomonobu, N.; Araki, K.; Shien, K.; et al. Newly Developed Anti-S100A8/A9 Monoclonal Antibody Efficiently Prevents Lung Tropic Cancer Metastasis. Int. J. Cancer 2019, 145, 569–575. [Google Scholar] [CrossRef]
- Tomonobu, N.; Kinoshita, R.; Wake, H.; Inoue, Y.; Ruma, I.M.W.; Suzawa, K.; Gohara, Y.; Komalasari, N.L.G.Y.; Jiang, F.; Murata, H.; et al. Histidine-Rich Glycoprotein Suppresses the S100A8/A9-Mediated Organotropic Metastasis of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 10300. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Yan, W.; Wang, C.; Liu, W.; Lin, X.; Zou, Z.; Sun, W.; Chen, Y. BRAF Inhibitor Resistance in Melanoma: Mechanisms and Alternative Therapeutic Strategies. Curr. Treat. Options Oncol. 2022, 23, 1503–1521. [Google Scholar] [CrossRef]
- Gopal, V.Y.N.; Gammon, S.; Prasad, R.; Knighton, B.; Pisaneschi, F.; Roszik, J.; Feng, N.; Johnson, S.; Pramanik, S.; Sudderth, J.; et al. A Novel Mitochondrial Inhibitor Blocks MAPK Pathway and Overcomes MAPK Inhibitor Resistance in Melanoma. Clin. Cancer Res. 2019, 25, 6429–6442. [Google Scholar] [CrossRef]
- Gouda, M.A.; Voss, M.H.; Tawbi, H.; Gordon, M.; Tykodi, S.S.; Lam, E.T.; Vaishampayan, U.; Tannir, N.M.; Chaves, J.; Nikolinakos, P.; et al. A Phase I/II Study of the Safety and Efficacy of Telaglenastat (CB-839) in Combination with Nivolumab in Patients with Metastatic Melanoma, Renal Cell Carcinoma, and Non-Small-Cell Lung Cancer. ESMO Open 2025, 10, 104536. [Google Scholar] [CrossRef]
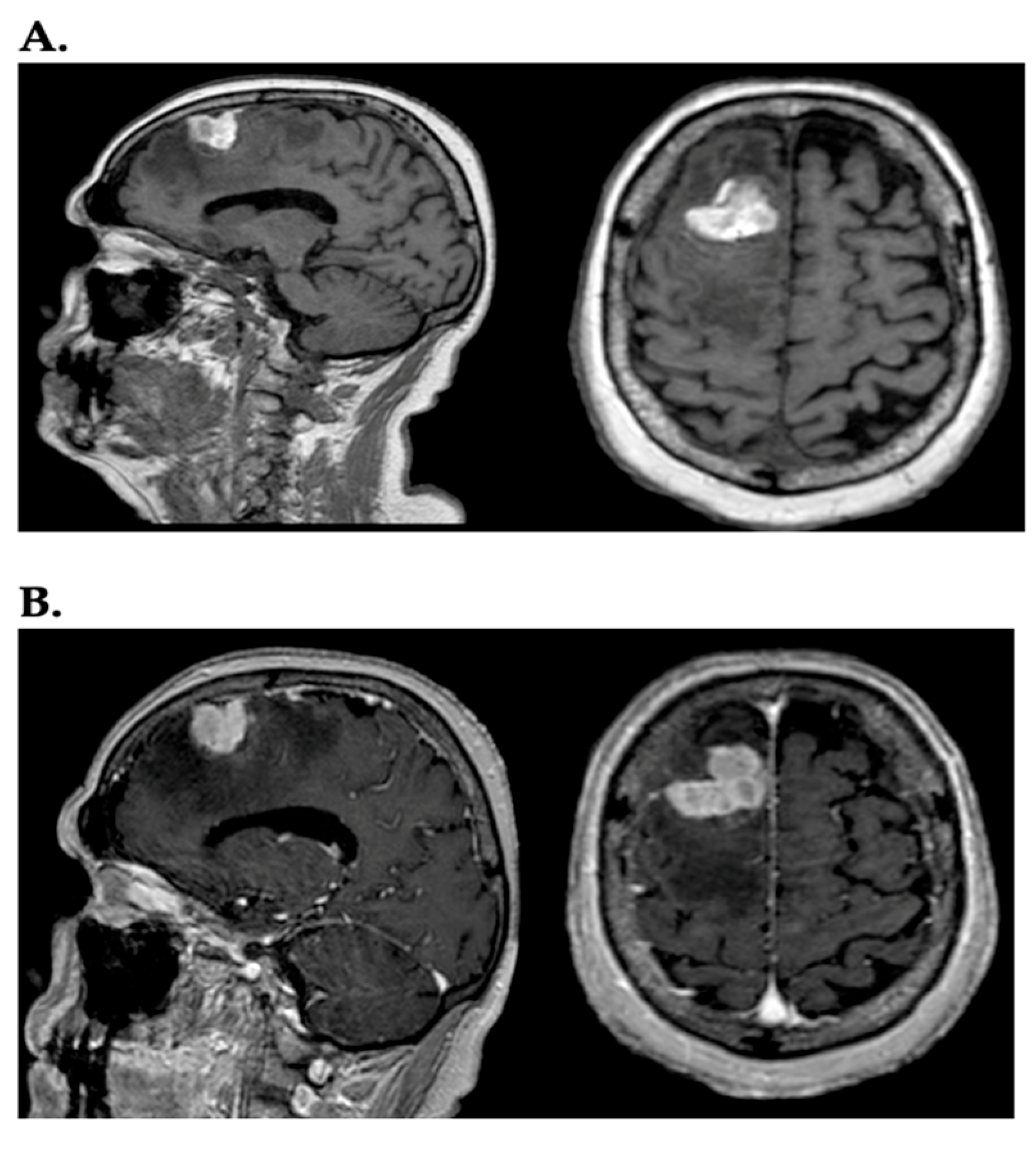
| Principal Target Therapies | Molecular Targets and Activities |
|---|---|
| Apatinib (YN 968N1) | Block VEGFR-2 reducing vascular mimicry |
| Norcantharidine | Inhibits MM2, vascular mimicry decreasing and tumor colonization. |
| Histidine-Rich Glycoprotein (HRG) | Block S100 A8/A9, reducing inflammatory loop making microenvironment not suitable for metastatic development |
| IACS-010759 | Block M-TOR, reducing oxidative phosphorylation, reducing glutamine concentration, increase immunocomponents |
| CB825 | Decreasing of glumatine, increase immunocomponents |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caruso, G.; Garcia Moreira, C.G.; Iaboni, E.; Tripodo, M.; Ferrarotto, R.; Abbritti, R.V.; Conte, L.; Caffo, M. Tumor Microenvironment in Melanoma Brain Metastasis: A New Potential Target? Int. J. Mol. Sci. 2025, 26, 5018. https://doi.org/10.3390/ijms26115018
Caruso G, Garcia Moreira CG, Iaboni E, Tripodo M, Ferrarotto R, Abbritti RV, Conte L, Caffo M. Tumor Microenvironment in Melanoma Brain Metastasis: A New Potential Target? International Journal of Molecular Sciences. 2025; 26(11):5018. https://doi.org/10.3390/ijms26115018
Chicago/Turabian StyleCaruso, Gerardo, Cristofer Gonzalo Garcia Moreira, Edvige Iaboni, Massimo Tripodo, Rosamaria Ferrarotto, Rosaria Viola Abbritti, Luana Conte, and Maria Caffo. 2025. "Tumor Microenvironment in Melanoma Brain Metastasis: A New Potential Target?" International Journal of Molecular Sciences 26, no. 11: 5018. https://doi.org/10.3390/ijms26115018
APA StyleCaruso, G., Garcia Moreira, C. G., Iaboni, E., Tripodo, M., Ferrarotto, R., Abbritti, R. V., Conte, L., & Caffo, M. (2025). Tumor Microenvironment in Melanoma Brain Metastasis: A New Potential Target? International Journal of Molecular Sciences, 26(11), 5018. https://doi.org/10.3390/ijms26115018






