Cell Type-Specific Expression of Purinergic P2X Receptors in the Hypothalamus
Abstract
1. Introduction
2. Nuclei and Neuron Types in the Hypothalamus
2.1. Supraoptic Nucleus (SON)
2.2. Paraventricular Nucleus (PVN)
2.3. Suprachiasmatic Nucleus (SCN)
2.4. Anteroventral Periventricular Nucleus (AVPV)
2.5. Anterior Hypothalamic Nucleus (AHN)
2.6. Arcuate Nucleus (ARC)
2.7. Ventromedial Hypothalamic Nucleus (VMH)
2.8. Dorsomedial Hypothalamic Nucleus (DMH)
2.9. Tuberomammilary Nucleus (TMN)
2.10. Lateral Hypothalamic Area (LHA)
3. Sources of Extracellular ATP in the Brain
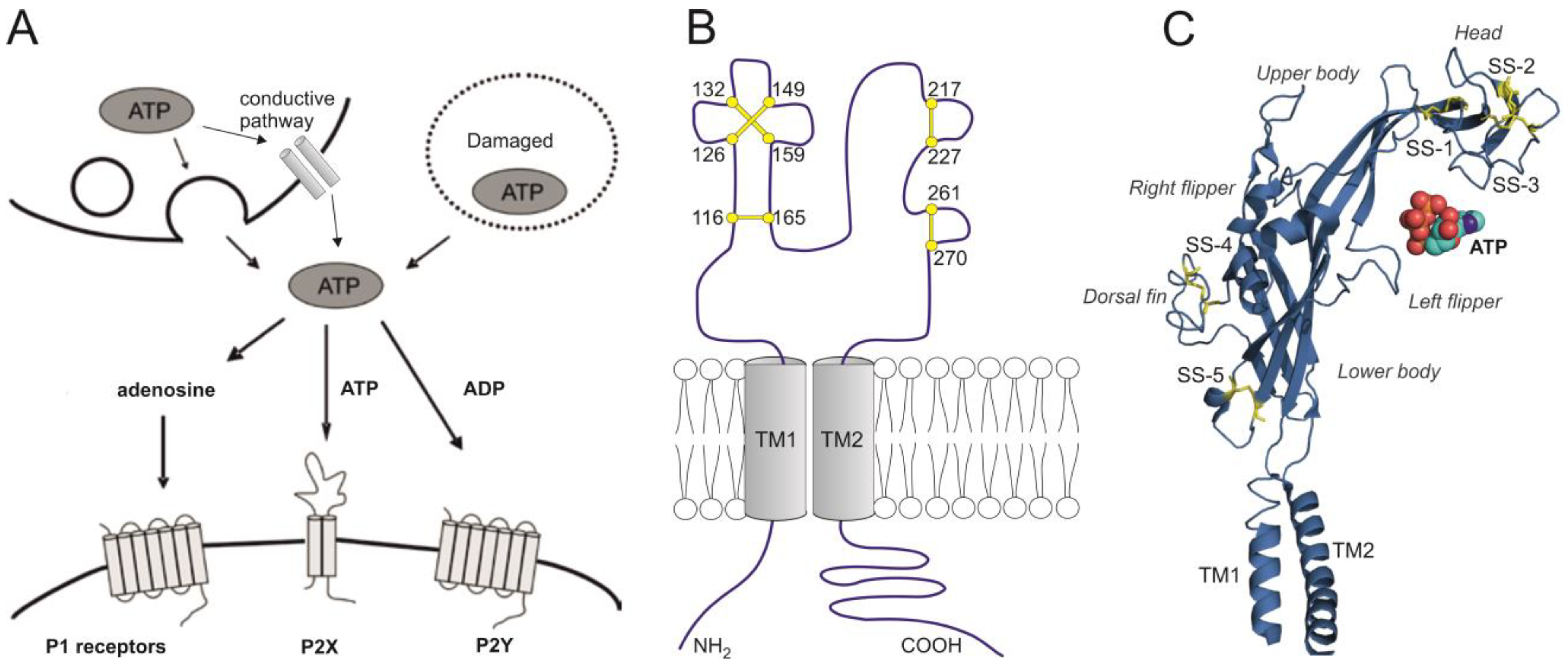
4. A Brief Overview of Purinergic P2X1-7 Receptors
| Receptor | Desensitization Rate | Ca2+ Permeability | αβme- ATP | Suramin PPADS | IVM | Acid pH | Large Pore |
|---|---|---|---|---|---|---|---|
| P2X1 | Fast | High | Yes | Yes | No | Inhibited | No |
| P2X2 | Slow | Medium | No | Yes | No | Potentiated | Yes |
| P2X3 | Fast | Low | Yes | Yes | No | Inhibited | No |
| P2X4 | Medium | High | No | Low | Yes | Inhibited | Yes |
| P2X5 | Slow | Low | No | Yes | No | No | No |
| P2X6 | N/D | N/D | N/D | N/D | N/D | N/D | N/D |
| P2X7 | None | High | No | Low | No * | Inhibited | Yes |
5. Expression and Function of P2X in Hypothalamic Nuclei
5.1. SON
5.1.1. Expression of P2X mRNA and Protein in SON
5.1.2. P2X Activity in SON Cells Studied by Intracellular Calcium Measurements
5.1.3. P2X Activity in SON Cells Studied by Using Electrophysiology
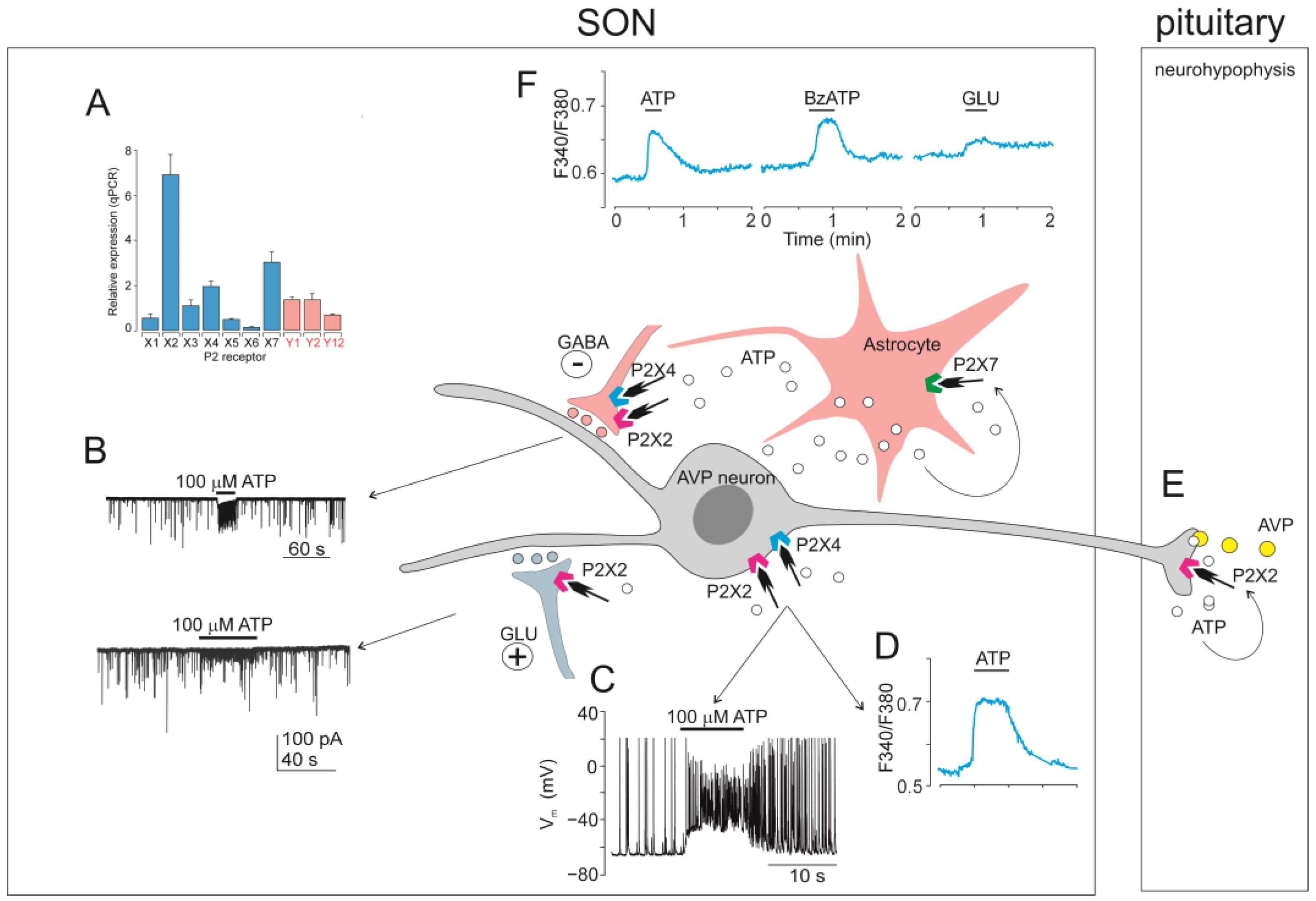
5.1.4. Functional Relevance of ATP Acting on P2X in the SON
5.2. PVN
5.2.1. Expression of P2X mRNA and Protein in PVN
5.2.2. P2X Activity in Magnocellular PVN Neurons Studied by Using Electrophysiology
5.2.3. P2X Activity in Parvocellular PVN Neurons Studied by Using Electrophysiology
5.2.4. Functional Relevance of ATP Acting at P2X in the PVN
5.3. SCN
5.3.1. Expression of P2X mRNA and Protein in the SCN
5.3.2. P2X Activity in SCN Cells Studied by Intracellular Calcium Measurements
5.3.3. P2X Activity in SCN Neurons Studied by Using Electrophysiology
5.3.4. Functional Relevance of ATP Acting at P2X in the SCN
5.4. AVPV
5.4.1. Expression of P2X mRNA and Protein in GnRH Neurons of AVPV
5.4.2. Expression of P2X mRNA and Protein in Kisspeptin Neurons of AVPV
5.4.3. P2X Activity in GnRH and Kisspeptin Neurons in AVPV Studied by Intracellular Calcium Measurements
5.4.4. P2X Activity in GnRH and Kisspeptin Neurons Studied by Using Electrophysiology
5.4.5. Functional Relevance of ATP Acting at P2X in the AVPV
5.5. AHN
5.5.1. Expression of P2X in the AHN
5.5.2. Functional Relevance of ATP in the AHN
5.6. ARC
5.6.1. Expression of P2X mRNA and Protein in ARC
5.6.2. P2X Activity in ARC Cells Studied by Intracellular Calcium Measurements
5.6.3. P2X Activity in ARC Cells Studied Using Electrophysiology
5.6.4. Role of ATP Acting in ARC Physiology
5.7. VMH
5.7.1. Expression of P2X mRNA and Protein in the VMH
5.7.2. P2X Activity in VMH Cells Studied by Intracellular Calcium Measurements
5.7.3. P2X Activity in VMH Cells Studied by Using Electrophysiology
5.7.4. Functional Role of ATP Acting at P2 Receptors in the VMH
5.8. DMH
5.8.1. Expression of P2X mRNA and Protein in DMH
5.8.2. P2X Activity in DMH Cells Studied by Intracellular Calcium Measurements
5.8.3. P2X Activity in DMH Cells Studied by Using Electrophysiology
5.8.4. Functional Relevance of ATP Acting on P2 Receptors in the DMH
5.9. TMN
5.9.1. Expression of P2X mRNA and Protein in TMN
5.9.2. P2X Activity in TMN Cells Studied by Electrophysiology
5.9.3. Functional Relevance of ATP Acting at P2X in the TMN
5.10. LHA
5.10.1. Expression of P2X mRNA and Protein in the LHA
5.10.2. P2X Activity in LHA Cells Studied by Electrophysiology
5.10.3. Functional Relevance of ATP Acting at P2X in the LHA
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADP | adenosine-5′-diphosphate |
| AgRP | agouti-related peptide |
| AMP | adenosine-5′-monophosphate |
| ATP | adenosine-5′-triphosphate |
| ATPγS | adenosine 5′-O-(2-thiotriphosphate) |
| 2MeSATP | 2-methylthio-adenosine triphosphate |
| AMPA | α-amino-3-hydroxy-5-methylisoxazole propionic acid |
| αβmeATP | α,β-methyleneadenosine 5′-triphosphate |
| AHN | anterior hypothalamic nucleus |
| ARC | arcuate nucleus |
| AVP | arginine vasopressin |
| AVPV | anteroventral periventricular nucleus |
| BBG | brilliant blue G |
| BzATP | 3’-O-(4-benzoyl)benzoyladenosine-5’-triphosphate |
| CNS | central nervous system |
| DMH | dorsomedial hypothalamic nucleus |
| DYN | dynorphin peptide |
| GABA | γ-aminobutyric acid |
| GABAA | GABA receptor type A |
| GFP | green fluorescent protein |
| GnRH | gonadotropin-releasing hormone |
| Kiss1R | kisspeptin receptor |
| LHA | lateral hypothalamic area |
| mIPSC | miniature inhibitory postsynaptic current |
| mEPSC | iniature excitatory postsynaptic current |
| MnPO | median preoptic nucleus |
| nNOS | neuronal nitric oxide synthase |
| NMDA | N-methyl-D-aspartate |
| MPOA | medial preoptic area |
| NPP1 | ecto-nucleotide pyrophosphatase/phosphodiesterase 1 |
| NPY | neuropeptide Y |
| P1 | purinergic receptor type 1 |
| P2X | purinergic receptor type 2 (ionotropic) |
| P2Y | purinergic receptor type 2 (metabotropic) |
| POA | preoptic area |
| PPADS | pyridoxalphosphate-6-azophenyl-2′, 4′-disulfonic acid |
| PVN | paraventricular nucleus |
| SCN | suprachiasmatic nucleus |
| SON | supraoptic nucleus |
| VMH | ventromedial hypothalamic nucleus |
| VTM | ventral tuberomammillary nucleus |
| TMN | tuberomammillary nucleus |
References
- Russell, J.A. Fifty Years of Advances in Neuroendocrinology. Brain Neurosci. Adv. 2018, 2, 2398212818812014. [Google Scholar] [CrossRef] [PubMed]
- Sanchez Jimenez, J.G.; De Jesus, O. Hypothalamic Dysfunction; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Steuernagel, L.; Lam, B.Y.H.; Klemm, P.; Dowsett, G.K.C.; Bauder, C.A.; Tadross, J.A.; Hitschfeld, T.S.; Del Rio Martin, A.; Chen, W.; de Solis, A.J.; et al. HypoMap-a unified single-cell gene expression atlas of the murine hypothalamus. Nat. Metab. 2022, 4, 1402–1419. [Google Scholar] [CrossRef]
- Collo, G.; North, R.A.; Kawashima, E.; Merlo-Pich, E.; Neidhart, S.; Surprenant, A.; Buell, G. Cloning OF P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996, 16, 2495–2507. [Google Scholar] [CrossRef]
- Tasker, J.G.; Oliet, S.H.; Bains, J.S.; Brown, C.H.; Stern, J.E. Glial regulation of neuronal function: From synapse to systems physiology. J. Neuroendocrinol. 2012, 24, 566–576. [Google Scholar] [CrossRef]
- Svobodova, I.; Bhattaracharya, A.; Ivetic, M.; Bendova, Z.; Zemkova, H. Circadian ATP Release in Organotypic Cultures of the Rat Suprachiasmatic Nucleus Is Dependent on P2X7 and P2Y Receptors. Front. Pharmacol. 2018, 9, 192. [Google Scholar] [CrossRef]
- Collo, G.; Neidhart, S.; Kawashima, E.; Kosco-Vilbois, M.; North, R.A.; Buell, G. Tissue distribution of the P2X7 receptor. Neuropharmacology 1997, 36, 1277–1283. [Google Scholar] [CrossRef]
- Genzen, J.R.; Platel, J.C.; Rubio, M.E.; Bordey, A. Ependymal cells along the lateral ventricle express functional P2X(7) receptors. Purinergic Signal. 2009, 5, 299–307. [Google Scholar] [CrossRef]
- Loesch, A. On P2X receptors in the brain: Microvessels. Dedicated to the memory of the late Professor Geoffrey Burnstock (1929–2020). Cell Tissue Res. 2021, 384, 577–588. [Google Scholar] [CrossRef]
- Caruso, V.; Zuccarini, M.; Di Iorio, P.; Muhammad, I.; Ronci, M. Metabolic Changes Induced by Purinergic Signaling: Role in Food Intake. Front. Pharmacol. 2021, 12, 655989. [Google Scholar] [CrossRef]
- Gao, Z.; Guan, J.; Yin, S.; Liu, F. The role of ATP in sleep-wake regulation: In adenosine-dependent and -independent manner. Sleep. Med. 2024, 119, 147–154. [Google Scholar] [CrossRef]
- Lemos, J.R.; Custer, E.E.; Ortiz-Miranda, S. Purinergic receptor types in the hypothalamic-neurohypophysial system. J. Neuroendocrinol. 2018, 30, e12588. [Google Scholar] [CrossRef] [PubMed]
- Zemkova, H.; Balik, A.; Jindrichova, M.; Vavra, V. Molecular structure of purinergic P2X receptors and their expression in the hypothalamus and pituitary. Physiol. Res. 2008, 57 (Suppl. 3), S23–S38. [Google Scholar] [CrossRef]
- Stojilkovic, S.S. Purinergic regulation of hypothalamopituitary functions. Trends Endocrinol. Metab. 2009, 20, 460–468. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Janjic, M.M.; Stojilkovic, S.S. Purinergic signaling pathways in endocrine system. Auton. Neurosci. 2015, 191, 102–116. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Zemkova, H. P2X receptor channels in endocrine glands. Wiley Interdiscip. Rev. Membr. Transp. Signal 2013, 2, 173–180. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic signalling in endocrine organs. Purinergic Signal. 2014, 10, 189–231. [Google Scholar]
- Ali, A.A.H.; Avakian, G.A.; Gall, C.V. The Role of Purinergic Receptors in the Circadian System. Int. J. Mol. Sci. 2020, 21, 3423. [Google Scholar] [CrossRef]
- Wang, X.; Dong, Y.T.; Hu, X.M.; Zhang, J.Z.; Shi, N.R.; Zuo, Y.Q.; Wang, X. The circadian regulation of extracellular ATP. Purinergic Signal. 2023, 19, 283–295. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, S.; Jin, S.Y.; Gao, T.M. Extracellular ATP Is a Homeostatic Messenger That Mediates Cell-Cell Communication in Physiological Processes and Psychiatric Diseases. Biol. Psychiatry 2024, 97, 41–53. [Google Scholar] [CrossRef]
- Moller, M.; Busch, J.R.; Jacobsen, C.; Lundemose, S.B.; Lynnerup, N.; Rath, M.F.; Banner, J. The accessory magnocellular neurosecretory system of the rostral human hypothalamus. Cell Tissue Res. 2018, 373, 487–498. [Google Scholar] [CrossRef]
- Voisin, D.L.; Herbison, A.E.; Poulain, D.A. Central inhibitory effects of muscimol and bicuculline on the milk ejection reflex in the anaesthetized rat. J. Physiol. 1995, 483 Pt 1, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Moos, F.C. GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J. Physiol. 1995, 488 Pt 1, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Brussaard, A.B.; Devay, P.; Leyting-Vermeulen, J.L.; Kits, K.S. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J. Physiol. 1999, 516 Pt 2, 513–524. [Google Scholar] [CrossRef]
- Jourdain, P.; Israel, J.M.; Dupouy, B.; Oliet, S.H.; Allard, M.; Vitiello, S.; Theodosis, D.T.; Poulain, D.A. Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J. Neurosci. 1998, 18, 6641–6649. [Google Scholar] [CrossRef]
- Oliet, S.H.; Bourque, C.W. Mechanosensitive channels transduce osmosensitivity in supraoptic neurons. Nature 1993, 364, 341–343. [Google Scholar] [CrossRef]
- Israel, J.M.; Poulain, D.A.; Oliet, S.H. Glutamatergic inputs contribute to phasic activity in vasopressin neurons. J. Neurosci. 2010, 30, 1221–1232. [Google Scholar] [CrossRef]
- Choe, K.Y.; Han, S.Y.; Gaub, P.; Shell, B.; Voisin, D.L.; Knapp, B.A.; Barker, P.A.; Brown, C.H.; Cunningham, J.T.; Bourque, C.W. High salt intake increases blood pressure via BDNF-mediated downregulation of KCC2 and impaired baroreflex inhibition of vasopressin neurons. Neuron 2015, 85, 549–560. [Google Scholar] [CrossRef]
- Pow, D.V.; Morris, J.F. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience 1989, 32, 435–439. [Google Scholar] [CrossRef]
- Sabatier, N.; Caquineau, C.; Dayanithi, G.; Bull, P.; Douglas, A.J.; Guan, X.M.; Jiang, M.; Van der Ploeg, L.; Leng, G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J. Neurosci. 2003, 23, 10351–10358. [Google Scholar] [CrossRef]
- Taylor, J.H.; Intorre, A.A.; French, J.A. Vasopressin and Oxytocin Reduce Food Sharing Behavior in Male, but Not Female Marmosets in Family Groups. Front. Endocrinol. 2017, 8, 181. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; Tortora, A.; Giusti, C.; Lisco, G.; Giagulli, V.A.; Guastamacchia, E.; De Pergola, G.; Triggiani, V. Oxytocin Signaling Pathway: From Cell Biology to Clinical Implications. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 91–110. [Google Scholar]
- Martucci, L.L.; Launay, J.M.; Kawakami, N.; Sicard, C.; Desvignes, N.; Dakouane-Giudicelli, M.; Spix, B.; Tetu, M.; Gilmaire, F.O.; Paulcan, S.; et al. Endolysosomal TPCs regulate social behavior by controlling oxytocin secretion. Proc. Natl. Acad. Sci. USA 2023, 120, e2213682120. [Google Scholar] [CrossRef]
- Hu, H.; Zarate, C.A., Jr.; Verbalis, J. Arginine vasopressin in mood disorders: A potential biomarker of disease pathology and a target for pharmacologic intervention. Psychiatry Clin. Neurosci. 2024, 78, 495–506. [Google Scholar] [CrossRef]
- Ludwig, M. Dendritic release of vasopressin and oxytocin. J. Neuroendocrinol. 1998, 10, 881–895. [Google Scholar] [CrossRef]
- Leng, G.; Ludwig, M. Neurotransmitters and peptides: Whispered secrets and public announcements. J. Physiol. 2008, 586, 5625–5632. [Google Scholar] [CrossRef]
- Kombian, S.B.; Hirasawa, M.; Mouginot, D.; Pittman, Q.J. Modulation of synaptic transmission by oxytocin and vasopressin in the supraoptic nucleus. Prog. Brain Res. 2002, 139, 235–246. [Google Scholar]
- Kombian, S.B.; Hirasawa, M.; Mouginot, D.; Chen, X.; Pittman, Q.J. Short-term potentiation of miniature excitatory synaptic currents causes excitation of supraoptic neurons. J. Neurophysiol. 2000, 83, 2542–2553. [Google Scholar] [CrossRef]
- Brussaard, A.B.; Kits, K.S. Changes in GABAA receptor-mediated synaptic transmission in oxytocin neurons during female reproduction: Plasticity in a neuroendocrine context. Ann. N. Y. Acad. Sci. 1999, 868, 677–680. [Google Scholar] [CrossRef]
- Li, C.; Tripathi, P.K.; Armstrong, W.E. Differences in spike train variability in rat vasopressin and oxytocin neurons and their relationship to synaptic activity. J. Physiol. 2007, 581 Pt 1, 221–240. [Google Scholar] [CrossRef]
- Sladek, C.D.; Kapoor, J.R. Neurotransmitter/neuropeptide interactions in the regulation of neurohypophyseal hormone release. Exp. Neurol. 2001, 171, 200–209. [Google Scholar] [CrossRef]
- Armstrong, W.E. The neurophysiology of neurosecretory cells. J. Physiol. 2007, 585 Pt 3, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Brown, C.H.; Russell, J.A. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Progress. Neurobiol. 1999, 57, 625–655. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Bourque, C.W. Functional N-Methyl-D-Aspartate and Non-N-Methyl-D-Aspartate Receptors are Expressed by Rat Supraoptic Neurosecretory Cells in vitro. J. Neuroendocrinol. 1991, 3, 509–514. [Google Scholar] [CrossRef]
- Shibuya, I.; Kabashima, N.; Ibrahim, N.; Setiadji, S.V.; Ueta, Y.; Yamashita, H. Pre- and postsynaptic modulation of the electrical activity of rat supraoptic neurones. Exp. Physiol. 2000, 85, 145S–151S. [Google Scholar] [CrossRef]
- Wuarin, J.P.; Dudek, F.E. Patch-clamp analysis of spontaneous synaptic currents in supraoptic neuroendocrine cells of the rat hypothalamus. J. Neurosci. 1993, 13, 2323–2331. [Google Scholar] [CrossRef]
- Decavel, C.; Curras, M.C. Increased expression of the N-methyl-D-aspartate receptor subunit, NR1, in immunohistochemically identified magnocellular hypothalamic neurons during dehydration. Neuroscience 1997, 78, 191–202. [Google Scholar] [CrossRef]
- Boudaba, C.; Di, S.; Tasker, J.G. Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J. Neuroendocrinol. 2003, 15, 803–810. [Google Scholar] [CrossRef]
- Iremonger, K.J.; Benediktsson, A.M.; Bains, J.S. Glutamatergic synaptic transmission in neuroendocrine cells: Basic principles and mechanisms of plasticity. Front. Neuroendocrinol. 2010, 31, 296–306. [Google Scholar] [CrossRef]
- Vilhena-Franco, T.; Valentim-Lima, E.; Reis, L.C.; Elias, L.L.K.; Antunes-Rodrigues, J.; Mecawi, A.S. Role of AMPA and NMDA receptors on vasopressin and oxytocin secretion induced by hypertonic extracellular volume expansion. J. Neuroendocrinol. 2018, 30, e12633. [Google Scholar] [CrossRef]
- Brown, C.H. Magnocellular Neurons and Posterior Pituitary Function. Compr. Physiol. 2016, 6, 1701–1741. [Google Scholar] [CrossRef]
- Ferguson, A.V.; Latchford, K.J.; Samson, W.K. The paraventricular nucleus of the hypothalamus-a potential target for integrative treatment of autonomic dysfunction. Expert. Opin. Ther. Targets 2008, 12, 717–727. [Google Scholar] [CrossRef]
- Rasiah, N.P.; Loewen, S.P.; Bains, J.S. Windows into stress: A glimpse at emerging roles for CRH(PVN) neurons. Physiol. Rev. 2023, 103, 1667–1691. [Google Scholar] [CrossRef]
- Sawchenko, P.E.; Brown, E.R.; Chan, R.K.; Ericsson, A.; Li, H.Y.; Roland, B.L.; Kovacs, K.J. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog. Brain Res. 1996, 107, 201–222. [Google Scholar]
- Simmons, D.M.; Swanson, L.W. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: Toward a global 3D model. J. Comp. Neurol. 2009, 516, 423–441. [Google Scholar] [CrossRef]
- Yang, Z.; Coote, J.H. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J. Physiol. 1998, 513, 521–530. [Google Scholar] [CrossRef]
- Shafton, A.D.; Ryan, A.; Badoer, E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998, 801, 239–243. [Google Scholar] [CrossRef]
- Sanders, J.; Nemeroff, C. The CRF System as a Therapeutic Target for Neuropsychiatric Disorders. Trends Pharmacol. Sci. 2016, 37, 1045–1054. [Google Scholar] [CrossRef]
- Marsh, N.; Marsh, A.A.; Lee, M.R.; Hurlemann, R. Oxytocin and the Neurobiology of Prosocial Behavior. Neuroscientist 2021, 27, 604–619. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Cuesta-Marti, C.; Lopez-Salas, A.; Chruscicka-Smaga, B.; Crespo-Ramirez, M.; Tesoro-Cruz, E.; Palacios-Lagunas, D.A.; Perez de la Mora, M.; Schellekens, H.; Fuxe, K. The oxytocin receptor represents a key hub in the GPCR heteroreceptor network: Potential relevance for brain and behavior. Front. Mol. Neurosci. 2022, 15, 1055344. [Google Scholar] [CrossRef]
- Santoso, P.; Nakata, M.; Ueta, Y.; Yada, T. Suprachiasmatic vasopressin to paraventricular oxytocin neurocircuit in the hypothalamus relays light reception to inhibit feeding behavior. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E478–E488. [Google Scholar] [CrossRef]
- Vargas, Y.; Castro Tron, A.E.; Rodriguez Rodriguez, A.; Uribe, R.M.; Joseph-Bravo, P.; Charli, J.L. Thyrotropin-Releasing Hormone and Food Intake in Mammals: An Update. Metabolites 2024, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Nunn, N.; Womack, M.; Dart, C.; Barrett-Jolley, R. Function and pharmacology of spinally-projecting sympathetic pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Curr. Neuropharmacol. 2011, 9, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Strecker, G.J.; Wuarin, J.P.; Dudek, F.E. GABAA-mediated local synaptic pathways connect neurons in the rat suprachiasmatic nucleus. J. Neurophysiol. 1997, 78, 2217–2220. [Google Scholar] [CrossRef]
- Moore, R.Y.; Eichler, V.B. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972, 42, 201–206. [Google Scholar] [CrossRef]
- Stephan, F.K.; Zucker, I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. USA 1972, 69, 1583–1586. [Google Scholar] [CrossRef]
- Sumova, A.; Travnickova, Z.; Peters, R.; Schwartz, W.J.; Illnerova, H. The rat suprachiasmatic nucleus is a clock for all seasons. Proc. Natl. Acad. Sci. USA 1995, 92, 7754–7758. [Google Scholar] [CrossRef]
- Reppert, S.M. A clockwork explosion! Neuron 1998, 21, 1–4. [Google Scholar] [CrossRef]
- Lee, H.S.; Billings, H.J.; Lehman, M.N. The suprachiasmatic nucleus: A clock of multiple components. J. Biol. Rhythm. 2003, 18, 435–449. [Google Scholar] [CrossRef]
- Moore, R.Y.; Card, J.P. Visual pathways and the entrainment of circadian rhythms. Ann. N. Y. Acad. Sci. 1985, 453, 123–133. [Google Scholar] [CrossRef]
- Jacomy, H.; Burlet, A.; Bosler, O. Vasoactive intestinal peptide neurons as synaptic targets for vasopressin neurons in the suprachiasmatic nucleus. Double-label immunocytochemical demonstration in the rat. Neuroscience 1999, 88, 859–870. [Google Scholar] [CrossRef]
- Brancaccio, M.; Enoki, R.; Mazuski, C.N.; Jones, J.; Evans, J.A.; Azzi, A. Network-mediated encoding of circadian time: The suprachiasmatic nucleus (SCN) from genes to neurons to circuits, and back. J. Neurosci. 2014, 34, 15192–15199. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.T.; Kawamura, H. Persistence of circadian rhythmicity in a mammalian hypothalamic island containing the suprachiasmatic nucleus. Proc. Natl. Acad. Sci. USA 1979, 76, 5962–5966. [Google Scholar] [CrossRef]
- Groos, G.; Hendriks, J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci. Lett. 1982, 34, 283–288. [Google Scholar] [CrossRef]
- Pennartz, C.M.; de Jeu, M.T.; Bos, N.P.; Schaap, J.; Geurtsen, A.M. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 2002, 416, 286–290. [Google Scholar] [CrossRef]
- Stephens, S.B.Z.; Kauffman, A.S. Estrogen Regulation of the Molecular Phenotype and Active Translatome of AVPV Kisspeptin Neurons. Endocrinology 2021, 162, bqab080. [Google Scholar] [CrossRef]
- Spergel, D.J. Modulation of Gonadotropin-Releasing Hormone Neuron Activity and Secretion in Mice by Non-peptide Neurotransmitters, Gasotransmitters, and Gliotransmitters. Front. Endocrinol. 2019, 10, 329. [Google Scholar] [CrossRef]
- Fu, J.; Yu, Q.; Guo, W.; He, C.; Burnstock, G.; Xiang, Z. P2X receptors are expressed on neurons containing luteinizing hormone-releasing hormone in the mouse hypothalamus. Neurosci. Lett. 2009, 458, 32–36. [Google Scholar] [CrossRef]
- Sarkar, D.K.; Chiappa, S.A.; Fink, G.; Sherwood, N.M. Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 1976, 264, 461–463. [Google Scholar] [CrossRef]
- Baca-Alonso, J.J.A.; Quintanar, J.L. The effect of gonadotropin-releasing hormone on the nervous system. Neuro Endocrinol. Lett. 2024, 45, 188–196. [Google Scholar]
- Bakker, J. Can kisspeptin be a new treatment for sexual dysfunction? Trends Endocrinol. Metab. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Messager, S.; Chatzidaki, E.E.; Ma, D.; Hendrick, A.G.; Zahn, D.; Dixon, J.; Thresher, R.R.; Malinge, I.; Lomet, D.; Carlton, M.B.; et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. USA 2005, 102, 1761–1766. [Google Scholar] [CrossRef] [PubMed]
- de Roux, N.; Genin, E.; Carel, J.C.; Matsuda, F.; Chaussain, J.L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed]
- Sliwowska, J.H.; Woods, N.E.; Alzahrani, A.R.; Paspali, E.; Tate, R.J.; Ferro, V.A. Kisspeptin a potential therapeutic target in treatment of both metabolic and reproductive dysfunction. J. Diabetes 2024, 16, e13541. [Google Scholar] [CrossRef]
- Boulant, J.A.; Hardy, J.D. The effect of spinal and skin temperatures on the firing rate and thermosensitivity of preoptic neurones. J. Physiol. 1974, 240, 639–660. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef]
- Martins, A.B.; Brownlow, M.L.; Araujo, B.B.; Garnica-Siqueira, M.C.; Zaia, D.A.M.; Leite, C.M.; Zaia, C.; Uchoa, E.T. Arcuate nucleus of the hypothalamus contributes to the hypophagic effect and plasma metabolic changes induced by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Neurochem. Int. 2022, 155, 105300. [Google Scholar] [CrossRef]
- Fioramonti, X.; Lorsignol, A.; Taupignon, A.; Penicaud, L. A new ATP-sensitive K+ channel-independent mechanism is involved in glucose-excited neurons of mouse arcuate nucleus. Diabetes 2004, 53, 2767–2775. [Google Scholar] [CrossRef]
- Mehay, D.; Silberman, Y.; Arnold, A.C. The Arcuate Nucleus of the Hypothalamus and Metabolic Regulation: An Emerging Role for Renin-Angiotensin Pathways. Int. J. Mol. Sci. 2021, 22, 7050. [Google Scholar] [CrossRef]
- Dos Santos, K.M.; Saunders, S.E.; Antunes, V.R.; Boychuk, C.R. Insulin activates parasympathetic hepatic-related neurons of the paraventricular nucleus of the hypothalamus through mTOR signaling. J. Neurophysiol. 2025, 133, 320–332. [Google Scholar] [CrossRef]
- Clarkson, J.; Han, S.Y.; Piet, R.; McLennan, T.; Kane, G.M.; Ng, J.; Porteous, R.W.; Kim, J.S.; Colledge, W.H.; Iremonger, K.J.; et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. USA 2017, 114, E10216–E10223. [Google Scholar] [CrossRef]
- Goto, T.; Hagihara, M.; Miyamichi, K. Dynamics of pulsatile activities of arcuate kisspeptin neurons in aging female mice. eLife 2023, 12, e82533. [Google Scholar] [CrossRef] [PubMed]
- Koysombat, K.; Tsoutsouki, J.; Patel, A.H.; Comninos, A.N.; Dhillo, W.S.; Abbara, A. Kisspeptin and Neurokinin B: Roles in reproductive health. Physiol. Rev. 2025, 105, 707–764. [Google Scholar] [CrossRef]
- Sawchenko, P.E.; Swanson, L.W. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982, 257, 275–325. [Google Scholar] [CrossRef]
- Mezey, E.; Kiss, J.Z.; Mueller, G.P.; Eskay, R.; O’Donohue, T.L.; Palkovits, M. Distribution of the pro-opiomelanocortin derived peptides, adrenocorticotrope hormone, alpha-melanocyte-stimulating hormone and beta-endorphin (ACTH, alpha-MSH, beta-END) in the rat hypothalamus. Brain Res. 1985, 328, 341–347. [Google Scholar] [CrossRef]
- Morgane, P.J. Electrophysiological studies of feeding and satiety centers in the rat. Am. J. Physiol. 1961, 201, 838–844. [Google Scholar] [CrossRef]
- King, B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol. Behav. 2006, 87, 221–244. [Google Scholar] [CrossRef]
- Jo, Y.H.; Donier, E.; Martinez, A.; Garret, M.; Toulme, E.; Boue-Grabot, E. Crosstalk between P2X4 and GABA-A receptors determines synaptic efficacy at central synapses. J. Biol. Chem. 2011, 256, 19993–20004. [Google Scholar] [CrossRef]
- Cheung, C.C.; Kurrasch, D.M.; Liang, J.K.; Ingraham, H.A. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. J. Comp. Neurol. 2013, 521, 1268–1288. [Google Scholar] [CrossRef]
- Sapkota, S.; Roy, S.C.; Briski, K.P. Dorsomedial Ventromedial Hypothalamic Nucleus Growth Hormone-Releasing Hormone Neuron Steroidogenic Factor-1 Gene Targets in Female Rat. ASN Neuro 2024, 16, 2403345. [Google Scholar] [CrossRef]
- Fosch, A.; Zagmutt, S.; Casals, N.; Rodriguez-Rodriguez, R. New Insights of SF1 Neurons in Hypothalamic Regulation of Obesity and Diabetes. Int. J. Mol. Sci. 2021, 22, 6186. [Google Scholar] [CrossRef]
- Bellinger, L.L.; Bernardis, L.L. The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: Lessons learned from lesioning studies. Physiol. Behav. 2002, 76, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Brasil, T.F.S.; Lopes-Azevedo, S.; Belem-Filho, I.J.A.; Fortaleza, E.A.T.; Antunes-Rodrigues, J.; Correa, F.M.A. The Dorsomedial Hypothalamus Is Involved in the Mediation of Autonomic and Neuroendocrine Responses to Restraint Stress. Front. Pharmacol. 2019, 10, 1547. [Google Scholar] [CrossRef]
- DiMicco, J.A.; Samuels, B.C.; Zaretskaia, M.V.; Zaretsky, D.V. The dorsomedial hypothalamus and the response to stress: Part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002, 71, 469–480. [Google Scholar] [CrossRef]
- Sakai, K.; Takahashi, K.; Anaclet, C.; Lin, J.S. Sleep-waking discharge of ventral tuberomammillary neurons in wild-type and histidine decarboxylase knock-out mice. Front. Behav. Neurosci. 2010, 4, 53. [Google Scholar] [CrossRef]
- Wada, H.; Inagaki, N.; Itowi, N.; Yamatodani, A. Histaminergic neuron system in the brain: Distribution and possible functions. Brain Res. Bull. 1991, 27, 367–370. [Google Scholar] [CrossRef]
- Mickelsen, L.E.; Bolisetty, M.; Chimileski, B.R.; Fujita, A.; Beltrami, E.J.; Costanzo, J.T.; Naparstek, J.R.; Robson, P.; Jackson, A.C. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 2019, 22, 642–656. [Google Scholar] [CrossRef]
- Collden, G.; Mangano, C.; Meister, B. P2X2 purinoreceptor protein in hypothalamic neurons associated with the regulation of food intake. Neuroscience 2010, 171, 62–78. [Google Scholar] [CrossRef]
- Sandoval-Caballero, C.; Jara, J.; Luarte, L.; Jimenez, Y.; Teske, J.A.; Perez-Leighton, C. Control of motivation for sucrose in the paraventricular hypothalamic nucleus by dynorphin peptides and the kappa opioid receptor. Appetite 2024, 200, 107504. [Google Scholar] [CrossRef]
- Bernardis, L.L.; Bellinger, L.L. The lateral hypothalamic area revisited: Neuroanatomy, body weight regulation, neuroendocrinology and metabolism. Neurosci. Biobehav. Rev. 1993, 17, 141–193. [Google Scholar] [CrossRef]
- Bonnavion, P.; Mickelsen, L.E.; Fujita, A.; de Lecea, L.; Jackson, A.C. Hubs and spokes of the lateral hypothalamus: Cell types, circuits and behaviour. J. Physiol. 2016, 594, 6443–6462. [Google Scholar] [CrossRef]
- Samson, W.K.; Taylor, M.M.; Follwell, M.; Ferguson, A.V. Orexin actions in hypothalamic paraventricular nucleus: Physiological consequences and cellular correlates. Regul. Pept. 2002, 104, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Young, S.L.; Cox, V.C. Analgesia for formalin-induced pain by lateral hypothalamic stimulation. Brain Res. 1991, 563, 1–6. [Google Scholar] [CrossRef]
- Khan, R.; Lee, B.; Inyang, K.; Bemis, H.; Bugescu, R.; Laumet, G.; Leinninger, G. Neurotensin-expressing lateral hypothalamic neurons alleviate neuropathic and inflammatory pain via neurotensin receptor signaling. Neurobiol. Pain. 2024, 16, 100172. [Google Scholar] [CrossRef]
- Beamer, E.; Conte, G.; Engel, T. ATP release during seizures-A critical evaluation of the evidence. Brain Res. Bull. 2019, 151, 65–73. [Google Scholar] [CrossRef]
- Juranyi, Z.; Sperlagh, B.; Vizi, E.S. Involvement of P2 purinoceptors and the nitric oxide pathway in [3H]purine outflow evoked by short-term hypoxia and hypoglycemia in rat hippocampal slices. Brain Res. 1999, 823, 183–190. [Google Scholar] [CrossRef]
- Godoy, P.A.; Ramirez-Molina, O.; Fuentealba, J. Exploring the Role of P2X Receptors in Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Guthrie, P.B.; Knappenberger, J.; Segal, M.; Bennett, M.V.; Charles, A.C.; Kater, S.B. ATP released from astrocytes mediates glial calcium waves. J. Neurosci. 1999, 19, 520–528. [Google Scholar] [CrossRef]
- Fields, R.D.; Stevens, B. ATP: An extracellular signaling molecule between neurons and glia. Trends Neurosci. 2000, 23, 625–633. [Google Scholar] [CrossRef]
- Inoue, K.; Koizumi, S.; Tsuda, M. The role of nucleotides in the neuron—Glia communication responsible for the brain functions. J. Neurochem. 2007, 102, 1447–1458. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Lalo, U.; Palygin, O.; Verkhratsky, A.; Grant, S.G.; Pankratov, Y. ATP from synaptic terminals and astrocytes regulates NMDA receptors and synaptic plasticity through PSD-95 multi-protein complex. Sci. Rep. 2016, 6, 33609. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Crosby, K.M.; Murphy-Royal, C.; Wilson, S.A.; Gordon, G.R.; Bains, J.S.; Pittman, Q.J. Cholecystokinin Switches the Plasticity of GABA Synapses in the Dorsomedial Hypothalamus via Astrocytic ATP Release. J. Neurosci. 2018, 38, 8515–8525. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, T.; Li, X.J.; Li, S. Mutant Huntingtin Impairs BDNF Release from Astrocytes by Disrupting Conversion of Rab3a-GTP into Rab3a-GDP. J. Neurosci. 2016, 36, 8790–8801. [Google Scholar] [CrossRef]
- Lazarowski, E.R.; Sesma, J.I.; Seminario-Vidal, L.; Kreda, S.M. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv. Pharmacol. 2011, 61, 221–261. [Google Scholar]
- Lazarowski, E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012, 8, 359–373. [Google Scholar] [CrossRef]
- Burnstock, G.; Fredholm, B.B.; Verkhratsky, A. Adenosine and ATP receptors in the brain. Curr. Top. Med. Chem. 2011, 11, 973–1011. [Google Scholar] [CrossRef]
- Jo, Y.H.; Schlichter, R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat. Neurosci. 1999, 2, 241–245. [Google Scholar] [CrossRef]
- Norenberg, W.; Illes, P. Neuronal P2X receptors: Localisation and functional properties. Naunyn. Schmiedebergs Arch. Pharmacol. 2000, 362, 324–339. [Google Scholar] [CrossRef]
- Jang, I.S.; Rhee, J.S.; Kubota, H.; Akaike, N. Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J. Physiol. 2001, 536 Pt 2, 505–519. [Google Scholar] [CrossRef]
- Rodrigues, R.J.; Almeida, T.; Richardson, P.J.; Oliveira, C.R.; Cunha, R.A. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 2005, 25, 6286–6295. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic cotransmission. Exp. Physiol. 2009, 94, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Vavra, V.; Bhattacharya, A.; Zemkova, H. Facilitation of glutamate and GABA release by P2X receptor activation in supraoptic neurons from freshly isolated rat brain slices. Neuroscience 2011, 188, 1–12. [Google Scholar] [CrossRef]
- Gordon, G.R.; Baimoukhametova, D.V.; Hewitt, S.A.; Rajapaksha, W.R.; Fisher, T.E.; Bains, J.S. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 2005, 8, 1078–1086. [Google Scholar] [CrossRef]
- Fumagalli, M.; Brambilla, R.; D’Ambrosi, N.; Volonte, C.; Matteoli, M.; Verderio, C.; Abbracchio, M.P. Nucleotide-mediated calcium signaling in rat cortical astrocytes: Role of P2X and P2Y receptors. Glia 2003, 43, 218–230. [Google Scholar] [CrossRef]
- Pascual, O.; Casper, K.B.; Kubera, C.; Zhang, J.; Revilla-Sanchez, R.; Sul, J.Y.; Takano, H.; Moss, S.J.; McCarthy, K.; Haydon, P.G. Astrocytic purinergic signaling coordinates synaptic networks. Science 2005, 310, 113–116. [Google Scholar] [CrossRef]
- Pangrsic, T.; Potokar, M.; Stenovec, M.; Kreft, M.; Fabbretti, E.; Nistri, A.; Pryazhnikov, E.; Khiroug, L.; Giniatullin, R.; Zorec, R. Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 2007, 282, 28749–28758. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 2008, 1, ra6. [Google Scholar] [CrossRef]
- Iglesias, R.; Dahl, G.; Qiu, F.; Spray, D.C.; Scemes, E. Pannexin 1: The molecular substrate of astrocyte “hemichannels”. J. Neurosci. 2009, 29, 7092–7097. [Google Scholar] [CrossRef]
- Li, S.; Bjelobaba, I.; Yan, Z.; Kucka, M.; Tomic, M.; Stojilkovic, S.S. Expression and roles of pannexins in ATP release in the pituitary gland. Endocrinology 2011, 152, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Kinoshita, M.; Hirayama, Y.; Fujishita, K.; Shibata, K.; Shinozaki, Y.; Shigetomi, E.; Takeda, A.; Le, H.P.N.; Hayashi, H.; Hiasa, M.; et al. Anti-Depressant Fluoxetine Reveals its Therapeutic Effect Via Astrocytes. EBioMedicine 2018, 32, 72–83. [Google Scholar] [CrossRef]
- Kawate, T.; Michel, J.C.; Birdsong, W.T.; Gouaux, E. Crystal structure of the ATP-gated P2X(4) ion channel in the closed state. Nature 2009, 460, 592–598. [Google Scholar] [CrossRef]
- Hattori, M.; Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 2012, 485, 207–212. [Google Scholar] [CrossRef]
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef]
- Nicke, A.; Baumert, H.G.; Rettinger, J.; Eichele, A.; Lambrecht, G.; Mutschler, E.; Schmalzing, G. P2X1 and P2X3 receptors form stable trimers: A novel structural motif of ligand-gated ion channels. EMBO J. 1998, 17, 3016–3028. [Google Scholar] [CrossRef]
- Pelegrin, P.; Surprenant, A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006, 25, 5071–5082. [Google Scholar] [CrossRef]
- Boue-Grabot, E.; Toulme, E.; Emerit, M.B.; Garret, M. Subunit-specific coupling between gamma-aminobutyric acid type A and P2X2 receptor channels. J. Biol. Chem. 2004, 279, 52517–52525. [Google Scholar] [CrossRef]
- Boue-Grabot, E.; Emerit, M.B.; Toulme, E.; Seguela, P.; Garret, M. Cross-talk and co-trafficking between rho1/GABA receptors and ATP-gated channels. J. Biol. Chem. 2004, 279, 6967–6975. [Google Scholar] [CrossRef]
- Xia, R.; Mei, Z.-Z.; Milligan, C.; Jiang, L.H. Inhibitory interaction between P2X4 and GABA(C) rho1 receptors. Biochem. Biophys. Res. Commun. 2008, 375, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Queme, L.F.; Weyler, A.A.; Cohen, E.R.; Hudgins, R.C.; Jankowski, M.P. A dual role for peripheral GDNF signaling in nociception and cardiovascular reflexes in the mouse. Proc. Natl. Acad. Sci. USA 2020, 117, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat. Rev. Neurosci. 2001, 2, 165–174. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tomic, M.; He, M.L.; Yan, Z.; Koshimizu, T.A.; Zemkova, H. Molecular dissection of purinergic P2X receptor channels. Ann. N. Y. Acad. Sci. 2005, 1048, 116–130. [Google Scholar] [CrossRef]
- Samways, D.S.; Li, Z.; Egan, T.M. Principles and properties of ion flow in P2X receptors. Front. Cell Neurosci. 2014, 8, 6. [Google Scholar] [CrossRef]
- Khakh, B.S.; Proctor, W.R.; Dunwiddie, T.V.; Labarca, C.; Lester, H.A. Allosteric control of gating and kinetics at P2X(4) receptor channels. J. Neurosci. 1999, 19, 7289–7299. [Google Scholar] [CrossRef]
- Coddou, C.; Yan, Z.; Obsil, T.; Huidobro-Toro, J.P.; Stojilkovic, S.S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 2011, 63, 641–683. [Google Scholar] [CrossRef]
- Coddou, C.; Stojilkovic, S.S.; Huidobro-Toro, J.P. Allosteric modulation of ATP-gated P2X receptor channels. Rev. Neurosci. 2011, 22, 335–354. [Google Scholar] [CrossRef]
- Stokes, L.; Bidula, S.; Bibic, L.; Allum, E. To Inhibit or Enhance? Is There a Benefit to Positive Allosteric Modulation of P2X Receptors? Front. Pharmacol. 2020, 11, 627. [Google Scholar] [CrossRef]
- Illes, P.; Muller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Sivcev, S.; Kudova, E.; Zemkova, H. Neurosteroids as positive and negative allosteric modulators of ligand-gated ion channels: P2X receptor perspective. Neuropharmacology 2023, 234, 109542. [Google Scholar] [CrossRef] [PubMed]
- Norenberg, W.; Sobottka, H.; Hempel, C.; Plotz, T.; Fischer, W.; Schmalzing, G.; Schaefer, M. Positive allosteric modulation by ivermectin of human but not murine P2X7 receptors. Br. J. Pharmacol. 2012, 167, 48–66. [Google Scholar] [CrossRef]
- Bianchi, B.R.; Lynch, K.J.; Touma, E.; Niforatos, W.; Burgard, E.C.; Alexander, K.M.; Park, H.S.; Yu, H.; Metzger, R.; Kowaluk, E.; et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur. J. Pharmacol. 1999, 376, 127–138. [Google Scholar] [CrossRef]
- Oury, C.; Toth-Zsamboki, E.; Van Geet, C.; Thys, C.; Wei, L.; Nilius, B.; Vermylen, J.; Hoylaerts, M.F. A natural dominant negative P2X1 receptor due to deletion of a single amino acid residue. J. Biol. Chem. 2000, 275, 22611–22614. [Google Scholar] [CrossRef]
- Khakh, B.S.; Bao, X.R.; Labarca, C.; Lester, H.A. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat. Neurosci. 1999, 2, 322–330. [Google Scholar] [CrossRef]
- Lynch, K.J.; Touma, E.; Niforatos, W.; Kage, K.L.; Burgard, E.C.; van Biesen, T.; Kowaluk, E.A.; Jarvis, M.F. Molecular and functional characterization of human P2X(2) receptors. Mol. Pharmacol. 1999, 56, 1171–1181. [Google Scholar]
- Clyne, J.D.; LaPointe, L.D.; Hume, R.I. The role of histidine residues in modulation of the rat P2X(2) purinoceptor by zinc and pH. J. Physiol. 2002, 539 Pt 2, 347–359. [Google Scholar] [CrossRef]
- Zhong, Y.; Dunn, P.M.; Xiang, Z.; Bo, X.; Burnstock, G. Pharmacological and molecular characterization of P2X receptors in rat pelvic ganglion neurons. Br. J. Pharmacol. 1998, 125, 771–781. [Google Scholar] [CrossRef]
- Haustein, M.D.; Kracun, S.; Lu, X.H.; Shih, T.; Jackson-Weaver, O.; Tong, X.; Xu, J.; Yang, X.W.; O’Dell, T.J.; Marvin, J.S.; et al. Conditions and constraints for astrocyte calcium signaling in the hippocampal mossy fiber pathway. Neuron 2014, 82, 413–429. [Google Scholar] [CrossRef]
- Kim, S.H.; Bahia, P.K.; Patil, M.; Sutton, S.; Sowells, I.; Hadley, S.H.; Kollarik, M.; Taylor-Clark, T.E. Development of a Mouse Reporter Strain for the Purinergic P2X(2) Receptor. eNeuro 2020, 7, 1–15. [Google Scholar] [CrossRef]
- Grohmann, M.; Schumacher, M.; Gunther, J.; Singheiser, S.M.; Nussbaum, T.; Wildner, F.; Gerevich, Z.; Jabs, R.; Hirnet, D.; Lohr, C.; et al. BAC transgenic mice to study the expression of P2X2 and P2Y(1) receptors. Purinergic Signal. 2021, 17, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek-Hajek, K.; Lorinczi, E.; Hausmann, R.; Nicke, A. Molecular and functional properties of P2X receptors—Recent progress and persisting challenges. Purinergic Signal. 2012, 8, 375–417. [Google Scholar] [PubMed]
- Hugel, S.; Schlichter, R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J. Neurosci. 2000, 20, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Khakh, B.S.; Gittermann, D.; Cockayne, D.A.; Jones, A. ATP modulation of excitatory synapses onto interneurons. J. Neurosci. 2003, 23, 7426–7437. [Google Scholar] [CrossRef]
- Lewis, C.; Neidhart, S.; Holy, C.; North, R.A.; Buell, G.; Surprenant, A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature 1995, 377, 432–435. [Google Scholar] [CrossRef]
- Finger, T.E.; Danilova, V.; Barrows, J.; Bartel, D.L.; Vigers, A.J.; Stone, L.; Hellekant, G.; Kinnamon, S.C. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005, 310, 1495–1499. [Google Scholar] [CrossRef]
- Cao, X.; Li, L.P.; Wang, Q.; Wu, Q.; Hu, H.H.; Zhang, M.; Fang, Y.Y.; Zhang, J.; Li, S.J.; Xiong, W.C.; et al. Astrocyte-derived ATP modulates depressive-like behaviors. Nat. Med. 2013, 19, 773–777. [Google Scholar] [CrossRef]
- Gerevich, Z.; Zadori, Z.S.; Koles, L.; Kopp, L.; Milius, D.; Wirkner, K.; Gyires, K.; Illes, P. Dual effect of acid pH on purinergic P2X3 receptors depends on the histidine 206 residue. J. Biol. Chem. 2007, 282, 33949–33957. [Google Scholar] [CrossRef]
- Mansoor, S.E.; Lu, W.; Oosterheert, W.; Shekhar, M.; Tajkhorshid, E.; Gouaux, E. X-ray structures define human P2X(3) receptor gating cycle and antagonist action. Nature 2016, 538, 66–71. [Google Scholar] [CrossRef]
- Chen, C.C.; Akopian, A.N.; Sivilotti, L.; Colquhoun, D.; Burnstock, G.; Wood, J.N. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 1995, 377, 428–431. [Google Scholar] [CrossRef]
- Cook, S.P.; McCleskey, E.W. Desensitization, recovery and Ca(2+)-dependent modulation of ATP-gated P2X receptors in nociceptors. Neuropharmacology 1997, 36, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- North, R.A. P2X3 receptors and peripheral pain mechanisms. J. Physiol. 2004, 554 Pt 2, 301–308. [Google Scholar] [CrossRef]
- Ding, S.; Zhu, L.; Tian, Y.; Zhu, T.; Huang, X.; Zhang, X. P2X3 receptor involvement in endometriosis pain via ERK signaling pathway. PLoS ONE 2017, 12, e0184647. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.B.; Zhang, Y.L.; Li, Q.; Liu, Y.G.; Wang, X.D.; Yang, B.L.; Zhu, G.C.; Zhou, C.F.; Gao, Y.; Liu, Z.X. Effects of 1,8-cineole on neuropathic pain mediated by P2X2 receptor in the spinal cord dorsal horn. Sci. Rep. 2019, 9, 7909. [Google Scholar] [CrossRef]
- North, R.A. P2X receptors. Philos. Trans. R Soc. Lond. B Biol. Sci. 2016, 371, 20150427. [Google Scholar] [CrossRef]
- Silva-Ramos, M.; Silva, I.; Faria, M.; Ferreirinha, F.; Correia-de-Sa, P. Activation of Prejunctional P2x2/3 Heterotrimers by ATP Enhances the Cholinergic Tone in Obstructed Human Urinary Bladders. J. Pharmacol. Exp. Ther. 2020, 372, 63–72. [Google Scholar] [CrossRef]
- Khakh, B.S.; North, R.A. P2X receptors as cell-surface ATP sensors in health and disease. Nature 2006, 442, 527–532. [Google Scholar] [CrossRef]
- Qureshi, O.S.; Paramasivam, A.; Yu, J.C.; Murrell-Lagnado, R.D. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007, 120 Pt 21, 3838–3849. [Google Scholar] [CrossRef]
- Huang, P.; Zou, Y.; Zhong, X.Z.; Cao, Q.; Zhao, K.; Zhu, M.X.; Murrell-Lagnado, R.; Dong, X.P. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J. Biol. Chem. 2014, 289, 17658–17667. [Google Scholar] [CrossRef]
- Stoop, R.; Surprenant, A.; North, R.A. Different sensitivities to pH of ATP-induced currents at four cloned P2X receptors. J. Neurophysiol. 1997, 78, 1837–1840. [Google Scholar] [CrossRef]
- Jelinkova, I.; Vavra, V.; Jindrichova, M.; Obsil, T.; Zemkova, H.W.; Zemkova, H.; Stojilkovic, S.S. Identification of P2X(4) receptor transmembrane residues contributing to channel gating and interaction with ivermectin. Pflug. Arch. 2008, 456, 939–950. [Google Scholar] [CrossRef]
- Buell, G.; Lewis, C.; Collo, G.; North, R.A.; Surprenant, A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996, 15, 55–62. [Google Scholar] [CrossRef]
- Jelinkova, I.; Yan, Z.; Liang, Z.; Moonat, S.; Teisinger, J.; Stojilkovic, S.S.; Zemkova, H. Identification of P2X(4) receptor-specific residues contributing to the ivermectin effects on channel deactivation. Biochem. Biophys. Res. Commun. 2006, 349, 619–625. [Google Scholar] [CrossRef]
- Le, K.T.; Babinski, K.; Seguela, P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 1998, 18, 7152–7159. [Google Scholar] [CrossRef]
- Seguela, P.; Haghighi, A.; Soghomonian, J.J.; Cooper, E. A novel neuronal P2x ATP receptor ion channel with widespread distribution in the brain. J. Neurosci. 1996, 16, 448–455. [Google Scholar] [CrossRef]
- Srivastava, P.; Cronin, C.G.; Scranton, V.L.; Jacobson, K.A.; Liang, B.T.; Verma, R. Neuroprotective and neuro-rehabilitative effects of acute purinergic receptor P2X4 (P2X4R) blockade after ischemic stroke. Exp. Neurol. 2020, 329, 113308. [Google Scholar] [CrossRef]
- Montilla, A.; Mata, G.P.; Matute, C.; Domercq, M. Contribution of P2X4 Receptors to CNS Function and Pathophysiology. Int. J. Mol. Sci. 2020, 21, 5562. [Google Scholar] [CrossRef]
- Kotnis, S.; Bingham, B.; Vasilyev, D.V.; Miller, S.W.; Bai, Y.; Yeola, S.; Chanda, P.K.; Bowlby, M.R.; Kaftan, E.J.; Samad, T.A.; et al. Genetic and functional analysis of human P2X5 reveals a distinct pattern of exon 10 polymorphism with predominant expression of the nonfunctional receptor isoform. Mol. Pharmacol. 2010, 77, 953–960. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Liu, X.; Burnstock, G.; Xiang, Z.; He, C. Developmental expression of P2X5 receptors in the mouse prenatal central and peripheral nervous systems. Purinergic Signal. 2013, 9, 239–248. [Google Scholar] [CrossRef]
- Guo, W.; Xu, X.; Gao, X.; Burnstock, G.; He, C.; Xiang, Z. Expression of P2X5 receptors in the mouse CNS. Neuroscience 2008, 156, 673–692. [Google Scholar] [CrossRef]
- Ryten, M.; Dunn, P.M.; Neary, J.T.; Burnstock, G. ATP regulates the differentiation of mammalian skeletal muscle by activation of a P2X5 receptor on satellite cells. J. Cell Biol. 2002, 158, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kajikawa, T.; Walsh, M.C.; Takegahara, N.; Jeong, Y.H.; Hajishengallis, G.; Choi, Y. The purinergic receptor P2X5 contributes to bone loss in experimental periodontitis. BMB Rep. 2018, 51, 468–473. [Google Scholar] [CrossRef]
- Soto, F.; Garcia-Guzman, M.; Karschin, C.; Stuhmer, W. Cloning and tissue distribution of a novel P2X receptor from rat brain. Biochem. Biophys. Res. Commun. 1996, 223, 456–460. [Google Scholar] [CrossRef]
- North, R. P2X receptors: A third major class of ligand-gated ion channels. Ciba Found. Symp. 1996, 198, 91–105. [Google Scholar]
- King, B.F.; Townsend-Nicholson, A.; Wildman, S.S.; Thomas, T.; Spyer, K.M.; Burnstock, G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 2000, 20, 4871–4877. [Google Scholar] [CrossRef]
- Torres, G.E.; Egan, T.M.; Voigt, M.M. Identification of a domain involved in ATP-gated ionotropic receptor subunit assembly. J. Biol. Chem. 1999, 274, 22359–22365. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Virginio, C.; MacKenzie, A.; Rassendren, F.A.; North, R.A.; Surprenant, A. Pore dilation of neuronal P2X receptor channels. Nat. Neurosci. 1999, 2, 315–321. [Google Scholar] [CrossRef]
- Cheewatrakoolpong, B.; Gilchrest, H.; Anthes, J.C.; Greenfeder, S. Identification and characterization of splice variants of the human P2X7 ATP channel. Biochem. Biophys. Res. Commun. 2005, 332, 17–27. [Google Scholar] [CrossRef]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandona, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The Elusive P2X7 Macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Gelin, C.F.; Bhattacharya, A.; Letavic, M.A. P2X7 receptor antagonists for the treatment of systemic inflammatory disorders. Prog. Med. Chem. 2020, 59, 63–99. [Google Scholar] [PubMed]
- Miras-Portugal, M.T.; Sebastian-Serrano, A.; de Diego Garcia, L.; Diaz-Hernandez, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef]
- Kopp, R.; Krautloher, A.; Ramirez-Fernandez, A.; Nicke, A. P2X7 Interactions and Signaling-Making Head or Tail of It. Front. Mol. Neurosci. 2019, 12, 183. [Google Scholar] [CrossRef]
- Bartlett, R.; Stokes, L.; Sluyter, R. The P2X7 receptor channel: Recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 2014, 66, 638–675. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Lord, B.; Grigoleit, J.S.; He, Y.; Fraser, I.; Campbell, S.N.; Taylor, N.; Aluisio, L.; O’Connor, J.C.; Papp, M.; et al. Neuropsychopharmacology of JNJ-55308942, evaluation of a clinical candidate targeting P2X7 ion channels in animal models of neuroinflammation and anhedonia. Neuropsychopharmacology 2018, 43, 2586–2596. [Google Scholar] [CrossRef]
- Czamara, D.; Muller-Myhsok, B.; Lucae, S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 82, 272–277. [Google Scholar] [CrossRef]
- Deussing, J.M.; Arzt, E. P2X7 Receptor: A Potential Therapeutic Target for Depression? Trends Mol. Med. 2018, 24, 736–747. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Biber, K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016, 64, 1772–1787. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Ceusters, M. Targeting neuroinflammation with brain penetrant P2X7 antagonists as novel therapeutics for neuropsychiatric disorders. Neuropsychopharmacology 2020, 45, 234–235. [Google Scholar] [CrossRef]
- Illes, P.; Verkhratsky, A.; Tang, Y. Pathological ATPergic Signaling in Major Depression and Bipolar Disorder. Front. Mol. Neurosci. 2019, 12, 331. [Google Scholar] [CrossRef]
- Liu, J.; Liu, T.T.; Mou, L.; Zhang, Y.; Chen, X.; Wang, Q.; Deng, B.L.; Liu, J. P2X7 receptor: A potential target for treating comorbid anxiety and depression. Purinergic Signal. 2024, 1–11. [Google Scholar] [CrossRef]
- Vereczkei, A.; Abdul-Rahman, O.; Halmai, Z.; Nagy, G.; Szekely, A.; Somogyi, A.; Faludi, G.; Nemoda, Z. Association of purinergic receptor P2RX7 gene polymorphisms with depression symptoms. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 207–216. [Google Scholar] [CrossRef]
- Zorina-Lichtenwalter, K.; Ase, A.R.; Verma, V.; Parra, A.I.M.; Komarova, S.; Khadra, A.; Seguela, P.; Diatchenko, L. Characterization of Common Genetic Variants in P2RX7 and Their Contribution to Chronic Pain Conditions. J. Pain 2024, 25, 545–556. [Google Scholar] [CrossRef]
- Jimenez-Pacheco, A.; Diaz-Hernandez, M.; Arribas-Blazquez, M.; Sanz-Rodriguez, A.; Olivos-Ore, L.A.; Artalejo, A.R.; Alves, M.; Letavic, M.; Miras-Portugal, M.T.; Conroy, R.M.; et al. Transient P2X7 Receptor Antagonism Produces Lasting Reductions in Spontaneous Seizures and Gliosis in Experimental Temporal Lobe Epilepsy. J. Neurosci. 2016, 36, 5920–5932. [Google Scholar] [CrossRef]
- Kaczmarek-Hajek, K.; Zhang, J.; Kopp, R.; Grosche, A.; Rissiek, B.; Saul, A.; Bruzzone, S.; Engel, T.; Jooss, T.; Krautloher, A.; et al. Re-evaluation of neuronal P2X7 expression using novel mouse models and a P2X7-specific nanobody. eLife 2018, 7, e36217. [Google Scholar] [CrossRef]
- Ortega, F.; Gomez-Villafuertes, R.; Benito-Leon, M.; Martinez de la Torre, M.; Olivos-Ore, L.A.; Arribas-Blazquez, M.; Gomez-Gaviro, M.V.; Azcorra, A.; Desco, M.; Artalejo, A.R.; et al. Salient brain entities labelled in P2rx7-EGFP reporter mouse embryos include the septum, roof plate glial specializations and circumventricular ependymal organs. Brain Struct. Funct. 2021, 226, 715–741. [Google Scholar] [CrossRef]
- Shibuya, I.; Tanaka, K.; Hattori, Y.; Uezono, Y.; Harayama, N.; Noguchi, J.; Ueta, Y.; Izumi, F.; Yamashita, H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J. Physiol. 1999, 514 Pt 2, 351–367. [Google Scholar] [CrossRef]
- Housley, G.D.; Kanjhan, R.; Raybould, N.P.; Greenwood, D.; Salih, S.G.; Jarlebark, L.; Burton, L.D.; Setz, V.C.; Cannell, M.B.; Soeller, C.; et al. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: Implications for sound transduction and auditory neurotransmission. J. Neurosci. 1999, 19, 8377–8388. [Google Scholar] [CrossRef]
- Vulchanova, L.; Arvidsson, U.; Riedl, M.; Wang, J.; Buell, G.; Surprenant, A.; North, R.A.; Elde, R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. USA 1996, 93, 8063–8067. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Bo, X.; Oglesby, I.; Ford, A.; Burnstock, G. Localization of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamus. Brain Res. 1998, 813, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Vavra, V.; Svobodova, I.; Bendova, Z.; Vereb, G.; Zemkova, H. Potentiation of inhibitory synaptic transmission by extracellular ATP in rat suprachiasmatic nuclei. J. Neurosci. 2013, 33, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Lommen, J.; Stahr, A.; Ingenwerth, M.; Ali, A.A.H.; von Gall, C. Time-of-day-dependent expression of purinergic receptors in mouse suprachiasmatic nucleus. Cell Tissue Res. 2017, 369, 579–590. [Google Scholar] [CrossRef]
- Loesch, A.; Miah, S.; Burnstock, G. Ultrastructural localisation of ATP-gated P2X2 receptor immunoreactivity in the rat hypothalamo-neurohypophysial system. J. Neurocytol. 1999, 28, 495–504. [Google Scholar] [CrossRef]
- Knott, T.K.; Velazquez-Marrero, C.; Lemos, J.R. ATP elicits inward currents in isolated vasopressinergic neurohypophysial terminals via P2X2 and P2X3 receptors. Pflug. Arch. 2005, 450, 381–389. [Google Scholar] [CrossRef]
- Guo, W.; Sun, J.; Xu, X.; Bunstock, G.; He, C.; Xiang, Z. P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurons in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem. Cell Biol. 2009, 131, 29–41. [Google Scholar] [CrossRef]
- Knott, T.K.; Hussy, N.; Cuadra, A.E.; Lee, R.H.; Ortiz-Miranda, S.; Custer, E.E.; Lemos, J.R. Adenosine trisphosphate appears to act via different receptors in terminals versus somata of the hypothalamic neurohypophysial system. J. Neuroendocrinol. 2012, 24, 681–689. [Google Scholar] [CrossRef]
- Xiang, Z.; He, C.; Burnstock, G. P2X5 receptors are expressed on neurons containing arginine vasopressin and nitric oxide synthase in the rat hypothalamus. Brain Res. 2006, 1099, 56–63. [Google Scholar] [CrossRef]
- Loesch, A.; Burnstock, G. Immunoreactivity to P2X(6) receptors in the rat hypothalamo- neurohypophysial system: An ultrastructural study with extravidin and colloidal gold-silver labelling. Neuroscience 2001, 106, 621–631. [Google Scholar] [CrossRef]
- Gomes, D.A.; Song, Z.; Stevens, W.; Sladek, C.D. Sustained stimulation of vasopressin and oxytocin release by ATP and phenylephrine requires recruitment of desensitization-resistant P2X purinergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, R940–R949. [Google Scholar] [CrossRef]
- Cuadra, A.E.; Custer, E.E.; Bosworth, E.L.; Lemos, J.R. P2X7 receptors in neurohypophysial terminals: Evidence for their role in arginine-vasopressin secretion. J. Cell Physiol. 2014, 229, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Levy, A.; Lightman, S.L. Activation of specific ATP receptors induces a rapid increase in intracellular calcium ions in rat hypothalamic neurons. Brain Res. 1994, 641, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Troadec, J.D.; Thirion, S.; Nicaise, G.; Lemos, J.R.; Dayanithi, G. ATP-evoked increases in [Ca2+]i and peptide release from rat isolated neurohypophysial terminals via a P2X2 purinoceptor. J. Physiol. 1998, 511 Pt 1, 89–103. [Google Scholar] [CrossRef]
- Song, Z.; Vijayaraghavan, S.; Sladek, C.D. ATP increases intracellular calcium in supraoptic neurons by activation of both P2X and P2Y purinergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R423–R431. [Google Scholar] [CrossRef]
- Espallergues, J.; Solovieva, O.; Techer, V.; Bauer, K.; Alonso, G.; Vincent, A.; Hussy, N. Synergistic activation of astrocytes by ATP and norepinephrine in the rat supraoptic nucleus. Neuroscience 2007, 148, 712–723. [Google Scholar] [CrossRef]
- Sladek, C.D.; Song, Z. Diverse roles of G-protein coupled receptors in the regulation of neurohypophyseal hormone secretion. J. Neuroendocrinol. 2012, 24, 554–565. [Google Scholar] [CrossRef]
- Day, T.A.; Sibbald, J.R.; Khanna, S. ATP mediates an excitatory noradrenergic neuron input to supraoptic vasopressin cells. Brain Res. 1993, 607, 341–344. [Google Scholar] [CrossRef]
- Hiruma, H.; Bourque, C.W. P2 purinoceptor-mediated depolarization of rat supraoptic neurosecretory cells in vitro. J. Physiol. 1995, 489 Pt 3, 805–811. [Google Scholar] [CrossRef]
- Ivetic, M.; Bhattacharyya, A.; Zemkova, H. P2X2 Receptor Expression and Function Is Upregulated in the Rat Supraoptic Nucleus Stimulated Through Refeeding After Fasting. Front. Cell Neurosci. 2019, 13, 284. [Google Scholar] [CrossRef]
- Mori, M.; Tsushima, H.; Matsuda, T. Antidiuretic effects of ATP induced by microinjection into the hypothalamic supraoptic nucleus in water-loaded and ethanol-anesthetized rats. Jpn. J. Pharmacol. 1994, 66, 445–450. [Google Scholar] [CrossRef]
- Kapoor, J.R.; Sladek, C.D. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J. Neurosci. 2000, 20, 8868–8875. [Google Scholar] [CrossRef] [PubMed]
- Buller, K.M.; Khanna, S.; Sibbald, J.R.; Day, T.A. Central noradrenergic neurons signal via ATP to elicit vasopressin responses to haemorrhage. Neuroscience 1996, 73, 637–642. [Google Scholar] [CrossRef]
- Sperlagh, B.; Mergl, Z.; Juranyi, Z.; Vizi, E.S.; Makara, G.B. Local regulation of vasopressin and oxytocin secretion by extracellular ATP in the isolated posterior lobe of the rat hypophysis. J. Endocrinol. 1999, 160, 343–350. [Google Scholar] [CrossRef]
- Knott, T.K.; Marrero, H.G.; Custer, E.E.; Lemos, J.R. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J. Cell Physiol. 2008, 217, 155–161. [Google Scholar] [CrossRef]
- Custer, E.E.; Knott, T.K.; Cuadra, A.E.; Ortiz-Miranda, S.; Lemos, J.R. P2X purinergic receptor knockout mice reveal endogenous ATP modulation of both vasopressin and oxytocin release from the intact neurohypophysis. J. Neuroendocrinol. 2012, 24, 674–680. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ohkubo, J.; Katoh, A.; Ohno, M.; Ishikura, T.; Kakuma, T.; Yoshimatsu, H.; Murphy, D.; Ueta, Y. A c-fos-monomeric red fluorescent protein 1 fusion transgene is differentially expressed in rat forebrain and brainstem after chronic dehydration and rehydration. J. Neuroendocrinol. 2013, 25, 478–487. [Google Scholar] [CrossRef]
- Edgar, R.S.; Green, E.W.; Zhao, Y.; van Ooijen, G.; Olmedo, M.; Qin, X.; Xu, Y.; Pan, M.; Valekunja, U.K.; Feeney, K.A.; et al. Peroxiredoxins are conserved markers of circadian rhythms. Nature 2012, 485, 459–464. [Google Scholar] [CrossRef]
- Gottlieb, H.B.; Ji, L.L.; Cunningham, J.T. Role of superior laryngeal nerve and Fos staining following dehydration and rehydration in the rat. Physiol. Behav. 2011, 104, 1053–1058. [Google Scholar] [CrossRef]
- Carreno, F.R.; Walch, J.D.; Dutta, M.; Nedungadi, T.P.; Cunningham, J.T. Brain-derived neurotrophic factor-tyrosine kinase B pathway mediates NMDA receptor NR2B subunit phosphorylation in the supraoptic nuclei following progressive dehydration. J. Neuroendocrinol. 2011, 23, 894–905. [Google Scholar] [CrossRef]
- Lucio-Oliveira, F.; Traslavina, G.A.; Borges, B.D.; Franci, C.R. Modulation of the activity of vasopressinergic neurons by estrogen in rats refed with normal or sodium-free food after fasting. Neuroscience 2015, 284, 325–336. [Google Scholar] [CrossRef]
- Yao, S.T.; Gourine, A.V.; Spyer, K.M.; Barden, J.A.; Lawrence, A.J. Localisation of P2X2 receptor subunit immunoreactivity on nitric oxide synthase expressing neurones in the brain stem and hypothalamus of the rat: A fluorescence immunohistochemical study. Neuroscience 2003, 121, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Cham, J.L.; Owens, N.C.; Barden, J.A.; Lawrence, A.J.; Badoer, E. P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp. Physiol. 2006, 91, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Neto, H.C.; Ribeiro, I.M.; Moreira, T.S.; Yao, S.T.; Antunes, V.R. Purinergic P2 receptors in the paraventricular nucleus of the hypothalamus are involved in hyperosmotic-induced sympathoexcitation. Neuroscience 2017, 349, 253–263. [Google Scholar] [CrossRef]
- Du, D.; Jiang, M.; Liu, M.; Wang, J.; Xia, C.; Guan, R.; Shen, L.; Ji, Y.; Zhu, D. Microglial P2X(7) receptor in the hypothalamic paraventricular nuclei contributes to sympathoexcitatory responses in acute myocardial infarction rat. Neurosci. Lett. 2015, 587, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Jacques-Silva, M.C.; Bernardi, A.; Rodnight, R.; Lenz, G. ERK, PKC and PI3K/Akt pathways mediate extracellular ATP and adenosine-induced proliferation of U138-MG human glioma cell line. Oncology 2004, 67, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Bains, J.S.; Oliet, S.H. Glia: They make your memories stick! Trends Neurosci. 2007, 30, 417–424. [Google Scholar] [CrossRef]
- Gordon, G.R.; Iremonger, K.J.; Kantevari, S.; Ellis-Davies, G.C.; MacVicar, B.A.; Bains, J.S. Astrocyte-mediated distributed plasticity at hypothalamic glutamate synapses. Neuron 2009, 64, 391–403. [Google Scholar] [CrossRef]
- Ferreira-Neto, H.C.; Antunes, V.R.; Stern, J.E. Purinergic P2 and glutamate NMDA receptor coupling contributes to osmotically driven excitability in hypothalamic magnocellular neurosecretory neurons. J. Physiol. 2021, 599, 3531–3547. [Google Scholar] [CrossRef]
- Whitlock, A.; Burnstock, G.; Gibb, A.J. The single-channel properties of purinergic P2X ATP receptors in outside-out patches from rat hypothalamic paraventricular parvocells. Pflug. Arch. 2001, 443, 115–122. [Google Scholar] [CrossRef]
- Ferreira-Neto, H.C.; Antunes, V.R.; Stern, J.E. ATP stimulates rat hypothalamic sympathetic neurons by enhancing AMPA receptor-mediated currents. J. Neurophysiol. 2015, 114, 159–169. [Google Scholar] [CrossRef]
- Xu, J.; Bernstein, A.M.; Wong, A.; Lu, X.H.; Khoja, S.; Yang, X.W.; Davies, D.L.; Micevych, P.; Sofroniew, M.V.; Khakh, B.S. P2X4 Receptor Reporter Mice: Sparse Brain Expression and Feeding-Related Presynaptic Facilitation in the Arcuate Nucleus. J. Neurosci. 2016, 36, 8902–8920. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Tsushima, H.; Matsuda, T. Antidiuretic effects of purinoceptor agonists injected into the hypothalamic paraventricular nucleus of water-loaded, ethanol-anesthetized rats. Neuropharmacology 1992, 31, 585–592. [Google Scholar] [CrossRef]
- Cruz, J.C.; Bonagamba, L.G.; Machado, B.H. Modulation of arterial pressure by P2 purinoceptors in the paraventricular nucleus of the hypothalamus of awake rats. Auton. Neurosci. Basic Clin. 2010, 158, 79–85. [Google Scholar] [CrossRef]
- Minczuk, K.; Schlicker, E.; Krzyzewska, A.; Malinowska, B. Angiotensin 1-7 injected into the rat paraventricular nucleus of hypothalamus increases blood pressure and heart rate via various receptors. Neuropharmacology 2024, 266, 110279. [Google Scholar] [CrossRef]
- Busnardo, C.; Ferreira-Junior, N.C.; Cruz, J.C.; Machado, B.H.; Correa, F.M.; Resstel, L.B. Cardiovascular responses to ATP microinjected into the paraventricular nucleus are mediated by nitric oxide and NMDA glutamate receptors in awake rats. Exp. Physiol. 2013, 98, 1411–1421. [Google Scholar] [CrossRef]
- Ferreira-Neto, H.C.; Yao, S.T.; Antunes, V.R. Purinergic and glutamatergic interactions in the hypothalamic paraventricular nucleus modulate sympathetic outflow. Purinergic Signal. 2013, 9, 337–349. [Google Scholar] [CrossRef]
- Barad, Z.; Jacob-Tomas, S.; Sobrero, A.; Lean, G.; Hicks, A.I.; Yang, J.; Choe, K.Y.; Prager-Khoutorsky, M. Unique Organization of Actin Cytoskeleton in Magnocellular Vasopressin Neurons in Normal Conditions and in Response to Salt-Loading. eNeuro 2020, 7. [Google Scholar] [CrossRef]
- Balapattabi, K.; Little, J.T.; Farmer, G.E.; Cunningham, J.T. High salt loading increases brain derived neurotrophic factor in supraoptic vasopressin neurones. J. Neuroendocrinol. 2018, 30, e12639. [Google Scholar] [CrossRef]
- Martins Sa, R.W.; Theparambil, S.M.; Dos Santos, K.M.; Christie, I.N.; Marina, N.; Cardoso, B.V.; Hosford, P.S.; Antunes, V.R. Salt-loading promotes extracellular ATP release mediated by glial cells in the hypothalamic paraventricular nucleus of rats. Mol. Cell Neurosci. 2023, 124, 103806. [Google Scholar] [CrossRef]
- Haam, J.; Halmos, K.C.; Di, S.; Tasker, J.G. Nutritional state-dependent ghrelin activation of vasopressin neurons via retrograde trans-neuronal-glial stimulation of excitatory GABA circuits. J. Neurosci. 2014, 34, 6201–6213. [Google Scholar] [CrossRef]
- Wei, B.; Cheng, G.; Bi, Q.; Lu, C.; Sun, Q.; Li, L.; Chen, N.; Hu, M.; Lu, H.; Xu, X.; et al. Microglia in the hypothalamic paraventricular nucleus sense hemodynamic disturbance and promote sympathetic excitation in hypertension. Immunity 2024, 57, 2030–2042. [Google Scholar] [CrossRef] [PubMed]
- Kanjhan, R.; Housley, G.D.; Burton, L.D.; Christie, D.L.; Kippenberger, A.; Thorne, P.R.; Luo, L.; Ryan, A.F. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J. Comp. Neurol. 1999, 407, 11–32. [Google Scholar] [CrossRef]
- Lommen, J.; Detken, J.; Harr, K.; von Gall, C.; Ali, A.A.H. Analysis of Spatial and Temporal Distribution of Purinergic P2 Receptors in the Mouse Hippocampus. Int. J. Mol. Sci. 2021, 22, 8078. [Google Scholar] [CrossRef] [PubMed]
- Cipolla-Neto, J.; Skorupa, A.L.; Ribeiro-Barbosa, E.R.; Bartol, I.; Mota, S.R.; Afeche, S.C.; Delagrange, P.; Guardiola-Lemaitre, B.; Canteras, N.S. The role of the retrochiasmatic area in the control of pineal metabolism. Neuroendocrinology 1999, 69, 97–104. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ishida, Y.; Inouye, S. Circadian rhythms of adenosine triphosphate contents in the suprachiasmatic nucleus, anterior hypothalamic area and caudate putamen of the rat—Negative correlation with electrical activity. Brain Res. 1994, 664, 237–240. [Google Scholar] [CrossRef]
- Womac, A.D.; Burkeen, J.F.; Neuendorff, N.; Earnest, D.J.; Zoran, M.J. Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur. J. Neurosci. 2009, 30, 869–876. [Google Scholar] [CrossRef]
- Hastings, M.H.; Maywood, E.S.; Brancaccio, M. The Mammalian Circadian Timing System and the Suprachiasmatic Nucleus as Its Pacemaker. Biology 2019, 8, 13. [Google Scholar] [CrossRef]
- McArthur, A.J.; Hunt, A.E.; Gillette, M.U. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: Activation of protein kinase C at dusk and dawn. Endocrinology 1997, 138, 627–634. [Google Scholar] [CrossRef]
- Marpegan, L.; Swanstrom, A.E.; Chung, K.; Simon, T.; Haydon, P.G.; Khan, S.K.; Liu, A.C.; Herzog, E.D.; Beaule, C. Circadian regulation of ATP release in astrocytes. J. Neurosci. 2011, 31, 8342–8350. [Google Scholar] [CrossRef]
- Burkeen, J.F.; Womac, A.D.; Earnest, D.J.; Zoran, M.J. Mitochondrial calcium signaling mediates rhythmic extracellular ATP accumulation in suprachiasmatic nucleus astrocytes. J. Neurosci. 2011, 31, 8432–8440. [Google Scholar] [CrossRef]
- Dworak, M.; McCarley, R.W.; Kim, T.; Kalinchuk, A.V.; Basheer, R. Sleep and brain energy levels: ATP changes during sleep. J. Neurosci. 2010, 30, 9007–9016. [Google Scholar] [CrossRef] [PubMed]
- Buell, G.; Collo, G.; Rassendren, F. P2X receptors: An emerging channel family. Eur. J. Neurosci. 1996, 8, 2221–2228. [Google Scholar] [CrossRef]
- Terasawa, E.; Keen, K.L.; Grendell, R.L.; Golos, T.G. Possible role of 5′-adenosine triphosphate in synchronization of Ca2+ oscillations in primate luteinizing hormone-releasing hormone neurons. Mol. Endocrinol. 2005, 19, 2736–2747. [Google Scholar] [CrossRef]
- Vastagh, C.; Rodolosse, A.; Solymosi, N.; Liposits, Z. Altered Expression of Genes Encoding Neurotransmitter Receptors in GnRH Neurons of Proestrous Mice. Front. Cell Neurosci. 2016, 10, 230. [Google Scholar] [CrossRef]
- Bjelobaba, I.; Nedeljkovic, N.; Subasic, S.; Lavrnja, I.; Pekovic, S.; Stojkov, D.; Rakic, L.; Stojiljkovic, M. Immunolocalization of ecto-nucleotide pyrophosphatase/phosphodiesterase 1 (NPP1) in the rat forebrain. Brain Res. 2006, 1120, 54–63. [Google Scholar] [CrossRef]
- Inoue, N.; Hazim, S.; Tsuchida, H.; Dohi, Y.; Ishigaki, R.; Takahashi, A.; Otsuka, Y.; Yamada, K.; Uenoyama, Y.; Tsukamura, H. Hindbrain Adenosine 5-Triphosphate (ATP)-Purinergic Signaling Triggers LH Surge and Ovulation via Activation of AVPV Kisspeptin Neurons in Rats. J. Neurosci. 2023, 43, 2140–2152. [Google Scholar] [CrossRef]
- Constantin, S.; Klenke, U.; Wray, S. The calcium oscillator of GnRH-1 neurons is developmentally regulated. Endocrinology 2010, 151, 3863–3873. [Google Scholar] [CrossRef]
- Bosma, M.M. Ion channel properties and episodic activity in isolated immortalized gonadotropin-releasing hormone (GnRH) neurons. J. Membr. Biol. 1993, 136, 85–96. [Google Scholar] [CrossRef]
- Koshimizu, T.; Tomic, M.; Koshimizu, M.; Stojilkovic, S.S. Identification of amino acid residues contributing to desensitization of the P2X2 receptor channel. J. Biol. Chem. 1998, 273, 12853–12857. [Google Scholar] [CrossRef]
- Barnea, A.; Cho, G.; Katz, B.M. A putative role for extracellular, A.T.P. facilitation of 67copper uptake and of copper stimulation of the release of luteinizing hormone-releasing hormone from median eminence explants. Brain Res. 1991, 541, 93–97. [Google Scholar] [CrossRef]
- Zsarnovszky, A.; Bartha, T.; Frenyo, L.V.; Diano, S. NTPDases in the neuroendocrine hypothalamus: Possible energy regulators of the positive gonadotrophin feedback. Reprod. Biol. Endocrinol. 2009, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Gonzalez-Iglesias, A.E.; Tomic, M.; Stojilkovic, S.S. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1-3 in hypothalamic and pituitary cells. Purinergic Signal. 2005, 1, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Allen-Worthington, K.; Xie, J.; Brown, J.L.; Edmunson, A.M.; Dowling, A.; Navratil, A.M.; Scavelli, K.; Yoon, H.; Kim, D.G.; Bynoe, M.S.; et al. The F0F1 ATP Synthase Complex Localizes to Membrane Rafts in Gonadotrope Cells. Mol. Endocrinol. 2016, 30, 996–1011. [Google Scholar] [CrossRef]
- Chen, Z.P.; Kratzmeier, M.; Levy, A.; McArdle, C.A.; Poch, A.; Day, A.; Mukhopadhyay, A.K.; Lightman, S.L. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc. Natl. Acad. Sci. USA 1995, 92, 5219–5223. [Google Scholar] [CrossRef]
- Tomic, M.; Jobin, R.M.; Vergara, L.A.; Stojilkovic, S.S. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J. Biol. Chem. 1996, 271, 21200–21208. [Google Scholar] [CrossRef]
- Zemkova, H.; Balik, A.; Jiang, Y.; Kretschmannova, K.; Stojilkovic, S.S. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol. Endocrinol. 2006, 20, 1423–1436. [Google Scholar] [CrossRef]
- Gourine, A.V.; Melenchuk, E.V.; Poputnikov, D.M.; Gourine, V.N.; Spyer, K.M. Involvement of purinergic signalling in central mechanisms of body temperature regulation in rats. Br. J. Pharmacol. 2002, 135, 2047–2055. [Google Scholar] [CrossRef]
- Klir, J.J.; McClellan, J.L.; Kluger, M.J. Interleukin-1 beta causes the increase in anterior hypothalamic interleukin-6 during LPS-induced fever in rats. Am. J. Physiol. 1994, 266 Pt 2, R1845–R1848. [Google Scholar] [CrossRef]
- Gourine, A.V.; Dale, N.; Gourine, V.N.; Spyer, K.M. Fever in systemic inflammation: Roles of purines. Front. Biosci. 2004, 9, 1011–1022. [Google Scholar] [CrossRef]
- Mehta, V.B.; Hart, J.; Wewers, M.D. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J. Biol. Chem. 2001, 276, 3820–3826. [Google Scholar] [CrossRef]
- Hide, I.; Tanaka, M.; Inoue, A.; Nakajima, K.; Kohsaka, S.; Inoue, K.; Nakata, Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J. Neurochem. 2000, 75, 965–972. [Google Scholar] [CrossRef]
- Gourine, A.V.; Poputnikov, D.M.; Zhernosek, N.; Melenchuk, E.V.; Gerstberger, R.; Spyer, K.M.; Gourine, V.N. P2 receptor blockade attenuates fever and cytokine responses induced by lipopolysaccharide in rats. Br. J. Pharmacol. 2005, 146, 139–145. [Google Scholar] [CrossRef]
- Gourine, A.V.; Dale, N.; Llaudet, E.; Poputnikov, D.M.; Spyer, K.M.; Gourine, V.N. Release of ATP in the central nervous system during systemic inflammation: Real-time measurement in the hypothalamus of conscious rabbits. J. Physiol. 2007, 585 Pt 1, 305–316. [Google Scholar] [CrossRef]
- Seidel, B.; Bigl, M.; Franke, H.; Kittner, H.; Kiess, W.; Illes, P.; Krugel, U. Expression of purinergic receptors in the hypothalamus of the rat is modified by reduced food availability. Brain Res. 2006, 1089, 143–152. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Timper, K.; Engstrom Ruud, L.; Evers, N.; Paeger, L.; Bremser, S.; Kloppenburg, P.; Bruning, J.C. Inhibition of P2Y6 Signaling in AgRP Neurons Reduces Food Intake and Improves Systemic Insulin Sensitivity in Obesity. Cell Rep. 2017, 18, 1587–1597. [Google Scholar] [CrossRef]
- Pollatzek, E.; Hitzel, N.; Ott, D.; Raisl, K.; Reuter, B.; Gerstberger, R. Functional expression of P2 purinoceptors in a primary neuroglial cell culture of the rat arcuate nucleus. Neuroscience 2016, 327, 95–114. [Google Scholar] [CrossRef]
- Wakamori, M.; Sorimachi, M. Properties of native P2X receptors in large multipolar neurons dissociated from rat hypothalamic arcuate nucleus. Brain Res. 2004, 1005, 51–59. [Google Scholar] [CrossRef]
- Steculorum, S.M.; Paeger, L.; Bremser, S.; Evers, N.; Hinze, Y.; Idzko, M.; Kloppenburg, P.; Bruning, J.C. Hypothalamic UDP Increases in Obesity and Promotes Feeding via P2Y6-Dependent Activation of AgRP Neurons. Cell 2015, 162, 1404–1417. [Google Scholar] [CrossRef]
- Sorimachi, M.; Ishibashi, H.; Moritoyo, T.; Akaike, N. Excitatory effect of ATP on acutely dissociated ventromedial hypothalamic neurons of the rat. Neuroscience 2001, 105, 393–401. [Google Scholar] [CrossRef]
- Kittner, H.; Franke, H.; Harsch, J.I.; El-Ashmawy, I.M.; Seidel, B.; Krugel, U.; Illes, P. Enhanced food intake after stimulation of hypothalamic P2Y1 receptors in rats: Modulation of feeding behaviour by extracellular nucleotides. Eur. J. Neurosci. 2006, 24, 2049–2056. [Google Scholar] [CrossRef]
- Burnstock, G.; Gentile, D. The involvement of purinergic signalling in obesity. Purinergic Signal. 2018, 14, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; He, X.; Zhang, J.; Tang, C.; Rong, P. Transcutaneous auricular vagal nerve stimulation inhibits hypothalamic P2Y1R expression and attenuates weight gain without decreasing food intake in Zucker diabetic fatty rats. Sci. Prog. 2021, 104, 368504211009669. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sorimachi, M.; Akaike, N. Excitatory effects of ATP on rat dorsomedial hypothalamic neurons. Brain Res. 2004, 1009, 234–237. [Google Scholar] [CrossRef]
- Kittner, H.; Franke, H.; Fischer, W.; Schultheis, N.; Krugel, U.; Illes, P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: Implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology 2003, 28, 435–444. [Google Scholar] [CrossRef]
- Vorobjev, V.S.; Sharonova, I.N.; Haas, H.L.; Sergeeva, O.A. Expression and function of P2X purinoceptors in rat histaminergic neurons. Br. J. Pharmacol. 2003, 138, 1013–1019. [Google Scholar] [CrossRef]
- Vorobjev, V.S.; Sharonova, I.N.; Sergeeva, O.A.; Haas, H.L. Modulation of ATP-induced currents by zinc in acutely isolated hypothalamic neurons of the rat. Br. J. Pharmacol. 2003, 139, 919–926. [Google Scholar] [CrossRef]
- Furukawa, K.; Ishibashi, H.; Akaike, N. ATP-induced inward current in neurons freshly dissociated from the tuberomammillary nucleus. J. Neurophysiol. 1994, 71, 868–873. [Google Scholar] [CrossRef]
- Wollmann, G.; Acuna-Goycolea, C.; van den Pol, A.N. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J. Neurophysiol. 2005, 94, 2195–2206. [Google Scholar] [CrossRef]
- Florenzano, F.; Viscomi, M.T.; Mercaldo, V.; Longone, P.; Bernardi, G.; Bagni, C.; Molinari, M.; Carrive, P. P2X2R purinergic receptor subunit mRNA and protein are expressed by all hypothalamic hypocretin/orexin neurons. J. Comp. Neurol. 2006, 498, 58–67. [Google Scholar] [CrossRef]
- Jo, Y.H.; Role, L.W. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J. Neurosci. 2002, 22, 4794–4804. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cihakova, J.; Ivetic, M.; Zemkova, H. Cell Type-Specific Expression of Purinergic P2X Receptors in the Hypothalamus. Int. J. Mol. Sci. 2025, 26, 5007. https://doi.org/10.3390/ijms26115007
Cihakova J, Ivetic M, Zemkova H. Cell Type-Specific Expression of Purinergic P2X Receptors in the Hypothalamus. International Journal of Molecular Sciences. 2025; 26(11):5007. https://doi.org/10.3390/ijms26115007
Chicago/Turabian StyleCihakova, Jana, Milorad Ivetic, and Hana Zemkova. 2025. "Cell Type-Specific Expression of Purinergic P2X Receptors in the Hypothalamus" International Journal of Molecular Sciences 26, no. 11: 5007. https://doi.org/10.3390/ijms26115007
APA StyleCihakova, J., Ivetic, M., & Zemkova, H. (2025). Cell Type-Specific Expression of Purinergic P2X Receptors in the Hypothalamus. International Journal of Molecular Sciences, 26(11), 5007. https://doi.org/10.3390/ijms26115007






