1. Introduction
Antibody–drug conjugates (ADCs) are novel cancer treatments that combine the specificity of monoclonal antibodies and the efficacy of cytotoxic drugs [
1,
2,
3]. ADCs target specific antigens on the surface of cancer cells via monoclonal antibodies and transport the linked cytotoxin to the intracellular space in targeted cells to selectively kill tumor cells while minimizing damage to normal tissues [
4,
5,
6]. Recent advances in biotechnology and in-depth clinical research contributed to the development of ADCs with significant efficacy as anti-tumor immunological therapies [
7,
8]. However, ADC therapy has limitations, including ocular toxicity, particularly in patients who receive long-term or multiple drug administrations [
9,
10]. Among the 15 commercially available ADCs, five drugs cause ocular toxicity, characterized by corneal epithelial damage, conjunctivitis, and dry eye syndrome [
11,
12]. Ocular toxicity severely affects the quality of life in patients and may lead to vision impairment [
13]. The mechanisms of ADC-induced ocular toxicity are poorly understood. Most studies concerning ADC-induced ocular toxicity are limited to the discovery and characterization of clinical cases. Understanding the mechanisms of ADC-induced ocular toxicity is essential for optimizing therapeutic protocols and improving patient safety and tolerance.
Enfortumab vedotin, an anti-Nectin4-VcMMAE (MMAE, monomethyl auristatin E) drug to treat urothelial cancer, obtained accelerated approval from the U.S. Food and Drug Administration in December 2019 [
14,
15]. Enfortumab, a human IgG1 monoclonal antibody targeting Nectin-4, and monomethyl auristatin E (MMAE), a microtubule inhibitor, are conjugated via a cleavable linker to form anti-Nectin4-VcMMAE. Anti-Nectin4-VcMMAE (enfortumab vedotin) is the first approved programmed cell death protein 1 (PD-1) + ADC combination drug and has a unique competitive advantage in treating urothelial cancer, offering new therapeutic options for patients [
15,
16]. Since approval and marketing, the sales revenue of anti-Nectin4-VcMMAE increased quickly, reaching 1.03 billion US dollars. Despite the significant clinical success of enfortumab vedotin, the potential ocular toxicity limits its use. Warnings and precautions on the drug label stress that using anti-Nectin4-VcMMAE may cause ocular diseases. Most ocular symptoms occur in the cornea, manifesting as dry eye syndrome and related symptoms. In a study of 310 patients receiving anti-Nectin4-VcMMAE therapy, 46% developed ocular diseases. Adverse effects in the cornea include keratitis, blurred vision, limbal stem cell deficiency, and other dry eye-related events [
17].
Human Nectin-4 is 94% homologous to
Macaca fascicularis and rats. Interestingly, the binding affinity of ADC for Nectin-4 is similar in humans,
Macaca fascicularis, and rats. Thus, these animal models are suitable for toxicological studies [
18]. Pre-clinical 4-week repeated dose toxicity studies in rats and
Macaca fascicularis and 13-week studies in rats demonstrated that the antibodies alone did not cause ocular diseases. Thus, the ocular toxicity is related to the whole ADC [
18].
The human eye is a complex organ with six corneal layers. The surrounding tissue and limbus of the cornea are filled with immunoglobulins, complements, and diverse components for cellular immunity [
19]. Therefore, the eye is an immune-privileged tissue, and large molecules have limited access. Only small molecules with specific physicochemical properties, such as monomethyl auristatin F and maytansinoid DM4, can enter the eye. The blood–aqueous barrier inhibits the entrance of ADCs into the aqueous humor, reducing the risk of cornea exposure to ADCs via the front aqueous humor. Furthermore, pre-clinical and clinical studies showed that ADC-related corneal damage is limited to the epithelial layer [
20,
21]. Anti-Nectin4-VcMMAE is linked via valine-citrulline-p-aminobenzyl carbamate (Val-Cit-PABC). Dipeptide Val-Cit is the most common cleavable linker in ADCs, exhibiting favorable plasma stability, release events, and chemical tractability [
22]. MMAE does not accumulate easily in the eye. Therefore, the mechanism for anti-Nectin4-VcMMAE entry into the ocular cornea to induce toxicity is the key question of interest in the present study.
We aimed to investigate the molecular mechanisms of anti-Nectin4-VcMMAE-induced ocular toxicity. An in vivo rat model mimicking human ocular reactions and human corneal epithelial cell-transformed (HCE-T) cells was employed to clarify the key pathways for anti-Nectin4-VcMMAE entry into corneal cells and other factors affecting ocular toxicity. Additionally, potential molecular modifications to reduce the ocular toxicity of anti-Nectin4-VcMMAE were assessed. The results of this study will provide valuable insights into the reasonable design of ADCs to improve clinical safety.
3. Discussion
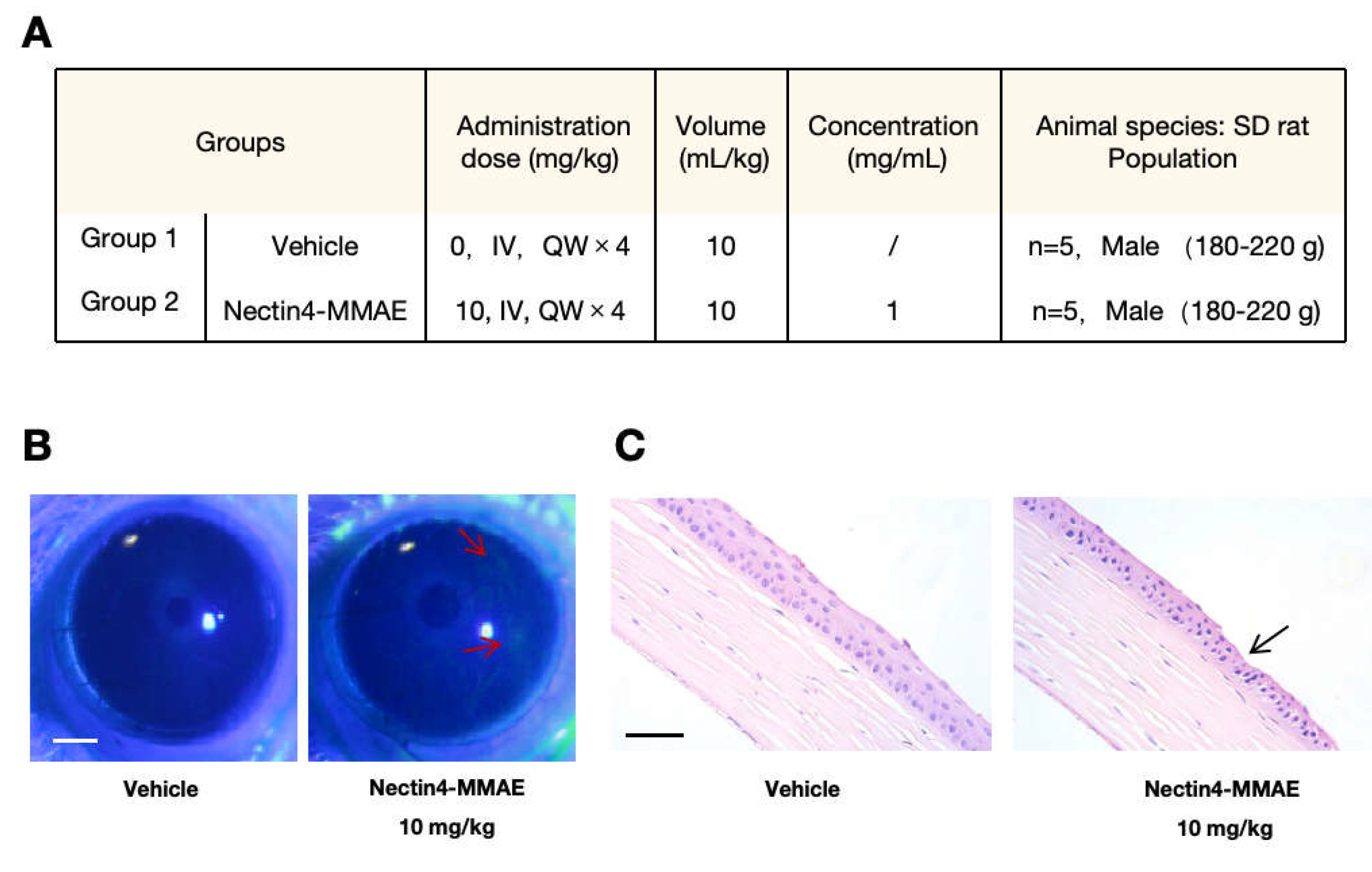
The mechanisms of enfortumab vedotin-induced ocular toxicity were examined. Our results clarify the mechanisms of ADC-induced ocular toxicity and lay a strong foundation for subsequent drug design optimization with translational potential. The cornerstone of the present study is the successful establishment of a rat model of enfortumab vedotin-induced ocular toxicity. The ocular structure and sensitivity in rats are more like humans than rabbits and Macaca fascicularis; thus, the possible side effects of enfortumab vedotin in rats are similar to the side effects in humans. Degeneration and necrosis of the corneal epithelium were observed in rats after weekly intravenous injection of 10 mg/kg enfortumab vedotin for 4 weeks. The ocular surface damage, as shown by sodium fluorescein staining, was consistent with the cornea-related pathology described in clinical reports in patients treated with enfortumab vedotin. Our rat model of enfortumab vedotin-induced ocular toxicity provides a valid platform for subsequent in-depth studies on the entry of drugs and drug-induced toxicity, filling the gap between the clinical phenotype and fundamental mechanistic research.
ADC drugs exert cytotoxicity via two mechanisms, on-target-off-tumor and off-target-off-tumor. The on-target-off-tumor mechanism occurs when the antibody-targeted antigen is expressed in other sites besides the tumor; ADC targets normal organs, leading to cytotoxic effects. The second mechanism, which is more common, is the off-target-off-tumor mechanism [
29]. On-target-off-tumor effects are mediated by receptors on the cell surface, including FcγR, neonatal Fc receptors, and C-type lectin receptors. Non-specific endocytosis, such as macropinocytosis and micropinocytosis, also contributes to toxicity. The endocytosis efficiency correlates with the charge heterogeneity, i.e., the hydrophilicity/hydrophobicity [
23,
24,
25,
26]. Additionally, if an unstable linker is used in the ADC, the small molecule may break from the antibody before entering cells via passive diffusion [
30]. The bystander effect may also contribute to cytotoxicity. After ADC kills cells, cell membrane disruption releases the lysed antibody and small molecules, which enter cells via passive diffusion [
31]. Our results demonstrate that enfortumab vedotin enters corneal epithelial cells via the Nectin-4-mediated on-target-off tumor mechanism and non-specific endocytosis.
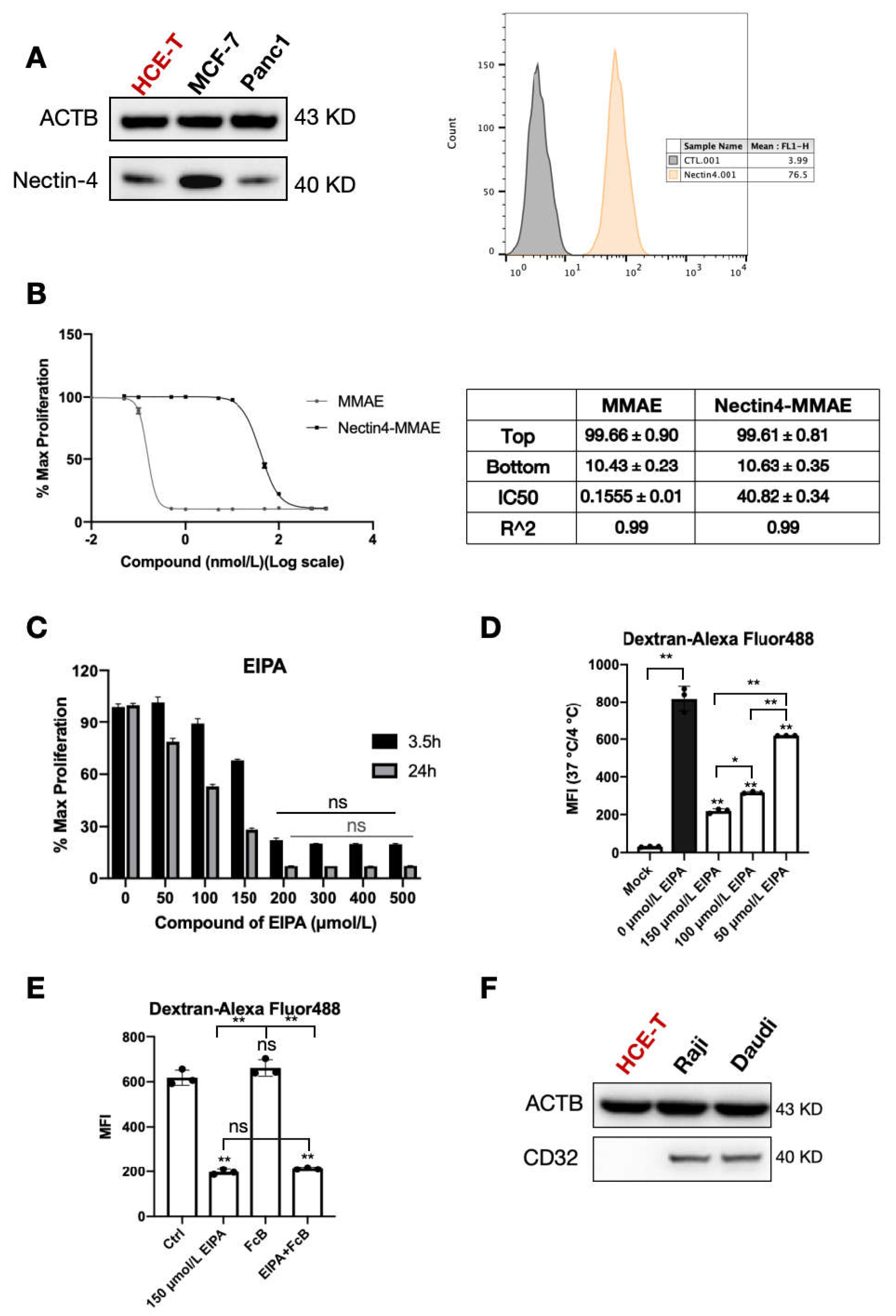
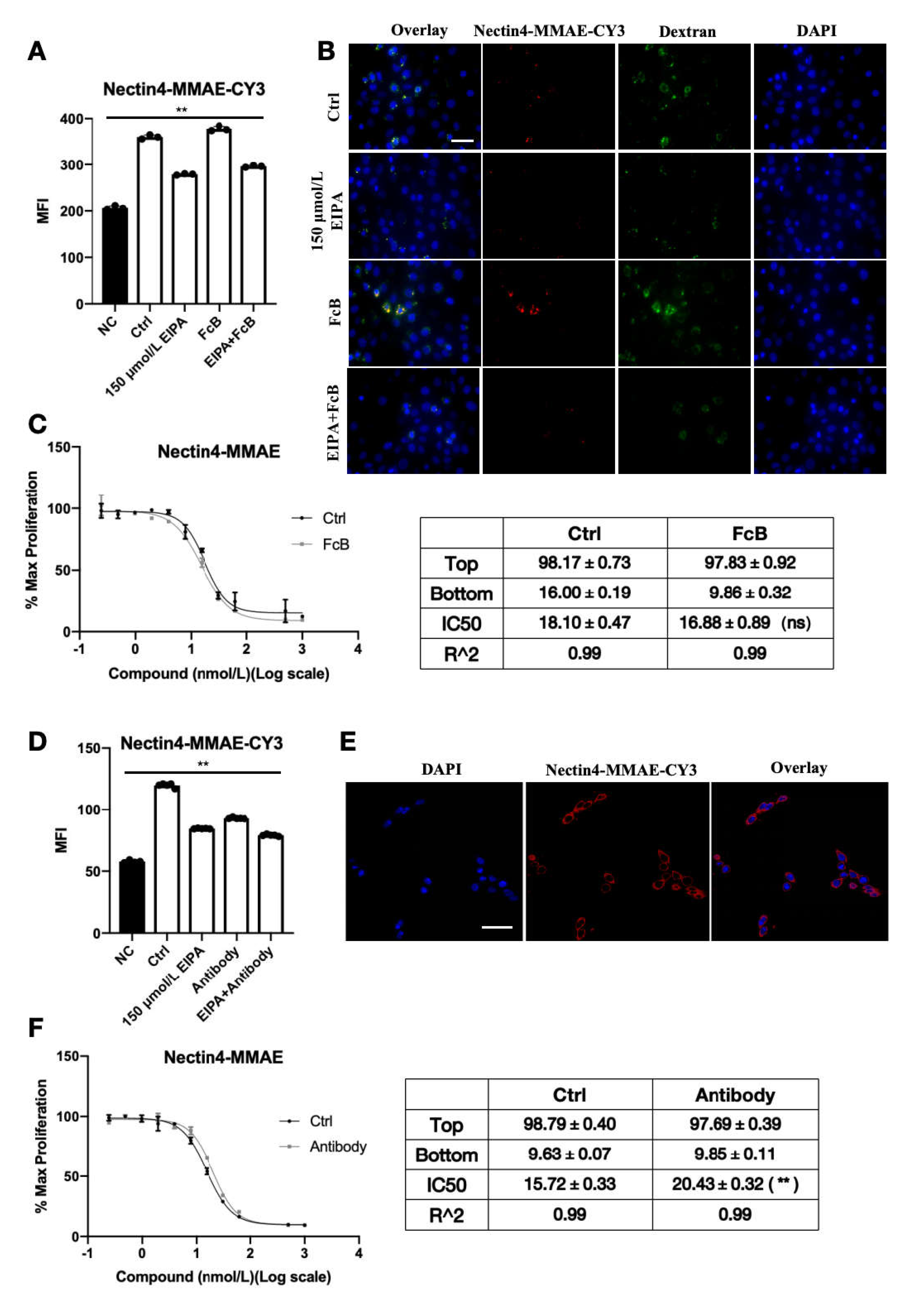
Nectin-4 is stably expressed in the corneal epithelial cell HCE-T lineage. When nectin-4 was pre-blocked with naked antibody, the endocytosis efficiency of ADC declined significantly, and the cytotoxic IC50 increased significantly. Fluorescence microscopy showed that ADC binds to the cell membrane surface. These results provide conclusive evidence that Nectin-4 plays a central role in enfortumab vedotin uptake by HCE-T cells. As an antigen, enfortumab vedotin binds specifically to cell surface Nectin-4 to form a complex and initiate endocytosis, allowing the drug to enter cells and exert its effects. This process is similar to the initial step of targeted killing of tumor cells but causes ocular toxicity.
Non-specific pinocytosis may facilitate enfortumab vedotin entry into corneal epithelial cells. In the present study, enfortumab vedotin and dextran endocytosis were significantly inhibited by EIPA, indicating that non-specific pinocytosis contributes to the entry of enfortumab vedotin into HCE-T cells. Although the relative contribution of pinocytosis is difficult to quantify, the presence of pinocytosis indicates that multiple redundant mechanisms are involved in the cellular uptake of drugs. Thus, drugs may still enter corneal epithelial cells if one pathway is blocked, highlighting the complexity of ocular toxicity.
To reduce the ocular toxicity of enfortumab vedotin, we constructed ADC variants with point mutations in the Fc fragment, referred to as Mu-anti-Nectin4-VcMMAE. The cytotoxicity of Mu-anti-Nectin4-VcMMAE in corneal cells was lower than the cytotoxicity of anti-Nectin4-VcMMAE without mutations. In PC3-AGS22 cells stably expressing Nectin-4, the biological activity of the mutated ADC was similar to the un-mutated drug, indicating that Mu-anti-Nectin4-VcMMAE retained the targeted anti-tumor activity but ocular toxicity was reduced. Endocytosis of the mutant molecule was consistent with the unmutated drug but significantly reduced. In addition, iCIEF showed that the charge heterogeneity of the mutated drug was altered. Therefore, altered charge distribution due to point mutations of the Fc fragment may have affected non-specific interactions with the cell membrane, resulting in reduced non-specific pinocytosis and, consequently, reduced unnecessary ocular uptake of the drug. Our results highlight a new strategy for ADC drug design to low ocular toxicity.
The mechanisms of enfortumab vedotin-induced ocular toxicity revealed in the present study provide theoretical guidance for the rational design of ADC drugs. Given the involvement of non-specific pinocytosis, future research should focus on modifying the physicochemical properties of ADC molecules, including adjusting charge and hydrophilicity/hydrophobicity to reduce non-specific cellular uptake. Point mutation of the Fc fragment provides an example of engineering ADC modifications to balance immunomodulation and toxicity.
For patients currently using or about to use ADC drugs such as enfortumab vedotin, physicians should be aware of the occurrence of ocular toxicity, particularly in patients undergoing long-term or high-dosage treatments. Routine ocular detection should include regular monitoring to identify early ocular pathogenesis and facilitate timely intervention. Once ocular toxicity is diagnosed, adjusting the dosage and frequency should be considered to minimize the effects of ocular toxicity on the quality of life.
Although the present study helps clarify the mechanism of ADC-mediated ocular toxicity, several study limitations should be considered. First, animal models may mimic human status but there are taxonomic differences. The dynamic processes, long-term impact, and individual variations in enfortumab vedotin-induced ocular toxicity in humans are impossible to fully recapitulate in a rat model. In future studies, human ocular tissue specimens or organoids should be used to mimic the real condition. Second, investigations into the mechanisms of Fc fragment point mutations in reducing ocular toxicity were limited to the charge heterogeneity. In-depth molecular structure-function studies using techniques such as cryo-electron microscopy and molecular dynamic simulations should be conducted to guide mutation design. Overall, our results clarify the mechanism of ADC-induced ocular toxicity. Future investigations should consider integrating multi-disciplinary techniques to balance the efficacy and safety of ADC drugs.
4. Materials and Methods
4.1. Cell Culture
HCE-T cells (Cat# CL-0743) and culture medium (Cat# CM-0743) were purchased from Wuhan Pricella Biotechnology Co., Ltd. (Wuhan, China). MCF-7, Panc1, Raji, and Daudi cells were purchased from the American Type Culture Collection (Manassas, VA, USA). MCF-7 and Panc1 cells were cultured in DMEM (Cat#SH30243.01, Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Cat#16000077, Gibco, Waltham, MA, USA). Raji and Daudi cells were cultured in RPMI 1640 Medium (Life Technologies, Waltham, MA, USA, Cat# 21875-034) with 10% fetal bovine serum (Gibco). PC3-AGS22 cells, which are human prostate cancer cells stably expressing Nectin-4, were purchased from Astellas Pharma Inc. (Tokyo, Japan). and cultured in RPMI 1640 Medium (Life Technologies, Cat# 21875-034) with 10% FBS (Gibco). All cells were cultured at 37 °C with 5% CO2. When the cell density exceeds 80%, trypsin (Gibco) will be used for subculture treatment.
4.2. Construction of the ADC
Anti-Nectin4-VcMMAE (product number 166211, batch number 4001867, 30 mg specification) was provided by Astellas. During the experiment, it was still within the expiration date. It was stored in a dry powder state at 4 °C and diluted with normal saline before use. According to the instruction manual, its drug–antibody ratio (DAR) is between 3.7 and 4.1. The anti-Nectin4 antibody was provided by Agensys (batch number DT1583, 1.77 mg/mL, Shohola, PA, USA). It was aliquoted and stored at −80 °C. After thawing, it should be immediately stored at 2–8 °C and can be stored for up to 9 weeks. MMAE was purchased from MedChemExpress (Cat# HY-15162, Monmouth Junction, NJ, USA).
Anti-Nectin4-VcMMAE with point mutations in the Fc fragment was produced by stably expressing the point mutation-containing heavy and light chain in hamster ovary cells and harvesting the antibody.
Experimental Details of the Conjugation Process: The antibody buffer system was replaced with PBS7.0/EDTA, and the antibody solution was prepared at a concentration of 10 mg/mL. This solution was placed in an EP tube, and 10 mM TCEP aqueous solution (2.5 equivalents relative to one molecule of antibody) was added to it. The mixture was incubated at 30 °C for 1 h to reduce the interchain disulfide bonds of the antibody.
At room temperature, a 10 mM DMSO solution of vcMMAE (5 equivalents relative to one molecule of antibody) was added to the above solution, and the mixture was thoroughly mixed. The reaction was allowed to proceed at room temperature for 30 min to conjugate vcMMAE to the antibody. Subsequently, a 100 mM L-cysteine hydrochloride solution (3 equivalents relative to one molecule of antibody) was added, and then the reaction was allowed to continue at room temperature for 20 min to terminate the conjugation reaction. The mutated ADC (referred to as Mu-anti-Nectin4-VcMMAE) was chemically conjugated to the small molecule MMAE via a Val-Cit linker to produce the final ADC drug with a drug-to-antibody ratio (DAR) of 3.8.
Anti-Nectin4-VcMMAE-CY3 and Mu-anti-Nectin4-VcMMAE-CY3 were prepared and provided by the laboratory of Dr. Peng Guo from the Chinese Academy of Sciences, Hangzhou. Anti-Nectin4-VcMMAE was covalently coupled to the CY3 molecule to form a stable complex. To prepare the fluorescently labeled ADCs, 1 mg of ADC was reacted with Cyanine3 N-hydroxysuccinimide ester (CY3-NHS ester) dye at a molar ratio of 1:5 (antibody: CY3). The reaction was performed in PBS buffer (pH 8.5) to a final volume of 500 μL and incubated at room temperature for 4 h. After the reaction, unbound CY3 dye was removed by purification through a PD MiniTrap G-25 column (Cat#28918007, Cytiva, Washington, DC, USA). Both Anti-Nectin4-Vc-MMAE-CY3 and Mu-Anti-Nectin4-Vc-MMAE-CY3 conjugates were successfully prepared.
The analytical data related to the synthesized ADCs, including their purity and drug-antibody ratio (DAR), can be found in the
Supplementary S3–S6.
4.3. Western Blotting
After reaching 90% confluence, cells were lysed in ice-cold RIPA buffer (Cat# 89901, Thermo Scientific, Waltham, MA, USA) containing protease inhibitors (Thermo Scientific, Cat# 78430). The cell lysate protein concentration was measured using a BCA Protein Assay Kit (Thermo Scientific, Cat# 23225). Proteins from cell lysates were separated on 10% SDS-PAGE gels and transferred to PVDF membranes. Subsequently, the membrane was blocked with 5% skim milk for 1 h. After incubating with primary and secondary antibodies, antibody binding on the membranes was detected using the ImageQuant LAS image system (GE, Marlborough, MA, USA). Primary antibodies included beta-actin (13E5) rabbit mAb (Cat# 4970S, CST, Danvers, MA, USA), recombinant anti-nectin-4 antibody (Cat# ab192033, Abcam, Cambridge, UK), and anti-CD32 antibody (Abcam, Cat# ab155972). The secondary antibody was goat anti-rabbit IgG (H + L) conjugated to HRP (Thermo Scientific, Cat# 31460). The dilution ratios of both the primary antibody and the secondary antibody were operated according to the instructions in the manual. The chemiluminescent luminescent solution and enhancement solution were SuperSignal™ West Pico PLUS (Thermo Scientific, Cat# 34577).
4.4. Flow Cytometry to Detect Nectin-4 Expression
Cells were seeded into 6-well plates and collected after 24 h of culture. The cells were treated with 50 nM anti-Nectin4-VcMMAE on ice for one hour. Cells were washed twice with PBS and stained with goat anti-human IgG (H + L) cross-adsorbed secondary antibody, Alexa Fluor™ 488 (Thermo Scientific, A-11013) for 30 min with the dilution ratio of 1:1000. The fluorescent signal was detected through the FL1 channel by using a BD FACS-Calibur flow cytometer (BD Bioscience, Franklin Lakes, NJ, USA). Data were analyzed using Flowjo 10 software.
4.5. Dose-Response Relationship
HCE-T cells (100 μL) were seeded into 96-well plates (Cat#354650, Corning, New York, NY, USA) at 1000 cells per well. After 8–12 h, different concentrations of ADC and small molecules were added. ADC and small molecules have a total of 12 concentration points: 0, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 50, 100, 500, and 1000 nM. After 4 days of treatment, the culture medium was removed and 100 μL of medium-diluted CCK-8 (Cat# CA1210-500T, Solarbio, Beijing, China) was added. After culturing cells for 4 h at 37 °C, the absorbance at 450 nm was measured using a microplate reader (Molecular Devices, San Jose, CA, USA, Spectramax M5). Data were normalized to the control data and analyzed in GraphPad Prism 8. The IC50 value was obtained using the log (inhibitor) vs. response with a variable slope (four parameters).
PC3-AGS22 cells were detected in a similar manner. On the first day, the cells were seeded at a density of 1.5 × 104 cells/mL, with 100 μL per well. On the second day, Nectin4-MMAE and Mu-Nectin4-MMAE were diluted and added to the assay plate. The diluted concentrations of the samples were 100, 25, 12.5, 6.25, 3.125, 1.563, 0.781, 0.391, 0.195, and 0.049 ng/mL, respectively. The assay plate was incubated for 120 h. On the seventh day, 20 μL of PrestoBlue® reagent (Life Technologies, Cat# A13262) was added to each well, and the microplate was incubated for another 2 h ± 30 min. The reduction of the dye could be determined by fluorescence measurement at an excitation wavelength of 540 nm and an emission wavelength of 590 nm. The dose–response curve was obtained through a four-parameter logistic fitting model, which plotted the graph with the relative fluorescence units (RFU) as the ordinate and the logarithmic concentration of the test sample as the abscissa. The relative potency of the test sample was calculated as the ratio of the C parameters derived from the four-parameter logistic fitting model.
4.6. Fluorescence Microscopy and Flow Cytometry Detection of ADC Endocytosis Efficiency
HCE-T cells (3 × 105 cells) were seeded into glass-bottom dishes (Cat# 150680, Nunc, Roskilde, Denmark). After culturing for 24 h, cells were treated with 150 μM ethylisopropylamiloride (EIPA) (Cat# HY-101840, MedChemExpress, NJ, USA) and Fc receptor binding inhibitor polyclonal antibody (Cat# 16-9161-73, eBioscience™, Santa Clara, CA, USA) with the dilution ratio of 1:5 for 30 min. Anti-Nectin4-VcMMAE-CY3 (50 nM) and 1 mg/mL Dextran-Alexa Fluor™ 488 (10,000 MW, Life Technologies, Cat# D22910) were added and the cells were cultured at 37 °C for 3 h. After washing with PBS, cells were fixed with 1 mL 4% paraformaldehyde (Cat# abs9179, absin, Shanghai, China) at room temperature for 20 min. Anti-fade mounting medium (ProLong™ Diamond, Thermo Scientific, Cat# P36962) containing 4′,6-diamidino-2-phenylindole (DAPI) was added to the cells. Cells were imaged using fluorescence microscopy (Nikon, Tokyo, Japan, Ts2R). The intracellular fluorescence intensity of ADC-CY3 was detected through the FL2 channel with a FACS-Calibur flow cytometer (BD Bioscience) and data were analyzed using Flowjo 10 software.
4.7. Imaged Capillary Isoelectric Focusing (iCIEF) Assay
Imaged capillary isoelectric focusing (iCIEF) is a separation technique. In the iCIEF process, a capillary is filled with a mixture of carrier ampholytes and the sample. When an electric field is applied, the ampholytes migrate to form a pH gradient within the capillary. The sample components then migrate to their respective isoelectric points in this gradient and focus. After focusing, the capillary is scanned, and the distribution of sample components is detected, generating a profile of charge heterogeneity.
The following chemicals were used: 1% methyl cellulose solution (Cat# 101876, Protein Simple, San Jose, CA, USA), 0.5% methyl cellulose solution (Protein Simple, Cat# 102505), Pharmalyte 8-10.5 (Cat# 17-0455-01, GE Healthcare Biosciences, Natick, MA, USA), Pharmalyte 5-8 (GE Healthcare, Cat# 17-0453-01), pI marker 7.65 (Protein Simple, Cat# 102407), pI marker 9.50 (Protein Simple, Cat# 101996), electrolyte kit (Protein Simple, Cat# 102506), 10 mM phosphate-buffered saline (pH 6.5), and 35 mM citric acid. To prepare the zwitterionic electrolyte solution, 1.20 g urea was dissolved in 1000 µL of 35 mM citric acid, 300 µL water, 2250 µL of 1% methyl cellulose solution, 100 µL Pharmalyte 5–8, 100 µL Pharmalyte 8-10.5, 25 µL pI marker 7.65, and 25 µL pI marker 9.50. Water was added to the volumetric flask to bring the volume to 5 mL. The test sample was diluted in 10 mM phosphate-buffered saline (pH 6.5). The samples were loaded, and data were collected and analyzed using a Protein Simple iCE3 analyzer.
4.8. Animal Experiment
Sprague-Dawley (SD) rats were intravenously injected with 10 mg/kg anti-Nectin4-VcMMAE. Each group consisted of 5 male SD rats, with a body weight of 180–220 g. The solvent control group received an equal volume of 5% glucose injection. The drug was administered once per week for 4 weeks. After treatment, 2 µL of 0.2% sodium fluorescein solution was instilled into the inferior conjunctival sac of the rat. The eyelid was gently closed for approximately 2 s and then rinsed with saline. The staining level of the ocular surface was examined under a slit-lamp microscope with cobalt blue diffuse light. After 4 weeks, eyes and optic nerves were collected, paraffin-embedded, hematoxylin–eosin stained, and examined under a microscope.
4.9. Data Analysis
All data were represented as the mean ± SEM. The experiments were performed at least three times. The one-way ANOVA test was used for analysis between groups. p < 0.05 indicates the distinction is significant.