VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration
Abstract
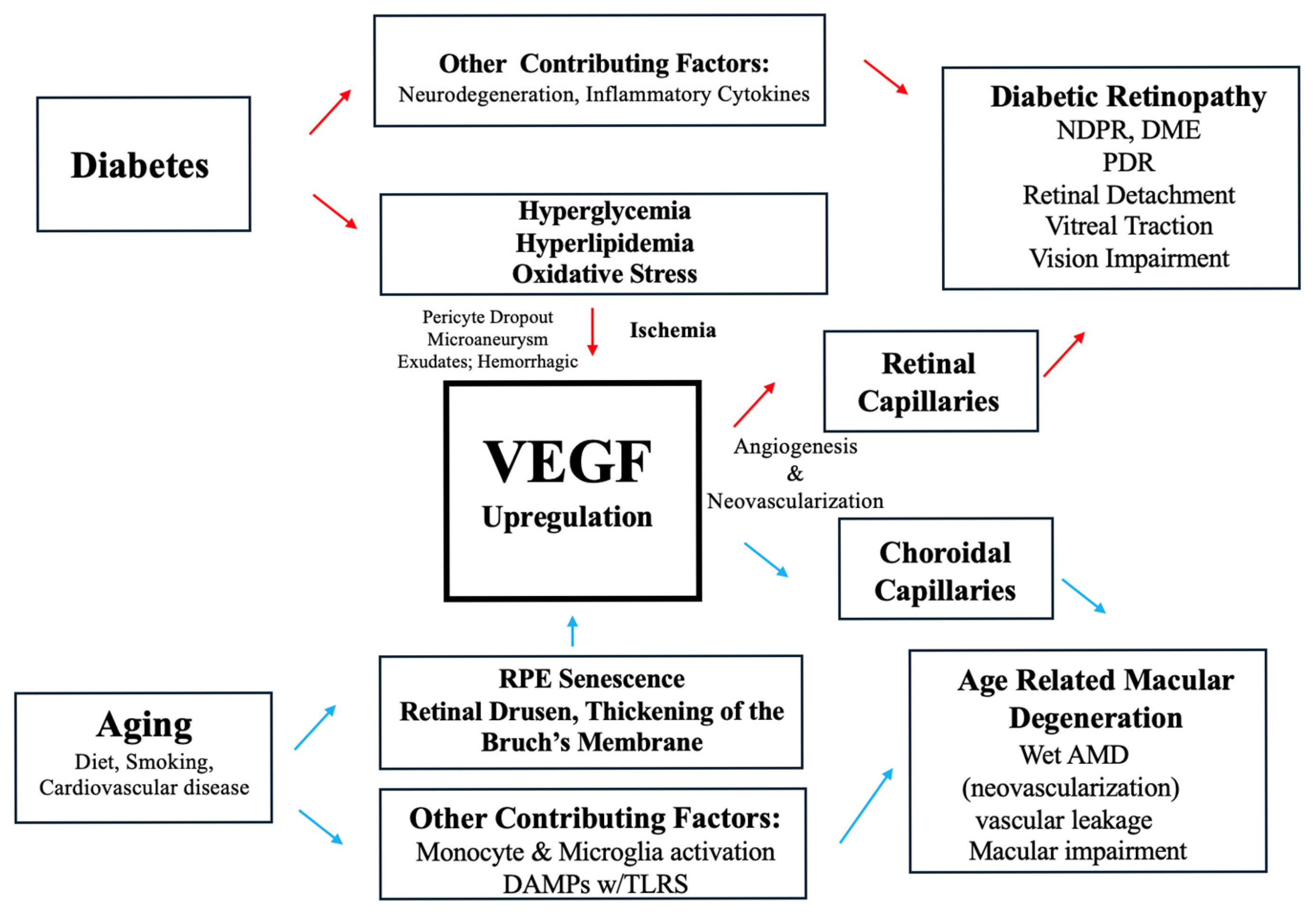
1. Introduction
2. Pathophysiology of DR and the Role of VEGF
2.1. Non-Proliferative Diabetic Retinopathy NPDR and VEGF
2.2. Proliferative Diabetic Retinopathy PDR and VEGF
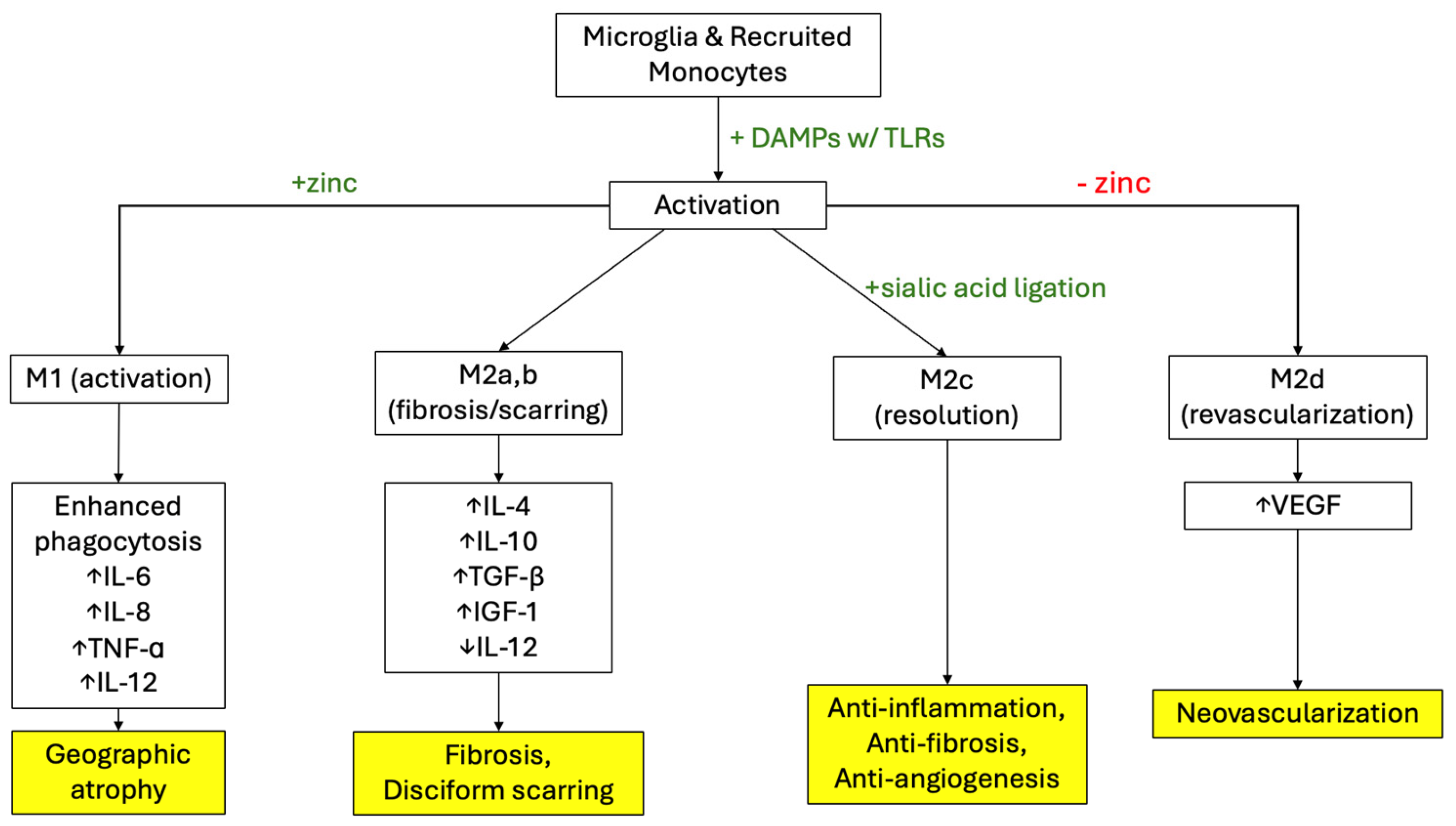
3. Pathophysiology of AMD and the Role of VEGF
3.1. Pathophysiology of Dry AMD and VEGF
3.2. Pathophysiology of Wet AMD and VEGF
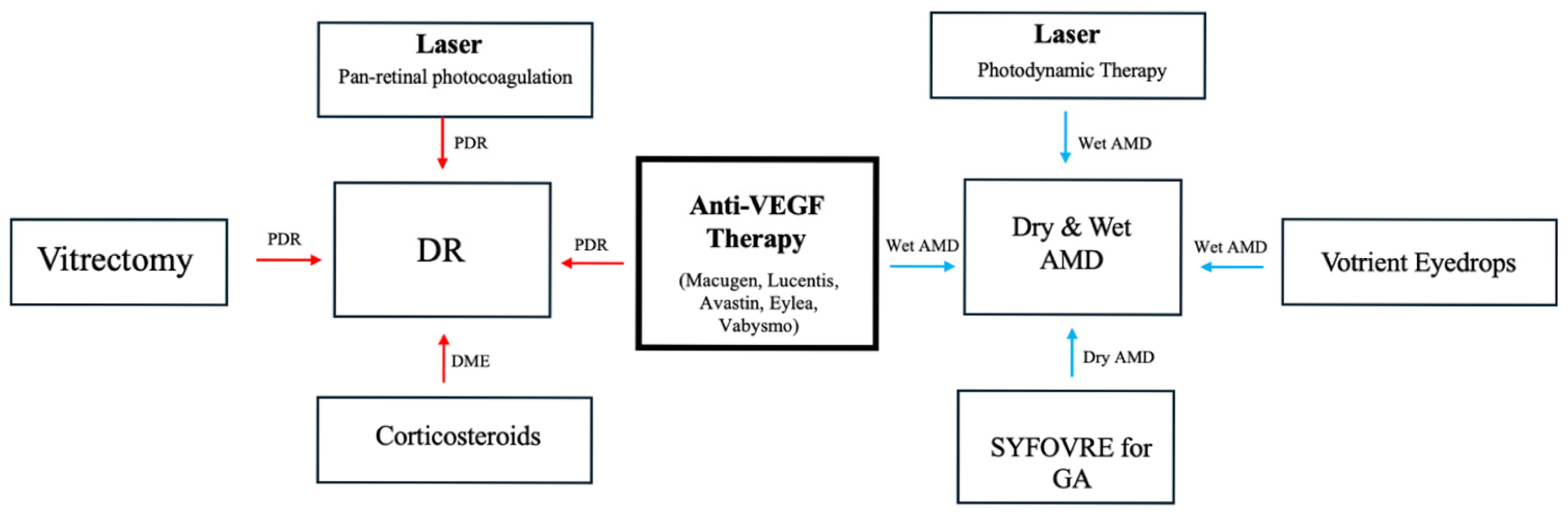
4. Anti-VEGF Therapy for DR and AMD
4.1. Anti-VEGF Therapy for NPDR
4.2. Anti-VEGF Therapy for PDR
4.3. Anti-VEGF Therapy for Dry AMD
4.4. Anti-VEGF Therapy for Wet AMD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar] [PubMed]
- Holmes, D.I.; Zachary, I. The vascular endothelial growth factor (VEGF) family: Angiogenic factors in health and disease. Genome Biol. 2005, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Acharya, K.R. Tying the knot: The cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J. 2011, 278, 4304–4322. [Google Scholar] [CrossRef]
- Antonetti, D.A.; Silva, P.S.; Stitt, A.W. Current understanding of the molecular and cellular pathology of diabetic retinopathy. Nat. Rev. Endocrinol. 2021, 17, 195–206. [Google Scholar] [CrossRef]
- Teo, Z.L.; Tham, Y.C.; Yu, M.; Chee, M.L.; Rim, T.H.; Cheung, N.; Bikbov, M.M.; Wang, Y.X.; Tang, Y.; Lu, Y.; et al. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology 2021, 128, 1580–1591. [Google Scholar] [CrossRef]
- Beltramo, E.; Porta, M. Pericyte loss in diabetic retinopathy: Mechanisms and consequences. Curr. Med. Chem. 2013, 20, 3218–3225. [Google Scholar] [CrossRef]
- Perais, J.; Agarwal, R.; Evans, J.R.; Loveman, E.; Colquitt, J.L.; Owens, D.; Hogg, R.E.; Lawrenson, J.G.; Takwoingi, Y.; Lois, N. Prognostic factors for the development and progression of proliferative diabetic retinopathy in people with diabetic retinopathy. Cochrane Database Syst. Rev. 2023, 2023, CD013775. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Zhang, C.; Zhang, J.; Gu, L.; Luo, D.; Qiu, Q. Diabetic Macular Edema: Current Understanding, Molecular Mechanisms and Therapeutic Implications. Cells 2022, 11, 3362. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Keenan, T.D.L.; Guymer, R.H.; Chakravarthy, U.; Schmitz-Valckenberg, S.; Klaver, C.C.; Wong, W.T.; Chew, E.Y. Age-related macular degeneration. Nat. Rev. Dis. Primers 2021, 7, 31. [Google Scholar] [CrossRef]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-related macular degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Knudtson, M.D.; Meuer, S.M.; Swift, M.; Gangnon, R.E. Fifteen-year cumulative incidence of age-related macular degeneration: The Beaver Dam Eye Study. Ophthalmology 2007, 114, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Lindsley, K.; Vedula, S.S.; Krzystolik, M.G.; Hawkins, B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst. Rev. 2019, 3, CD005139. [Google Scholar] [CrossRef]
- Ricci, F.; Bandello, F.; Navarra, P.; Staurenghi, G.; Stumpp, M.; Zarbin, M. Neovascular Age-Related Macular Degeneration: Therapeutic Management and New-Upcoming Approaches. Int. J. Mol. Sci. 2020, 21, 8242. [Google Scholar] [CrossRef]
- Heier, J.S.; Lad, E.M.; Holz, F.G.; Rosenfeld, P.J.; Guymer, R.H.; Boyer, D.; Grossi, F.; Baumal, C.R.; Korobelnik, J.-F.; Slakter, J.S.; et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): Two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet 2023, 402, 1434–1448. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Z. Mechanistic Pathogenesis of Endothelial Dysfunction in Diabetic Nephropathy and Retinopathy. Front. Endocrinol. 2022, 13, 816400. [Google Scholar] [CrossRef]
- Pande, G.S.; Tidake, P. Laser Treatment Modalities for Diabetic Retinopathy. Cureus 2022, 14, e30024. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-Inducible Factor-1 in Physiological and Pathophysiological Angiogenesis: Applications and Therapies. BioMed Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Fiorini, G.; Schofield, C.J. Biochemistry of the hypoxia-inducible factor hydroxylases. Curr. Opin. Chem. Biol. 2024, 79, 102428. [Google Scholar] [CrossRef]
- Duan, L.J.; Takeda, K.; Fong, G.H. Prolyl hydroxylase domain protein 2 (PHD2) mediates oxygen-induced retinopathy in neonatal mice. Am. J. Pathol. 2011, 178, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Eshaq, R.S.; Lee, M.; Kaur, G.; Harris, N.R. Retinal Physiology and Circulation: Effect of Diabetes. Compr. Physiol. 2020, 10, 933–974. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bini, S.; Midena, G.; Berton, M.; Pilotto, E.; Midena, E. Hyperreflective intraretinal spots in diabetics without and with nonproliferative diabetic retinopathy: An in vivo study using spectral domain OCT. J. Diabetes Res. 2013, 2013, 491835. [Google Scholar] [CrossRef]
- Stepp, M.A.; Menko, A.S. Immune responses to injury and their links to eye disease. Transl. Res. 2021, 236, 52–71. [Google Scholar] [CrossRef]
- Berry, S.; Lin, W.V.; Sadaka, A.; Lee, A.G. Nonarteritic anterior ischemic optic neuropathy: Cause, effect, and management. Eye Brain 2017, 9, 23–28. [Google Scholar] [CrossRef]
- Park, S.S. Cell Therapy Applications for Retinal Vascular Diseases: Diabetic Retinopathy and Retinal Vein Occlusion. Investig. Ophthalmol. Vis. Sci. 2016, 57, ORSFj1–ORSFj10. [Google Scholar] [CrossRef]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Boyce, M.; Xin, Y.; Chowdhury, O.; Shang, P.; Liu, H.; Koontz, V.; Strizhakova, A.; Nemani, M.; Hose, S.; Zigler, J.S., Jr.; et al. Microglia-Neutrophil Interactions Drive Dry AMD-like Pathology in a Mouse Model. Cells 2022, 11, 3535. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Increased mitochondrial DNA damage and down-regulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid. Mol. Vis. 2008, 14, 644–651. [Google Scholar] [PubMed]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Sennlaub, F.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Invest. 2007, 117, 2920–2928. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, M.J.; Tolentino, A.J. Investigational drugs in clinical trials for macular degeneration. Expert Opin. Investig. Drugs 2022, 31, 1067–1085. [Google Scholar] [CrossRef]
- Tolentino, M.J.; Tolentino, A.J.; Tolentino, E.M.; Krishnan, A.; Genead, M.A. Sialic Acid Mimetic Microglial Sialic Acid-Binding Immunoglobulin-like Lectin Agonism: Potential to Restore Retinal Homeostasis and Regain Visual Function in Age-Related Macular Degeneration. Pharmaceuticals 2023, 16, 1735. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and, E.; beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436, Erratum in Arch. Ophthalmol. 2008, 126, 1251. [Google Scholar] [CrossRef]
- Pyle, C.J.; Akhter, S.; Bao, S.; Dodd, C.E.; Schlesinger, L.S.; Knoell, D.L. Zinc Modulates Endotoxin-Induced Human Macrophage Inflammation through ZIP8 Induction and C/EBPβ Inhibition. PLoS ONE. 2017, 12, e0169531. [Google Scholar] [CrossRef]
- Yang, S.; Li, T.; Jia, H.; Gao, M.; Li, Y.; Wan, X.; Huang, Z.; Li, M.; Zhai, Y.; Li, X.; et al. Targeting C3b/C4b and VEGF with a bispecific fusion protein optimized for neovascular age-related macular degeneration therapy. Sci. Transl. Med. 2022, 14, eabj2177. [Google Scholar] [CrossRef]
- Tan, L.X.; Toops, K.A.; Lakkaraju, A. Protective responses to sublytic complement in the retinal pigment epithelium. Proc. Natl. Acad. Sci. USA 2016, 113, 8789–8794. [Google Scholar] [CrossRef]
- Peach, C.J.; Mignone, V.W.; Arruda, M.A.; Alcobia, D.C.; Hill, S.J.; Kilpatrick, L.E.; Woolard, J. Molecular Pharmacology of VEGF-A Isoforms: Binding and Signalling at VEGFR2. Int. J. Mol. Sci. 2018, 19, 1264. [Google Scholar] [CrossRef]
- Saint-Geniez, M.; Kurihara, T.; Sekiyama, E.; Maldonado, A.E.; D’Amore, P.A. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc. Natl. Acad. Sci. USA 2009, 106, 18751–18756. [Google Scholar] [CrossRef]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Garcia, S.; Corkhill, C.; Roubeix, C.; Davids, A.-M.; Kociok, N.; Strauss, O.; Joussen, A.M.; Reichhart, N. Inhibition of Placenta Growth Factor Reduces Subretinal Mononuclear Phagocyte Accumulation in Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4997–5006. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.T.; Harris, A.; Oddone, F.; Siesky, B.; Vercellin, A.V.; Ciulla, T.A. Disease progression pathways of wet AMD: Opportunities for new target discovery. Expert Opin. Ther. Targets 2022, 26, 5–12. [Google Scholar] [CrossRef]
- Cabral, T.; Lima, L.H.; Mello, L.G.M.; Polido, J.; Correa É, P.; Oshima, A.; Duong, J.; Serracarbassa, P.; Regatieri, C.V.; Belfort, R., Jr.; et al. Bevacizumab Injection in Patients with Neovascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol. Retina. 2018, 2, 31–37. [Google Scholar] [CrossRef]
- Zhou, L.B.; Zhou, Y.Q.; Zhang, X.Y. Blocking VEGF signaling augments interleukin-8 secretion via MEK/ERK/1/2 axis in human retinal pigment epithelial cells. Int. J. Ophthalmol. 2020, 13, 1039–1045. [Google Scholar] [CrossRef]
- Mounirou, B.A.M.; Adam, N.D.; Yakoura, A.K.H.; Aminou, M.S.M.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian J. Endocrinol. Metab. 2022, 26, 111–118. [Google Scholar] [CrossRef]
- Hobbs, S.D.; Tripathy, K.; Pierce, K. Wet Age-Related Macular Degeneration (AMD); StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Song, D.; Liu, P.; Shang, K.; Ma, Y. Application and mechanism of anti-VEGF drugs in age-related macular degeneration. Front. Bioeng. Biotechnol. 2022, 10, 943915. [Google Scholar] [CrossRef]
- Yorston, D. Anti-VEGF drugs in the prevention of blindness. Community Eye Health 2014, 27, 44–46. [Google Scholar]
- Liberski, S.; Wichrowska, M.; Kocięcki, J. Aflibercept versus Faricimab in the Treatment of Neovascular Age-Related Macular Degeneration and Diabetic Macular Edema: A Review. Int. J. Mol. Sci. 2022, 23, 9424. [Google Scholar] [CrossRef]
- Augsburger, M.; Sarra, G.-M.; Imesch, P. Treat and extend versus pro re nata regimens of ranibizumab and aflibercept in neovascular age-related macular degeneration: A comparative study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1889–1895. [Google Scholar] [CrossRef]
- Hirano, T.; Toriyama, Y.; Iesato, Y.; Imai, A.; Murata, T. Changes in plasma vascular endothelial growth factor level after intravitreal injection of bevacizumab, aflibercept, or ranibizumab for diabetic macular edema. Retina 2018, 38, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Khanani, A.M.; Quezada Ruiz, C.; Basu, K.; Ferrone, P.J.; Brittain, C.; Figueroa, M.S.; Lin, H.; Holz, F.G.; Patel, V.; et al. Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): Two randomised, double-masked, phase 3, non-inferiority trials. Lancet 2022, 399, 729–740. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Diabetic Retinopathy Preferred Practice Pattern®. Ophthalmology 2020, 127, P66–P145. [Google Scholar] [CrossRef]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Fawzi, A.; Lim, J.I.; Vemulakonda, G.A.; Ying, G.-S. Age-Related Macular Degeneration Preferred Practice Pattern®. Ophthalmology 2020, 127, P1–P65. [Google Scholar] [CrossRef]
- Hufendiek, K.; Pielen, A.; Framme, C. [Strategies of Intravitreal Injections with Anti-VEGF: “Pro re Nata versus Treat and Extend”]. Klin. Monatsblatter Augenheilkunde 2018, 235, 930–939. [Google Scholar]
- Bahr, T.A.; Bakri, S.J. Update on the Management of Diabetic Retinopathy: Anti-VEGF Agents for the Prevention of Complications and Progression of Nonproliferative and Proliferative Retinopathy. Life 2023, 13, 1098. [Google Scholar] [CrossRef]
- Maturi, R.K.; Glassman, A.R.; Josic, K.; Antoszyk, A.N.; Blodi, B.A.; Jampol, L.M.; Marcus, D.M.; Martin, D.F.; Melia, M.; Salehi-Had, H.; et al. Effect of Intravitreous Anti-Vascular Endothelial Growth Factor vs Sham Treatment for Prevention of Vision-Threatening Complications of Diabetic Retinopathy: The Protocol W Randomized Clinical Trial. JAMA Ophthalmol. 2021, 139, 701–712. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Modi, Y.S.; Kim, L.A.; Skondra, D.; Kim, J.E.; Wykoff, C.C. American Society of Retina Specialists Clinical Practice Guidelines: Management of Nonproliferative and Proliferative Diabetic Retinopathy Without Diabetic Macular Edema. J. Vitr. Dis. 2020, 4, 125–135. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Jampol, L.M.; Inusah, S.; Aiello, L.P.; Antoszyk, A.N.; Baker, C.W.; Berger, B.B.; Bressler, N.M.; Browning, D.; et al. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA 2015, 314, 2137–2146. [Google Scholar]
- Association USFaD. EYLEA (Afilbercept) Injection Label; Association USFaD: Silver Spring, MD, USA, 2011.
- Hattori, T.; Shimada, H.; Nakashizuka, H.; Mizutani, Y.; Mori, R.; Yuzawa, M. Dose of intravitreal bevacizumab (avastin) used as preoperative adjunct therapy for proliferative diabetic retinopathy. Retina 2010, 30, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Fromow-Guerra, J.; Quiroz-Mercado, H.; Sanchez, J.G.; Wu, L.; Maia, M.; Berrocal, M.H.; Solis-Vivanco, A.; Farah, M.E.; Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: Results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007, 114, 743–750. [Google Scholar] [CrossRef]
- Zhang, W.; Geng, J.; Sang, A. Effectiveness of Panretinal Photocoagulation Plus Intravitreal Anti-VEGF Treatment Against PRP Alone for Diabetic Retinopathy: A Systematic Review with Meta-Analysis. Front. Endocrinol. 2022, 13, 807687. [Google Scholar] [CrossRef]
- Rajar, R.M.; Shaikh, F.F.; Jatoi, S.M. Comparison of efficacy of combination therapy of an Intravitreal injection of bevacizumab and photocoagulation versus Pan Retinal Photocoagulation alone in High risk Proliferative Diabetic Retinopathy. Pak. J. Med. Sci. 2021, 37, 157–161. [Google Scholar] [CrossRef]
- Dervenis, P.; Dervenis, N.; Smith, J.M.; Steel, D.H. Anti-vascular endothelial growth factors in combination with vitrectomy for complications of proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2023, 2023, CD008214. [Google Scholar] [CrossRef]
- Gemenetzi, M.; Lotery, A.J.; Patel, P.J. Risk of geographic atrophy in age-related macular degeneration patients treated with intravitreal anti-VEGF agents. Eye 2017, 31, 1–9. [Google Scholar] [CrossRef]
- Śpiewak, D.; Drzyzga, Ł.; Dorecka, M.; Wyględowska-Promieńska, D. Summary of the Therapeutic Options for Patients with Dry and Neovascular AMD. J. Clin. Med. 2024, 13, 4227. [Google Scholar] [CrossRef]
- ElSheikh, R.H.; Chauhan, M.Z.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Neovascular Age-Related Macular Degeneration. Biomolecules 2022, 12, 1629. [Google Scholar] [CrossRef]
- Berg, K.; Pedersen, T.R.; Sandvik, L.; Bragadóttir, R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology 2015, 122, 146–152. [Google Scholar] [CrossRef]
- Böhni, S.C.; Bittner, M.; Howell, J.P.; Bachmann, L.M.; Faes, L.; Schmid, M.K. Comparison of Eylea® with Lucentis® as first-line therapy in patients with treatment-naïve neovascular age-related macular degeneration in real-life clinical practice: Retrospective case-series analysis. BMC Ophthalmol. 2015, 15, 109. [Google Scholar] [CrossRef]
- Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group; Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.-s.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., 3rd. Ranibizumab and Bevacizumab for Treatment of Neovascular Age-related Macular Degeneration: Two-Year Results. Ophthalmology 2020, 127, S135–S145. [Google Scholar] [CrossRef]
- Association USFaD. LUCENTIS (Ranibizumab Injection) Label; Association USFaD: Silver Spring, MD, USA, 2006.
- Stein, J.D.; Newman-Casey, P.A.; Mrinalini, T.; Lee, P.P.; Hutton, D.W. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology 2014, 121, 936–945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callan, A.; Heckman, J.; Tah, G.; Lopez, S.; Valdez, L.; Tsin, A. VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration. Int. J. Mol. Sci. 2025, 26, 4992. https://doi.org/10.3390/ijms26114992
Callan A, Heckman J, Tah G, Lopez S, Valdez L, Tsin A. VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2025; 26(11):4992. https://doi.org/10.3390/ijms26114992
Chicago/Turabian StyleCallan, Andrew, Justin Heckman, Giani Tah, Samantha Lopez, Laura Valdez, and Andrew Tsin. 2025. "VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration" International Journal of Molecular Sciences 26, no. 11: 4992. https://doi.org/10.3390/ijms26114992
APA StyleCallan, A., Heckman, J., Tah, G., Lopez, S., Valdez, L., & Tsin, A. (2025). VEGF in Diabetic Retinopathy and Age-Related Macular Degeneration. International Journal of Molecular Sciences, 26(11), 4992. https://doi.org/10.3390/ijms26114992






