Extracellular-Matrix-Mimetic Hydrogels by Using Nanomaterials
Abstract
1. Introduction
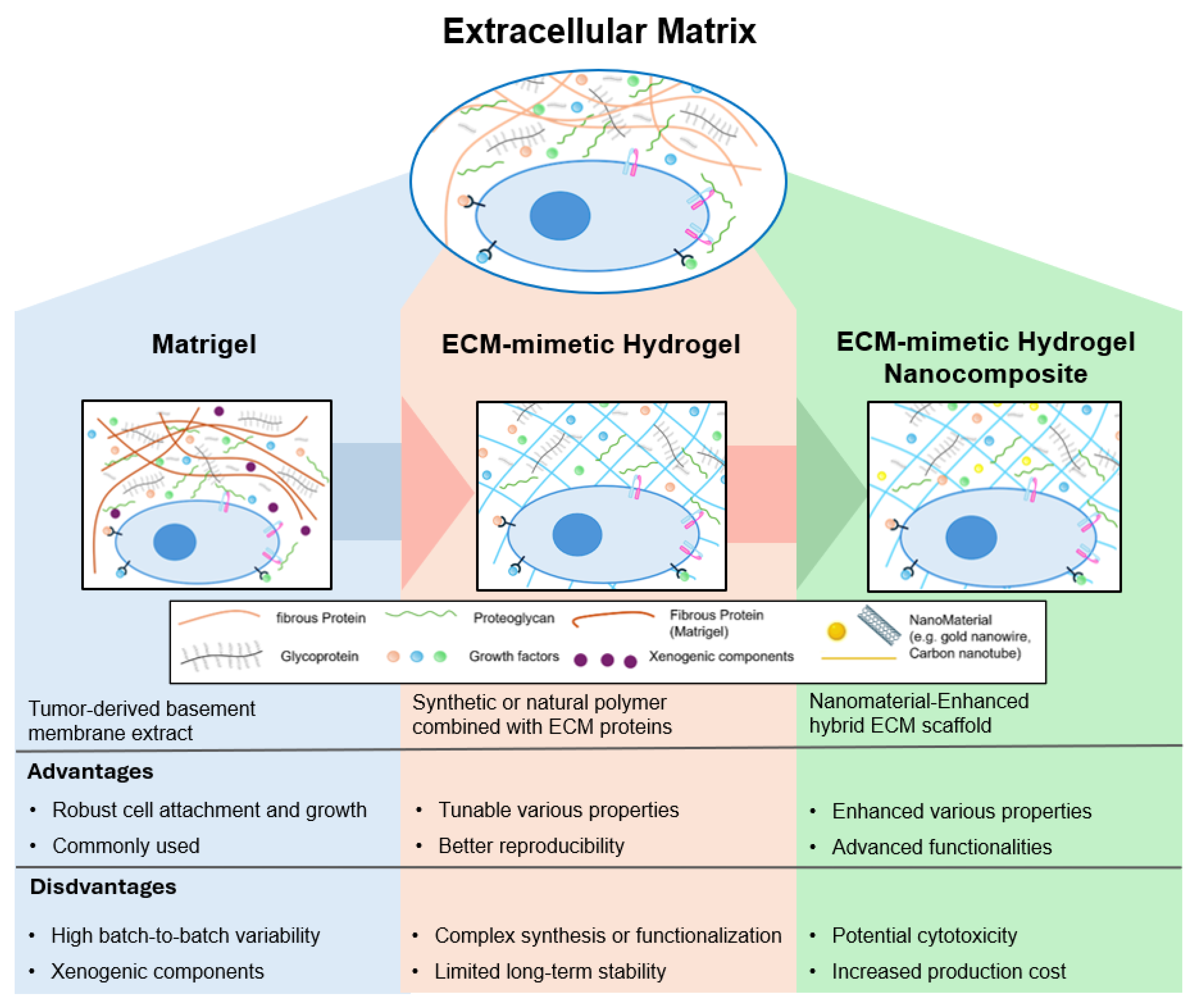
2. The Extracellular Matrix and the Evolution of Biomimetic Hydrogels
3. ECM-Mimetic Hydrogels: An Alternative Approach to Matrigel for ECM Mimicry
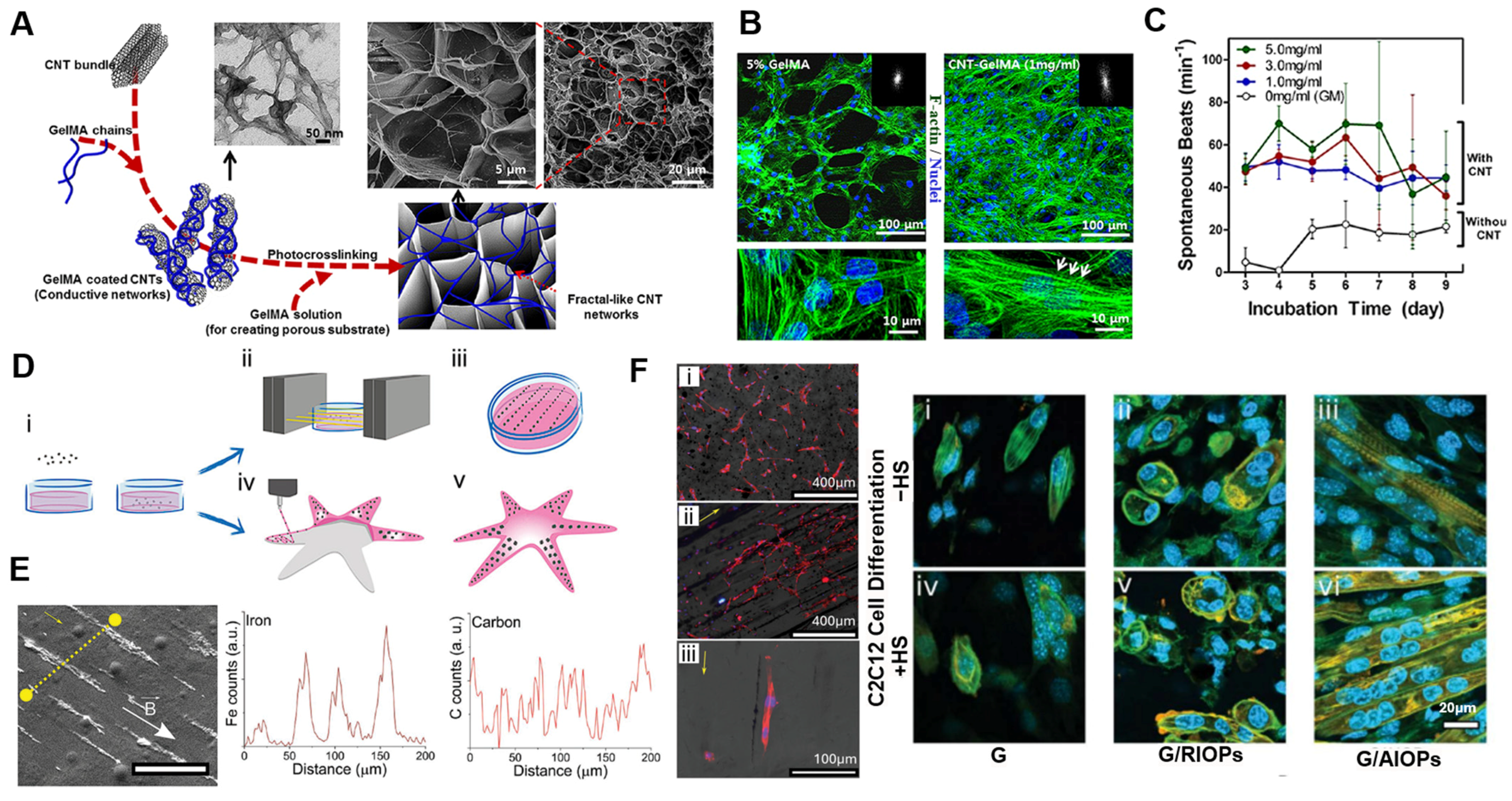
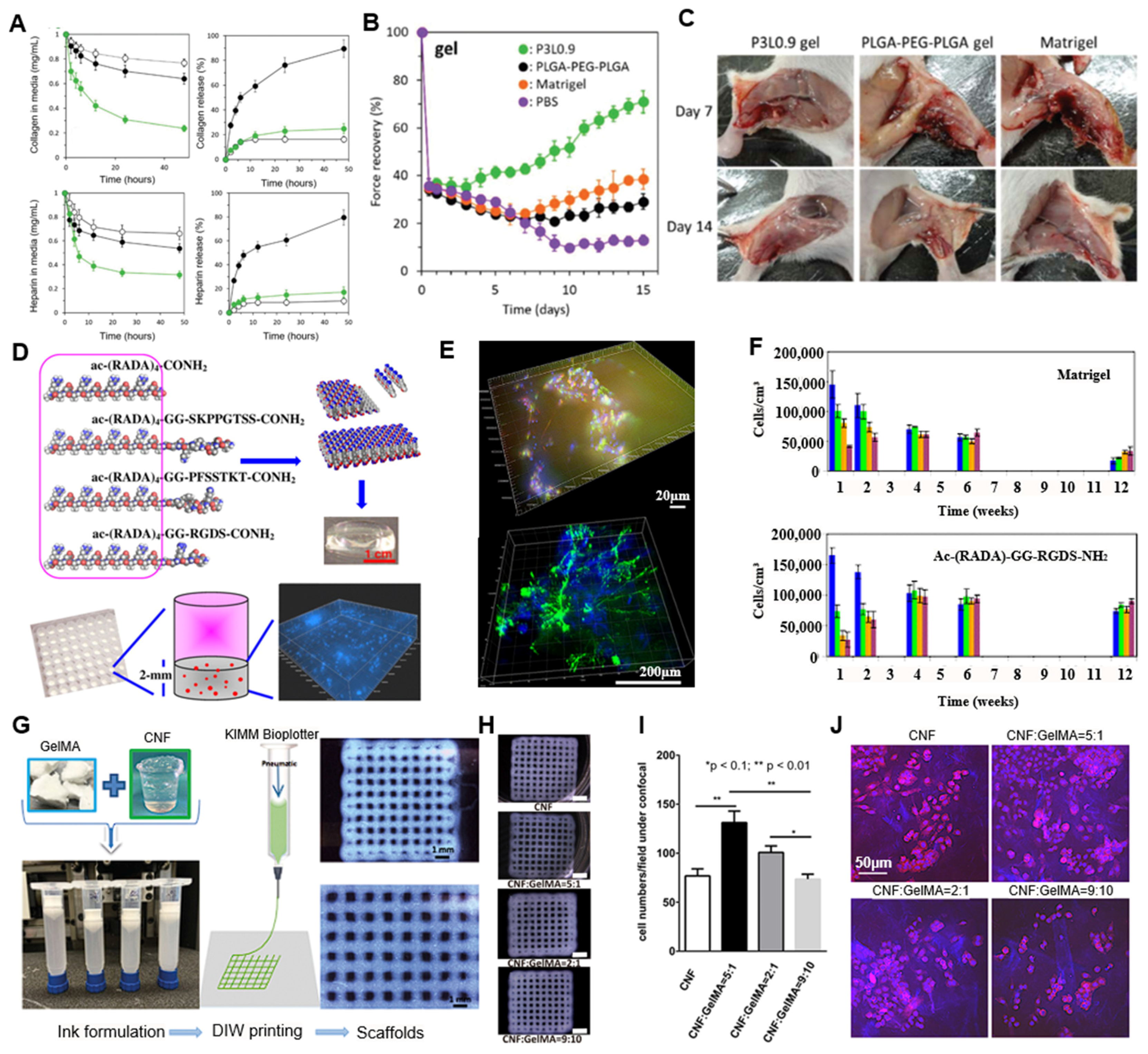
4. Application of Synthetic ECM-Mimetic Hydrogels
4.1. Cell Culture Modeling
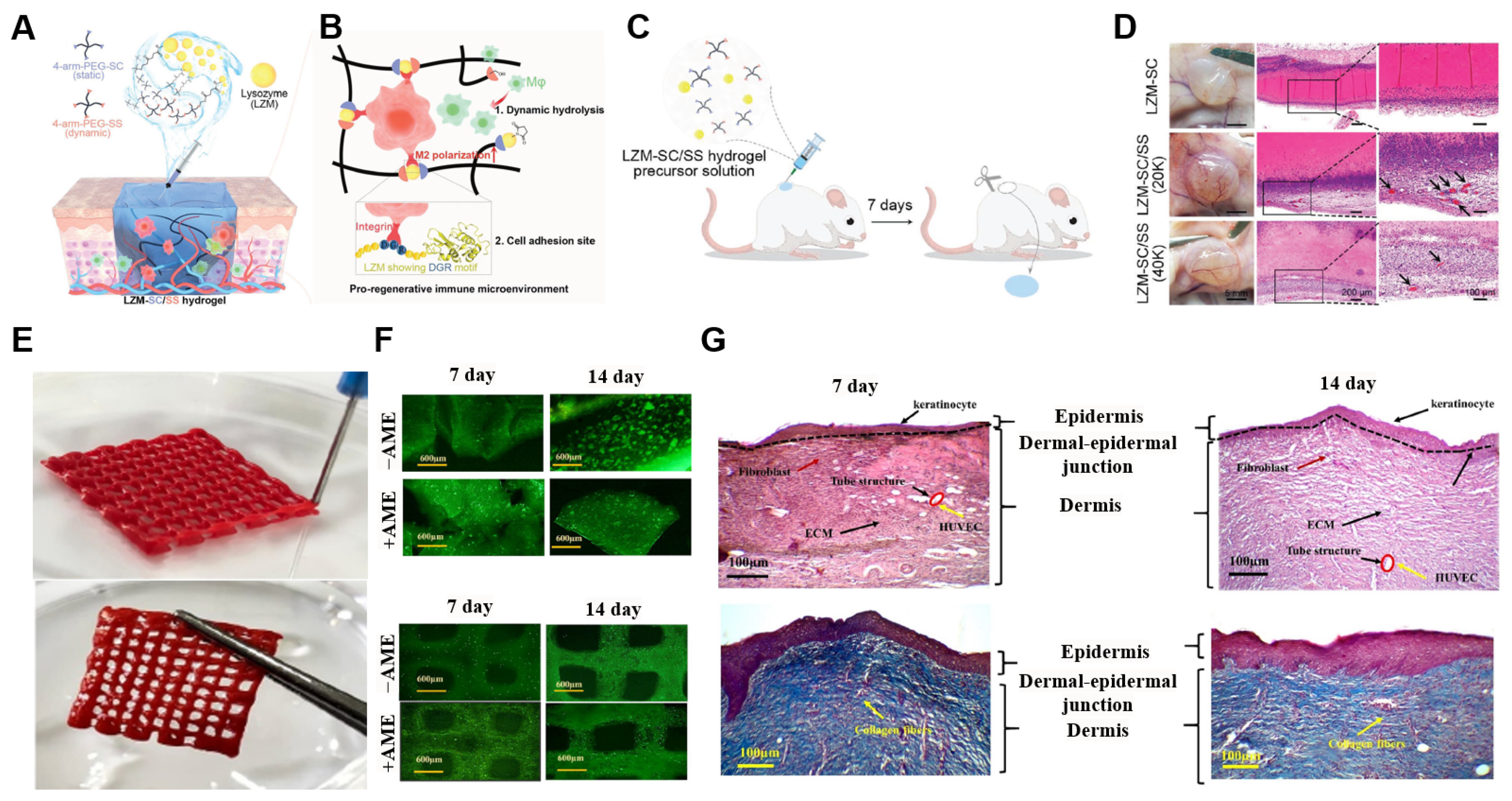
4.2. Tissue Engineering
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Parks, W.C.; Mecham, R.P. (Eds.) Extracellular Matrix Degradation; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Wheeldon, I.; Farhadi, A.; Bick, A.G.; Jabbari, E.; Khademhosseini, A. Nanoscale tissue engineering: Spatial control over cell-materials interactions. Nanotechnology 2011, 22, 212001. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef]
- Kubota, Y.; Kleinman, H.K.; Martin, G.R.; Lawley, T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J. Cell Biol. 1988, 107, 1589–1598. [Google Scholar] [CrossRef]
- Kleinman, H.K.; McGarvey, M.L.; Hassell, J.R.; Star, V.L.; Cannon, F.B.; Laurie, G.W.; Martin, G.R. Basement membrane complexes with biological activity. Biochemistry 1986, 25, 312–318. [Google Scholar] [CrossRef]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef]
- Corning Incorporated Life Sciences. Corning Matrigel Matrix: Frequently Asked Questions; Corning Incorporated Life Sciences: Corning, NY, USA, 2019. [Google Scholar]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 2014, 345, 124–125. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Vukicevic, S.; Kleinman, H.K.; Luyten, F.P.; Roberts, A.B.; Roche, N.S.; Reddi, A.H. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp. Cell Res. 1992, 202, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, E.; Leijten, J.; Xu, Q.; Khademhosseini, A. The matrix reloaded: The evolution of regenerative hydrogels. Mater. Today 2016, 19, 190–196. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.J.; Jia, S.R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part. B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Paul, G.R.; Malhotra, A.; Müller, R. Mechanical stimuli in the local in vivo environment in bone: Computational approaches linking organ-scale loads to cellular signals. Curr. Osteoporos. Rep. 2018, 16, 395–403. [Google Scholar] [CrossRef]
- Li, C.; Xu, Q. Mechanical stress-initiated signal transduction in vascular smooth muscle cells in vitro and in vivo. Cell. Signal. 2007, 19, 881–891. [Google Scholar] [CrossRef]
- Lee, W.H.; Yoon, C.-K.; Park, H.; Park, G.-H.; Jeong, J.H.; Cha, G.D.; Lee, B.-H.; Lee, J.; Lee, C.W.; Bootharaju, M.S. Highly efficient nitrogen-fixing microbial hydrogel device for sustainable solar hydrogen production. Adv. Mater. 2023, 35, 2306092. [Google Scholar] [CrossRef]
- Lee, W.H.; Lee, C.W.; Cha, G.D.; Lee, B.-H.; Jeong, J.H.; Park, H.; Heo, J.; Bootharaju, M.S.; Sunwoo, S.-H.; Kim, J.H.; et al. Floatable photocatalytic hydrogel nanocomposites for large-scale solar hydrogen production. Nat. Nanotechnol. 2023, 18, 754–762. [Google Scholar] [CrossRef]
- Reneman, R.S.; Arts, T.; Hoeks, A.P. Wall shear stress-an important determinant of endothelial cell function and structure-in the arterial system in vivo. J. Vasc. Res. 2006, 43, 251–259. [Google Scholar] [CrossRef]
- Zhao, M. Electrical fields in wound healing—An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef]
- Song, B.; Zhao, M.; Forrester, J.; McCaig, C. Nerve regeneration and wound healing are stimulated and directed by an endogenous electrical field in vivo. J. Cell Sci. 2004, 117, 4681–4690. [Google Scholar] [CrossRef]
- Hunter, R.H.F.; López-Gatius, F. Temperature gradients in the mammalian ovary and genital tract: A clinical perspective. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 382–386. [Google Scholar] [CrossRef]
- Arai, S.; Suzuki, M.; Park, S.J.; Yoo, J.S.; Wang, L.; Kang, N.Y.; Ha, H.H.; Chang, Y.T. Mitochondria-targeted fluorescent thermometer monitors intracellular temperature gradient. Chem. Commun. 2015, 51, 8044–8047. [Google Scholar] [CrossRef]
- Kim, J.; Cha, G.D.; Kim, M.; Lee, S.P.; Sunwoo, S.H.; Kim, D.H. Soft cardiac patch using a bifacial architecture of adhesive/low-impedance hydrogel nanocomposites and highly conductive elastomer nanocomposites. Adv. NanoBiomed Res. 2025, 5, 2400143. [Google Scholar] [CrossRef]
- Cha, G.D.; Lee, W.H.; Sunwoo, S.-H.; Kang, D.; Kang, T.; Cho, K.W.; Kim, M.; Park, O.K.; Jung, D.; Lee, J.; et al. Multifunctional injectable hydrogel for in vivo diagnostic and therapeutic applications. ACS Nano 2022, 16, 554–567. [Google Scholar] [CrossRef]
- Kang, T.; Cha, G.D.; Park, O.K.; Cho, H.R.; Kim, M.; Lee, J.; Kim, D.; Lee, B.; Chu, J.; Koo, S.; et al. Penetrative and sustained drug delivery using injectable hydrogel nanocomposites for postsurgical brain tumor treatment. ACS Nano 2023, 17, 5435–5447. [Google Scholar] [CrossRef]
- Marino, A.; Marino, A.; Arai, S.; Hou, Y.; Pellegrino, M.; Mazzolai, B.; Mattoli, V.; Suzuki, M.; Ciofani, G. Assessment of the effects of wireless neural stimulation mediated by piezoelectric nanoparticles. In Neuromethods; Humana Press: New York, NY, USA, 2018; pp. 109–120. [Google Scholar]
- Li, Y.; Huang, G.; Zhang, X.; Li, B.; Chen, Y.; Lu, T.; Lu, T.J.; Xu, F. Magnetic hydrogels and their potential biomedical applications. Adv. Funct. Mater. 2013, 23, 660–672. [Google Scholar] [CrossRef]
- Nicolas, J.; Magli, S.; Rabbachin, L.; Sampaolesi, S.; Nicotra, F.; Russo, L. 3D extracellular matrix mimics: Fundamental concepts and role of materials chemistry to influence stem cell fate. Biomacromolecules 2020, 21, 1968–1994. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent advances in surface modification for regulating cell adhesion and behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- Kshitiz Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Mondrinos, M.J.; Koutzaki, S.; Jiwanmall, E.; Li, M.; Dechadarevian, J.-P.; Lelkes, P.I.; Finck, C.M. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006, 12, 717–728. [Google Scholar] [CrossRef]
- Li, Z.; Guan, J. Hydrogels for cardiac tissue engineering. Polymers 2011, 3, 740–761. [Google Scholar] [CrossRef]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; MacKellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef]
- Semler, E.J.; Ranucci, C.S.; Moghe, P.V. Mechanochemical manipulation of hepatocyte aggregation can selectively induce or repress liver-specific function. Biotechnol. Bioeng. 2000, 69, 359–369. [Google Scholar] [CrossRef]
- Kane, K.I.W.; Lucumi Moreno, E.; Lehr, C.M.; Hachi, S.; Dannert, R.; Sanctuary, R.; Wagner, C.; Fleming, R.M.T.; Baller, J. Determination of the rheological properties of Matrigel for optimum seeding conditions in microfluidic cell cultures. AIP Adv. 2018, 8, 125332. [Google Scholar] [CrossRef]
- Thirumala, S.; Goebel, W.S.; Woods, E.J. Manufacturing and banking of mesenchymal stem cells. Expert Opin. Biol. Ther. 2013, 13, 673–691. [Google Scholar] [CrossRef]
- Halme, D.G.; Kessler, D.A. FDA regulation of stem-cell-based therapies. N. Engl. J. Med. 2006, 355, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Melkoumian, Z.; Weber, J.L.; Weber, D.M.; Fadeev, A.G.; Zhou, Y.; Dolley-Sonneville, P.; Yang, J.; Qiu, L.; Priest, C.A.; Shogbon, C.; et al. Synthetic peptide-acrylate differentiation of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 606–610. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Zhao, X.; Li, Q.; Ye, Z.; Li, Z.; Liu, Y.; Zhou, Y.; Ma, H.; Pan, G.; et al. Long-term self-renewal of human pluripotent stem cells on peptide-decorated poly(OEGMA-co-HEMA) brushes under fully defined conditions. Acta Biomater. 2013, 9, 8840–8850. [Google Scholar] [CrossRef]
- Ovadia, E.M.; Colby, D.W.; Kloxin, A.M. Designing well-defined photopolymerized synthetic matrices for three-dimensional culture and differentiation of induced pluripotent stem cells. Biomater. Sci. 2018, 6, 1358–1370. [Google Scholar] [CrossRef]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–352. [Google Scholar] [CrossRef]
- Huang, G.; Li, F.; Zhao, X.; Ma, Y.; Li, Y.; Lin, M.; Jin, G.; Lu, T.J.; Genin, G.M.; Xu, F.; et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017, 117, 12764–12850. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; Quirós, M.; Huang, S.; Siuda, D.; Spence, J.R.; Nusrat, A.; García, A.J. PEG-4MAL hydrogels for human organoid generation, culture, and in vivo delivery. Nat. Protoc. 2018, 13, 2102. [Google Scholar] [CrossRef]
- Collier, J.H.; Segura, T. Evolving the use of peptides as biomaterial components. Biomaterials 2011, 32, 4198–4204. [Google Scholar] [CrossRef]
- Carvalho, A.M.; da Costa, D.S.; Reis, R.L.; Pashkuleva, I. Influence of hyaluronan density on the behavior of breast cancer cells with different CD44 expression. Adv. Healthc. Mater. 2022, 11, e2101309. [Google Scholar] [CrossRef]
- Luo, T.; Tan, B.; Zhu, L.; Wang, Y.; Liao, J. A review on the design of hydrogels with different stiffness and their effects on tissue repair. Front. Bioeng. Biotechnol. 2022, 10, 817391. [Google Scholar] [CrossRef]
- Nam, M.; Lee, J.W.; Cha, G.D. Biomedical application of enzymatically crosslinked injectable hydrogels. Gels 2024, 10, 640. [Google Scholar] [CrossRef]
- Villa-Diaz, L.G.; Nandivada, H.; Ding, J.; Nogueira-de-Souza, N.C.; Krebsbach, P.H.; O’Shea, K.S.; Lahann, J.; Smith, G.D. Synthetic polymer coatings for long-term growth of human embryonic stem cells. Nat. Biotechnol. 2010, 28, 581–583. [Google Scholar] [CrossRef]
- Higuchi, A.; Kao, S.-H.; Ling, Q.-D.; Chen, Y.-M.; Li, H.-F.; Alarfaj, A.A.; Munusamy, M.A.; Murugan, K.; Chang, S.-C.; Lee, H.-C. Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity. Sci. Rep. 2015, 5, 18136. [Google Scholar] [CrossRef]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Toh, W.S.; Lim, T.C.; Kurisawa, M.; Spector, M. Modulation of mesenchymal stem cell chondrogenesis in a tunable hyaluronic acid hydrogel microenvironment. Biomaterials 2012, 33, 3835–3845. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef]
- Butcher, A.L.; Offeddu, G.S.; Oyen, M.L. Nanofibrous hydrogel composites as mechanically robust tissue engineering scaffolds. Trends Biotechnol. 2014, 32, 564–570. [Google Scholar] [CrossRef]
- Pournemati, B.; Tabesh, H.; Jenabi, A.; Aghdam, R.M.; Rezayan, A.H.; Poorkhalil, A.; Tafti, S.H.A.; Mottaghy, K. Injectable conductive nanocomposite hydrogels for cardiac tissue engineering: Focusing on carbon and metal-based nanostructures. Eur. Polym. J. 2022, 174, 111336. [Google Scholar] [CrossRef]
- Navaei, A.; Saini, H.; Christenson, W.; Sullivan, R.T.; Ros, R.; Nikkhah, M. Gold nanorod-incorporated gelatin-based conductive hydrogels for engineering cardiac tissue constructs. Acta Biomater. 2016, 41, 133–146. [Google Scholar] [CrossRef]
- Ranga, A.; Girgin, M.; Meinhardt, A.; Eberle, D.; Caiazzo, M.; Tanaka, E.M.; Lutolf, M.P. Neural tube morphogenesis in synthetic 3D microenvironments. Proc. Natl. Acad. Sci. USA 2016, 113, E6831–E6839. [Google Scholar] [CrossRef]
- Cruz-Acuña, R.; Quirós, M.; Farkas, A.E.; Dedhia, P.H.; Huang, S.; Siuda, D.; García-Hernández, V.; Miller, A.J.; Spence, J.R.; Nusrat, A.; et al. Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat. Cell Biol. 2017, 19, 1326–1335. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nature 2016, 539, 560–564. [Google Scholar] [CrossRef]
- Gjorevski, N.; Lutolf, M.P. Synthesis and characterization of well-defined hydrogel matrices and their application to intestinal stem cell and organoid culture. Nat. Protoc. 2017, 12, 2263–2274. [Google Scholar] [CrossRef]
- Musah, S.; Morin, S.A.; Wrighton, P.J.; Zwick, D.B.; Jin, S.; Kiessling, L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano 2012, 6, 10168–10177. [Google Scholar] [CrossRef]
- Tognato, R.; Armiento, A.R.; Bonfrate, V.; Levato, R.; Malda, J.; Alini, M.; Eglin, D.; Giancane, G.; Serra, T. A stimuli-responsive nanocomposite for 3D anisotropic cell-guidance and magnetic soft robotics. Adv. Funct. Mater. 2019, 29, 1804647. [Google Scholar] [CrossRef]
- Zhang, J.; Schwartz, M.P.; Hou, Z.; Bai, Y.; Ardalani, H.; Swanson, S.; Steill, J.; Ruotti, V.; Elwell, A.; Nguyen, B.K.; et al. A genome-wide analysis of human pluripotent stem cell-derived endothelial cells in 2D or 3D culture. Stem Cell Rep. 2017, 8, 907–918. [Google Scholar] [CrossRef]
- Rastin, H.; Zhang, B.; Mazinani, A.; Hassan, K.; Bi, J.; Tung, T.T.; Losic, D. 3D bioprinting of cell-laden electroconductive MXene nanocomposite bioinks. Nanoscale 2020, 12, 16069–16080. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef]
- Tay, A.; Sohrabi, A.; Poole, K.; Seidlits, S.; Di Carlo, D. A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. 2018, 30, 1800927. [Google Scholar] [CrossRef]
- Crocini, C.; Walker, C.J.; Anseth, K.S.; Leinwand, L.A. Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog. Biophys. Mol. Biol. 2020, 154, 71–79. [Google Scholar] [CrossRef]
- Fernandes, S.; Kuklok, S.; McGonigle, J.; Reinecke, H.; Murry, C.E. Synthetic matrices to serve as niches for muscle cell transplantation. Cells Tissues Organs 2011, 195, 48–59. [Google Scholar] [CrossRef]
- Zhu, W.; Harris, B.T.; Zhang, L.G. Gelatin methacrylamide hydrogel with graphene nanoplatelets for neural cell-laden 3D bioprinting. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Cebe, T.; Ahuja, N.; Monte, F.; Awad, K.; Vyavhare, K.; Aswath, P.; Huang, J.; Brotto, M.; Varanasi, V. Novel 3D-printed methacrylated chitosan-laponite nanosilicate composite scaffolds enhance cell growth and biomineral formation in MC3T3 pre-osteoblasts. J. Mater. Res. 2020, 35, 58–75. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage tissue engineering by 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Möller, T.; Amoroso, M.; Hägg, D.; Brantsing, C.; Rotter, N.; Apelgren, P.; Lindahl, A.; Kölby, L.; Gatenholm, P. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast. Reconstr. Surg. Glob. Open 2017, 5, e1227. [Google Scholar] [CrossRef]
- Shin, S.R.; Jung, S.M.; Zalabany, M.; Kim, K.; Zorlutuna, P.; Kim, S.B.; Nikkhah, M.; Khabiry, M.; Azize, M.; Kong, J.; et al. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano 2013, 7, 2369–2380. [Google Scholar] [CrossRef]
- Skaat, H.; Ziv-Polat, O.; Shahar, A.; Last, D.; Mardor, Y.; Margel, S. Magnetic scaffolds enriched with bioactive nanoparticles for tissue engineering. Adv. Healthc. Mater. 2012, 1, 168–171. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Nagase, H.; Fields, G.B. Human matrix metalloproteinase specificity studies using collagen sequence-based synthetic peptides. Peptide Sci. 1996, 40, 399–416. [Google Scholar] [CrossRef]
- Nguyen, E.H.; Daly, W.T.; Le, N.N.T.; Farnoodian, M.; Belair, D.G.; Schwartz, M.P.; Lebakken, C.S.; Ananiev, G.E.; Saghiri, M.A.; Knudsen, T.B.; et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 2017, 1, 0096. [Google Scholar] [CrossRef]
- Madri, J.A.; Williams, S.K. Capillary endothelial cell cultures: Phenotypic modulation by matrix components. J. Cell Biol. 1983, 97, 153–165. [Google Scholar] [CrossRef]
- Miyake, K.; Satomi, N.; Sasaki, S. Elastic modulus of polystyrene film from near surface to bulk measured by nanoindentation using atomic force microscopy. Appl. Phys. Lett. 2006, 89, 031925. [Google Scholar] [CrossRef]
- Bauer, J.; Margolis, M.; Schreiner, C.; Edgell, C.-J.; Azizkhan, J.; Lazarowski, E.; Juliano, R.L. In vitro model of angiogenesis using a human endothelium-derived permanent cell line: Contributions of induced gene expression, G-proteins, and integrins. J. Cell Physiol. 1992, 153, 437–449. [Google Scholar] [CrossRef]
- Belair, D.G.; Belair, D.G.; Whisler, J.A.; Valdez, J.; Velazquez, J.; Molenda, J.A.; Vickerman, V.; Lewis, R.; Daigh, C.; Hansen, T.D.; et al. Human vascular tissue models formed from human induced pluripotent stem cell-derived endothelial cells. Stem Cell Rev. Rep. 2015, 11, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Cha, G.D.; Kim, M.; Park, O.K.; Sunwoo, S.-H.; Kang, T.; Lee, W.H.; Nam, S.; Hyeon, T.; Choi, S.H.; Kim, D.-H. Minimally-invasive and in-vivo hydrogel patterning method for in situ fabrication of implantable hydrogel devices. Small Methods 2023, 7, 2300032. [Google Scholar] [CrossRef]
- Bhana, B.; Iyer, R.K.; Chen, W.L.K.; Zhao, R.; Sider, K.L.; Likhitpanichkul, M.; Simmons, C.A.; Radisic, M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol. Bioeng. 2010, 105, 1148–1160. [Google Scholar] [CrossRef]
- Mu, R.; Campos de Souza, S.; Liao, Z.; Dong, L.; Wang, C. Reprogramming the immune niche for skin tissue regeneration—From cellular mechanisms to biomaterials applications. Adv. Drug Deliv. Rev. 2022, 185, 114298. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Yao, Y.; Zhai, Z.; Duan, Y.; Zhang, D.; Mao, Z.; Lu, L.; Gao, C. Dimethyl itaconate-loaded nanofibers rewrite macrophage polarization, reduce inflammation, and enhance repair of myocardial infarction. Small 2021, 17, 2006992. [Google Scholar] [CrossRef]
- Wu, Z.; Bai, J.; Ge, G.; Wang, T.; Feng, S.; Ma, Q.; Liang, X.; Li, W.; Zhang, W.; Xu, Y.; et al. Regulating macrophage polarization in high glucose microenvironment using lithium-modified bioglass-hydrogel for diabetic bone regeneration. Adv. Healthc. Mater. 2022, 11, 2270079. [Google Scholar] [CrossRef]
- Wang, H.; Huang, R.; Bai, L.; Cai, Y.; Lei, M.; Bao, C.; Lin, S.; Ji, S.; Liu, C.; Qu, X. Extracellular matrix-mimetic immunomodulatory hydrogel for accelerating wound healing. Adv. Healthc. Mater. 2023, 12, 2301264. [Google Scholar] [CrossRef]
- Pazhouhnia, Z.; Huang, R.; Bai, L.; Cai, Y.; Lei, M.; Bao, C.; Lin, S.; Ji, S.; Liu, C.; Qu, X. 3D-bioprinted GelMA/gelatin/amniotic membrane extract (AME) scaffold loaded with keratinocytes, fibroblasts, and endothelial cells for skin tissue engineering. Sci. Rep. 2024, 14, 12670. [Google Scholar] [CrossRef]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Chitosan-collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. J. Biomed. Mater. Res. A 2013, 101, 3482–3492. [Google Scholar] [CrossRef]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-loaded sandwich-like nanofibrous membrane prepared by electrospinning technology as wound dressing for accelerated wound healing. Mater. Sci. Eng. C 2021, 127, 112245. [Google Scholar] [CrossRef] [PubMed]
- Belair, D.G.; Murphy, W.L. Specific VEGF sequestering to biomaterials: Influence of serum stability. Acta Biomater. 2013, 9, 8823–8831. [Google Scholar] [CrossRef][Green Version]
- Li, M.; Sun, L.; Liu, Z.; Shen, Z.; Cao, Y.; Han, L.; Sang, S.; Wang, J. 3D bioprinting of heterogeneous tissue-engineered skin containing human dermal fibroblasts and keratinocytes. Biomater. Sci. 2023, 11, 2461–2477. [Google Scholar] [CrossRef]
- Nagahama, K.; Oyama, N.; Ono, K.; Hotta, A.; Kawauchi, K.; Nishikata, T. Nanocomposite injectable gels capable of self-replenishing regenerative extracellular microenvironments for in vivo tissue engineering. Biomater. Sci. 2018, 6, 550–561. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Moore, E.M.; Ying, G.; West, J.L. Macrophages influence vessel formation in 3D bioactive hydrogels. Adv. Biosyst. 2017, 1, e1600021. [Google Scholar] [CrossRef]
- Hayashi, K.; Okamoto, F.; Hoshi, S.; Katashima, T.; Zujur, D.C.; Li, X.; Shibayama, M.; Gilbert, E.P.; Chung, U.-I.; Ohba, S.; et al. Fast-forming hydrogel with ultralow polymeric content as an artificial vitreous body. Nat. Biomed. Eng. 2017, 1, 0044. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial antioxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Koutsopoulos, S.; Zhang, S. Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, Matrigel and collagen I. Acta Biomater. 2013, 9, 5162–5169. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, B.; Cheng, F.; Molino, P.J.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On low-concentration inks formulated by nanocellulose assisted with gelatin methacrylate (GelMA) for 3D printing toward wound healing application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef]
- Herbert, C.B.; Bittner, G.D.; Hubbell, J.A. Effects of fibrinolysis on neurite growth from dorsal root ganglia cultured in two- and three-dimensional fibrin gels. J. Comp. Neurol. 1996, 365, 380–391. [Google Scholar] [CrossRef]
- Smith, L.A.; Liu, X.; Hu, J.; Wang, P.; Ma, P.X. Enhancing osteogenic differentiation of mouse embryonic stem cells by nanofibers. Tissue Eng. Part A 2009, 15, 1855–1864. [Google Scholar] [CrossRef]
- Gao, J.; Niklason, L.; Langer, R. Surface hydrolysis of poly(glycolic acid) meshes increases the seeding density of vascular smooth muscle cells. J. Biomed. Mater. Res. 1998, 42, 417–424. [Google Scholar] [CrossRef]
- Lu, Q.; Simionescu, A.; Vyavahare, N.R. Novel capillary channel fiber scaffolds for guided tissue engineering. Acta Biomater. 2005, 1, 607–614. [Google Scholar] [CrossRef]
- Li, V.C.F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci. Rep. 2017, 7, 8018. [Google Scholar] [CrossRef]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D printed scaffolds with gradient porosity based on a cellulose nanocrystal hydrogel. Nanoscale 2018, 10, 4421–4431. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Potential and limitations of nanocelluloses as components in biocomposite inks for three-dimensional bioprinting and for biomedical devices. Biomacromolecules 2018, 19, 701–711. [Google Scholar] [CrossRef]
- Li, T.; Qi, H.; Zhao, Y.; Kumar, P.; Zhao, C.; Li, Z.; Dong, X.; Guo, X.; Zhao, M.; Li, X.; et al. Robust and sensitive conductive nanocomposite hydrogel with bridge cross-linking–dominated hierarchical structural design. Sci. Adv. 2024, 10, eadk6643. [Google Scholar] [CrossRef]

| Field | Hydrogel Type | Nanomaterial Functionality | Hydrogel System | Target Tissue of Cell | Synthesis Method | Reference |
|---|---|---|---|---|---|---|
| Cell culture Modeling | ECM-mimetic Hydrogel | None | PEG-Laminin hydrogel | Neuroepithelial organoids | Ligand-functionalized polymer with enzymatic crosslinking | [60] |
| PEG-Maleimide hydrogel | Intestinal organoids | Enzymatic and in situ crosslinking with biofunctionalization | [61,62,63,64,65] | |||
| PEG-RGD hydrogel | iPSC-derived fibroblasts | Photo-crosslinkable synthetic polymer with adhesion motifs | [45,65,66] | |||
| ECM-mimetic Hydrogel Nanocomposite | Electromagnetic properties | Hyaluronic acid/Alginate + Ti3C2 MXene | Neural tissue | Physical mixing and photocrosslinking | [67] | |
| Magnetic hydrogel + gold NPs + filamentous phage | 3D cell culture platform | Magnetic labeling and levitation-based assembly | [68] | |||
| Stimuli responsiveness | GelMA + Iron oxide nanoparticles | Skeletal muscle (C2C12) | Field-assisted alignment during photocrosslinking | [65] | ||
| Hyaluronic acid + SPIONs | Neuronal cells | Microparticle incorporation with UV crosslinking | [69] | |||
| Tissue Engineering | ECM-mimetic Hydrogel | None | RGD peptide | Skeletal muscle (satellite cells) | Injectable chemically modified hydrogel (self-setting) | [70,71] |
| ECM-mimetic Hydrogel Nanocomposite | Electromagnetic properties | GelMA + Gold nanorods | Cardiac tissue | Nanoparticle dispersion followed by UV curing | [59] | |
| GelMA + Graphene nanoplatelets | Neural tissue scaffold | 3D bioprinting with photopolymerizable matrix | [72] | |||
| Mechanical reinforcement | ChiMA + Nanosilicate clay | Bone tissue scaffold | 3D printing of nanocomposite followed by UV curing | [73] | ||
| GelMA + CNF | Cartilage (chondrocytes/iPSC) | Blending and extrusion-based 3D bioprinting | [74,75,76] | |||
| Stimuli responsiveness | GelMA + CNTs | Cardiomyocytes | Nanomaterial dispersion and UV photocrosslinking | [77] | ||
| Magnetic fibrin + γ-Fe2O3 NPs conjugated with bFGF/NGF/GDNF | Nasal olfactory mucosa (NOM) | In situ enzymatic gelation with pre-conjugated nanoparticle | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.G.; Cha, G.D. Extracellular-Matrix-Mimetic Hydrogels by Using Nanomaterials. Int. J. Mol. Sci. 2025, 26, 4987. https://doi.org/10.3390/ijms26114987
Kim DG, Cha GD. Extracellular-Matrix-Mimetic Hydrogels by Using Nanomaterials. International Journal of Molecular Sciences. 2025; 26(11):4987. https://doi.org/10.3390/ijms26114987
Chicago/Turabian StyleKim, Do Gyun, and Gi Doo Cha. 2025. "Extracellular-Matrix-Mimetic Hydrogels by Using Nanomaterials" International Journal of Molecular Sciences 26, no. 11: 4987. https://doi.org/10.3390/ijms26114987
APA StyleKim, D. G., & Cha, G. D. (2025). Extracellular-Matrix-Mimetic Hydrogels by Using Nanomaterials. International Journal of Molecular Sciences, 26(11), 4987. https://doi.org/10.3390/ijms26114987







