Phenylalanine Ammonia-Lyase GhPAL9 Confers Resistance to Verticillium Wilt in Cotton
Abstract
1. Introduction
2. Results
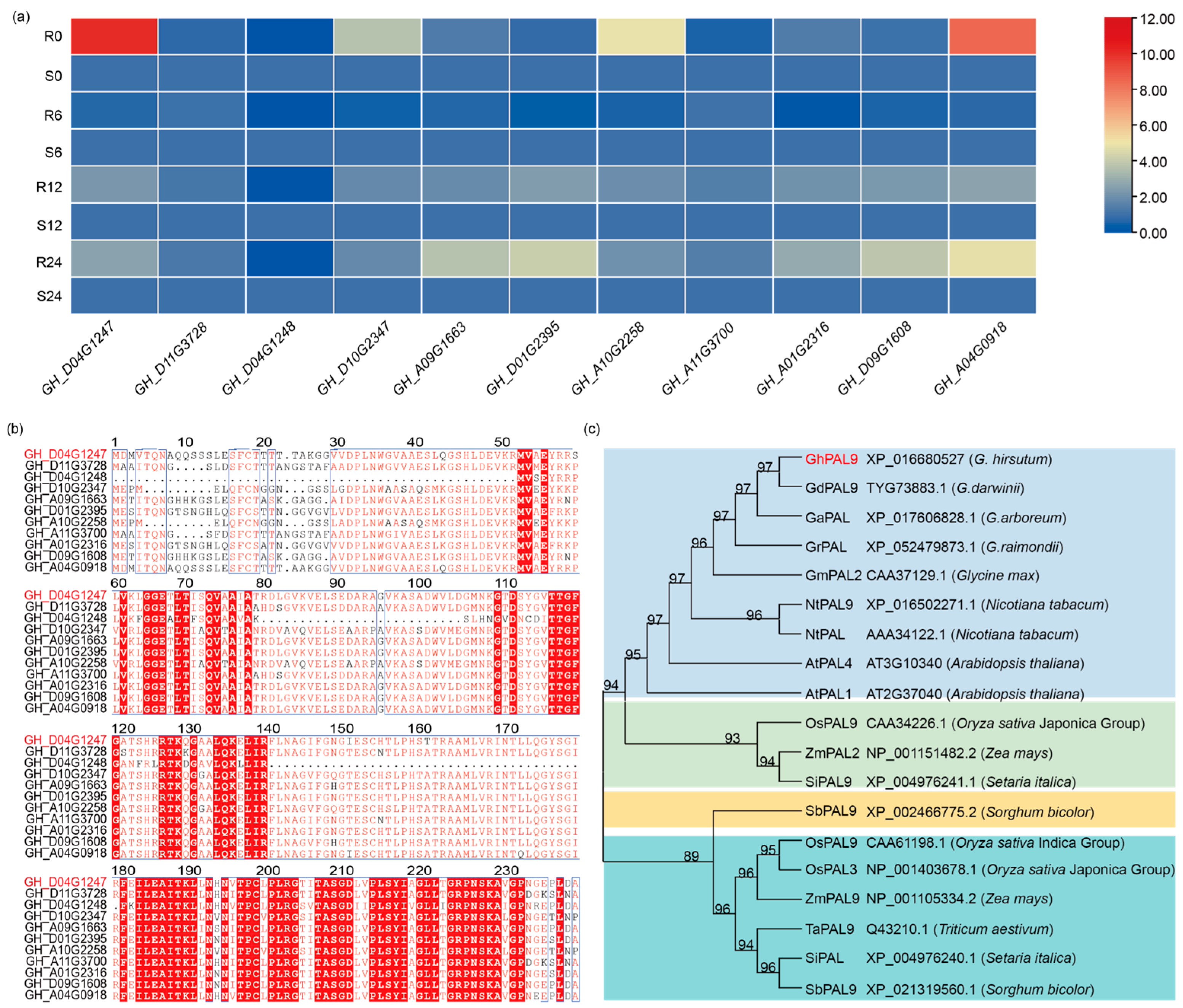
2.1. Identification of the GhPAL9 Gene in Cotton
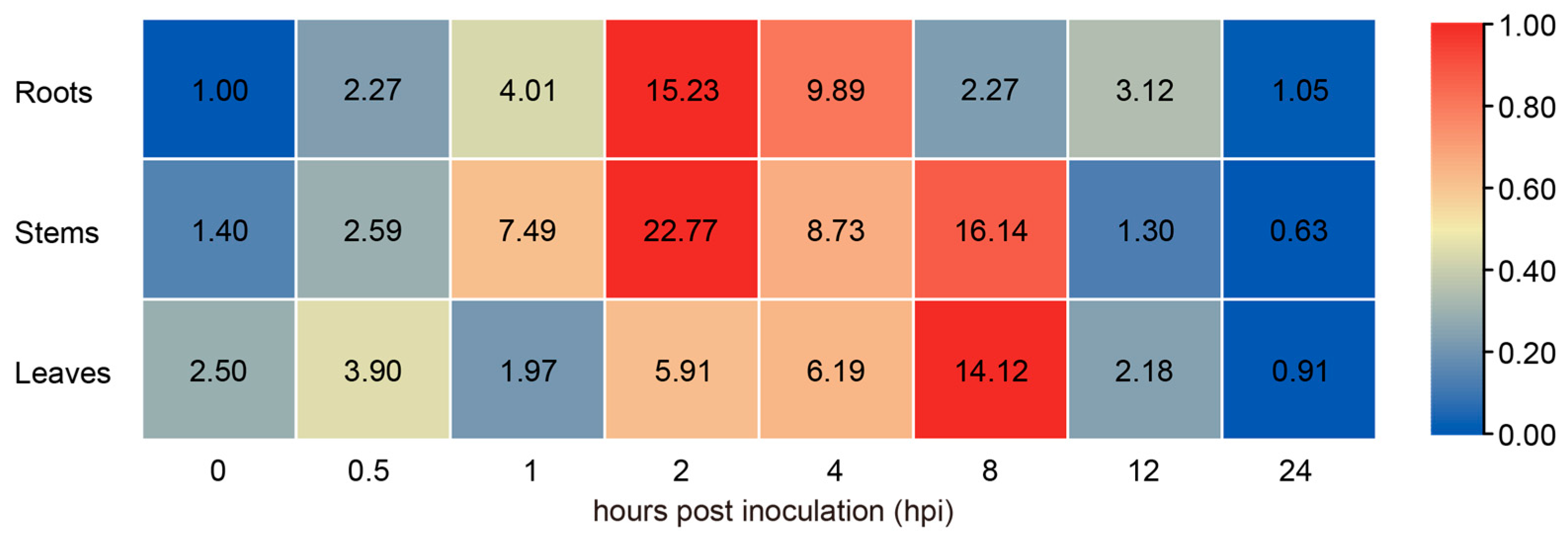
2.2. The Expression of GhPAL9 Was Upregulated in Response to V. dahliae Infection
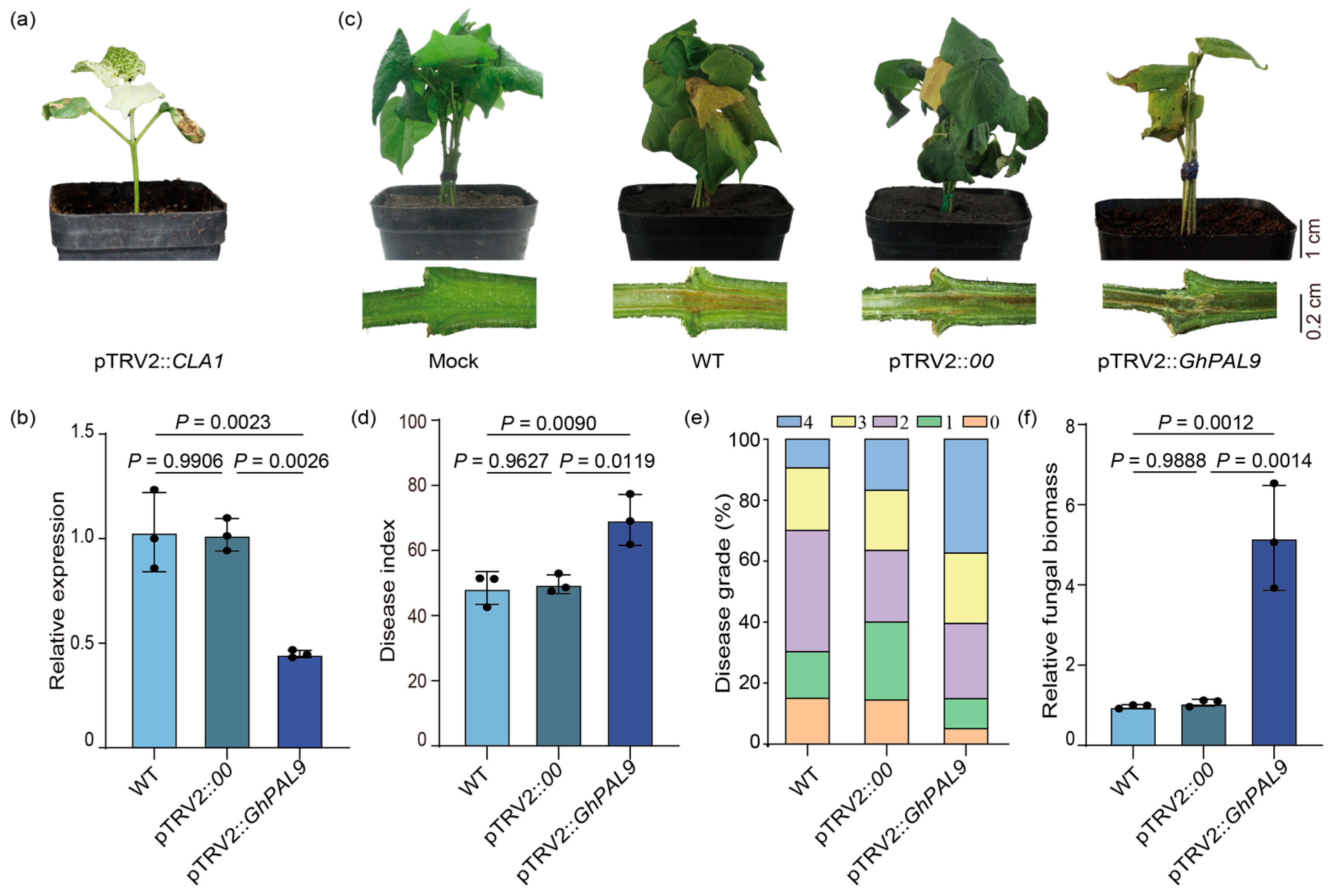
2.3. Silencing GhPAL9 Expression Decreased Cotton Resistance to V. dahliae
2.4. Decreased GhPAL9 Expression Affected Lignin Accumulation
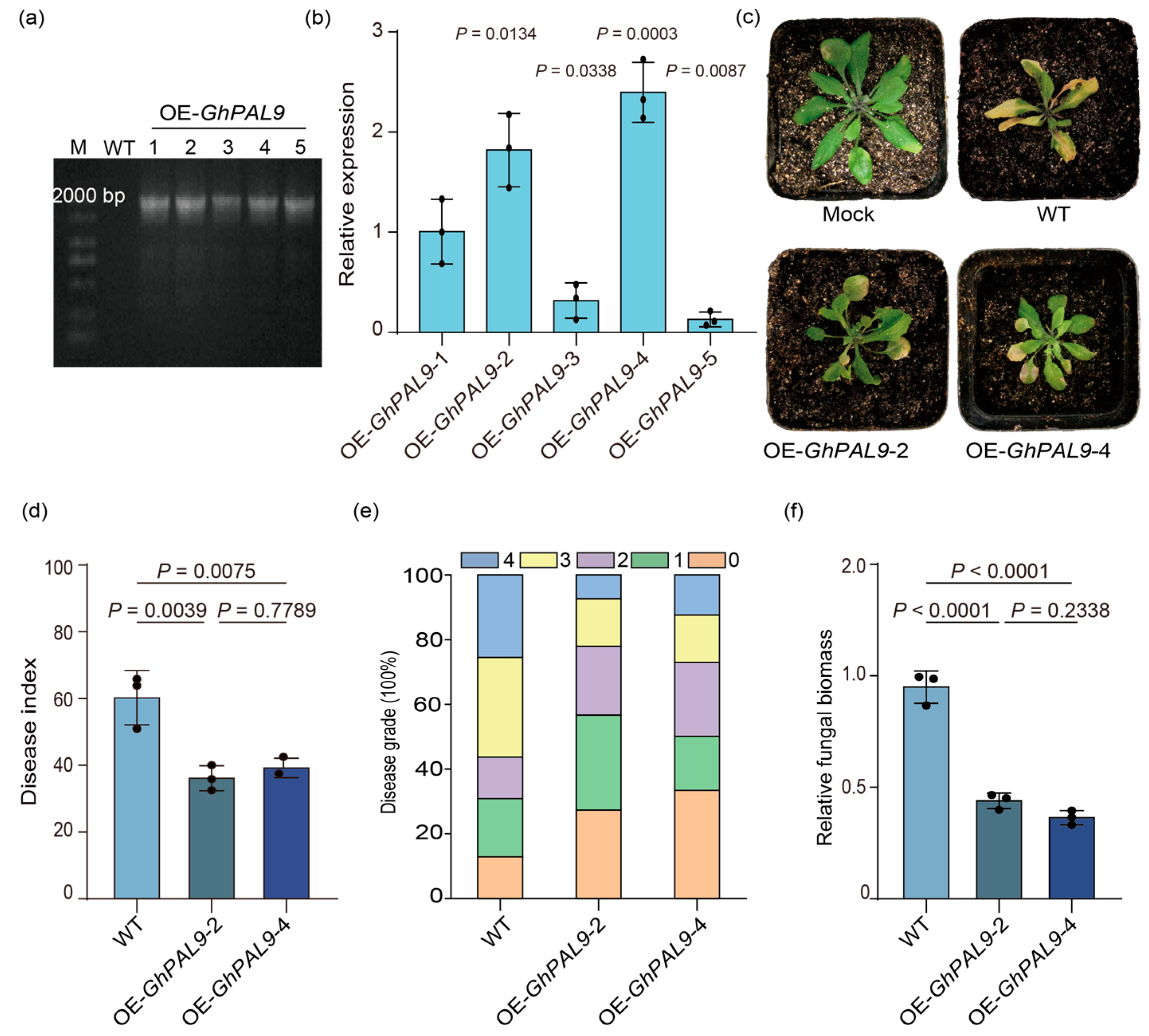
2.5. GhPAL9 as a Positive Regulator of Plant Resistance Against V. dahliae in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Growth of Plant Material and V. dahliae Cultures
4.2. Bioinformatic Analysis
4.3. Gene Expression Analysis
4.4. Virus-Induced Gene Silencing (VIGS)
4.5. Pathogenic Assay and Determination of Fungal Biomass
4.6. Histochemical Staining and Quantification of Lignin
4.7. Arabidopsis Transformation and Identification of Positive Transgenic Plants
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Gao, C.; Zhang, Y.; Yan, Q.; Hu, W.; Yang, L.; Wang, Z.; Li, F. Recent progression and future perspectives in cotton genomic breeding. J. Integr. Plant Biol. 2023, 65, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Qanmber, G.; Liu, Z.; Li, F.; Yang, Z. Brassinosteroids in cotton: Orchestrating fiber development. New Phytol. 2024, 244, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Li, J.; Zhang, L.; Chen, K.; Shao, J.; Zheng, B.; Yuan, H.; Qi, J.; Yue, L.; Hu, Q.; et al. Polyethyleneimine-coated MXene quantum dots improve cotton tolerance to Verticillium dahliae by maintaining ROS homeostasis. Nat. Commun. 2023, 14, 7392. [Google Scholar] [CrossRef]
- Xu, F.; Huang, L.; Wang, J.; Ma, C.; Tan, Y.; Wang, F.; Fan, Y.; Luo, M. Sphingolipid synthesis inhibitor fumonisin B1 causes verticillium wilt in cotton. J. Integr. Plant Biol. 2022, 64, 836–842. [Google Scholar] [CrossRef]
- Zhang, D.D.; Dai, X.F.; Klosterman, S.J.; Subbarao, K.V.; Chen, J.Y. The secretome of Verticillium dahliae in collusion with plant defence responses modulates Verticillium wilt symptoms. Biol. Rev. 2022, 97, 1810–1822. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Li, J.; Xie, C.; Jian, W.; Yang, X. An Overview of the Molecular Genetics of Plant Resistance to the Verticillium Wilt Pathogen Verticillium dahliae. Int. J. Mol. Sci. 2020, 21, 1120. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, M.; Li, T.; Wang, L.; Liao, C.; Liu, D.; Zhang, H.; Zhao, Y.; Liu, L.; Ge, X.; et al. Interactions between Verticillium dahliae and cotton: Pathogenic mechanism and cotton resistance mechanism to Verticillium wilt. Front. Plant Sci. 2023, 14, 1174281. [Google Scholar] [CrossRef]
- Umer, M.J.; Zheng, J.; Yang, M.; Batool, R.; Abro, A.A.; Hou, Y.; Xu, Y.; Gebremeskel, H.; Wang, Y.; Zhou, Z.; et al. Insights to Gossypium defense response against Verticillium dahliae: The Cotton Cancer. Funct. Integr. Genom. 2023, 23, 142. [Google Scholar] [CrossRef] [PubMed]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Asfaliza, R.; Latif, M.A. Blast resistance in rice: A review of conventional breeding to molecular approaches. Mol. Biol. Rep. 2013, 40, 2369–2388. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gao, C.; Zhang, Z.; Yuan, Y.; Zeng, X.; Hu, W.; Yang, L.; Li, F.; Yang, Z. Uncovering genomic and transcriptional variations facilitates utilization of wild resources in cotton disease resistance improvement. Theor. Appl. Genet. 2023, 136, 204. [Google Scholar] [CrossRef]
- Peng, S.; Li, P.; Li, T.; Tian, Z.; Xu, R. GhCNGC13 and 32 Act as Critical Links between Growth and Immunity in Cotton. Int. J. Mol. Sci. 2023, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yuan, Y.; Yang, C.; Geng, S.; Sun, Q.; Long, L.; Cai, C.; Chu, Z.; Liu, X.; Wang, G.; et al. Characterization, Expression, and Functional Analysis of a Novel NAC Gene Associated with Resistance to Verticillium Wilt and Abiotic Stress in Cotton. G3 Genes Genomes Genet. 2016, 6, 3951–3961. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, H.; Wang, Y.; Zhu, J.; Zhang, X.; Sun, J.; Liu, F.; Zhao, Y. Function analysis of GhWRKY53 regulating cotton resistance to Verticillium wilt by JA and SA signaling pathways. Front. Plant Sci. 2023, 14, 1203695. [Google Scholar] [CrossRef]
- Xiao, S.; Ming, Y.; Hu, Q.; Ye, Z.; Si, H.; Liu, S.; Zhang, X.; Wang, W.; Yu, Y.; Kong, J.; et al. GhWRKY41 forms a positive feedback regulation loop and increases cotton defence response against Verticillium dahliae by regulating phenylpropanoid metabolism. Plant Biotechnol. J. 2023, 21, 961–978. [Google Scholar] [CrossRef]
- Liu, F.; Cai, S.; Ma, Z.; Yue, H.; Xing, L.; Wang, Y.; Feng, S.; Wang, L.; Dai, L.; Wan, H.; et al. RVE2, a new regulatory factor in jasmonic acid pathway, orchestrates resistance to Verticillium wilt. Plant Biotechnol. J. 2023, 21, 2507–2524. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, T.; Kan, J.; Yao, Y.; Guo, D.; Yang, Y.; Ling, X.; Wang, J.; Zhang, B. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol. J. 2022, 20, 722–735. [Google Scholar] [CrossRef]
- Xiong, X.P.; Sun, S.C.; Zhu, Q.H.; Zhang, X.Y.; Liu, F.; Li, Y.J.; Xue, F.; Sun, J. Transcriptome Analysis and RNA Interference Reveal GhGDH2 Regulating Cotton Resistance to Verticillium Wilt by JA and SA Signaling Pathways. Front. Plant Sci. 2021, 12, 654676. [Google Scholar] [CrossRef]
- Gong, Q.; Yang, Z.; Wang, X.; Butt, H.I.; Chen, E.; He, S.; Zhang, C.; Zhang, X.; Li, F. Salicylic acid-related cotton (Gossypium arboreum) ribosomal protein GaRPL18 contributes to resistance to Verticillium dahliae. BMC Plant Biol. 2017, 17, 59. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, H.; Zhao, P.; Chen, W.; Li, X.; Sang, X.; Lu, J.; Wang, H. GhTBL34 Is Associated with Verticillium Wilt Resistance in Cotton. Int. J. Mol. Sci. 2021, 22, 9115. [Google Scholar] [CrossRef]
- Han, L.B.; Li, Y.B.; Wang, F.X.; Wang, W.Y.; Liu, J.; Wu, J.H.; Zhong, N.Q.; Wu, S.J.; Jiao, G.L.; Wang, H.Y.; et al. The Cotton Apoplastic Protein CRR1 Stabilizes Chitinase 28 to Facilitate Defense against the Fungal Pathogen Verticillium dahliae. Plant Cell 2019, 31, 520–536. [Google Scholar] [CrossRef]
- Lu, T.; Zhu, L.; Liang, Y.; Wang, F.; Cao, A.; Xie, S.; Chen, X.; Shen, H.; Wang, B.; Hu, M.; et al. Comparative Proteomic Analysis Reveals the Ascorbate Peroxidase-Mediated Plant Resistance to Verticillium dahliae in Gossypium barbadense. Front. Plant Sci. 2022, 13, 877146. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zuo, D.; Xiao, S.; Wang, Q.; Cheng, H.; Lv, L.; Zhang, Y.; Li, P.; Song, G. Genome-Wide Identification and Characterization of GhCOMT Gene Family during Fiber Development and Verticillium Wilt Resistance in Cotton. Plants 2021, 10, 2756. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cai, S.; Wu, P.; Dai, L.; Li, X.; Ai, N.; Feng, G.; Wang, N.; Zhou, B. General Regulatory Factor7 regulates innate immune signalling to enhance Verticillium wilt resistance in cotton. J. Exp. Bot. 2024, 75, 468–482. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Song, J.; Wang, H.; Ruan, C.; Zhang, W.; Guo, Z.; Li, W.; Guo, W. Cotton peroxisome-localized lysophospholipase counteracts the toxic effects of Verticillium dahliae NLP1 and confers wilt resistance. Plant J. 2023, 115, 452–469. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, X.; Zhou, J.; Wu, Y.; Zhang, X.; Feng, Z.; Wei, F.; Zhao, L.; Zhang, Y.; Shi, Y.; et al. Genome-Wide Analysis of Ribosomal Protein GhRPS6 and Its Role in Cotton Verticillium Wilt Resistance. Int. J. Mol. Sci. 2021, 22, 1795. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Miao, Y.; Ullah, A.; Khan, A.Q.; Menghwar, H.; Khan, A.H.; Ahmed, M.M.; Tabassum, M.A.; Zhu, L. Physiological and molecular mechanism of defense in cotton against Verticillium dahliae. Plant Physiol. Biochem. 2018, 125, 193–204. [Google Scholar] [CrossRef]
- Zhan, C.; Li, Y.; Li, H.; Wang, M.; Gong, S.; Ma, D.; Li, Y. Phylogenomic analysis of phenylalanine ammonia-lyase (PAL) multigene family and their differential expression analysis in wheat (Triticum aestivum L.) suggested their roles during different stress responses. Front. Plant Sci. 2022, 13, 982457. [Google Scholar] [CrossRef]
- Simpson, J.P.; Olson, J.; Dilkes, B.; Chapple, C. Identification of the Tyrosine- and Phenylalanine-Derived Soluble Metabolomes of Sorghum. Front. Plant Sci. 2021, 12, 714164. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Tonnessen, B.W.; Manosalva, P.; Lang, J.M.; Baraoidan, M.; Bordeos, A.; Mauleon, R.; Oard, J.; Hulbert, S.; Leung, H.; Leach, J.E. Rice phenylalanine ammonia-lyase gene OsPAL4 is associated with broad spectrum disease resistance. Plant Mol. Biol. 2015, 87, 273–286. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, T.; Du, K.; Chen, H.; Cao, Y.; Xie, J.; Li, M.; Carr, J.P.; Wu, B.; Fan, Z.; et al. Maize phenylalanine ammonia-lyases contribute to resistance to Sugarcane mosaic virus infection, most likely through positive regulation of salicylic acid accumulation. Mol. Plant Pathol. 2019, 20, 1365–1378. [Google Scholar] [CrossRef]
- Feduraev, P.; Riabova, A.; Skrypnik, L.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Assessment of the Role of PAL in Lignin Accumulation in Wheat (Triticum aestivum L.) at the Early Stage of Ontogenesis. Int. J. Mol. Sci. 2021, 22, 9848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Hwang, B.K. An important role of the pepper phenylalanine ammonia-lyase gene (PAL1) in salicylic acid-dependent signalling of the defence response to microbial pathogens. J. Exp. Bot. 2014, 65, 2295–2306. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Zhang, F.; Dong, L.; Wu, J.; Cheng, Q.; Qi, D.; Yan, X.; Jiang, L.; Fan, S.; et al. Phenylalanine ammonia-lyase2.1 contributes to the soybean response towards Phytophthora sojae infection. Sci. Rep. 2017, 7, 7242. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.; Zhu, Y.M.; Chen, Y.; Qiu, C.W.; Zhu, S.; Wu, F. Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol. Plant 2019, 165, 343–355. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, A.; Zhao, C.; Ma, X.; Zhang, X.; Li, Y.; Sun, J. Transcriptome Analysis Reveals Key Pathways and Genes Involved in Lodging Resistance of Upland Cotton. Plants 2024, 13, 3493. [Google Scholar] [CrossRef]
- Hu, Z.; Zhong, X.; Zhang, H.; Luo, X.; Wang, Y.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, X.; An, H.; et al. GhMYB18 confers Aphis gossypii Glover resistance through regulating the synthesis of salicylic acid and flavonoids in cotton plants. Plant Cell Rep. 2023, 42, 355–369. [Google Scholar] [CrossRef]
- Ojeda-Rivera, J.O.; Ulloa, M.; Perez-Zavala, F.G.; Najera-Gonzalez, H.R.; Roberts, P.A.; Yong-Villalobos, L.; Yadav, H.; Chavez Montes, R.A.; Herrera-Estrella, L.; Lopez-Arredondo, D. Enhanced phenylpropanoid metabolism underlies resistance to Fusarium oxysporum f. sp. vasinfectum race 4 infection in the cotton cultivar Pima-S6 (Gossypium barbadense L.). Front. Genet. 2024, 14, 1271200. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Farooq, M.A.; Zhao, T.; Wang, P.; Tabusam, J.; Wang, Y.; Xuan, S.; Zhao, J.; Chen, X.; Shen, S.; et al. Virus-Induced Gene Silencing (VIGS): A Powerful Tool for Crop Improvement and Its Advancement towards Epigenetics. Int. J. Mol. Sci. 2023, 24, 5608. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Tang, Y.; Hu, G.; Quan, Y.; Chen, A.; Zhong, N.; Peng, Q.; Wu, J. Cotton miR319b-Targeted TCP4-Like Enhances Plant Defense Against Verticillium dahliae by Activating GhICS1 Transcription Expression. Front. Plant Sci. 2022, 13, 870882. [Google Scholar] [CrossRef] [PubMed]
- Cesarino, I. Structural features and regulation of lignin deposited upon biotic and abiotic stresses. Curr. Opin. Biotechnol. 2019, 56, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kishi-Kaboshi, M.; Seo, S.; Takahashi, A.; Hirochika, H. The MAMP-Responsive MYB Transcription Factors MYB30, MYB55 and MYB110 Activate the HCAA Synthesis Pathway and Enhance Immunity in Rice. Plant Cell Physiol. 2018, 59, 903–915. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, L.; Wang, X.; Chen, B.; Zhao, J.; Cui, J.; Li, Z.; Yang, J.; Wu, L.; Wu, J.; et al. The cotton laccase gene GhLAC15 enhances Verticillium wilt resistance via an increase in defence-induced lignification and lignin components in the cell walls of plants. Mol. Plant Pathol. 2019, 20, 309–322. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Zhu, L.; Guo, H.; Cheng, H.; Su, X. Heat Shock Transcription Factor GhHSFB2a Is Crucial for Cotton Resistance to Verticillium dahliae. Int. J. Mol. Sci. 2023, 24, 1845. [Google Scholar] [CrossRef]
- Maldonado-Gonzalez, M.M.; Bakker, P.A.; Prieto, P.; Mercado-Blanco, J. Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front. Microbiol. 2015, 6, 266. [Google Scholar] [CrossRef]
- Liu, L.; Wang, D.; Zhang, C.; Liu, H.; Guo, H.; Cheng, H.; Liu, E.; Su, X. The heat shock factor GhHSFA4a positively regulates cotton resistance to Verticillium dahliae. Front. Plant Sci. 2022, 13, 1050216. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Zhang, G.; Cheng, G.; Wang, Q. Phenylalanine Ammonia-Lyase GhPAL9 Confers Resistance to Verticillium Wilt in Cotton. Int. J. Mol. Sci. 2025, 26, 4983. https://doi.org/10.3390/ijms26114983
Li C, Zhang G, Cheng G, Wang Q. Phenylalanine Ammonia-Lyase GhPAL9 Confers Resistance to Verticillium Wilt in Cotton. International Journal of Molecular Sciences. 2025; 26(11):4983. https://doi.org/10.3390/ijms26114983
Chicago/Turabian StyleLi, Chuanzong, Guoshuai Zhang, Guanfu Cheng, and Qi Wang. 2025. "Phenylalanine Ammonia-Lyase GhPAL9 Confers Resistance to Verticillium Wilt in Cotton" International Journal of Molecular Sciences 26, no. 11: 4983. https://doi.org/10.3390/ijms26114983
APA StyleLi, C., Zhang, G., Cheng, G., & Wang, Q. (2025). Phenylalanine Ammonia-Lyase GhPAL9 Confers Resistance to Verticillium Wilt in Cotton. International Journal of Molecular Sciences, 26(11), 4983. https://doi.org/10.3390/ijms26114983




