Inhibition of Ferroptosis Attenuates Neuron Damage and Improves Cognitive Impairment in Mice Surviving Severe Hypothermia
Abstract
1. Introduction
2. Results
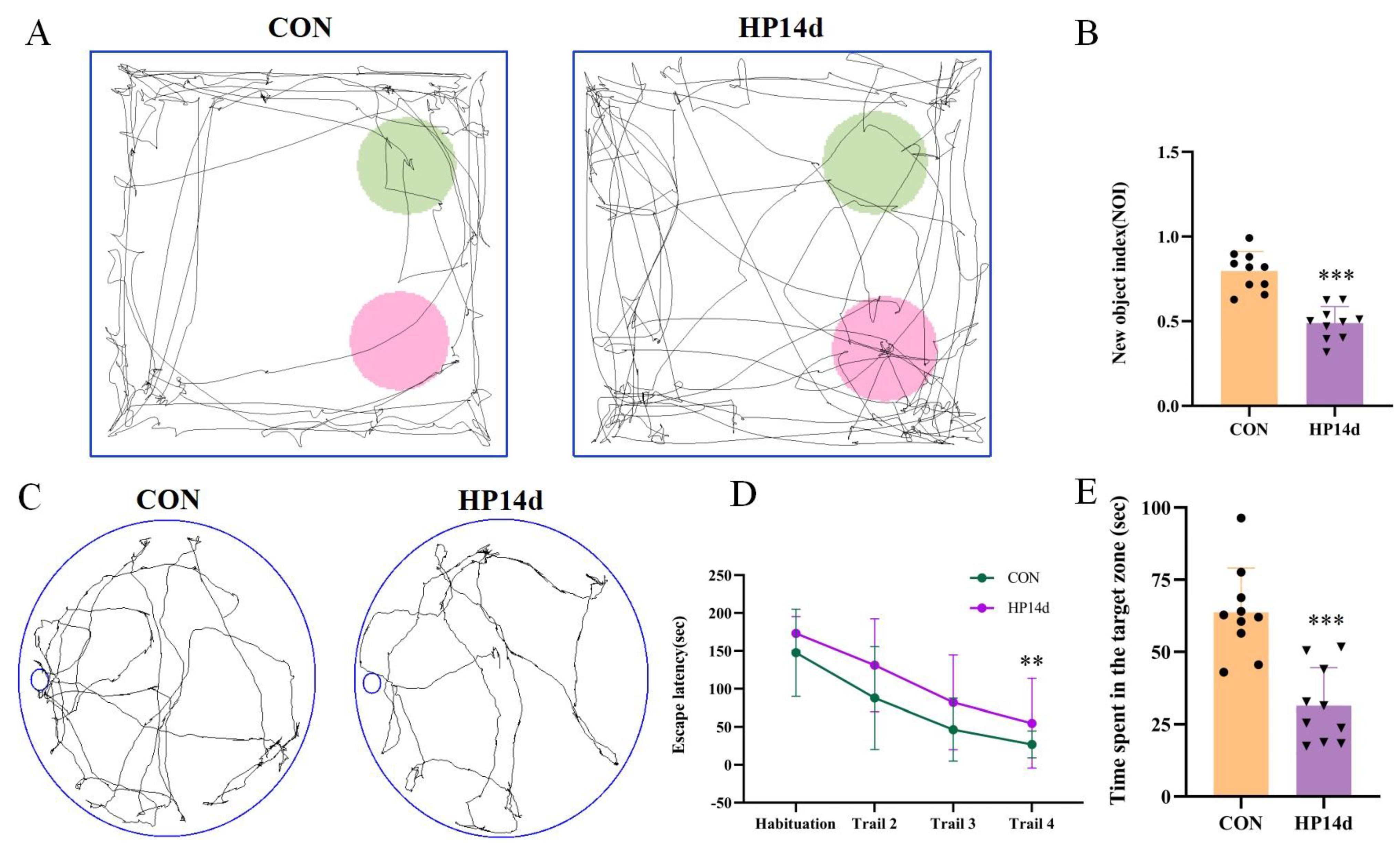
2.1. Cognitive Impairment Was Observed in Surviving Mice with Severe Hypothermia
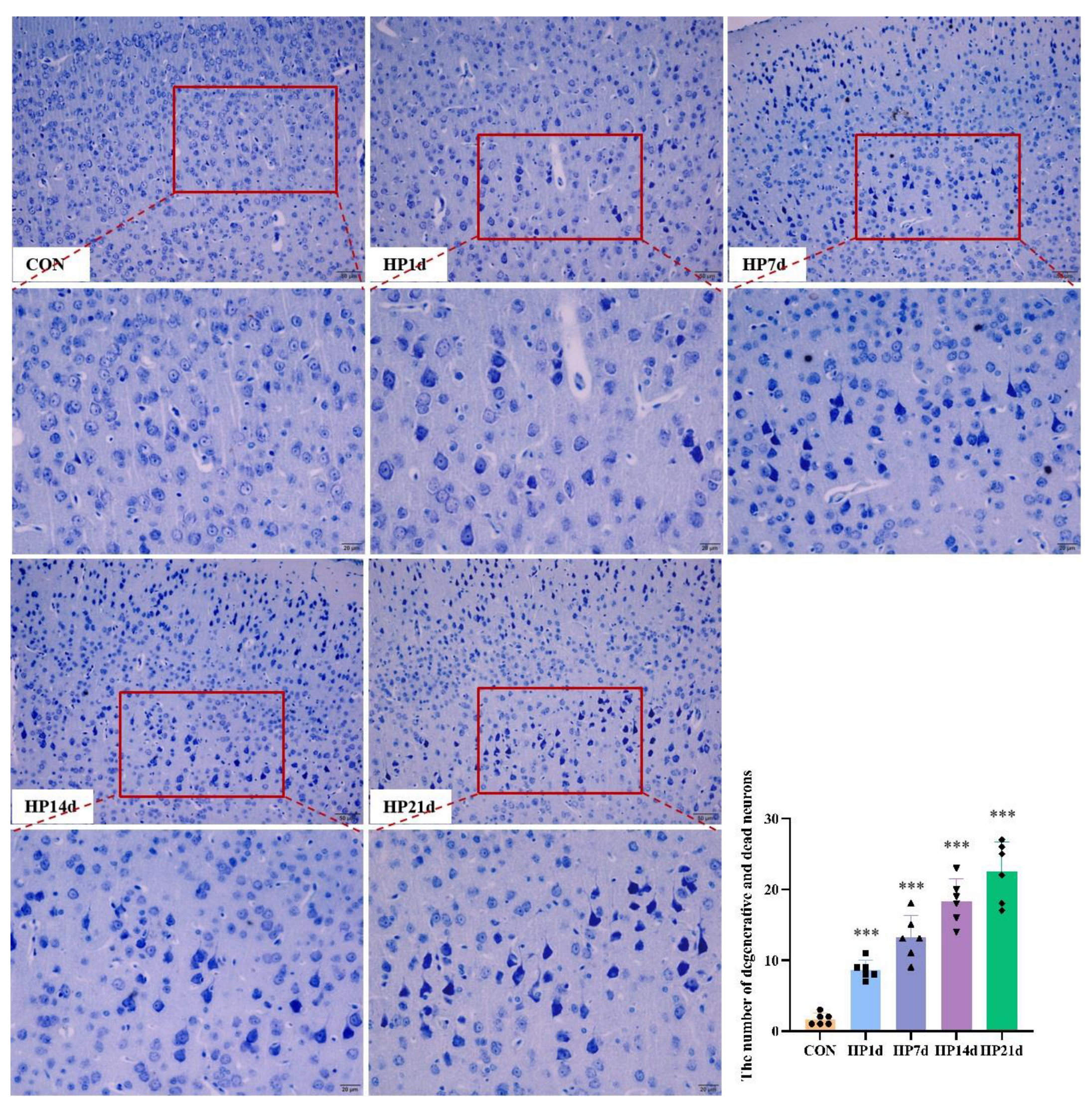
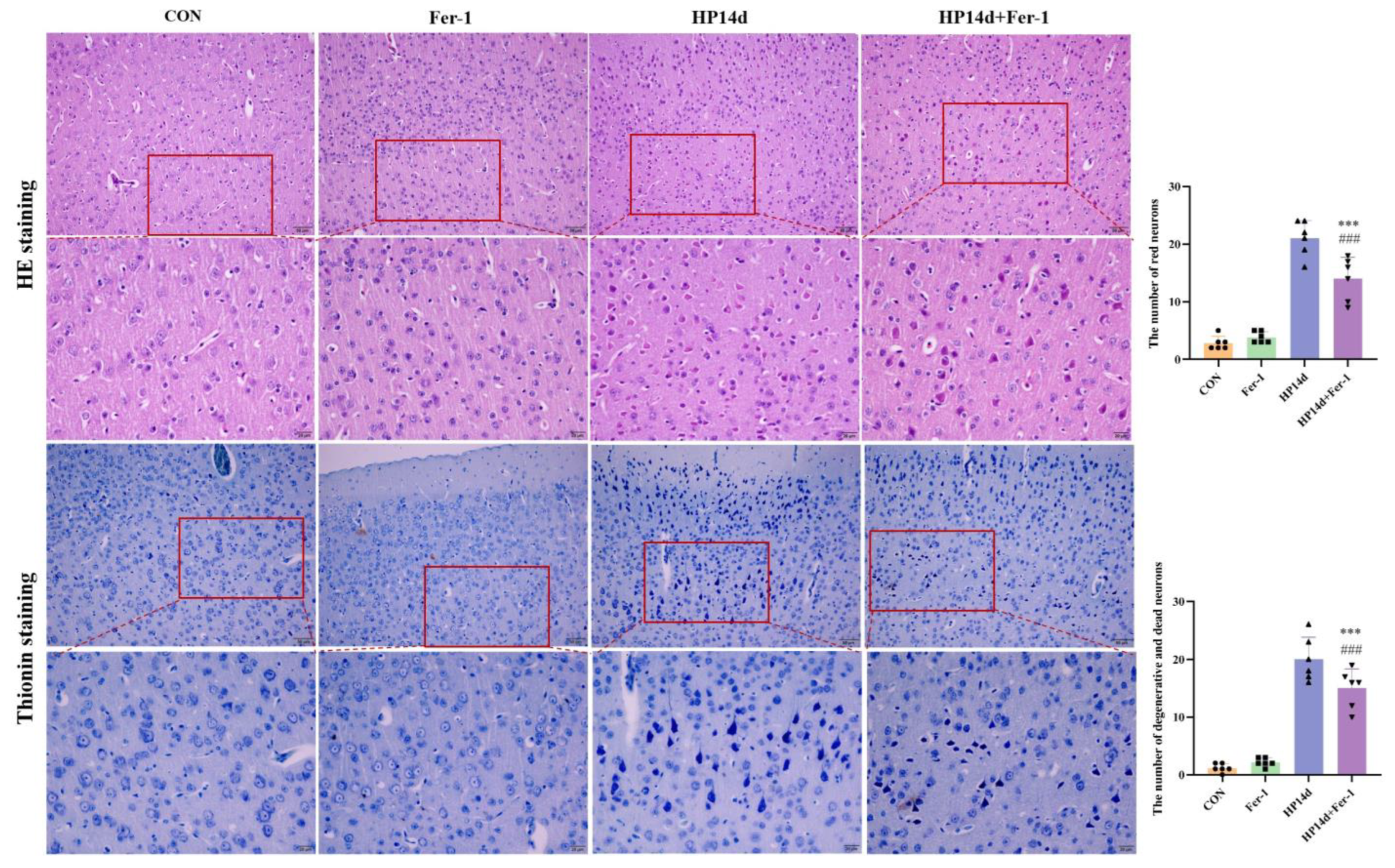
2.2. Degeneration and Death of Cortical Neurons in Surviving Mice with Severe Hypothermia
2.3. Ferroptosis Occurs in the Cerebral Cortex of Mice Surviving Severe Hypothermia
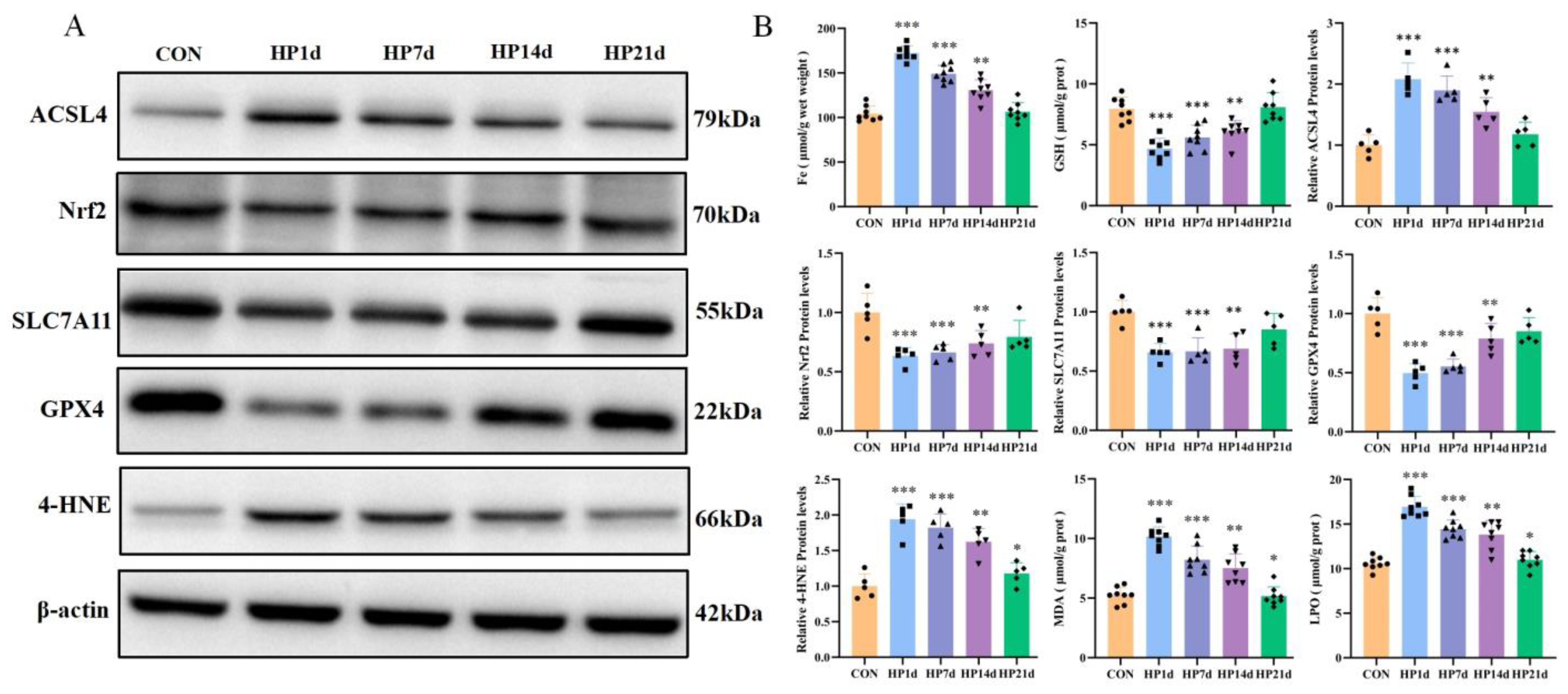
2.3.1. Ferrous Ion Accumulation in the Cerebral Cortex of Mice Surviving Severe Hypothermia
2.3.2. Pro-Ferroptosis Proteins Were Activated in the Cerebral Cortex of Mice Surviving Hypothermia Coma
2.3.3. Failure of the Antioxidant System in the Cerebral Cortex of Mice Surviving Hypothermia Coma
2.3.4. Increased Products of Lipid Peroxidation in the Cerebral Cortex of Surviving with Hypothermic Coma
2.4. Ferroptosis Inhibitors Mitigate Nerve Damage in the Cerebral Cortex in Mice Surviving Severe Hypothermia
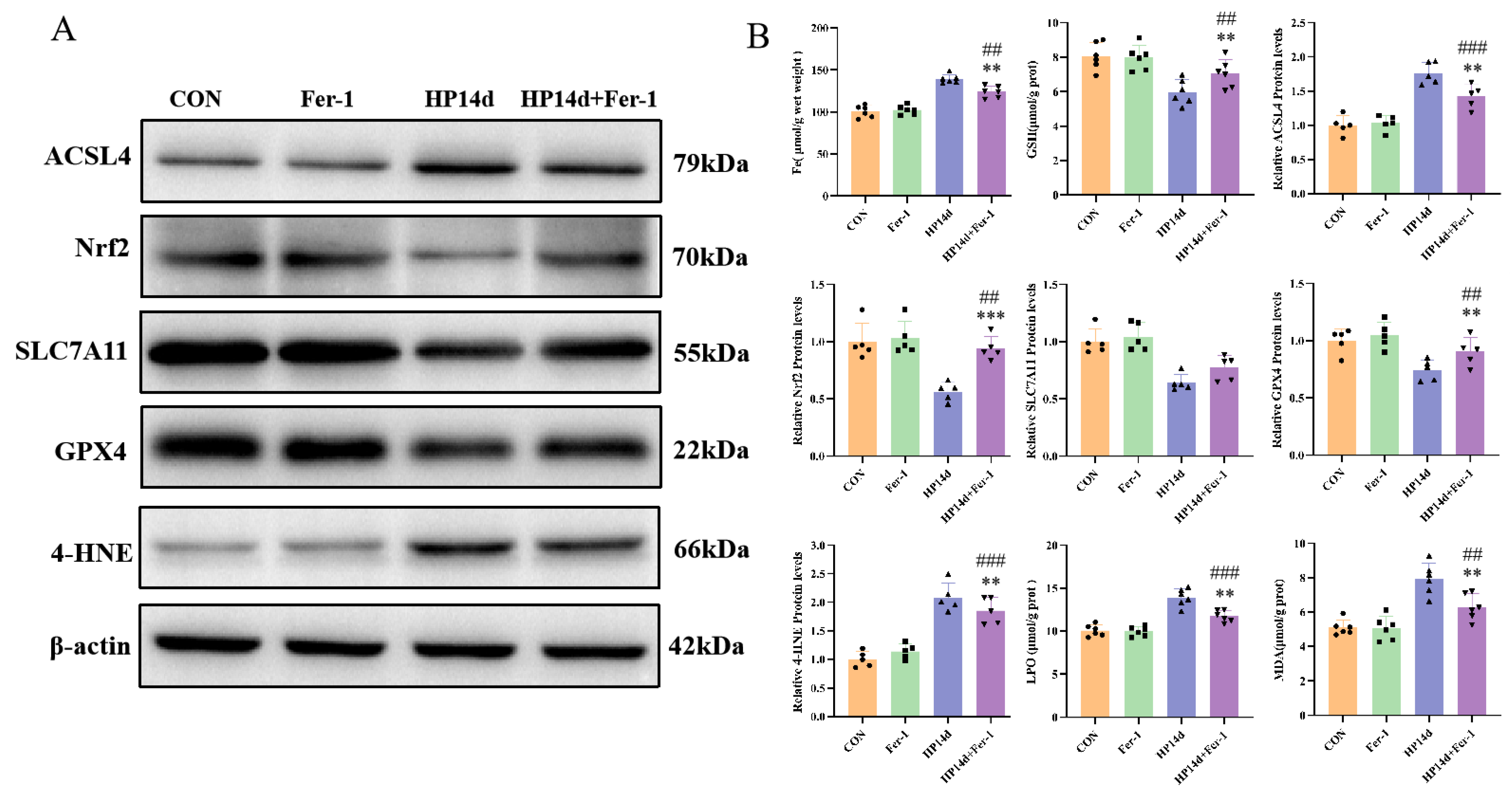
2.4.1. Ferroptosis Inhibitor Can Reduce the Expression of Ferroptosis-Related Proteins in Mice Surviving Severe Hypothermia
2.4.2. Ferroptosis Inhibitor (Fer-1) Alleviated Neuronal Degeneration and Death in Mice Surviving Severe Hypothermia
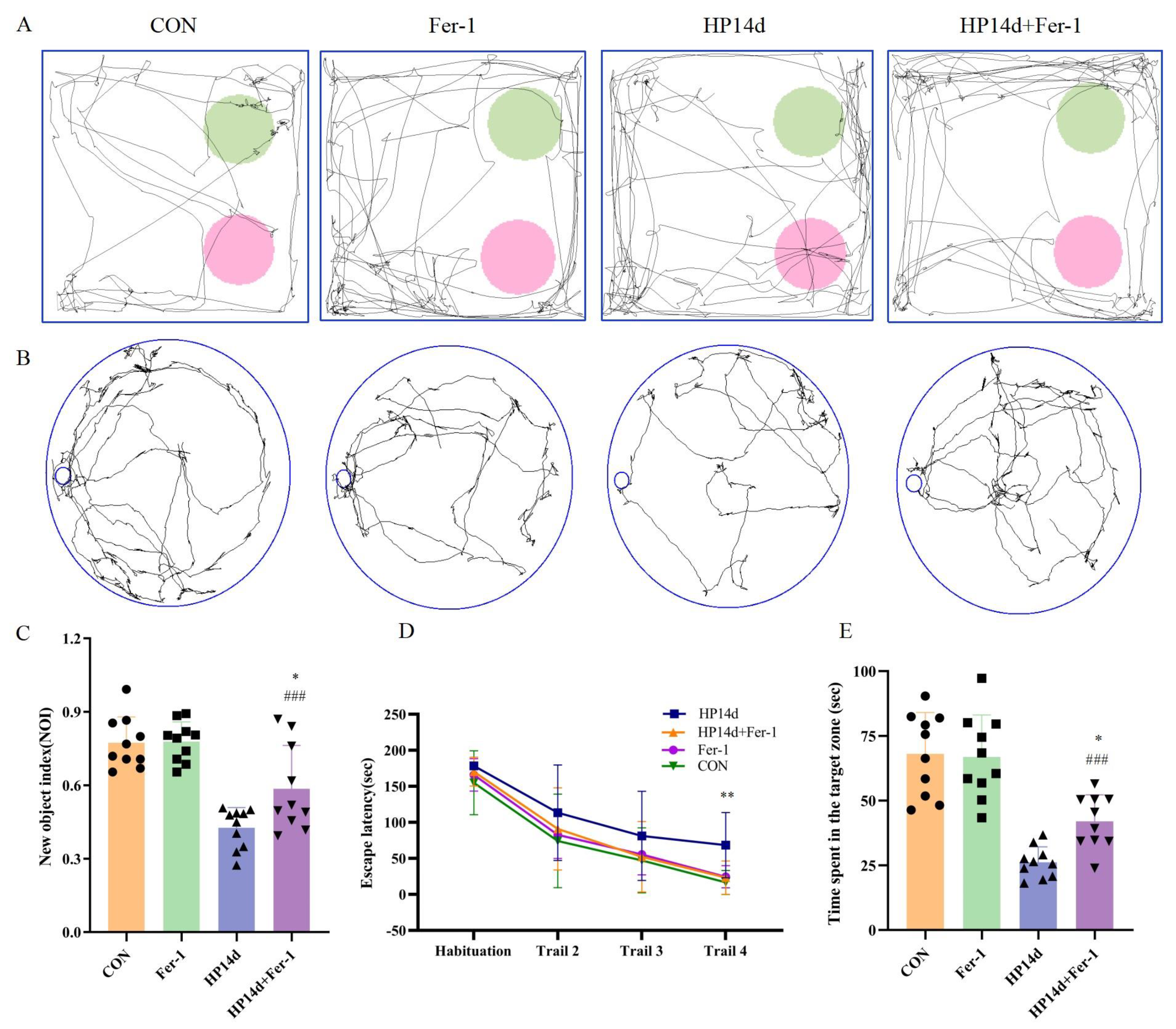
2.4.3. Ferroptosis Inhibitor (Fer-1) Improved Cognitive Function in Mice Surviving Severe Hypothermia
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Model Preparation and Sample Extraction
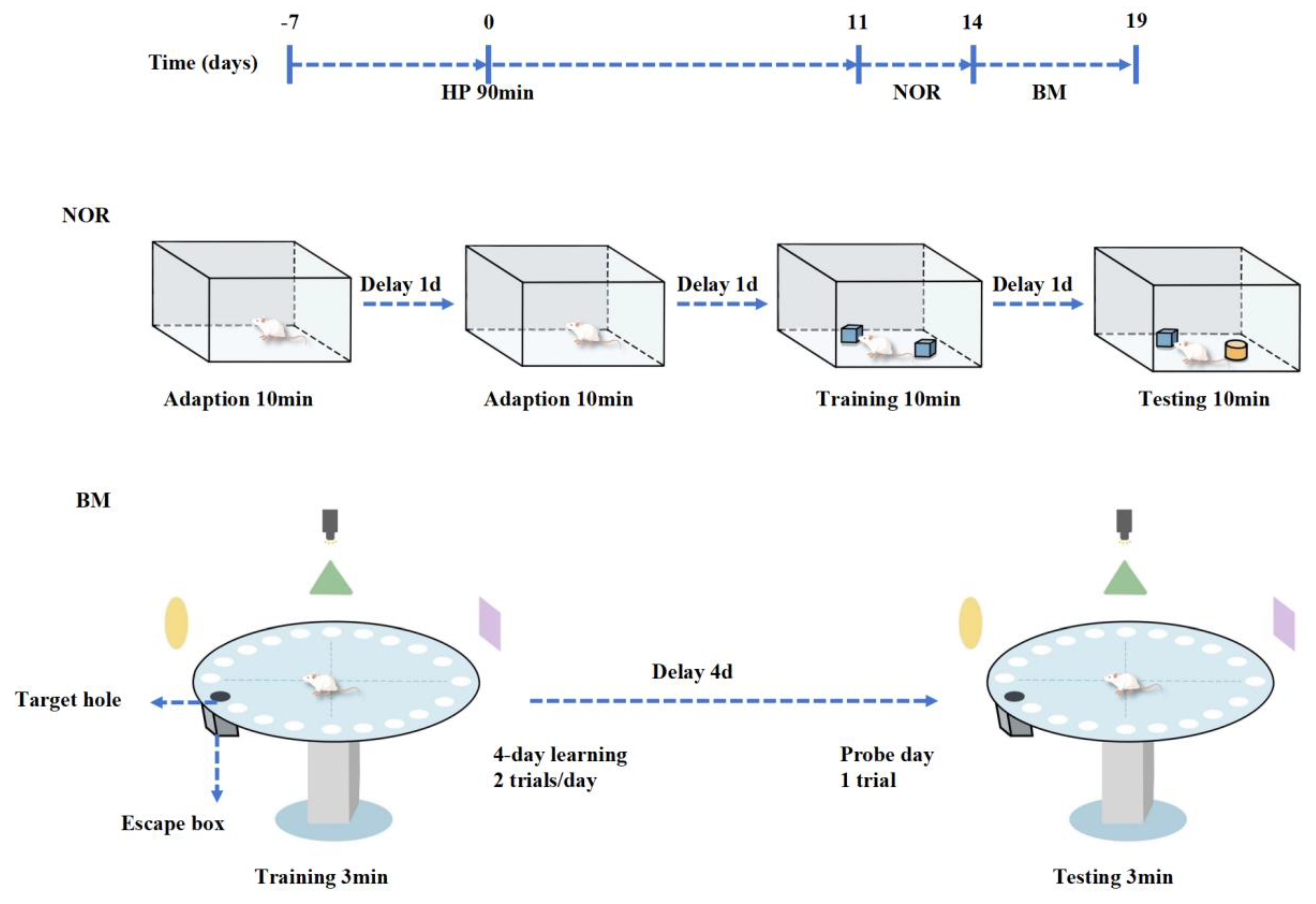
4.3. Behavioral Test
4.3.1. Novel Object Recognition
4.3.2. Barnes Maze
4.4. HE Staining
4.5. Thionin Staining
4.6. Western Blotting Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Iron Determination
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Xiong, K.; Wang, A.; Wang, Z.; Cui, Q.; Xie, H.; Yang, T.; Fan, X.; Jiang, W.; Tan, X.; et al. Cold stress causes liver damage by inducing ferroptosis through the p38 MAPK/Drp1 pathway. Cryobiology 2023, 113, 104563. [Google Scholar] [CrossRef]
- Weilnhammer, V.; Schmid, J.; Mittermeier, I.; Schreiber, F.; Jiang, L.; Pastuhovic, V.; Herr, C.; Heinze, S. Extreme weather events in europe and their health consequences—A systematic review. Int. J. Hyg. Environ. Health 2021, 233, 113688. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Mohammed, S.S.; Tammam, H.G.; Ibrahim Abdel-Karim, R.; Farag, M.M. Histopathological, histochemical and biochemical postmortem changes in induced fatal hypothermia in rats. Forensic Sci. Res. 2022, 7, 211–227. [Google Scholar] [CrossRef]
- Fudge, J. Preventing and Managing Hypothermia and Frostbite Injury. Sports Health 2016, 8, 133–139. [Google Scholar] [CrossRef]
- Lu, C.L.; Sha, J.J.; Ma, R.F.; Dong, X.T.; Su, X.R.; Cong, B.; Wang, S.J. Severe Hypothermia Induces Ferroptosis in Cerebral Cortical Nerve Cells. Int. J. Mol. Sci. 2024, 25, 8086. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. [Google Scholar] [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2022, 24, 449. [Google Scholar] [CrossRef]
- Shakya, A.; McKee, N.W.; Dodson, M.; Chapman, E.; Zhang, D.D. Anti-Ferroptotic Effects of Nrf2: Beyond the Antioxidant Response. Mol. Cells 2023, 46, 165–175. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Sun, G.; Su, W.; Bao, J.; Teng, T.; Song, X.; Wang, J.; Shi, B. Dietary full-fat rice bran prevents the risk of heart ferroptosis and imbalance of energy metabolism induced by prolonged cold stimulation. Food Funct. 2023, 14, 1530–1544. [Google Scholar] [CrossRef]
- Zou, X.F.; Wu, S.H.; Ma, J.G.; Yin, Z.Q.; Hu, Z.D.; Wang, Y.W.; Yang, J.; Guo, R.D. 3-O-Methyl-D-Glucose Blunts Cold Ischemia Damage in Kidney via Inhibiting Ferroptosis. Biomed. Pharmacother. 2024, 173, 116262. [Google Scholar] [CrossRef]
- Zhou, J.; Jin, Y.; Lei, Y.; Liu, T.; Wan, Z.; Meng, H.; Wang, H. Ferroptosis Is Regulated by Mitochondria in Neurodegenerative Diseases. Neurodegener. Dis. 2020, 20, 20–34. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Dirksen, R.T.; Wojtovich, A.P. Iron Dysregulation in Mitochondrial Dysfunction and Alzheimer’s Disease. Antioxidants 2022, 11, 692. [Google Scholar] [CrossRef]
- Li, C.; Wu, Z.; Xue, H.; Gao, Q.; Zhang, Y.; Wang, C.; Zhao, P. Ferroptosis contributes to hypoxic-ischemic brain injury in neonatal rats: Role of the SIRT1/Nrf2/GPx4 signaling pathway. CNS Neurosci. Ther. 2022, 28, 2268–2280. [Google Scholar] [CrossRef]
- Xie, B.S.; Wang, Y.Q.; Lin, Y.; Mao, Q.; Feng, J.F.; Gao, G.Y.; Jiang, J.Y. Inhibition of ferroptosis attenuates tissue damage and improves long-term outcomes after traumatic brain injury in mice. CNS Neurosci. Ther. 2019, 25, 465–475. [Google Scholar] [CrossRef]
- Du, J.; Wang, T.; Li, Y.; Zhou, Y.; Wang, X.; Yu, X.; Ren, X.; An, Y.; Wu, Y.; Sun, W.; et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic. Biol. Med. 2019, 131, 356–369. [Google Scholar] [CrossRef]
- Ide, K.; Souma, T. In Vivo Assessment of Ferroptosis and Ferroptotic Stress in Mice. Curr. Protoc. 2022, 2, e413. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Kim, K.J.; Gaschler, M.M.; Patel, M.; Shchepinov, M.S.; Stockwell, B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl. Acad. Sci. USA 2016, 113, E4966–E4975. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vuckovic, A.M.; Bosello Travain, V.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef]
- Muller, M.D.; Gunstad, J.; Alosco, M.L.; Miller, L.A.; Updegraff, J.; Spitznagel, M.B.; Glickman, E.L. Acute cold exposure and cognitive function: Evidence for sustained impairment. Ergonomics 2012, 55, 792–798. [Google Scholar] [CrossRef]
- Falla, M.; Micarelli, A.; Hufner, K.; Strapazzon, G. The Effect of Cold Exposure on Cognitive Performance in Healthy Adults: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 9725. [Google Scholar] [CrossRef]
- Ahmadian-Attar, M.M.; Ahmadiani, A.; Kamalinejad, M.; Dargahi, L.; Mosaddegh, M. Chronic Cold-Water-Induced Hypothermia Impairs Memory Retrieval and Nepeta menthoides as a Traditional “Hot” Herb Reverses the Impairment. Iran. J. Pharm. Res. 2014, 13, 185–193. [Google Scholar]
- Su, Y.; Jiao, Y.; Cai, S.; Xu, Y.; Wang, Q.; Chen, X. The molecular mechanism of ferroptosis and its relationship with Parkinson’s disease. Brain Res. Bull. 2024, 213, 110991. [Google Scholar] [CrossRef]
- Deng, L.; He, S.; Guo, N.; Tian, W.; Zhang, W.; Luo, L. Molecular mechanisms of ferroptosis and relevance to inflammation. Inflamm. Res. 2023, 72, 281–299. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Hu, J.; Tian, Y.; Fu, X. Ferroptosis at the nexus of metabolism and metabolic diseases. Theranostics 2024, 14, 5826–5852. [Google Scholar] [CrossRef]
- Sohn, S.H.; Chae, S.; Choi, J.W.; Nam, K.; Cho, Y.J.; Cho, J.Y.; Hwang, H.Y. Differences in Brain Metabolite Profiles Between Normothermia and Hypothermia. J. Korean Med. Sci. 2024, 39, e79. [Google Scholar] [CrossRef]
- Rousseau, G.; Chao de la Barca, J.M.; Rouge-Maillart, C.; Teresinski, G.; Jousset, N.; Dieu, X.; Chabrun, F.; Prunier-Mirabeau, D.; Simard, G.; Reynier, P.; et al. A serum metabolomics signature of hypothermia fatalities involving arginase activity, tryptophan content, and phosphatidylcholine saturation. Int. J. Leg. Med. 2019, 133, 889–898. [Google Scholar] [CrossRef]
- Elmsjo, A.; Ward, L.J.; Horioka, K.; Watanabe, S.; Kugelberg, F.C.; Druid, H.; Green, H. Biomarker patterns and mechanistic insights into hypothermia from a postmortem metabolomics investigation. Sci. Rep. 2024, 14, 18972. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, K.; Ma, L.; Qian, Z.; Tian, X.; Miao, Y.; Niu, Y.; Xu, X.; Guo, S.; Yang, Y.; et al. Endogenous glutamate determines ferroptosis sensitivity via ADCY10-dependent YAP suppression in lung adenocarcinoma. Theranostics 2021, 11, 5650–5674. [Google Scholar] [CrossRef]
- Conlon, M.; Poltorack, C.D.; Forcina, G.C.; Armenta, D.A.; Mallais, M.; Perez, M.A.; Wells, A.; Kahanu, A.; Magtanong, L.; Watts, J.L.; et al. A compendium of kinetic modulatory profiles identifies ferroptosis regulators. Nat. Chem. Biol. 2021, 17, 665–674. [Google Scholar] [CrossRef]
- Guo, X.; Guo, Y.; Li, J.; Liu, Q.; Wu, H. Arginine Expedites Erastin-Induced Ferroptosis through Fumarate. Int. J. Mol. Sci. 2023, 24, 14595. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pan, B.; Lv, X.; Chen, C.; Li, K.; Wang, Y.; Liu, J. Ferroptosis: Roles and molecular mechanisms in diabetic cardiomyopathy. Front. Endocrinol. 2023, 14, 1140644. [Google Scholar] [CrossRef]
- Bieri, S.; Moller, B.; Amsler, J. Ferroptosis in Arthritis: Driver of the Disease or Therapeutic Option? Int. J. Mol. Sci. 2024, 25, 8212. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, Z.; Zhou, X.; Zhao, Z.; Zhao, R.; Xu, X.; Kong, X.; Ren, J.; Yao, X.; Wen, Q.; et al. Microglia and macrophage exhibit attenuated inflammatory response and ferroptosis resistance after RSL3 stimulation via increasing Nrf2 expression. J. Neuroinflammation 2021, 18, 249. [Google Scholar] [CrossRef]
- Wu, Y.T.; Zhang, G.Y.; Li, L.; Liu, B.; Wang, R.Y.; Song, R.Q.; Hua, Y.; Bi, Y.M.; Han, X.; Zhang, F.; et al. Salvia miltiorrhiza suppresses cardiomyocyte ferroptosis after myocardial infarction by activating Nrf2 signaling. J. Ethnopharmacol. 2024, 330, 118214. [Google Scholar] [CrossRef]
- Zhu, X.; Guan, R.; Zou, Y.; Li, M.; Chen, J.; Zhang, J.; Luo, W. Cold-inducible RNA binding protein alleviates iron overload-induced neural ferroptosis under perinatal hypoxia insult. Cell Death Differ. 2024, 31, 524–539. [Google Scholar] [CrossRef]
- Xu, X.E.; Liu, L.; Wang, Y.C.; Wang, C.T.; Zheng, Q.; Liu, Q.X.; Li, Z.F.; Bai, X.J.; Liu, X.H. Caspase-1 inhibitor exerts brain-protective effects against sepsis-associated encephalopathy and cognitive impairments in a mouse model of sepsis. Brain Behav. Immun. 2019, 80, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Lavanco, G.; Feo, S.; D’Amico, C.; Micale, V.; Kuchar, M.; Plescia, F.; Brancato, A.; Cannizzaro, C. Prenatal Exposure to Delta9-Tetrahydrocannabinol Affects Hippocampus-Related Cognitive Functions in the Adolescent Rat Offspring: Focus on Specific Markers of Neuroplasticity. Pharmaceutics 2023, 15, 692. [Google Scholar] [CrossRef] [PubMed]
- Tork, Y.J.; Naseri, E.; Basir, H.S.; Komaki, A. Protective effects of L-carnitine against beta-amyloid-induced memory impairment and anxiety-like behavior in a rat model of Alzheimer’s disease. Eur. J. Pharmacol. 2024, 982, 176879. [Google Scholar] [CrossRef]
- Wang, S.; Shi, W.; Zhang, G.; Zhang, X.; Ma, C.; Zhao, K.; Cong, B.; Li, Y. Endoplasmic Reticulum Stress-Mediated Basolateral Amygdala GABAergic Neuron Injury Is Associated with Stress-Induced Mental Disorders in Rats. Front. Cell. Neurosci. 2019, 13, 511. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-X.; Dong, X.-T.; Zhang, F.; Wang, J.-Y.; Lu, C.-L.; Zhou, Z.-Q.; Gu, J.-Y.; Wang, S.-J. Inhibition of Ferroptosis Attenuates Neuron Damage and Improves Cognitive Impairment in Mice Surviving Severe Hypothermia. Int. J. Mol. Sci. 2025, 26, 4965. https://doi.org/10.3390/ijms26114965
Li W-X, Dong X-T, Zhang F, Wang J-Y, Lu C-L, Zhou Z-Q, Gu J-Y, Wang S-J. Inhibition of Ferroptosis Attenuates Neuron Damage and Improves Cognitive Impairment in Mice Surviving Severe Hypothermia. International Journal of Molecular Sciences. 2025; 26(11):4965. https://doi.org/10.3390/ijms26114965
Chicago/Turabian StyleLi, Wei-Xuan, Xue-Tong Dong, Fu Zhang, Jun-Yan Wang, Chao-Long Lu, Zhao-Qi Zhou, Jia-Yi Gu, and Song-Jun Wang. 2025. "Inhibition of Ferroptosis Attenuates Neuron Damage and Improves Cognitive Impairment in Mice Surviving Severe Hypothermia" International Journal of Molecular Sciences 26, no. 11: 4965. https://doi.org/10.3390/ijms26114965
APA StyleLi, W.-X., Dong, X.-T., Zhang, F., Wang, J.-Y., Lu, C.-L., Zhou, Z.-Q., Gu, J.-Y., & Wang, S.-J. (2025). Inhibition of Ferroptosis Attenuates Neuron Damage and Improves Cognitive Impairment in Mice Surviving Severe Hypothermia. International Journal of Molecular Sciences, 26(11), 4965. https://doi.org/10.3390/ijms26114965



