A Combination of Resveratrol and Quercetin Prevents Sarcopenic Obesity: Its Role as a Signaling Inhibitor of Myostatin/ActRIIA and ActRIIB/Smad and as an Enhancer of Insulin Actions
Abstract
1. Introduction
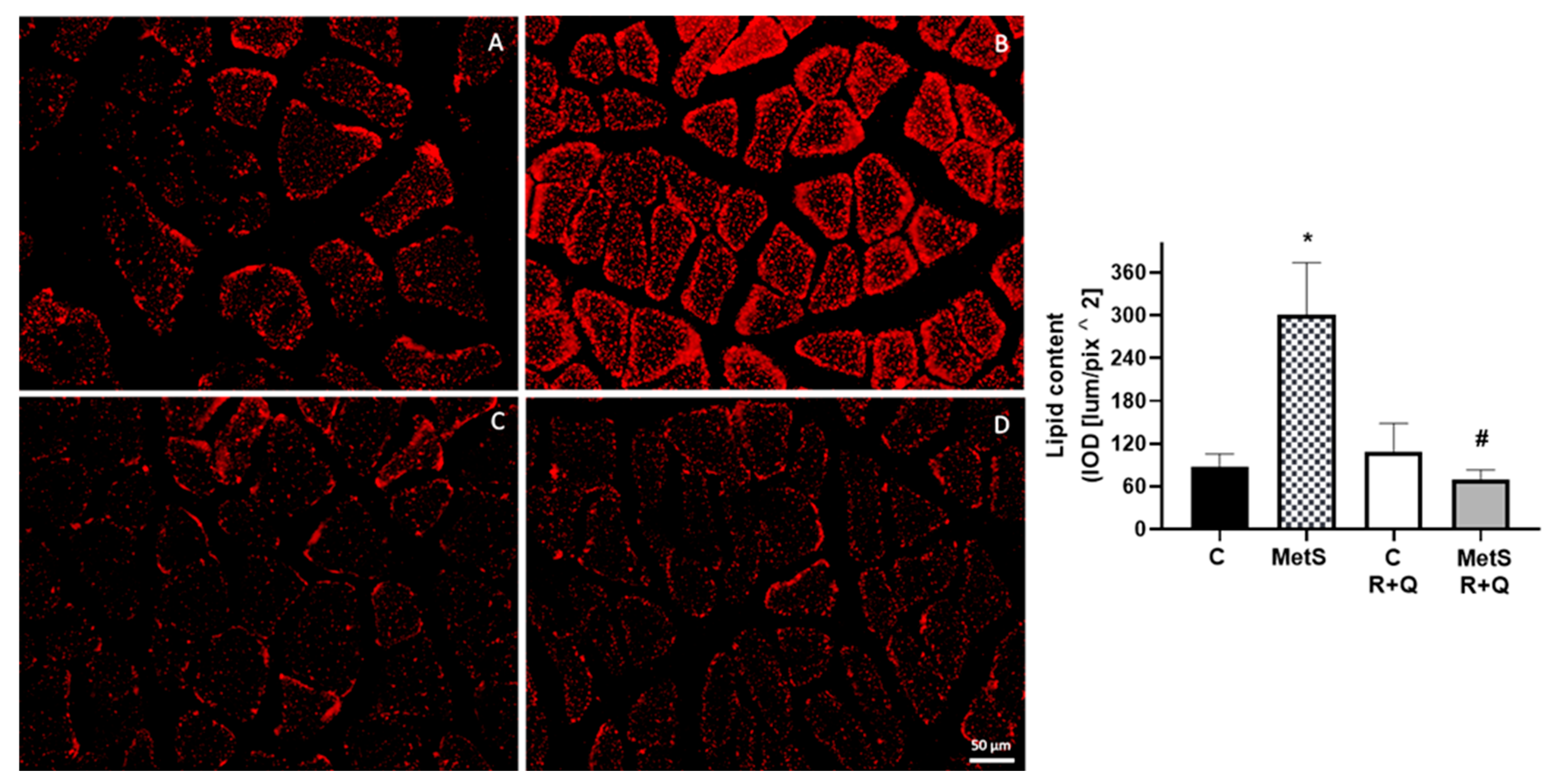
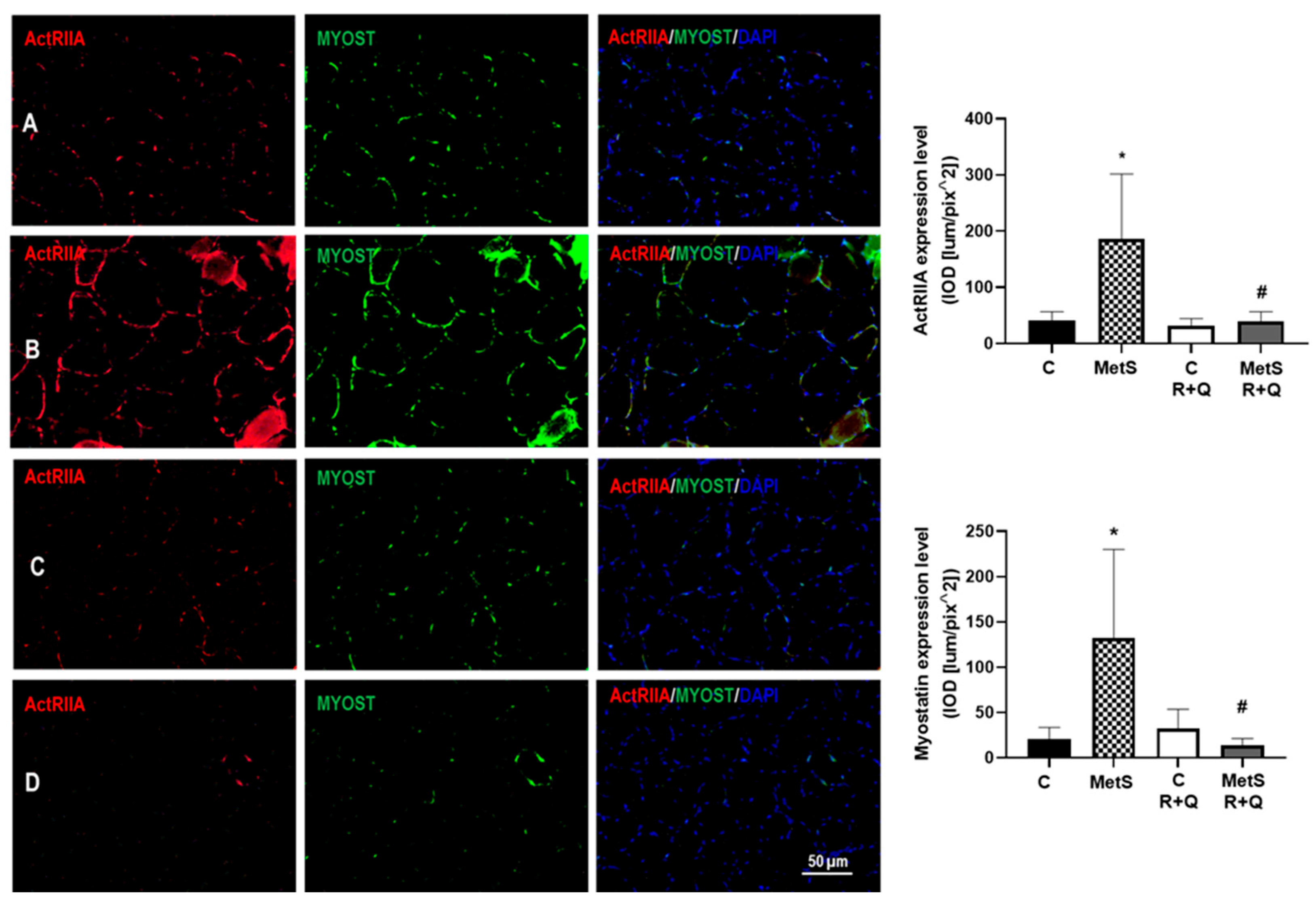
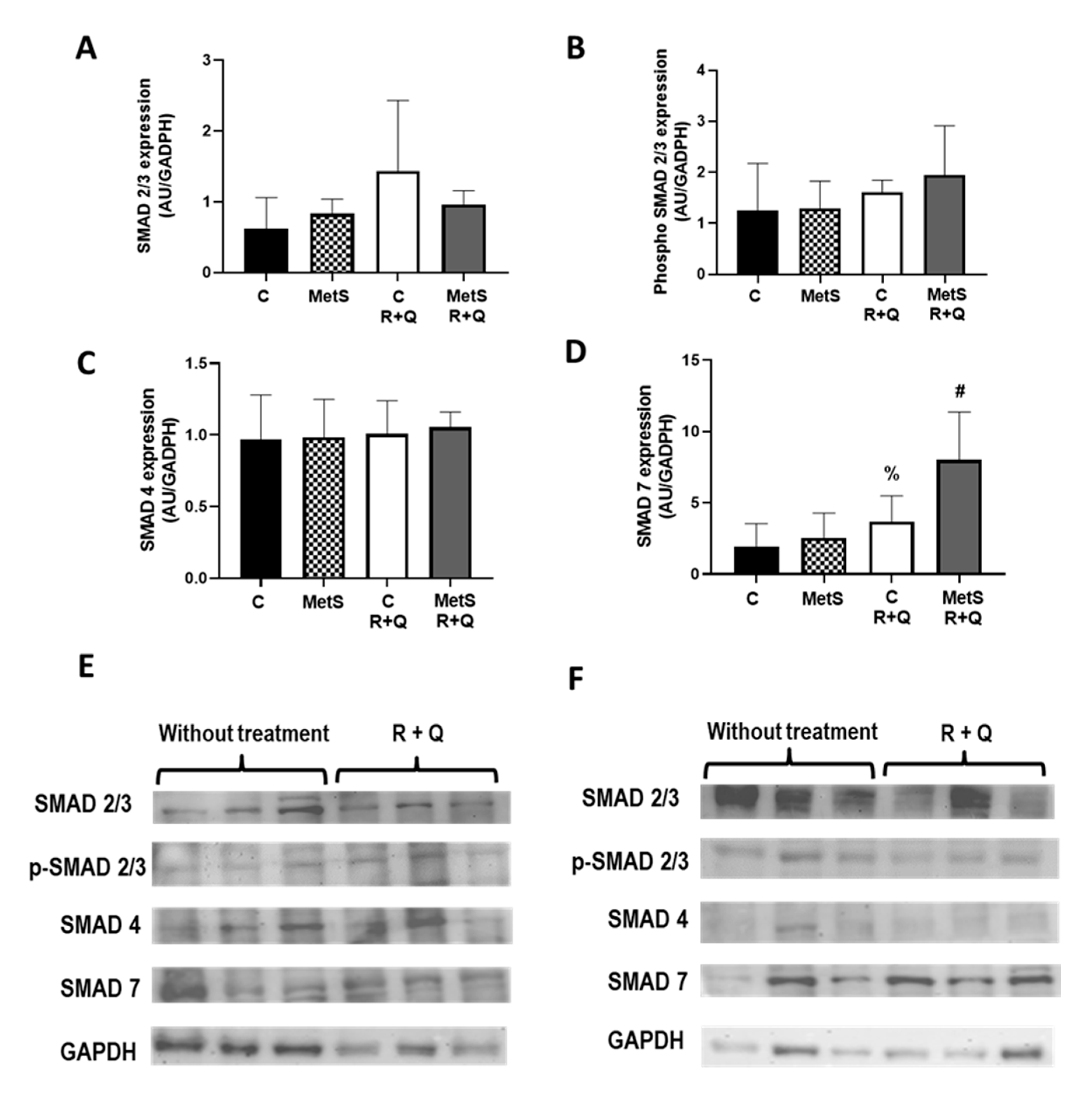
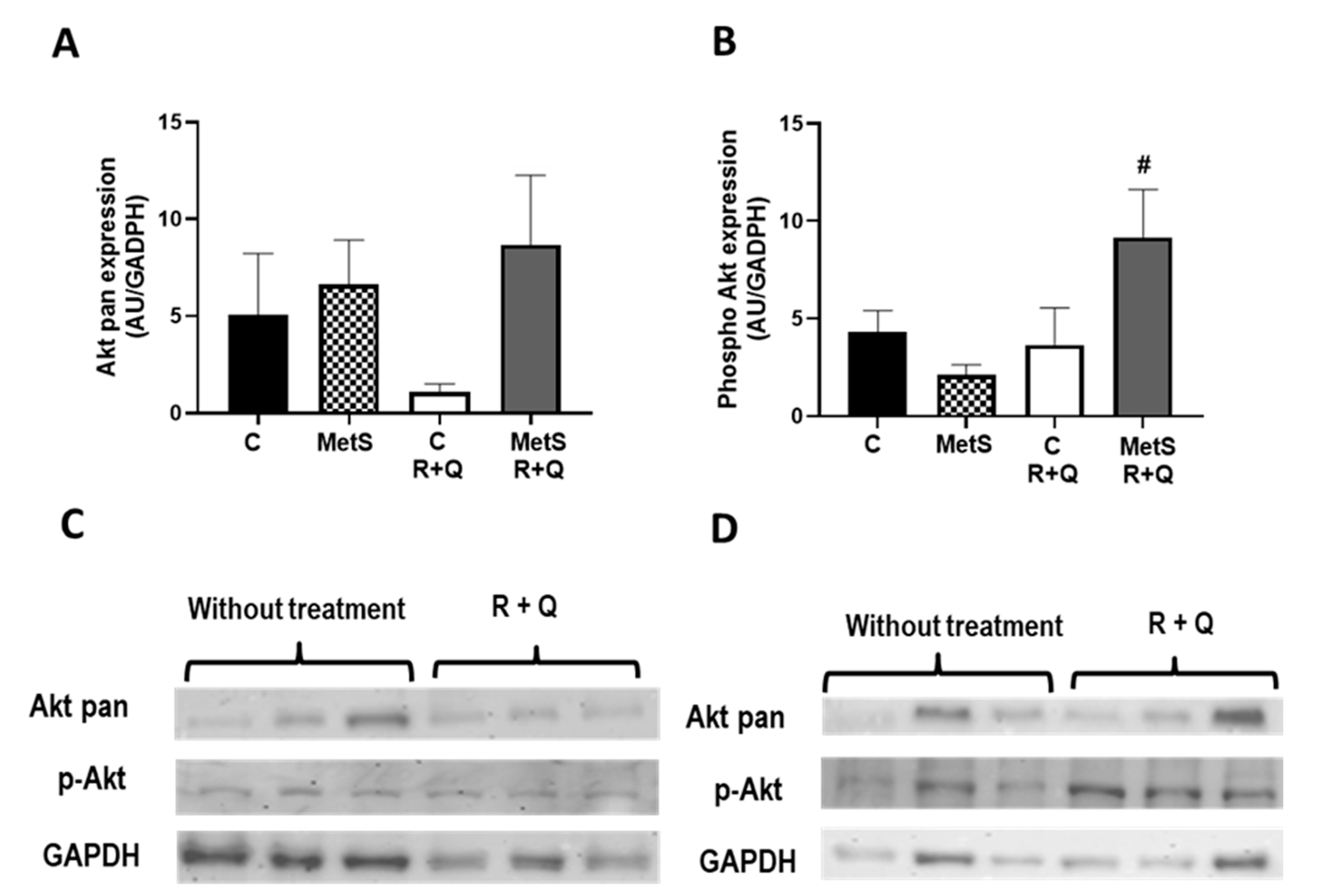
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Treatment
4.2. Serum Biochemical Assessment
4.3. Tissue Sampling
4.4. Western Blot Analysis
4.5. Histological Analysis
4.5.1. Oil Red Staining
4.5.2. Periodic Acid–Schiff (PAS) Staining
4.5.3. Immunolocalization: Immunofluorescence Assay
4.5.4. Image Acquisition and Label Quantification
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms Underlying Metabolic Syndrome-Related Sarcopenia and Possible Therapeutic Measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Hong, Y.P.; Shin, H.J.; Lee, W. Associations of Sarcopenia and Sarcopenic Obesity With Metabolic Syndrome Considering Both Muscle Mass and Muscle Strength. J. Prev. Med. Public Health 2016, 49, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.; Cumming, R.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Handelsman, D.J.; Seibel, M.; Waite, L.M.; Hirani, V. Associations of sarcopenic obesity with the metabolic syndrome and insulin resistance over five years in older men: The Concord Health and Ageing in Men Project. Exp. Gerontol. 2018, 108, 99–105. [Google Scholar] [CrossRef]
- Axelrod, C.L.; Dantas, W.S.; Kirwan, J.P. Sarcopenic obesity: Emerging mechanisms and therapeutic potential. Metabolism 2023, 146, 155639. [Google Scholar] [CrossRef]
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494. [Google Scholar] [CrossRef]
- Talbot, J.; Maves, L. Skeletal muscle fiber type: Using insights from muscle developmental biology to dissect targets for susceptibility and resistance to muscle disease. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 518–534. [Google Scholar] [CrossRef]
- Zurmanova, J.; Soukup, T. Comparison of myosin heavy chain mRNAs, protein isoforms and fiber type proportions in the rat slow and fast muscles. Physiol. Res. 2013, 62, 445–453. [Google Scholar] [CrossRef]
- Nishikawa, H.; Asai, A.; Fukunishi, S.; Nishiguchi, S.; Higuchi, K. Metabolic Syndrome and Sarcopenia. Nutrients 2021, 73, 3519. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Ribaldone, D.G.; Bugianesi, E. The Impact of Dysmetabolic Sarcopenia Among Insulin Sensitive Tissues: A Narrative Review. Front. Endocrinol. 2021, 12, 716533. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef]
- Eilers, W.; Chambers, D.; Cleasby, M.; Foster, K. Local myostatin inhibition improves skeletal muscle glucose uptake in insulin-resistant high-fat diet-fed mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E163–E174. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, A.; Saucier, C.; Bisbal, C.; Lambert, K. Grape Polyphenols in the Treatment of Human Skeletal Muscle Damage Due to Inflammation and Oxidative Stress during Obesity and Aging: Early Outcomes and Promises. Molecules 2022, 27, 6594. [Google Scholar] [CrossRef] [PubMed]
- Le, N.H.; Kim, C.S.; Park, T.; Park, J.H.; Sung, M.K.; Lee, D.G.; Hong, S.M.; Choe, S.Y.; Goto, T.; Kawada, T.; et al. Quercetin protects against obesity-induced skeletal muscle inflammation and atrophy. Mediat. Inflamm. 2014, 2014, 834294. [Google Scholar] [CrossRef]
- Goodman, C.A.; Hornberger, T.A. New roles for Smad signaling and phosphatidic acid in the regulation of skeletal muscle mass. F1000Prime Rep. 2014, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Kollias, H.D.; Perry, R.L.; Miyake, I.; Aziz, A.; McDermott, J.C. Smad7 promotes and enhances skeletal muscle differentiation. Mol. Cell. Biol. 2006, 26, 6248–6260. [Google Scholar] [CrossRef]
- Cohen, T.V.; Kollias, H.D.; Liu, N.; Ward, C.W.; Wagner, K.R. Genetic disruption of Smad7 impairs skeletal muscle growth and regeneration. J. Physiol. 2015, 593, 2479–2497. [Google Scholar] [CrossRef]
- Hittel, D.S.; Berggren, J.R.; Shearer, J.; Boyle, K.; Houmard, J.A. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009, 58, 30–38. [Google Scholar] [CrossRef]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef]
- Oh, M.; Kim, S.-Y.; Park, S.; Kim, K.-N.; Kim, S.H. Phytochemicals in Chinese Chive (Allium tuberosum) Induce the Skeletal Muscle Cell Proliferation via PI3K/Akt/mTOR and Smad Pathways in C2C12 Cells. Int. J. Mol. Sci. 2021, 22, 2296. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Tan, R.; Liu, Y. Effect of flavonoids on skeletal muscle mass, strength and physical performance in middle-aged and older adults with or without Sarcopenia: A meta-analysis of randomized controlled trials. Front. Nutr. 2022, 9, 1013449. [Google Scholar] [CrossRef]
- Jung, U.J. Sarcopenic Obesity: Involvement of Oxidative Stress and Beneficial Role of Antioxidant Flavonoids. Antioxidants 2023, 12, 1063. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, X.; Chen, K.; Lang, H.; Zhang, Y.; Hou, P.; Ran, L.; Zhou, M.; Zheng, J.; Yi, L.; et al. Resveratrol prevents sarcopenic obesity by reversing mitochondrial dysfunction and oxidative stress via the PKA/LKB1/AMPK pathway. Aging 2019, 11, 2217–2240. [Google Scholar] [CrossRef]
- Bai, C.H.; Alizargar, J.; Peng, C.Y.; Wu, J.P. Combination of exercise training and resveratrol attenuates obese sarcopenia in skeletal muscle atrophy. Chin. J. Physiol. 2020, 63, 101–112. [Google Scholar] [CrossRef]
- Wei, S.; Nguyen, I.T.; Zhang, Y.; Ryu, D.; Gariani, K. Sarcopenic Obesity: Epidemiology, Pathophysiology, Cardiovascular Disease, Mortality, and Management. Front. Endocrinol. 2023, 14, 1185221. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [CrossRef]
- Mo, X.; Cheng, R.; Shen, L.; Sun, Y.; Wang, P.; Jiang, G.; Wen, L.; Li, X.; Peng, X.; Liao, Y.; et al. High-Fat Diet Induces Sarcopenic Obesity in Natural Aging Rats Through the Gut–Trimethylamine N-Oxide–Muscle Axis. J. Adv. Res. 2025, 70, 405–422. [Google Scholar] [CrossRef]
- Cano-Martínez, A.; Bautista-Pérez, R.; Castrejón-Téllez, V.; Carreón-Torres, E.; Pérez-Torres, I.; Díaz-Diaz, E.; Flores-Estrada, I.; Guarner-Lans, V.; Rubio-Ruiz, M.E. Resveratrol and Quercetin as Regulators of Inflammatory and Purinergic Receptors to Attenuate Liver Damage Associated to Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 8939. [Google Scholar] [CrossRef] [PubMed]
- Castrejón-Téllez, V.; Rodríguez-Pérez, I.M.; Pérez-Torres, I.; Pérez-Hernández, N.; Cruz-Lagunas, A.; Guarner-Lans, V.; Vargas-Alarcón, G.; Rubio-Ruiz, M.E. The Effect of Resveratrol and Quercetin Treatment on PPAR Mediated Uncoupling Protein (UCP-) 1, 2, and 3 Expression in Visceral White Adipose Tissue from Metabolic Syndrome Rats. Int. J. Mol. Sci. 2016, 17, 1069. [Google Scholar] [CrossRef]
- Peredo-Escárcega, A.E.; Guarner-Lans, V.; Pérez-Torres, I.; Ortega-Ocampo, S.; Carreón-Torres, E.; Castrejón-Téllez, V.; Díaz-Díaz, E.; Rubio-Ruiz, M.E. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndrome in Rats by Modifying the Serum Fatty Acid Composition and by Upregulating SIRT1 and SIRT2 Expression in White Adipose Tissue. Evid.-Based Complement. Altern. Med. 2015, 2015, 474032. [Google Scholar] [CrossRef]
- Kim, J.G.; Sharma, A.R.; Lee, Y.H.; Chatterjee, S.; Choi, Y.T.; Raivansh, R.; Chakraborty, C.; Lee, S.S. Therapeutic Potential of Quercetin as an Antioxidant for Bone-Muscle-Tendon Regeneration and Aging. Aging Dis. 2024. online ahead of print. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Wolf, D. Skeletal Muscle Lipid Accumulation in Obesity, Insulin Resistance, and Type 2 Diabetes. Pediatr. Diabetes 2004, 5, 219–226. [Google Scholar] [CrossRef]
- Al Saedi, A.; Debruin, D.A.; Hayes, A.; Hamrick, M. Lipid Metabolism in Sarcopenia. Bone 2022, 164, 116539. [Google Scholar] [CrossRef]
- Bahramzadeh, A.; Bolandnazar, K.; Meshkani, R. Resveratrol as a Potential Protective Compound Against Skeletal Muscle Insulin Resistance. Heliyon 2023, 9, e21305. [Google Scholar] [CrossRef]
- Long, Y.; Wu, Y.; Zhong, Y.; Wu, Y.; Ye, H.; Luo, Y.; Xiao, L.; Ma, Y.; Wang, M. Resveratrol as a Potential Therapeutic Agent for Sarcopenic Obesity: Insights from In Vivo Experiments. Biomed. Pharmacother. 2024, 179, 117396. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, H.J.; Yang, G.; Kim, D.Y.; Kim, J.U.; Yook, T.H.; Lee, J.H.; Kim, H.J. A Novel Treatment Strategy Targeting Cellular Pathways with Natural Products to Alleviate Sarcopenia. Phytother. Res. 2024, 38, 5033–5051. [Google Scholar] [CrossRef]
- Russo, C.; Valle, M.S.; D’Angeli, F.; Surdo, S.; Malaguarnera, L. Resveratrol and Vitamin D: Eclectic Molecules Promoting Mitochondrial Health in Sarcopenia. Int. J. Mol. Sci. 2024, 25, 7503. [Google Scholar] [CrossRef]
- Allen, D.L.; Cleary, A.S.; Speaker, K.I.; Lindsay, S.F.; Uyenishi, J.; Reed, J.M.; Madden, M.C.; Mehan, R.S. Myostatin, Activin Receptor IIB, and Follistatin-Like-3 Gene Expression Are Altered in Adipose Tissue and Skeletal Muscle of Obese Mice. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E918–E927. [Google Scholar] [CrossRef]
- Dutra, D.B.; Bueno, P.G.; Silva, R.N.; Nakahara, N.H.; Selistre-Araújo, H.S.; Nonaka, K.O.; Leal, A.M. Expression of Myostatin, Myostatin Receptors and Follistatin in Diabetic Rats Submitted to Exercise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 417–422. [Google Scholar] [CrossRef]
- Toniolo, L.; Concato, M.; Giacomello, E. Resveratrol, a Multitasking Molecule That Improves Skeletal Muscle Health. Nutrients 2023, 15, 3413. [Google Scholar] [CrossRef]
- Bueno, P.G.; Bassi, D.; Contrera, D.G.; Carnielli, H.M.; Silva, R.N.; Nonaka, K.O.; Selistre-de-Araújo, H.S.; Leal, A.M. Post-Exercise Changes in Myostatin and Activin Expression in Obese Insulin-Resistant Rats. Mol. Cell. Endocrinol. 2011, 339, 159–164. [Google Scholar] [CrossRef]
- Winbanks, C.E.; Murphy, K.T.; Bernardo, B.C.; Qian, H.; Liu, Y.; Sepulveda, P.V.; Beyer, C.; Hagg, A.; Thomson, R.E.; Chen, I.L.; et al. Smad7 Gene Delivery Prevents Muscle Wasting Associated with Cancer Cachexia in Mice. Sci. Transl. Med. 2016, 8, 348ra98. [Google Scholar] [CrossRef]
- Aykaç, M.; Balkan, E.; Gedi Kli, S.; Öztürk, N. Resveratrol Treatment Ameliorates Hepatic Damage via the IGF-β/SMAD Signaling Pathway in a Phenobarbital/CCl₄-Induced Hepatic Fibrosis Model. Iran. J. Basic Med. Sci. 2024, 27, 1124–1133. [Google Scholar] [CrossRef]
- Zhai, X.X.; Ding, J.C.; Tang, Z.M. Resveratrol Inhibits Proliferation and Induces Apoptosis of Pathological Scar Fibroblasts Through the Mechanism Involving IGF-β1/Smads Signaling Pathway. Cell Biochem. Biophys. 2015, 71, 1267–1272. [Google Scholar] [CrossRef]
- Bilski, J.; Pinkas, M.; Wojcik-Grzybek, D.; Magierowski, M.; Korbut, E.; Mazur-Bialy, A.; Krzysiek-Mączka, G.; Kwiecień, S.; Magierowska, K.; Brzozowski, T. Role of Obesity, Physical Exercise, Adipose Tissue–Skeletal Muscle Crosstalk and Molecular Advances in Barrett’s Esophagus and Esophageal Adenocarcinoma. Int. J. Mol. Sci. 2022, 23, 3942. [Google Scholar] [CrossRef]
- Wang, J.; Dai, L.; Yu, T.; Xiao, J. Insights into the Progressive Impact of High-Fat-Diet Induced Insulin Resistance on Skeletal Muscle and Myocardium: A Comprehensive Study on C57BL6 Mice. PLoS ONE 2025, 20, e0310458. [Google Scholar] [CrossRef]
- Anhe, G.F.; Okamoto, M.M.; Kinote, A.; Sollon, C.; Lellis-Santos, C.; Anhe, F.F.; Lima, G.A.; Hirabara, S.M.; Velloso, L.A.; Bordin, S.; et al. Quercetin Decreases Inflammatory Response and Increases Insulin Action in Skeletal Muscle of ob/ob Mice and in L6 Myotubes. Eur. J. Pharmacol. 2012, 689, 285–293. [Google Scholar] [CrossRef]
- Park, S.; Jextovic, F.; Krassovskaia, P.M.; Chaves, A.B.; Zheng, D.; Treebak, J.T.; Houmard, J.A. Effect of Resveratrol on Insulin Action in Primary Myotubes from Lean Individuals and Individuals with Severe Obesity. Am. J. Physiol. Endocrinol. Metab. 2024, 326, E398–E406. [Google Scholar] [CrossRef]
- Eseberri, I.; Laurens, C.; Miranda, J.; Louche, K.; Lasa, A.; Moro, C.; Portillo, M.P. Effects of Physiological Doses of Resveratrol and Quercetin on Glucose Metabolism in Primary Myotubes. Int. J. Mol. Sci. 2021, 22, 1384. [Google Scholar] [CrossRef]
- Nagai, A.; Kaneda, Y.; Izumo, T.; Nakao, Y.; Honda, H.; Shimizu, K. Quercetin Induces a Slow Myofiber Phenotype in Engineered Human Skeletal Muscle Tissues. FASEB J. 2024, 38, e70009. [Google Scholar] [CrossRef]
- Gorza, L.; Germinario, E.; Vitadello, M.; Guerra, I.; De Majo, F.; Gasparella, F.; Caliceti, P.; Vitiello, L.; Danieli-Betto, D. Curcumin Administration Improves Force of mdx Dystrophic Diaphragm by Acting on Fiber-Type Composition, Myosin Nitrotyrosination and SERCA1 Protein Levels. Antioxidants 2023, 12, 1181. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 Activator, Attenuates Aging-Associated Alterations in Skeletal Muscle and Heart in Mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Pellegrinelli, V.; Rouault, C.; Rodriguez-Cuenca, S.; Albert, V.; Edom-Vovard, F.; Vidal-Puig, A.; Clément, K.; Butler-Browne, G.S.; Lacasa, D. Human Adipocytes Induce Inflammation and Atrophy in Muscle Cells During Obesity. Diabetes 2015, 64, 3121–3134. [Google Scholar] [CrossRef]
- Kubota, Y.; Umegaki, K.; Kagota, S.; Tanaka, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29, 1756–1758. [Google Scholar] [CrossRef]
- Ibrahim, J.; Berk, B.C.; Hughes, A.D. Comparison of simultaneous measurements of blood pressure by tail-cuff and carotid arterial methods in conscious spontaneously hypertensive and Wistar-Kyoto rats. Clin. Exp. Hypertens. 2006, 28, 57–72. [Google Scholar] [CrossRef]
- Jiménez, I.U.; Díaz-Díaz, E.; Castro, J.S.; Ramos, I.P.; León, M.C.; Alvarado Ríos, J.A.; Auriostigue Bautista, J.C.; Correa-Rotter, R.; Aguilar Salinas, C.A.; Larrea, F. Circulating Concentrations of Advanced Glycation End Products, its Association With the Development of Diabetes Mellitus. Arch. Med. Res. 2017, 48, 360–369. [Google Scholar] [CrossRef]
- Pérez Méndez, O.; García Hernández, L. El tamaño y la composición de las lipoproteínas de alta densidad (HDL) se modifican en la rata por una dieta suplementada con aguacate “Hass” (Persea americana Miller). Arch. Cardiol. Mex. 2007, 77, 17–22. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Babcock, L.W.; Knoblauch, M.; Clarke, M.S. The Role of Myostatin and Activin Receptor IIB in the Regulation of Unloading-Induced Myofiber Type-Specific Skeletal Muscle Atrophy. J. Appl. Physiol. (1985) 2015, 119, 633–642. [Google Scholar] [CrossRef]

| Control | Control Plus R+Q | MetS | MetS Plus R+Q | |
|---|---|---|---|---|
| Weight (g) | 457.2 ± 13.7 | 514.2 ± 20.9 | 598.1 ± 9.1 * | 481.8 ± 7.7 # |
| Abdominal fat (g) | 4.9 ± 0.3 | 6.3 ± 0.9 | 12.9 ± 0.3 * | 7.8 ± 1.0 # |
| Blood pressure (mm Hg) | 102.8 ± 0.8 | 110.8 ± 3.9 | 141.8 ± 0.9 * | 115.4 ± 2.9 # |
| Total cholesterol (mg/dL) | 53.7 ± 2.9 | 60.3 ± 3.5 | 63.5 ± 3.2 | 61.3 ± 1.5 |
| HDL-c (mg/dL) | 29.2 ± 1.9 | 28.4 ± 1.3 | 15.8 ± 1.2 * | 22.3 ± 1.9 # |
| Non-HDL-c (mg/dL) | 24.2 ± 1.4 | 20.5 ± 0.8 | 37.1 ± 4.2 * | 20.1 ± 1.9 # |
| Triglycerides (mg/dL) | 83.6 ± 6.7 | 78.9 ± 4.2 | 145.2 ± 6.2 * | 98.3 ± 5.2 # |
| Glucose (mg/dL) | 93.0 ± 3.3 | 91.1 ± 5.2 | 95.5 ± 2.4 | 90.1 ± 2.2 |
| Insulin (μU/mL) | 0.14 ± 0.03 | 0.12 ± 0.02 | 0.45 ± 0.05 * | 0.16 ± 0.01 # |
| HOMA-IR | 0.81 ± 0.19 | 0.56 ± 0.10 | 2.16 ± 0.30 * | 0.60 ± 0.07 # |
| Muscle-to-body mass ratio (mg/g) | 0.51 ± 0.05 | 0.49 ± 0.02 | 0.32 ± 0.03 * | 0.44 ± 0.01 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Martínez, A.; Méndez-Castro, J.A.; García-Vázquez, V.E.; Carreón-Torres, E.; Díaz-Díaz, E.; Sánchez-Aguilar, M.; Castrejón-Téllez, V.; Rubio-Ruíz, M.E. A Combination of Resveratrol and Quercetin Prevents Sarcopenic Obesity: Its Role as a Signaling Inhibitor of Myostatin/ActRIIA and ActRIIB/Smad and as an Enhancer of Insulin Actions. Int. J. Mol. Sci. 2025, 26, 4952. https://doi.org/10.3390/ijms26104952
Cano-Martínez A, Méndez-Castro JA, García-Vázquez VE, Carreón-Torres E, Díaz-Díaz E, Sánchez-Aguilar M, Castrejón-Téllez V, Rubio-Ruíz ME. A Combination of Resveratrol and Quercetin Prevents Sarcopenic Obesity: Its Role as a Signaling Inhibitor of Myostatin/ActRIIA and ActRIIB/Smad and as an Enhancer of Insulin Actions. International Journal of Molecular Sciences. 2025; 26(10):4952. https://doi.org/10.3390/ijms26104952
Chicago/Turabian StyleCano-Martínez, Agustina, Jimena Alejandra Méndez-Castro, Viviana Estefanía García-Vázquez, Elizabeth Carreón-Torres, Eulises Díaz-Díaz, María Sánchez-Aguilar, Vicente Castrejón-Téllez, and María Esther Rubio-Ruíz. 2025. "A Combination of Resveratrol and Quercetin Prevents Sarcopenic Obesity: Its Role as a Signaling Inhibitor of Myostatin/ActRIIA and ActRIIB/Smad and as an Enhancer of Insulin Actions" International Journal of Molecular Sciences 26, no. 10: 4952. https://doi.org/10.3390/ijms26104952
APA StyleCano-Martínez, A., Méndez-Castro, J. A., García-Vázquez, V. E., Carreón-Torres, E., Díaz-Díaz, E., Sánchez-Aguilar, M., Castrejón-Téllez, V., & Rubio-Ruíz, M. E. (2025). A Combination of Resveratrol and Quercetin Prevents Sarcopenic Obesity: Its Role as a Signaling Inhibitor of Myostatin/ActRIIA and ActRIIB/Smad and as an Enhancer of Insulin Actions. International Journal of Molecular Sciences, 26(10), 4952. https://doi.org/10.3390/ijms26104952





