Biopolymers of Polycaprolactone Loaded with Caffeic Acid and Trametes versicolor Extract Induced Proliferation in Human Coronary Artery Endothelial Cells and Inhibited Platelet Activity
Abstract
1. Introduction
2. Results
2.1. Morphological Characterization
2.2. Determination of Contact Angle
2.3. Quantification of Total Polyphenol and Protein Delivery from Coated Fibers
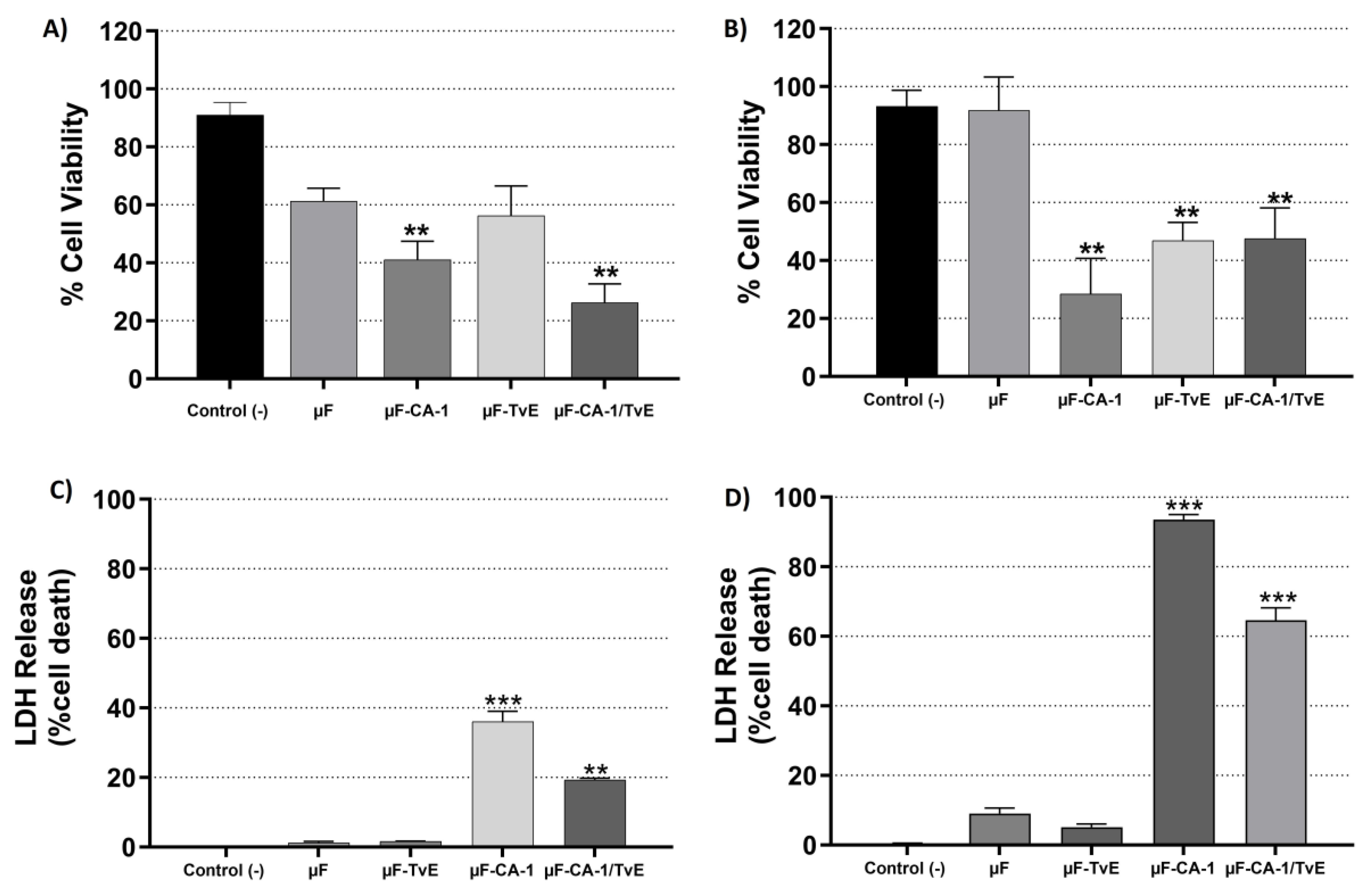
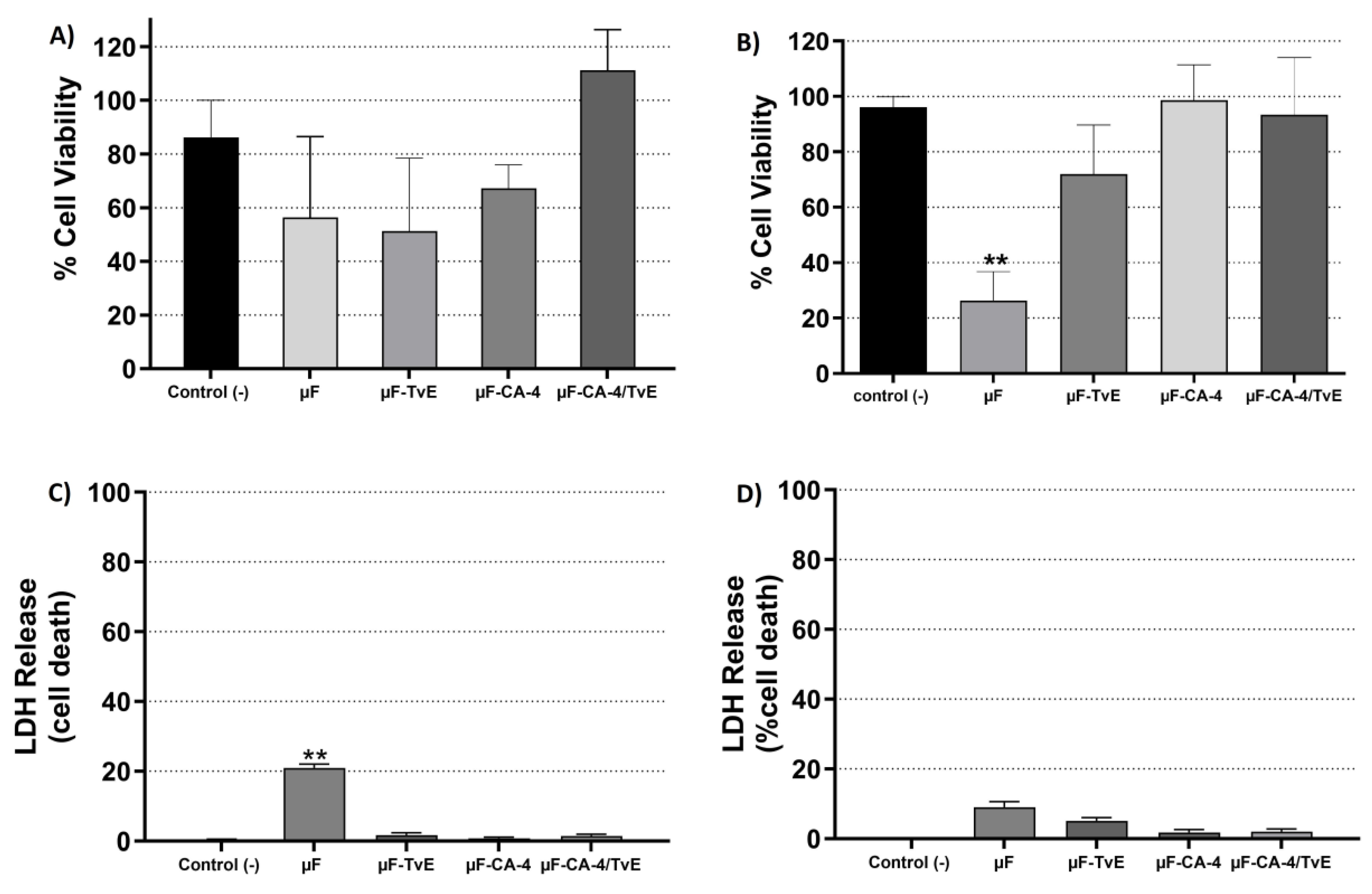
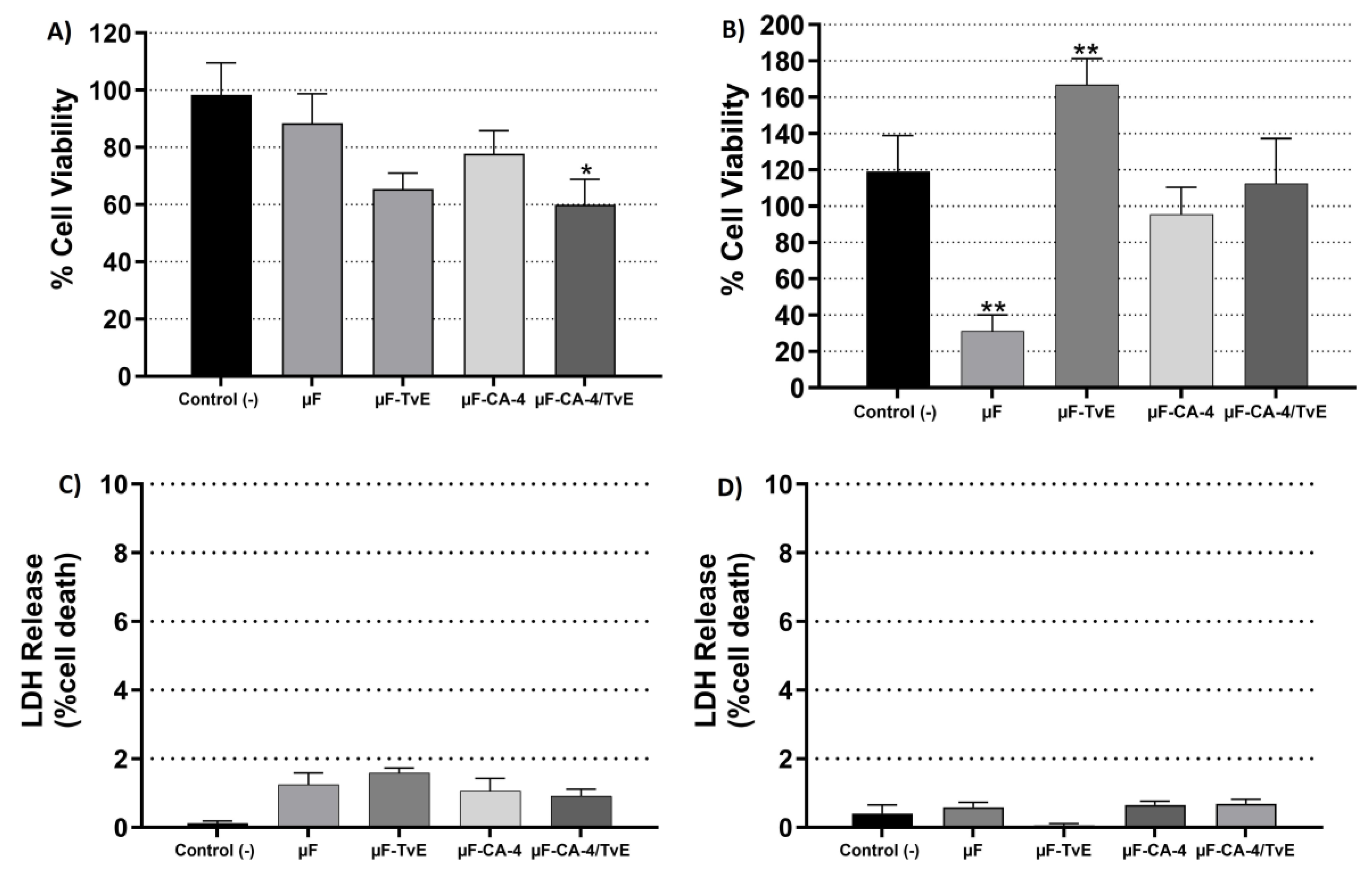
2.4. Cytotoxic Effects of µF-TvE and/or µF-CA on CASMC and HCAEC Cultures
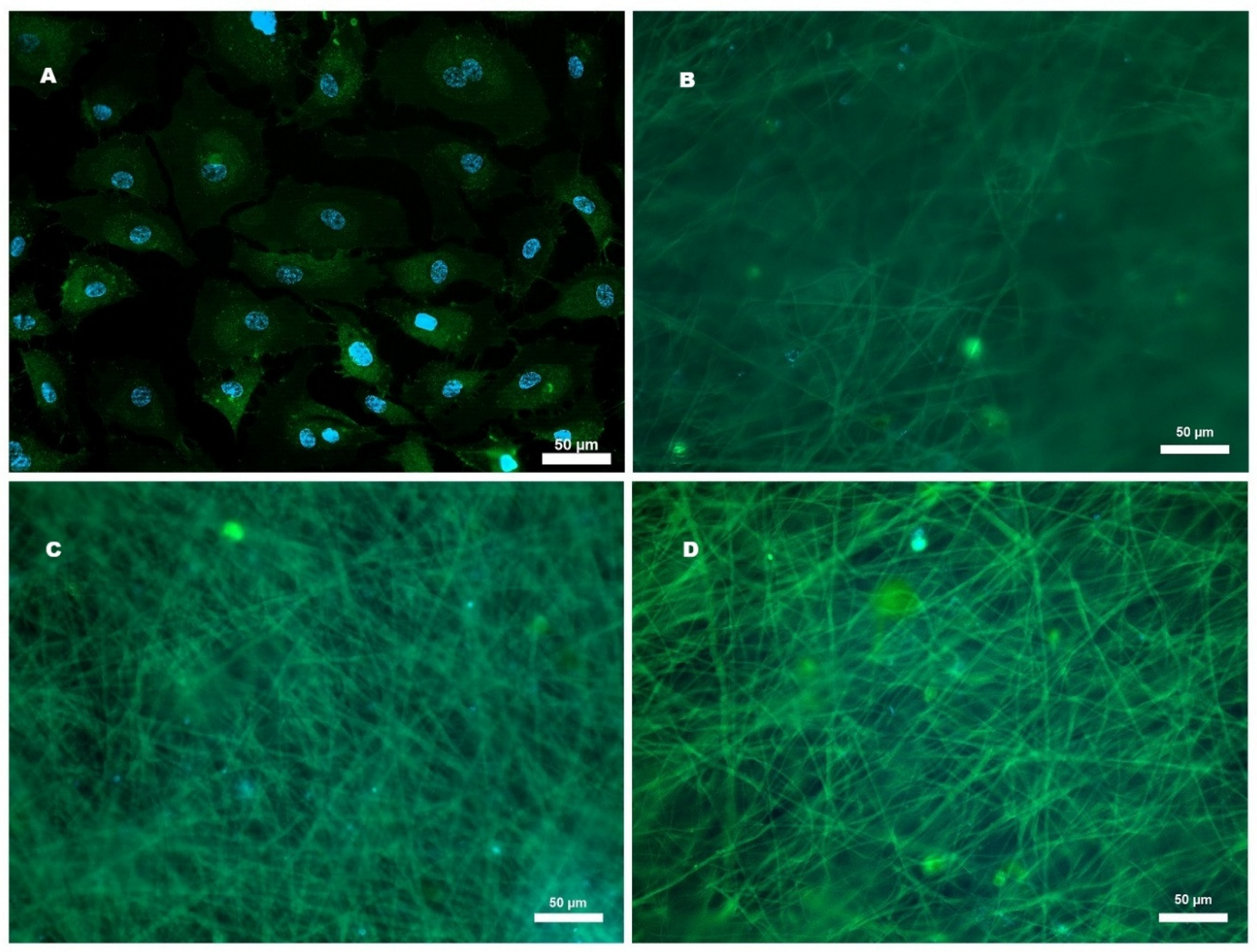
2.5. Cell Adhesion of HCAECs to Microfibers
2.6. Effect of Microfibers on HCAEC Proliferation
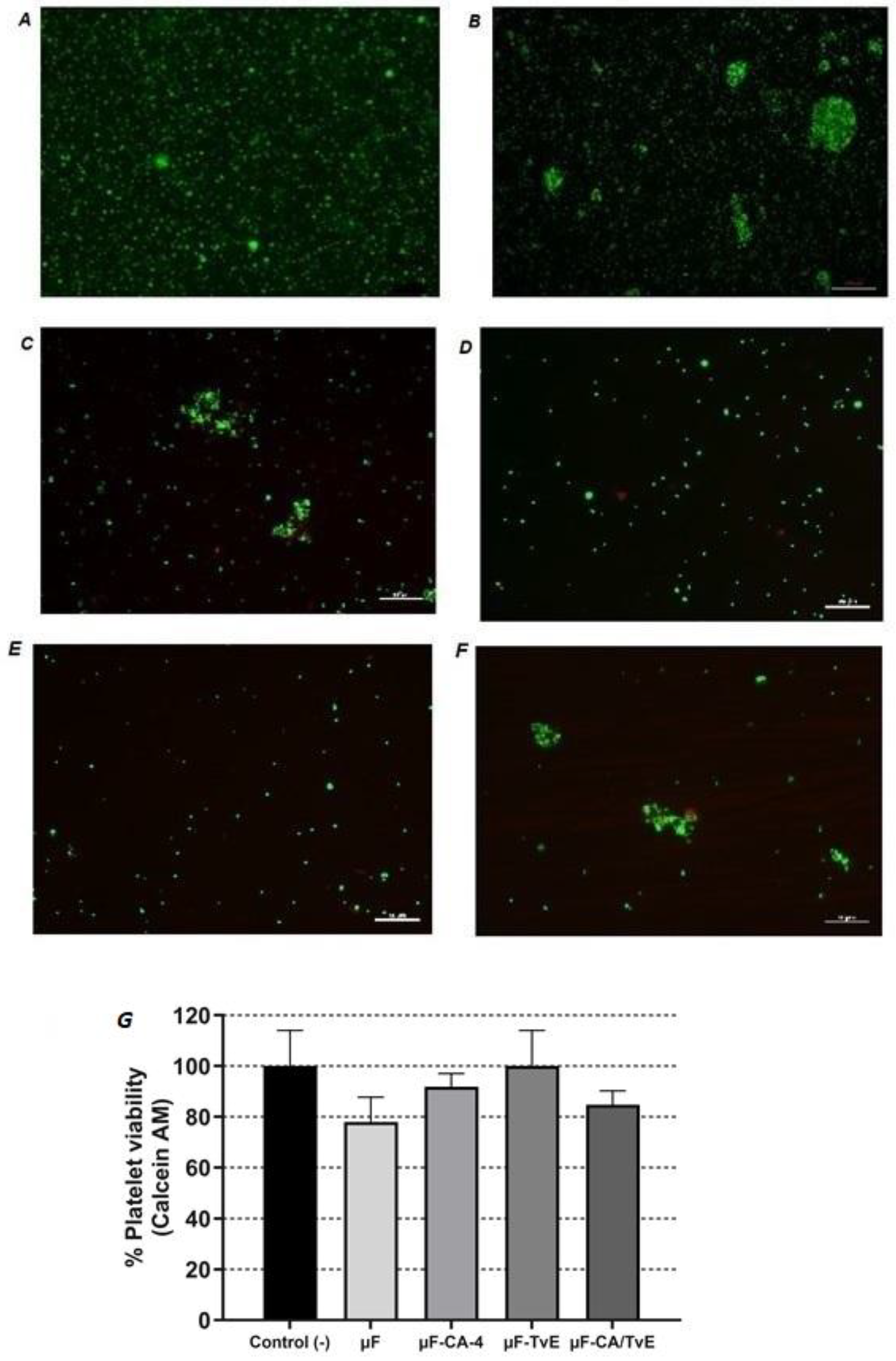
2.7. Effect of Microfibers on Platelet Viability
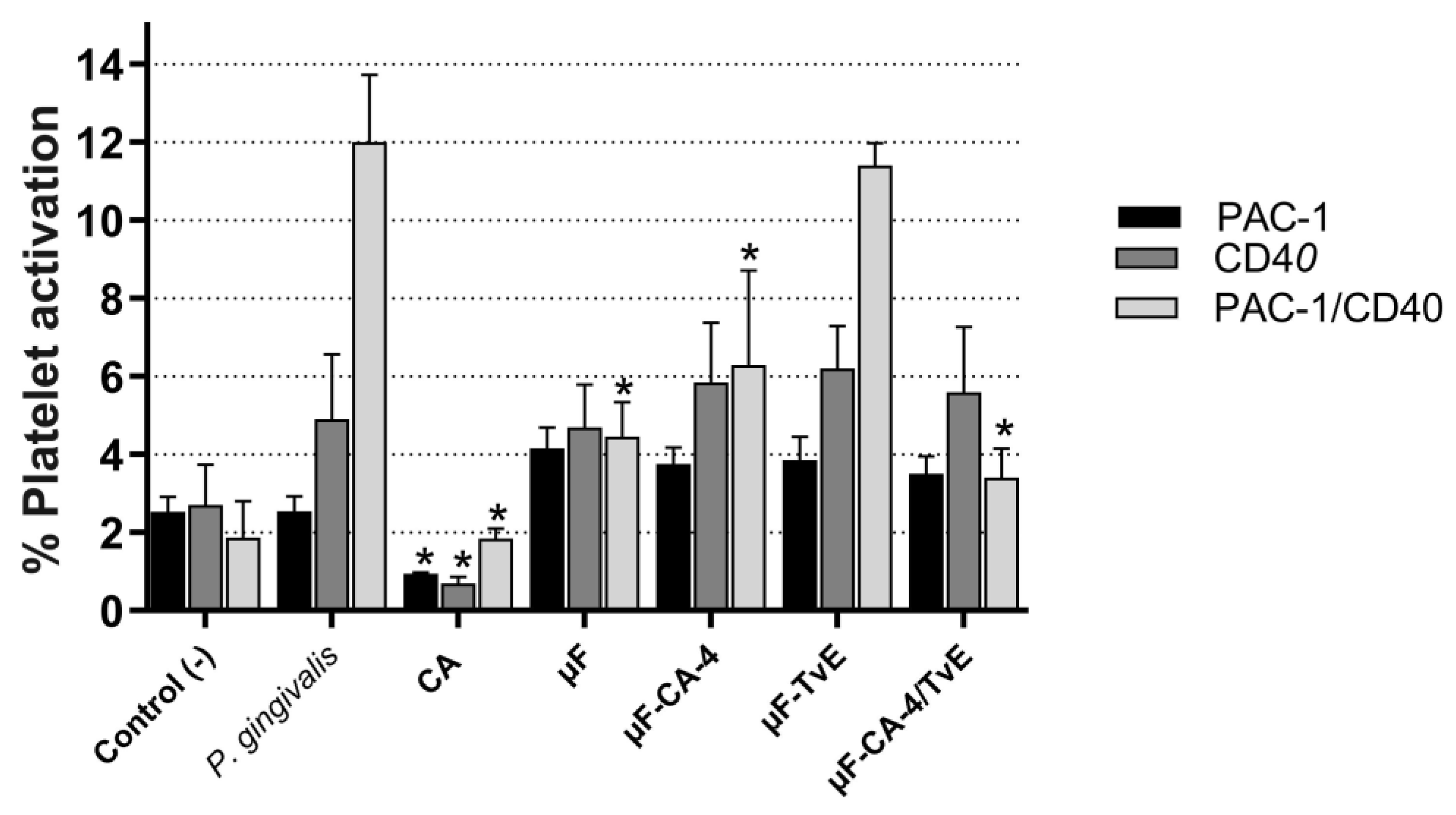
2.8. Inhibition of Platelet Activation Treated with Microfibers
2.9. Effect of Microfibers on Platelet Adhesion
3. Discussion
4. Materials and Methods
4.1. Preparation of Solutions for Producing PCL Fibers and the Process of Electrospinning
4.2. Physicochemical Characterization of Fibers
4.2.1. Scanning Electron Microscopy
4.2.2. Contact Angle Measurement
4.3. Culture of HCAECs and CASMCs
4.4. Stimulation of HCAECs and CASMCs with Microfibers Loaded with TvE and CA
4.5. Cell Viability Assay
4.6. Lactate Dehydrogenase (LDH) Activity Assay
4.7. Adhesion of Endothelial Cells to CA-Loaded PCL Systems
4.8. Cell Proliferation Assay
4.9. Platelet Stimulation Model Using LPS from Porphyromonas Gingivalis W83
4.10. Evaluation of Platelet Viability
4.11. Determination of the Expression of Platelet Adhesion and Activation Molecules
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, J.; Niehaus, A.; Nichols, S.; Lee, D.; Koepsel, J.; Anderson, D.; Lannutti, J. Electrospun PCL in Vitro: A Microstructural Basis for Mechanical Property Changes. J. Biomater. Sci. Polym. Ed. 2009, 20, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Suárez, D.F.; Pinzón-García, A.D.; Sinisterra, R.D.; Dussan, A.; Mesa, F.; Ramírez-Clavijo, S. Uniaxial and Coaxial Nanofibers Pcl/alginate or Pcl/gelatine Transport and Release Tamoxifen and Curcumin Affecting the Viability of MCF7 Cell Line. Nanomaterials 2022, 12, 3348. [Google Scholar] [CrossRef]
- Pinzón-García, A.D.; Cassini-Vieira, P.; Ribeiro, C.C.; De Matos Jensen, C.E.; Barcelos, L.S.; Cortes, M.E.; Sinisterra, R.D. Efficient Cutaneous Wound Healing Using Bixin-loaded PCL Nanofibers in Diabetic Mice. J. Biomed. Mater. Res. 2017, 105, 1938–1949. [Google Scholar] [CrossRef]
- Dias, A.M.; Da Silva, F.G.; Monteiro, A.P.D.F.; Pinzón-García, A.D.; Sinisterra, R.D.; Cortés, M.E. Polycaprolactone Nanofibers Loaded Oxytetracycline Hydrochloride and Zinc Oxide for Treatment of Periodontal Disease. Mater. Sci. Eng. C 2019, 103, 109798. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Adamu, B.F.; Gao, J.; Jhatial, A.K.; Kumelachew, D.M. A Review of Medicinal Plant-based Bioactive Electrospun Nano Fibrous Wound Dressings. Mater. Des. 2021, 209, 109942. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloids Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Son, S.; Lewis, B.A. Free Radical Scavenging and Antioxidative Activity of Caffeic Acid Amide and Ester Analogues: Structure−activity Relationship. J. Agric. Food Chem. 2002, 50, 468–472. [Google Scholar] [CrossRef]
- Narayanan, V.; Alam, M.; Ahmad, N.; Balakrishnan, S.B.; Ganesan, V.; Shanmugasundaram, E.; Rajagopal, B.; Thambusamy, S. Electrospun Poly (vinyl Alcohol) Nanofibers Incorporating Caffeic Acid/cyclodextrins Through the Supramolecular Assembly for Antibacterial Activity. Spectrochim. Acta Part A 2021, 249, 119308. [Google Scholar] [CrossRef]
- Li, P.; Peng, Y.; Ma, Q.; Li, Z.; Zhang, X. Study on the Formation of Antihypertensive Twin Drugs by Caffeic Acid and Ferulic Acid with Telmisartan. Drug Des. Dev. Ther. 2020, 14, 977–992. [Google Scholar] [CrossRef]
- Sun, R.; Wu, T.; Xing, S.; Wei, S.; Bielicki, J.K.; Pan, X.; Zhou, M.; Chen, J. Caffeic Acid Protects Against Atherosclerotic Lesions and Cognitive Decline in Apoe−/− Mice. J. Pharmacol. Sci. 2023, 151, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.S.; Nam, K.-S.; Park, H.-J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Q.; Liu, Y.-Y.; Sun, K.; Fan, J.-Y.; Wang, C.-S.; Han, J.-Y. Inhibitory Effect of Caffeic Acid on Adp-induced Thrombus Formation and Platelet Activation Involves Mitogen-activated Protein Kinases. Sci. Rep. 2015, 5, 13824. [Google Scholar] [CrossRef]
- Nam, G.S.; Park, H.-J.; Nam, K.-S. The Antithrombotic Effect of Caffeic Acid Is Associated with a Camp-dependent Pathway and Clot Retraction in Human Platelets. Thromb. Res. 2020, 195, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Trametes Versicolor (synn. Coriolus Versicolor) Polysaccharides in Cancer Therapy: Targets and Efficacy. Biomedicines 2020, 8, 135. [Google Scholar] [CrossRef]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal Mushrooms as an Attractive New Source of Natural Compounds for Future Cancer Therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef]
- Jędrzejewski, T.; Sobocińska, J.; Pawlikowska, M.; Dzialuk, A.; Wrotek, S. Extract from the Coriolus Versicolor Fungus as an Anti-inflammatory Agent with Cytotoxic Properties Against Endothelial Cells and Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9063. [Google Scholar] [CrossRef]
- Lowenthal, R.; Taylor, M.; Gidden, J.A.; Heflin, B.; Lay, J.O.; Avaritt, N.; Tackett, A.J.; Urbaniak, A. The Mycelium of the Trametes Versicolor Synn. Coriolus Versicolor (turkey Tail Mushroom) Exhibit Anti-melanoma Activity in Vitro. Biomed. Pharmacother. 2023, 161, 114424. [Google Scholar] [CrossRef]
- Bains, A.; Chawla, P. In Vitro Bioactivity, Antimicrobial and Anti-inflammatory Efficacy of Modified Solvent Evaporation Assisted Trametes Versicolor Extract. 3 Biotech. 2020, 10, 404. [Google Scholar] [CrossRef]
- Nikolic, M.; Lazarevic, N.; Novakovic, J.; Jeremic, N.; Jakovljevic, V.; Zivkovic, V.; Bradic, J.; Pecarski, D.; Tel-Çayan, G.; Glamocija, J. Characterization, in Vitro Biological Activity and in Vivo Cardioprotective Properties of Trametes Versicolor (l.:fr.) Quél. Heteropolysaccharides in a Rat Model of Metabolic Syndrome. Pharmaceuticals 2023, 16, 787. [Google Scholar] [CrossRef]
- GBD. Global Burden of 369 Diseases and Injuries in 204 Countries and Territories, 1990-2019: A Systematic Analysis for the Global Burden of Disease Study. Lancet 2019, 396, 1204–1222. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhao, Y.; Chen, Y.; Huang, R. Global and National Burden of Atherosclerosis from 1990 to 2019: Trend Analysis Based on the Global Burden of Disease Study 2019. Chin. Med. J. 2019, 136, 2442–2450. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Chrissobolis, S.; Diep, H.; Chan, C.T.; Ferens, D.; Drummond, G.R.; Sobey, C.G. Advanced Atherosclerosis Is Associated with Inflammation, Vascular Dysfunction and Oxidative Stress, but Not Hypertension. Pharmacol. Res. 2017, 116, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Gualtero, D.F.; Lafaurie, G.I.; Buitrago, D.M.; Castillo, Y.; Vargas-Sanchez, P.K.; Castillo, D.M. Oral Microbiome Mediated Inflammation, a Potential Inductor of Vascular Diseases: A Comprehensive Review. Front. Cardiovasc. Med. 2023, 10, 1250263. [Google Scholar] [CrossRef]
- Otsuka, F.; Yasuda, S.; Noguchi, T.; Ishibashi-Ueda, H. Pathology of Coronary Atherosclerosis and Thrombosis. Cardiovasc. Diagn. Ther. 2016, 6, 396–408. [Google Scholar] [CrossRef]
- Schulz, C.; Massberg, S. Platelets in Atherosclerosis and Thrombosis. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 111–133. [Google Scholar] [CrossRef]
- Guzmán-Oyarzo, D.; Plaza, T.; Recio-Sánchez, G.; Abdalla, D.S.P.; Salazar, L.A.; Hernández-Montelongo, J. Use of Npsi-βcd Composite Microparticles for the Controlled Release of Caffeic Acid and Pinocembrin, Two Main Polyphenolic Compounds Found in a Chilean Propolis. Pharmaceutics 2019, 11, 289. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, W.; Deng, Y.; Gu, N.; Qiu, Z.; Deng, C.; Yang, S.; Pan, L.; Long, S.; Wang, Y. Non-target Lesion Progression: Unveiling Critical Predictors and Outcomes in Patients with In-stent Restenosis. Int. J. Cardiol. 2024, 416, 132451. [Google Scholar] [CrossRef]
- Guo, S.; Bi, C.; Wang, X.; Lv, T.; Zhang, Z.; Chen, X.; Yan, J.; Mao, D.; Huang, W.; Ye, M. Comparative Efficacy of Interventional Therapies and Devices for Coronary In-stent Restenosis: A Systematic Review and Network Meta-analysis of Randomized Controlled Trials. Heliyon 2024, 10, e27521. [Google Scholar] [CrossRef]
- Zong, X.; Kim, K.; Fang, D.; Ran, S.; Hsiao, B.S.; Chu, B. Structure and Process Relationship of Electrospun Bioabsorbable Nanofiber Membranes. Polymer 2020, 43, 4403–4412. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of Atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Migliori, M.; Cantaluppi, V.; Mannari, C.; Bertelli, A.A.E.; Medica, D.; Quercia, A.D.; Navarro, V.; Scatena, A.; Giovannini, L.; Biancone, L. Caffeic Acid, a Phenol Found in White Wine, Modulates Endothelial Nitric Oxide Production and Protects from Oxidative Stress-associated Endothelial Cell Injury. PLoS ONE 2015, 10, e0117530. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.; Lopes, N.M.F. Cardiovascular Effects of Caffeic Acid and Its Derivatives: A Comprehensive Review. Front. Physiol. 2020, 11, 595516. [Google Scholar] [CrossRef] [PubMed]
- Teymoorian, S.K.; Nouri, H.; Moghimi, H. In-vivo and In-vitro Wound Healing and Tissue Repair Effect of Trametes Versicolor Polysaccharide Extract. Sci. Rep. 2024, 14, 3796. [Google Scholar] [CrossRef]
- Interdonato, L.; Impellizzeri, D.; D’amico, R.; Cordaro, M.; Siracusa, R.; D’agostino, M.; Genovese, T.; Gugliandolo, E.; Crupi, R.; Fusco, R. Modulation of Tlr4/nfκb Pathways in Autoimmune Myocarditis. Antioxidants 2023, 12, 1507. [Google Scholar] [CrossRef]
- Han, D.G.; Ahn, C.B.; Lee, J.-H.; Hwang, Y.; Kim, J.H.; Park, K.Y.; Lee, J.W.; Son, K.H. Optimization of Electrospun Poly(caprolactone) Fiber Diameter for Vascular Scaffolds to Maximize Smooth Muscle Cell Infiltration and Phenotype Modulation. Polymers 2019, 11, 643. [Google Scholar] [CrossRef]
- Guarino, V.; Cirillo, V.; Taddei, P.; Alvarez-perez, M.A.; Ambrosio, L. Tuning Size Scale and Crystallinity of PCL Electrospun Fibres via Solvent Permittivity to Address Hmsc Response. Macromol. Biosci. 2011, 11, 1694–1705. [Google Scholar] [CrossRef]
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and Characterization of Pcl/gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228–1235. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Anulekha, K.H.; Girish, C.M.; Prasanth, R.; Nair, S.V.; Jayakumar, R. Single Step Electrospinning of Chitosan/poly(caprolactone) Nanofibers Using Formic Acid/acetone Solvent Mixture. Carbohydr. Polym. 2010, 80, 413–419. [Google Scholar] [CrossRef]
- Madhaiyan, K.; Sridhar, R.; Sundarrajan, S.; Venugopal, J.R.; Ramakrishna, S. Vitamin B12 Loaded Polycaprolactone Nanofibers: A Novel Transdermal Route for the Water Soluble Energy Supplement Delivery. Int. J. Pharm. 2013, 444, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Tiyek, I.; Gunduz, A.; Yalcinkaya, F.; Chaloupek, J. Influence of Electrospinning Parameters on the Hydrophilicity of Electrospun Polycaprolactone Nanofibres. J. Nanosci. Nanotechnol. 2019, 19, 7251–7260. [Google Scholar] [CrossRef]
- Huang, F.L.; Wang, Q.Q.; Wei, Q.F.; Gao, W.D.; Shou, H.Y.; Jiang, S.D. Dynamic Wettability and Contact Angles of Poly(vinylidene Fluoride) Nanofiber Membranes Grafted with Acrylic Acid. Express Polym. Lett. 2010, 4, 551–558. [Google Scholar] [CrossRef]
- Huang, Y. Cytotoxic Effects of Poly(ε-caprolactone) on Vascular Smooth Muscle Cells. Biomaterials 2010, 31, 1913–1921. [Google Scholar]
- Schoolaert, E.; Cossu, L.; Becelaere, J.; Van Guyse, J.F.R.; Tigrine, A.; Vergaelen, M.; Hoogenboom, R.; De Clerck, K. Nanofibers with a Tunable Wettability by Electrospinning and Physical Crosslinking of Poly(2-n-propyl-2-oxazoline). Mater. Des. 2020, 192, 108747. [Google Scholar] [CrossRef]
- Almeida, H. Biodegradation of Polycaprolactone: A Review. Mater. Sci. Eng. C 2015, 54, 97–106. [Google Scholar]
- Kumar, A. Polycaprolactone: A Potential Biodegradable Polymer for Medical Applications. Curr. Drug Deliv. 2012, 9, 193–202. [Google Scholar]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Cytotoxicity of Poly(ε-caprolactone) and Poly(lactic-co-glycolic Acid) in Different Cell Types. Biomaterials 2012, 33, 4660–4672. [Google Scholar]
- Li, X.; Zhang, S.; Zhang, X.; Xie, S.; Zhao, G.; Zhang, L. Biocompatibility and Physicochemical Characteristics of Poly(ɛ-caprolactone)/poly(lactide-co-glycolide)/nano-hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Mater. Des. 2017, 114, 149–160. [Google Scholar] [CrossRef]
- Cao, Y. Cytotoxicity of Caffeic Acid on Human Vascular Endothelial Cells. J. Funct. Foods 2017, 33, 107–114. [Google Scholar]
- Secme, M.; Mutlu, D.; Elmas, L.; Arslan, S. Assessing Effects of Caffeic Acid on Cytotoxicity, Apoptosis, Invasion, GST Enzyme Activity, Oxidant, Antioxidant Status and Micro-rna Expressions in HCT116 Colorectal Cancer Cells. S. Afr. J. Bot. 2023, 157, 19–26. [Google Scholar] [CrossRef]
- Huang, Y. Biocompatibility of Polycaprolactone Nanofibers Loaded with Caffeic Acid on Human Endothelial Cells. Mater. Sci. Eng. C 2018, 91, 317–326. [Google Scholar]
- Bellosta, S.; Selmin, F.; Magri, G.; Castiglioni, S.; Procacci, P.; Sartori, P.; Scarpa, E.; Tolva, V.; Rossi, C.; Puoci, F. Caffeic Acid-grafted PLGA as a Novel Material for the Design of Fluvastatin-eluting Nanoparticles for the Prevention of Neointimal Hyperplasia. Mol. Pharm. 2022, 19, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. The Effects of Caffeic Acid-loaded PCL Nanofibers on Smooth Muscle Cell Proliferation and Viability. Biomater. Sci. 2020, 8, 233–242. [Google Scholar]
- Khan, M.I. Antioxidant and Cytotoxic Activities of Trametes Versicolor Extracts Against Human Cancer Cell Lines. J. Ethnopharmacol. 2019, 242, 112036. [Google Scholar]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar]
- Lau, C.B.; Ho, C.Y.; Kim, C.F.; Leung, K.N.; Fung, K.P.; Tse, T.F.; Chan, H.H.; Chow, M.S. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004, 75, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.C.; Chang, H.C.; Ting, C.T.; Kuo, C.Y.; Yang, V.C. Caffeic Acid Phenethyl Ester Inhibits Proliferation and Migration, and Induces Apoptosis in Platelet-derived Growth Factor-bb-stimulated Human Coronary Smooth Muscle Cells. J. Vasc. Res. 2012, 49, 24–32. [Google Scholar] [CrossRef]
- Mcnicol, A.; Israels, S.J. Mechanisms of Oral Bacteria-induced Platelet Activation. Can. J. Physiol. Pharmacol. 2010, 88, 510–524. [Google Scholar] [CrossRef]
- Mcnicol, A.; Zhu, R.; Pesun, R.; Pampolina, C.; Jackson, E.C.; Bowden, G.H.W.; Zelinski, T. A Role for Immunoglobulin G in Donor-specific Streptococcus Sanguis-induced Platelet Aggregation. Thromb. Haemost. 2006, 95, 288–293. [Google Scholar] [CrossRef]
- Jin, C.; Chen, D.; Zhu, T.; Chen, S.; Du, J.; Zhang, H.; Dong, W. Poly(ferulic Acid)-hybrid Nanofibers for Reducing Thrombosis and Restraining Intimal Hyperplasia in Vascular Tissue Engineering. Biomater. Adv. 2023, 146, 213278. [Google Scholar] [CrossRef]
- Kamiyama, M.; Horiuchi, M.; Umano, K.; Kondo, K.; Otsuka, Y.; Shibamoto, T. Antioxidant/anti-inflammatory Activities and Chemical Composition of Extracts from the Mushroom Trametes Versicolor. Int. J. Nutr. Food Sci. 2013, 2, 85. [Google Scholar] [CrossRef]
- Nguyen, T.M.H.; Le, H.L.; Ha, T.T.; Bui, B.H.; Le, N.T.; Nguyen, V.H.; Nguyen, T.V.A. Inhibitory Effect on Human Platelet Aggregation and Coagulation and Antioxidant Activity of C. Edulis Ker Gawl Rhizome and Its Secondary Metabolites. J. Ethnopharmacol. 2020, 263, 113136. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]

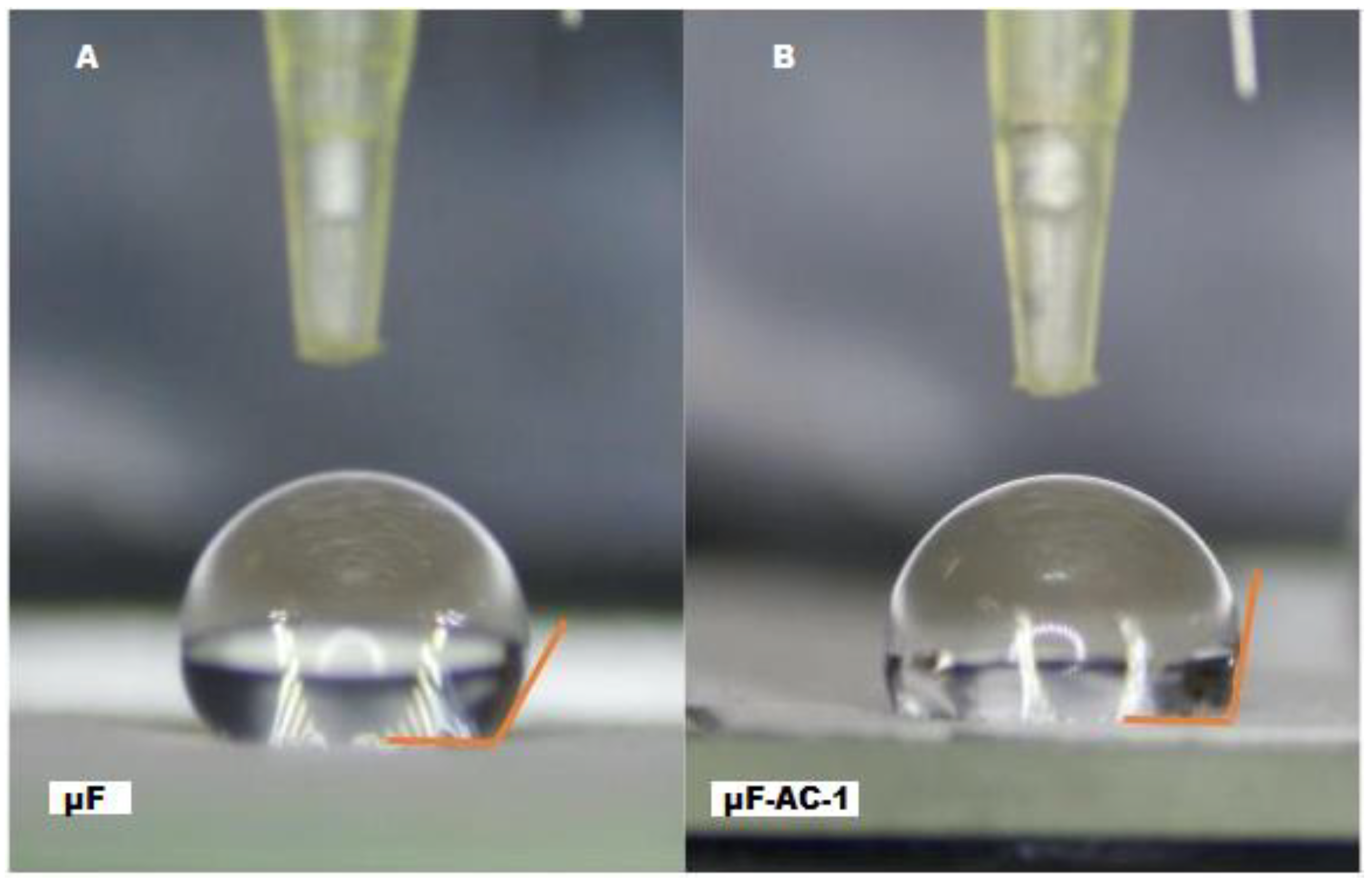
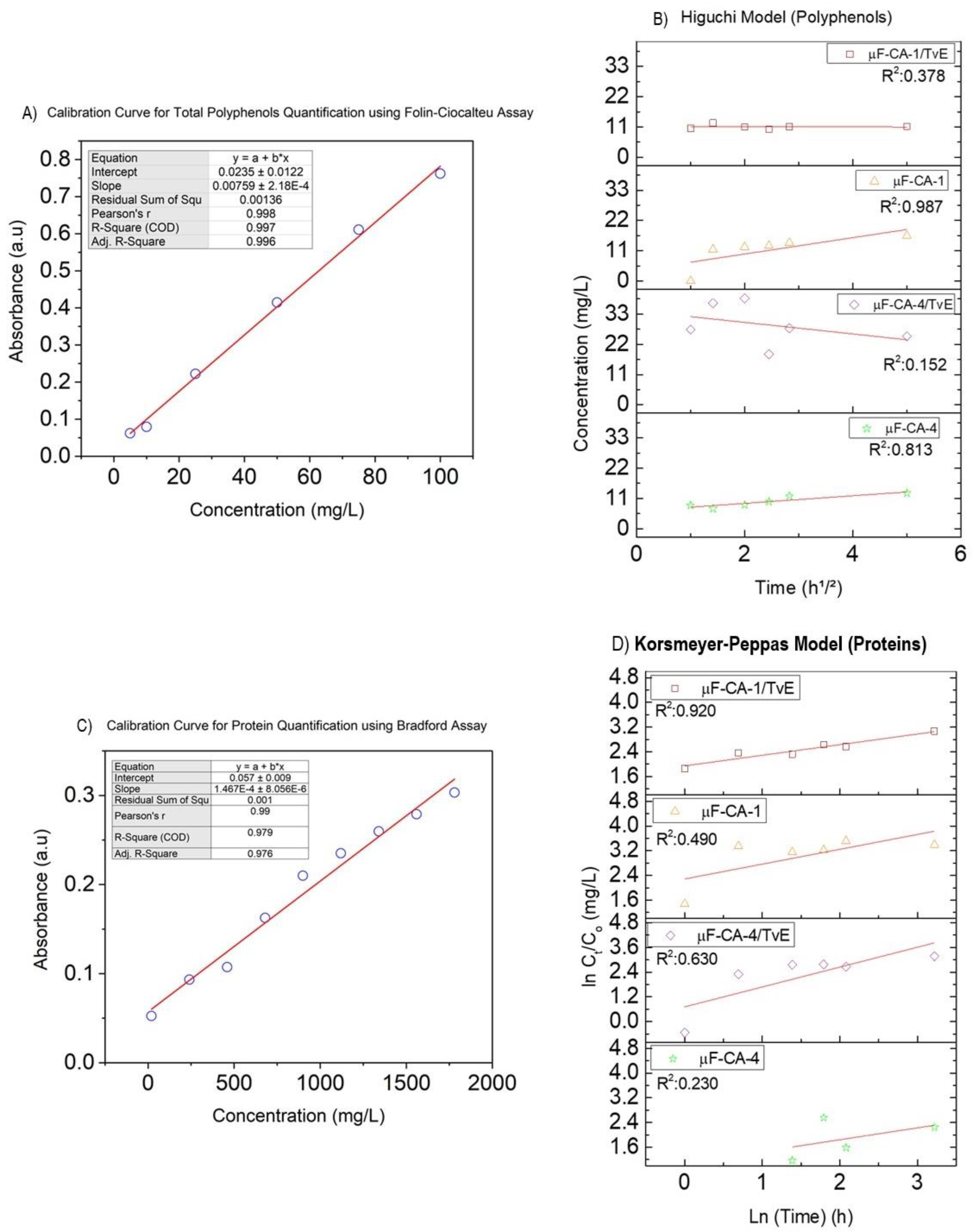

| System | Fiber Diameter Media ± S.D (µm) | D (min) (µm) | D (max) (µm) | Contact Angle |
|---|---|---|---|---|
| µF | 2.50 ± 1.12 | 0.48 | 4.60 | 105.5° ± 3.25° (<90°) |
| µF-CA-1 | 1.96 ± 0.80 | 0.50 | 2.10 | 78.32° ± 2.84° (<90°) |
| µF-TvE | 2.41 ± 1.53 | 0.95 | 4.21 | not determined |
| µF-CA-1/TvE | 2.03 ± 1.05 | 0.75 | 3.25 | not determined |
| Treatment | PAC-1 (%) | CD40 (%) | PAC-1/CD40 (%) |
|---|---|---|---|
| Control (−) | 2.52 ± 0.025 | 2.70 ± 1.3 | 1.87 ± 1.0 |
| P. gingivalis LPS | 2.55 ± 1.5 | 4.90 ± 0.8 | 12 ± 0.4 |
| Caffeic acid | 0.95 ± 1.8 * | 0.70 ± 2.2 * | 1.85 ± 0.5 * |
| μF | 4.15 ± 1.3 | 4.70 ± 2.9 | 4.45 ± 0.9 |
| μF-CA-4 | 3.75 ± 0.8 | 5.85 ± 2.4 | 6.30 ± 1.4 * |
| μF-TvE | 3.85 ±2.2 | 6.2 ± 3.8 | 11.4 ± 2.2 |
| μF-CA-4/TvE | 3.5 ± 1.6 | 5.6 ± 0.5 | 3.4 ± 2.0 * |
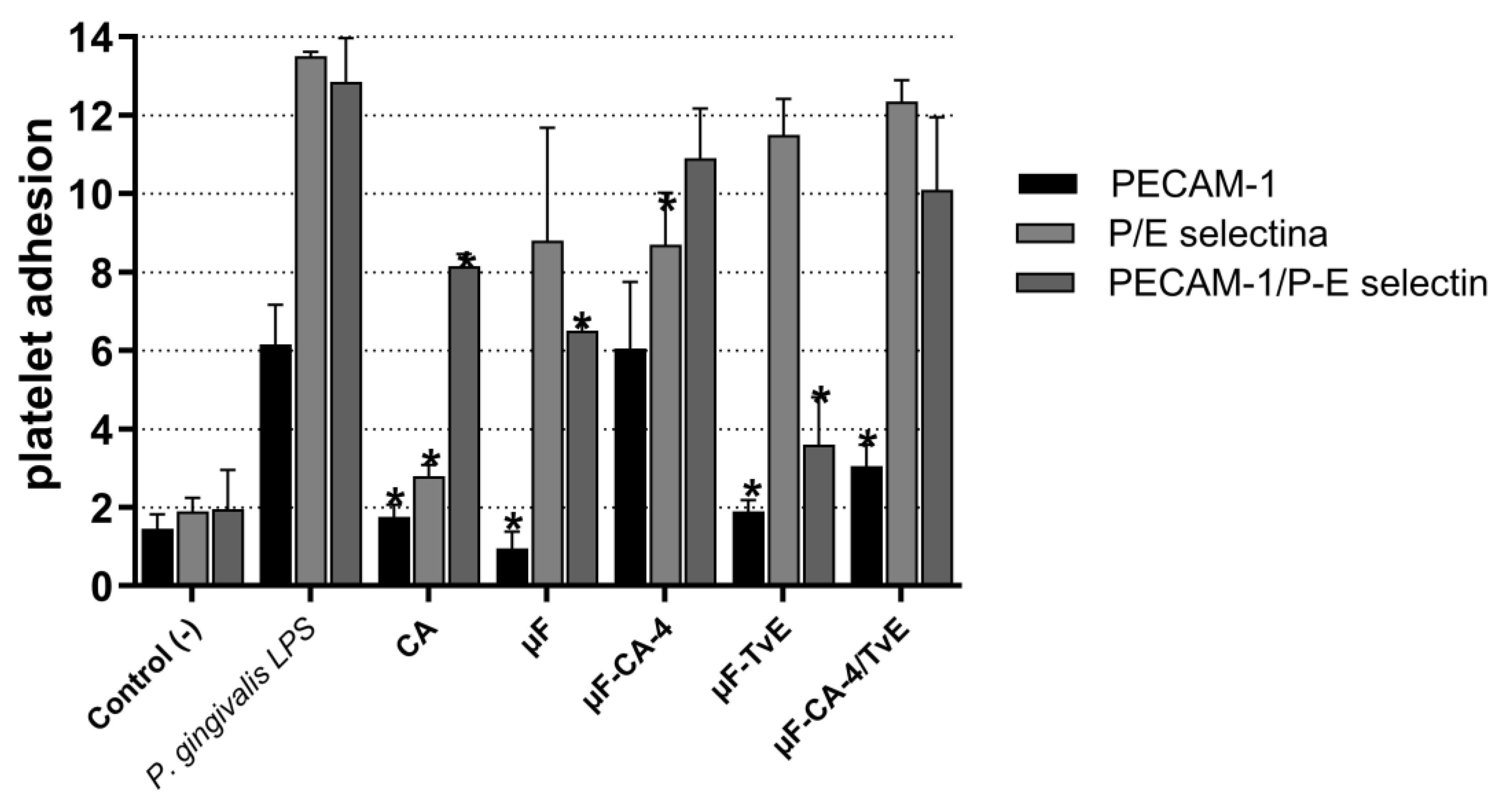
| Treatment | PECAM-1 (%) | P/E Selectina (%) | PECAM-1/P-E Selectina (%) |
|---|---|---|---|
| Control (+) | 1.45 ± 0.3 | 1.90 ± 0.15 | 1.95 ± 0.55 |
| P. gingivalis LPS | 6.15 ± 2.4 | 13.5 ± 2.3 | 12.8 ± 2.0 |
| Caffeic acid | 1.75 ± 0.15 * | 2.80 ± 2.1 * | 8.15 ± 2.5 * |
| μF | 0.95 ± 0.3 * | 8.8 ± 3.4 | 6.5 ± 1.1 * |
| μF-CA-4 | 6.0 ± 0.25 | 8.75 ± 0.25 * | 10.9 ± 1.4 |
| μF-TvE | 1.70 ± 0.3 * | 11.5 ± 4.4 | 3.60 ± 4.4 * |
| μF-CA-4/TvE | 3.0 ± 2.2 * | 12.3 ± 0.9 | 10.0 ± 0.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gualtero, D.F.; Buitrago, D.M.; Pinzón-García, A.D.; Cely Veloza, W.F.; Figueroa-Ariza, L.T.; Torres-Morales, S.; Rodriguez-Navarrete, J.D.; Jimenez, V.J.; Lafaurie, G.I. Biopolymers of Polycaprolactone Loaded with Caffeic Acid and Trametes versicolor Extract Induced Proliferation in Human Coronary Artery Endothelial Cells and Inhibited Platelet Activity. Int. J. Mol. Sci. 2025, 26, 4949. https://doi.org/10.3390/ijms26104949
Gualtero DF, Buitrago DM, Pinzón-García AD, Cely Veloza WF, Figueroa-Ariza LT, Torres-Morales S, Rodriguez-Navarrete JD, Jimenez VJ, Lafaurie GI. Biopolymers of Polycaprolactone Loaded with Caffeic Acid and Trametes versicolor Extract Induced Proliferation in Human Coronary Artery Endothelial Cells and Inhibited Platelet Activity. International Journal of Molecular Sciences. 2025; 26(10):4949. https://doi.org/10.3390/ijms26104949
Chicago/Turabian StyleGualtero, Diego Fernando, Diana Marcela Buitrago, Ana Delia Pinzón-García, Willy Fernando Cely Veloza, Leydy Tatiana Figueroa-Ariza, Santiago Torres-Morales, Juan David Rodriguez-Navarrete, Victor Junior Jimenez, and Gloria Inés Lafaurie. 2025. "Biopolymers of Polycaprolactone Loaded with Caffeic Acid and Trametes versicolor Extract Induced Proliferation in Human Coronary Artery Endothelial Cells and Inhibited Platelet Activity" International Journal of Molecular Sciences 26, no. 10: 4949. https://doi.org/10.3390/ijms26104949
APA StyleGualtero, D. F., Buitrago, D. M., Pinzón-García, A. D., Cely Veloza, W. F., Figueroa-Ariza, L. T., Torres-Morales, S., Rodriguez-Navarrete, J. D., Jimenez, V. J., & Lafaurie, G. I. (2025). Biopolymers of Polycaprolactone Loaded with Caffeic Acid and Trametes versicolor Extract Induced Proliferation in Human Coronary Artery Endothelial Cells and Inhibited Platelet Activity. International Journal of Molecular Sciences, 26(10), 4949. https://doi.org/10.3390/ijms26104949








