1. Introduction
Mucopolysaccharidoses (MPSs) are a group of inherited lysosomal storage disorders caused by deficiencies in enzymes required to degrade glycosaminoglycans (GAGs), which are integral to connective tissue. The inability to break down GAGs results in accumulation in multiple tissues and organs, leading to systemic dysfunction. Depending on the specific enzyme deficiency, MPSs are classified into several subtypes [
1]. In some instances, a phenomenon known as pseudodeficiency can potentially complicate diagnosis and prognosis, highlighting the importance of biomarkers that accurately reflect the stage and prognosis of the disease [
2].
Among these, MPS IVA (Morquio A syndrome) is characterized by a deficiency in the N-acetylgalactosamine-6-sulfate sulfatase (GALNS) enzyme, which leads to the accumulation of keratan sulfate (KS) and chondroitin-6-sulfate (C6S) [
3]. MPS IVA is a rare genetic disorder that causes growth impairment and skeletal abnormalities with an estimated incidence of 0.11 to 1.32 per 100,000 live births, depending on the region [
4,
5]. The resultant skeletal dysplasia is primarily driven by the accumulation of GAGs (KS and C6S), which disrupts the function of chondrocytes in the growth plates, impairs cartilage matrix development, and inhibits bone matrix mineralization [
6]. The severity of bone and cartilage damage differs by the severity of the phenotype (attenuated or severe) and the type of bone affected [
7]. Bone growth by endochondral ossification is affected more severely than other types of MPS. Thus, it is a type of skeletal dysplasia affecting bone and cartilage growth. The hallmark feature of MPS IVA is progressive and characterized by incomplete or defective endochondral ossification and a successive imbalance of growth [
3,
8,
9,
10]. The skeletal-related symptoms of MPS IVA include prominent forehead, abnormal face with a large mandible, disproportionate short-trunk dwarfism with short neck, cervical spine instability with odontoid hypoplasia, cervical spinal cord compression, pectus carinatum, tracheal deviation and obstruction, restrictive lung, flaring of the rib cage, kyphoscoliosis, platyspondyly, hip dysplasia with coxa valga, genu valgum, hypermobile joints, waddling gait, and pes planus. The degree of imbalance in growth between bone and other organs and tissues contributes to the unique skeletal dysplasia and clinical severity, including the narrowing of the trachea. Respiratory failure has been shown as the primary cause of death in patients (63%), followed by cardiac failure (11%), post-traumatic organ failure (11%), complications of surgery (11%), and myocardial infarction (4%) [
11].
Identifying therapeutic surrogate biomarkers for patients with MPS remains a significant challenge. Characterization of GAGs in urine, blood, and cerebrospinal fluid has been performed for several decades. The previous studies indicated no correlation between urine and serum GAG levels in MPS II and MPS IVA patients [
12]. We found that blood and urine KS levels follow different age-dependent patterns and sulfation levels [
13,
14]. Another report in MPS I suggested that the oligosaccharide storage pattern (the ratio of heparan sulfate to dermatan sulfate) in urine reflects the storage in the kidney, which is different from the storage pattern in serum, liver, and brain [
15]. These findings suggest that urinary GAG is derived primarily from GAGs stored or reabsorbed in the kidney. Some urinary GAG may be derived from small GAG fragments (i.e., oligosaccharides rather than a large saccharide chain) that filter through the kidneys from the circulation. Thus, blood GAGs would more closely reflect GAGs stored in the whole body and may be a more informative indicator of systemic disease burden than urinary GAGs.
In MPS IVA, blood and urinary KS levels correlate to some extent with clinical severity in the early and progressive stages of the disease, before the growth plate is destroyed, since KS synthesis decreases rapidly after the teenage years, and KS levels in MPS IVA patients are naturally normalized or subnormal by the age of 20 years [
8,
13,
14,
16,
17,
18,
19]. Therefore, it can only be useful as a biomarker before the teenage years [
8,
20]. In addition, even before the teenage years, there was a lack of reliable biomarkers that correlate closely with skeletal symptoms, as described below.
Blood KS in MPS IVA directly indicates growth and/or repair of the cartilage, where it is mainly synthesized [
20,
21]. Most blood KS is derived from chondrocytes and their ECM; therefore, its reduction would be a more direct indicator of bone improvement. However, there is an overlap in blood KS levels between patients and age-matched controls [
22,
23]. On the other hand, urinary KS can distinguish more MPS IVA patients from age-matched controls than blood KS; however, there was no correlation between urinary KS reduction and clinical improvement of skeletal lesions during enzyme replacement therapy (ERT) [
24,
25]. After starting ERT, most patients showed a decrease in urinary KS levels because the enzyme is delivered to the kidney and digests KS stored in the kidney [
26], but a concomitant decrease in blood KS levels has not yet been reported because urinary KS does not reflect blood KS coming directly from bone and cartilage, as explained above. Thus, urinary KS is functional in demonstrating the pharmacodynamic effects of therapy, but it does not provide a valuable surrogate biomarker of skeletal or clinical improvement during these therapies for MPS IVA [
26]. In summary, blood KS reflects overall skeletal symptoms more accurately than urinary KS, particularly in patients undergoing ERT. However, it should be noted that blood KS is less sensitive than urinary KS when diagnosing patients. Conversely, urinary KS presents a challenge in effectively monitoring therapeutic response in patients receiving ERT.
Due to the current limitations described above, developing novel biomarkers remains an unmet challenge, prompting us to explore alternative biomarkers that can more accurately reflect the skeletal pathology of MPS IVA. In this study, we investigated N-terminal pro-C-type natriuretic peptide (NT-proCNP), collagen type I, and collagen type II as candidate biomarkers in human blood because of their importance in the skeletal system, particularly in the epiphyseal plate, connective tissues, and cartilage, respectively. Additionally, we separately evaluated two types of KS, mono-sulfated KS (Galβ1 → 4GlcNAc(6S)) and di-sulfated KS (Galβ1(6S) → 4GlcNAc(6S)), to elucidate their respective significance.
In addition to its role in the cardiovascular and neurological systems [
27,
28], C-type natriuretic peptide (CNP) plays a critical role in skeletal development [
29]. CNP is synthesized in various tissues, including cartilage [
30,
31,
32,
33,
34,
35], and acts through the natriuretic peptide receptor B (NPR-B) on chondrocytes to activate the receptor’s guanylyl cyclase domain, increasing intracellular cGMP levels [
36]. Elevated cGMP then activates protein kinase G (PKG), which phosphorylates various proteins controlling gene expression and metabolic processes. This phosphorylation influences transcription factors and other regulators, promoting chondrocyte maturation, extracellular matrix production, and balanced cell proliferation [
36,
37]. Through these coordinated intracellular events, CNP ensures the proper progression of chondrocyte differentiation and healthy growth plate development. As a result, the skeletal structure can expand and mature, underscoring CNP’s critical role in maintaining the equilibrium between chondrocyte growth and its progression to a fully differentiated state [
36,
38,
39]. These processes are crucial for endochondral ossification in the growth plates, facilitating longitudinal bone growth. NT-proCNP, consisting of 50 amino acids, is the biologically inactive cleavage product of proCNP [
40,
41] and is secreted in equimolar amounts with CNP [
42]. Based on the higher concentration of NT-proCNP in blood than that of CNP [
43,
44,
45,
46], NT-proCNP is thought to have a longer half-life than CNP in circulation, reflecting the systemic expression level of CNP better than CNP itself.
Collagen type I is the most abundant collagen in the human body, forming a critical structural component of bone, skin, tendons, and other connective tissues [
47,
48,
49]. It provides the scaffold for bone mineralization and contributes to the strength and rigidity of the skeletal system [
50,
51,
52]. Interestingly, studies have shown that the expression of mRNA encoding collagen type I increased in chondrocytes derived from MPS IVA patients [
53,
54].
Collagen type II, on the other hand, is predominantly found in cartilage [
55] and serves as a marker for chondrocyte activity [
56,
57], especially in the proliferative zone in growth plates [
58]. It is essential for maintaining the tensile strength and structural integrity of cartilage, including the growth plate [
55,
59]. As a critical component of the extracellular matrix, collagen type II supports the mechanical properties of cartilage and facilitates chondrocyte differentiation and matrix organization [
60].
In this study, we focused on these three biomarkers (NT-proCNP, collagen types I and II) to elucidate their relationship with the skeletal manifestations of MPS IVA. By investigating these biomarkers with GAGs (C6S and KS) levels and genotyping, we aimed to identify a novel biomarker that can more accurately predict clinical severity, disease prognosis, and therapeutic efficacy relevant to improvements in bone lesions. Our findings have provided valuable insights into the pathophysiology of MPS IVA and informed the development of potential surrogate biomarkers.
3. Discussion
In this study, we measured several biomarkers in 60 MPS IVA patients enrolled in the Morquio A natural history program (clinicaltrial.gov: NCT05284006) to evaluate the significance of each biomarker concerning growth failure. Fifty-one patients (85%) received ERT, and two (3.3%) received HSCT. Two (3.3%) pediatric patients had not yet started ERT because they were newly diagnosed. Six (10%) adult patients had never received ERT despite its approval in the United States. Judging from their heights, ERT did not show apparent effects for skeletal dysplasia, including bone growth (
Figure 1,
Table 8), as indicated by the previous reports [
62,
63,
64,
65,
66,
67]. However, it should be noted that we could not statistically compare with and without ERT in pediatric patients due to the limited number of pediatric patients without ERT, which is a limitation of our research. We identified four novel mutations through genetic testing: one was a deletion resulting in a frameshift, another was a mutation at a splice donor site, and the remaining two were missense mutations predicted to be disease-causing by two bioinformatics tools. In 10 patients, we found only one mutation on one allele. We sequenced only the exons and exon-intron boundaries of the
GALNS gene and did not search for large deletions, thereby limiting our genetic testing.
CNP is significantly involved in both vascular homeostasis and bone growth, which is mainly controlled by CNP secreted from vascular endothelial cells [
68] and chondrocytes in growth plates [
35], respectively. It plays a key role in endochondral ossification, crucial for forming and growing long bones [
29]. CNP primarily affects bone growth by signaling through the chondrocyte receptor NPR-B, encoded by the
NPR2 gene, which induces cell division and differentiation [
38].
Recent research indicated that CNP and NT-proCNP levels are significantly elevated in several skeletal dysplasias (achondroplasia, hypochondroplasia, thanatophoric dysplasia, and Maroteaux type of acromesomelic dysplasia) caused by mutations in the
FGFR3 (fibroblast growth factor receptor 3) or
NPR2 (natriuretic peptide receptor 2) gene [
69]. These genes are closely related to each other and to extracellular CNP, playing a crucial role in cell proliferation and differentiation. Substantial progress has been made in recent years in the study of CNP and their role in skeletal disorders, particularly achondroplasia, which is caused by a gain-of-function mutation in
FGFR3 that results in constitutive activation of FGFR3 and the downstream MEK/ERK MAPK pathway [
70,
71]. The MEK/ERK MAPK pathway is a signaling pathway that regulates various cellular processes, including cell growth, proliferation, and differentiation [
72,
73,
74]. Elevated levels of CNP and NT-proCNP in achondroplasia are thought to be a natural response of the human body to this overactivation of the MEK/ERK MAPK pathway in chondrocytes, which functionally inhibits the effect of CNP on chondrocytes via NPR-B encoded by
NPR2 [
69,
75]. In this paper, we have identified the elevation of NT-proCNP in MPS IVA patients. The gene responsible for MPS IVA is
GALNS, which is not closely related to
FGFR3 or
NPR2. Therefore, this is the first report to identify the elevation of NT-proCNP in patients with a disease unrelated to mutations in the
FGFR3 or
NPR2 genes.
Recent research has not only identified NT-proCNP as a potential biomarker but also highlighted the therapeutic potential of CNP. The development of drugs such as Vosoritide, a CNP analog approved to induce bone growth in achondroplasia [
70,
71,
76], underscores the potential of CNP as a therapeutic agent against the growth impairment of MPS. This potential has already been demonstrated in MPS IVA and VII mouse models [
77,
78]. Furthermore, a Phase I/II clinical trial of the CNP analog in MPS IVA and VI patients has been initiated to explore further these therapeutic possibilities (ClinicalTrials.gov ID: NCT05845749) and inform the scientific community about the latest developments in MPS research.
NT-proCNP is a peptide fragment derived from the precursor protein of CNP. The
NPPC (natriuretic peptide precursor C) gene expresses a precursor protein known as preproCNP. PreproCNP undergoes proteolytic cleavage to remove the signal peptide and to form proCNP [
42]. Furin, one of the proprotein convertases, cleaves NT-proCNP from proCNP in the trans-Golgi network to produce the active CNP [
40,
41,
79,
80,
81]. After the cleavage of proCNP, active CNP is secreted into the extracellular space via exocytosis [
42]. For NT-proCNP, we found no clear mechanism of how it is secreted by cells; however, these processes should occur primarily in neurons, glial cells [
30,
31,
32], vascular endothelial cells [
33], and chondrocytes in cartilage [
34,
35]. The half-life of CNP in the blood is short, about 2–3 min, as NPRs take up CNP, and such a short half-life helps to adapt to frequent changes in circulatory status [
41,
43,
82]. In the case of NT-proCNP, there is no clear pathway to remove it. As a result, the normal concentration ranges of these two molecules in healthy children are significantly different: CNP is 0.5~3 pmol/L, and NT-proCNP is 15~60 pmol/L (74.8~299 pg/mL) [
45]. This difference makes NT-proCNP easier to detect and quantify than CNP. Considering that bone growth is a slower and more gradual phenomenon than the adaptation of the circulatory system, NT-proCNP is expected to be a better biomarker than CNP for correlating human skeletal symptoms in MPS. This leads us to select NT-proCNP as a potential surrogate biomarker for future studies.
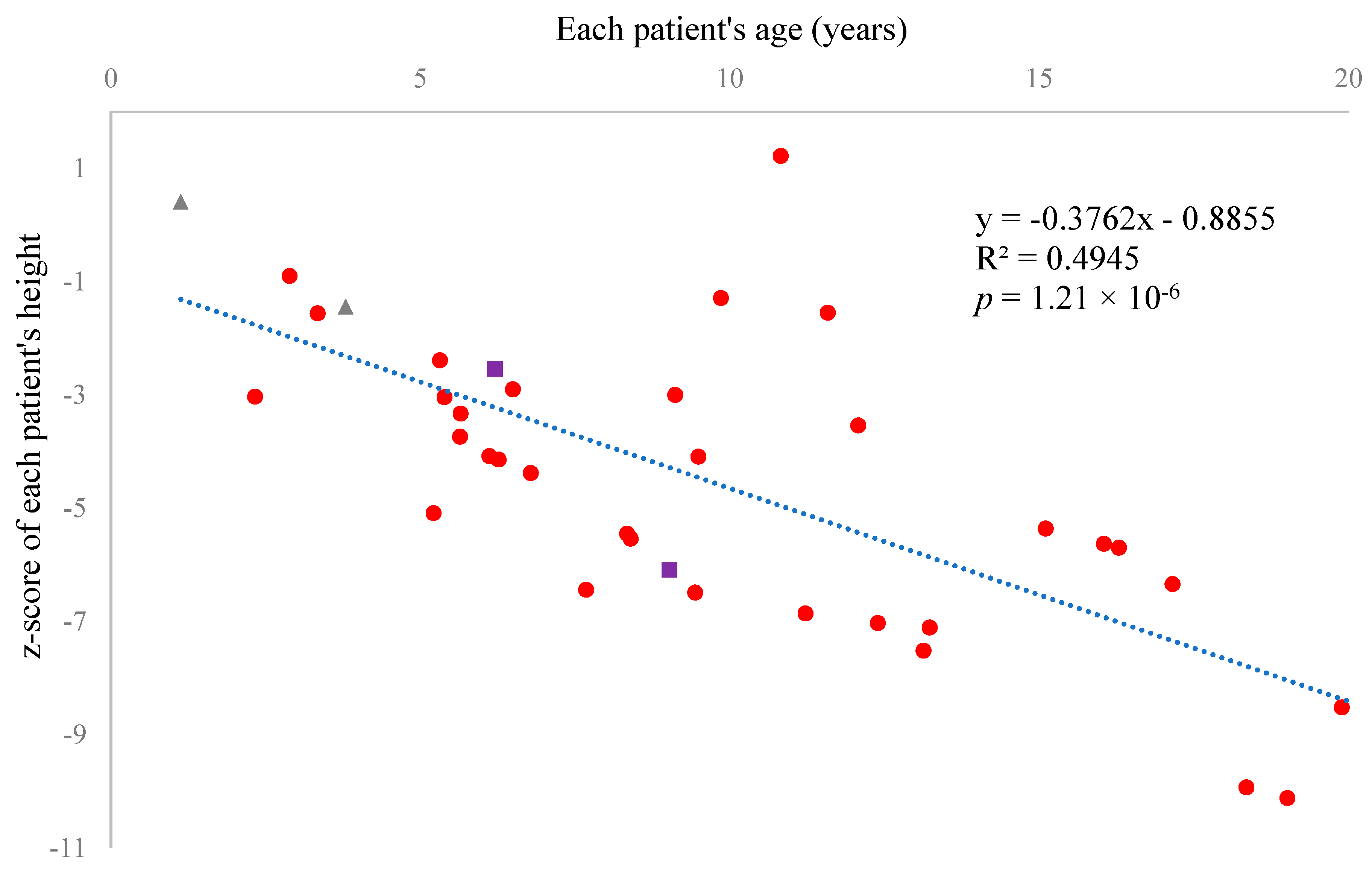
Our data demonstrated that NT-proCNP was significantly elevated in the plasma of MPS IVA patients in all comparable age groups. In addition, the concentrations in patients negatively correlated with height z-score, the most reliable indicator of skeletal growth failure [
83,
84]. The source of this elevated NT-proCNP (presumably, CNP as well) remains unknown. The primary sources of these peptides are neurons, glial cells [
30,
31,
32], vascular endothelial cells [
33], and chondrocytes in cartilage [
34,
35], but the exact percentage of production at each site under normal conditions remains uncertain. The CNP production by growth plate chondrocytes is the most critical factor for bone growth, as evidenced by the growth impairment observed in mice where the
Nppc gene has been selectively knocked out in chondrocytes [
35]. Even when plasma CNP levels were significantly reduced by knocking out the
Nppc gene selectively in mouse vascular endothelial cells, Moyes et al. did not observe any growth failure [
68].
Based on previous and current results, we propose potential hypotheses (factors) contributing to plasma NT-proCNP elevation in MPS IVA patients.
The expression of the
NPPC gene is upregulated in MPS IVA patients to compensate for growth impairment or tissue resistance to CNP, as seen in achondroplasia, hypochondroplasia, and thanatophoric dysplasia [
69]. A feedback mechanism in patients with growth failure contributes to the higher expression of CNP. This feedback mechanism could involve vascular endothelial cells as a source of excessive CNP and NT-proCNP secretion.
CNP and NT-proCNP are leaking from damaged chondrocytes in MPS IVA patients. The secretion or leakage of NT-proCNP from damaged chondrocytes in the growth plates can enter the bloodstream, increasing its concentration in the blood.
NT-proCNP clearance is delayed due to a systemic disorder of MPS IVA.
A combination of multiple factors may account for the elevation of NT-proCNP. With age, the contribution factor could vary. Further research is needed to investigate the regulation of CNP expression (at both mRNA and protein levels) and clarify this paradoxical phenomenon.
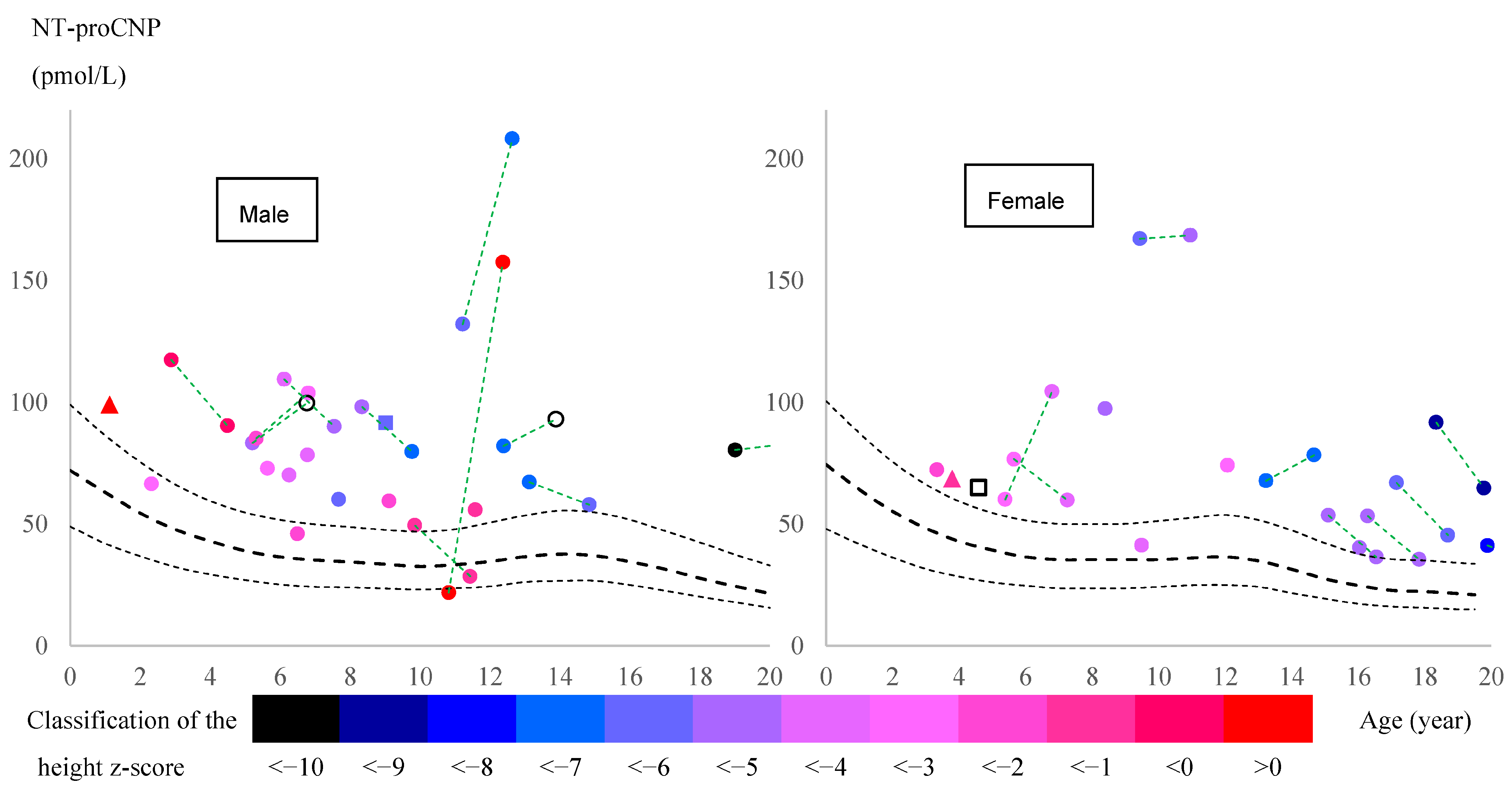
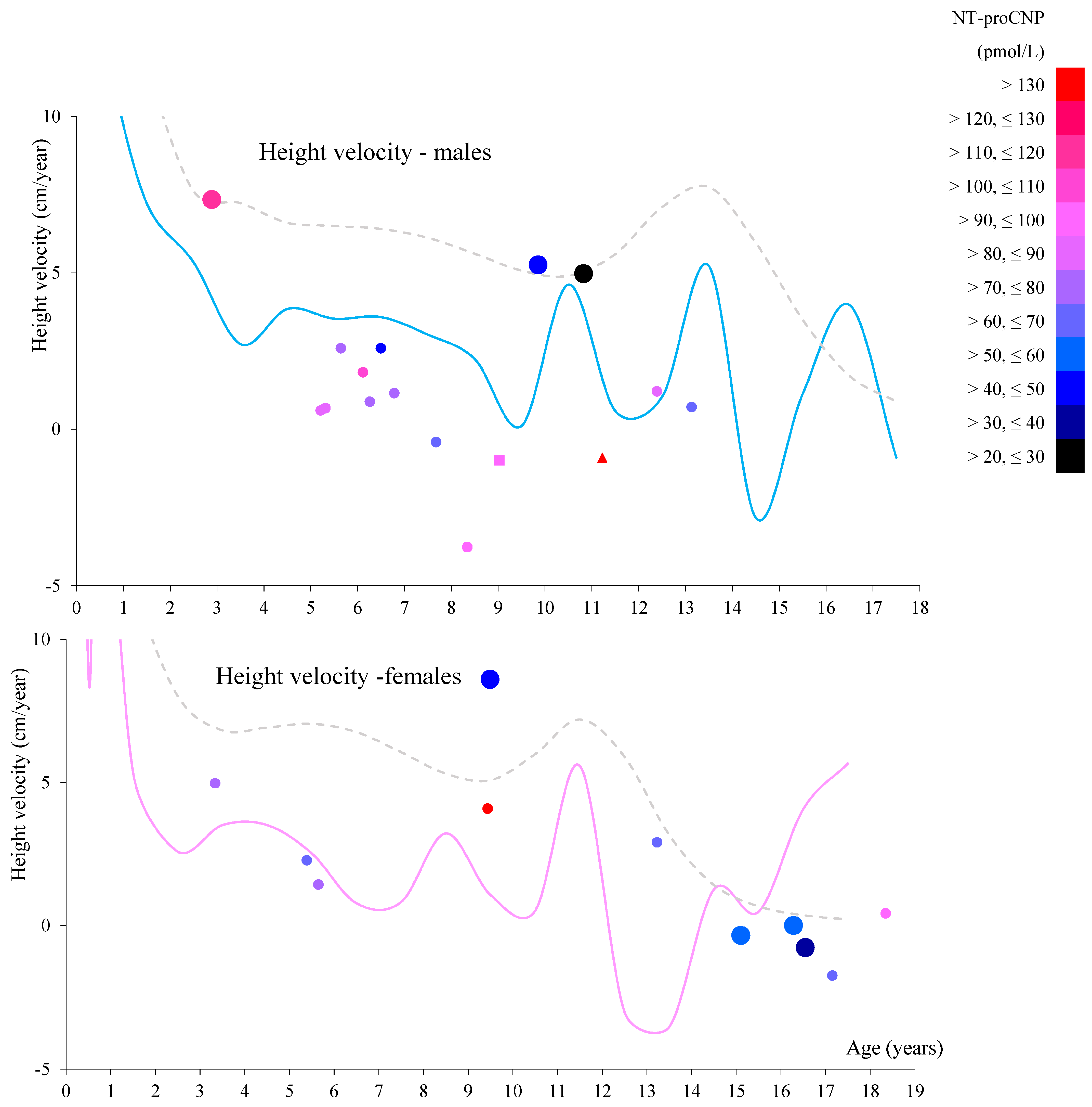
Focusing on patients who had two data points at different ages prior to peak pubertal growth spurt (all of whom had received ERT during these two periods), NT-proCNP levels increased in 5 of 10 boys and 2 of 3 girls (
Figure 2) while urinary mono-sulfated KS was reduced in 9 out of 10 patients, as shown in
Table 8. This finding indicates that plasma NT-proCNP is not a pharmacodynamic biomarker like urinary KS prior to the onset of pubertal growth spurt. Therefore, we hypothesize that reduced NT-proCNP can be more directly linked to improving bone growth than reducing total urinary KS. Further studies will confirm whether NT-proCNP is an excellent surrogate biomarker that correlates with clinical improvement in the skeletal system.
Collagen type I is the most abundant collagen in the human body. It is a fibrous protein that forms a significant part of the extracellular matrix in various tissues, including skin, tendons, ligaments, and, importantly, bone [
47,
48,
49]. It provides a scaffold for bone mineralization and forms a fibrous network that serves as the structural framework for bone tissue [
50,
51,
52]. This network supports the deposition of hydroxyapatite crystals, primarily composed of calcium and phosphate [
85,
86]. The interaction between collagen fibers and these crystals gives bones strength and rigidity. Procollagen Type I N-Terminal Propeptide (P1NP) and C-Terminal Telopeptide of Type I Collagen (CTX-I) are two major peptides well studied in several diseases related to collagen type I metabolism. P1NP reflects collagen synthesis [
87], while CTX-I reflects collagen degradation [
88]. Elevated levels of these peptides in human blood or urine are associated with diseases or conditions related to increased bone turnover (such as Paget’s disease [
89,
90], osteoporosis [
91], or hyperparathyroidism [
92,
93]), fibrotic processes (such as liver fibrosis and cirrhosis [
94,
95]), or cancer (bone metastases [
96,
97] or multiple myeloma [
98]).
Several papers reported that the expression level of mRNA encoding collagen type I increased in chondrocytes from human MPS IVA patients [
53,
54]. Comprehensive proteomic analysis of 6-week-old mouse femurs also showed that collagen alpha-1 and 2 (Uniprot IDs: P11087 and Q01149) increased in MPS IVA [
99]. Therefore, we predicted that patients would present higher concentration levels than the control group. However, only adult patients showed such differences. Other age groups showed lower concentrations in patients. Since human collagen type I has a molecular weight of approximately 300 kDa, much larger than NT-proCNP, surrounded by hydroxyapatite in bone [
85,
86,
100], it could be difficult for collagen type I to escape from bone and cartilage and enter the bloodstream compared to NT-proCNP, thereby preventing higher concentrations in the blood of patients. We should have measured smaller peptides related to collagen type I, as in other diseases described above.
Collagen type II is a specific type of collagen found predominantly in cartilage [
55] and is a marker for chondrocytes [
56,
57]. It is also present in the vitreous humor of the eye and in the nucleus pulposus of intervertebral discs [
101]. Like other collagens, type II collagen is a fibrous protein that forms a triple-helical structure, providing tensile strength and structural integrity to tissues [
59]. Its primary role in forming and maintaining cartilage makes it crucial for joint health, enabling smooth and pain-free movement [
102,
103]. Additionally, its presence on the intervertebral discs and the vitreous body of the eye underscores its importance in maintaining flexibility and structural support in these tissues. Blood procollagen II C-terminal propeptide (CPII) and/or urinary C-terminal telopeptide of type II collagen (CTX-II) are currently the two main biomarkers of type II collagen metabolism in osteoarthritis [
104,
105,
106], joint injuries [
107], and Kashin–Beck disease (KBD) [
108]. CPII reflects the synthesis of type II collagen during cartilage repair and remodeling [
109,
110,
111], while CTX-II is a marker of type II collagen breakdown, reflecting cartilage catabolism [
106,
112].
De Franceschi et al. and Dvorak-Ewell et al. reported decreased expression of mRNA encoding collagen type II in chondrocytes from human MPS IVA patients, in contrast to collagen type I [
53,
54]. The same phenomenon was also observed in mouse models of MPS type I and type VII [
113,
114]. These findings suggest that the decrease in collagen type II may be a potential biomarker for MPS types I, IVA, and VII. In contrast, proteomic analysis of 6-week-old mice revealed a statistically significant increase in collagen alpha-1(II) chain (Uniprot ID: P28481) in the MPS IVA model [
99]. This discrepancy in collagen type II expression levels between humans and mice warrants further investigation. Our results showed a statistically significant increase in patients aged 5 to 10 years and over 20 years. This seems to support the results of the latter proteomic analysis [
99]. However, due to the poor correlation between the measured concentration in serum and plasma, as shown in
Figure S4, it is too early to draw conclusions for patients aged 5 to 10 years.
We also calculated the correlation coefficient between collagen type II and NT-proCNP, controlling for age, to evaluate the effect of excessive CNP expression on chondrocytes in patients. NT-proCNP concentration would indicate the total expression level of CNP in a subject’s body. Collagen type II is a marker of chondrocytes [
56,
57]. Therefore, elevated NT-proCNP levels could correlate positively with collagen type II levels. However, we found no correlation (
r = 0.042,
p = 0.69), suggesting that the increased CNP secretion in patients is insufficient to compensate for the damage to chondrocytes in growth plates.
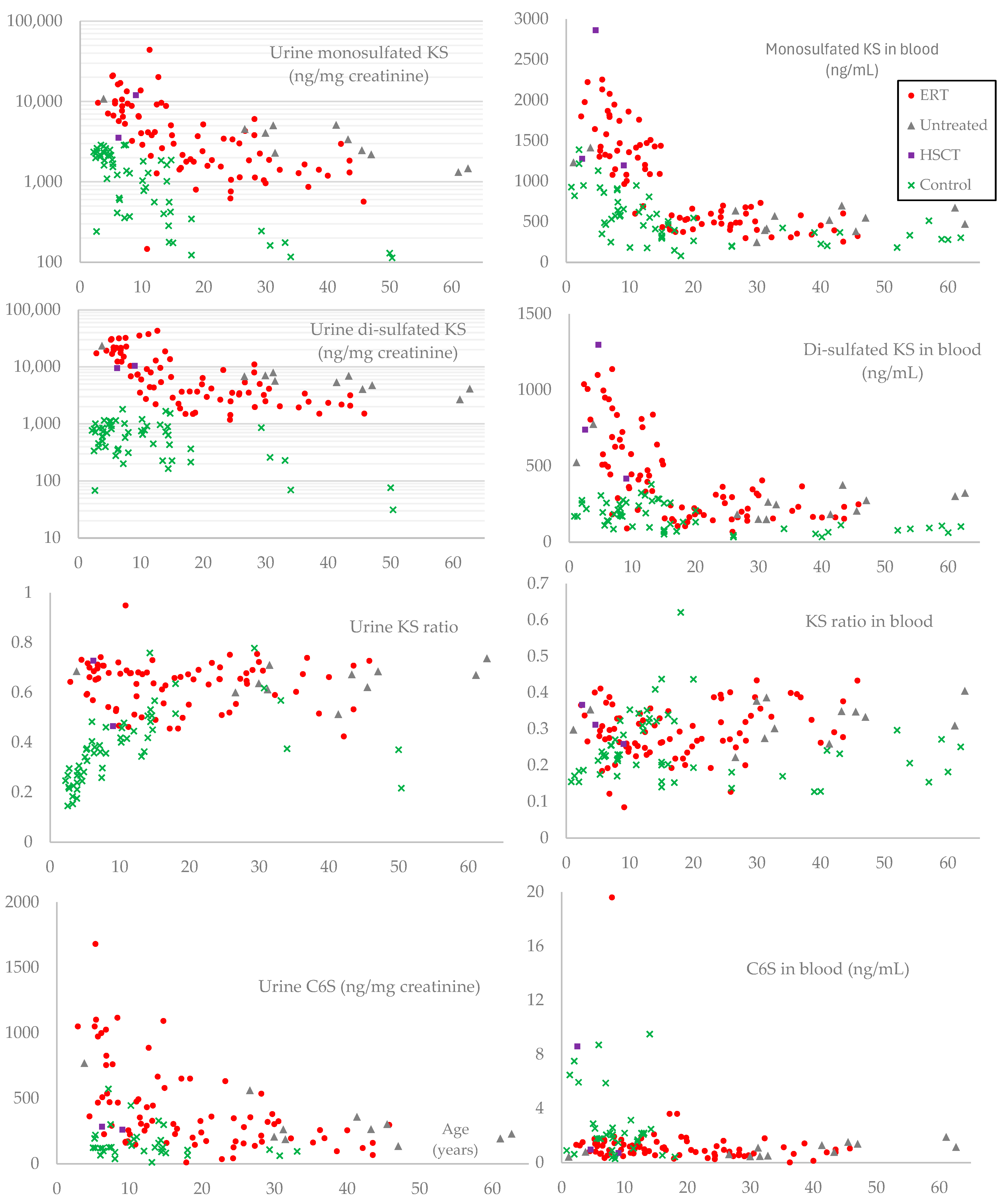
GAG levels are well-established biomarkers for MPS, and the difference between plasma and serum is negligible, making our comparison between controls and patients reasonable [
115]. Our results were consistent with the previous reports [
22,
116]. However, to our knowledge, we could not find any prior report that quantitatively correlates GAG levels with the severity of skeletal symptoms. Therefore, we correlated each GAG with the z-score of height, one of the quantitative indicators of growth failure. Since each GAG level except for plasma C6S tended to decrease with age up to 20 years (
Figure 4), we calculated partial correlation coefficients controlling for age, then found a moderate negative correlation in urinary mono- and di-sulfated KS (
Table 6). These two biomarkers also showed statistical differences between adult patients with and without ERT (
Table 7), making urinary KS a more important prognostic biomarker than C6S in MPS IVA.
We established the KS assay by LC-MS/MS, measuring both mono- and di-sulfated KS in age-matched controls and MPS IVA patients [
13,
14,
18,
115]. Our previous studies identified differences in age and sulfation between urine and blood KS in MPS IVA and healthy controls, suggesting the importance of measuring both types of KS in MPS IVA [
8,
14]. Urinary KS is considered a pharmacodynamic biomarker that does not accurately reflect the therapeutic effect on skeletal symptoms in patients receiving ERT [
26]. Hendriksz et al. presumably measured both KSs (mono-sulfated and di-sulfated) and calculated the total KS [
117]. In this study, we observed the transition of these two types of KS during ERT or after HSCT in the most critical age group for treatment of skeletal growth, namely, those under 10 years old. Interestingly, a decrease in urinary di-sulfated KS was observed in 5 out of 10 patients, whereas 9 out of 10 patients showed a decrease in mono-sulfated KS (
Table 8). Based on this result, we assume that urinary di-sulfated KS may not be a pharmacodynamic biomarker like mono-sulfated KS. Furthermore, blood di-sulfated KS showed more pronounced differences from controls in most age groups (
Figure 4). Further investigations are needed to clarify the significance of di-sulfated KS.
4. Materials and Methods
Subjects: MPS IVA patients receiving care at Nemours Children’s Health in Delaware and participating in the natural history program (Non-invasive Functional Assessment and Pathogenesis of Morquio A, clinicaltrial.gov: NCT05284006, NIH funding number: 1R01HD102545-01A1) were enrolled. The diagnosis of MPS IVA was confirmed by deficient enzyme activity of <5% of normal activity measured in plasma, leukocytes, or fibroblasts before enrollment in this clinical trial. Before any study procedures, written informed consent was obtained from all participants. A total of 60 participants formalized their consent for the research study. The z-score of each patient’s height during sample collection was calculated based on the CDC Growth Chart [
84]. It was also categorized into percentiles based on the growth chart constructed from the accumulated data of Morquio A patients without ERT or HSCT (hematopoietic stem cell transplantation) [
7]. If a patient had two or more height measurements taken at different times, the height growth rate (annual height velocity) was calculated using two data points.
Genotyping: Genomic DNA was extracted from the white blood cells
and/or skin fibroblasts. The 14 exons of the
GALNS gene were amplified by polymerase chain reaction (PCR) and sequenced using the Sanger method to identify pathogenic mutations. PCR conditions and primer pairs used are summarized in
Table S3. Mutations were annotated based on the reference sequence NM_000512.5.
Control Specimens: Serum or urine samples from individuals without skeletal dysplasia at Shimane University Hospital (Japan) were provided as control samples for this research. The age ranged from 0 days to 18 years for serum and from 5 to 33 years for urine. Additionally, blood samples were collected from 7 adults without skeletal dysplasia working in our laboratory, and both plasma and serum were tested to confirm the correlation between serum and plasma for collagen type I and II levels. Written informed consent was obtained from all individuals (IRB# 750932).
ELISA-based method for the measurement of collagen type I, II, and NT-proCNP: The Human Collagen Type I (COL1) ELISA Kit (Cat# EKU03297-96T, BIOMATIK, Kitchener, Canada), the Human Collagen Type II, Col II ELISA Kit (Cat# EKC40379, BIOMATIK), and the NT-proCNP ELISA kit (Cat# BI-20812, BIOMEDICA, Vienna, Austria) were utilized to measure the concentration of each biomarker in patient plasma and serum, control serum from children, and control plasma and serum from adults according to the manufacturer’s instructions. Each kit used a quantitative sandwich enzyme immunoassay technique. The absorbance of each well was measured using FLUOstar Omega (BMG LABTECH, Ortenberg, Germany).
Glycosaminoglycans (GAGs) Analysis by LC-MS/MS: Mono-sulfated KS, di-sulfated KS, and C6S in the plasma and urine samples from patients were measured according to our established method [
22,
118]. Briefly, 10 μL of each serum, plasma, urine sample, or standard was mixed with 90 μL of 50 mM Tris-hydrochloric acid buffer (pH 7.0) in wells of AcroPrep™Advance 96 Well Filter Plates equipped with ultrafiltration Omega10K membrane filters (PALL Corporation, Port Washington, NY, USA). A cocktail of 30 μL of recombinant chondroitinase ABC and keratanase II (each enzyme at 1 mU/sample) and internal standard solution (5 μg/mL) was added to each well, followed by the addition of 70 μL of 50 mM Tris-hydrochloric acid buffer. After overnight incubation at 37 °C, the filter plate was centrifuged for 20 min at 2500×
g. The filtered samples were injected into our 1290 Infinity liquid chromatography system with a 6460 triple quad mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Levels of GAGs in urine samples were normalized to creatinine, which was measured with a Creatinine (urinary) Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI, USA). The serum and urine of control subjects were also measured in the same way.
Effect of ERT on Each Biomarker: We compared the difference in each biomarker between patients treated with ERT and patients who had not been treated with ERT. Since most patients in the latter group were between 25 and 50 years old, we used only this age range.
Transitions in skeletal growth and biomarkers in pediatric patients younger than 12 years with more than two hospital visits: To explore a correlation between significant biomarkers, skeletal development, and treatment (ERT or HSCT) in growing pediatric patients, we summarized the data from patients who provided their first specimen before the age of 10 years in
Table 8.
Statistical Analysis: For urinary mono- and di-sulfated KS, the Mann–Whitney U test was used to determine statistical differences between subjects and controls. For other biomarkers, Welch’s
t-test was performed. Both tests were calculated with GraphPad Prism version 10.2.3. R-4.4.3 for Windows was used to calculate correlation coefficients or partial correlation coefficients, controlling for age, to reveal the relationship between each factor [
119,
120]. To evaluate correlations involving urinary mono- or di-sulfated KS, the Spearman correlation coefficient was calculated. For correlations not involving these two biomarkers, the Pearson correlation coefficient was used.