Noise-Induced Hearing Loss: Overview and Future Prospects for Research on Oxidative Stress
Abstract
1. Overview of Noise-Induced Hearing Loss
2. Clinical Features of NIHL
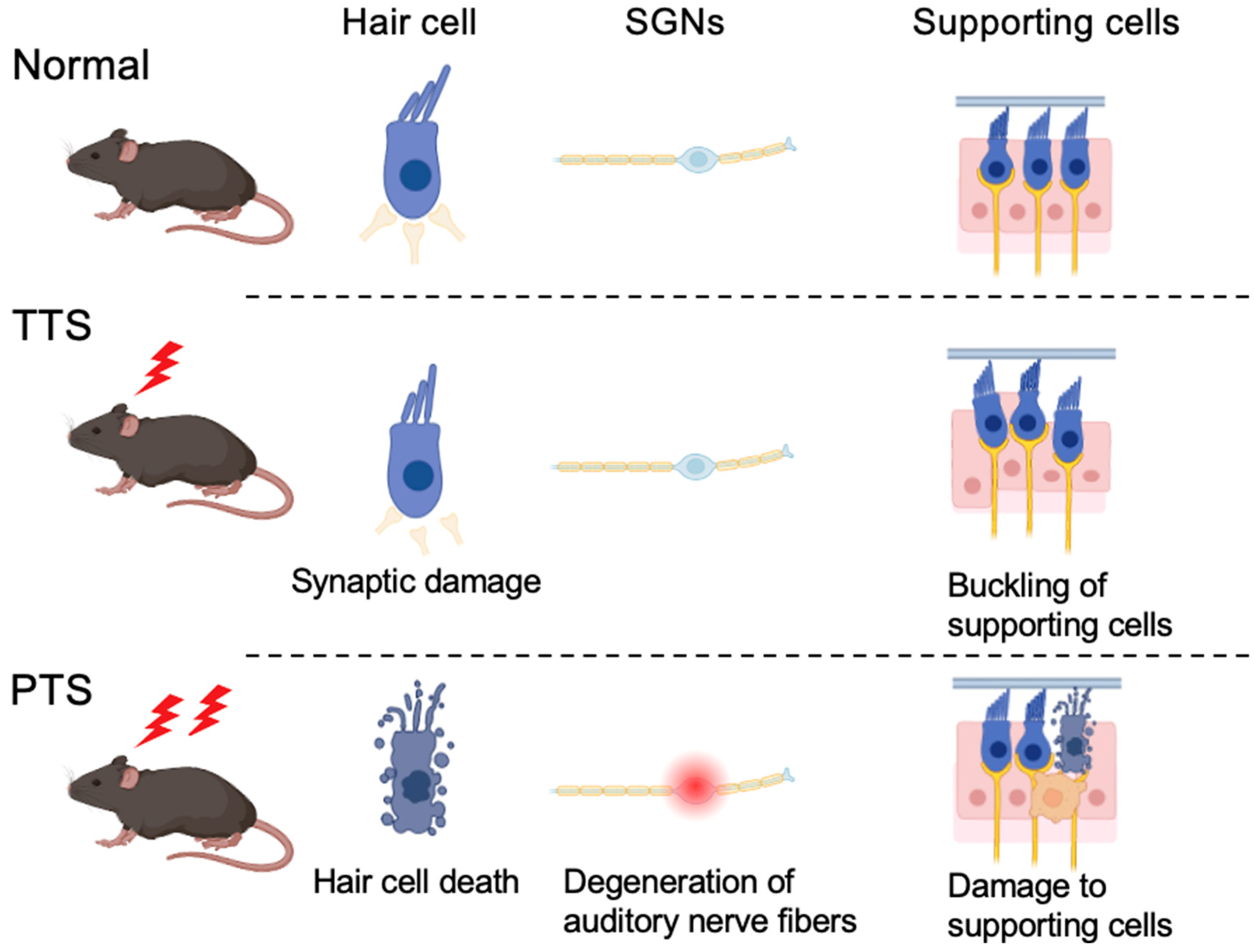
3. Histopathological Changes in the Cochlea in NIHL
3.1. PTS
- (i)
- Hair Cell Death
- (ii)
- Degeneration of Auditory Nerve Fibers
- (iii)
- Damage to Supporting Cells and Cochlear Structures
3.2. TTS
- (i)
- Synaptic Damage
- (ii)
- Buckling of Supporting Cells
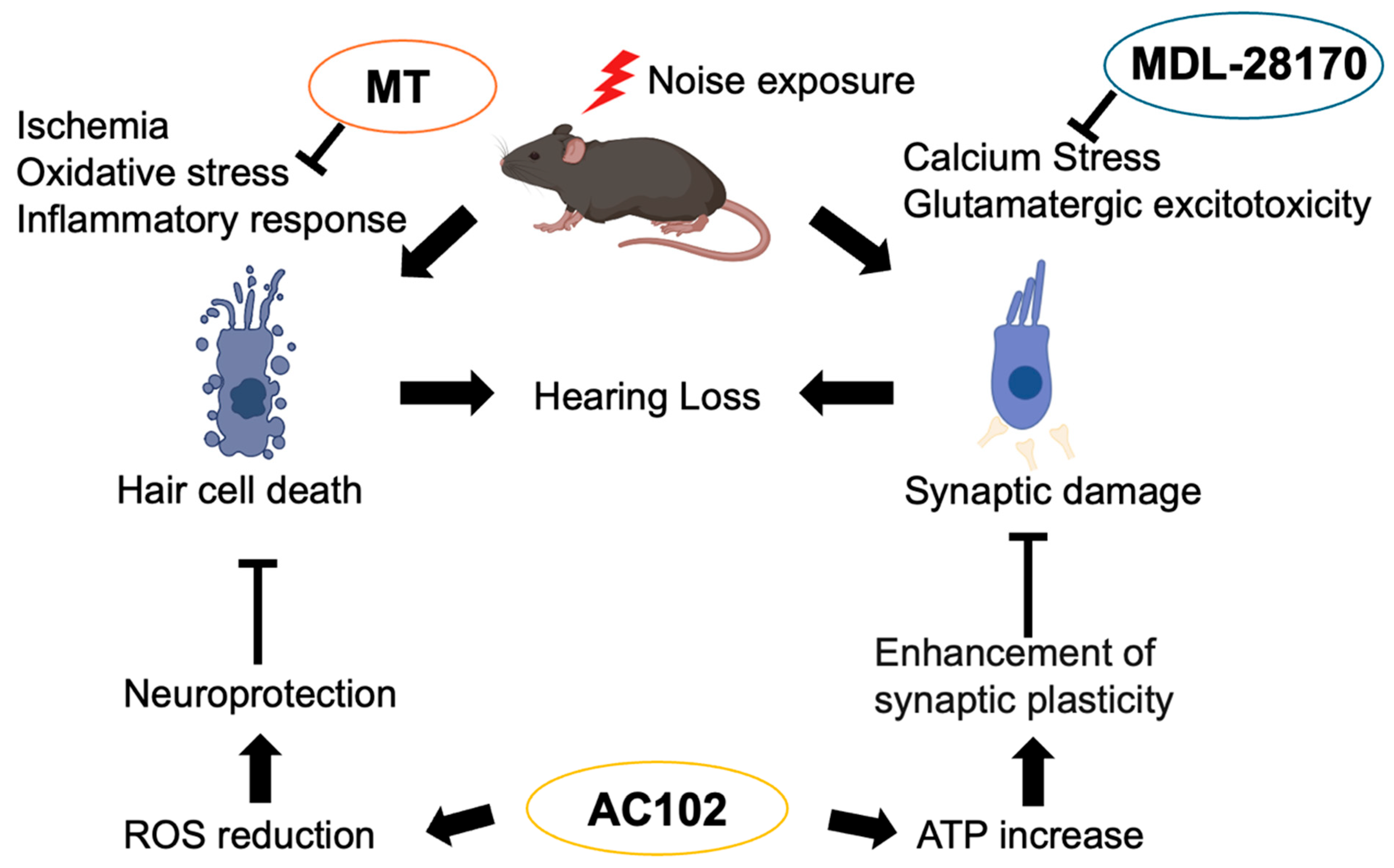
4. Molecular Mechanisms Underlying NIHL
4.1. Oxidative Stress
4.2. Calcium Stress and Glutamatergic Excitotoxicity
4.3. Inflammatory Response
4.4. Endocochlear Potential (EP) Reduction
4.5. Ischemia
4.6. Changes in Auditory Cortex
5. Therapeutic Strategies: Potential of Antioxidant Therapy in NIHL
5.1. MT (mito-TEMPO)
5.2. 6-fluoro-9-methyl-pyridoindole (AC102)
5.3. Calpain Inhibitor (MDL-28170)
6. Suggesting New Avenues for Future Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIHL | noise-induced hearing loss |
| TTS | temporary threshold shift |
| PTS | permanent threshold shift |
| IHC | inner hair cell |
| EP | endocochlear potential |
| ROS | reactive oxygen species |
References
- Metidieri, M.M.; Rodrigues, H.F.; Filho, F.J.; Ferraz, D.P.; Neto, A.F.; Torres, S. Noise-Induced Hearing Loss (NIHL): Literature review with a focus on occupational medicine. Int. Arch. Otorhinolaryngol. 2013, 17, 208–212. [Google Scholar] [CrossRef]
- Moon, I.S.; Park, S.Y.; Park, H.J.; Yang, H.S.; Hong, S.J.; Lee, W.S. Clinical characteristics of acoustic trauma caused by gunshot noise in mass rifle drills without ear protection. J. Occup. Environ. Hyg. 2011, 8, 618–623. [Google Scholar] [CrossRef]
- Brookhouser, P.E. Prevention of noise-induced hearing loss. Prev. Med. 1994, 23, 665–669. [Google Scholar] [CrossRef]
- Oishi, N.; Schacht, J. Emerging treatments for noise-induced hearing loss. Expert Opin. Emerg. Drugs 2011, 16, 235–245. [Google Scholar] [CrossRef]
- Daniell, W.E.; Fulton-Kehoe, D.; Smith-Weller, T.; Franklin, G.M. Occupational hearing loss in Washington state, 1984–1991: II. Morbidity and associated costs. Am. J. Ind. Med. 1998, 33, 529–536. [Google Scholar] [CrossRef]
- Fligor, B.J.; Cox, L.C. Output levels of commercially available portable compact disc players and the potential risk to hearing. Ear Hear. 2004, 25, 513–527. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kimizuka, T.; Kataoka, C.; Tazoe, M.; Sato, Y.; Hosoya, M.; Shimanuki, M.N.; Wakabayashi, T.; Ueno, M.; Ozawa, H.; et al. Relationship between hearing thresholds and cognitive function in hearing aid non-users and long-term users post-midlife. npj Aging 2025, 11, 14. [Google Scholar] [CrossRef]
- Dawes, P.; Munro, K.J. Hearing Loss and Dementia: Where to From Here? Ear Hear. 2024, 45, 529–536, Erratum in Ear Hear. 2024, 45, 1088. [Google Scholar] [CrossRef]
- Lawrence, B.J.; Jayakody, D.M.P.; Bennett, R.J.; Eikelboom, R.H.; Gasson, N.; Friedland, P.L. Hearing Loss and Depression in Older Adults: A Systematic Review and Meta-analysis. Gerontologist 2020, 60, e137–e154. [Google Scholar] [CrossRef]
- Wang, Y.; Hirose, K.; Liberman, M.C. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J. Assoc. Res. Otolaryngol. 2002, 3, 248–268. [Google Scholar] [CrossRef]
- Ohlemiller, K.K. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006, 1091, 89–102. [Google Scholar] [CrossRef]
- Le Prell, C.G.; Henderson, D.; Fay, R.R.; Popper, A.N. Noise-Induced Hearing Loss: Scientific Advances; Springer: New York, NY, USA, 2012. [Google Scholar]
- Yamane, H.; Nakai, Y.; Takayama, M.; Iguchi, H.; Nakagawa, T.; Kojima, A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur. Arch. Otorhinolaryngol. 1995, 252, 504–508. [Google Scholar] [CrossRef]
- Fetoni, A.R.; Paciello, F.; Rolesi, R.; Paludetti, G.; Troiani, D. Targeting dysregulation of redox homeostasis in noise-induced hearing loss: Oxidative stress and ROS signaling. Free. Radic. Biol. Med. 2019, 135, 46–59. [Google Scholar] [CrossRef]
- Kujawa, S.G.; Liberman, M.C. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef]
- Chen, H.; Shi, L.; Liu, L.; Yin, S.; Aiken, S.; Wang, J. Noise-induced Cochlear Synaptopathy and Signal Processing Disorders. Neuroscience 2019, 407, 41–52. [Google Scholar] [CrossRef]
- Ryan, A.; Bone, R.C. Noise-induced threshold shift and cochlear pathology in the Mongolian gerbil. J. Acoust. Soc. Am. 1978, 63, 1145–1151. [Google Scholar] [CrossRef]
- Clark, W.W.; Bohne, B.A. Effects of noise on hearing. JAMA 1999, 281, 1658–1659. [Google Scholar] [CrossRef]
- Morata, T.C.; Dunn, D.E.; Kretschmer, L.W.; Lemasters, G.K.; Keith, R.W. Effects of occupational exposure to organic solvents and noise on hearing. Scand. J. Work. Environ. Health 1993, 19, 245–254. [Google Scholar] [CrossRef]
- Lobarinas, E.; Spankovich, C.; Le Prell, C.G. Evidence of “hidden hearing loss” following noise exposures that produce robust TTS and ABR wave-I amplitude reductions. Hear. Res. 2017, 349, 155–163. [Google Scholar] [CrossRef]
- Lonsbury-Martin, B.L.; Martin, G.K.; Bohne, B.A. Repeated TTS exposures in monkeys: Alterations in hearing, cochlear structure, and single-unit thresholds. J. Acoust. Soc. Am. 1987, 81, 1507–1518. [Google Scholar] [CrossRef]
- Early, S.; Du, E.; Boussaty, E.; Friedman, R. Genetics of noise-induced hearing loss in the mouse model. Hear. Res. 2022, 425, 108505. [Google Scholar] [CrossRef]
- Escabi, C.D.; Frye, M.D.; Trevino, M.; Lobarinas, E. The rat animal model for noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 3692. [Google Scholar] [CrossRef]
- Bielefeld, E.C.; Harrison, R.T.; Riley DeBacker, J. Pharmaceutical otoprotection strategies to prevent impulse noise-induced hearing loss. J. Acoust. Soc. Am. 2019, 146, 3790. [Google Scholar] [CrossRef]
- Han, E.; Lee, D.H.; Park, S.; Rah, Y.C.; Park, H.C.; Choi, J.W.; Choi, J. Noise-induced hearing loss in zebrafish model: Characterization of tonotopy and sex-based differences. Hear. Res. 2022, 418, 108485. [Google Scholar] [CrossRef]
- Rommelspacher, H.; Bera, S.; Brommer, B.; Ward, R.; Kwiatkowska, M.; Zygmunt, T.; Theden, F.; Usekes, B.; Eren, N.; Nieratschker, M.; et al. A single dose of AC102 restores hearing in a guinea pig model of noise-induced hearing loss to almost prenoise levels. Proc. Natl. Acad. Sci. USA 2024, 121, e2314763121. [Google Scholar] [CrossRef]
- Burton, J.A.; Valero, M.D.; Hackett, T.A.; Ramachandran, R. The use of nonhuman primates in studies of noise injury and treatment. J. Acoust. Soc. Am. 2019, 146, 3770. [Google Scholar] [CrossRef]
- Nordmann, A.S.; Bohne, B.A.; Harding, G.W. Histopathological differences between temporary and permanent threshold shift. Hear. Res. 2000, 139, 13–30. [Google Scholar] [CrossRef]
- Shi, L.; Liu, K.; Wang, H.; Zhang, Y.; Hong, Z.; Wang, M.; Wang, X.; Jiang, X.; Yang, S. Noise induced reversible changes of cochlear ribbon synapses contribute to temporary hearing loss in mice. Acta Otolaryngol. 2015, 135, 1093–1102. [Google Scholar] [CrossRef]
- Valero, M.D.; Burton, J.A.; Hauser, S.N.; Hackett, T.A.; Ramachandran, R.; Liberman, M.C. Noise-induced cochlear synaptopathy in rhesus monkeys (Macaca mulatta). Hear. Res. 2017, 353, 213–223. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Wright, J.S.; Dugan, L.L. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol. Neurootol. 1999, 4, 229–236. [Google Scholar] [CrossRef]
- Fan, B.; Lu, F.; Du, W.J.; Chen, J.; An, X.G.; Wang, R.F.; Li, W.; Song, Y.L.; Zha, D.J.; Chen, F.Q. PTEN inhibitor bisperoxovanadium protects against noise-induced hearing loss. Neural Regen. Res. 2023, 18, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.; Bielefeld, E.C.; Harris, K.C.; Hu, B.H. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006, 27, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Bottger, E.C.; Schacht, J. The mitochondrion: A perpetrator of acquired hearing loss. Hear. Res. 2013, 303, 12–19. [Google Scholar] [CrossRef]
- Shi, X.; Han, W.; Yamamoto, H.; Omelchenko, I.; Nuttall, A. Nitric oxide and mitochondrial status in noise-induced hearing loss. Free. Radic. Res. 2007, 41, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, H.S.; Song, J.J.; Chang, S.O.; Oh, S.H. Increased activity of mitochondrial respiratory chain complex in noise-damaged rat cochlea. Acta Otolaryngol. 2012, 132 (Suppl. S1), S134–S141. [Google Scholar] [CrossRef]
- Coleman, J.K.; Kopke, R.D.; Liu, J.; Ge, X.; Harper, E.A.; Jones, G.E.; Cater, T.L.; Jackson, R.L. Pharmacological rescue of noise induced hearing loss using N-acetylcysteine and acetyl-L-carnitine. Hear Res. 2007, 226, 104–113. [Google Scholar] [CrossRef]
- Suzuki, M.; Yamasoba, T.; Ishibashi, T.; Miller, J.M.; Kaga, K. Effect of noise exposure on blood-labyrinth barrier in guinea pigs. Hear Res. 2002, 164, 12–18. [Google Scholar] [CrossRef]
- Nicotera, T.M.; Hu, B.H.; Henderson, D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 466–477. [Google Scholar] [CrossRef]
- Yamashita, D.; Miller, J.M.; Jiang, H.Y.; Minami, S.B.; Schacht, J. AIF and EndoG in noise-induced hearing loss. Neuroreport 2004, 15, 2719–2722. [Google Scholar]
- Yuan, H.; Wang, X.; Hill, K.; Chen, J.; Lemasters, J.; Yang, S.M.; Sha, S.H. Autophagy attenuates noise-induced hearing loss by reducing oxidative stress. Antioxid. Redox Signal. 2015, 22, 1308–1324. [Google Scholar] [CrossRef]
- Zuo, H.; Cui, B.; She, X.; Wu, M. Changes in Guinea pig cochlear hair cells after sound conditioning and noise exposure. J. Occup. Health 2008, 50, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pignol, B.; Chabrier, P.E.; Saido, T.; Lloyd, R.; Tang, Y.; Lenoir, M.; Puel, J.L. A novel dual inhibitor of calpains and lipid peroxidation (BN82270) rescues the cochlea from sound trauma. Neuropharmacology 2007, 52, 1426–1437. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yoneyama, M.; Ogita, K. Calpain inhibitor alleviates permanent hearing loss induced by intense noise by preventing disruption of gap junction-mediated intercellular communication in the cochlear spiral ligament. Eur. J. Pharmacol. 2017, 803, 187–194. [Google Scholar] [CrossRef]
- Wood, D.E.; Newcomb, E.W. Caspase-dependent activation of calpain during drug-induced apoptosis. J. Biol. Chem. 1999, 274, 8309–8315. [Google Scholar] [CrossRef] [PubMed]
- McCollum, A.T.; Nasr, P.; Estus, S. Calpain activates caspase-3 during UV-induced neuronal death but only calpain is necessary for death. J. Neurochem. 2002, 82, 1208–1220. [Google Scholar] [CrossRef]
- Bing, D.; Lee, S.C.; Campanelli, D.; Xiong, H.; Matsumoto, M.; Panford-Walsh, R.; Wolpert, S.; Praetorius, M.; Zimmermann, U.; Chu, H.; et al. Cochlear NMDA receptors as a therapeutic target of noise-induced tinnitus. Cell. Physiol. Biochem. 2015, 35, 1905–1923. [Google Scholar] [CrossRef]
- Chamorro, A.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, Y.; Fujimoto, C.; Kashio, A.; Kondo, K.; Yamasoba, T. Macrophage recruitment, but not interleukin 1 beta activation, enhances noise-induced hearing damage. Biochem. Biophys. Res. Commun. 2017, 493, 894–900. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, H.; Pyykko, I.; Zou, J. The TLR-4/NF-kappaB signaling pathway activation in cochlear inflammation of rats with noise-induced hearing loss. Hear Res. 2019, 379, 59–68. [Google Scholar] [CrossRef]
- Fujioka, M.; Kanzaki, S.; Okano, H.J.; Masuda, M.; Ogawa, K.; Okano, H. Proinflammatory cytokines expression in noise-induced damaged cochlea. J. Neurosci. Res. 2006, 83, 575–583. [Google Scholar] [CrossRef]
- Tan, W.J.; Thorne, P.R.; Vlajkovic, S.M. Characterisation of cochlear inflammation in mice following acute and chronic noise exposure. Histochem. Cell Biol. 2016, 146, 219–230. [Google Scholar] [CrossRef]
- Pan, J.; Wang, K.; Qu, J.; Chen, D.; Chen, A.; You, Y.; Tang, J.; Zhang, H. Activated tissue-resident macrophages contribute to hair cell insults in noise-induced hearing loss in mice. Commun. Biol. 2024, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Sai, N.; Zhou, Y.; Yu, N.; Jiang, Q.Q.; Sun, W.; Han, W.J.; Guo, W. CD38 Coordinates with NF-kappaB to Promote Cochlear Inflammation in Noise-Induced Hearing Loss: The Protective Effect of Apigenin. Mol. Neurobiol. 2024, 62, 6166–6178. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Chen, X.; Liu, H.; Li, M.; Zheng, L.; Yong, P.; Huang, M.; Shi, X.; Xu, Y.; Chen, S.; et al. Exploring the efficacy of (R)-PFI-2 hydrochloride in mitigating noise-induced hearing loss by targeting NLRP3 inflammasome and NF-kappaB pathway to reduce inner ear inflammation. J. Otol. 2024, 19, 200–206. [Google Scholar] [CrossRef]
- Mantel, P.Y.; Schmidt-Weber, C.B. Transforming growth factor-beta: Recent advances on its role in immune tolerance. Methods Mol. Biol. 2011, 677, 303–338. [Google Scholar] [CrossRef]
- Murillo-Cuesta, S.; Rodriguez-de la Rosa, L.; Contreras, J.; Celaya, A.M.; Camarero, G.; Rivera, T.; Varela-Nieto, I. Transforming growth factor beta1 inhibition protects from noise-induced hearing loss. Front. Aging Neurosci. 2015, 7, 32. [Google Scholar] [CrossRef][Green Version]
- Sanjabi, S.; Zenewicz, L.A.; Kamanaka, M.; Flavell, R.A. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr. Opin. Pharmacol. 2009, 9, 447–453. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Pro- and Anti-Inflammatory Prostaglandins and Cytokines in Humans: A Mini Review. Int. J. Mol. Sci. 2023, 24, 9647. [Google Scholar] [CrossRef]
- Satoh, H.; Billings, P.; Firestein, G.S.; Harris, J.P.; Keithley, E.M. Transforming growth factor beta expression during an inner ear immune response. Ann. Otol. Rhinol. Laryngol. 2006, 115, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, Y.; Hu, H.; Fan, C.; Zhang, A.; Ding, R.; Ye, B.; Xiang, M. Systematic Transcriptome Analysis of Noise-Induced Hearing Loss Pathogenesis Suggests Inflammatory Activities and Multiple Susceptible Molecules and Pathways. Front. Genet. 2020, 11, 968. [Google Scholar] [CrossRef]
- Jamesdaniel, S.; Hu, B.; Kermany, M.H.; Jiang, H.; Ding, D.; Coling, D.; Salvi, R. Noise induced changes in the expression of p38/MAPK signaling proteins in the sensory epithelium of the inner ear. J. Proteom. 2011, 75, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Lutze, R.D.; Kelmann, R.G.; Kresock, D.F.; Marsh, J.D.; Quevedo, R.V.; Zuo, J.; Teitz, T. KSR1 Knockout Mouse Model Demonstrates MAPK Pathway’s Key Role in Cisplatin- and Noise-induced Hearing Loss. J. Neurosci. 2024, 44, e2174232024. [Google Scholar] [CrossRef]
- Hirose, K.; Liberman, M.C. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J. Assoc. Res. Otolaryngol. 2003, 4, 339–352. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Nagashima, R.; Yoneyama, M.; Shiba, T.; Ogita, K. Disruption of ion-trafficking system in the cochlear spiral ligament prior to permanent hearing loss induced by exposure to intense noise: Possible involvement of 4-hydroxy-2-nonenal as a mediator of oxidative stress. PLoS ONE 2014, 9, e102133. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Kaur, T.; Warchol, M.E.; Withnell, R.H. The endocochlear potential as an indicator of reticular lamina integrity after noise exposure in mice. Hear Res. 2018, 361, 138–151. [Google Scholar] [CrossRef]
- Lamm, K.; Arnold, W. Successful treatment of noise-induced cochlear ischemia, hypoxia, and hearing loss. Ann. N. Y. Acad. Sci. 1999, 884, 233–248. [Google Scholar] [CrossRef]
- Miller, J.M.; Brown, J.N.; Schacht, J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol. Neurootol. 2003, 8, 207–221. [Google Scholar] [CrossRef]
- Shin, S.A.; Lyu, A.R.; Jeong, S.H.; Kim, T.H.; Park, M.J.; Park, Y.H. Acoustic Trauma Modulates Cochlear Blood Flow and Vasoactive Factors in a Rodent Model of Noise-Induced Hearing Loss. Int. J. Mol. Sci. 2019, 20, 5316. [Google Scholar] [CrossRef]
- Ohlemiller, K.K.; Dugan, L.L. Elevation of reactive oxygen species following ischemia-reperfusion in mouse cochlea observed in vivo. Audiol. Neurootol. 1999, 4, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rieger, J.M.; Shah, A.R.; Gidday, J.M. Ischemia-reperfusion injury of retinal endothelium by cyclooxygenase- and xanthine oxidase-derived superoxide. Exp. Eye Res. 2002, 74, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Walder, C.E.; Green, S.P.; Darbonne, W.C.; Mathias, J.; Rae, J.; Dinauer, M.C.; Curnutte, J.T.; Thomas, G.R. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke 1997, 28, 2252–2258. [Google Scholar] [CrossRef]
- Tran, T.P.; Tu, H.; Liu, J.; Muelleman, R.L.; Li, Y.L. Mitochondria-derived superoxide links to tourniquet-induced apoptosis in mouse skeletal muscle. PLoS ONE 2012, 7, e43410. [Google Scholar] [CrossRef][Green Version]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.A.; Zweier, J.L. Hypoxia and reoxygenation induce endothelial nitric oxide synthase uncoupling in endothelial cells through tetrahydrobiopterin depletion and S-glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef]
- Picciotti, P.M.; Fetoni, A.R.; Paludetti, G.; Wolf, F.I.; Torsello, A.; Troiani, D.; Ferraresi, A.; Pola, R.; Sergi, B. Vascular endothelial growth factor (VEGF) expression in noise-induced hearing loss. Hear. Res. 2006, 214, 76–83. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.S.; Zinsmaier, A.K.; Patterson, G.; Leptich, E.J.; Shoemaker, S.L.; Yatskievych, T.A.; Gibboni, R.; Pace, E.; Luo, H.; et al. Neuroinflammation mediates noise-induced synaptic imbalance and tinnitus in rodent models. PLoS Biol. 2019, 17, e3000307. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, K.W.; Lee, D.H.; Lee, S.M.; Kim, S.Y. Overexpression of the receptor for advanced glycation end-products in the auditory cortex of rats with noise-induced hearing loss. BMC Neurosci. 2021, 22, 38. [Google Scholar] [CrossRef]
- Imig, T.J.; Durham, D. Effect of unilateral noise exposure on the tonotopic distribution of spontaneous activity in the cochlear nucleus and inferior colliculus in the cortically intact and decorticate rat. J. Comp. Neurol. 2005, 490, 391–413. [Google Scholar] [CrossRef]
- Pienkowski, M.; Eggermont, J.J. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hear. Res. 2009, 257, 24–40. [Google Scholar] [CrossRef]
- Eggermont, J.J. Acquired hearing loss and brain plasticity. Hear. Res. 2017, 343, 176–190. [Google Scholar] [CrossRef]
- Nguyen, A.; Khaleel, H.M.; Razak, K.A. Effects of noise-induced hearing loss on parvalbumin and perineuronal net expression in the mouse primary auditory cortex. Hear. Res. 2017, 350, 82–90. [Google Scholar] [CrossRef]
- Manohar, S.; Chen, G.D.; Ding, D.; Liu, L.; Wang, J.; Chen, Y.C.; Chen, L.; Salvi, R. Unexpected Consequences of Noise-Induced Hearing Loss: Impaired Hippocampal Neurogenesis, Memory, and Stress. Front. Integr. Neurosci. 2022, 16, 871223. [Google Scholar] [CrossRef]
- Hayes, S.H.; Patel, S.V.; Arora, P.; Zhao, L.; Schormans, A.L.; Whitehead, S.N.; Allman, B.L. Neurophysiological, structural, and molecular alterations in the prefrontal and auditory cortices following noise-induced hearing loss. Neurobiol. Dis. 2024, 200, 106619. [Google Scholar] [CrossRef]
- Han, M.A.; Back, S.A.; Kim, H.L.; Park, S.Y.; Yeo, S.W.; Park, S.N. Therapeutic Effect of Dexamethasone for Noise-induced Hearing Loss: Systemic Versus Intratympanic Injection in Mice. Otol. Neurotol. 2015, 36, 755–762. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxid. Med. Cell. Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Xu, G.; McCarter, K.D.; Li, J.; Mayhan, W.G. Mito-Tempo prevents nicotine-induced exacerbation of ischemic brain damage. J. Appl. Physiol. 2018, 125, 49–57. [Google Scholar] [CrossRef]
- Du, K.; Farhood, A.; Jaeschke, H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 2017, 91, 761–773. [Google Scholar] [CrossRef]
- Chen, J.W.; Ma, P.W.; Yuan, H.; Wang, W.L.; Lu, P.H.; Ding, X.R.; Lun, Y.Q.; Yang, Q.; Lu, L.J. mito-TEMPO Attenuates Oxidative Stress and Mitochondrial Dysfunction in Noise-Induced Hearing Loss via Maintaining TFAM-mtDNA Interaction and Mitochondrial Biogenesis. Front. Cell. Neurosci. 2022, 16, 803718. [Google Scholar] [CrossRef]
- Wernicke, C.; Hellmann, J.; Zieba, B.; Kuter, K.; Ossowska, K.; Frenzel, M.; Dencher, N.A.; Rommelspacher, H. 9-Methyl-beta-carboline has restorative effects in an animal model of Parkinson’s disease. Pharmacol. Rep. 2010, 62, 35–53. [Google Scholar] [CrossRef]
- Northington, F.J.; Chavez-Valdez, R.; Martin, L.J. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 2011, 69, 743–758. [Google Scholar] [CrossRef]
- Lai, R.; Fang, Q.; Wu, F.; Pan, S.; Haque, K.; Sha, S.H. Prevention of noise-induced hearing loss by calpain inhibitor MDL-28170 is associated with upregulation of PI3K/Akt survival signaling pathway. Front. Cell. Neurosci. 2023, 17, 1199656. [Google Scholar] [CrossRef]
- Tona, Y.; Hamaguchi, K.; Ishikawa, M.; Miyoshi, T.; Yamamoto, N.; Yamahara, K.; Ito, J.; Nakagawa, T. Therapeutic potential of a gamma-secretase inhibitor for hearing restoration in a guinea pig model with noise-induced hearing loss. BMC Neurosci. 2014, 15, 66. [Google Scholar] [CrossRef]
- Umugire, A.; Lee, S.; Kim, D.; Choi, M.; Kim, H.S.; Cho, H.H. Avenanthramide-C prevents noise- and drug-induced hearing loss while protecting auditory hair cells from oxidative stress. Cell Death Discov. 2019, 5, 115. [Google Scholar] [CrossRef]
- Fetoni, A.R.; De Bartolo, P.; Eramo, S.L.; Rolesi, R.; Paciello, F.; Bergamini, C.; Fato, R.; Paludetti, G.; Petrosini, L.; Troiani, D. Noise-induced hearing loss (NIHL) as a target of oxidative stress-mediated damage: Cochlear and cortical responses after an increase in antioxidant defense. J. Neurosci. 2013, 33, 4011–4023. [Google Scholar] [CrossRef]
- Shi, X. Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear. Res. 2016, 338, 52–63. [Google Scholar] [CrossRef]
- An, X.; Zha, D. Development of nanoparticle drug-delivery systems for the inner ear. Nanomedicine 2020, 15, 1981–1993. [Google Scholar] [CrossRef]
- Horie, R.T.; Sakamoto, T.; Nakagawa, T.; Ishihara, T.; Higaki, M.; Ito, J. Stealth-nanoparticle strategy for enhancing the efficacy of steroids in mice with noise-induced hearing loss. Nanomedicine 2010, 5, 1331–1340. [Google Scholar] [CrossRef]
- Xu, X.; Lin, K.; Wang, Y.; Xu, K.; Sun, Y.; Yang, X.; Yang, M.; He, Z.; Zhang, Y.; Zheng, H.; et al. A metal-organic framework based inner ear delivery system for the treatment of noise-induced hearing loss. Nanoscale 2020, 12, 16359–16365. [Google Scholar] [CrossRef]
- Suzuki, J.; Hashimoto, K.; Xiao, R.; Vandenberghe, L.H.; Liberman, M.C. Cochlear gene therapy with ancestral AAV in adult mice: Complete transduction of inner hair cells without cochlear dysfunction. Sci. Rep. 2017, 7, 45524. [Google Scholar] [CrossRef]
- Yoshimura, H.; Shibata, S.B.; Ranum, P.T.; Smith, R.J.H. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci. Rep. 2018, 8, 2980. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kuroiwa, M.; Oakden, W.; Paul, B.T.; Noman, A.; Chen, J.; Lin, V.; Dimitrijevic, A.; Stanisz, G.; Le, T.N. Local magnetic delivery of adeno-associated virus AAV2(quad Y-F)-mediated BDNF gene therapy restores hearing after noise injury. Mol. Ther. 2022, 30, 519–533. [Google Scholar] [CrossRef]
- Tan, F.; Chu, C.; Qi, J.; Li, W.; You, D.; Li, K.; Chen, X.; Zhao, W.; Cheng, C.; Liu, X.; et al. AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 2019, 10, 3733. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, L.; Wang, D.; Chen, B.; Shu, Y. Approaches and Vectors for Efficient Cochlear Gene Transfer in Adult Mouse Models. Biomolecules 2022, 13, 38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitama, T.; Nishiyama, T.; Hosoya, M.; Shimanuki, M.N.; Ueno, M.; You, F.; Ozawa, H.; Oishi, N. Noise-Induced Hearing Loss: Overview and Future Prospects for Research on Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 4927. https://doi.org/10.3390/ijms26104927
Kitama T, Nishiyama T, Hosoya M, Shimanuki MN, Ueno M, You F, Ozawa H, Oishi N. Noise-Induced Hearing Loss: Overview and Future Prospects for Research on Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(10):4927. https://doi.org/10.3390/ijms26104927
Chicago/Turabian StyleKitama, Tsubasa, Takanori Nishiyama, Makoto Hosoya, Marie N. Shimanuki, Masafumi Ueno, Fukka You, Hiroyuki Ozawa, and Naoki Oishi. 2025. "Noise-Induced Hearing Loss: Overview and Future Prospects for Research on Oxidative Stress" International Journal of Molecular Sciences 26, no. 10: 4927. https://doi.org/10.3390/ijms26104927
APA StyleKitama, T., Nishiyama, T., Hosoya, M., Shimanuki, M. N., Ueno, M., You, F., Ozawa, H., & Oishi, N. (2025). Noise-Induced Hearing Loss: Overview and Future Prospects for Research on Oxidative Stress. International Journal of Molecular Sciences, 26(10), 4927. https://doi.org/10.3390/ijms26104927






