BDNF/proBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases
Abstract
1. Introduction
1.1. The Neurotrophic System
1.2. Neurotrophins
1.3. Neurotrophins and Trk Receptors
1.4. Neurotrophins and p75NTR Receptors
| Neurotrophin | Receptors |
|---|---|
| NGF | TrkA [18,19,20] |
| p75NTR [29,33] | |
| BDNF | TrkB [21] |
| p75NTR [29] | |
| TrkBT1 [25] | |
| NT-3 | TrkC [22,23] |
| TrkA and TrkB [24] | |
| p75NTR [29] | |
| TrkbT1 [25] | |
| NT-4/5 | TrkB [21] |
| TrkA [24] | |
| p75NTR [29] | |
| TrkbT1 [25] | |
| NT-6 | Heparan sulfate proteoglycans [26] |
| NT-7 | Rat TrkA and human p75NTR [27] |
| proNGF | Sortillin/p75NTR [29,34,35] |
| proBDNF | |
| pro NT-3 | |
| pro NT4/5 |
1.5. Regulation of Neuronal Apoptosis by Neurotrophins
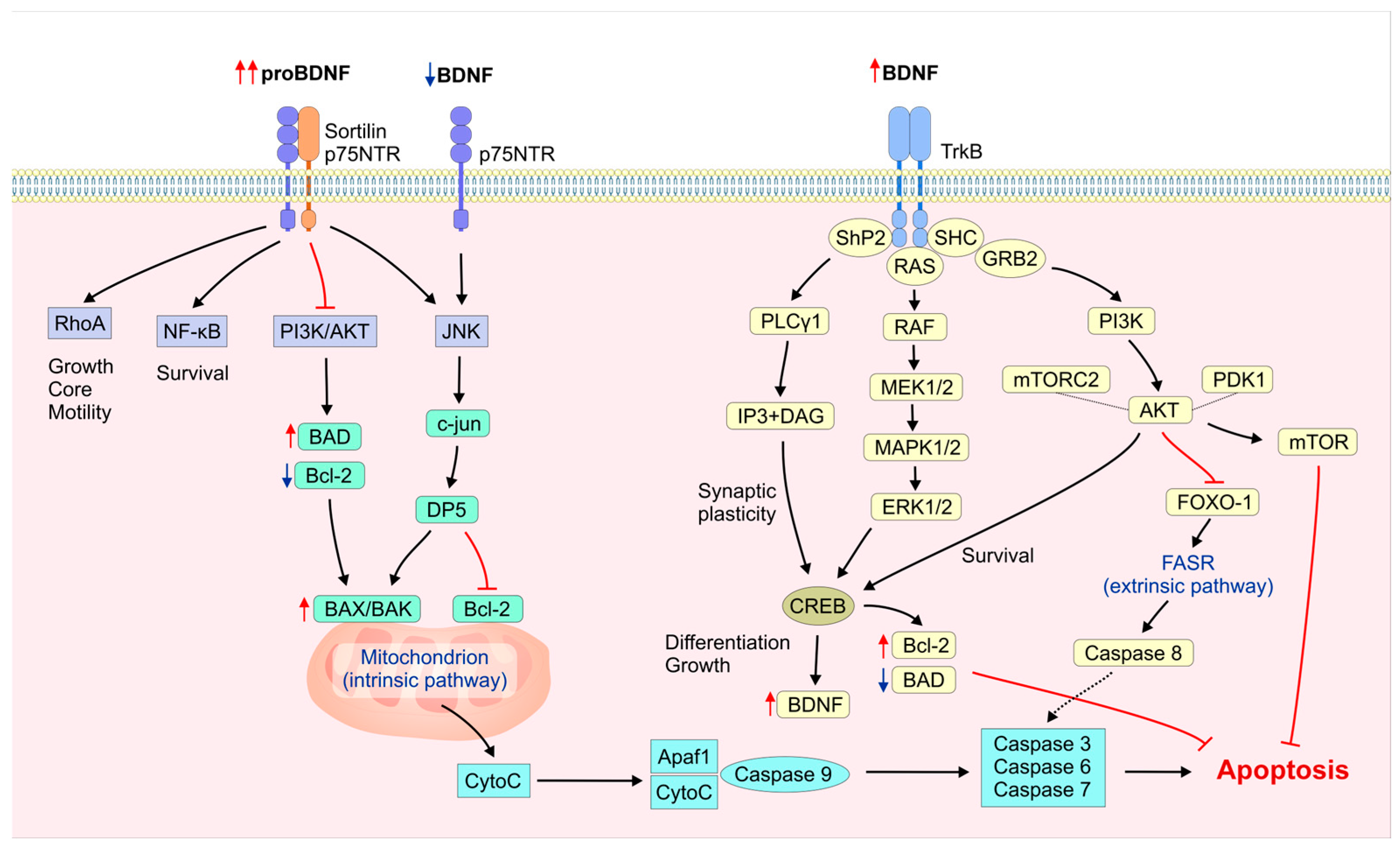
2. Molecular Mechanisms of BDNF and proBDNF in Neuronal Apoptosis Regulation
2.1. BDNF/proBDNF Origin and Synthesis
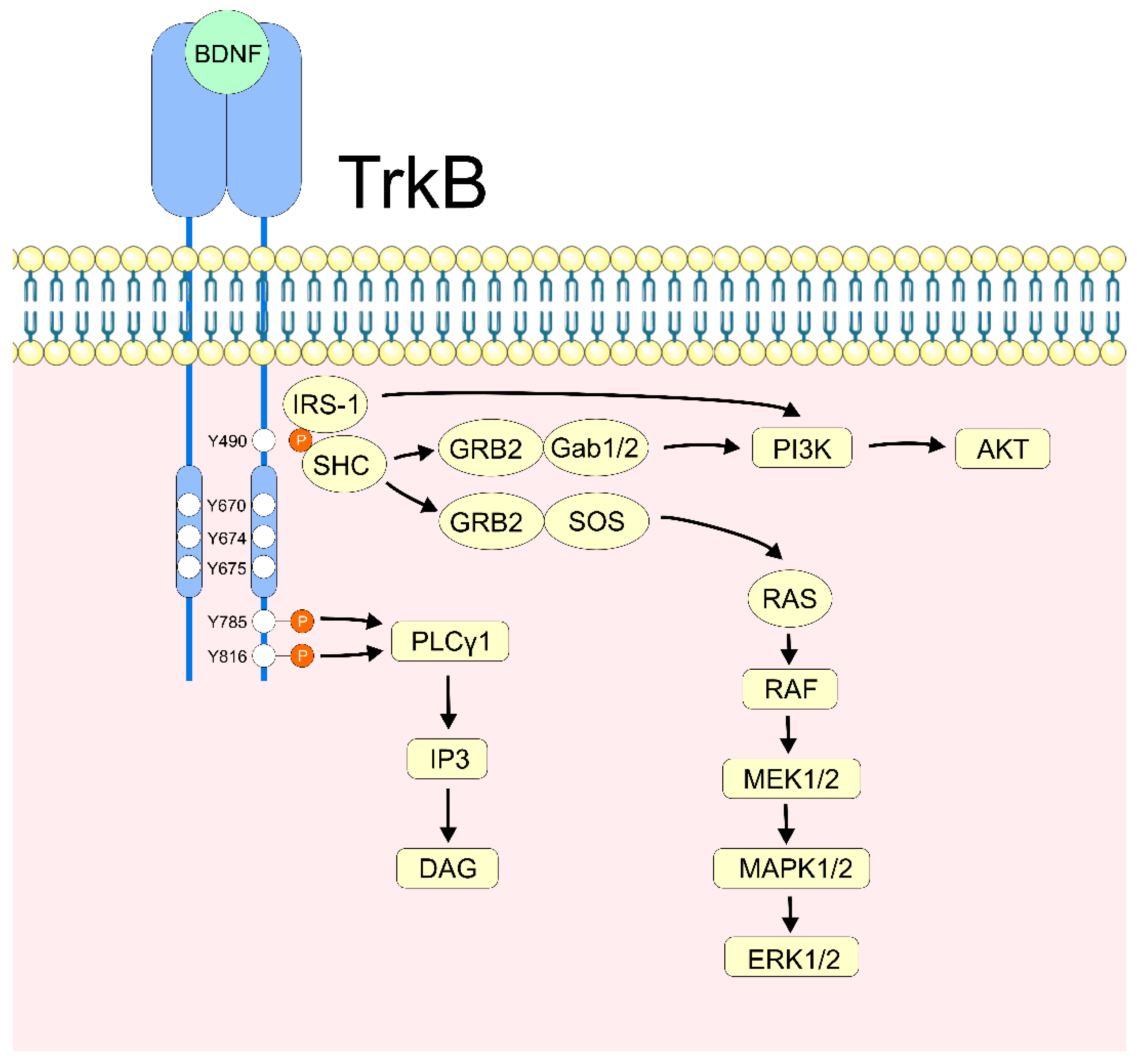
2.2. BDNF/TrkB Activation
2.3. BDNF’s Neuronal Apoptotic Regulation via PI3K/Akt
2.4. BDNF’s Neuronal Apoptotic Regulation via MAPK/ERK and MAPK/p38
2.5. Regulation of Neuronal Apoptosis via BDNF/Akt and BDNF/ERK Cross-Talk
2.6. BDNF/PLCγ Signaling in Neuronal Survival and Apoptosis
2.7. The Role of ProBDNF in Neuronal Apoptosis
2.8. The Role of BDNF/proBDNF Interplay in Neuronal Survival and Apoptosis
3. Factors Regulating BDNF/proBDNF Balance
3.1. BDNF Gene Transcription
3.2. proBDNF/BDNF Proteolytic Cleavage
3.3. TrkB and p75NTR/Sortillin Receptor Expression
4. The Clinical Implications of Dysregulated BDNF/proBDNF Interplay in Apoptotic-Related Neurodegenerative Disorders
4.1. BDNF/proBDNF in the Apoptotic Mechanisms Associated with Alzheimer’s Disease
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [195] (hippocampal and cortical rat cell lines) | BDNF ↓ | - | Apoptotic rate ↑ | Dexmedetomidine (specific agonist of α2-adrenoceptor) | BDNF ↑ Apoptotic rate ↓ |
| [196] (human neuroblastoma SH-SY5Y cells) | BDNF ↓ proBDNF ↑ | pTrkB ↑ TrkB ↓ | Bax ↑ Bcl-2 ↓ Caspase-1 and -6 ↑ Neuronal growth ↓ | ZN-015, VB-030, and VB-037 (anti-inflammatory, antioxidant, and antimicrobial) | BDNF/proBDNF ↑ TrkB/pTrkB ↑ Bax ↓ Bcl-2 ↑ Caspase-1 and -6 ↓ Neuronal growth ↑ |
| [197] (human neuroblastoma SH-SY5Y cells) | BDNF ↓ | pTrkB ↑ | Pro-caspase-9 ↓ Caspase-9 ↑ Neurite length ↓ | α-GPC (cholinergic transmission stimulator) | BDNF ↑ pTrkB ↓ Pro-caspase-9 ↑ Caspase-9 ↓ Neurite length ↑ |
| [198] (human neuroblastoma SH-SY5Y cells) | BDNF ↓ | - | Caspase-3/7 ↑ Neurite length ↓ | Retinoic acid plus exogenous BDNF | BDNF ↑ Caspase-3/7 ↓ Neurite length ↑ |
| [199] (mouse hippocampal HT22 cell line) | BDNF ↓ | pTrkB ↑ TrkB ↓ | Caspase-3 ↑ Neuronal cell death ↑ | Astragaloside IV (PPARγ agonist) | BDNF ↑ TrkB/pTrkB ↑ Caspase-3 ↓ Neuronal cell death ↓ |
| [200] (mouse hippocampal HT22 cell line) | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ Apoptotic rate ↑ | Icaritin (anti-inflammatory) | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Apoptotic rate ↓ |
| [202] (SH-SY5Y cells and mouse hippocampal neurons) | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Apoptotic cells ↑ | - | BDNF ↑ Bax ↓ Bcl-2 ↑ Apoptotic cells ↓ |
| [203] (PC12 cells—rat pheochromocytoma cell line) | BDNF ↓ | - | Bax ↑ Caspase-3 ↑ Bcl-2 ↓ Cell viability ↓ | Long non-coding RNA BDNF-AS | BDNF ↑ Bax ↓ Caspase-3 ↓ Bcl-2 ↑ Cell viability ↑ |
| [204] (THP-1 and BV-2 cells) | BDNF ↓ | - | Caspase-3/7 ↑ | GLP-1 GIP | BDNF ↑ Caspase-3/7 ↓ |
| [205] (N2A cells—mouse neuroblasts) | BDNF ↓ | - | Apoptotic cells ↑ | Paeoniflorin (PDK3 blocker) | BDNF ↑ Apoptotic cells ↓ |
| [206] (ndSH-SY5Y cells) | BDNF ↓ | - | Neurite length ↓ Cell viability ↓ Neurite fragmentation ↑ | Exogenous BDNF | BDNF ↑ Neurite length ↑ Cell viability ↑ Neurite fragmentation ↓ |
| [207] (mouse neural stem cells) | BDNF ↓ | - | Bax ↑ Caspase-3 ↑ Bcl-2 ↓ Apoptotic cells ↑ | Lycopene (antioxidant) | BDNF ↑ Bax ↓ Caspase-3 ↓ Bcl-2 ↑ Apoptotic cells ↓ |
| [208] (SH-SY5Y cells) | BDNF ↓ | TrkB ↓ | Bax ↑ Caspase-3 ↑ Bcl-2 ↓ Cell viability ↓ | Pandalus borealis extract | BDNF n.c. TrkB n.c. Bax ↓ Caspase-3 ↓ Bcl-2 ↑ Cell viability ↑ |
| [209] (mouse cortical neurons) | BDNF ↓ | - | Bax/Bcl-2 ratio ↑ Cleaved caspase-3/caspase-3 ratio ↑ Cell viability ↓ | Berberine (anti-inflammatory, antioxidant, Ach-esterase inhibitor) | BDNF ↑ Bax/Bcl-2 ratio ↓ Cleaved caspase-3/caspase-3 ratio ↓ Cell viability ↑ |
| [210] (hippocampal neural stem cells from APP/PS1 transgenic mice) | proBDNF ↑ | p75 ↑ sortilin ↑ | Apoptotic cells ↑ | CSF of patients with AD | proBDNF ↑↑ p75 ↑↑ Sortilin ↑↑ Apoptotic cells ↑↑ |
| [211] (5xFAD mouse neuronal cells) | BDNF ↓ | pTrkB ↓ | Cell viability ↓ Nuclear apoptosis ↑ Cleaved caspase-3 ↑ | Exogenous BDNF | BDNF ↑ Cell viability ↑ Nuclear apoptosis ↓ Cleaved caspase-3 ↓ |
4.2. Role of BDNF/proBDNF in Apoptotic Mechanisms Involved in Parkinson’s Disease
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [212] (rat stereotaxic 6-OHDA model) | - | - | Bax/Bcl-2 ratio ↑ P38 ↑ Akt ↓ Number of striatal DA neurons ↓ | BHME (BDNF mimetic, antioxidant) | Bax/Bcl-2 ratio ↓ P38 ↓ Akt ↑ Number of striatal DA neurons ↑ |
| [213] (rat stereotaxic 6-OHDA model) | BDNF ↓ | TrkB ↓ | Number of SN and ST DA neurons ↓ | Exogenous BDNF (umbilical stem cells) | BDNF ↑ TrkB ↑ Number of SN and ST DA neurons ↑ |
| [214] (rat stereotaxic rotenone model) | BDNF ↓ | - | Number of striatal DA neurons ↓ | Apigenin (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF ↑ Number of striatal DA neurons ↑ |
| [215] (rat rotenone systemic administration model) | BDNF ↓ | TrkB ↓ | Caspase-3 ↑ Striatal neuron degeneration ↓ | Roflumilast (PDE4 inhibitor) | BDNF ↑ TrkB ↑ Caspase-3 ↓ Striatal neuron degeneration ↑ |
| [216] (rat stereotaxic rotenone model) | BDNF ↓ | - | Number of striatal neurons ↓ | Agmatine (antioxidant, anti-inflammatory, anticonvulsant, antidepressant) | BDNF ↑ Number of striatal neurons ↑ |
| [217] (mouse rotenone systemic administration model) | BDNF ↓ | pTrkB ↓ | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ Loss of SN and ST DA neurons ↑ | Oleuropein (antioxidant) | BDNF ↑ pTrkB ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Loss of SN and ST DA neurons ↓ |
| [218] (mouse MPTP model and MPP+ rat model) | BDNF ↓ | - | Bcl-2 ↓ Number of striatal neurons ↓ | Electroacupuncture | BDNF ↑ Bcl-2 ↑ Number of striatal neurons ↑ |
| [219] (mouse MPTP model) | BDNF n.c. | TrkB n.c. | TH-positive neurons in SN ↓ Apoptotic cells in SN ↑ | K252a (TrkB antagonist) NaHS (H2S donor) | ↑TH-positive neurons in SN Apoptotic cells in SN ↓ |
| [220] (mouse MPTP model) | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Number of striatal neurons ↓ | DA-JC1 (GLP-1 and GIP agonist) | BDNF ↑ Bax ↓ Bcl-2 ↑ Number of striatal neurons ↑ |
| [221] (mouse MPTP model) | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ TH-positive neurons in SN ↓ | Wuzi Yanzong pill (antioxidant, anti-apoptotic) | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ TH-positive neurons in SN ↑ |
| [222] (suppression of NA stimulatory effect) | BDNF ↓ | pTrkB ↓ TrkB ↓ | Neuronal survival ↓ | BDNF stimulation from LC | BDNF ↑ pTrkB ↑ TrkB ↑ Neuronal survival ↑ |
| [223] (mouse stereotaxic 6-OHDA model) | BDNF ↓ | - | Caspase-3 ↑ Caspase-9 ↑ Striatal degeneration and gliosis ↑ | Schisandra chinensis extract (antioxidant, antidepressant) | BDNF ↑ Caspase-3 ↓ Caspase-9 ↓ Striatal degeneration and gliosis ↓ |
| [224] (MPTP mouse model and MPP+ SH-SY5Y cell model) | BDNF ↓ | - | DA cell apoptotic rate ↑ | miR-125b-5p | BDNF ↑ DA cell apoptotic rate ↓ |
4.3. Role of BDNF/proBDNF in Apoptotic Mechanisms Involved in Huntington’s Disease
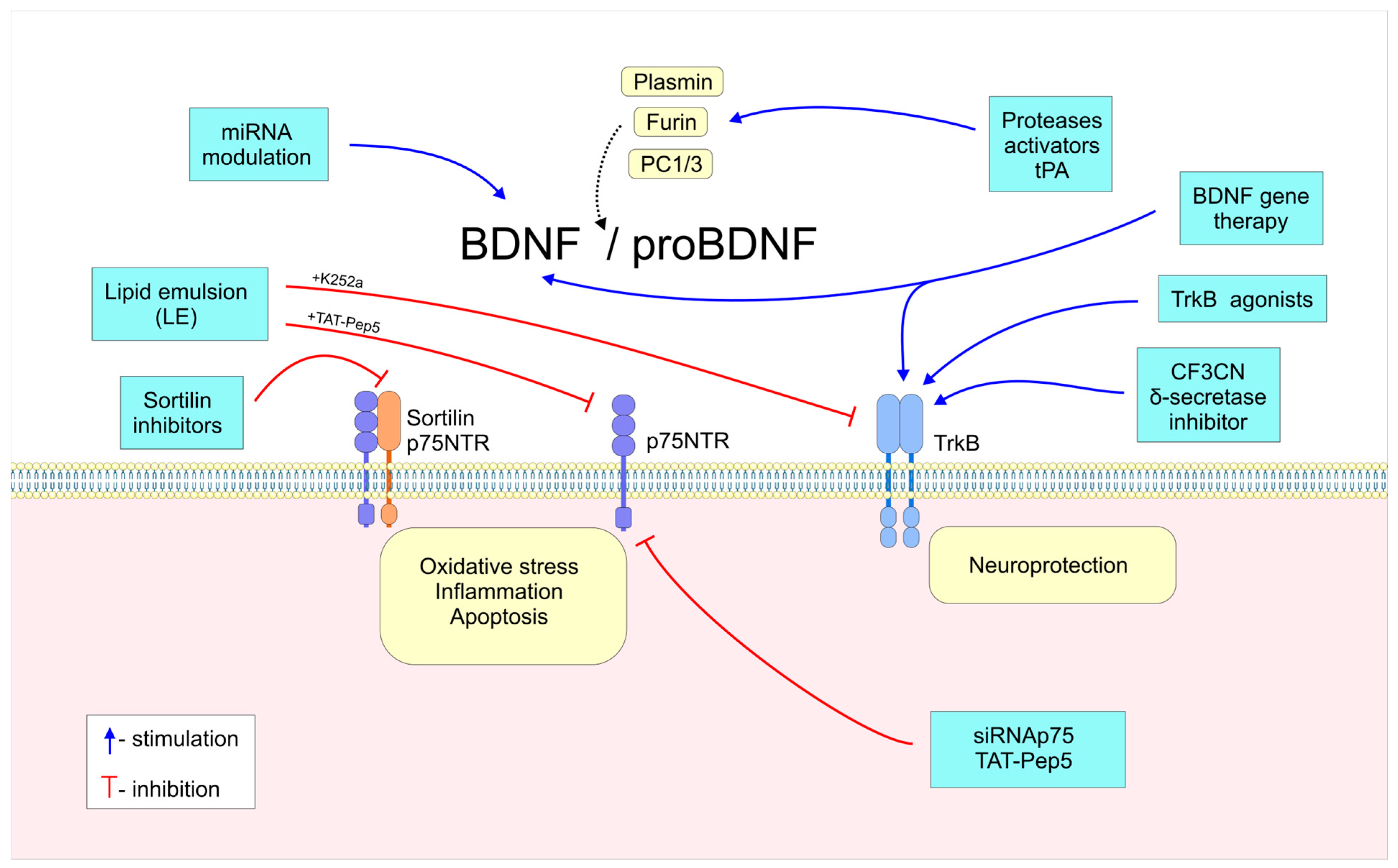
5. Emerging Therapeutic Strategies for Neurodegenerative Diseases: Targeting BDNF and proBDNF Apoptotic Signaling
6. Conclusions
7. Future Directions: Translational Application of BDNF/proBDNF Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| PI3K/Akt | Phosphatidylinositol 3-kinase/Protein kinase B |
| MAPK/ERK | Mitogen-activated protein kinase/Extracellular signal-regulated kinase |
| JNK | Jun N-terminal kinase |
| Rho | RAS homolog |
| GTPase | Small or monomeric guanine nucleotide-binding regulatory proteins |
| TRAF6 | TNF receptor-associated factor 6 |
| AD | Alzheimer’s disease |
| PD | Parkinson’s disease |
| HD | Huntington’s disease |
| NTFs | Neurotrophic factors |
| CNS | Central nervous system |
| PNS | Peripheral nervous system |
| NGF | Nerve growth factor |
| CDNF/MANF | Cerebral dopamine neurotrophic factor/Mesencephalic astrocyte-derived |
| NT | Neurotrophin |
| Trk | Tropomyosin receptor kinase |
| P75NTR | P75 neurotrophic receptor |
| PLCγ | Phospholipase C-γ |
| TNF | Tumor necrosis factor |
| NFkB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| MAG | Myelin-associated glycoprotein |
| Omg | Oligodendrocyte-myelin glycoprotein |
| Bcl-2 | B-cell lymphoma 2 |
| BAX | BCL2-associated X |
| PTB | Phosphotyrosine-binding |
| SH-2 | src-homology-2 |
| DAG/IP | Diacylglycerol/Inositol trisphosphate |
| GRB2/SOS | Growth-factor-receptor-binding protein 2/Son of Sevenless |
| IRS1 | Insulin receptor substrate 1 |
| PDK1 | Pyruvate dehydrogenase kinase 1 |
| PIP2 | Phosphatidylinositol-4,5-bisphosphate |
| PIP3 | Phosphatidylinositol-3,4,5-trisphosphate |
| PDK | Phosphoinositide-dependent kinase-1 |
| mTORC2 | Mammalian target of rapamycin complex 2 |
| GSK3β | Glycogen synthase kinase 3 beta |
| FOXO-1 | Forkhead box protein O1 |
| CREB | Cyclic AMP-responsive element-binding protein |
| PCNA | Proliferating cell nuclear antigen |
| NMDA-R | N-methyl-D-aspartate receptor |
| RSKs | Ribosomal S6 kinases |
| MAPKAPK2 | MAP kinase-activated protein kinase 2 |
| ATF2 | Activating transcription factor 2 |
| PKC | Protein kinase C |
| SGCs | Satellite glial cells |
| mRNAs | Non-coding RNAs |
| CRTC1 | CREB-regulated transcription coactivator-1 |
| CBP | CREB-binding protein |
| DMN | Default mode network |
| MEF-2 | Myocyte enhancer factor 2 |
| PAI-1 | Plasminogen activator inhibitor-1 |
| ApoE | Apolipoprotein E |
| SPNs | Spiny projection neurons |
| BAI1 | Brain-specific angiogenesis inhibitor-1 |
| STAT3/EZH2 | Signal transducer and activator of transcription 3/Enhancer of zeste homolog 2 |
| BDNF-AS | lncRNA BDNF antisense |
| ΔΨm | Mitochondrial membrane potential |
| MWM | Morris water maze |
| MPP | 1-Methyl-4-phenyl-pyridinium |
| MPTP | 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MMPs | Matrix metalloproteinases |
| EPA-pPE | EPA-enriched ethanolamine plasmalogen |
| EPA-PEEPA | Enriched phosphatidylethanolamine |
| AchE | Acetylcholinesterase |
| DISS-3,6′ | Disinapoyl sucrose P |
| PUFA | Polyunsaturated fatty acid |
| APP/PS1 | Double-transgenic mice expressing a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant human presenilin 1 (PS1-dE9) |
| α-GPC | Choline alfoscerate |
| PPARγ | Proliferator-activated receptor |
| FXR | Farnesoid X receptor |
| GLP-1 | Glucagon-like peptide-1 |
| GIP | Glucose-dependent insulinotropic polypeptide |
| 6-OHDA | 6-Hydroxydopamine hydrobromide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| THSG | 2,3,5,4′-Tetrahydroxystilbene-2-O-β-D-glucoside |
| FGF2 | Fibroblast growth factor 2 |
| PPX | Pramipexole |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| GDN | Lacrimal glial cell line-derived neurotrophic factor |
| LPSs | Lipopolysaccharides |
| XIAP | X-linked inhibitor of apoptosis protein |
| PARP1 | Poly(ADP-ribose) polymerase |
| PACAP | Pituitary adenylate cyclase-activating polypeptide |
| PAC1 | Pituitary AC-activating polypeptide (PACAP) receptor (ADCAYP1R1) |
| 7,8-DHF | 7,8-Dihydroxyflavone |
| 3-NP | 3-Nitropropionic acid |
| 4-MTBITC | 4-(Methylthio)butyl isothiocyanate |
References
- Ito, K.; Enomoto, H. Retrograde transport of neurotrophic factor signaling: Implications in neuronal development and pathogenesis. J. Biochem. 2016, 160, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D. Neurotrophic factors: An overview. Methods Mol. Biol. 2017, 1727, 1–17. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Deshmukh, R. Neurotrophic factors and Parkinson’s disease. Clin. Investig. 2018, 8, 53–62. [Google Scholar] [CrossRef]
- Kim, J.; He, M.J.; Widmann, A.K.; Lee, F.S. The role of neurotrophic factors in novel, rapid psychiatric treatments. Neuropsychopharmacology 2023, 49, 227–245. [Google Scholar] [CrossRef]
- El Ouaamari, Y.; Van den Bos, J.; Willekens, B.; Cools, N.; Wens, I. Neurotrophic factors as regenerative therapy for neurodegenerative diseases: Current status, challenges and future perspectives. Int. J. Mol. Sci. 2023, 24, 3866. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Ai, M.; Wang, J.; Chen, J.; Wang, W.; Xu, X.; Gan, Y.; Li, X.; Gou, X.; Cao, J.; Lv, Z.; et al. Plasma brain-derived neurotrophic factor (BDNF) concentration and the BDNF Val66Met polymorphism in suicide: A prospective study in patients with depressive disorder. Pharmacogenom. Pers. Med. 2019, 12, 97–106. [Google Scholar] [CrossRef]
- Rejdak, K.; Sienkiewicz-Jarosz, H.; Bienkowski, P.; Alvarez, A. Modulation of neurotrophic factors in the treatment of dementia, stroke and TBI: Effects of Cerebrolysin. Med. Res. Rev. 2023, 43, 1668–1700. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Yang, J.H.; Barba, D.U.H.; Bakay, R.A.; Pay, M.M.; Masliah, E.; Conner, J.M.; Kobalka, P.; Roy, S.; Nagahara, A.H. Nerve growth factor gene therapy. JAMA Neurol. 2015, 72, 1139. [Google Scholar] [CrossRef]
- Stansberry, W.M.; Pierchala, B.A. Neurotrophic factors in the physiology of motor neurons and their role in the pathobiology and therapeutic approach to amyotrophic lateral sclerosis. Front. Mol. Neurosci. 2023, 16, 1238453. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in neuronal development and function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.V. Neurotrophins and their receptors: A convergence point for many signalling pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Jindal, A.; Badu, N.Y.; Katiki, C.; Ponnapalli, V.J.; Desai, K.J.; Nassar, S.T. Role of Neurotrophins in the development and treatment of neurodegenerative diseases: A systematic review. Cureus 2024, 16, e74048. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Ultsch, M.H.; Wiesmann, C.; Simmons, L.C.; Henrich, J.; Yang, M.; Reilly, D.; Bass, S.H.; De Vos, A.M. Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC 1 1Edited by I. A. Wilson. J. Mol. Biol. 1999, 290, 149–159. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef]
- Angeletti, P.U.; Levi-Montalcini, R.; Calissano, P. The nerve growth factor (NGF): Chemical properties and metabolic effects. Adv. Enzymol.-Relat. Areas Mol. Biol. 1968, 31, 51–75. [Google Scholar] [CrossRef]
- Stoleru, B.; Popescu, A.M.; Tache, D.E.; Neamtu, O.M.; Emami, G.; Tataranu, L.G.; Buteica, A.S.; Dricu, A.; Purcaru, S.O. Tropomyosin-receptor-kinases signaling in the nervous system. Maedica 2013, 8, 43–48. Available online: https://pubmed.ncbi.nlm.nih.gov/24023598/ (accessed on 10 April 2025).
- Wiesmann, C.; de Vos, A. Nerve growth factor: Structure and function. Cell. Mol. Life Sci. 2001, 58, 748–759. [Google Scholar] [CrossRef]
- Sahay, A.; Kale, A.; Joshi, S. Role of neurotrophins in pregnancy and offspring brain development. Neuropeptides 2020, 83, 102075. [Google Scholar] [CrossRef]
- Manti, S.; Xerra, F.; Spoto, G.; Butera, A.; Gitto, E.; Di Rosa, G.; Nicotera, A.G. Neurotrophins: Expression of brain–lung Axis development. Int. J. Mol. Sci. 2023, 24, 7089. [Google Scholar] [CrossRef]
- Zhou, L.; Baumgartner, B.J.; Hill-Felberg, S.J.; McGowen, L.R.; Shine, H.D. Neurotrophin-3 ExpressedIn SituInduces Axonal plasticity in the adult injured spinal cord. J. Neurosci. 2003, 23, 1424–1431. [Google Scholar] [CrossRef]
- Oudega, M.; Hao, P.; Shang, J.; Haggerty, A.E.; Wang, Z.; Sun, J.; Liebl, D.J.; Shi, Y.; Cheng, L.; Duan, H.; et al. Validation study of neurotrophin-3-releasing chitosan facilitation of neural tissue generation in the severely injured adult rat spinal cord. Exp. Neurol. 2019, 312, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M. Ngf, bdnf, nt3, and NT4. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 3–15. [Google Scholar] [CrossRef]
- Tessarollo, L.; Yanpallewar, S. TrkB truncated Isoform receptors as transducers and determinants of BDNF functions. Front. Neurosci. 2022, 16, 847572. [Google Scholar] [CrossRef]
- Li, X.; Franz, J.; Lottspeich, F.; Götz, R. Recombinant fish neurotrophin-6 is a heparin-binding glycoprotein: Implications for a role in axonal guidance. Biochem. J. 1997, 324, 461–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nilsson, A.; Fainzilber, M.; Falck, P.; Ibáñez, C.F. Neurotrophin-7: A novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998, 424, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Skeldal, S.; Coulson, E. Signaling and function of death receptors of the tumor necrosis factor receptor superfamily. Encycl. Cell Biol. 2016, 3, 67–75. [Google Scholar] [CrossRef]
- Malik, S.C.; Sozmen, E.G.; Baeza-Raja, B.; Le Moan, N.; Akassoglou, K.; Schachtrup, C. In vivo functions of p75NTR: Challenges and opportunities for an emerging therapeutic target. Trends Pharmacol. Sci. 2021, 42, 772–788. [Google Scholar] [CrossRef]
- Yoon, S.O.; Casaccia-Bonnefil, P.; Carter, B.; Chao, M.V. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998, 18, 3273–3281. [Google Scholar] [CrossRef]
- Bibel, M.; Barde, Y. Neurotrophins: Key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000, 14, 2919–2937. [Google Scholar] [CrossRef] [PubMed]
- Friedman, W.J. Neurotrophins induce death of hippocampal neurons via the p75 receptor. J. Neurosci. 2000, 20, 6340–6346. [Google Scholar] [CrossRef]
- Conroy, J.N.; Coulson, E.J. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef]
- Chen, Z.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005, 25, 6156–6166. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, J.; Fleury, S.; Boukhatem, I.; Bélanger, J.; Welman, M.; Lordkipanidzé, M. Platelets selectively regulate the release of BDNF, but not that of its precursor protein, proBDNF. Front. Immunol. 2020, 11, 575607. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.K.; Teng, K.K.; Lee, R.; Wright, S.; Tevar, S.; Almeida, R.D.; Kermani, P.; Torkin, R.; Chen, Z.; Lee, F.S.; et al. ProBDNF induces neuronal Apoptosis via activation of a receptor complex of p75NTR and Sortilin. J. Neurosci. 2005, 25, 5455–5463. [Google Scholar] [CrossRef]
- Troy, C.M.; Friedman, J.E.; Friedman, W.J. Mechanisms of p75-mediated death of hippocampal neurons. J. Biol. Chem. 2002, 277, 34295–34302. [Google Scholar] [CrossRef]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Lebrun-Julien, F.; Bertrand, M.J.; De Backer, O.; Stellwagen, D.; Morales, C.R.; Di Polo, A.; Barker, P.A. ProNGF induces tnfα-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 3817–3822. [Google Scholar] [CrossRef]
- Wang, K.C.; Kim, J.A.; Sivasankaran, R.; Segal, R.; He, Z. P75 interacts with the Nogo receptor as a Co-receptor for Nogo, MAG and OMgp. Nature 2002, 420, 74–78. [Google Scholar] [CrossRef]
- Bharti, A.; Mittal, P.; Bora, K.S. Neurotrophic Factors as Antiapoptotic Agents—A Review. Res. J. Pharm. Technol. 2022, 15, 5327–5330. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal cell death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Hardwick, J.M.; Soane, L. Multiple functions of BCL-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008722. [Google Scholar] [CrossRef] [PubMed]
- Deckwerth, T.L.; Elliott, J.L.; Knudson, C.; Johnson, E.M.; Snider, W.D.; Korsmeyer, S.J. BAX is required for neuronal death after trophic factor deprivation and during development. Neuron 1996, 17, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.; Lautrette, C.; Bessette, B.; Decourt, C.; Mathonnet, M.; Jauberteau, M. Modulation of Fas-induced apoptosis by p75 neurotrophin receptor in a human neuroblastoma cell line. Apoptiosis 2005, 10, 1271–1283. [Google Scholar] [CrossRef]
- Yu, L.; Saarma, M.; Arumäe, U. Death receptors and Caspases but not mitochondria are activated in the GDNF- or BDNF-deprived Dopaminergic neurons. J. Neurosci. 2008, 28, 7467–7475. [Google Scholar] [CrossRef]
- Kuan, C.; Roth, K.A.; Flavell, R.A.; Rakic, P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000, 23, 291–297. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Miura, M. Programmed cell death in Neurodevelopment. Dev. Cell 2015, 32, 478–490. [Google Scholar] [CrossRef]
- Ceni, C.; Unsain, N.; Zeinieh, M.P.; Barker, P.A. Neurotrophins in the regulation of cellular survival and death. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 220, pp. 193–221. [Google Scholar] [CrossRef]
- Oppenheim, R.W.; Milligan, C.E.; Von Bartheld, C.S. Programmed cell death and neurotrophic factors. Fundam. Neurosci. 2013, 13, 405–435. [Google Scholar] [CrossRef]
- Montroull, L.E.; Danelon, V.; Cragnolini, A.B.; Mascó, D.H. Loss of TrkB signaling due to status Epilepticus induces a probdnf-dependent cell death. Front. Cell. Neurosci. 2019, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Rosenfeld, R.; Matheson, C.; Hawkins, N.; Lopez, O.; Bennett, L.; Welcher, A. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience 1997, 78, 431–448. [Google Scholar] [CrossRef]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J. Cell Biol. 2012, 196, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Harte-Hargrove, L.; Siao, C.; Marinic, T.; Clarke, R.; Ma, Q.; Jing, D.; LaFrancois, J.; Bath, K.; Mark, W.; et al. ProBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014, 7, 796–806. [Google Scholar] [CrossRef]
- Komori, T.; Okamura, K.; Ikehara, M.; Yamamuro, K.; Endo, N.; Okumura, K.; Yamauchi, T.; Ikawa, D.; Ouji-Sageshima, N.; Toritsuka, M.; et al. Brain-derived neurotrophic factor from microglia regulates neuronal development in the medial prefrontal cortex and its associated social behavior. Mol. Psychiatry 2024, 29, 1338–1349. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.M.; Riva, M.A. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef]
- Anastasia, A.; Deinhardt, K.; Chao, M.V.; Will, N.E.; Irmady, K.; Lee, F.S.; Hempstead, B.L.; Bracken, C. Val66Met polymorphism of BDNF alters prodomain structure to induce neuronal growth cone retraction. Nat. Commun. 2013, 4, 2490. [Google Scholar] [CrossRef]
- Lei, M.; Liu, Q.; Nie, J.; Huang, R.; Yan, M.; Pan, D.; Chen, Y.; Liu, W. Impact and Mechanisms of Action of BDNF on Neurological Disorders, Cancer, and Cardiovascular Diseases. CNS Neurosci. Ther. 2024, 30, e70138. [Google Scholar] [CrossRef] [PubMed]
- De Vincenti, A.P.; Ríos, A.S.; Paratcha, G.; Ledda, F. Mechanisms that modulate and diversify BDNF functions: Implications for hippocampal synaptic plasticity. Front. Cell. Neurosci. 2019, 13, 135. [Google Scholar] [CrossRef]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. A dose-dependent facilitation and inhibition of peripheral nerve regeneration by brain-derived neurotrophic factor. Eur. J. Neurosci. 2002, 15, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef] [PubMed]
- He, X.P.; Pan, E.; Sciarretta, C.; Minichiello, L.; McNamara, J.O. Disruption of trkb-mediated Phospholipase Cγ signaling inhibits limbic Epileptogenesis. J. Neurosci. 2010, 30, 6188–6196. [Google Scholar] [CrossRef]
- Schirò, G.; Iacono, S.; Ragonese, P.; Aridon, P.; Salemi, G.; Balistreri, C.R. A brief overview on BDNF-trk pathway in the nervous system: A potential biomarker or possible target in treatment of multiple sclerosis? Front. Neurol. 2022, 13, 917527. [Google Scholar] [CrossRef]
- Brunet, A.; Datta, S.R.; Greenberg, M.E. Transcription-dependent and -independent control of neuronal survival by the PI3K–akt signaling pathway. Curr. Opin. Neurobiol. 2001, 11, 297–305. [Google Scholar] [CrossRef]
- Sánchez-Alegría, K.; Flores-León, M.; Avila-Muñoz, E.; Rodríguez-Corona, N.; Arias, C. PI3K signaling in neurons: A central node for the control of multiple functions. Int. J. Mol. Sci. 2018, 19, 3725. [Google Scholar] [CrossRef]
- Guo, N.; Wang, X.; Xu, M.; Bai, J.; Yu, H.; Le, Z. PI3K/AKT signaling pathway: Molecular mechanisms and therapeutic potential in depression. Pharmacol. Res. 2024, 206, 107300. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, Y.; He, T.; Li, W.; Li, Q.; Miao, Y. Brain-derived neurotrophic factor inhibits hyperglycemia-induced apoptosis and downregulation of synaptic plasticity-related proteins in hippocampal neurons via the PI3K/Akt pathway. Int. J. Mol. Med. 2019, 43, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, X.; Zhong, S.; Liu, J.; Chen, C.; Wu, X. Knockdown of long noncoding antisense RNA brain-derived neurotrophic factor attenuates hypoxia/reoxygenation-induced nerve cell apoptosis through the BDNF–trkb–PI3K/Akt signaling pathway. NeuroReport 2017, 28, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Ma, Y.; Liu, J.; Zhu, Y.; Xie, K.; Yu, L.; Zhang, H. Brain-derived neurotrophic factor alleviates ropivacaine-induced neuronal damage by enhancing the Akt signaling pathway. Med. Sci. Monit. 2019, 25, 10154–10163. [Google Scholar] [CrossRef]
- Kim, J.H. Brain-derived neurotrophic factor exerts neuroprotective actions against amyloid β-induced apoptosis in neuroblastoma cells. Exp. Ther. Med. 2014, 8, 1891–1895. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Malik, M.; Wen, G.; Chauhan, A.; Chauhan, V.; Gong, C.; Liu, F.; Brown, W.T.; Li, X. BDNF-akt-Bcl2 antiapoptotic signaling pathway is compromised in the brain of autistic subjects. J. Neurosci. Res. 2010, 88, 2641–2647. [Google Scholar] [CrossRef]
- Pérez-Navarro, E.; Gavaldà, N.; Gratacòs, E.; Alberch, J. Brain-derived neurotrophic factor prevents changes in bcl-2 family members and caspase-3 activation induced by excitotoxicity in the striatum. J. Neurochem. 2005, 92, 678–691. [Google Scholar] [CrossRef]
- Geng, X.; Zou, Y.; Li, J.; Li, S.; Qi, R.; Yu, H.; Zhong, L. BDNF alleviates Parkinson’s disease by promoting STAT3 phosphorylation and regulating neuronal autophagy. Cell Tissue Res. 2023, 393, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Ikeuchi, T.; Hatanaka, H. Brain-Derived Neurotrophic Factor Accelerates Nitric Oxide Donor-Induced Apoptosis of Cultured Cortical Neurons. J. Neurochem. 2002, 75, 494–502. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 6, 1164–1178. [Google Scholar] [CrossRef]
- Kharebava, G.; Makonchuk, D.Y.; Kalita, K.; Zheng, J.-J.; Hetman, M. Requirement of 3-Phosphoinositide-Dependent Protein Kinase-1 for BDNF-Mediated Neuronal Survival. J. Neurosci. 2008, 28, 11409–11420. [Google Scholar] [CrossRef]
- Asih, P.R.; Prikas, E.; Stefanoska, K.; Tan, A.R.; Ahel, H.I.; Ittner, A. Functions of p38 MAP kinases in the central nervous system. Front. Mol. Neurosci. 2020, 13, 570586. [Google Scholar] [CrossRef]
- Mullen, L.M.; Pak, K.K.; Chavez, E.; Kondo, K.; Brand, Y.; Ryan, A.F. Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res. 2012, 1430, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Olvera, A.; Pérez-Martínez, L.; Pedraza-Alva, G. Bdnf mitigates Tnf and Β-amyloid peptide-induced Neurodegeneration in cholinergic neurons via P38 mapk-mediated Jnk inhibition. Neuroscience 2024. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Almeida, S.; Laço, M.; Cunha-Oliveira, T.; Oliveira, C.R.; Rego, A.C. BDNF regulates BIM expression levels in 3-nitropropionic acid-treated cortical neurons. Neurobiol. Dis. 2009, 35, 448–456. [Google Scholar] [CrossRef]
- Chen, S.-D.; Wu, C.L.; Hwang, W.C.; Yang, D.I. More Insight into BDNF against Neurodegeneration: Anti-Apoptosis, Anti-Oxidation, and Suppression of Autophagy. Int. J. Mol. Sci. 2017, 18, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Bijur, G.N.; Styles, N.A.; Li, X. Regulation of FOXO3a by brain-derived neurotrophic factor in differentiated human SH-SY5Y neuroblastoma cells. Mol. Brain Res. 2004, 126, 45–56. [Google Scholar] [CrossRef]
- Saba, J.; Turati, J.; Ramírez, D.; Carniglia, L.; Durand, D.E.; Lasaga, M.; Caruso, C.M. Astrocyte truncated tropomyosin receptor kinase B mediates brain-derived neurotrophic factor anti-apoptotic effect leading to neuroprotection. J. Neurochem. 2018, 146, 686–702. [Google Scholar] [CrossRef]
- Silva, A.; Naia, L.; Dominguez, A.; Ribeiro, M.; Rodrigues, J.; Vieira, O.V.; Lessmann, V.; Rego, A.C. Overexpression of BDNF and full-length TrkB receptor ameliorate Striatal neural survival in Huntington’s disease. Neurodegener. Dis. 2015, 15, 207–218. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, Neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Moya-Alvarado, G.; Valero-Peña, X.; Aguirre-Soto, A.; Bustos, F.J.; Lazo, O.M.; Bronfman, F.C. PLC-γ-Ca2+ pathway regulates axonal TrkB endocytosis and is required for long-distance propagation of BDNF signaling. Front. Mol. Neurosci. 2024, 17, 1009404. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Li, X.; Zhang, Y. Effect of acupuncture on BDNF signaling pathways in several nervous system diseases. Front. Neurol. 2023, 14, 1248348. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Odaka, H.; Adachi, N. Actions of brain-derived Neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int. J. Mol. Sci. 2018, 19, 3650. [Google Scholar] [CrossRef]
- Jerónimo-Santos, A.; Fonseca-Gomes, J.; Guimarães, D.A.; Tanqueiro, S.R.; Ramalho, R.M.; Ribeiro, J.A.; Sebastião, A.M.; Diógenes, M.J. Brain-derived neurotrophic factor mediates neuroprotection against aβ-induced toxicity through a mechanism independent on adenosine 2a receptor activation. Growth Factors 2015, 33, 298–308. [Google Scholar] [CrossRef]
- Wang, H.; Han, S.; Xie, J.; Zhao, R.; Li, S.; Li, J. IL-17A exacerbates caspase-12-dependent neuronal apoptosis following ischemia through the src-plcγ-calpain pathway. Exp. Neurol. 2024, 379, 114863. [Google Scholar] [CrossRef]
- Li, Q.; Hu, Y.; Gao, S.; Wang, P.; Hu, Z.; Dai, R. ProBDNF and its receptors in immune-mediated inflammatory diseases: Novel insights into the regulation of metabolism and mitochondria. Front. Immunol. 2023, 14, 1155333. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, G.; Tu, B.; Hu, Z.; Luo, C.; Cui, Y.; Xu, Y.; Li, F.; Dai, R.; Bi, F.; et al. Corticosterone induced the increase of proBDNF in primary hippocampal neurons via endoplasmic Reticulum stress. Neurotox. Res. 2020, 38, 370–384. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Nie, Y.; Wei, W.; Xiong, W. ProBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wu, L.L.; Li, H.; Wang, Y.; Zhou, X. Differential effects of pro-BDNF on sensory neurons after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 2008, 27, 2380–2390. [Google Scholar] [CrossRef]
- Wang, X.; Wei, M.; Wang, T.; Yang, J.-W.; Wu, Z.; Liu, K.; Dai, Y.; Zang, C.; Wei, L.; Liu, J.; et al. BDNF-TrkB and proBDNF-p75NTR/Sortilin Signaling Pathways are Involved in Mitochondria-Mediated Neuronal Apoptosis in Dorsal Root Ganglia after Sciatic Nerve Transection. Cns Neurol. Disord.-Drug Targets 2020, 19, 66–82. [Google Scholar] [CrossRef]
- Koshimizu, H.; Hazama, S.; Hara, T.; Ogura, A.; Kojima, M. Distinct signaling pathways of precursor BDNF and mature BDNF in cultured cerebellar granule neurons. Neurosci. Lett. 2010, 473, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From synaptic plasticity to Neurodegeneration: BDNF as a transformative target in medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Speidell, A.; Bin Abid, N.; Yano, H. Brain-derived neurotrophic factor dysregulation as an essential pathological feature in Huntington’s disease: Mechanisms and potential therapeutics. Biomedicines 2023, 11, 2275. [Google Scholar] [CrossRef] [PubMed]
- Garzon, D.; Yu, G.; Fahnestock, M. A new brain-derived neurotrophic factor transcript and decrease inbrain-derived neurotrophic factor transcripts 1, 2 and 3 in Alzheimer’s disease parietal cortex. J. Neurochem. 2002, 82, 1058–1064. [Google Scholar] [CrossRef]
- Pisani, A.; Paciello, F.; Del Vecchio, V.; Malesci, R.; De Corso, E.; Cantone, E.; Fetoni, A.R. The role of BDNF as a biomarker in cognitive and sensory Neurodegeneration. J. Pers. Med. 2023, 13, 652. [Google Scholar] [CrossRef]
- Numakawa, T.; Odaka, H. Brain-derived neurotrophic factor signaling in the pathophysiology of Alzheimer’s disease: Beneficial effects of flavonoids for Neuroprotection. Int. J. Mol. Sci. 2021, 22, 5719. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- You, H.J.; Park, J.H.; Pareja-Galeano, H.; Lucia, A.; Shin, J.I. Targeting MicroRNAs involved in the BDNF signaling impairment in neurodegenerative diseases. NeuroMolecular Med. 2016, 18, 540–550. [Google Scholar] [CrossRef]
- Chen, K.; Chen, L. Epigenetic regulation of BDNF gene during development and diseases. Int. J. Mol. Sci. 2017, 18, 571. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, J.; Wang, Y.; Wang, G.; Tang, P.; Zhang, Y.; Ouyang, L. Targeting epigenetic modifications in Parkinson’s disease therapy. Med. Res. Rev. 2023, 43, 1748–1777. [Google Scholar] [CrossRef] [PubMed]
- Esvald, E.; Tuvikene, J.; Moistus, A.; Rannaste, K.; Kõomägi, S.; Timmusk, T. Differential regulation of the BDNF gene in cortical and hippocampal neurons. J. Neurosci. 2022, 42, 9110–9128. [Google Scholar] [CrossRef]
- Yanev, P.; Scheel, N.; Zhang, R.; Zhu, D.C.; Stowe, A.; Trout, A.L. Influence of BDNF polymorphisms on the neuronal-enriched extracellular vesicle proBDNF/BDNF ratio and DMN activity in participants of the rrAD trial. Alzheimers Dement. 2024, 20 (Suppl. S2), e091552. [Google Scholar] [CrossRef]
- Cagni, F.C.; Campêlo, C.L.; Coimbra, D.G.; Barbosa, M.R.; Júnior, L.G.; Neto, A.B.; Ribeiro, A.M.; Júnior, C.D.; Gomes de Andrade, T.; Silva, R.H. Association of BDNF Val66MET polymorphism with Parkinson’s disease and depression and anxiety symptoms. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Przybylska, I.; Marusiak, J.; Toczyłowska, B.; Stępień, A.; Brodacki, B.; Langfort, J.; Chalimoniuk, M. Association between the Val66Met (rs6265) polymorphism of the brain-derived neurotrophic factor (BDNF) gene, BDNF protein level in the blood and the risk of developing early onset Parkinson’s disease. Acta Neurobiol. Exp. 2024, 84, 296–308. [Google Scholar] [CrossRef]
- Wang, J. Myocyte enhancer factor-2 (MEF2) in diseases of central nervous system: A mini review. Explor. Res. Hypothesis Med. 2016, 1, 2–6. [Google Scholar] [CrossRef][Green Version]
- Chen, L.F.; Lyons, M.R.; Liu, F.; Green, M.V.; Hedrick, N.G.; Williams, A.B.; Narayanan, A.; Yasuda, R.; West, A.E. The NMDA receptor subunit GluN3A regulates synaptic activity-induced and myocyte enhancer factor 2C (MEF2C)-dependent transcription. J. Biol. Chem. 2020, 295, 8613–8627. [Google Scholar] [CrossRef]
- Avarlaid, A.; Falkenberg, K.; Lehe, K.; Mudò, G.; Belluardo, N.; Di Liberto, V.; Frinchi, M.; Tuvikene, J.; Timmusk, T. An upstream enhancer and MEF2 transcription factors fine-tune the regulation of the Bdnf gene in cortical and hippocampal neurons. J. Biol. Chem. 2024, 300, 107411. [Google Scholar] [CrossRef]
- Cao, J.; Guo, B.; Li, S.; Zhang, X.; Zhang, X.; Zhang, G.; Sun, Y.; Wang, Y.; Song, X.; Zhang, Z. Neuroprotection against 1-Methyl-4-phenylpyridinium-induced cytotoxicity by naturally occurring polydatin through activation of transcription factor MEF2D. NeuroReport 2021, 32, 1065–1072. [Google Scholar] [CrossRef]
- Chen, H.; Cao, J.; Zha, L.; Wang, P.; Liu, Z.; Guo, B.; Zhang, G.; Sun, Y.; Zhang, Z.; Wang, Y. Neuroprotective and neurogenic effects of novel tetramethylpyrazine derivative T-006 in Parkinson’s disease models through activating the MEF2-pgc1α and BDNF/CREB pathways. Aging 2020, 12, 14897–14917. [Google Scholar] [CrossRef]
- Delezie, J.; Handschin, C. Endocrine crosstalk between skeletal muscle and the brain. Front. Neurol. 2018, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Andreska, T.; Rauskolb, S.; Schukraft, N.; Lüningschrör, P.; Sasi, M.; Signoret-Genest, J.; Behringer, M.; Blum, R.; Sauer, M.; Tovote, P.; et al. Induction of BDNF expression in layer II/III and layer V neurons of the motor cortex is essential for motor learning. J. Neurosci. 2020, 40, 6289–6308. [Google Scholar] [CrossRef] [PubMed]
- Kaagman, D.G.; Van Wegen, E.E.; Cignetti, N.; Rothermel, E.; Vanbellingen, T.; Hirsch, M.A. Effects and mechanisms of exercise on brain-derived neurotrophic factor (BDNF) levels and clinical outcomes in people with Parkinson’s disease: A systematic review and meta-analysis. Brain Sci. 2024, 14, 194. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, X.; Senthil Kumar, S.P.; Zhang, J.; Shi, H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm. Behav. 2013, 63, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Deb, P.; Chini, A.; Guha, P.; Rishi, A.; Bhan, A.; Brady, B.; Perrotti, L.I.; Mandal, S.S. Dynamic regulation of BDNF gene expression by estradiol and lncRNA HOTAIR. Gene 2024, 897, 148055. [Google Scholar] [CrossRef]
- Wang, M.; Xie, Y.; Qin, D. Proteolytic cleavage of proBDNF to mBDNF in neuropsychiatric and neurodegenerative diseases. Brain Res. Bull. 2021, 166, 172–184. [Google Scholar] [CrossRef]
- Cai, M.; Jung, I.; Kwon, H.; Cho, E.; Jeon, J.; Yun, J.; Lee, Y.C.; Kim, D.H.; Ryu, J.H. Spinosin attenuates Alzheimer’s disease-associated synaptic dysfunction via regulation of plasmin activity. Biomol. Ther. 2020, 28, 131–136. [Google Scholar] [CrossRef]
- Mou, X.; Peterson, C.B.; Prosser, R.A. Tissue-type plasminogen activator-plasmin-BDNF modulate glutamate-induced phase-shifts of the mouse suprachiasmatic circadian clock in vitro. Eur. J. Neurosci. 2009, 30, 1451–1460. [Google Scholar] [CrossRef]
- Rodriguez, G.; Eren, M.; Haupfear, I.; Viola, K.L.; Cline, E.N.; Miyata, T.; Klein, W.L.; Vaughan, D.E.; Dong, H. Pharmacological inhibition of plasminogen activator inhibitor-1 prevents memory deficits and reduces neuropathology in APP/PS1 mice. Psychopharmacology 2023, 240, 2641–2655. [Google Scholar] [CrossRef]
- Liu, C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE4 and Aβ Oligomers reduce BDNF expression via HDAC nuclear translocation. J. Neurosci. 2015, 35, 7538–7551. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Nelson, T.J.; Alkon, D.L. ApoE isoforms differentially regulates cleavage and secretion of BDNF. Mol. Brain 2017, 10, 19. [Google Scholar] [CrossRef]
- Stoilov, P.; Castren, E.; Stamm, S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem. Biophys. Res. Commun. 2002, 290, 1054–1065. [Google Scholar] [CrossRef]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Allen, S.J.; Dawbarn, D.; Eckford, S.D.; Wilcock, G.K.; Ashcroft, M.; Colebrook, S.M.; Feeney, R.; MacGowan, S.H. Cloning of a non-catalytic form of human trkB and distribution of messenger RNA for trkB in human brain. Neuroscience 1994, 60, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Tejeda, G.S.; Díaz-Guerra, M. Integral Characterization of Defective BDNF/TrkB Signalling in Neurological and Psychiatric Disorders Leads the Way to New Therapies. Int. J. Mol. Sci. 2017, 18, 268. [Google Scholar] [CrossRef]
- Allen, S.J.; Wilcock, G.K.; Dawbarn, D. Profound and selective loss of catalytic TrkB immunoreactivity in Alzheimer’s disease. Biochem. Biophys. Res. Commun. 1999, 264, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Garner, B.; Halliday, G.M.; Kwok, J.B. Srp20 regulates TrkB pre-mRNA splicing to generate TrkB-Shc transcripts with implications for Alzheimer’s disease. J. Neurochem. 2012, 123, 159–171. [Google Scholar] [CrossRef]
- Fenner, M.E.; Achim, C.L.; Fenner, B.M. Expression of full-length and truncated trkB in human striatum and substantia nigra neurons: Implications for Parkinson’s disease. J. Mol. Histol. 2013, 45, 349–361. [Google Scholar] [CrossRef]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alexiou, A.; Papadakis, M.; AlAseeri, A.A.; Alruwaili, M.; Saad, H.M.; Batiha, G.E. BDNF/TrkB activators in Parkinson’s disease: A new therapeutic strategy. J. Cell. Mol. Med. 2024, 28, e18368. [Google Scholar] [CrossRef]
- Ding, Y.; Xia, Y.; Jiao, X.; Duan, L.; Yu, J.; Wang, X.; Chen, L. The trkb-positive Dopaminergic neurons are less sensitive to MPTP insult in the Substantia Nigra of adult C57/BL mice. Neurochem. Res. 2011, 36, 1759–1766. [Google Scholar] [CrossRef]
- He, K.; Nie, L.; Dai, Z.; Li, S.; Yang, X.; Liu, G. The role of TrkB receptor signaling in Alzheimer’s disease. Hum. Brain 2023, 2. [Google Scholar] [CrossRef]
- Avrutsky, M.I.; Troy, C.M. Caspase-9: A Multimodal Therapeutic Target With Diverse Cellular Expression in Human Disease. Front. Pharmacol. 2021, 12, 701301. [Google Scholar] [CrossRef]
- Hallbook, F.; Ayer-leleivre, C.; Ebendal, T.; Persson, H. Expression of nerve growth factor receptor mRNA during early development of the chicken embryo: Emphasis on cranial ganglia. Development 1990, 108, 693–704. [Google Scholar] [CrossRef]
- Springer, J.E.; Robbins, E.; Meyer, S.; Baldino, F.; Lewis, M.E. Localization of nerve growth factor receptor mRNA in the rat basal forebrain with in situ hybridization histochemistry. Cell. Mol. Neurobiol. 1990, 10, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.W.; Chong, Y.S.; Lin, W.; Kisiswa, L.; Sim, E.; Ibanez, C.F.; Sajikumar, S. Age-related changes in hippocampal-dependent synaptic plasticity and memory mediated by p75 neurotrophin receptor. Aging Cell 2021, 20, e13305. [Google Scholar] [CrossRef] [PubMed]
- Cade, S.; Zhou, X.F.; Bobrovskaya, L. The role of brain-derived neurotrophic factor and the neurotrophin receptor p75NTR in age-related brain atrophy and the transition to Alzheimer’s disease. Rev. Neurosci. 2022, 33, 515–529. [Google Scholar] [CrossRef]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. TrkB receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef]
- Meeker, R.; Williams, K. Dynamic nature of the p75 Neurotrophin receptor in response to injury and disease. J. Neuroimmune Pharmacol. 2014, 9, 615–628. [Google Scholar] [CrossRef]
- Chakravarthy, B.; Ménard, M.; Ito, S.; Gaudet, C.; Dal Prà, I.; Armato, U.; Whitfield, J. Hippocampal membrane-associated p75NTR levels are increased in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2012, 30, 675–684. [Google Scholar] [CrossRef]
- Sotthibundhu, A.; Sykes, A.M.; Fox, B.; Underwood, C.K.; Thangnipon, W.; Coulson, E.J. Beta-amyloid(1–42) induces neuronal death through the p75 neurotrophin receptor. J. Neurosci. 2008, 28, 3941–3946. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.H.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Alnaaim, S.A.; Saad, H.M.; Batiha, G.E. The Molecular Pathway of p75 Neurotrophin Receptor (p75NTR) in Parkinson’s Disease: The Way of New Inroads. Mol. Neurobiol. 2024, 61, 2469–2480. [Google Scholar] [CrossRef]
- Lozano-Ureña, A.; Frade, J.M. Differential contribution of TrkB and p75NTR to BDNF-dependent self-renewal, proliferation, and differentiation of adult neural stem cells. Front. Mol. Neurosci. 2023, 16, 1271820. [Google Scholar] [CrossRef] [PubMed]
- Andreska, T.; Lüningschrör, P.; Wolf, D.; McFleder, R.L.; Ayon-Olivas, M.; Rattka, M.; Drechsler, C.; Perschin, V.; Blum, R.; Aufmkolk, S.; et al. DRD1 signaling modulates TrkB turnover and BDNF sensitivity in direct pathway striatal medium spiny neurons. Cell Rep. 2023, 42, 112575. [Google Scholar] [CrossRef]
- Cade, S.; Zhou, X.; Bobrovskaya, L. An imbalance in the pro/mature BDNF ratio occurs in multiple brain regions during normal ageing in wild-type mice. J. Mol. Neurosci. 2023, 73, 469–484. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The global burden of neurological disorders: Translating evidence into policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association Report. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Du, Q.; Zhu, X.; Si, J. Angelica polysaccharide ameliorates memory impairment in Alzheimer’s disease rat through activating BDNF/TrkB/CREB pathway. Exp. Biol. Med. 2020, 245, 1–10. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, L. The regulation of BAI1 in astrocytes through the STAT3/EZH2 axis relieves neuronal apoptosis in rats with Alzheimer’s disease. Brain Res. 2024, 1839, 149007. [Google Scholar] [CrossRef]
- De Assis, G.G.; Murawska-Ciałowicz, E. BDNF modulation by microRNAs: An update on the experimental evidence. Cells 2024, 13, 880. [Google Scholar] [CrossRef]
- Ren, H.; Qiu, W.; Zhu, B.; Li, Q.; Peng, C.; Chen, X. The long non-coding RNA BDNF-AS induces neuronal cell apoptosis by targeting miR-125b-5p in Alzheimer’s disease models. Adv. Clin. Exp. Med. 2024, 33, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Kan, B.; Shi, H.; Han, J. miR-22 exerts anti-alzheimic effects via the regulation of apoptosis of hippocampal neurons. Cell. Mol. Biol. 2018, 64, 84–89. [Google Scholar] [CrossRef]
- Babaei, H.; Kheirollah, A.; Ranjbaran, M.; Cheraghzadeh, M.; Sarkaki, A.; Adelipour, M. Preconditioning adipose-derived mesenchymal stem cells with dimethyl fumarate promotes their therapeutic efficacy in the brain tissues of rats with Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2023, 672, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.H.; Shao, S.J.; Yang, J.J.; Liu, J.R.; Guo, H.D. Designer Self-Assemble Peptides Maximize the Therapeutic Benefits of Neural Stem Cell Transplantation for Alzheimer’s Disease via Enhancing Neuron Differentiation and Paracrine Action. Mol. Neurobiol. 2016, 53, 1108–1123. [Google Scholar] [CrossRef]
- Hu, W.; Feng, Z.; Xu, J.; Jiang, Z.; Feng, M. Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cells-derived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer’s disease rats. Brain Res. 2019, 1710, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Lv, X.; Guo, Y.; Qi, M.; Ge, B. Total flavonoids of Cynomorium songaricum attenuates cognitive defects in an Aβ 1-42 -induced Alzheimer’s disease rat model by activating BDNF/TrkB signaling transduction. Neuroreport 2023, 34, 825–833. [Google Scholar] [CrossRef]
- Sharma, S.; Saini, A.; Nehru, B. Neuroprotective effects of carbenoxolone against amyloid-beta 1-42 oligomer-induced neuroinflammation and cognitive decline in rats. Neurotoxicology 2021, 83, 89–105. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.K.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, D.L.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2018, 22, 111–122. [Google Scholar] [CrossRef]
- Wang, Y.L.; Chio, C.C.; Kuo, S.C.; Yeh, C.H.; Ma, J.T.; Liu, W.P.; Lin, M.T.; Lin, K.C.; Chang, C.P. Exercise Rehabilitation and/or Astragaloside Attenuate Amyloid-beta Pathology by Reversing BDNF/TrkB Signaling Deficits and Mitochondrial Dysfunction. Mol. Neurobiol. 2022, 59, 3091–3109. [Google Scholar] [CrossRef]
- Liu, S.; Li, X.; Gao, J.; Liu, Y.; Shi, J.; Gong, Q. Icariside II, a Phosphodiesterase-5 Inhibitor, Attenuates Beta-Amyloid-Induced Cognitive Deficits via BDNF/TrkB/CREB Signaling. Cell. Physiol. Biochem. 2018, 49, 985. [Google Scholar] [CrossRef]
- Che, H.; Zhang, L.; Ding, L.; Xie, W.; Jiang, X.; Xue, C.; Zhang, T.; Wang, Y. EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine enhance BDNF/TrkB/CREB signaling and inhibit neuronal apoptosis in vitro and in vivo. Food Funct. 2020, 11, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.Y.; Kim, H.B.; Hwang, E.S.; Park, H.S.; Cho, J.M.; Ham, Y.K.; Kim, J.H.; Mun, M.K.; Maeng, S.; Park, J.H. Naringin enhances long-term potentiation and recovers learning and memory deficits of amyloid-beta induced Alzheimer’s disease-like behavioral rat model. Neurotoxicology 2023, 95, 35–45. [Google Scholar] [CrossRef]
- Xiang, X.; Xia, S.; Li, S.; Zeng, Y.; Wang, L.; Zhou, Y. Study on the role and mechanism of Tan IIA in Alzheimer’s disease based on CREB-BDNF-TrkB pathway. Neurosci. Lett. 2024, 830, 137769. [Google Scholar] [CrossRef]
- Helli, B.; Navabi, S.P.; Hosseini, S.A.; Sabahi, A.; Khorsandi, L.; Amirrajab, N.; Mahdavinia, M.; Rahmani, S.; Dehghani, M.A. The Protective Effects of Syringic Acid on Bisphenol A-Induced Neurotoxicity Possibly Through AMPK/PGC-1α/Fndc5 and CREB/BDNF Signaling Pathways. Mol. Neurobiol. 2024, 61, 7767–7784. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.S.; Abdelkader, N.F.; Abd El-Rahman, S.S.; Emara, M.; Zaki, H.F.; Khattab, M.M. Stimulation of ACE2/ANG(1-7)/Mas Axis by Diminazene Ameliorates Alzheimer’s Disease in the D-Galactose-Ovariectomized Rat Model: Role of PI3K/Akt Pathway. Mol. Neurobiol. 2018, 55, 8188–8202. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Lin, L.; Chen, L.; Xiao, W.; Gong, Z. l-Theanine Ameliorates d-Galactose-Induced Brain Damage in Rats via Inhibiting AGE Formation and Regulating Sirtuin1 and BDNF Signaling Pathways. Oxidative Med. Cell. Longev. 2021, 2021, 8850112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Q.; Lu, L.; Su, Y.; Shi, W.; Zhang, H.; Liu, R.; Pu, Y.; Yin, L. Copper Induces Cognitive Impairment in Mice via Modulation of Cuproptosis and CREB Signaling. Nutrients 2023, 15, 972. [Google Scholar] [CrossRef]
- Lou, S.; Gong, D.; Yang, M.; Qiu, Q.; Luo, J.; Chen, T. Curcumin Improves Neurogenesis in Alzheimer’s Disease Mice via the Upregulation of Wnt/β-Catenin and BDNF. Int. J. Mol. Sci. 2024, 25, 5123. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, H.; Wang, S.; Li, Y.; Yang, P.; Xu, L.; Liu, Y.; Liu, M. PRG ameliorates cognitive impairment in Alzheimer’s disease mice by regulating β-amyloid and targeting the ERK pathway. Phytomedicine 2024, 130, 155671. [Google Scholar] [CrossRef]
- Lian, W.W.; Zhou, W.; Zhang, B.Y.; Jia, H.; Xu, L.J.; Liu, A.L.; Du, G.H. DL0410 ameliorates cognitive disorder in SAMP8 mice by promoting mitochondrial dynamics and the NMDAR-CREB-BDNF pathway. Acta Pharmacol. Sin. 2021, 42, 1055–1068. [Google Scholar] [CrossRef]
- Yuan, J.; He, M.; Dai, X.; Huo, Q.; Chang, P.; Zhang, J.; Wang, S.; Sun, Y. 3,6’-Disinapoyl sucrose alleviates cognitive deficits in APP/PS1 transgenic mice. J. Neurophysiol. 2023, 130, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Zhao, F.; Deng, F.; Wang, Y. Regulating effect of Qifu Yin on intestinal microbiota in mice with memory impairment induced by scopolamine hydrobromide. J. Ethnopharmacol. 2024, 333, 118445. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.W.; Kim, E.J.; Kim, D.; Jeong, J.Y.; Kim, G.H.; Lim, K.; Moon, M.; Yoon, K.A.; Choi, D.E.; Kim, J.J. Fat-1 expression enhance hippocampal memory in scopolamine-induced amnesia. J. Nutr. Biochem. 2020, 82, 108394. [Google Scholar] [CrossRef]
- Ikram, M.; Jo, M.H.; Choe, K.; Khan, A.; Ahmad, S.; Saeed, K.; Kim, M.W.; Kim, M.O. Cycloastragenol, a Triterpenoid Saponin, Regulates Oxidative Stress, Neurotrophic Dysfunctions, Neuroinflammation and Apoptotic Cell Death in Neurodegenerative Conditions. Cells 2021, 10, 2719. [Google Scholar] [CrossRef]
- Anand, A.; Khurana, N.; Kaur, S.; Ali, N.; AlAsmari, A.F.; Waseem, M.; Iqbal, M.; Alzahrani, F.M.; Sharma, N. The multifactorial role of vanillin in amelioration of aluminium chloride and D-galactose induced Alzheimer’s disease in mice. Eur. J. Pharmacol. 2023, 954, 175832. [Google Scholar] [CrossRef]
- Broderick, T.L.; Rasool, S.; Li, R.; Zhang, Y.; Anderson, M.; Al-Nakkash, L.; Plochocki, J.H.; Geetha, T.; Babu, J.R. Neuroprotective Effects of Chronic Resveratrol Treatment and Exercise Training in the 3xTg-AD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 7337. [Google Scholar] [CrossRef]
- Gerenu, G.; Martisova, E.; Ferrero, H.; Carracedo, M.; Rantamäki, T.; Ramirez, M.J.; Gil-Bea, F.J. Modulation of BDNF cleavage by plasminogen-activator inhibitor-1 contributes to Alzheimer’s neuropathology and cognitive deficits. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, Y.; Zhang, C.; Xiang, Z.; Ding, J.; Han, X. Learning and memory deficits and alzheimer’s disease-like changes in mice after chronic exposure to microcystin-LR. J. Hazard. Mater. 2019, 373, 504–518. [Google Scholar] [CrossRef]
- Muhammad, T.; Ali, T.; Ikram, M.; Khan, A.; Alam, S.I.; Kim, M.O. Melatonin Rescue Oxidative Stress-Mediated Neuroinflammation/Neurodegeneration and Memory Impairment in Scopolamine-Induced Amnesia Mice Model. J. Neuroimmune Pharmacol. 2019, 14, 278–294. [Google Scholar] [CrossRef]
- Hong, S.M.; Yoon, D.H.; Lee, M.K.; Lee, J.K.; Kim, S.Y. A Mixture of Ginkgo biloba L. Leaf and Hericium erinaceus (Bull.) Pers. Fruit Extract Attenuates Scopolamine-Induced Memory Impairments in Mice. Oxidative Med. Cell. Longev. 2022, 2022, 9973678. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, Q.; Wu, C.; Du, G.; Zeng, Y.; Song, Z.; Jiang, X.; Jiang, X.; Zhuo, R.; et al. Propane-2-sulfonic acid octadec-9-enyl-amide, a novel PPARα/γ dual agonist, attenuates molecular pathological alterations in learning memory in AD mice. Neurol. Res. 2024, 46, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Prabhakar, S.; Kalra, J.; Anand, A. Effect of human umbilical cord blood derived lineage negative stem cells transplanted in amyloid-β induced cognitive impaired mice. Behav. Brain Res. 2015, 291, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chen, J.; Li, X.; Mao, J.; Wu, Y.; Zhuo, P.; Zhang, Y.; Liu, W.; Huang, J.; Tao, J.; et al. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic factor in APP/PS1 transgenic mice. Mol. Med. Rep. 2016, 13, 1611–1617. [Google Scholar] [CrossRef]
- Barbereau, C.; Yehya, A.; Silhol, M.; Cubedo, N.; Verdier, J.M.; Maurice, T.; Rossel, M. Neuroprotective brain-derived neurotrophic factor signaling in the TAU-P301L tauopathy zebrafish model. Pharmacol. Res. 2020, 158, 104865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, A.; Ma, W. Dexmedetomidine attenuates the toxicity of β amyloid on neurons and astrocytes by increasing BDNF production under the regulation of HDAC2 and HDAC5. Mol. Med. Rep. 2019, 19, 533–540. [Google Scholar] [CrossRef]
- Weng, Z.K.; Lin, T.H.; Chang, K.H.; Chiu, Y.J.; Lin, C.H.; Tseng, P.H.; Sun, Y.C.; Lin, W.; Lee-Chen, G.J.; Chen, C.M. Using ΔK280 TauRD Folding Reporter Cells to Screen TRKB Agonists as Alzheimer’s Disease Treatment Strategy. Biomolecules 2023, 13, 219. [Google Scholar] [CrossRef]
- Catanesi, M.; d’Angelo, M.; Antonosante, A.; Castelli, V.; Alfonsetti, M.; Benedetti, E.; Desideri, G.; Ferri, C.; Cimini, A. Neuroprotective potential of choline alfoscerate against β-amyloid injury: Involvement of neurotrophic signals. Cell Biol. Int. 2020, 44, 1734–1744. [Google Scholar] [CrossRef]
- Krishtal, J.; Bragina, O.; Metsla, K.; Palumaa, P.; Tõugu, V. In situ fibrillizing amyloid-beta 1-42 induces neurite degeneration and apoptosis of differentiated SH-SY5Y cells. PLoS ONE 2017, 12, e0186636. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Chen, H.; Li, W.; Li, W.; Zhu, G. Astragaloside IV prevents Aβ1-42 oligomers-induced memory impairment and hippocampal cell apoptosis by promoting PPARγ/BDNF signaling pathway. Brain Res. 2020, 1747, 147041. [Google Scholar] [CrossRef]
- Tang, C.; Liu, X.; Zhu, H.; Lu, Q. Antagonizing effect of icaritin on apoptosis and injury of hippocampal neurocytes induced by amyloid beta via GR/BDNF signaling pathway. J. Recept. Signal Transduct. 2020, 40, 550–559. [Google Scholar] [CrossRef]
- Huang, Y.N.; Lin, C.I.; Liao, H.; Liu, C.Y.; Chen, Y.H.; Chiu, W.C.; Lin, S.H. Cholesterol overload induces apoptosis in SH-SY5Y human neuroblastoma cells through the up regulation of flotillin-2 in the lipid raft and the activation of BDNF/Trkb signaling. Neuroscience 2016, 328, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ma, H.; Guo, X.; Liu, J.; Gui, T.; Gai, Z. Farnesoid X Receptor (FXR) Aggravates Amyloid-β-Triggered Apoptosis by Modulating the cAMP-Response Element-Binding Protein (CREB)/Brain-Derived Neurotrophic Factor (BDNF) Pathway In Vitro. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 9335–9345. [Google Scholar] [CrossRef]
- Guo, C.C.; Jiao, C.H.; Gao, Z.M. Silencing of LncRNA BDNF-AS attenuates Aβ25-35-induced neurotoxicity in PC12 cells by suppressing cell apoptosis and oxidative stress. Neurol. Res. 2018, 40, 795–804. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur. J. Cell Biol. 2017, 96, 240–253. [Google Scholar] [CrossRef]
- Pinky Anwar, S.; Neha Parvez, S. Paeoniflorin inhibits pyruvate dehydrogenase kinase 3 and promotes BDNF activity by modulating neuronal activity and TNF-α. Brain Res. 2025, 1851, 149476. [Google Scholar] [CrossRef] [PubMed]
- Tagai, N.; Tanaka, A.; Sato, A.; Uchiumi, F.; Tanuma, S.I. Low Levels of Brain-Derived Neurotrophic Factor Trigger Self-aggregated Amyloid β-Induced Neuronal Cell Death in an Alzheimer’s Cell Model. Biol. Pharm. Bull. 2020, 43, 1073–1080. [Google Scholar] [CrossRef]
- Huang, C.; Gan, D.; Fan, C.; Wen, C.; Li, A.; Li, Q.; Zhao, J.; Wang, Z.; Zhu, L.; Lu, D. The Secretion from Neural Stem Cells Pretreated with Lycopene Protects against tert-Butyl Hydroperoxide-Induced Neuron Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 5490218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiao, G.; Song, C.; Gu, S.; Brown, R.E.; Zhang, J.; Zhang, P.; Gagnon, J.; Locke, S.; Stefanova, R.; et al. An Extract from Shrimp Processing By-Products Protects SH-SY5Y Cells from Neurotoxicity Induced by Aβ25-35. Mar. Drugs 2017, 15, 83. [Google Scholar] [CrossRef]
- Wen, C.; Huang, C.; Yang, M.; Fan, C.; Li, Q.; Zhao, J.; Gan, D.; Li, A.; Zhu, L.; Lu, D. The Secretion from Bone Marrow Mesenchymal Stem Cells Pretreated with Berberine Rescues Neurons with Oxidative Damage Through Activation of the Keap1-Nrf2-HO-1 Signaling Pathway. Neurotox. Res. 2020, 38, 59–73. [Google Scholar] [CrossRef]
- Fleitas, C.; Piñol-Ripoll, G.; Marfull, P.; Rocandio, D.; Ferrer, I.; Rampon, C.; Egea, J.; Espinet, C. ProBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol. Brain 2018, 11, 68. [Google Scholar] [CrossRef]
- Song, M.S.; Learman, C.R.; Ahn, K.C.; Baker, G.B.; Kippe, J.; Field, E.M.; Dunbar, G.L. In vitro validation of effects of BDNF-expressing mesenchymal stem cells on neurodegeneration in primary cultured neurons of APP/PS1 mice. Neuroscience 2015, 307, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Firouzan, B.; Iravanpour, F.; Abbaszadeh, F.; Akparov, V.; Zaringhalam, J.; Ghasemi, R.; Maghsoudi, N. Dipeptide mimetic of BDNF ameliorates motor dysfunction and striatal apoptosis in 6-OHDA-induced Parkinson’s rat model: Considering Akt and MAPKs signaling. Behav. Brain Res. 2023, 452, 114585. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, J.; Sun, G.; Feng, M. BDNF-modified human umbilical cord mesenchymal stem cells-derived dopaminergic-like neurons improve rotation behavior of Parkinson’s disease rats through neuroprotection and anti-neuroinflammation. Mol. Cell. Neurosci. 2022, 123, 103784. [Google Scholar] [CrossRef] [PubMed]
- Anusha, C.; Sumathi, T.; Joseph, L.D. Protective role of apigenin on rotenone induced rat model of Parkinson’s disease: Suppression of neuroinflammation and oxidative stress mediated apoptosis. Chem.-Biol. Interact. 2017, 269, 67–79. [Google Scholar] [CrossRef]
- Farid, H.A.; Sayed, R.H.; El-Shamarka, M.E.; Abdel-Salam, O.M.E.; El Sayed, N.S. PI3K/AKT signaling activation by roflumilast ameliorates rotenone-induced Parkinson’s disease in rats. Inflammopharmacology 2024, 32, 1421–1437. [Google Scholar] [CrossRef]
- Bilge, S.S.; Günaydin, C.; Önger, M.E.; Bozkurt, A.; Avci, B. Neuroprotective action of agmatine in rotenone-induced model of Parkinson’s disease: Role of BDNF/cREB and ERK pathway. Behav. Brain Res. 2020, 392, 112692. [Google Scholar] [CrossRef]
- Singh, R.; Zahra, W.; Singh, S.S.; Birla, H.; Rathore, A.S.; Keshri, P.K.; Dilnashin, H.; Singh, S.; Singh, S.P. Oleuropein confers neuroprotection against rotenone-induced model of Parkinson’s disease via BDNF/CREB/Akt pathway. Sci. Rep. 2023, 13, 2452. [Google Scholar] [CrossRef]
- Lin, J.G.; Chen, C.J.; Yang, H.B.; Chen, Y.H.; Hung, S.Y. Electroacupuncture Promotes Recovery of Motor Function and Reduces Dopaminergic Neuron Degeneration in Rodent Models of Parkinson’s Disease. Int. J. Mol. Sci. 2017, 18, 1846. [Google Scholar] [CrossRef] [PubMed]
- Hacioglu, G.; Cirrik, S.; Tezcan Yavuz, B.; Tomruk, C.; Keskin, A.; Uzunoglu, E.; Takir, S. The BDNF-TrkB signaling pathway is partially involved in the neuroprotective effects of hydrogen sulfide in Parkinson’s disease. Eur. J. Pharmacol. 2023, 944, 175595. [Google Scholar] [CrossRef]
- Ji, C.; Xue, G.F.; Lijun, C.; Feng, P.; Li, D.; Li, L.; Li, G.; Hölscher, C. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing expression of BNDF. Brain Res. 2016, 1634, 1–11. [Google Scholar] [CrossRef]
- Hang, W.; Fan, H.J.; Li, Y.R.; Xiao, Q.; Jia, L.; Song, L.J.; Gao, Y.; Jin, X.M.; Xiao, B.G.; Yu, J.Z.; et al. Wuzi Yanzong pill attenuates MPTP-induced Parkinson’s Disease via PI3K/Akt signaling pathway. Metab. Brain Dis. 2022, 37, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Hassani, O.K.; Rymar, V.V.; Nguyen, K.Q.; Huo, L.; Cloutier, J.F.; Miller, F.D.; Sadikot, A.F. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: Implications for development and Parkinson’s disease. Neurobiol. Aging 2020, 85, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Mao, Q.; Zhang, X.; Wu, B.; Bi, K.; He, B.; Jia, Y. Schisandra chinensis protects against dopaminergic neuronal oxidative stress, neuroinflammation and apoptosis via the BDNF/Nrf2/NF-κB pathway in 6-OHDA-induced Parkinson’s disease mice. Food Funct. 2021, 12, 4079–4091. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhao, X.; Lu, K.; Cheng, G. LncRNA BDNF-AS promotes autophagy and apoptosis in MPTP-induced Parkinson’s disease via ablating microRNA-125b-5p. Brain Res. Bull. 2020, 157, 119–127. [Google Scholar] [CrossRef]
- Nadig, A.P.R.; Huwaimel, B.; Alobaida, A.; Khafagy, E.S.; Alotaibi, H.F.; Moin, A.; Lila, A.S.A.; Suman, M.S.; Krishna, K.L. Manganese chloride (MnCl2) induced novel model of Parkinson’s disease in adult Zebrafish; Involvement of oxidative stress, neuroinflammation and apoptosis pathway. Biomed. Pharmacother. 2022, 155, 113697. [Google Scholar] [CrossRef]
- Yu, Y.; Lang, X.Y.; Li, X.X.; Gu, R.Z.; Liu, Q.S.; Lan, R.; Qin, X.Y. 2,3,5,4’-Tetrahydroxystilbene-2-O-β-d-glucoside attenuates MPP+/MPTP-induced neurotoxicity in vitro and in vivo by restoring the BDNF-TrkB and FGF2-Akt signaling axis and inhibition of apoptosis. Food Funct. 2019, 10, 6009–6019. [Google Scholar] [CrossRef]
- Deng, C.; Zhu, J.; Yuan, J.; Xiang, Y.; Dai, L. Pramipexole Inhibits MPP+-Induced Neurotoxicity by miR-494-3p/BDNF. Neurochem. Res. 2020, 45, 268–277. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Raza, M.U.; Zhan, Y.; Fan, Y. Norepinephrine upregulates the expression of tyrosine hydroxylase and protects dopaminegic neurons against 6-hydrodopamine toxicity. Neurochem. Int. 2019, 131, 104549. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death. Int. J. Mol. Sci. 2022, 23, 16176. [Google Scholar] [CrossRef]
- Cheon, M.J.; Lee, N.K.; Paik, H.D. Neuroprotective Effects of Heat-Killed Lactobacillus plantarum 200655 Isolated from Kimchi Against Oxidative Stress. Probiotics Antimicrob. Proteins 2021, 13, 788–795. [Google Scholar] [CrossRef]
- Chen, Y.F.; Wu, S.N.; Gao, J.M.; Liao, Z.Y.; Tseng, Y.T.; Fülöp, F.; Chang, F.R.; Lo, Y.C. The Antioxidant, Anti-Inflammatory, and Neuroprotective Properties of the Synthetic Chalcone Derivative AN07. Molecules 2020, 25, 2907. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lin, L.Y.; Zhang, Y.; Lim, Y.; Rahman, M.; Beck, A.; Al-Hawwas, M.; Feng, S.; Bobrovskaya, L.; Zhou, X.F. Pro-BDNF Knockout Causes Abnormal Motor Behaviours and Early Death in Mice. Neuroscience 2020, 438, 145–157. [Google Scholar] [CrossRef]
- Wehner, A.B.; Milen, A.M.; Albin, R.L.; Pierchala, B.A. The p75 neurotrophin receptor augments survival signaling in the striatum of pre-symptomatic Q175(WT/HD) mice. Neuroscience 2016, 324, 297–306. [Google Scholar] [CrossRef]
- Cardinale, A.; Paldino, E.; Giampà, C.; Bernardi, G.; Fusco, F.R. PARP-1 Inhibition Is Neuroprotective in the R6/2 Mouse Model of Huntington’s Disease. PLoS ONE 2015, 10, e0134482. [Google Scholar] [CrossRef] [PubMed]
- Smail, S.; Bahga, D.; McDole, B.; Guthrie, K. Increased Olfactory Bulb BDNF Expression Does Not Rescue Deficits in Olfactory Neurogenesis in the Huntington’s Disease R6/2 Mouse. Chem. Senses 2016, 41, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Solés-Tarrés, I.; Cabezas-Llobet, N.; Lefranc, B.; Leprince, J.; Alberch, J.; Vaudry, D.; Xifró, X. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Protects Striatal Cells and Improves Motor Function in Huntington’s Disease Models: Role of PAC1 Receptor. Front. Pharmacol. 2022, 12, 797541. [Google Scholar] [CrossRef]
- Ahmed, S.; Kwatra, M.; Gawali, B.; Panda, S.R.; Naidu, V.G. Potential role of TrkB agonist in neuronal survival by promoting CREB/BDNF and PI3K/Akt signaling in vitro and in vivo model of 3-nitropropionic acid (3-NP)-induced neuronal death. Apoptosis 2021, 26, 52–70. [Google Scholar] [CrossRef]
- Abdelfattah, M.S.; Badr, S.E.A.; Lotfy, S.A.; Attia, G.H.; Aref, A.M.; Abdel Moneim, A.E.; Kassab, R.B. Rutin and Selenium Co-administration Reverse 3-Nitropropionic Acid-Induced Neurochemical and Molecular Impairments in a Mouse Model of Huntington’s Disease. Neurotox. Res. 2020, 37, 77–92. [Google Scholar] [CrossRef]
- Ibrahim, W.W.; Abdel Rasheed, N.O. Diapocynin neuroprotective effects in 3-nitropropionic acid Huntington’s disease model in rats: Emphasis on Sirt1/Nrf2 signaling pathway. Inflammopharmacology 2022, 30, 1745–1758. [Google Scholar] [CrossRef]
- Gendy, A.M.; Soubh, A.; Elnagar, M.R.; Hamza, E.; Ahmed, K.A.; Aglan, A.; El-Haddad, A.E.; Farag, M.A.; El-Sadek, H.M. New insights into the role of berberine against 3-nitropropionic acid-induced striatal neurotoxicity: Possible role of BDNF-TrkB-PI3K/Akt and NF-κB signaling. Food Chem. Toxicol. 2023, 175, 113721. [Google Scholar] [CrossRef]
- Kaur, P.; Attri, S.; Singh, D.; Rashid, F.; Singh, S.; Kumar, A.; Kaur, H.; Bedi, N.; Arora, S. Neuromodulatory effect of 4-(methylthio)butyl isothiocyanate against 3-nitropropionic acid induced oxidative impairments in human dopaminergic SH-SY5Y cells via BDNF/CREB/TrkB pathway. Sci. Rep. 2023, 13, 4461. [Google Scholar] [CrossRef] [PubMed]
- Üremiş, N.; Üremiş, M.M. Oxidative/Nitrosative stress, Apoptosis, and redox signaling: Key players in neurodegenerative diseases. J. Biochem. Mol. Toxicol. 2025, 39, e70133. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Wilson, K.; Saharan, A.; Gautam, R.K.; Chopra, H.; Gupta, S.; Amjad Kamal, M. Insights on aspects of apoptosis in neurodegenerative disorders: A comprehensive review. Explor. Med. 2024, 5, 89–100. [Google Scholar] [CrossRef]
- Giarratana, A.O.; Teng, S.; Reddi, S.; Zheng, C.; Adler, D.; Thakker-Varia, S.; Alder, J. BDNF Val66Met genetic polymorphism results in poor recovery following repeated mild traumatic brain injury in a mouse model and treatment with AAV-BDNF improves outcomes. Front. Neurol. 2019, 10, 1175. [Google Scholar] [CrossRef] [PubMed]
- Health, USD. First-In-Human Clinical Trial to Assess Gene Therapy for Alzheimer’s Disease; Health, USD: Tokyo, Japan, 2021; Available online: https://health.ucsd.edu/news/releases/Pages/2021-02-18-first-in-human-clinical-trial-to-assess-gene-therapy-for-alzheimers-disease.aspx (accessed on 10 April 2025).
- Bennett, J.; Wellman, J.; Marshall, K.A.; McCague, S.; Ashtari, M.; DiStefano-Pappas, J.; Elci, O.U.; Chung, D.C.; Sun, J.; Wright, J.F.; et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: A follow-on phase 1 trial. Lancet 2016, 388, 661–672. [Google Scholar] [CrossRef]
- Long, S.; Wang, Y.; Cheng, R.; Deng, L.; Chen, L.; Dong, Y. Different IL-1β levels differentially mediate neuroprotection or neurodegeneration and may be related to BDNF. Cytokine 2025, 188, 156877. [Google Scholar] [CrossRef]
- Head, B.; Patel, H.; Niesman, I.; Drummond, J.; Roth, D.; Patel, P. Inhibition of p75 Neurotrophin receptor attenuates isoflurane-mediated neuronal Apoptosis in the neonatal central nervous system. Anesthesiology 2009, 110, 813–825. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, Z.; Liu, X.; Kang, S.S.; Zhang, Y.; Ye, K. The prodrug of 7,8-dihydroxyflavone development and therapeutic efficacy for treating Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, 578–583. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Kang, S.S.; Wu, Z.; Liu, X.; Edgington-Mitchell, L.; Ye, K. Treating Parkinson’s disease via activation of BDNF/TrkB signaling pathways and inhibition of delta-secretase. Neurotherapeutics 2022, 19, 1283–1297. [Google Scholar] [CrossRef]
- Jia, D.; Wang, F.; Bai, Z.; Chen, X. BDNF-trkb/probdnf-p75ntr pathway regulation by lipid emulsion rescues bupivacaine-induced central neurotoxicity in rats. Sci. Rep. 2023, 13, 18364. [Google Scholar] [CrossRef] [PubMed]
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [158] | BDNF ↓ | TrkB ↓ | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Angelica sinensis polysaccharide (antioxidant) | BDNF/TrkB/CREB pathway ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [159] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Brain-specific angiogenesis inhibitor-1 | STAT3/EZH2 pathway ↑ BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [161] | BDNF-AS ↑ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | miR-125b-5p ↑ BDNF-AS ↓ | Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [162] | BDNF ↓ | Bax ↑ Bcl-2 ↓ | miR-22 | BDNF ↑ Bax ↓ Bcl-2 ↑ | |
| [163] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Mesenchymal stem cell application | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [164] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Neural stem cells | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [165] | BDNF ↓ | - | Positive apoptotic neuronal cells ↑ | BDNF-modified hUC-MSCs | BDNF ↑ Positive apoptotic neuronal cells ↓ |
| [166] | BDNF ↓ | TrkB ↓ | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ Caspase-9 ↑ | Cynomorium songaricum (antioxidant, anti-inflammatory) | BDNF ↑ TrkB ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Caspase-9 ↓ |
| [167] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ Caspase-9 ↑ | Carbenoxolone (anti-inflammatory) | BDNF/TrkB/CREB pathway ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Caspase-9 ↓ |
| [168] | BDNF ↓ | - | DNA fragmentation ↑ | Lactuca capensis (anti-inflammatory) | BDNF ↑ DNA fragmentation ↓ |
| [169] | BDNF ↓ | TrkB ↓ | DNA fragmentation ↑ | Astragaloside Exercise rehabilitation | BDNF ↑ TrkB ↑ DNA fragmentation ↓ |
| [170] | BDNF ↓ | TrkB ↓ | Bax ↑ Bcl-2 ↓ Pro-caspase-3 ↓ Active caspase-3 ↑ | Icariside II (antioxidant, anti-inflammatory) | BDNF/TrkB/CREB pathway ↑ Bax ↓ Bcl-2 ↑ Pro-caspase-3 ↑ Active caspase-3 ↓ |
| [171] | BDNF ↓ | TrkB ↓ | Number of neuronal Nissl bodies ↓ | EPA-enriched ethanolamine plasmalogen EPA-enriched phosphatidylethanolamine | BDNF/TrkB/CREB pathway ↑ Number of neuronal Nissl bodies ↑ |
| [172] | BDNF ↓ | TrkB ↓ | Bax ↑ Bcl-2 ↓ | Naringin (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF ↑ TrkB ↑ Bax ↓ Bcl-2 ↑ |
| [173] | BDNF ↓ | TrkB ↓ | Neuronal apoptosis in CA1 ↑ | Tan II (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF/TrkB/CREB pathway ↑ |
| [174] | BDNF ↓ | TrkB ↑ | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Syringic acid (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF/TrkB/CREB pathway ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [175] | BDNF ↓ | - | Caspase-3 ↑ | Diminazene aceturate | BDNF ↑ Caspase-3 ↓ |
| [176] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | L-theanine (antioxidant, anti-inflammatory) | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [177] | BDNF ↓ | TrkB ↓ | Neuronal nuclear protein ↓ ↑ Apoptosis | - | - |
| [178] | BDNF ↓ | - | Caspase-3 ↑ | Curcumin (anti-inflammatory, neuroprotective) | BDNF ↑ Caspase-3 ↓ |
| [179] | BDNF ↓ | - | Number of apoptotic cells ↑ Amyloid accumulation ↑ | Polysaccharide fraction of Phellinus ribis (neurotrophic, neuroprotective) | BDNF ↑ Number of apoptotic cells ↓ Amyloid accumulation ↓ |
| [180] | BDNF ↓ | TrkB ↓ | Bcl-2/Bax ratio ↓ | DL0410 (AChE inhibitor, antioxidant, anti-inflammatory) | BDNF ↑ TrkB ↑ Bcl-2/Bax ratio ↑ |
| [181] | BDNF ↓ | - | Neuronal cell loss and injury ↑ Bax/Bcl-2 ratio ↑ | 3,6′-Disinapoyl sucrose (antidepressant, antioxidant) | BDNF ↑ Neuronal cell loss and injury ↓ Bax/Bcl-2 ratio ↓ |
| [182] | BDNF ↓ | - | Apoptosis signal-regulating kinase-1 ↑ c-Jun N-terminal kinase mRNA ↑ | Qifu Yin (antioxidant, anti-apoptotic) | BDNF ↑ Apoptosis signal-regulating kinase-1 ↓ c-Jun N-terminal kinase mRNA ↓ |
| [183] | BDNF ↓ | - | Caspase-3 ↑ Bax ↑ Phosphorylated p53 ↑ | (Gene regulating) conversion of PUFA-6 to PUFA-3 | BDNF ↑ Caspase-3 ↓ Bax ↓ Phosphorylated p53 ↓ |
| [184] | BDNF ↓ | TrkB ↓ | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ | Cycloastragenol (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF ↑ TrkB ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ |
| [185] | BDNF ↓ | - | Caspase-3 ↑ | Vanillin (antioxidant) | BDNF ↑ Caspase-3 ↓ |
| [186] | BDNF ↓ | - | Caspase-3 ↑ Caspase-7 ↑ Caspase-9 ↑ Adam-10 ↑ | Resveratrol (antioxidant) Exercise training | BDNF ↑ Caspase-3 ↓ Caspase-7 ↓ Caspase-9 ↓ Adam-10 ↓ |
| [187] | proBDNF ↑ BDNF ↓ | TrkB ↓ | Neuronal cell loss and injury ↑ JNK/c-Jun ↑ | PAI-039 | proBDNF ↓ BDNF ↑ TrkB ↑ Neuronal cell loss and injury ↓ JNK/c-Jun ↓ |
| [188] | proBDNF ↑ BDNF ↓ | TrkB ↓ | tPA/mBDNF/TrkB signaling pathway ↑ proBDNF/BDNF ↑ | - | - |
| [189] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Pro-caspase-3 ↑ Caspase-3 ↑ | Melatonin (antioxidant, anti-apoptotic, anti-inflammatory) | Akt/ERK/CREB signaling Bax ↓ Bcl-2 ↑ Pro-caspase-3 ↓ Caspase-3 ↓ |
| [190] | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Caspase-3 ↑ Hippocampal BDNF-immunoreactive neurons ↓ | Ginkgo biloba L. leaf extract Hericium erinaceus (Bull.) Pers. fruit extract (antioxidant, anti-inflammatory) | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Hippocampal BDNF-immunoreactive neurons ↑ |
| [191] | BDNF ↓ | - | Adam-10 ↓ Presenilin-1 ↑ β-secretase-1 ↑ | Propane-2-sulfonic acid octadec-9-enyl-amide (antioxidant, anti-inflammatory, anti-apoptotic) | BDNF ↑ Presenilin-1 ↓ β-secretase-1 ↓ Adam-10 ↑ |
| [192] | BDNF ↓ | Fas-L ↑ | Human umbilical cord blood-derived stem cells | BDNF ↑ Fas-L ↓ CREB ↑ | |
| [193] | proBDNF ↑ BDNF ↓ | pTrkB ↑ TrkB ↓ p75 ↑ | Hippocampal neuronal cell loss ↑ | Electroacupuncture | proBDNF ↓ BDNF ↑ TrkB ↑ pTrkB ↓ p75 ↓ Hippocampal neuronal cell loss ↓ |
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [226] (rat mesencephalic dopamine neurons and SH-SY5Y cell line) | BDNF ↓ | TrkB ↓ | Cell viability↓ Apoptotic rate ↑ | THSG (antioxidant, anti-aging, anti-inflammatory) | BDNF ↑ Apoptotic rate ↓ Bcl-2 ↑ Cleaved caspase-3 ↓ |
| [227] (human neuroblastoma SK-N-SH and CHP 212 cells) | BDNF ↓ | - | Bax ↑ Bcl-2 ↓ Cleaved caspase-3 ↑ | Pramipexole (PPX) (selective dopamine D2 receptor agonist) | BDNF↑ Bax ↓ Bcl-2 ↑ Cleaved caspase-3 ↓ |
| [228] (rat ventral mesencephalon and dopaminergic cell line MN9D) | BDNF ↓ | - | Apoptotic rate ↑ | Norepinephrine | BDNF ↑ Tyrosine hydroxylase ↑ pERK1/2 ↑ |
| [204] (human monocytic THP-1 cells, murine BV-2 microglia cell line, murine NSC-34 neuronal cells, and cDNA from human primary microglia and astrocytes) | - | - | Caspase-3/7 ↑ | GLP-1 (glucagon-like peptide-1), GIP (glucose-dependent insulinotropic polypeptide) | BDNF ↑ Caspase-3/7 ↓ |
| [229] (SH-SY5Y cell line) | BDNF ↓ | - | Mitochondria damage ↑ Cell viability ↓ | EPA (eicosapentaenoic acid) | BDNF ↑ Mitochondria damage ↓ Cell viability ↑ |
| [230] (HT-29 (human colon adenocarcinoma) and SH-SY5Y) | BDNF ↓ | - | Bax/Bcl-2 ratio ↑ Caspase-3 ↑ | Lactobacillus plantarum 200655 | BDNF ↑ Bax/Bcl-2 ratio ↓ Caspase-3 ↓ Apoptotic rate ↓ |
| [231] (SH-SY5Y cells and murine macrophage cell line RAW 264.7) | BDNF ↓ | - | Bcl-2 ↓ Cytochrome c ↑ | Hydroxy-4′-methoxychalcone (AN07), a synthetic chalcone derivative | BDNF ↑ Bcl-2 ↑ Cytochrome c ↓ |
| BDNF/proBDNF | Neurotrophin Receptor | Apoptotic Mechanism | Treatment | Effect | |
|---|---|---|---|---|---|
| [232] (pro-BDNF knockout mouse model) | BDNF ↓ | TrkB n.c. p75 n.c. sortilin n.c. | Caspase-3 ↑ Bcl-2 ↓ Cell density ↓ Nuclear pyknosis ↑ | - | - |
| [233] (Q175 knock-in mouse model) | - | TrkB n.c. p75 n.c. | Bcl-XL ↓ XIAP ↓ Striatal volume ↓ | - | - |
| [234] (R6/2 mutant mice) | BDNF ↓ | - | Bax/Bcl-2 ratio ↑ Striatal volume ↓ Number of striatal neurons ↓ | INO-1001 (PARP-1 inhibitor) | BDNF ↑ Bax/Bcl-2 ratio ↓ Striatal volume ↑ Number of striatal neurons ↑ |
| [235] (R6/2 mutant mice) | BDNF ↓ | - | Number of striatal neurons ↓ Granule cell survival ↓ | Exogenous BDNFE7 | BDNF ↑ Number of striatal neurons ↑ Granule cell survival n.c. |
| [236] (R6/1 mutant mice) | BDNF ↓ | Caspase-3 ↑ | PACAP | BDNF ↑ Caspase-3 ↓ | |
| [237] (3-NP mouse and cell line model) | BDNF ↓ | pTrkB/TrkB ↓ | Caspase-3 ↑ Number of striatal neurons ↓ | 7,8-DHF (TrkB agonist) | BDNF ↑ pTrkB/TrkB ↑ Caspase-3 ↓ Number of striatal neurons ↑ |
| [238] (3-NP mouse model) | BDNF ↓ | - | Bax ↑ Caspase-3 ↑ Bcl-2 ↓ Striatal nuclear pyknosis ↑ | Rutin and selen (antioxidant, anti-apoptotic, anti-inflammatory) | BDNF ↑ Bax ↓ Bcl-2 ↑ Caspase-3 ↓ Striatal nuclear pyknosis ↓ |
| [239] (3-NP rat model) | BDNF ↓ | - | Bax ↑ p53 ↑ Bcl-2 ↓ Number of striatal neurons ↓ | Diapocynin (NADPH oxidase inhibitor) | BDNF ↑ Bax ↓ Bcl-2 ↑ p53 ↓ Number of striatal neurons ↑ |
| [240] (3-NP rat model) | BDNF ↓ | pTrkB/TrkB ↓ | Caspase-3 ↑ Bcl-2 ↓ Striatal lesion score ↑ | Berberine (anti-inflammatory, antioxidant) | BDNF ↑ pTrkB/TrkB ↑ Caspase-3 ↓ Bcl-2 ↑ Striatal lesion score ↓ |
| [241] (3-NP on SH-SY5Y cells) | BDNF ↓ | TrkB ↓ | Caspase-3 ↑ Apoptosis (flow cytometry) ↑ | 4-MTBITC (structural analog of sulforaphane) | BDNF TrkB ↑ Caspase-3 ↓ Apoptosis (flow cytometry) ↓ |
| Therapeutic Strategy | Mechanism of Action | Targeted Pathway | Key Limitations |
|---|---|---|---|
| BDNF Gene Therapy [244] | AAV viral vector-mediated overexpression of BDNF | Endogenous BDNF/TrkB signaling | Delivery precision, safety concerns, immune response |
| TrkB Agonists [249,251] | Mimic BDNF and selectively activate TrkB signaling | PI3K/Akt and MAPK/ERK survival pathways | Poor pharmacokinetics, short half-life, limited BBB permeability |
| p75NTR Antagonists [252] | Block proBDNF-induced apoptosis | JNK and caspase-3 pro-apoptotic pathway | Interference with normal neurodevelopment and plasticity |
| Protease Activators [248] | Enhance conversion of proBDNF to BDNF | BDNF maturation and PI3K/Akt survival pathway | Limited specificity, risk of off-target shedding, lack of brain-selective activators |
| Sortilin Inhibitors [99] | Prevent formation of proBDNF–p75NTR apoptotic complex | ProBDNF binding and apoptotic pathway inhibition | Lack of clinical-grade inhibitors, unknown long-term safety |
| miRNA Modulation (e.g., miR-125b-5p) [161] | Restore BDNF translation by inhibiting BDNF-suppressing miRNAs | Post-transcriptional regulation and inhibition of Bax/Bcl-2 mitochondrial apoptosis | Delivery challenges, off-target gene effects |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrovic, M.; Selakovic, D.; Jovicic, N.; Ljujic, B.; Rosic, G. BDNF/proBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 4926. https://doi.org/10.3390/ijms26104926
Mitrovic M, Selakovic D, Jovicic N, Ljujic B, Rosic G. BDNF/proBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(10):4926. https://doi.org/10.3390/ijms26104926
Chicago/Turabian StyleMitrovic, Marina, Dragica Selakovic, Nemanja Jovicic, Biljana Ljujic, and Gvozden Rosic. 2025. "BDNF/proBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 10: 4926. https://doi.org/10.3390/ijms26104926
APA StyleMitrovic, M., Selakovic, D., Jovicic, N., Ljujic, B., & Rosic, G. (2025). BDNF/proBDNF Interplay in the Mediation of Neuronal Apoptotic Mechanisms in Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(10), 4926. https://doi.org/10.3390/ijms26104926





