Capsaicinoid Profiles, Phenolic Content, and Antioxidant Properties of Chili Peppers Grown in Urban Settings
Abstract
1. Introduction
2. Results
2.1. Pepper Fruit Quality Characteristics over Two Growing Seasons
2.2. Capsaicinoid Profiles of 47 Pepper Cultivars Grown During Year 2
2.3. Total Phenolic Content
2.4. Antioxidant Activity
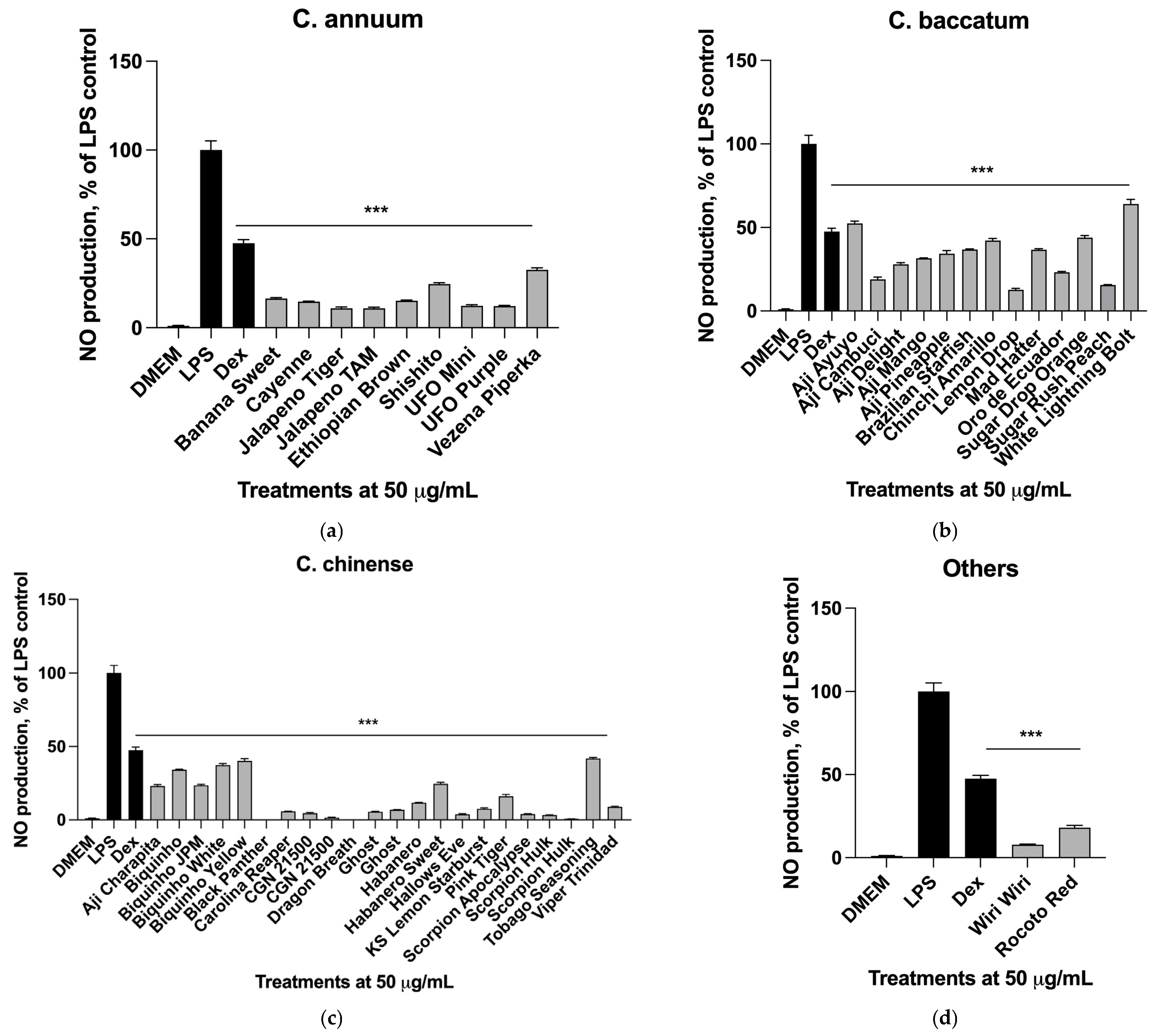
2.5. Nitric Oxide Production in Macrophages
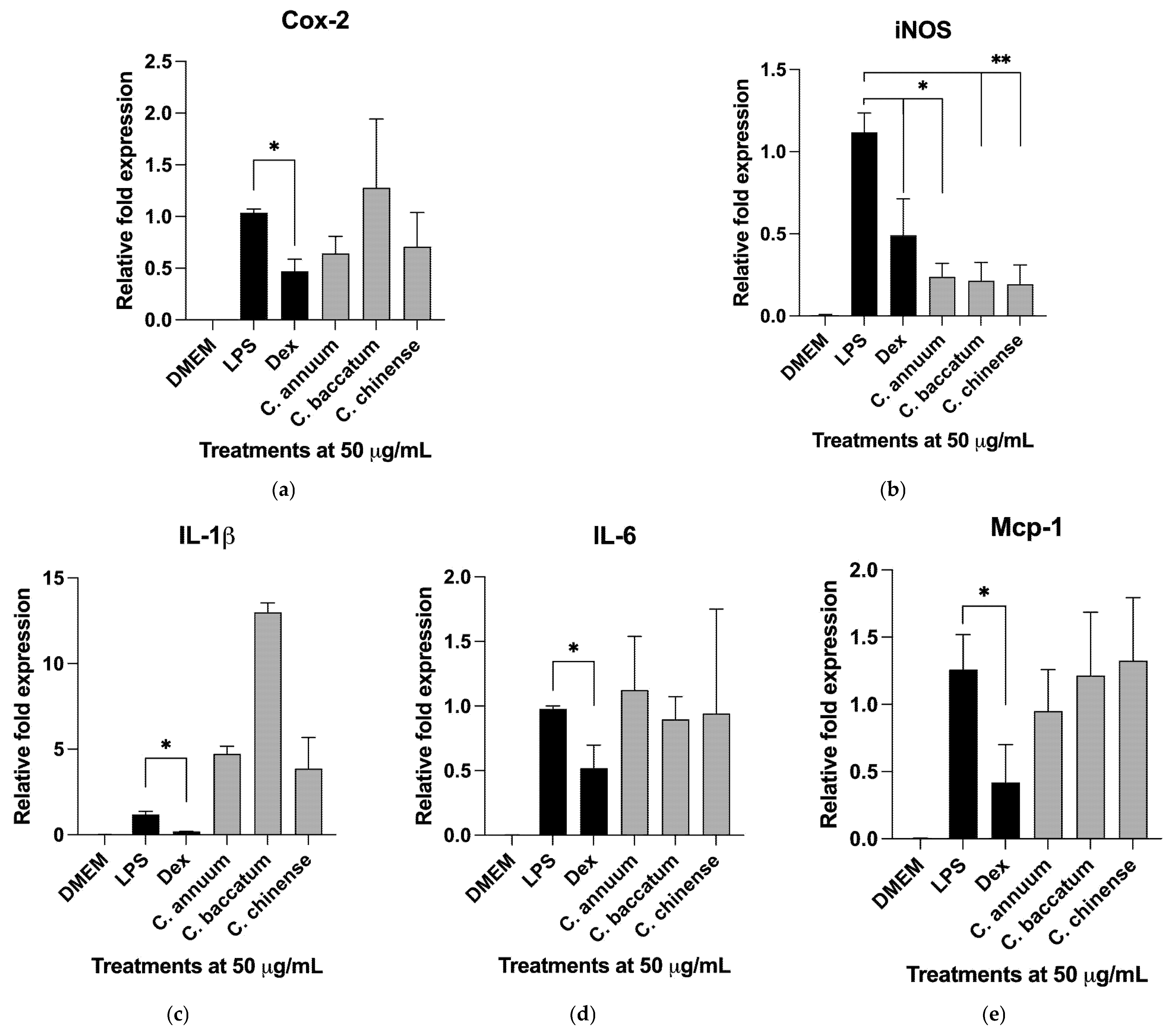
2.6. Gene Expression Profiles in Activated Macrophages
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Acetonitrile Extracts and Capsaicin Profiles by HPLC
4.3. Metanolic Extracts for Phenolic, Antioxidant, and Cell Culture Assays
4.4. Total Phenolics Assay
4.5. Free Radical Scavenging (ABTS/TEAC) Assay
4.6. DPPH Antioxidant Assay
4.7. Ferric-Reducing Antioxidant Power (FRAP) Assay
4.8. Cell Culture
4.9. Nitric Oxide Production
4.10. RNA Extraction, cDNA Synthesis, and qPCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bosland, P.W. Chiles: History, Cultivation, and Uses. Dev. Food Sci. 1994, 34, 347. [Google Scholar]
- Alonso-Villegas, R.; González-Amaro, R.M.; Figueroa-Hernández, C.Y.; Rodríguez-Buenfil, I.M. The Genus Capsicum: A Review of Bioactive Properties of Its Polyphenolic and Capsaicinoid Composition. Molecules 2023, 28, 4239. [Google Scholar] [CrossRef] [PubMed]
- Carrizo García, C.; Barfuss, M.H.J.; Sehr, E.M.; Barboza, G.E.; Samuel, R.; Moscone, E.A.; Ehrendorfer, F. Phylogenetic Relationships, Diversification and Expansion of Chili Peppers (Capsicum, Solanaceae). Ann. Bot. 2016, 118, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Kraft, K.H.; Brown, C.H.; Nabhan, G.P.; Luedeling, E.; Luna Ruiz, J.d.J.; Coppens d’Eeckenbrugge, G.; Hijmans, R.J.; Gepts, P. Multiple Lines of Evidence for the Origin of Domesticated Chili Pepper, Capsicum Annuum, in Mexico. Proc. Natl. Acad. Sci. USA 2014, 111, 6165–6170. [Google Scholar] [CrossRef]
- McClung de Tapia, E. The Origins of Agriculture in Mesoamerica and Central America. Orig. Agric. Int. Perspect. 1992, 40, 143–171. [Google Scholar]
- Perry, L.; Flannery, K.V. Precolumbian Use of Chili Peppers in the Valley of Oaxaca, Mexico. Proc. Natl. Acad. Sci. USA 2007, 104, 11905–11909. [Google Scholar] [CrossRef]
- Hernández-Verdugo, S.; Dávila, P.; Oyama, K. Síntesis Del Conocimiento Taxonómico, Origen y Domesticación Del Género Capsicum. Bot. Sci. 1999, 64, 65–84. [Google Scholar] [CrossRef][Green Version]
- Magdy, M.; Ou, L.; Yu, H.; Chen, R.; Zhou, Y.; Hassan, H.; Feng, B.; Taitano, N.; van der Knaap, E.; Zou, X.; et al. Pan-Plastome Approach Empowers the Assessment of Genetic Variation in Cultivated Capsicum Species. Hortic. Res. 2019, 6, 108. [Google Scholar] [CrossRef]
- Carrizo García, C.; Barboza, G.E.; Palombo, N.; Weiss-Schneeweiss, H. Diversification of Chiles (Capsicum, Solanaceae) through Time and Space: New Insights from Genome-Wide RAD-Seq Data. Front. Genet. 2022, 13, 1030536. [Google Scholar] [CrossRef]
- González-Pérez, S.; Garcés-Claver, A.; Mallor, C.; Sáenz de Miera, L.E.; Fayos, O.; Pomar, F.; Merino, F.; Silvar, C. New Insights into Capsicum Spp. Relatedness and the Diversification Process of Capsicum Annuum in Spain. PLoS ONE 2014, 9, e116276. [Google Scholar] [CrossRef]
- Komarnytsky, S.; Retchin, S.; Vong, C.I.; Lila, M.A. Gains and Losses of Agricultural Food Production: Implications for the Twenty-First Century. Annu. Rev. Food Sci. Technol. 2022, 13, 239–261. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Dias, L.; Vilanova, S.; Fita, A.; Prohens, J.; Rodríguez-Burruezo, A. Genetic Diversity, Population Structure, and Relationships in a Collection of Pepper (Capsicum spp.) Landraces from the Spanish Centre of Diversity Revealed by Genotyping-by-Sequencing (GBS). Hortic. Res. 2019, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Kantar, M.B.; Anderson, J.E.; Lucht, S.A.; Mercer, K.; Bernau, V.; Case, K.A.; Le, N.C.; Frederiksen, M.K.; DeKeyser, H.C.; Wong, Z.-Z.; et al. Vitamin Variation in Capsicum Spp. Provides Opportunities to Improve Nutritional Value of Human Diets. PLoS ONE 2016, 11, e0161464. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hassan, N.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Gómez-Caravaca, A.M.; López de Andrés, J.; Voltes-Martínez, A.; Zamora, A.; Pérez-Molina, G.M.; Castro, D.J.; Marchal, J.A.; Verardo, V. Evaluation of Phenolic Compounds and Pigments Content in Yellow Bell Pepper Wastes. Antioxidants 2022, 11, 557. [Google Scholar] [CrossRef]
- Lahbib, K.; Bnejdi, F.; Pandino, G.; Lombardo, S.; El-Gazzah, M.; El-Bok, S.; Dabbou, S. Changes in Yield-Related Traits, Phytochemical Composition, and Antioxidant Activity of Pepper (Capsicum Annuum) Depending on Its Variety, Fruit Position, and Ripening Stage. Foods 2023, 12, 3948. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Jin, J.S.; Cho, Y.A.; Lee, J.H.; Park, S.; Jeong, S.W.; Kim, Y.-H.; Lim, C.-S.; El-Aty, A.M.A.; Kim, G.-S.; et al. Determination of Polyphenols in Three Capsicum annuum L. (Bell Pepper) Varieties Using High-Performance Liquid Chromatography-Tandem Mass Spectrometry: Their Contribution to Overall Antioxidant and Anticancer Activity. J. Sep. Sci. 2011, 34, 2967–2974. [Google Scholar] [CrossRef]
- González-Paramás, A.M.; Ayuda-Durán, B.; Martínez, S.; González-Manzano, S.; Santos-Buelga, C. The Mechanisms Behind the Biological Activity of Flavonoids. Curr. Med. Chem. 2019, 26, 6976–6990. [Google Scholar] [CrossRef]
- Mazourek, M.; Pujar, A.; Borovsky, Y.; Paran, I.; Mueller, L.; Jahn, M.M. A Dynamic Interface for Capsaicinoid Systems Biology. Plant Physiol. 2009, 150, 1806–1821. [Google Scholar] [CrossRef]
- Stewart, C.; Mazourek, M.; Stellari, G.M.; O’Connell, M.; Jahn, M. Genetic Control of Pungency in C. Chinense via the Pun1 Locus. J. Exp. Bot. 2007, 58, 979–991. [Google Scholar] [CrossRef]
- Cervantes-Hernández, F.; Alcalá-González, P.; Martínez, O.; Ordaz-Ortiz, J.J. Placenta, Pericarp, and Seeds of Tabasco Chili Pepper Fruits Show a Contrasting Diversity of Bioactive Metabolites. Metabolites 2019, 9, 206. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, R.D.; Banks, C.E. Electroanalytical Overview: The Pungency of Chile and Chilli Products Determined via the Sensing of Capsaicinoids. Analyst 2021, 146, 2769–2783. [Google Scholar] [CrossRef]
- Willis, W.D. The Role of TRPV1 Receptors in Pain Evoked by Noxious Thermal and Chemical Stimuli. Exp. Brain Res. 2009, 196, 5–11. [Google Scholar] [CrossRef]
- Simon, S.A.; de Araujo, I.E. The Salty and Burning Taste of Capsaicin. J. Gen. Physiol. 2005, 125, 531–534. [Google Scholar] [CrossRef]
- Haar, R.J.; Iacopino, V.; Ranadive, N.; Weiser, S.D.; Dandu, M. Health Impacts of Chemical Irritants Used for Crowd Control: A Systematic Review of the Injuries and Deaths Caused by Tear Gas and Pepper Spray. BMC Public Health 2017, 17, 831. [Google Scholar] [CrossRef]
- Fernández-Carvajal, A.; Fernández-Ballester, G.; Ferrer-Montiel, A. TRPV1 in Chronic Pruritus and Pain: Soft Modulation as a Therapeutic Strategy. Front. Mol. Neurosci. 2022, 15, 930964. [Google Scholar] [CrossRef]
- Reyes-Escogido, M.d.L.; Gonzalez-Mondragon, E.G.; Vazquez-Tzompantzi, E. Chemical and Pharmacological Aspects of Capsaicin. Molecules 2011, 16, 1253–1270. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.-K. Fight Fire with Fire: Neurobiology of Capsaicin-Induced Analgesia for Chronic Pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin Induces Browning of White Adipose Tissue and Counters Obesity by Activating TRPV1 Channel-dependent Mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef]
- Torres-Narváez, J.C.; Pérez-Torres, I.; Castrejón-Téllez, V.; Varela-López, E.; Oidor-Chan, V.H.; Guarner-Lans, V.; Vargas-González, Á.; Martínez-Memije, R.; Flores-Chávez, P.; Cervantes-Yañez, E.Z.; et al. The Role of the Activation of the TRPV1 Receptor and of Nitric Oxide in Changes in Endothelial and Cardiac Function and Biomarker Levels in Hypertensive Rats. Int. J. Environ. Res. Public Health 2019, 16, 3576. [Google Scholar] [CrossRef]
- Blum, E.; Mazourek, M.; O’Connell, M.; Curry, J.; Thorup, T.; Liu, K.; Jahn, M.; Paran, I. Molecular Mapping of Capsaicinoid Biosynthesis Genes and Quantitative Trait Loci Analysis for Capsaicinoid Content in Capsicum. Theor. Appl. Genet. 2003, 108, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Harvell, K.P.; Bosland, P.W. The Environment Produces a Significant Effect on Pungency of Chiles. HortScience 1997, 32, 1292. [Google Scholar] [CrossRef]
- Das, S.; Sarkar, S.; Das, M.; Banik, P.; Bhattacharya, S.S. Influence of Soil Quality Factors on Capsaicin Biosynthesis, Pungency, Yield, and Produce Quality of Chili: An Insight on Csy1, Pun1, and Pun12 Signaling Responses. Plant Physiol. Biochem. 2021, 166, 427–436. [Google Scholar] [CrossRef]
- Siebert, E.; Lee, S.-Y.; Prescott, M.P. Chili Pepper Preference Development and Its Impact on Dietary Intake: A Narrative Review. Front. Nutr. 2022, 9, 1039207. [Google Scholar] [CrossRef]
- Ebenso, B.; Otu, A.; Giusti, A.; Cousin, P.; Adetimirin, V.; Razafindralambo, H.; Effa, E.; Gkisakis, V.; Thiare, O.; Levavasseur, V.; et al. Nature-Based One Health Approaches to Urban Agriculture Can Deliver Food and Nutrition Security. Front. Nutr. 2022, 9, 773746. [Google Scholar] [CrossRef]
- Ju, J.-H.; Yoon, Y.-H.; Shin, S.-H.; Ju, S.-Y.; Yeum, K.-J. Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods. Horticulturae 2022, 8, 767. [Google Scholar] [CrossRef]
- Sridonpai, P.; Kongprapun, P.; Sungayuth, N.; Sukprasansap, M.; Chimkerd, C.; Judprasong, K. Nutritive Values and Phytochemical Compositions of Edible Indigenous Plants in Thailand. Front. Sustain. Food Syst. 2022, 6, 870147. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Liu, Y.; Li, M.-Y.; Ge, Y.-Y.; Geng, F.; He, X.-Q.; Xia, Y.; Guo, B.-L.; Gan, R.-Y. Antioxidant Capacity, Phytochemical Profiles, and Phenolic Metabolomics of Selected Edible Seeds and Their Sprouts. Front. Nutr. 2022, 9, 1067597. [Google Scholar] [CrossRef]
- Rathinasabapathy, T.; Sakthivel, L.P.; Komarnytsky, S. Plant-Based Support of Respiratory Health during Viral Outbreaks. J. Agric. Food Chem. 2022, 70, 2064–2076. [Google Scholar] [CrossRef]
- Ahmad, F.; Kusumiyati, K.; Soleh, M.A.; Khan, M.R.; Sundari, R.S. Chili Crop Innovation: Exploring Enclosed Growing Designs for Varied Varieties—A Review. Agrosystems Geosci. Environ. 2024, 7, e20491. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E. Preference for Hot Pepper: A Complex Interplay of Personal, Cultural, and Pharmacological Effects. Temperature 2016, 3, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Gudzune, K.A.; Welsh, C.; Lane, E.; Chissell, Z.; Anderson Steeves, E.; Gittelsohn, J. Increasing Access to Fresh Produce by Pairing Urban Farms with Corner Stores: A Case Study in a Low-Income Urban Setting. Public Health Nutr. 2015, 18, 2770–2774. [Google Scholar] [CrossRef]
- Hernández-Pérez, T.; Gómez-García, M.D.R.; Valverde, M.E.; Paredes-López, O. Capsicum Annuum (Hot Pepper): An Ancient Latin-American Crop with Outstanding Bioactive Compounds and Nutraceutical Potential. A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2972–2993. [Google Scholar] [CrossRef]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin-the Major Bioactive Ingredient of Chili Peppers: Bio-Efficacy and Delivery Systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef]
- Barboza, G.E.; García, C.C.; Bianchetti, L.d.B.; Romero, M.V.; Scaldaferro, M. Monograph of Wild and Cultivated Chili Peppers (Capsicum L., Solanaceae). PhytoKeys 2022, 200, 1. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, C.; Ye, Q.; Liu, C.; Wan, H.; Ruan, M.; Zhou, G.; Wang, R.; Li, Z.; Diao, M.; et al. The Influence of Different Factors on the Metabolism of Capsaicinoids in Pepper (Capsicum annuum L.). Plants 2024, 13, 2887. [Google Scholar] [CrossRef]
- Lozada, D.N.; Coon, D.L.; Guzmán, I.; Bosland, P.W. Heat Profiles of ‘Superhot’ and New Mexican Type Chile Peppers (Capsicum spp.). Sci. Hortic. 2021, 283, 110088. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Palma, J.M.; Terán, F.; Contreras-Ruiz, A.; Rodríguez-Ruiz, M.; Corpas, F.J. Antioxidant Profile of Pepper (Capsicum Annuum L.) Fruits Containing Diverse Levels of Capsaicinoids. Antioxidants 2020, 9, 878. [Google Scholar] [CrossRef]
- Lee, Y.; Howard, L.R.; Villalón, B. Flavonoids and Antioxidant Activity of Fresh Pepper (Capsicum annuum) Cultivars. J. Food Sci. 1995, 60, 473–476. [Google Scholar] [CrossRef]
- Materska, M. Flavone C-Glycosides from Capsicum Annuum L.: Relationships between Antioxidant Activity and Lipophilicity. Eur. Food Res. Technol. 2015, 240, 549–557. [Google Scholar] [CrossRef]
- Yang, D.; Luo, Z.; Ma, S.; Wong, W.T.; Ma, L.; Zhong, J.; He, H.; Zhao, Z.; Cao, T.; Yan, Z.; et al. Activation of TRPV1 by Dietary Capsaicin Improves Endothelium-Dependent Vasorelaxation and Prevents Hypertension. Cell Metab. 2010, 12, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-W.; Lee, S.T.; Wu, W.T.; Fu, W.-M.; Ho, F.-M.; Lin, W.W. Signal Transduction for Inhibition of Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Induction by Capsaicin and Related Analogs in Macrophages. Br. J. Pharmacol. 2003, 140, 1077–1087. [Google Scholar] [CrossRef]
- Tang, J.; Luo, K.; Li, Y.; Chen, Q.; Tang, D.; Wang, D.; Xiao, J. Capsaicin Attenuates LPS-Induced Inflammatory Cytokine Production by Upregulation of LXRα. Int. Immunopharmacol. 2015, 28, 264–269. [Google Scholar] [CrossRef]
- Herbert, M.K.; Holzer, P. Interleukin-1 Beta Enhances Capsaicin-Induced Neurogenic Vasodilatation in the Rat Skin. Br. J. Pharmacol. 1994, 111, 681–686. [Google Scholar] [CrossRef]
- Kim, H.B.; Na, E.Y.; Yun, S.J.; Lee, J.-B. The Effect of Capsaicin on Neuroinflammatory Mediators of Rosacea. Ann. Dermatol. 2022, 34, 261–269. [Google Scholar] [CrossRef]
- Bret-Dibat, J.-L.; Creminon, C.; Couraud, J.-Y.; Kelley, K.W.; Dantzer, R.; Kent, S. Systemic Capsaicin Pretreatment Fails to Block the Decrease in Food-Motivated Behavior Induced by Lipopolysaccharide and Interleukin-1β. Brain Res. Bull. 1997, 42, 443–449. [Google Scholar] [CrossRef]
- Salib, A.-M.N.; Crane, M.J.; Lee, S.H.; Wainger, B.J.; Jamieson, A.M.; Lipscombe, D. Interleukin-1α Links Peripheral CaV2.2 Channel Activation to Rapid Adaptive Increases in Heat Sensitivity in Skin. Sci. Rep. 2024, 14, 9051. [Google Scholar] [CrossRef]
- Lv, Z.; Xu, X.; Sun, Z.; Yang, Y.X.; Guo, H.; Li, J.; Sun, K.; Wu, R.; Xu, J.; Jiang, Q.; et al. TRPV1 Alleviates Osteoarthritis by Inhibiting M1 Macrophage Polarization via Ca2+/CaMKII/Nrf2 Signaling Pathway. Cell Death Dis. 2021, 12, 504. [Google Scholar] [CrossRef]
- Collins, M.D.; Wasmund, L.M.; Bosland, P.W. Improved Method for Quantifying Capsaicinoids in Capsicum Using High-Performance Liquid Chromatography. HortScience 1995, 30, 137–139. [Google Scholar] [CrossRef]
- Miletic, N.; Popović, B.; Mitrović, O.; Kandić, M. Phenolic Content and Antioxidant Capacity of Fruits of Plum Cv. “stanley” (Prunus Domestica L.) as Influenced by Maturity Stage and on-Tree Ripening. Aust. J. Crop Sci. 2012, 6, 681–687. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M.M. Comparison of DPPH and ABTS Assays for Determining Antioxidant Potential of Water and Methanol Extracts of Spirulina platensis. Indian J. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of Radical Scavenging Activity of Some Medicinal and Aromatic Plant Extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Tomasina, F.; Carabio, C.; Celano, L.; Thomson, L. Analysis of Two Methods to Evaluate Antioxidants. Biochem. Mol. Biol. Educ. 2012, 40, 266–270. [Google Scholar] [CrossRef]
| Species | Cultivar | Internal Code | Growing Year | Length, mm | Width, mm | Fresh Weight, g | Moisture Content, % |
|---|---|---|---|---|---|---|---|
| C. annuum | Banana Sweet | 14 (136) | 1 | 95 ± 13 a | 32 ± 3 a | 27.1 ± 2.8 a | 90.7 ± 8.1 ab |
| 2 | 106 ± 8 a | 35 ± 5 a | 33.4 ± 6.1 a | 89.1 ± 7.6 a | |||
| Cayenne | 93 (138) | 1 | 76 ± 4 b | 13 ± 1 c | 3.1 ± 0.3 e | 80.9 ± 11.4 b | |
| 2 | 66 ± 2 c | 12 ± 1 d | 3.6 ± 1.4 e | 82.2 ± 9.3 c | |||
| Jalapeno TAM | 57 | 1 | 66 ± 2 c | 25 ± 2 b | 15.6 ± 2.3 b | 86.6 ± 6.2 ab | |
| 2 | 66 ± 2 c | 24 ± 5 b | 14.3 ± 4.1 c | 84.2 ± 7.3 b c | |||
| C. baccatum | Aji Delight | 51 | 1 | 66 ± 2 c | 19 ± 1 b | 7.7 ± 0.5 d | 78.6 ± 6.3 c |
| 2 | 62 ± 12 c | 20 ± 2 b | 8.2 ± 0.9 d | 81.9 ± 4.4 c | |||
| Lemon Drop | 52 | 1 | 62 ± 5 c | 17 ± 1 b | 3.5 ± 0.2 e | 82.3 ± 12.7 b | |
| 2 | 59 ± 7 c | 16 ± 1 c | 3.9 ± 0.4 e | 80.5 ± 9.7 c | |||
| Sugar Rush Peach | 62 | 1 | 67 ± 1 c | 22 ± 1 b | 9.9 ± 0.6 c | 84.9 ± 5.9 b | |
| 2 | 64 ± 11 c | 19 ± 1 b | 9.2 ± 1.6 d | 82.4 ± 4.0 c | |||
| C. chinense | Aji Charapita | 50 | 1 | 13 ± 1 f | 10 ± 1 c | 0.6 ± 0.02 f | 83.5 ± 3.3 b |
| 2 | 12 ± 1 e | 11 ± 1 d | 0.5 ± 0.06 g | 81.6 ± 3.9 c | |||
| Biquinho | 53 | 1 | 24 ± 1 f | 13 ± 1 c | 1.3 ± 0.1 f | 83.8 ± 1.9 b | |
| 2 | 21 ± 2 d | 14 ± 1 c | 1.5 ± 0.2 f | 85.9 ± 4.5 b | |||
| Habanero | 59 | 1 | 49 ± 2 d | 18 ± 1 b | 5.1 ± 0.7 e | 87.7 ± 4.4 ab | |
| 2 | 44 ± 7 c | 16 ± 1 c | 4.9 ± 0.4 e | 88.4 ± 5.1 a | |||
| C. frutescens | Wiri Wiri | 95 (137) | 1 | 16 ± 1 f | 15 ± 1 bc | 2.4 ± 0.9 e | 76.7 ± 13.4 c |
| 2 | 14 ± 2 d | 17 ± 1 bc | 2.2 ± 1.1 ef | 81.4 ± 9.8 c | |||
| C. pubescens | Rocoto Red | 49 (154) | 1 | 37 ± 1 e | 37 ± 3 a | 18.5 ± 1.1 b | 93.3 ± 2.7 a |
| 2 | 41 ± 4 c | 34 ± 6 a | 20.9 ± 3.8 b | 92.3 ± 4.1 a |
| Species | Cultivar | Internal Code | C, μg/g 1 | DHC, μg/g 2 | NDHC, μg/g 3 | Pungency, SHU 4 |
|---|---|---|---|---|---|---|
| C. annuum | Banana Sweet | 14 (136) | 0 | 0 | 3 | 28 |
| Cayenne | 93 (138) | 3452 | 2026 | 399 | 91,907 | |
| Jalapeno Tiger | 57D | 1690 | 610 | 148 | 38,406 | |
| Jalapeno TAM | 57E | 891 | 252 | 35 | 18,728 | |
| Ethiopian Brown | 201 | 5057 | 1849 | 401 | 114,916 | |
| Shishito | 193 | 819 | 385 | 62 | 19,961 | |
| UFO Mini Black | 199 | 5667 | 1809 | 318 | 123,321 | |
| UFO Purple | 199A | 1300 | 557 | 160 | 31,386 | |
| Vezena Piperka | 139 | 5 | 2 | 0 | 113 | |
| C. baccatum | Aji Ayuyo | 159 | 940 | 496 | 189 | 24,877 |
| Aji Cambuci | 56 | 15 | 8 | 0 | 370 | |
| Aji Delight | 51 | 0 | 0 | 0 | 0 | |
| Aji Mango | 171 | 1320 | 615 | 212 | 33,125 | |
| Aji Pineapple | 172 | 2173 | 1050 | 222 | 53,955 | |
| Brazilian Starfish | 176 | 1541 | 269 | 92 | 29,997 | |
| Chinchi Amarillo | 174 | 744 | 262 | 116 | 17,275 | |
| Lemon Drop | 52 | 2315 | 1131 | 176 | 57,117 | |
| Mad Hatter | 175 | 4 | 1 | 0 | 81 | |
| Oro de Ecuador | 177 | 625 | 182 | 123 | 14,137 | |
| Sugar Rush Peach | 62 | 580 | 522 | 100 | 18,672 | |
| Sugar Drop Orange | 154 | 1137 | 1023 | 223 | 36,850 | |
| White Lightning Bolt | 170 | 807 | 479 | 116 | 21,783 | |
| White Lightning Bolt | 170A | 909 | 891 | 167 | 30,533 | |
| C. chinense | Aji Charapita | 50 | 3690 | 466 | 628 | 72,752 |
| Biquinho | 53 | 2473 | 419 | 83 | 47,333 | |
| Biquinho JPM | 53A | 3 | 3 | 0 | 97 | |
| Biquinho White | 53B | 1847 | 229 | 124 | 34,577 | |
| Biquinho Yellow | 53C | 2678 | 669 | 91 | 54,733 | |
| Black Panther | 135 | 15,741 | 7205 | 365 | 372,825 | |
| Carolina Reaper | 160 | 19,636 | 11,969 | 1017 | 518,299 | |
| CGN 21500 | 133A | 10,204 | 2147 | 565 | 204,106 | |
| CGN 21500 | 133B | 8379 | 1672 | 114 | 162,881 | |
| Dragon Breath | 162 | 17,991 | 10,004 | 786 | 458,029 | |
| Ghost | 61A | 7381 | 1451 | 100 | 143,125 | |
| Ghost | 61B | 9027 | 1925 | 152 | 177,741 | |
| Habanero | 59C | 11,214 | 1848 | 656 | 216,399 | |
| Habanero Sweet | 59B | 4 | 0 | 0 | 64 | |
| Hallows Eve | 129 | 14,751 | 5870 | 327 | 335,039 | |
| KS Lemon Starburst | 130 | 10,752 | 2947 | 49 | 221,010 | |
| Pink Tiger | 134 | 7424 | 1234 | 309 | 142,268 | |
| Scorpion Apocalypse | 161 | 463 | 136 | 36 | 9979 | |
| Scorpion Hulk | 164 | 14,424 | 7397 | 305 | 354,155 | |
| Scorpion Hulk | 164A | 10,011 | 4156 | 286 | 230,749 | |
| Tobago Seasoning | 156 | 6 | 2 | 0 | 129 | |
| Viper Trinidad | 163 | 14,143 | 5594 | 329 | 320,825 | |
| C. frutescens | Wiri Wiri | 95 (137) | 7718 | 2574 | 144 | 167,040 |
| C. pubescens | Rocoto Red | 49 (54) | 1137 | 2023 | 223 | 52,950 |
| Species | Cultivar | Internal Code | Phenolics, mg/g DW | ABTS µM TE/g DW | DPPH µM TE/g DW | FRAP µM TE/g DW |
|---|---|---|---|---|---|---|
| C. annuum | Banana Sweet | 14 (136) | 1.150 ± 0.079 kr | 11.583 ± 0.322 m–p | 6.097 ± 0.386 hp | 10.715 ± 0.439 jl |
| Cayenne | 93 (138) | 1.618 ± 0.099 ek | 16.167 ± 0.411 ik | 8.380 ± 0.404 bf–i | 18.421 ± 0.077 h | |
| Jalapeno Tiger | 57D | 1.740 ± 0.174 d–i | 17.703 ± 0.393 h–j | 6.820 ± 0.615 f–o | 15.689 ± 0.302 i | |
| Jalapeno TAM | 57E | 1.881 ± 0.142 d–g | 10.041 ± 0.109 nq–s | 7.091 ± 0.211 e-n | 9.864 ± 0.177 kl | |
| Ethiopian Brown | 201 | 1.602 ± 0.098 mr | 13.038 ± 0.362 l–n | 5.641 ± 0.271 kp | 11.887 ± 0.048 jk | |
| Shishito | 193 | 1.425 ± 0.015 gk–n | 8.021 ± 0.007 qw | 8.396 ± 0.047 bf–i | 6.631 ± 0.049 nq | |
| UFO Mini Black | 199 | 1.381 ± 0.029 hk–n | 16.472 ± 0.542 ik | 6.351 ± 0.397 fp | 12.550 ± 0.504 j | |
| UFO Purple | 199A | 1.348 ± 0.083 nr | 11.720 ± 0.385 m–o | 5.848 ± 0.478 jp | 8.775 ± 0.148 ln | |
| Vezena Piperka | 139 | 1.312 ± 0.032 i–o | 7.490 ± 0.011 sw | 7.177 ± 0.083 e–n | 5.613 ± 0.101 oq–t | |
| C. baccatum | Aji Ayuyo | 159 | 1.022 ± 0.043 lr | 8.066 ± 0.286 qw | 5.945 ± 0.093 ip | 4.456 ± 0.100 rw |
| Aji Cambuci | 56 | 1.156 ± 0.086 kr | 6.460 ± 0.256 tw | 4.873 ± 0.985 np | 4.229 ± 0.035 rw | |
| Aji Delight | 51 | 0.879 ± 0.070 or | 6.077 ± 0.060 uw | 4.559 ± 0.378 op | 3.256 ± 0.028 vw | |
| Aji Mango | 171 | 1.089 ± 0.025 lr | 6.759 ± 0.640 tw | 5.666 ± 0.844 jp | 3.865 ± 0.078 sw | |
| Aji Pineapple | 172 | 1.142 ± 0.048 kr | 7.719 ± 0.449 rw | 5.731 ± 0.805 jp | 4.577 ± 0.076 qw | |
| Brazilian Starfish | 176 | 1.124 ± 0.121 lr | 6.388 ± 0.572 tw | 6.745 ± 0.136 f–o | 3.609 ± 0.057 tw | |
| Chinchi Amarillo | 174 | 0.862 ± 0.036 or | 6.350 ± 0.316 tw | 4.566 ± 0.911 op | 3.252 ± 0.058 vw | |
| Lemon Drop | 52 | 1.013 ± 0.097 lr | 7.263 ± 0.016 sw | 5.214 ± 0.319 lp | 7.194 ± 0.135 m–p | |
| Mad Hatter | 175 | 1.405 ± 0.028 gk–n | 6.305 ± 0.833 tw | 7.298 ± 0.385 d–n | 3.081 ± 0.061 w | |
| Oro de Ecuador | 177 | 0.664 ± 0.043 r | 4.995 ± 0.202 w | 4.861 ± 0.159 np | 3.210 ± 0.057 vw | |
| Sugar Rush Peach | 62 | 1.499 ± 0.122 fkl | 8.504 ± 0.108 p–v | 7.540 ± 0.262 d–l | 7.592 ± 0.055 m–o | |
| Sugar Drop Orange | 154 | 1.013 ± 0.089 lr | 7.550 ± 0.164 sw | 3.994 ± 0.498 p | 5.319 ± 0.107 p–v | |
| White Lightning Bolt | 170 | 0.813 ± 0.004 pr | 5.574 ± 0.259 vw | 4.040 ± 0.765 p | 3.196 ± 0.043 vw | |
| White Lightning Bolt | 170 A | 0.799 ± 0.017 pr | 5.885 ± 0.067 vw | 4.970 ± 0.011 mp | 3.590 ± 0.031 tw | |
| C. chinense | Aji Charapita | 50 | 1.069 ± 0.032 lr | 15.074 ± 0.263 j–l | 5.723 ± 0.075 jp | 11.658 ± 0.162 jk |
| Biquinho | 53 | 1.496 ± 0.046 fkl | 17.381 ± 0.185 ij | 6.574 ± 0.581 f–o | 15.029 ± 1.109 i | |
| Biquinho JPM | 53A | 1.445 ± 0.032 g k–m | 8.439 ± 0.5056 q–v | 4.952 ± 0.589 m p | 5.491 ± 0.092 o q–u | |
| Biquinho White | 53B | 1.238 ± 0.066 j–q | 9.023 ± 0.032 oq–u | 6.565 ± 0.625 f–o | 8.833 ± 0.122 lm | |
| Biquinho Yellow | 53C | 1.406 ± 0.049 g–n | 16.963 ± 0.805 ij | 7.898 ± 0.092 cf–k | 12.162 ± 0.185 j | |
| Black Panther | 135 | 1.291 ± 0.047 i–p | 32.163 ± 0.121 e | 5.411 ± 0.043 kp | 39.290 ± 0.431 e | |
| Carolina Reaper | 160 | 3.078 ± 0.021 a | 77.847 ± 0.193 a | 11.641 ± 0.064 a | 111.777 ± 0.321 a | |
| CGN 21500 | 133A | 1.307 ± 0.008 i–o | 18.996 ± 0.331 hi | 7.425 ± 0.047 d–m | 17.947 ± 0.377 h | |
| CGN 21500 | 133B | 1.753 ± 0.137 d–i | 20.567 ± 1.593 h | 10.252 ± 0.134 a–c | 18.770 ± 0.147 gh | |
| Dragon Breath | 162 | 2.382 ± 0.182 bc | 64.680 ± 0.716 b | 9.372 ± 0.159 a–e | 98.194 ± 0.361 b | |
| Ghost | 61A | 2.710 ± 0.171 ab | 27.680 ± 0.164 f | 11.778 ± 0.876 a | 23.643 ± 0.430 f | |
| Ghost | 61B | 1.722 ± 0.124 d–j | 26.617 ± 0.376 fg | 8.169 ± 0.337 bf–j | 25.109 ± 0.136 f | |
| Habanero | 59C | 1.131 ± 0.016 kr | 23.975 ± 0.193 g | 5.049 ± 0.010 l–p | 20.039 ± 1.092 gh | |
| Habanero Sweet | 59B | 1.070 ± 0.037 lr | 9.209 ± 0.472 oq–t | 7.156 ± 0.097 e–n | 6.383 ± 0.119 oqr | |
| Hallows Eve | 129 | 2.050 ± 0.080 ce | 51.604 ± 1.812 c | 9.737 ± 0.255 a–d | 54.011 ± 0.825 c | |
| KS Lemon Starburst | 130 | 2.141 ± 0.084 cd | 10.792 ± 0.322 mqr | 8.689 ± 0.641 bfg | 11.691 ± 0.242 jk | |
| Pink Tiger | 134 | 1.223 ± 0.056 k–q | 7.430 ± 0.020 sw | 6.211 ± 0.082 gp | 6.008 ± 0.056 oq–s | |
| Scorpion Apocalypse | 161 | 1.145 ± 0.094 kr | 5.626 ± 0.140 vw | 5.042 ± 0.219 lp | 3.408 ± 0.063 uw | |
| Scorpion Hulk | 164 | 2.681 ± 0.155 ab | 51.861 ± 0.878 c | 10.451 ± 0.272 ab | 55.895 ± 1.036 c | |
| Scorpion Hulk | 164A | 0.980 ± 0.026 mr | 13.493 ± 0.418 km | 3.915 ± 0.119 p | 12.609 ± 0.260 j | |
| Tobago Seasoning | 156 | 0.793 ± 0.013 qr | 5.077 ± 0.123 w | 5.069 ± 0.080 lp | 3.485 ± 0.049 tw | |
| Viper Trinidad | 163 | 1.967 ± 0.051 cef | 43.542 ± 0.340 d | 8.778 ± 0.110 bf | 51.365 ± 0.927 d | |
| C. frutescens | Wiri Wiri | 95 (137) | 1.331 ± 0.099 i–o | 18.045 ± 0.259 h–j | 6.283 ± 0.292 fp | 20.761 ± 0.213 g |
| C. pubescens | Rocoto Red | 49 (54) | 1.869 ± 0.018 d–h | 10.975 ± 0.218 mq | 8.478 ± 0.577 bf–h | 11.643 ± 0.151 jk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alghamdi, M.; Rathinasabapathy, T.; Komarnytsky, S. Capsaicinoid Profiles, Phenolic Content, and Antioxidant Properties of Chili Peppers Grown in Urban Settings. Int. J. Mol. Sci. 2025, 26, 4916. https://doi.org/10.3390/ijms26104916
Alghamdi M, Rathinasabapathy T, Komarnytsky S. Capsaicinoid Profiles, Phenolic Content, and Antioxidant Properties of Chili Peppers Grown in Urban Settings. International Journal of Molecular Sciences. 2025; 26(10):4916. https://doi.org/10.3390/ijms26104916
Chicago/Turabian StyleAlghamdi, Malak, Thirumurugan Rathinasabapathy, and Slavko Komarnytsky. 2025. "Capsaicinoid Profiles, Phenolic Content, and Antioxidant Properties of Chili Peppers Grown in Urban Settings" International Journal of Molecular Sciences 26, no. 10: 4916. https://doi.org/10.3390/ijms26104916
APA StyleAlghamdi, M., Rathinasabapathy, T., & Komarnytsky, S. (2025). Capsaicinoid Profiles, Phenolic Content, and Antioxidant Properties of Chili Peppers Grown in Urban Settings. International Journal of Molecular Sciences, 26(10), 4916. https://doi.org/10.3390/ijms26104916






