Acetazolamide-Loaded Nanoparticle Based on Modified Hyaluronic Acid as Delivery System to Target Carbonic Anhydrases in Escherichia coli
Abstract
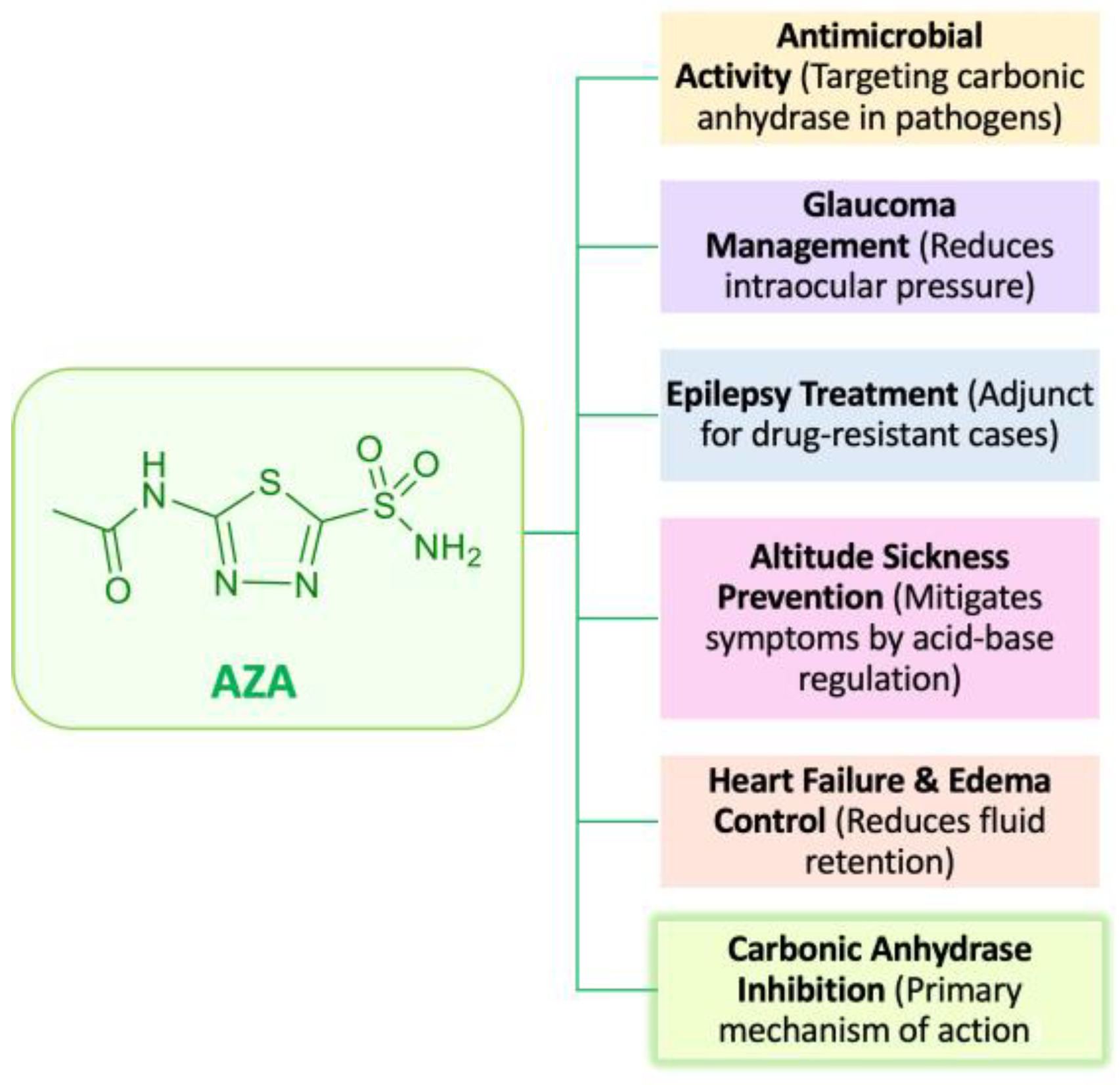
1. Introduction
2. Results
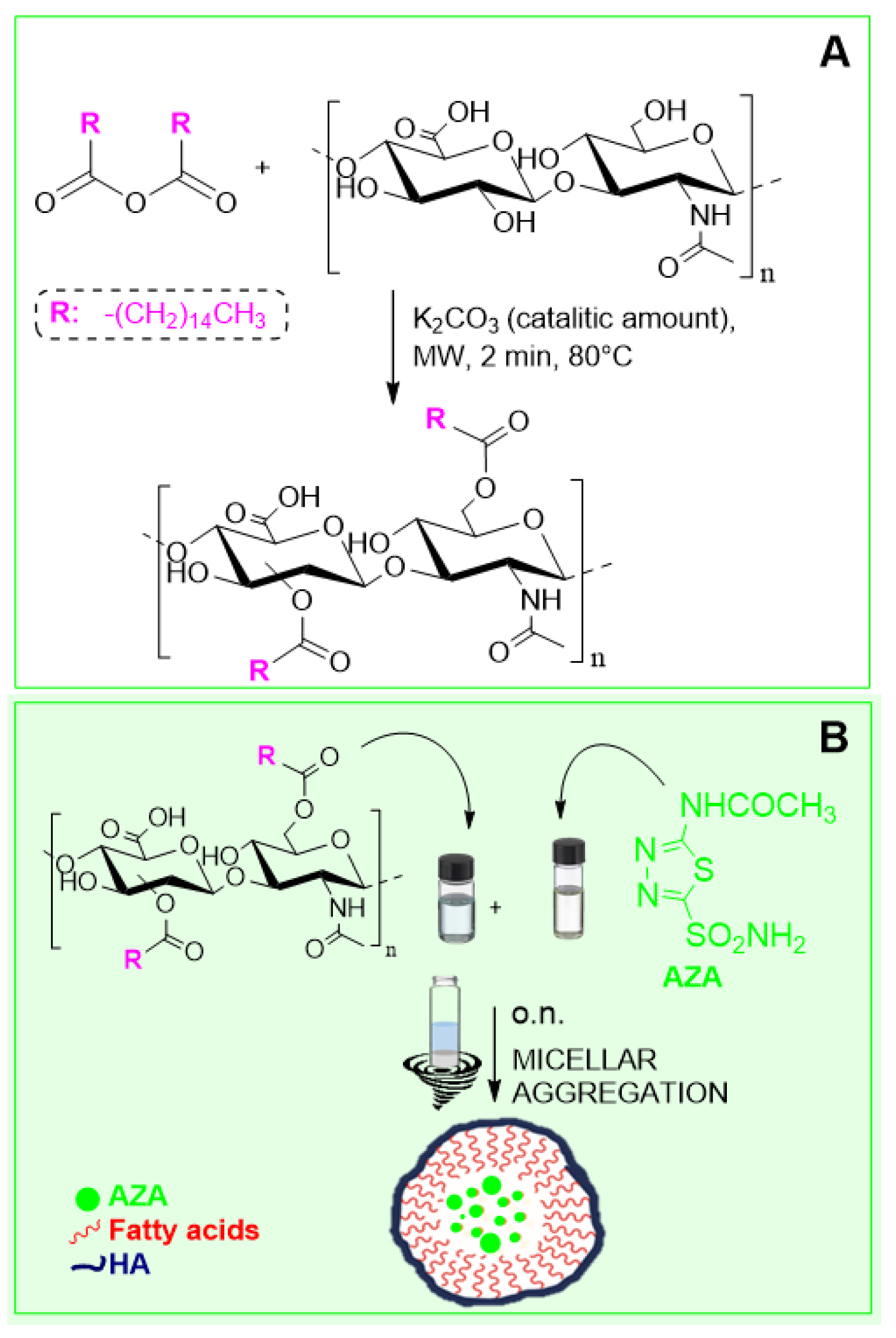
2.1. Synthesis and Characterization of AZA Loaded HA–Palmitate Nanoparticles
2.2. Enhanced Efficacy of AZA–HA–Palmitate Nanoparticles
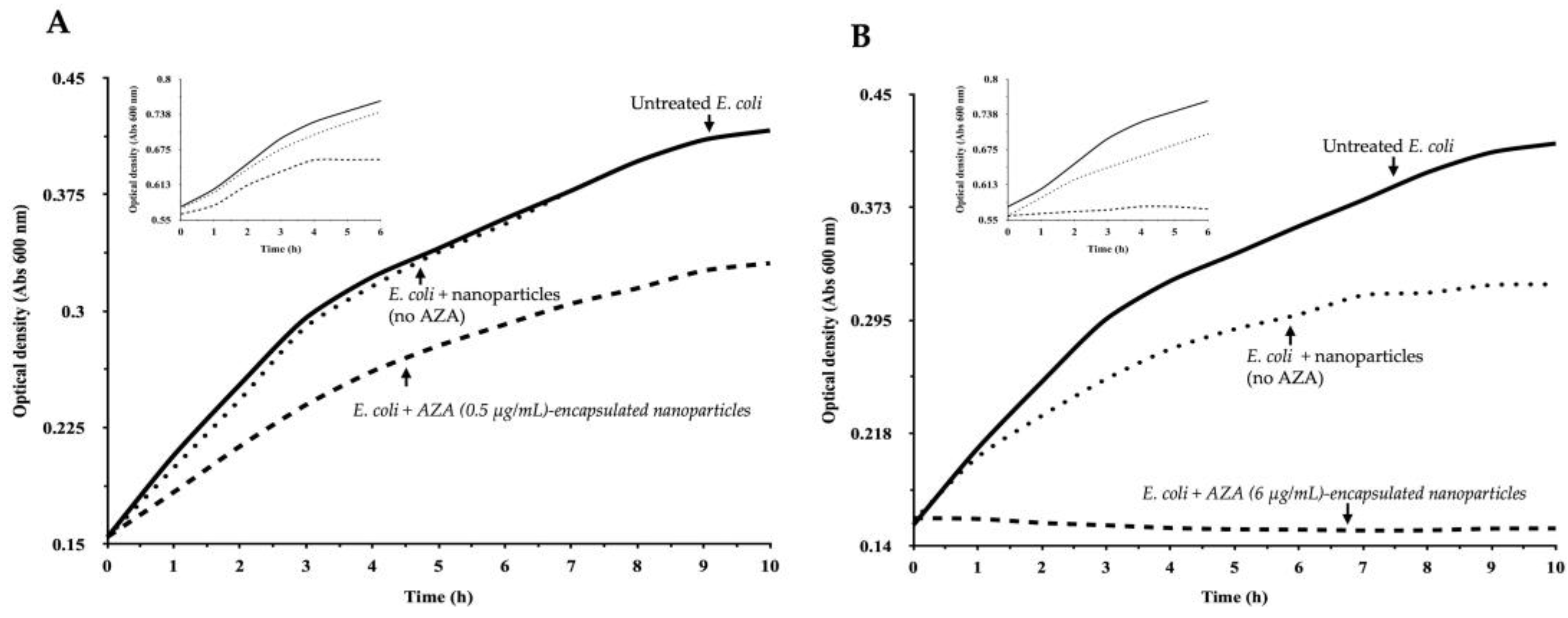
2.2.1. Effect of AZA–HA–Palmitate Nanoparticles on E. coli Growth
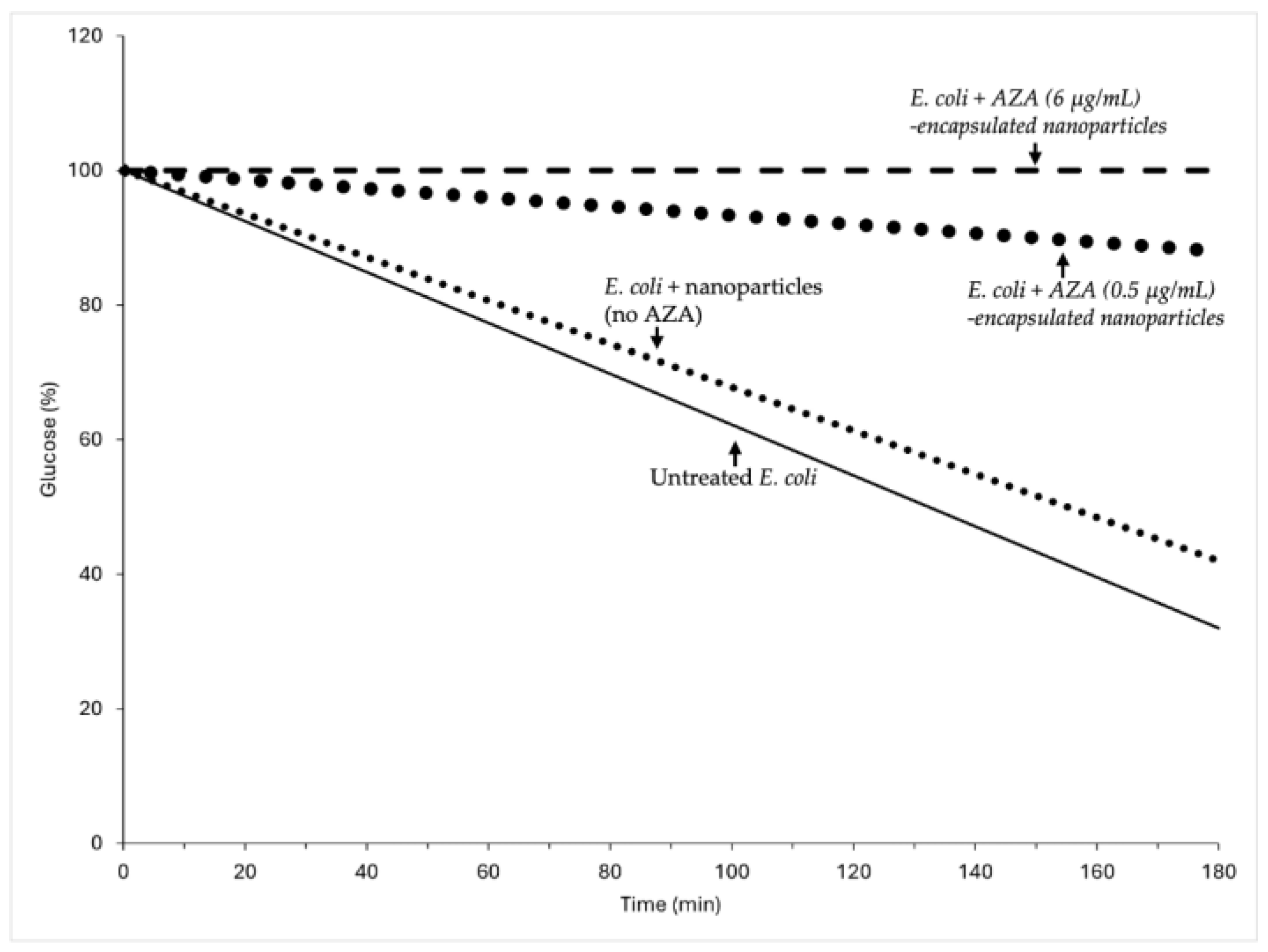
2.2.2. Impact of AZA-Loaded HA–Palmitate Nanoparticles on Glucose Consumption
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of HA–Palmitate Conjugates
4.3. Preparation and Size Distribution Characterization of AZA-Loaded HA–Palmitate Micelles
4.4. Characterization of AZA Loaded HA–Palmitate Nanoparticles: Encapsulation Efficiency
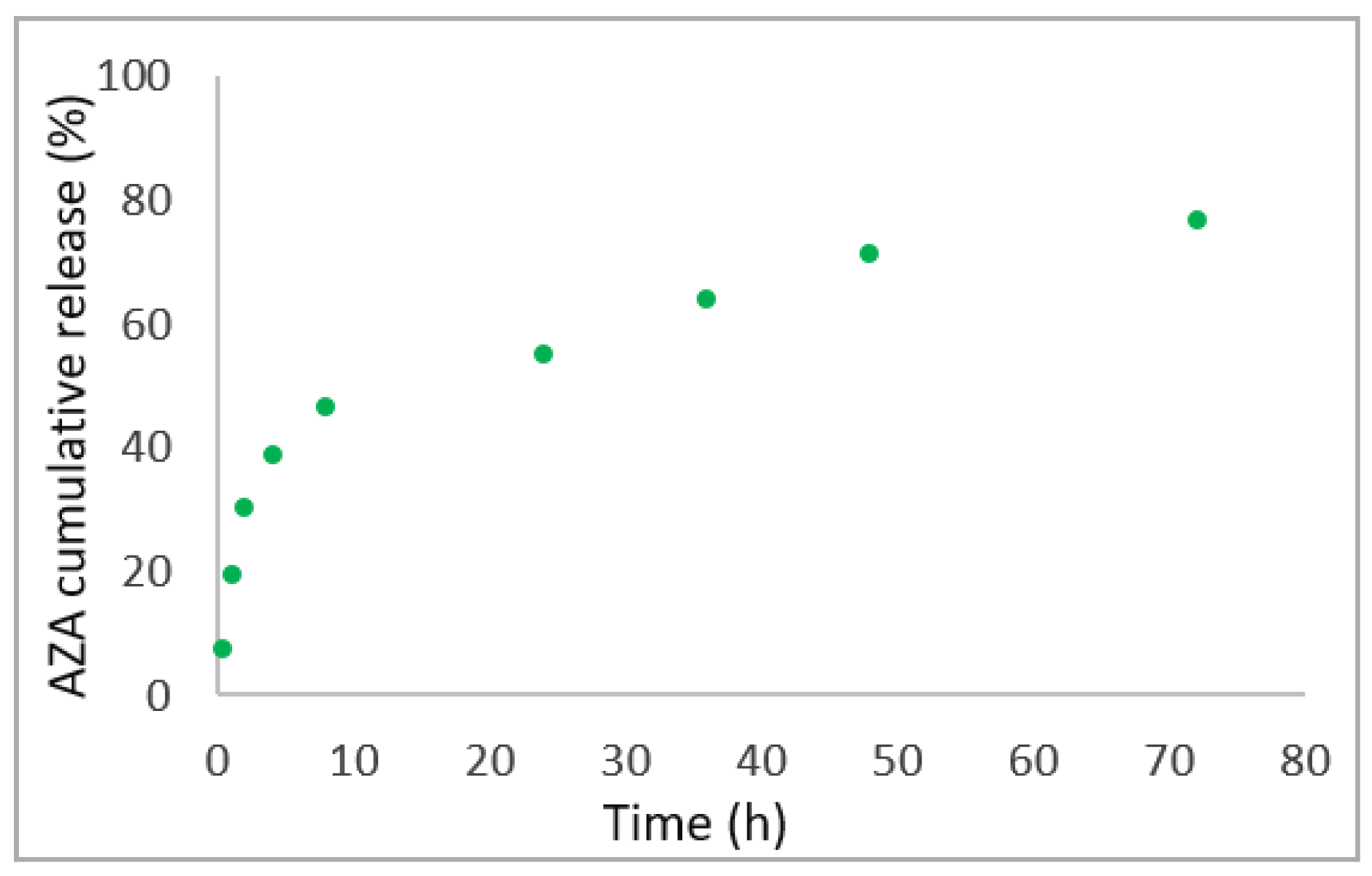
4.5. In Vitro AZA Release Study
4.6. Bacterial Strains and Culture Conditions
4.7. Treatment with AZA-Loaded HA-Based Nanocarrier
4.7.1. Bacterial Growth Monitoring and Treatment Conditions
4.7.2. Effect on Glucose Consumption
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stoner, A.; Harris, A.; Oddone, F.; Belamkar, A.; Verticchio Vercellin, A.C.; Shin, J.; Januleviciene, I.; Siesky, B. Topical carbonic anhydrase inhibitors and glaucoma in 2021: Where do we stand? Br. J. Ophthalmol. 2022, 106, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, H.D.; Colcher, H.; Hollander, F. Carbonic anhydrase inhibition and gastric secretion. Trans. N. Y. Acad. Sci. 1952, 15, 54–55. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, H.D.; Colcher, H.; Hollander, F. Inhibition of gastric secretion of acid in dogs by carbonic anhydrase inhibitor, 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide. Am. J. Physiol. 1952, 171, 325–330. [Google Scholar] [CrossRef]
- Janowitz, H.D.; Colcher, H.; Hollander, F. Inhibition of gastric secretion of hydrochloric acid in vivo by carbonic anhydrase inhibition. Nature 1952, 170, 499. [Google Scholar] [CrossRef]
- Buzas, G.M.; Supuran, C.T. The history and rationale of using carbonic anhydrase inhibitors in the treatment of peptic ulcers. In memoriam Ioan Puscas (1932–2015). J. Enzym. Inhib. Med. Chem. 2016, 31, 527–533. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. Biomedical applications of prokaryotic carbonic anhydrases: An update. Expert Opin. Ther. Pat. 2024, 34, 351–363. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. Overview on bacterial carbonic anhydrase genetic families. Enzymes 2024, 55, 1–29. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. Bacterial iota-CAs. Enzymes 2024, 55, 121–142. [Google Scholar] [CrossRef]
- Kumar, S.; Rulhania, S.; Jaswal, S.; Monga, V. Recent advances in the medicinal chemistry of carbonic anhydrase inhibitors. Eur. J. Med. Chem. 2021, 209, 112923. [Google Scholar] [CrossRef]
- D’Ambrosio, K.; Di Fiore, A.; Alterio, V.; Langella, E.; Monti, S.M.; Supuran, C.T.; De Simone, G. Multiple Binding Modes of Inhibitors to Human Carbonic Anhydrases: An Update on the Design of Isoform-Specific Modulators of Activity. Chem. Rev. 2025, 125, 150–222. [Google Scholar] [CrossRef]
- Supuran, C.T.; Scozzafava, A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg. Med. Chem. 2007, 15, 4336–4350. [Google Scholar] [CrossRef] [PubMed]
- Canepa, E.; Parodi-Rullan, R.; Vazquez-Torres, R.; Gamallo-Lana, B.; Guzman-Hernandez, R.; Lemon, N.L.; Angiulli, F.; Debure, L.; Ilies, M.A.; Ostergaard, L.; et al. Correction to “FDA-approved carbonic anhydrase inhibitors reduce amyloid beta pathology and improve cognition, by ameliorating cerebrovascular health and glial fitness”. Alzheimers Dement. 2024, 20, 2297. [Google Scholar] [CrossRef]
- Garcia-Llorca, A.; Carta, F.; Supuran, C.T.; Eysteinsson, T. Carbonic anhydrase, its inhibitors and vascular function. Front. Mol. Biosci. 2024, 11, 1338528. [Google Scholar] [CrossRef] [PubMed]
- Hilvo, M.; Supuran, C.T.; Parkkila, S. Characterization and inhibition of the recently discovered carbonic anhydrase isoforms CA XIII, XIV and XV. Curr. Top. Med. Chem. 2007, 7, 893–899. [Google Scholar] [CrossRef]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Shukralla, A.A.; Dolan, E.; Delanty, N. Acetazolamide: Old drug, new evidence? Epilepsia Open 2022, 7, 378–392. [Google Scholar] [CrossRef]
- Malik, B.A.; Nnodebe, I.; Fayaz, A.; Inayat, H.; Murtaza, S.F.; Umer, M.; Zaidi, S.A.T.; Amin, A. Effect of Acetazolamide as Add-On Diuretic Therapy in Patients with Heart Failure: A Meta-Analysis. Cureus J. Med. Sci. 2023, 15, e37792. [Google Scholar] [CrossRef]
- Mutschler, T.; Furian, M.; Lichtblau, M.; Buergin, A.; Schneider, S.R.; Appenzeller, P.; Mayer, L.; Muralt, L.; Mademilov, M.; Abdyraeva, A.; et al. Effect of altitude and acetazolamide on postural control in healthy lowlanders 40 years of age or older. Randomized, placebo-controlled trial. Front. Physiol. 2023, 14, 1274111. [Google Scholar] [CrossRef]
- Moviat, M.; Pickkers, P.; van der Voort, P.H.; van der Hoeven, J.G. Acetazolamide-mediated decrease in strong ion difference accounts for the correction of metabolic alkalosis in critically ill patients. Crit. Care 2006, 10, R14. [Google Scholar] [CrossRef]
- Abutaleb, N.S.; Elhassanny, A.E.M.; Nocentini, A.; Hewitt, C.S.; Elkashif, A.; Cooper, B.R.; Supuran, C.T.; Seleem, M.N.; Flaherty, D.P. Repurposing FDA-approved sulphonamide carbonic anhydrase inhibitors for treatment of Neisseria gonorrhoeae. J. Enzym. Inhib. Med. Chem. 2022, 37, 51–61. [Google Scholar] [CrossRef]
- Abutaleb, N.S.; Elkashif, A.; Flaherty, D.P.; Seleem, M.N. In Vivo Antibacterial Activity of Acetazolamide. Antimicrob. Agents Chemother. 2021, 65, aac.01715-20. [Google Scholar] [CrossRef] [PubMed]
- Chilambi, G.S.; Wang, Y.H.; Wallace, N.R.; Obiwuma, C.; Evans, K.M.; Li, Y.; Shalaby, M.W.; Flaherty, D.P.; Shields, R.K.; Doi, Y.; et al. Carbonic Anhydrase Inhibition as a Target for Antibiotic Synergy in Enterococci. Microbiol. Spectr. 2023, 11, e0396322. [Google Scholar] [CrossRef]
- An, W.; Holly, K.J.; Nocentini, A.; Imhoff, R.D.; Hewitt, C.S.; Abutaleb, N.S.; Cao, X.; Seleem, M.N.; Supuran, C.T.; Flaherty, D.P. Structure-activity relationship studies for inhibitors for vancomycin-resistant Enterococcus and human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2022, 37, 1838–1844. [Google Scholar] [CrossRef]
- Abutaleb, N.S.; Shrinidhi, A.; Bandara, A.B.; Seleem, M.N.; Flaherty, D.P. Evaluation of 1,3,4-Thiadiazole Carbonic Anhydrase Inhibitors for Gut Decolonization of Vancomycin-Resistant Enterococci. ACS Med. Chem. Lett. 2023, 14, 487–492. [Google Scholar] [CrossRef]
- Holly, K.J.; Youse, M.S.; Flaherty, D.P. Enterococci carbonic anhydrase inhibition. Enzymes 2024, 55, 283–311. [Google Scholar] [CrossRef]
- Granero, G.E.; Longhi, M.R. Promising complexes of acetazolamide for topical ocular administration. Expert. Opin. Drug Deliv. 2010, 7, 943–953. [Google Scholar] [CrossRef]
- Schmickl, C.N.; Owens, R.L.; Orr, J.E.; Edwards, B.A.; Malhotra, A. Side effects of acetazolamide: A systematic review and meta-analysis assessing overall risk and dose dependence. BMJ Open Respir. Res. 2020, 7, e000557. [Google Scholar] [CrossRef]
- Iqbal, H.; Razzaq, A.; Zhou, D.; Lou, J.; Xiao, R.; Lin, F.; Liang, Y. Nanomedicine in glaucoma treatment; Current challenges and future perspectives. Mater. Today Bio 2024, 28, 101229. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef] [PubMed]
- Matalqah, S.; Lafi, Z.; Asha, S.Y. Hyaluronic Acid in Nanopharmaceuticals: An Overview. Curr. Issues Mol. Biol. 2024, 46, 10444–10461. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Devnarain, N.; Elhassan, E.; Govender, T. Exploring the applications of hyaluronic acid-based nanoparticles for diagnosis and treatment of bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 14, e1799. [Google Scholar] [CrossRef]
- Smejkalova, D.; Muthny, T.; Nesporova, K.; Hermannova, M.; Achbergerova, E.; Huerta-Angeles, G.; Svoboda, M.; Cepa, M.; Machalova, V.; Luptakova, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Pepe, G.; Calce, E.; Verdoliva, V.; Saviano, M.; Maglione, V.; Di Pardo, A.; De Luca, S. Curcumin-Loaded Nanoparticles Based on Amphiphilic Hyaluronan-Conjugate Explored as Targeting Delivery System for Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8846. [Google Scholar] [CrossRef]
- Calce, E.; Mercurio, F.A.; Leone, M.; Saviano, M.; De Luca, S. Eco-friendly microwave-assisted protocol to prepare hyaluronan-fatty acid conjugates and to induce their self-assembly process. Carbohyd Polym. 2016, 143, 84–89. [Google Scholar] [CrossRef]
- De Luca, V.; Carginale, V.; Supuran, C.T.; Capasso, C. The gram-negative bacterium Escherichia coli as a model for testing the effect of carbonic anhydrase inhibition on bacterial growth. J. Enzym. Inhib. Med. Chem. 2022, 37, 2092–2098. [Google Scholar] [CrossRef]
- Verdoliva, V.; Bedini, E.; De Luca, S. Sustainable Chemical Modification of Natural Polysaccharides: Mechanochemical, Solvent-Free Conjugation of Pectins and Hyaluronic Acid Promoted by Microwave Radiations. Biomacromolecules 2024, 25, 6217–6228. [Google Scholar] [CrossRef]
- Calce, E.; Ringhieri, P.; Mercurio, F.A.; Leone, M.; Bugatti, V.; Saviano, M.; Vittoria, V.; De Luca, S. A biocompatible process to prepare hyaluronan-based material able to self-assemble into stable nano-particles. RSC Adv. 2015, 5, 29573–29576. [Google Scholar] [CrossRef]
- Lin, T.Y.; Zhu, T.T.; Xun, Y.; Tao, Y.S.; Yang, Y.Q.; Xie, J.L.; Zhang, X.M.; Chen, S.X.; Ding, B.J.; Chen, W.D. A novel drug delivery system of mixed micelles based on poly(ethylene glycol)-poly(lactide) and poly(ethylene glycol)-poly(varepsilon-caprolactone) for gambogenic acid. Kaohsiung J. Med. Sci. 2019, 35, 757–764. [Google Scholar] [CrossRef]
- Yu, M.; Yuan, W.; Li, D.; Schwendeman, A.; Schwendeman, S.P. Predicting drug release kinetics from nanocarriers inside dialysis bags. J. Controllled Release 2019, 315, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Astasov-Frauenhoffer, M.; Waltimo, T.; Bonkat, G. A Review of Methods to Determine Viability, Vitality, and Metabolic Rates in Microbiology. Front. Microbiol. 2020, 11, 547458. [Google Scholar] [CrossRef] [PubMed]
- Solokhina, A.; Bruckner, D.; Bonkat, G.; Braissant, O. Metabolic activity of mature biofilms of Mycobacterium tuberculosis and other non-tuberculous mycobacteria. Sci. Rep. 2017, 7, 9225. [Google Scholar] [CrossRef] [PubMed]
- Neijssel, O.M.; Buurman, E.T.; Demattos, M.J.T. The Role of Futile Cycles in the Energetics of Bacterial-Growth. Biochim. Biophys. Acta 1990, 1018, 252–255. [Google Scholar] [CrossRef]
- Russell, J.B.; Cook, G.M. Energetics of Bacterial-Growth—Balance of Anabolic and Catabolic Reactions. Microbiol. Rev. 1995, 59, 48–62. [Google Scholar] [CrossRef]
- Qian, H.; Beard, D.A. Metabolic futile cycles and their functions: A systems analysis of energy and control. Syst. Biol. 2006, 153, 192–200. [Google Scholar] [CrossRef]
- Liao, W.; Badri, W.; Dumas, E.; Ghnimi, S.; Elaissari, A.; Saurel, R.; Gharsallaoui, A. Nanoencapsulation of Essential Oils as Natural Food Antimicrobial Agents: An Overview. Appl. Sci. 2021, 11, 5778. [Google Scholar] [CrossRef]
- Nair, A.; Greeny, A.; Nandan, A.; Sah, R.K.; Jose, A.; Dyawanapelly, S.; Junnuthula, V.; Athira, K.V.; Sadanandan, P. Advanced drug delivery and therapeutic strategies for tuberculosis treatment. J. Nanobiotechnol. 2023, 21, 414. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Le Guevel, X.; Fleury, J.B.; Hanssen, E.; Nguyen, T.H.P.; Juodkazis, S.; Bryant, G.; Crawford, R.J.; Stoodley, P.; et al. Antibacterial Action of Nanoparticles by Lethal Stretching of Bacterial Cell Membranes. Adv. Mater. 2020, 32, e2005679. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Ye, J.; Chen, Y.; Wang, R.; Jin, D.; Liu, Y. Mechanism Study on Nanoparticle Negative Surface Charge Modification by Ascorbyl Palmitate and Its Improvement of Tumor Targeting Ability. Molecules 2022, 27, 4408. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Abedi Dorcheh, K.; Akbari, A.; Jabbari, E. Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances. Nanomaterials 2021, 11, 1755. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, R.; Ayan, M.; Hamblin, M.R.; Ranjbar, R.; Rashki, S. Hyaluronic Acid-Based Nanomaterials as a New Approach to the Treatment and Prevention of Bacterial Infections. Front. Bioeng. Biotechnol. 2022, 10, 913912. [Google Scholar] [CrossRef] [PubMed]
- Gamarra, A.; Forés, E.; Morató, J.; Muñoz-Guerra, S. Amphiphilic ionic complexes of hyaluronic acid with organophosphonium compounds and their antimicrobial activity. Int. J. Biol. Macromol. 2018, 118, 2021–2031. [Google Scholar] [CrossRef]
- Osman, A.I.; Zhang, Y.B.; Farghali, M.; Rashwan, A.K.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Mohamed, I.M.A.; Badr, M.M.; Ihara, I.; Rooney, D.W.; et al. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: A review. Env. Chem. Lett. 2024, 22, 841–887. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Lin, M.; Qi, X.R. Purification Method of Drug-Loaded Liposome; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Aliabadi, H.M.; Elhasi, S.; Mahmud, A.; Gulamhusein, R.; Mahdipoor, P.; Lavasanifar, A. Encapsulation of hydrophobic drugs in polymeric micelles through co-solvent evaporation: The effect of solvent composition on micellar properties and drug loading. Int. J. Pharm. 2007, 329, 158–165. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdoliva, V.; De Luca, V.; Supuran, C.T.; De Luca, S.; Capasso, C. Acetazolamide-Loaded Nanoparticle Based on Modified Hyaluronic Acid as Delivery System to Target Carbonic Anhydrases in Escherichia coli. Int. J. Mol. Sci. 2025, 26, 4908. https://doi.org/10.3390/ijms26104908
Verdoliva V, De Luca V, Supuran CT, De Luca S, Capasso C. Acetazolamide-Loaded Nanoparticle Based on Modified Hyaluronic Acid as Delivery System to Target Carbonic Anhydrases in Escherichia coli. International Journal of Molecular Sciences. 2025; 26(10):4908. https://doi.org/10.3390/ijms26104908
Chicago/Turabian StyleVerdoliva, Valentina, Viviana De Luca, Claudiu T. Supuran, Stefania De Luca, and Clemente Capasso. 2025. "Acetazolamide-Loaded Nanoparticle Based on Modified Hyaluronic Acid as Delivery System to Target Carbonic Anhydrases in Escherichia coli" International Journal of Molecular Sciences 26, no. 10: 4908. https://doi.org/10.3390/ijms26104908
APA StyleVerdoliva, V., De Luca, V., Supuran, C. T., De Luca, S., & Capasso, C. (2025). Acetazolamide-Loaded Nanoparticle Based on Modified Hyaluronic Acid as Delivery System to Target Carbonic Anhydrases in Escherichia coli. International Journal of Molecular Sciences, 26(10), 4908. https://doi.org/10.3390/ijms26104908








