Adverse Effect of Polystyrene Nanoplastics in Impairing Glucose Metabolism in Liver Injury
Abstract
1. Introduction
2. Results
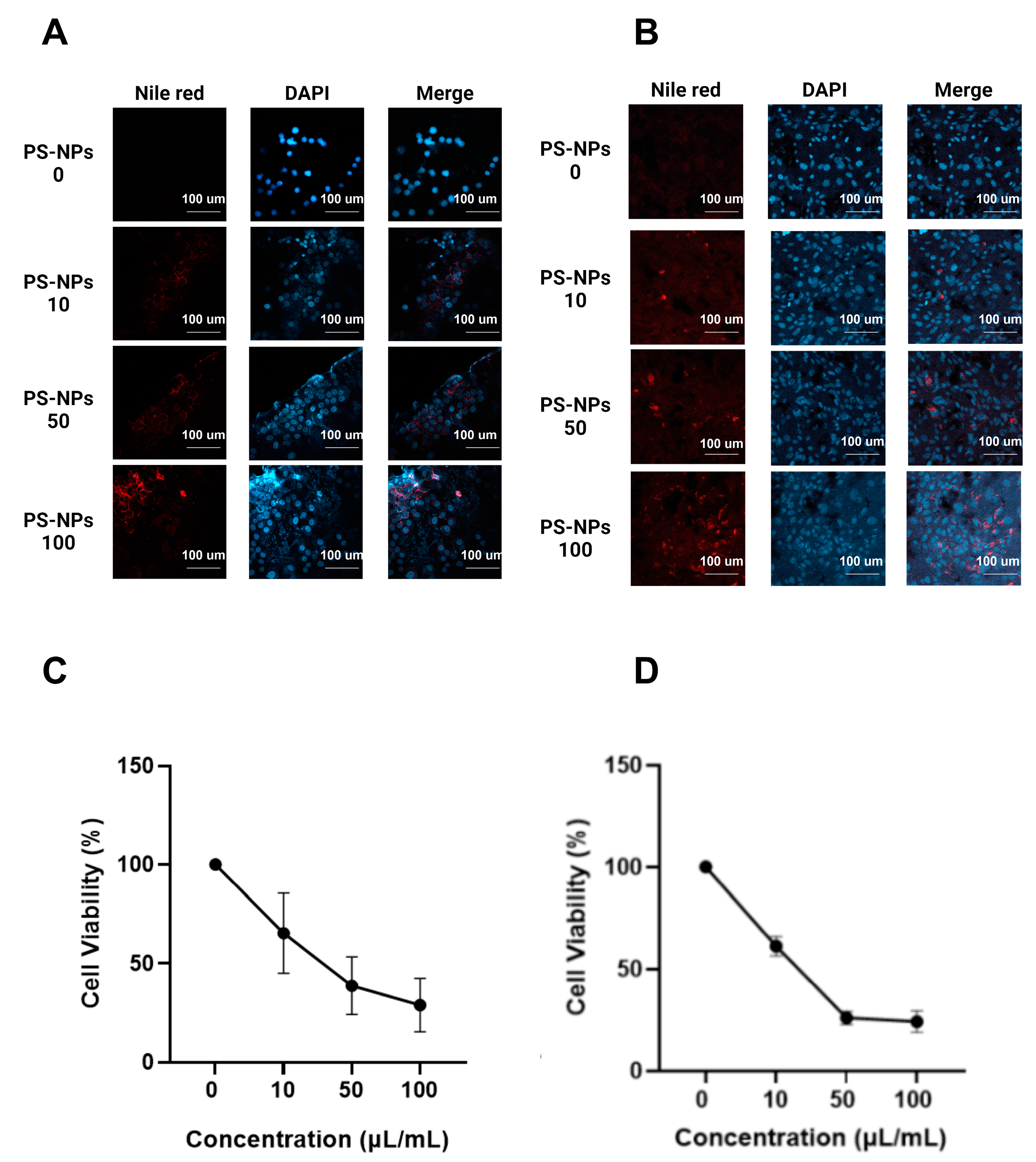
2.1. Effect of PS-NPs on Cell Lines
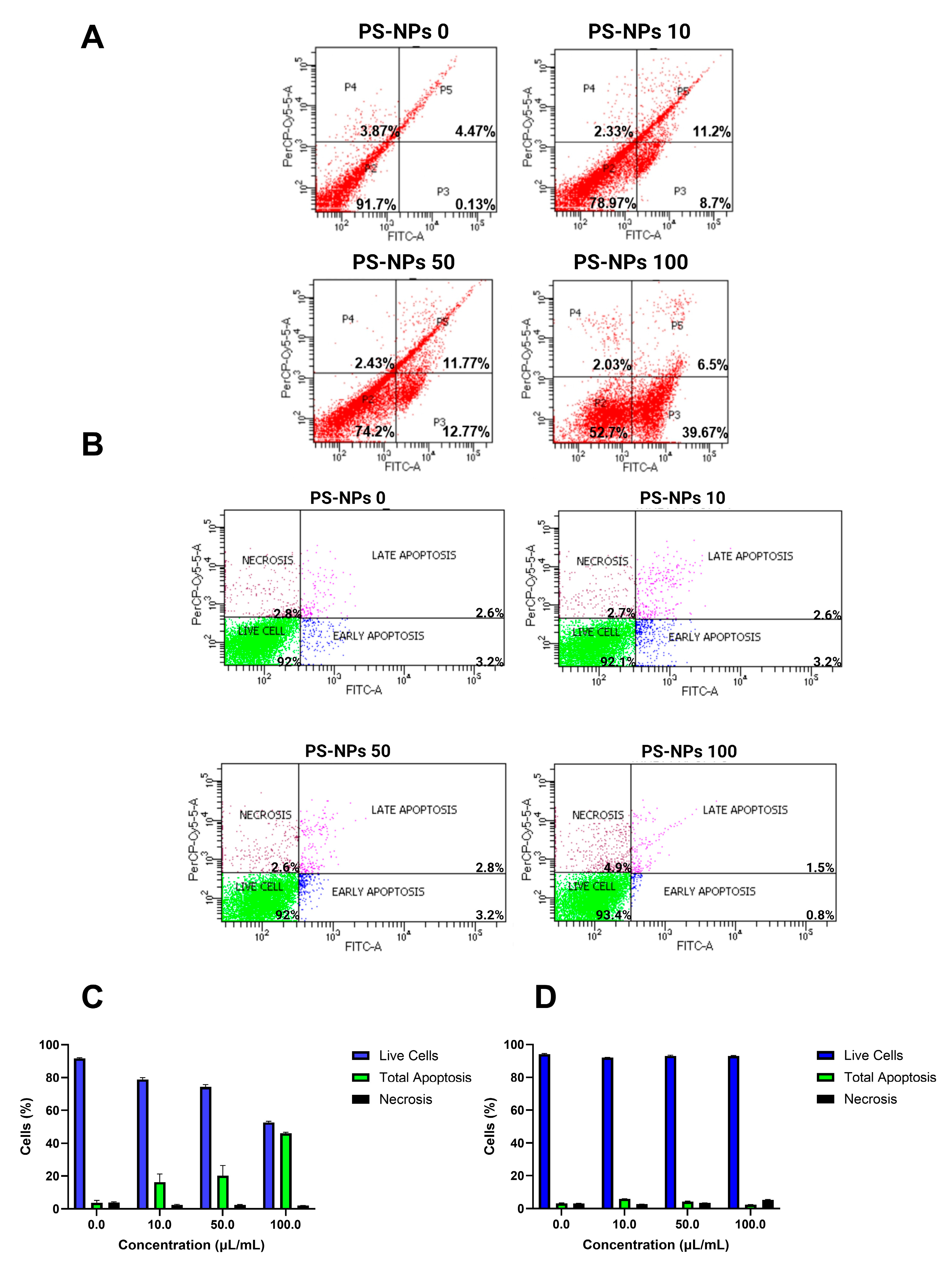
2.2. Effect of PS-NPs on Flow Cytometry Assay
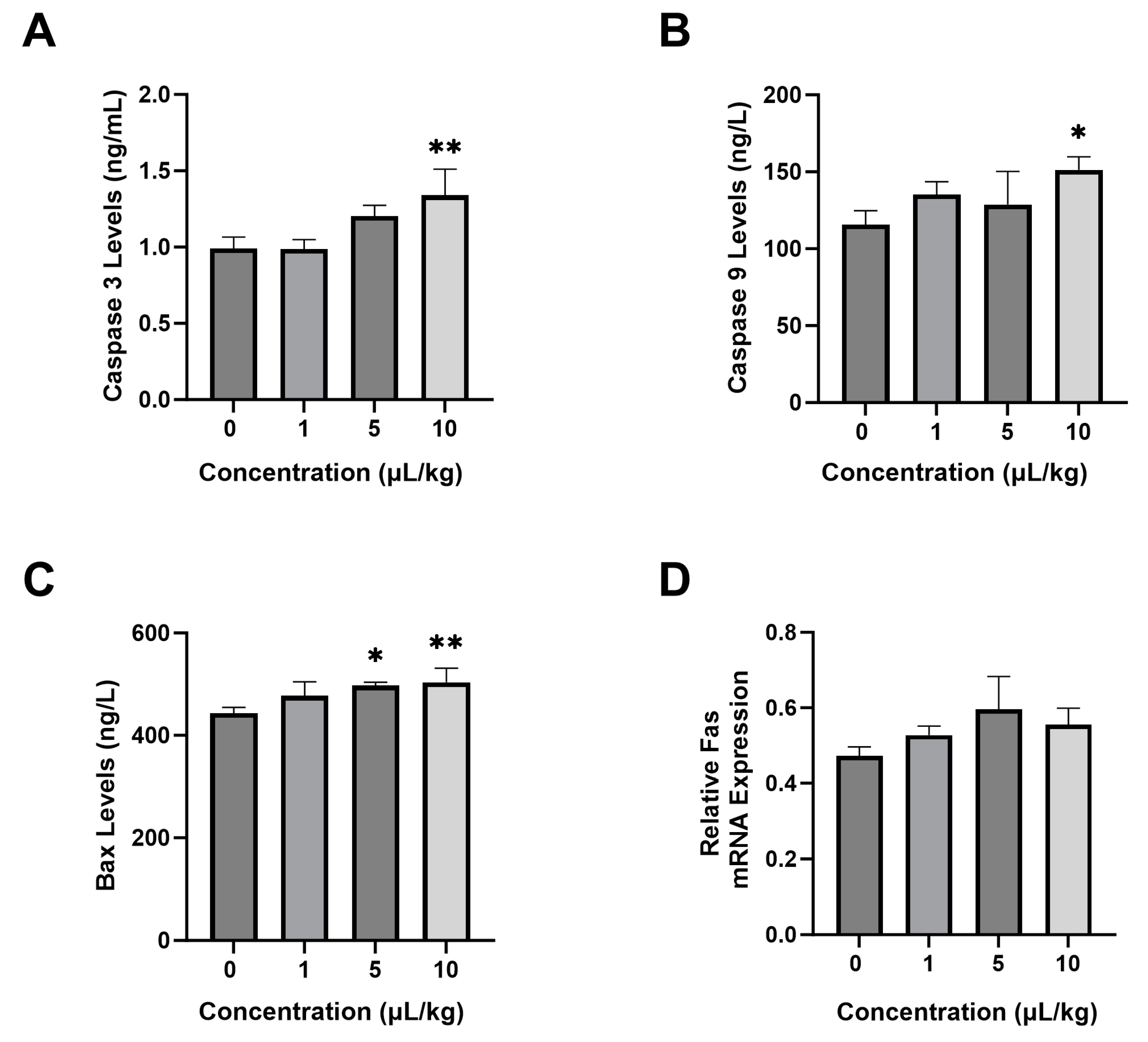
2.3. Effect of PS-NPs on Apoptosis Parameters
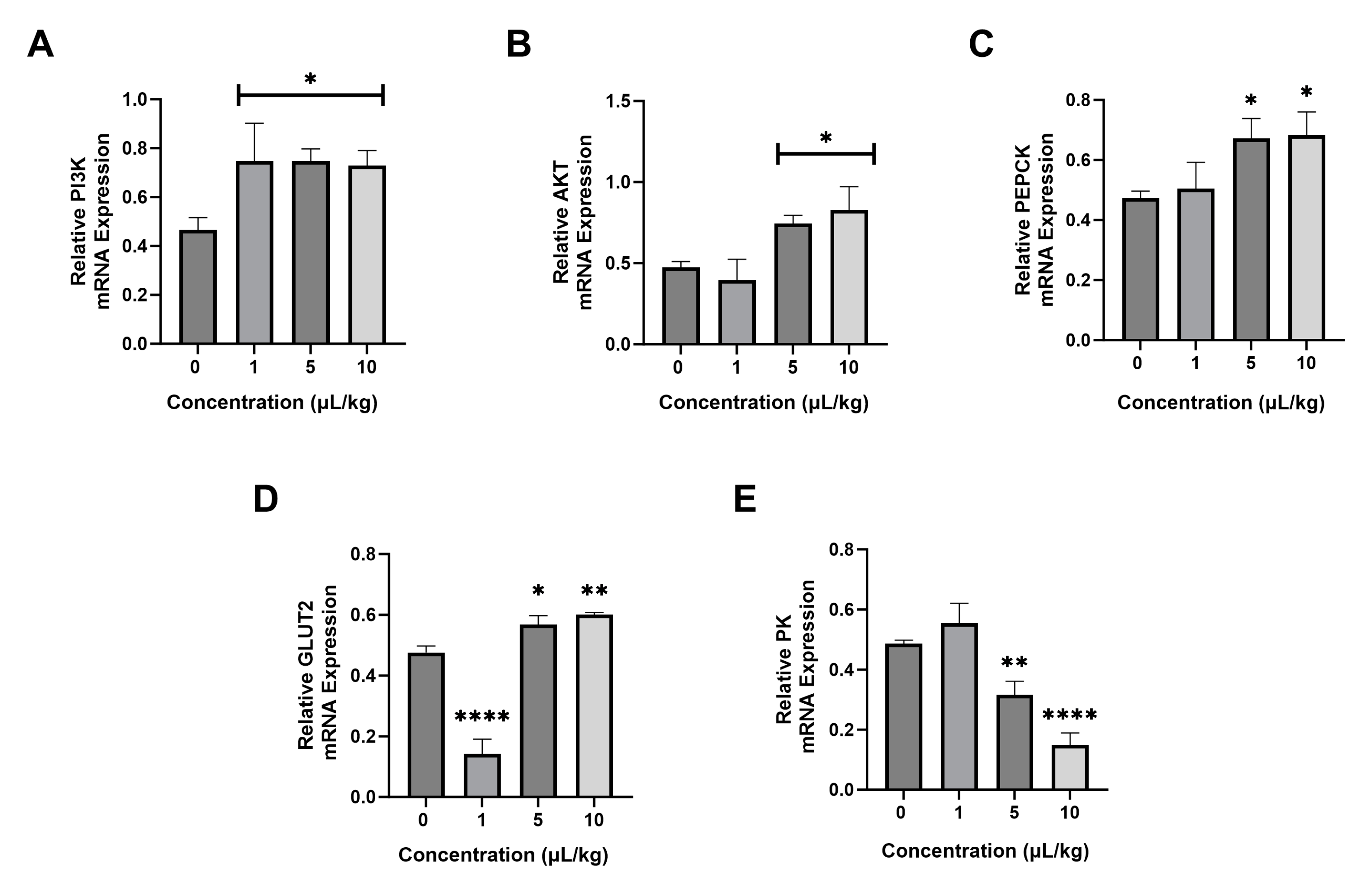
2.4. Effect of PS-NPs on the mRNA Expression of Glucose Metabolism Genes
2.5. Effect of PS-NPs on Liver Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture (In Vitro Experiment)
4.2. Preparation of Confocal Microscope
4.3. Cell Viability Assay
4.4. Flow Cytometry Assay
4.5. Animals (In Vivo Experiment)
4.6. Experimental Design
4.7. ELISA Test
4.8. Real-Time PCR Analysis
4.9. Histology Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nayanathara Thathsarani Pilapitiya, P.G.C.; Ratnayake, A.S. The world of plastic waste: A review. Clean. Mater. 2024, 11, 100220. [Google Scholar] [CrossRef]
- Esterhuizen, M.; Kim, Y.J. Effects of polypropylene, polyvinyl chloride, polyethylene terephthalate, polyurethane, high-density polyethylene, and polystyrene microplastic on Nelumbo nucifera (Lotus) in water and sediment. Environ. Sci. Pollut. Res. 2022, 29, 17580–17590. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Singh, S.; Bahmid, N.A.; Shyu, D.J.H.; Domínguez, R.; Lorenzo, J.M.; Pereira, J.A.M.; Câmara, J.S. Polystyrene microplastic particles in the food chain: Characteristics and toxicity—A review. Sci. Total Environ. 2023, 892, 164531. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, S.; Alqahtani, S.; Saquib, Q.; Mohiddin, F. Toxicological impact of microplastics and nanoplastics on humans: Understanding the mechanistic aspect of the interaction. Front. Toxicol. 2023, 5, 1193386. [Google Scholar] [CrossRef] [PubMed]
- Kögel, T.; Bjorøy, Ø.; Toto, B.; Bienfait, A.M.; Sanden, M. Micro- and nanoplastic toxicity on aquatic life: Determining factors. Sci. Total Environ. 2020, 709, 136050. [Google Scholar] [CrossRef]
- Xu, M.; Halimu, G.; Zhang, Q.; Song, Y.; Fu, X.; Li, Y.; Li, Y.; Zhang, H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Sci. Total Environ. 2019, 694, 133794. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Lynch, I.; Ejtehadi, M.R.; Monopoli, M.P.; Bombelli, F.B.; Laurent, S. Protein−Nanoparticle Interactions: Opportunities and Challenges. Chem. Rev. 2011, 111, 5610–5637. [Google Scholar] [CrossRef]
- Ali, N.; Katsouli, J.; Marczylo, E.L.; Gant, T.W.; Wright, S.; Bernardino De La Serna, J. The potential impacts of micro-and-nano plastics on various organ systems in humans. eBioMedicine 2024, 99, 104901. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef]
- Li, L.; Wan, T.; Wan, M.; Liu, B.; Cheng, R.; Zhang, R. The effect of the size of fluorescent dextran on its endocytic pathway. Cell Biol. Int. 2015, 39, 531–539. [Google Scholar] [CrossRef]
- Xiao, M.; Li, X.; Zhang, X.; Duan, X.; Lin, H.; Liu, S.; Sui, G. Assessment of cancer-related signaling pathways in responses to polystyrene nanoplastics via a kidney-testis microfluidic platform (KTP). Sci. Total Environ. 2023, 857, 159306. [Google Scholar] [CrossRef] [PubMed]
- Kaksonen, M.; Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2018, 19, 313–326. [Google Scholar] [CrossRef]
- Mani, I.; Pandey, K.N. Emerging concepts of receptor endocytosis and concurrent intracellular signaling: Mechanisms of guanylyl cyclase/natriuretic peptide receptor-A activation and trafficking. Cell. Signal. 2019, 60, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Fazlollahi, F.; Angelow, S.; Yacobi, N.R.; Marchelletta, R.; Yu, A.S.L.; Hamm-Alvarez, S.F.; Borok, Z.; Kim, K.-J.; Crandall, E.D. Polystyrene nanoparticle trafficking across MDCK-II. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 588–594. [Google Scholar] [CrossRef]
- Leslie, H.A.; Van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Prüst, M.; Meijer, J.; Westerink, R.H.S. The plastic brain: Neurotoxicity of micro- and nanoplastics. Part. Fibre Toxicol. 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.; Valiyaveettil, S.; Tang, B. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Hayati, A.; Pramudya, M.; Soepriandono, H.; Astri, A.R.; Kusuma, M.R.; Maulidah, S.; Adriansyah, W.; Dewi, F.R.P. Assessing the recovery of steroid levels and gonadal histopathology of tilapia exposed to polystyrene particle pollution by supplementary feed. Vet. World 2022, 15, 517–523. [Google Scholar] [CrossRef]
- Hu, M.; Palić, D. Micro- and nano-plastics activation of oxidative and inflammatory adverse outcome pathways. Redox Biol. 2020, 37, 101620. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Triwahyudi, H.; Soehargo, L.; Muniroh, L.; Qolbi, R.N.; Aini, T.Q.; Kurnia, R.F.Z.; Putra, P.A.D.; Pramudya, M.; Muchtaromah, B.; Hayati, A. Potential of Red Seaweed (Dichotomania obtusata) on Immune Response and Histopathology of Rat Testis Exposed to Nanoplastics. Trop. J. Nat. Prod. Res. 2023, 7, 2969–2973. [Google Scholar] [CrossRef]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxid. Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Su, S.; Shang, R.; Yang, L.; Zhao, X.; Wu, C.; Yang, Z.; Zhang, H.; Wu, L.; Liu, Y.; et al. Optogenetic induction of caspase-8 mediated apoptosis by employing Arabidopsis cryptochrome 2. Sci. Rep. 2023, 13, 23067. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Liu, M.; Bai, C.; Ruan, Y.; Wang, Y.; Qiu, L.; Hong, Y.; Wang, X.; Li, L.; Li, B. Caspase-8 Induces Lysosome-Associated Cell Death in Cancer Cells. Mol. Ther. 2020, 28, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta BBA—Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Lu, H.; Hou, L.; Zhang, Y.; Guo, T.; Wang, Y.; Xing, M. Polystyrene microplastics mediate cell cycle arrest, apoptosis, and autophagy in the G2/M phase through ROS in grass carp kidney cells. Environ. Toxicol. 2024, 39, 1923–1935. [Google Scholar] [CrossRef]
- Esteves, F.; Rueff, J.; Kranendonk, M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics 2021, 11, 94–114. [Google Scholar] [CrossRef]
- Allameh, A.; Niayesh-Mehr, R.; Aliarab, A.; Sebastiani, G.; Pantopoulos, K. Oxidative Stress in Liver Pathophysiology and Disease. Antioxidants 2023, 12, 1653. [Google Scholar] [CrossRef]
- Yin, K.; Wang, D.; Zhang, Y.; Lu, H.; Wang, Y.; Xing, M. Dose-effect of polystyrene microplastics on digestive toxicity in chickens (Gallus gallus): Multi-omics reveals critical role of gut-liver axis. J. Adv. Res. 2023, 52, 3–18. [Google Scholar] [CrossRef]
- Han, H.-S.; Kang, G.; Kim, J.S.; Choi, B.H.; Koo, S.-H. Regulation of glucose metabolism from a liver-centric perspective. Exp. Mol. Med. 2016, 48, e218. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Garcia Whitlock, A.E.; Sostre-Colón, J.; Gavin, M.; Martin, N.D.; Baur, J.A.; Sims, C.A.; Titchenell, P.M. Loss of FOXO transcription factors in the liver mitigates stress-induced hyperglycemia. Mol. Metab. 2021, 51, 101246. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Q.; Yan, H.; Zhang, K.; Guo, X.; Xu, Z.; Yang, W.; Qi, Y.; Guo, C.A.; Hornsby, C.; et al. Novel Mechanism of Foxo1 Phosphorylation in Glucagon Signaling in Control of Glucose Homeostasis. Diabetes 2018, 67, 2167–2182. [Google Scholar] [CrossRef] [PubMed]
- Link, W.; Fernandez-Marcos, P.J. FOXO transcription factors at the interface of metabolism and cancer. Int. J. Cancer 2017, 141, 2379–2391. [Google Scholar] [CrossRef]
- Santos, B.F.; Grenho, I.; Martel, P.J.; Ferreira, B.I.; Link, W. Correction: FOXO family isoforms. Cell Death Dis. 2023, 14, 797. [Google Scholar] [CrossRef]
- Teaney, N.A.; Cyr, N.E. FoxO1 as a tissue-specific therapeutic target for type 2 diabetes. Front. Endocrinol. 2023, 14, 1286838. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Zhao, H.; Xu, H.-T.; Han, X.-D.; Wu, Y.-S.; Xu, F.-F.; Yang, X.-B.; Göransson, U.; Liu, B. Kaempferia galanga L.: Progresses in Phytochemistry, Pharmacology, Toxicology and Ethnomedicinal Uses. Front. Pharmacol. 2021, 12, 675350. [Google Scholar] [CrossRef]
- Zając, M.; Kotyńska, J.; Zambrowski, G.; Breczko, J.; Deptuła, P.; Cieśluk, M.; Zambrzycka, M.; Święcicka, I.; Bucki, R.; Naumowicz, M. Exposure to polystyrene nanoparticles leads to changes in the zeta potential of bacterial cells. Sci. Rep. 2023, 13, 9552. [Google Scholar] [CrossRef]
- Ducoli, S.; Kalčíková, G.; Velimirovic, M.; Depero, L.E.; Federici, S. Production, characterization, and toxicology of environmentally relevant nanoplastics: A review. Environ. Chem. Lett. 2025, 23, 649–675. [Google Scholar] [CrossRef]
- Wang, H.; Shi, X.; Gao, Y.; Zhang, X.; Zhao, H.; Wang, L.; Zhang, X.; Chen, R. Polystyrene nanoplastics induce profound metabolic shift in human cells as revealed by integrated proteomic and metabolomic analysis. Environ. Int. 2022, 166, 107349. [Google Scholar] [CrossRef]
- Li, L.; Sun, S.; Tan, L.; Wang, Y.; Wang, L.; Zhang, Z.; Zhang, L. Polystyrene Nanoparticles Reduced ROS and Inhibited Ferroptosis by Triggering Lysosome Stress and TFEB Nucleus Translocation in a Size-Dependent Manner. Nano Lett. 2019, 19, 7781–7792. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Li, T.; Chen, K.; Deng, X.; Zhang, Q.; Tang, H.; Shi, Z.; Zhu, T.; Zhu, J. PS-NPs Induced Neurotoxic Effects in SHSY-5Y Cells via Autophagy Activation and Mitochondrial Dysfunction. Brain Sci. 2022, 12, 952. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Jiang, X.; Yi, L.; Li, C.; Wang, H.; Xiong, W.; Li, Z.; Shen, J. Mitochondria Energy Metabolism Depression as Novel Adjuvant to Sensitize Radiotherapy and Inhibit Radiation Induced-Pulmonary Fibrosis. Adv. Sci. 2024, 11, 2401394. [Google Scholar] [CrossRef]
- Brillo, V.; Chieregato, L.; Leanza, L.; Muccioli, S.; Costa, R. Mitochondrial Dynamics, ROS, and Cell Signaling: A Blended Overview. Life 2021, 11, 332. [Google Scholar] [CrossRef]
- McArthur, K.; Whitehead, L.W.; Heddleston, J.M.; Li, L.; Padman, B.S.; Oorschot, V.; Geoghegan, N.D.; Chappaz, S.; Davidson, S.; San Chin, H.; et al. BAK/BAX macropores facilitate mitochondrial herniation and mtDNA efflux during apoptosis. Science 2018, 359, eaao6047. [Google Scholar] [CrossRef]
- Chaudhry, G.-S.; Md Akim, A.; Sung, Y.Y.; Sifzizul, T.M.T. Cancer and apoptosis: The apoptotic activity of plant and marine natural products and their potential as targeted cancer therapeutics. Front. Pharmacol. 2022, 13, 842376. [Google Scholar] [CrossRef]
- Yuan, H.; Yi, N.; Li, D.; Xu, C.; Yin, G.-R.; Zhuang, C.; Wang, Y.-J.; Ni, S. PPARγ regulates osteoarthritis chondrocytes apoptosis through caspase-3 dependent mitochondrial pathway. Sci. Rep. 2024, 14, 11237. [Google Scholar] [CrossRef]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Sharma, S.; Carmona, A.; Skowronek, A.; Yu, F.; Collins, M.O.; Naik, S.; Murzeau, C.M.; Tseng, P.-L.; Erdmann, K.S. Apoptotic signalling targets the post-endocytic sorting machinery of the death receptor Fas/CD95. Nat. Commun. 2019, 10, 3105. [Google Scholar] [CrossRef] [PubMed]
- Su, W.Y.; Tian, L.Y.; Guo, L.P.; Huang, L.Q.; Gao, W.Y. PI3K signaling-regulated metabolic reprogramming: From mechanism to application. Biochim. Biophys. Acta BBA—Rev. Cancer 2023, 1878, 188952. [Google Scholar] [CrossRef]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef]
- Sun, B.; Chen, H.; Xue, J.; Li, P.; Fu, X. The role of GLUT2 in glucose metabolism in multiple organs and tissues. Mol. Biol. Rep. 2023, 50, 6963–6974. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Young, J.; Wang, K.; Qian, Y.; Cai, L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol. Med. Rep. 2020, 22, 603–611. [Google Scholar] [CrossRef]
- Dong, S.; Ma, S.; Chen, H.; Tang, Z.; Song, W.; Deng, M. Nucleobase-crosslinked poly(2-oxazoline) nanoparticles as paclitaxel carriers with enhanced stability and ultra-high drug loading capacity for breast cancer therapy. Asian J. Pharm. Sci. 2022, 17, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2019, 9, 802. [Google Scholar] [CrossRef]
- Onyango, A.N. Excessive gluconeogenesis causes the hepatic insulin resistance paradox and its sequelae. Heliyon 2022, 8, e12294. [Google Scholar] [CrossRef]
- Hora, S.; Wuestefeld, T. Liver Injury and Regeneration: Current Understanding, New Approaches, and Future Perspectives. Cells 2023, 12, 2129. [Google Scholar] [CrossRef] [PubMed]
- Hayaza, S.; Wahyuningsih, S.P.A.; Susilo, R.J.K.; Husen, S.A.; Winarni, D.; Doong, R.; Darmanto, W. Dual role of immunomodulation by crude polysaccharide from okra against carcinogenic liver injury in mice. Heliyon 2021, 7, e06183. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Deng, S.; Wang, B.; Zhang, F.; Luo, T.; Kuang, H.; Kuang, X.; Yuan, Y.; Huang, J.; Zhang, D. Exposure to polystyrene nanoplastics induces hepatotoxicity involving NRF2-NLRP3 signaling pathway in mice. Ecotoxicol. Environ. Saf. 2024, 278, 116439. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| GAPDH | 5′-TGC ACC ACC AAC TGC TTA GC-3′ | 5′-GGA TGC AGG GAT GAT GTT CT-3′ |
| AKT | 5′-GGA GCT CTG TTA GCA CCG TT-3′ | 5′-AGT GGA AAT CCA GTT CCG AGC-3′ |
| PI3K | 5′-ACA TCG ACC TAC ACT TGG GG-3′ | 5′-TCC CCT CTC CCC AGT AGT TT-3′ |
| FAS | 5′-CAG GAA CAA CTC ATC CGT TCT CT-3′ | 5′-GGA CCG AGT AAT GCC GTT CA-3′ |
| GLUT2 | 5′-CCA GCA CAT ACG ACA CCA GAC G-3′ | 5′-CCA ACA TGG CTT TGA TCC TTC C-3′ |
| PK | 5′-CGT GGA CGA TGG GCT CAT CT-3′ | 5′-AGG TTC ACG CCC TTC TTG CT-3′ |
| PEPCK | 5′-GTC CCC CTT GTC TAC GAA GC-3′ | 5′-TGC ATG ATG ACC TTG CCC TTA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susilo, R.J.K.; Pramudya, M.; Dewi, F.R.P.; Seftiarini, W.; Hidayati, D.; Aunurohim, A.; Lim, V.; Herdiansyah, M.A.; Hayati, A. Adverse Effect of Polystyrene Nanoplastics in Impairing Glucose Metabolism in Liver Injury. Int. J. Mol. Sci. 2025, 26, 4870. https://doi.org/10.3390/ijms26104870
Susilo RJK, Pramudya M, Dewi FRP, Seftiarini W, Hidayati D, Aunurohim A, Lim V, Herdiansyah MA, Hayati A. Adverse Effect of Polystyrene Nanoplastics in Impairing Glucose Metabolism in Liver Injury. International Journal of Molecular Sciences. 2025; 26(10):4870. https://doi.org/10.3390/ijms26104870
Chicago/Turabian StyleSusilo, Raden Joko Kuncoroningrat, Manikya Pramudya, Firli Rahmah Primula Dewi, Windy Seftiarini, Dewi Hidayati, Aunurohim Aunurohim, Vuanghao Lim, Mochammad Aqilah Herdiansyah, and Alfiah Hayati. 2025. "Adverse Effect of Polystyrene Nanoplastics in Impairing Glucose Metabolism in Liver Injury" International Journal of Molecular Sciences 26, no. 10: 4870. https://doi.org/10.3390/ijms26104870
APA StyleSusilo, R. J. K., Pramudya, M., Dewi, F. R. P., Seftiarini, W., Hidayati, D., Aunurohim, A., Lim, V., Herdiansyah, M. A., & Hayati, A. (2025). Adverse Effect of Polystyrene Nanoplastics in Impairing Glucose Metabolism in Liver Injury. International Journal of Molecular Sciences, 26(10), 4870. https://doi.org/10.3390/ijms26104870







