Therapeutic Mechanisms of Exercise in Parkinson’s Disease
Abstract
1. Introduction
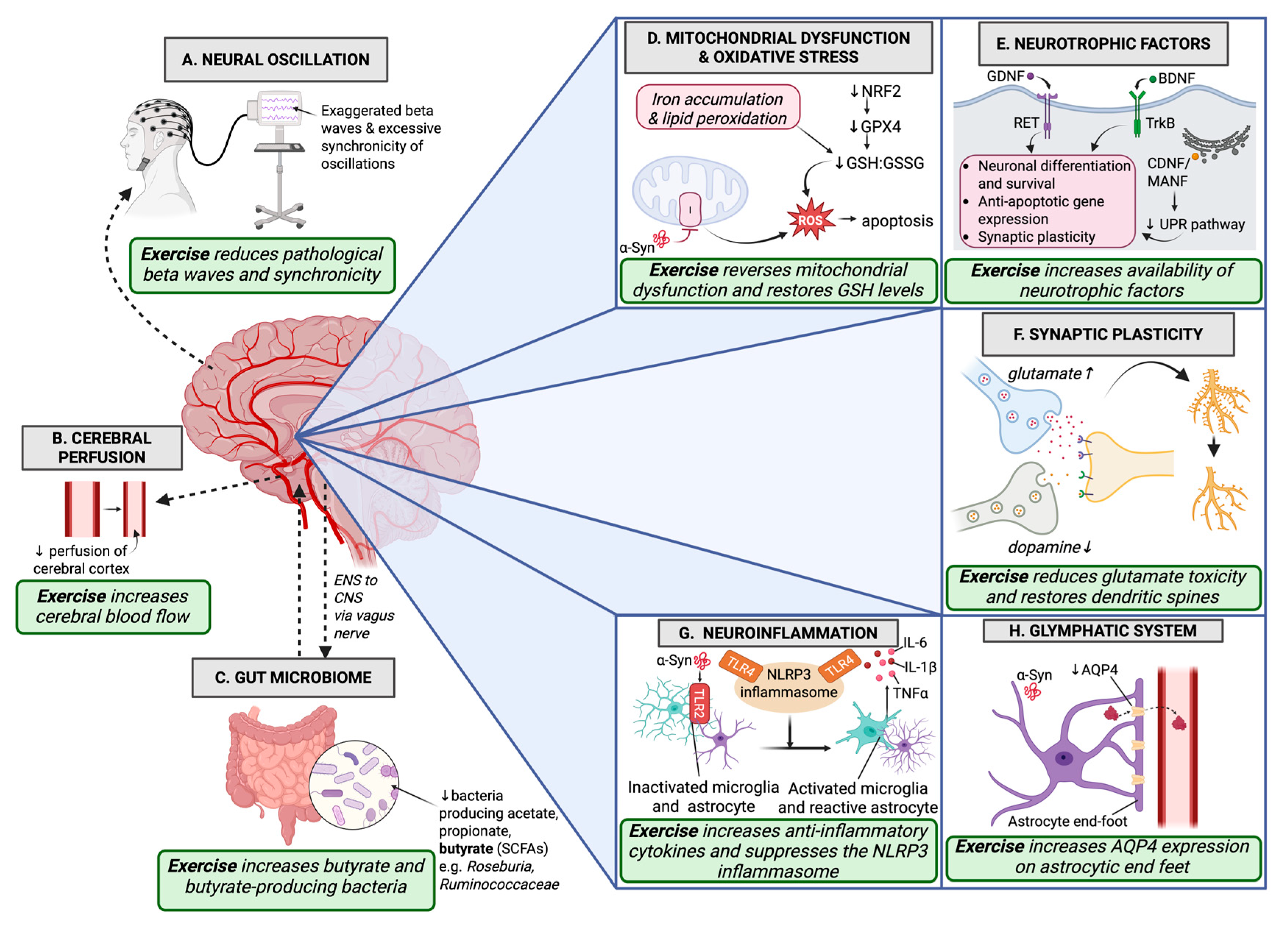
2. Neurotrophic Factors
3. Synaptic Regulation
4. Neural Oscillation
5. Cerebral Perfusion
6. Glymphatic System
7. Neuroinflammation
8. Gut Microbiome
9. Mitochondrial Dysfunction and Oxidative Stress
10. Irisin: The Molecular Mediator?
11. Discussion
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Mai, D.; Qu, S. Molecular Mechanisms of Glutamate Toxicity in Parkinson’s Disease. Front. Neurosci. 2020, 14, 585584. [Google Scholar] [CrossRef] [PubMed]
- Dong-Chen, X.; Yong, C.; Yang, X.; Chen-Yu, S.; Li-Hua, P. Signaling pathways in Parkinson’s disease: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 73. [Google Scholar] [CrossRef]
- Farrow, S.L.; Cooper, A.A.; O’Sullivan, J.M. Redefining the hypotheses driving Parkinson’s diseases research. npj Parkinson’s Dis. 2022, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Bennett, D.A.; Beckett, L.A.; Murray, A.M.; Shannon, K.M.; Goetz, C.G.; Pilgrim, D.M.; Evans, D.A. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N. Engl. J. Med. 1996, 334, 71–76. [Google Scholar] [CrossRef]
- Dorsey, E.R.; De Miranda, B.R.; Horsager, J.; Borghammer, P. The Body, the Brain, the Environment, and Parkinson’s Disease. J. Parkinson’s Dis. 2024, 14, 363–381. [Google Scholar] [CrossRef]
- Zapanta, K.; Schroeder, E.T.; Fisher, B.E. Rethinking Parkinson Disease: Exploring Gut-Brain Interactions and the Potential Role of Exercise. Phys. Ther. 2022, 102, pzac022. [Google Scholar] [CrossRef]
- Feng, Y.S.; Yang, S.D.; Tan, Z.X.; Wang, M.M.; Xing, Y.; Dong, F.; Zhang, F. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020, 245, 117345. [Google Scholar] [CrossRef]
- Bispo, D.; Lins, C.; Hawkes, K.L.; Tripp, S.; Khoo, T.K. The Positive Effects of Physical Activity on Quality of Life in Parkinson’s Disease: A Systematic Review. Geriatrics 2024, 9, 94. [Google Scholar] [CrossRef]
- Amara, A.W.; Memon, A.A. Effects of Exercise on Non-motor Symptoms in Parkinson’s Disease. Clin. Ther. 2018, 40, 8–15. [Google Scholar] [CrossRef]
- Dauwan, M.; Begemann, M.J.H.; Slot, M.I.E.; Lee, E.H.M.; Scheltens, P.; Sommer, I.E.C. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. J. Neurol. 2021, 268, 1222–1246. [Google Scholar] [CrossRef] [PubMed]
- Sujkowski, A.; Hong, L.; Wessells, R.J.; Todi, S.V. The protective role of exercise against age-related neurodegeneration. Ageing Res. Rev. 2022, 74, 101543. [Google Scholar] [CrossRef] [PubMed]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Albert, K.; Raymundo, D.P.; Panhelainen, A.; Eesmaa, A.; Shvachiy, L.; Araujo, G.R.; Chmielarz, P.; Yan, X.; Singh, A.; Cordeiro, Y.; et al. Cerebral dopamine neurotrophic factor reduces alpha-synuclein aggregation and propagation and alleviates behavioral alterations in vivo. Mol. Ther. 2021, 29, 2821–2840. [Google Scholar] [CrossRef]
- Lohelaid, H.; Saarma, M.; Airavaara, M. CDNF and ER stress: Pharmacology and therapeutic possibilities. Pharmacol. Ther. 2024, 254, 108594. [Google Scholar] [CrossRef]
- Houlton, J.; Abumaria, N.; Hinkley, S.F.R.; Clarkson, A.N. Therapeutic Potential of Neurotrophins for Repair After Brain Injury: A Helping Hand From Biomaterials. Front. Neurosci. 2019, 13, 790. [Google Scholar] [CrossRef]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Wysocka, A.; Stepniewska, A.; Niewiadomska, G. Exercise-Induced Neuroprotection and Recovery of Motor Function in Animal Models of Parkinson’s Disease. Front. Neurol. 2019, 10, 1143. [Google Scholar] [CrossRef]
- Ateaque, S.; Merkouris, S.; Barde, Y.A. Neurotrophin signalling in the human nervous system. Front. Mol. Neurosci. 2023, 16, 1225373. [Google Scholar] [CrossRef]
- Kaplan, D.R.; Miller, F.D. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Bonanni, R.; Cariati, I.; Tarantino, U.; D’Arcangelo, G.; Tancredi, V. Physical Exercise and Health: A Focus on Its Protective Role in Neurodegenerative Diseases. J. Funct. Morphol. Kinesiol. 2022, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Bjorklund, A.; Gash, D.M.; Whone, A.; Van Laar, A.; Kordower, J.H.; Bankiewicz, K.; Kieburtz, K.; Saarma, M.; Booms, S.; et al. GDNF and Parkinson’s Disease: Where Next? A Summary from a Recent Workshop. J. Parkinson’s Dis. 2020, 10, 875–891. [Google Scholar] [CrossRef] [PubMed]
- Pakarinen, E.; Lindholm, P. CDNF and MANF in the brain dopamine system and their potential as treatment for Parkinson’s disease. Front. Psychiatry 2023, 14, 1188697. [Google Scholar] [CrossRef]
- Leem, Y.H.; Park, J.S.; Park, J.E.; Kim, D.Y.; Kim, H.S. Suppression of neuroinflammation and alpha-synuclein oligomerization by rotarod walking exercise in subacute MPTP model of Parkinson’s disease. Neurochem. Int. 2023, 165, 105519. [Google Scholar] [CrossRef]
- Da Silva, W.A.B.; Ferreira Oliveira, K.; Caroline Vitorino, L.; Ferreira Romao, L.; Allodi, S.; Lourenco Correa, C. Physical exercise increases the production of tyrosine hydroxylase and CDNF in the spinal cord of a Parkinson’s disease mouse model. Neurosci. Lett. 2021, 760, 136089. [Google Scholar] [CrossRef]
- Palasz, E.; Niewiadomski, W.; Gasiorowska, A.; Mietelska-Porowska, A.; Niewiadomska, G. Neuroplasticity and Neuroprotective Effect of Treadmill Training in the Chronic Mouse Model of Parkinson’s Disease. Neural Plast. 2019, 2019, 8215017. [Google Scholar] [CrossRef]
- Fontanesi, C.; Kvint, S.; Frazzitta, G.; Bera, R.; Ferrazzoli, D.; Di Rocco, A.; Rebholz, H.; Friedman, E.; Pezzoli, G.; Quartarone, A.; et al. Intensive Rehabilitation Enhances Lymphocyte BDNF-TrkB Signaling in Patients with Parkinson’s Disease. Neurorehabil. Neural Repair. 2016, 30, 411–418. [Google Scholar] [CrossRef]
- Paterno, A.; Polsinelli, G.; Federico, B. Changes of brain-derived neurotrophic factor (BDNF) levels after different exercise protocols: A systematic review of clinical studies in Parkinson’s disease. Front. Physiol. 2024, 15, 1352305. [Google Scholar] [CrossRef]
- Fallah Mohammadi, Z.; Falah Mohammadi, H.; Patel, D.I. Comparing the effects of progressive and mild intensity treadmill running protocols on neuroprotection of parkinsonian rats. Life Sci. 2019, 229, 219–224. [Google Scholar] [CrossRef]
- McCullough, M.J.; Gyorkos, A.M.; Spitsbergen, J.M. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience 2013, 240, 258–268. [Google Scholar] [CrossRef]
- Petzinger, G.M.; Holschneider, D.P.; Fisher, B.E.; McEwen, S.; Kintz, N.; Halliday, M.; Toy, W.; Walsh, J.W.; Beeler, J.; Jakowec, M.W. The Effects of Exercise on Dopamine Neurotransmission in Parkinson’s Disease: Targeting Neuroplasticity to Modulate Basal Ganglia Circuitry. Brain Plast. 2015, 1, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Huang, Z.; Zhu, Y.; Liu, B.; Chen, Z.; Chen, T.; Jia, L.; Li, Y.; Lei, W. Increase in Glutamatergic Terminals in the Striatum Following Dopamine Depletion in a Rat Model of Parkinson’s Disease. Neurochem. Res. 2019, 44, 1079–1089. [Google Scholar] [CrossRef]
- Deutch, A.Y.; Colbran, R.J.; Winder, D.J. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat. Disord. 2007, 13 (Suppl. S3), S251–S258. [Google Scholar] [CrossRef]
- Segal, M.; Andersen, P. Dendritic spines shaped by synaptic activity. Curr. Opin. Neurobiol. 2000, 10, 582–586. [Google Scholar] [CrossRef]
- Shin, M.S.; Jeong, H.Y.; An, D.I.; Lee, H.Y.; Sung, Y.H. Treadmill exercise facilitates synaptic plasticity on dopaminergic neurons and fibers in the mouse model with Parkinson’s disease. Neurosci. Lett. 2016, 621, 28–33. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, S.; Sun, J. Exercise increases striatal Glu reuptake and improves motor dysfunction in 6-OHDA-induced Parkinson’s disease rats. Exp. Brain Res. 2021, 239, 3277–3287. [Google Scholar] [CrossRef]
- Alarcon, T.A.; Presti-Silva, S.M.; Simoes, A.P.T.; Ribeiro, F.M.; Pires, R.G.W. Molecular mechanisms underlying the neuroprotection of environmental enrichment in Parkinson’s disease. Neural Regen. Res. 2023, 18, 1450–1456. [Google Scholar]
- De Laat, B.; Hoye, J.; Stanley, G.; Hespeler, M.; Ligi, J.; Mohan, V.; Wooten, D.W.; Zhang, X.; Nguyen, T.D.; Key, J.; et al. Intense exercise increases dopamine transporter and neuromelanin concentrations in the substantia nigra in Parkinson’s disease. npj Parkinson’s Dis. 2024, 10, 34. [Google Scholar] [CrossRef]
- Kasanga, E.A.; Soto, I.; Centner, A.; McManus, R.; Shifflet, M.K.; Navarrete, W.; Han, Y.; Lisk, J.; Ehrhardt, T.; Wheeler, K.; et al. Moderate intensity aerobic exercise alleviates motor deficits in 6-OHDA lesioned rats and reduces serum levels of biomarkers of Parkinson’s disease severity without recovery of striatal dopamine or tyrosine hydroxylase. Exp. Neurol. 2024, 379, 114875. [Google Scholar] [CrossRef]
- Kintz, N.; Petzinger, G.M.; Jakowec, M.W. Treadmill exercise modifies dopamine receptor expression in the prefrontal cortex of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of Parkinson’s disease. Neuroreport 2017, 28, 987–995. [Google Scholar] [CrossRef] [PubMed]
- VanLeeuwen, J.E.; Petzinger, G.M.; Walsh, J.P.; Akopian, G.K.; Vuckovic, M.; Jakowec, M.W. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J. Neurosci. Res. 2010, 88, 650–668. [Google Scholar] [CrossRef] [PubMed]
- Gergin, S.; Kirazli, O.; Boraci, H.; Yildiz, S.D.; Yananli, H.R.; Sehirli, U.S. The effects of regular swimming exercise and melatonin on the neurons localized in the striatum of hemiparkinsonian rats. Anat. Sci. Int. 2023, 98, 204–219. [Google Scholar] [CrossRef]
- Shi, K.; Liu, X.; Hou, L.; Qiao, D.; Peng, Y. Exercise Improves Movement by Regulating the Plasticity of Cortical Function in Hemiparkinsonian Rats. Front. Aging Neurosci. 2021, 13, 695108. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Madadi Asl, M.; Vahabie, A.H.; Valizadeh, A. The Origin of Abnormal Beta Oscillations in the Parkinsonian Corticobasal Ganglia Circuits. Parkinson’s Dis. 2022, 2022, 7524066. [Google Scholar] [CrossRef]
- Galvan, A.; Devergnas, A.; Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 2015, 9, 5. [Google Scholar] [CrossRef]
- Costa, R.M.; Lin, S.C.; Sotnikova, T.D.; Cyr, M.; Gainetdinov, R.R.; Caron, M.G.; Nicolelis, M.A. Rapid alterations in corticostriatal ensemble coordination during acute dopamine-dependent motor dysfunction. Neuron 2006, 52, 359–369. [Google Scholar] [CrossRef]
- Mallet, N.; Pogosyan, A.; Sharott, A.; Csicsvari, J.; Bolam, J.P.; Brown, P.; Magill, P.J. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008, 28, 4795–4806. [Google Scholar] [CrossRef]
- Halje, P.; Brys, I.; Mariman, J.J.; da Cunha, C.; Fuentes, R.; Petersson, P. Oscillations in cortico-basal ganglia circuits: Implications for Parkinson’s disease and other neurologic and psychiatric conditions. J. Neurophysiol. 2019, 122, 203–231. [Google Scholar] [CrossRef]
- Simpson, T.G.; Godfrey, W.; Torrecillos, F.; He, S.; Herz, D.M.; Oswal, A.; Muthuraman, M.; Pogosyan, A.; Tan, H. Cortical beta oscillations help synchronise muscles during static posture holding in healthy motor control. Neuroimage 2024, 298, 120774. [Google Scholar] [CrossRef]
- Bougou, V.; Vanhoyland, M.; Decramer, T.; Van Hoylandt, A.; Smeijers, S.; Nuttin, B.; De Vloo, P.; Vandenberghe, W.; Nieuwboer, A.; Janssen, P.; et al. Active and Passive Cycling Decrease Subthalamic beta Oscillations in Parkinson’s Disease. Mov. Disord. 2024, 39, 85–93. [Google Scholar] [CrossRef]
- Chaire, A.; Becke, A.; Duzel, E. Effects of Physical Exercise on Working Memory and Attention-Related Neural Oscillations. Front. Neurosci. 2020, 14, 239. [Google Scholar] [CrossRef] [PubMed]
- Firbank, M.J.; Molloy, S.; McKeith, I.G.; Burn, D.J.; O’Brien, J.T. Longitudinal change in 99mTcHMPAO cerebral perfusion SPECT in Parkinson’s disease over one year. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1448–1451. [Google Scholar] [CrossRef] [PubMed]
- Rane, S.; Koh, N.; Oakley, J.; Caso, C.; Zabetian, C.P.; Cholerton, B.; Montine, T.J.; Grabowski, T. Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 2020, 76, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Wang, H.; Xu, D.; You, H.; Zuo, Z.; Feng, F. Altered cerebral perfusion and microstructure in advanced Parkinson’s disease and their associations with clinical features. Neurol. Res. 2022, 44, 47–56. [Google Scholar] [CrossRef]
- Pelizzari, L.; Lagana, M.M.; Di Tella, S.; Rossetto, F.; Bergsland, N.; Nemni, R.; Clerici, M.; Baglio, F. Combined Assessment of Diffusion Parameters and Cerebral Blood Flow Within Basal Ganglia in Early Parkinson’s Disease. Front. Aging Neurosci. 2019, 11, 134. [Google Scholar] [CrossRef]
- Erro, R.; Ponticorvo, S.; Manara, R.; Barone, P.; Picillo, M.; Scannapieco, S.; Cicarelli, G.; Squillante, M.; Volpe, G.; Esposito, F.; et al. Subcortical atrophy and perfusion patterns in Parkinson disease and multiple system atrophy. Parkinsonism Relat. Disord. 2020, 72, 49–55. [Google Scholar] [CrossRef]
- Liu, J.; Min, L.; Liu, R.; Zhang, X.; Wu, M.; Di, Q.; Ma, X. The effect of exercise on cerebral blood flow and executive function among young adults: A double-blinded randomized controlled trial. Sci. Rep. 2023, 13, 8269. [Google Scholar] [CrossRef]
- Issidorides, M.R. Neuronal vascular relationships in the zona compacta of normal and parkinsonian substantia nigra. Brain Res. 1971, 25, 289–299. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Wang, X.; Chen, C.; Zhao, R.; Lu, H.; Zhu, H.; Xue, B.; Liang, H.; Sethi, S.K.; et al. Vascular, flow and perfusion abnormalities in Parkinson’s disease. Parkinsonism Relat. Disord. 2020, 73, 8–13. [Google Scholar] [CrossRef]
- Smith, J.C.; Paulson, E.S.; Cook, D.B.; Verber, M.D.; Tian, Q. Detecting changes in human cerebral blood flow after acute exercise using arterial spin labeling: Implications for fMRI. J. Neurosci. Methods 2010, 191, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Secher, N.H.; Seifert, T.; Van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Mekari, S.; Neyedli, H.F.; Fraser, S.; O’Brien, M.W.; Martins, R.; Evans, K.; Earle, M.; Aucoin, R.; Chiekwe, J.; Hollohan, Q.; et al. High-Intensity Interval Training Improves Cognitive Flexibility in Older Adults. Brain Sci. 2020, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Ogoh, S.; Ainslie, P.N. Regulatory mechanisms of cerebral blood flow during exercise: New concepts. Exerc. Sport Sci. Rev. 2009, 37, 123–129. [Google Scholar] [CrossRef]
- Viboolvorakul, S.; Patumraj, S. Exercise training could improve age-related changes in cerebral blood flow and capillary vascularity through the upregulation of VEGF and eNOS. BioMed Res. Int. 2014, 2014, 230791. [Google Scholar] [CrossRef]
- Kwak, S.E.; Lee, J.H.; Zhang, D.; Song, W. Angiogenesis: Focusing on the effects of exercise in aging and cancer. J. Exerc. Nutr. Biochem. 2018, 22, 21–26. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Plog, B.A.; Nedergaard, M. The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. 2018, 13, 379–394. [Google Scholar] [CrossRef]
- Szlufik, S.; Kopec, K.; Szleszkowski, S.; Koziorowski, D. Glymphatic System Pathology and Neuroinflammation as Two Risk Factors of Neurodegeneration. Cells 2024, 13, 286. [Google Scholar] [CrossRef]
- Mestre, H.; Hablitz, L.M.; Xavier, A.L.; Feng, W.; Zou, W.; Pu, T.; Monai, H.; Murlidharan, G.; Castellanos Rivera, R.M.; Simon, M.J.; et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. eLife 2018, 7, e40070. [Google Scholar] [CrossRef]
- Wood, K.H.; Nenert, R.; Miften, A.M.; Kent, G.W.; Sleyster, M.; Memon, R.A.; Joop, A.; Pilkington, J.; Memon, A.A.; Wilson, R.N.; et al. Diffusion Tensor Imaging-Along the Perivascular-Space Index Is Associated with Disease Progression in Parkinson’s Disease. Mov. Disord. 2024, 39, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of Perivascular Localization of Aquaporin-4 with Cognition and Alzheimer Disease in Aging Brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, C.; He, X.Z.; Li, Z.H.; Meng, J.C.; Mao, R.T.; Li, X.; Xue, R.; Gui, Q.; Zhang, G.X.; et al. Interaction Between the Glymphatic System and alpha-Synuclein in Parkinson’s Disease. Mol. Neurobiol. 2023, 60, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef]
- Von Holstein-Rathlou, S.; Petersen, N.C.; Nedergaard, M. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett. 2018, 662, 253–258. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Li, L.; Zhang, L.; Zuo, Z.; Feng, Y.; He, X.; Hu, X. Voluntary wheel exercise improves glymphatic clearance and ameliorates colitis-associated cognitive impairment in aged mice by inhibiting TRPV4-induced astrocytic calcium activity. Exp. Neurol. 2024, 376, 114770. [Google Scholar] [CrossRef]
- Jessen, N.A.; Munk, A.S.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef]
- Dolezal, B.A.; Neufeld, E.V.; Boland, D.M.; Martin, J.L.; Cooper, C.B. Interrelationship between Sleep and Exercise: A Systematic Review. Adv. Prev. Med. 2017, 2017, 1364387. [Google Scholar]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, 691. [Google Scholar] [CrossRef]
- Qu, Y.; Li, J.; Qin, Q.; Wang, D.; Zhao, J.; An, K.; Mao, Z.; Min, Z.; Xiong, Y.; Li, J.; et al. A systematic review and meta-analysis of inflammatory biomarkers in Parkinson’s disease. npj Parkinson’s Dis. 2023, 9, 18. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Wijeyekoon, R.; Yarnall, A.J.; Lawson, R.A.; Breen, D.P.; Evans, J.R.; Cummins, G.A.; Duncan, G.W.; Khoo, T.K.; Burn, D.J.; et al. Serum immune markers and disease progression in an incident Parkinson’s disease cohort (ICICLE-PD). Mov. Disord. 2016, 31, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Gillardon, F.; Schmid, R.; Draheim, H. Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience 2012, 208, 41–48. [Google Scholar] [CrossRef]
- Wang, S.; Chu, C.H.; Stewart, T.; Ginghina, C.; Wang, Y.; Nie, H.; Guo, M.; Wilson, B.; Hong, J.S.; Zhang, J. alpha-Synuclein, a chemoattractant, directs microglial migration via H2O2-dependent Lyn phosphorylation. Proc. Natl. Acad. Sci. USA 2015, 112, E1926–E1935. [Google Scholar] [PubMed]
- Fellner, L.; Irschick, R.; Schanda, K.; Reindl, M.; Klimaschewski, L.; Poewe, W.; Wenning, G.K.; Stefanova, N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia 2013, 61, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Ho, D.H.; Suk, J.E.; You, S.; Michael, S.; Kang, J.; Joong Lee, S.; Masliah, E.; Hwang, D.; Lee, H.J.; et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013, 4, 1562. [Google Scholar] [CrossRef]
- Wang, W.; Lv, Z.; Gao, J.; Liu, M.; Wang, Y.; Tang, C.; Xiang, J. Treadmill exercise alleviates neuronal damage by suppressing NLRP3 inflammasome and microglial activation in the MPTP mouse model of Parkinson’s disease. Brain Res. Bull. 2021, 174, 349–358. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Real, C.C.; Garcia, P.C.; Britto, L.R.G. Treadmill Exercise Prevents Increase of Neuroinflammation Markers Involved in the Dopaminergic Damage of the 6-OHDA Parkinson’s Disease Model. J. Mol. Neurosci. 2017, 63, 36–49. [Google Scholar] [CrossRef]
- Szymura, J.; Kubica, J.; Wiecek, M.; Pera, J. The Immunomodulary Effects of Systematic Exercise in Older Adults and People with Parkinson’s Disease. J. Clin. Med. 2020, 9, 184. [Google Scholar] [CrossRef]
- Zhou, X.; Spittau, B.; Krieglstein, K. TGFbeta signalling plays an important role in IL4-induced alternative activation of microglia. J. Neuroinflamm. 2012, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Koo, J.H.; Kwon, I.; Kang, E.B.; Um, H.S.; Soya, H.; Lee, Y.; Cho, J.Y. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017, 1655, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Huang, P.; Cui, S.S.; Tan, Y.Y.; He, Y.C.; Shen, X.; Jiang, Q.Y.; Huang, P.; He, G.Y.; Li, B.Y.; et al. Mechanisms of motor symptom improvement by long-term Tai Chi training in Parkinson’s disease patients. Transl. Neurodegener. 2022, 11, 6. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.F.; Pelletier, A.; Montplaisir, J. Prodromal autonomic symptoms and signs in Parkinson’s disease and dementia with Lewy bodies. Mov. Disord. 2013, 28, 597–604. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Vedders, L.; Lue, L.; White Iii, C.L.; Akiyama, H.; Caviness, J.N.; Shill, H.A.; Sabbagh, M.N.; et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010, 119, 689–702. [Google Scholar] [CrossRef]
- Goehler, L.E.; Busch, C.R.; Tartaglia, N.; Relton, J.; Sisk, D.; Maier, S.F.; Watkins, L.R. Blockade of cytokine induced conditioned taste aversion by subdiaphragmatic vagotomy: Further evidence for vagal mediation of immune-brain communication. Neurosci. Lett. 1995, 185, 163–166. [Google Scholar] [CrossRef]
- Aho, V.T.E.; Houser, M.C.; Pereira, P.A.B.; Chang, J.; Rudi, K.; Paulin, L.; Hertzberg, V.; Auvinen, P.; Tansey, M.G.; Scheperjans, F. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol. Neurodegener. 2021, 16, 6. [Google Scholar] [CrossRef]
- Cirstea, M.S.; Yu, A.C.; Golz, E.; Sundvick, K.; Kliger, D.; Radisavljevic, N.; Foulger, L.H.; Mackenzie, M.; Huan, T.; Finlay, B.B.; et al. Microbiota Composition and Metabolism Are Associated with Gut Function in Parkinson’s Disease. Mov. Disord. 2020, 35, 1208–1217. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Kidd, S.K.; Schneider, J.S. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010, 1354, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Chen, C.C.; Liao, H.Y.; Lin, Y.T.; Wu, Y.W.; Liou, J.M.; Wu, M.S.; Kuo, C.H.; Lin, C.H. Association of Fecal and Plasma Levels of Short-Chain Fatty Acids with Gut Microbiota and Clinical Severity in Patients with Parkinson Disease. Neurology 2022, 98, e848–e858. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Yang, G.; Meng, B.; Yi, Z.; Yang, G.; Chen, M.; Hou, P.; Wang, H.; Xu, X. Moderate-Intensity Physical Exercise Affects the Exercise Performance and Gut Microbiota of Mice. Front. Cell Infect. Microbiol. 2021, 11, 712381. [Google Scholar] [CrossRef]
- Munukka, E.; Ahtiainen, J.P.; Puigbo, P.; Jalkanen, S.; Pahkala, K.; Keskitalo, A.; Kujala, U.M.; Pietila, S.; Hollmen, M.; Elo, L.; et al. Six-Week Endurance Exercise Alters Gut Metagenome That Is not Reflected in Systemic Metabolism in Over-weight Women. Front. Microbiol. 2018, 9, 2323. [Google Scholar] [CrossRef]
- Mitchell, C.M.; Davy, B.M.; Hulver, M.W.; Neilson, A.P.; Bennett, B.J.; Davy, K.P. Does Exercise Alter Gut Microbial Composition? A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 160–167. [Google Scholar] [CrossRef]
- Batacan, R.B.; Fenning, A.S.; Dalbo, V.J.; Scanlan, A.T.; Duncan, M.J.; Moore, R.J.; Stanley, D. A gut reaction: The combined influence of exercise and diet on gastrointestinal microbiota in rats. J. Appl. Microbiol. 2017, 122, 1627–1638. [Google Scholar] [CrossRef]
- Matsumoto, M.; Inoue, R.; Tsukahara, T.; Ushida, K.; Chiji, H.; Matsubara, N.; Hara, H. Voluntary running exercise alters microbiota composition and increases n-butyrate concentration in the rat cecum. Biosci. Biotechnol. Biochem. 2008, 72, 572–576. [Google Scholar] [CrossRef]
- Wang, Y.; Pu, Z.; Zhang, Y.; Du, Z.; Guo, Z.; Bai, Q. Exercise training has a protective effect in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice model with improved neural and intestinal pathology and modified intestinal flora. Behav. Brain Res. 2023, 439, 114240. [Google Scholar] [CrossRef]
- Bycura, D.; Santos, A.C.; Shiffer, A.; Kyman, S.; Winfree, K.; Sutliffe, J.; Pearson, T.; Sonderegger, D.; Cope, E.; Caporaso, J.G. Impact of Different Exercise Modalities on the Human Gut Microbiome. Sports 2021, 9, 14. [Google Scholar] [CrossRef]
- Moore, J.H.; Smith, K.S.; Chen, D.; Lamb, D.A.; Smith, M.A.; Osburn, S.C.; Ruple, B.A.; Morrow, C.D.; Huggins, K.W.; McDonald, J.R.; et al. Exploring the Effects of Six Weeks of Resistance Training on the Fecal Microbiome of Older Adult Males: Secondary Analysis of a Peanut Protein Supplemented Randomized Controlled Trial. Sports 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Boytar, A.N.; Skinner, T.L.; Wallen, R.E.; Jenkins, D.G.; Dekker Nitert, M. The Effect of Exercise Prescription on the Human Gut Microbiota and Comparison between Clinical and Apparently Healthy Populations: A Systematic Review. Nutrients 2023, 15, 1534. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. Influence of Foods and Nutrition on the Gut Microbiome and Implications for Intestinal Health. Int. J. Mol. Sci. 2022, 23, 9588. [Google Scholar] [CrossRef] [PubMed]
- Henrich, M.T.; Oertel, W.H.; Surmeier, D.J.; Geibl, F.F. Mitochondrial dysfunction in Parkinson’s disease—A key disease hallmark with therapeutic potential. Mol. Neurodegener. 2023, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Alhazmi, H.A.; Hassani, R.; Khuwaja, G.; Maheshkumar, V.P.; Aldahish, A.; Chidambaram, K. Role of ferroptosis pathways in neuroinflammation and neurological disorders: From pathogenesis to treatment. Heliyon 2024, 10, e24786. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef]
- Niu, C.; Dong, M.; Niu, Y. Role of Glutathione in Parkinson’s Disease Pathophysiology and Therapeutic Potential of Polyphenols. Phytother. Res. 2024, 38, 5567–5582. [Google Scholar] [CrossRef]
- Johnson, D.A.; Johnson, J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015, 88, 253–267. [Google Scholar] [CrossRef]
- Suh, J.H.; Shenvi, S.V.; Dixon, B.M.; Liu, H.; Jaiswal, A.K.; Liu, R.M.; Hagen, T.M. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc. Natl. Acad. Sci. USA 2004, 101, 3381–3386. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport. Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free Radic. Biol. Med. 2016, 98, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Chien, C.Y.; Pan, C.Y.; Tseng, Y.T.; Wang, T.C.; Lin, T.K. Effects of long-term Tai Chi vs. aerobic exercise on antioxidant activity and cognitive function in individuals with Parkinson’s disease. Behav. Brain Res. 2025, 476, 115274. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Schilling, B.K.; Karlage, R.E.; Ledoux, M.S.; Pfeiffer, R.F.; Callegari, J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med. Sci. Sports Exerc. 2008, 40, 1385–1389. [Google Scholar] [CrossRef]
- Monir, D.M.; Mahmoud, M.E.; Ahmed, O.G.; Rehan, I.F.; Abdelrahman, A. Forced exercise activates the NrF2 pathway in the striatum and ameliorates motor and behavioral manifestations of Parkinson’s disease in rotenone-treated rats. Behav. Brain Funct. 2020, 16, 9. [Google Scholar] [CrossRef]
- Koo, J.H.; Cho, J.Y.; Lee, U.B. Treadmill exercise alleviates motor deficits and improves mitochondrial import machinery in an MPTP-induced mouse model of Parkinson’s disease. Exp. Gerontol. 2017, 89, 20–29. [Google Scholar] [CrossRef]
- Chuang, C.S.; Chang, J.C.; Cheng, F.C.; Liu, K.H.; Su, H.L.; Liu, C.S. Modulation of mitochondrial dynamics by treadmill training to improve gait and mitochondrial deficiency in a rat model of Parkinson’s disease. Life Sci. 2017, 191, 236–244. [Google Scholar] [CrossRef]
- Tutakhail, A.; Nazary, Q.A.; Lebsir, D.; Kerdine-Romer, S.; Coudore, F. Induction of brain Nrf2-HO-1 pathway and antinociception after different physical training paradigms in mice. Life Sci. 2018, 209, 149–156. [Google Scholar] [CrossRef]
- Tung, Y.T.; Liao, Y.C.; Yeh, T.H.; Tsao, S.P.; Chang, C.C.; Shih, W.T.; Huang, H.Y. 10 weeks low intensity treadmill exercise intervention ameliorates motor deficits and sustains muscle mass via decreasing oxidative damage and increasing mitochondria function in a rat model of Parkinson’s disease. Life Sci. 2024, 350, 122733. [Google Scholar] [CrossRef]
- Pinho, R.A.; Aguiar, A.S., Jr.; Radak, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, 529. [Google Scholar] [CrossRef]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Avgerinos, K.I.; Liu, J.; Dalamaga, M. Could exercise hormone irisin be a therapeutic agent against Parkinson’s and other neurodegenerative diseases? Metabol. Open 2023, 17, 100233. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Martinez, A.; Ruiz-Lozano, P.; Chien, K.R. Mouse PeP: A novel peroxisomal protein linked to myoblast differentiation and development. Dev. Dyn. 2002, 224, 154–167. [Google Scholar] [CrossRef]
- Mitchell, A.K.; Bliss, R.R.; Church, F.C. Exercise, Neuroprotective Exerkines, and Parkinson’s Disease: A Narrative Review. Biomolecules 2024, 14, 1241. [Google Scholar] [CrossRef]
- Qiu, R.; Sun, W.; Su, Y.; Sun, Z.; Fan, K.; Liang, Y.; Lin, X.; Zhang, Y. Irisin’s emerging role in Parkinson’s disease research: A review from molecular mechanisms to therapeutic prospects. Life Sci. 2024, 357, 123088. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Yang, D.; Li, J.; Li, Z.; Zhao, M.; Wang, D.; Gou, F.; Wang, J.; Dai, Y.; et al. Irisin regulates oxidative stress and mitochondrial dysfunction through the UCP2-AMPK pathway in prion diseases. Cell Death Dis. 2025, 16, 66. [Google Scholar] [CrossRef]
- Islam, M.R.; Valaris, S.; Young, M.F.; Haley, E.B.; Luo, R.; Bond, S.F.; Mazuera, S.; Kitchen, R.R.; Caldarone, B.J.; Bettio, L.E.B.; et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat. Metab. 2021, 3, 1058–1070. [Google Scholar] [CrossRef]
- Shahabi, S.; Esfarjani, F.; Zamani, S.; Rarani, F.Z.; Rashidi, B. Evaluating the Efficacy of Irisin Injection in Mimicking the Molecular Responses Induced by Endurance Exercise in Mouse Liver Tissue. Int. J. Prev. Med. 2024, 15, 66. [Google Scholar] [CrossRef]
- Ernst, M.; Folkerts, A.K.; Gollan, R.; Lieker, E.; Caro-Valenzuela, J.; Adams, A.; Cryns, N.; Monsef, I.; Dresen, A.; Roheger, M.; et al. Physical exercise for people with Parkinson’s disease: A systematic review and network meta-analysis. Cochrane Database Syst. Rev. 2024, 4, CD013856. [Google Scholar]
- Cancela-Carral, J.M.; Campo-Prieto, P.; Rodriguez-Fuentes, G. The IntegraPark Study: An Opportunity to Facilitate High-Intensity Exercise with Immersive Virtual Reality in Parkinson’s Disease Patients. J. Funct. Morphol. Kinesiol. 2024, 9, 156. [Google Scholar] [CrossRef]
- Skrzatek, A.; Nuic, D.; Cherif, S.; Beranger, B.; Gallea, C.; Bardinet, E.; Welter, M.L. Brain modulation after exergaming training in advanced forms of Parkinson’s disease: A randomized controlled study. J. Neuroeng. Rehabil. 2024, 21, 133. [Google Scholar] [CrossRef]
- Hardeman, L.E.S.; Geerse, D.J.; Hoogendoorn, E.M.; Nonnekes, J.; Roerdink, M. Remotely prescribed, monitored, and tailored home-based gait-and-balance exergaming using augmented reality glasses: A clinical feasibility study in people with Parkinson’s disease. Front. Neurol. 2024, 15, 1373740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, W.; Bai, Q.; Gao, S. The therapeutic effects of yoga in people with Parkinson’s disease: A mini-review. Ann. Med. 2023, 55, 2294935. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ru, Q.; Chen, L.; Xu, G.; Wu, Y. Advances in animal models of Parkinson’s disease. Brain Res. Bull. 2024, 215, 111024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, A.C.; Pountney, D.L.; Khoo, T.K. Therapeutic Mechanisms of Exercise in Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 4860. https://doi.org/10.3390/ijms26104860
Wilson AC, Pountney DL, Khoo TK. Therapeutic Mechanisms of Exercise in Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(10):4860. https://doi.org/10.3390/ijms26104860
Chicago/Turabian StyleWilson, Alice C., Dean L. Pountney, and Tien K. Khoo. 2025. "Therapeutic Mechanisms of Exercise in Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 10: 4860. https://doi.org/10.3390/ijms26104860
APA StyleWilson, A. C., Pountney, D. L., & Khoo, T. K. (2025). Therapeutic Mechanisms of Exercise in Parkinson’s Disease. International Journal of Molecular Sciences, 26(10), 4860. https://doi.org/10.3390/ijms26104860





