Harnessing Mammalian- and Plant-Derived Exosomes for Drug Delivery: A Comparative Review
Abstract
1. Introduction
2. Milk-Derived Exosomes
2.1. Biogenesis
Key Regulatory Factors in Mammalian Exosome Formation and Release
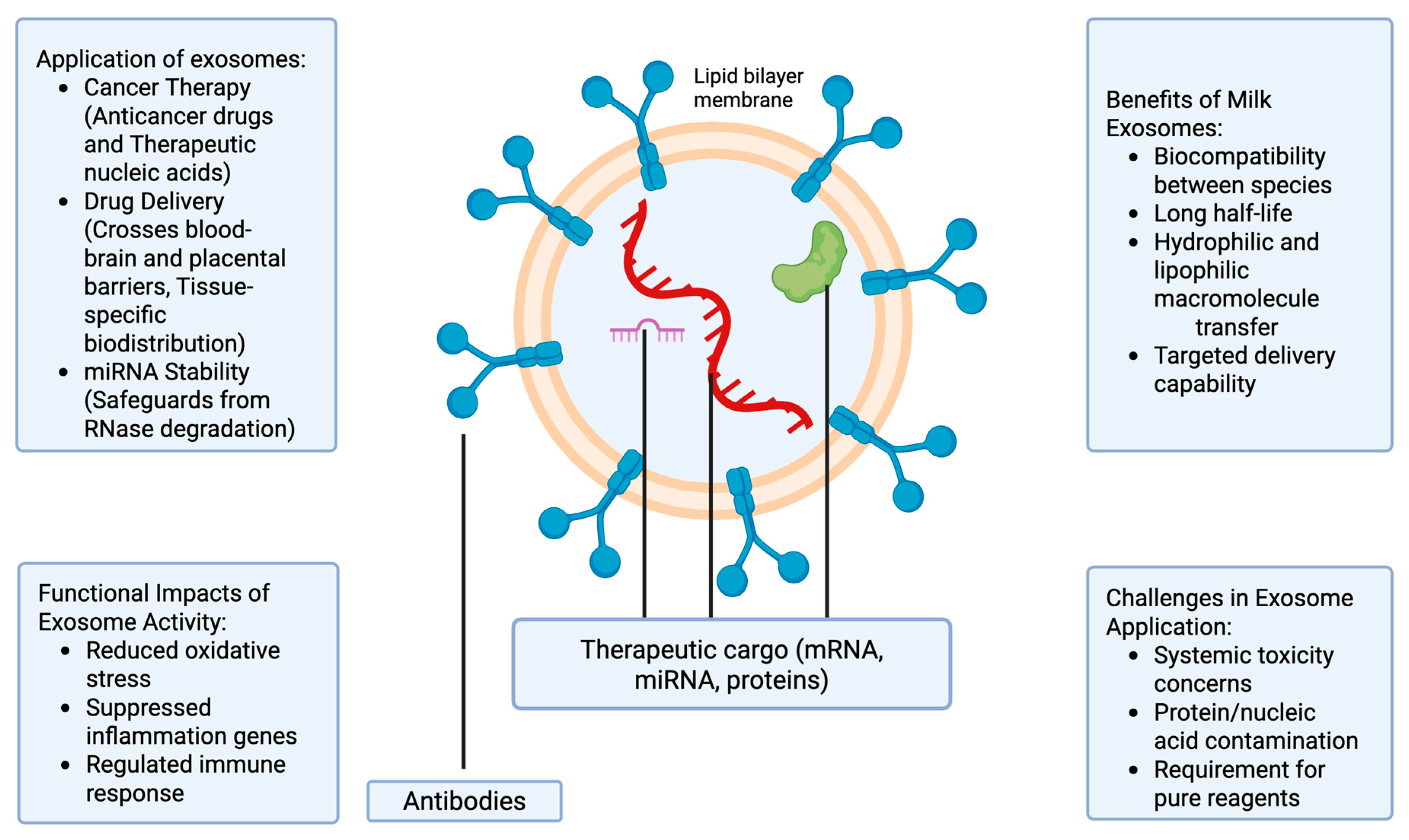
2.2. Therapeutic Use of Milk-Derived Exosomes
Mechanisms Underlying Superior Targeting by Milk-Derived Exosomes
3. Promising Developments in Biomedical Applications of Milk-Derived Exosomes
3.1. Impact on Nervous System
3.2. Antiviral Potential of Milk-Derived Exosomes
3.3. Milk-Derived Exosomes Promote Hair Growth
3.4. Milk-Derived Exosomes and Bone Health
3.5. Cosmetics Applications of Milk-Derived Exosomes
4. Plant-Derived Exosome-like Nanoparticles
4.1. Biogenesis
| Function | Milk-Derived | References | PDENs | References |
|---|---|---|---|---|
| Cellular communication | They mediate cell–cell communication via transferring molecular signals such proteins, lipids, and RNAs. | [68] | They facilitate cellular signaling by transferring lipids, proteins, and RNAs between different cells during stress responses or developmental processes. | [60] |
| Biological response regulation | They are involved in immune response including antigen presentation and the modulation of immune cell activity. | [69,70] | They are reported to regulate cellular responses to drought, salt stress, and immunity. | [63] |
| Disease modulation | They play a role in cancer progression. Tumor-derived exosomes might facilitate metastasis. | [71,72] | They regulate plant pathogen immune responses by transferring immune-related molecules like plant-specific small RNAs. | [59] |

Key Regulatory Factors in Plant-Derived Nanoparticle Formation
4.2. Therapeutic Use of PDENs
Disease Models and Clinical Translation Potential of PDENs
5. Promising Developments in Biomedical Applications of PDENs
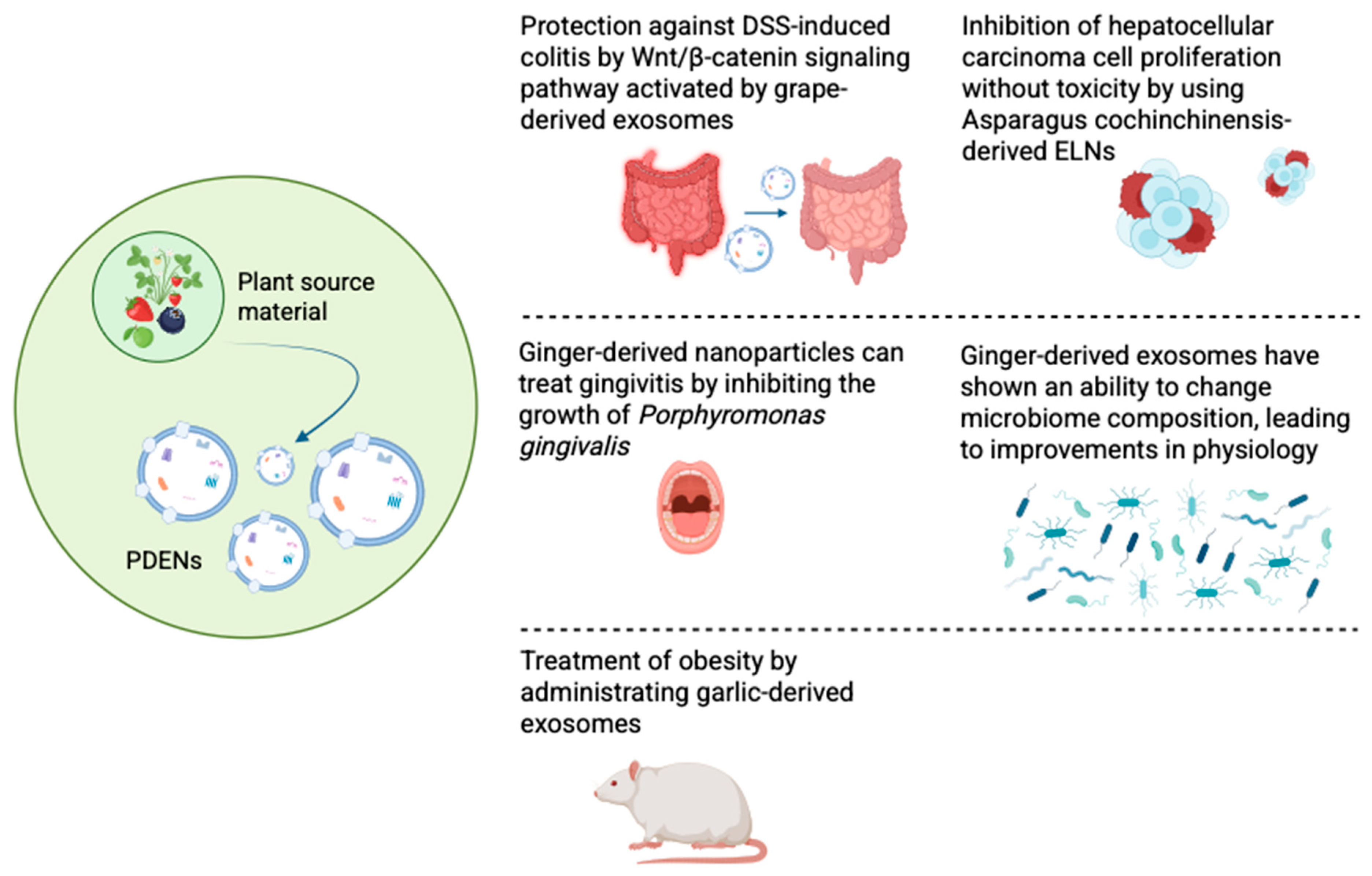
5.1. Anticancer Effect of Plant-Derived Exosomes
5.2. Treatment of Periodontitis
5.3. Alteration in Microbiome Composition
5.4. Treatment of Obesity
5.5. Treatment of Colitis
6. Use of Extracellular Vesicles as Drug-Delivery Systems in Diseases
7. Discussion
8. Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine Milk Contains MicroRNA and Messenger RNA That Are Stable under Degradative Conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Tsuda, M.; Sato, Y.; Kosaka, N.; Ochiya, T.; Iwamoto, H.; Namba, K.; Takeda, Y. Bovine Milk Exosomes Contain MicroRNA and MRNA and Are Taken up by Human Macrophages. J. Dairy Sci. 2015, 98, 2920–2933. [Google Scholar] [CrossRef]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 15, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Shiff, Y.E.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and Biological Function of Milk-Derived MiRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and Fat Composition of Mare’s Milk: Some Nutritional Remarks with Reference to Human and Cow’s Milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Reif, S.; Shiff, Y.E.; Golan-Gerstl, R. Milk-Derived Exosomes (MDEs) Have a Different Biological Effect on Normal Fetal Colon Epithelial Cells Compared to Colon Tumor Cells in a MiRNA-Dependent Manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef]
- Trams, E.G.; Lauter, C.J.; Salem, J.N.; Heine, U. Exfoliation of Membrane Ecto-Enzymes in the Form of Micro-Vesicles. BBA-Biomembranes 1981, 645, 63–70. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Malhotra, H.; Sheokand, N.; Kumar, S.; Chauhan, A.S.; Kumar, M.; Jakhar, P.; Boradia, V.M.; Raje, C.I.; Raje, M. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 2016, 12, 1101–1114. [Google Scholar] [CrossRef]
- Hood, J.L. Post Isolation Modification of Exosomes for Nanomedicine Applications. Nanomedicine 2016, 11, 1745–1756. [Google Scholar] [CrossRef]
- Pieters, B.C.H.; Arntz, O.J.; Bennink, M.B.; Broeren, M.G.A.; Van Caam, A.P.M.; Koenders, M.I.; Van Lent, P.L.E.M.; Van Den Berg, W.B.; De Vries, M.; Van Der Kraan, P.M.; et al. Commercial Cow Milk Contains Physically Stable Extracellular Vesicles Expressing Immunoregulatory TGF-β. PLoS ONE 2015, 10, e0121123. [Google Scholar] [CrossRef]
- Groenen, M.A.M.; van der Poel, J.J. Regulation of Expression of Milk Protein Genes: A ReviewRégulation de l’expression Des Génes de BacterioprotéinesRegulation de Expression van Milchprotein-Genen. Livest. Prod. Sci. 1994, 38, 61–78. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine Milk-Derived Exosomes for Drug Delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Betker, J.L.; Angle, B.M.; Graner, M.W.; Anchordoquy, T.J. The Potential of Exosomes From Cow Milk for Oral Delivery. J. Pharm. Sci. 2019, 108, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Vaswani, K.; Mitchell, M.D.; Holland, O.J.; Koh, Y.Q.; Hill, R.J.; Harb, T.; Davies, P.S.W.; Peiris, H. A Method for the Isolation of Exosomes from Human and Bovine Milk. J. Nutr. Metab. 2019, 2019, 5764740. [Google Scholar] [CrossRef]
- Joseph, B. Mammalian Milk Is Loaded with Exosomes. Available online: https://cen.acs.org/business/start-ups/Meet-exosome-rising-star-drug/96/i31 (accessed on 5 October 2024).
- Iriawati, I.; Vitasasti, S.; Rahmadian, F.N.A.; Barlian, A. Isolation and Characterization of Plant-Derived Exosome-like Nanoparticles from Carica papaya L. Fruit and Their Potential as Anti-Inflammatory Agent. PLoS ONE 2024, 19, e0304335. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Feng, X.; Chen, X.; Zheng, X.; Zhu, H.; Qi, Q.; Liu, S.; Zhang, H.; Che, J. Latest Trend of Milk Derived Exosomes: Cargos, Functions, and Applications. Front. Nutr. 2021, 8, 747294. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Baixauli, F.; Gutiérrez-Vázquez, C.; Sánchez-Madrid, F.; Mittelbrunn, M. Sorting It out: Regulation of Exosome Loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef]
- Sedykh, S.; Kuleshova, A.; Nevinsky, G. Milk Exosomes: Perspective Agents for Anticancer Drug Delivery. Int. J. Mol. Sci. 2020, 21, 6646. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.K.; Aqil, F.; Jeyabalan, J.; Spencer, W.A.; Beck, J.; Gachuki, B.W.; Alhakeem, S.S.; Oben, K.; Munagala, R.; Bondada, S.; et al. Milk-Derived Exosomes for Oral Delivery of Paclitaxel. Nanomedicine 2017, 13, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Patel, T. Milk-Derived Extracellular Vesicles for Therapeutic Delivery of Small Interfering RNAs. Methods Mol. Biol. 2018, 1740, 187–197. [Google Scholar] [CrossRef]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Agrawal, A.K.; Kyakulaga, A.H.; Wilcher, S.A.; Gupta, R.C. Milk Exosomes—Natural Nanoparticles for SiRNA Delivery. Cancer Lett. 2019, 449, 186–195. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Lázaro-Ibáñez, E.; Viitala, T.; Vuorimaa-Laukkanen, E.; Siljander, P.; Yliperttula, M. Microvesicle- and Exosome-Mediated Drug Delivery Enhances the Cytotoxicity of Paclitaxel in Autologous Prostate Cancer Cells. J. Control. Release 2015, 220, 727–737. [Google Scholar] [CrossRef]
- Burkova, E.E.; Grigor’eva, A.E.; Bulgakov, D.V.; Dmitrenok, P.S.; Vlassov, V.V.; Ryabchikova, E.I.; Sedykh, S.E.; Nevinsky, G.A. Extra Purified Exosomes from Human Placenta Contain An Unpredictable Small Number of Different Major Proteins. Int. J. Mol. Sci. 2019, 20, 2434. [Google Scholar] [CrossRef]
- Antimisiaris, S.G.; Mourtas, S.; Marazioti, A. Exosomes and Exosome-Inspired Vesicles for Targeted Drug Delivery. Pharmaceutics 2018, 10, 218. [Google Scholar] [CrossRef]
- Sato, Y.T.; Umezaki, K.; Sawada, S.; Mukai, S.A.; Sasaki, Y.; Harada, N.; Shiku, H.; Akiyoshi, K. Engineering Hybrid Exosomes by Membrane Fusion with Liposomes. Sci. Rep. 2016, 6, 21933. [Google Scholar] [CrossRef]
- Del Pozo-Acebo, L.; de las Hazas, M.C.L.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for Mirna-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Aryani, A.; Denecke, B. Exosomes as a Nanodelivery System: A Key to the Future of Neuromedicine? Mol. Neurobiol. 2016, 53, 818–834. [Google Scholar] [CrossRef]
- Elliott, R.O.; He, M. Unlocking the Power of Exosomes for Crossing Biological Barriers in Drug Delivery. Pharmaceutics 2021, 13, 122. [Google Scholar] [CrossRef]
- Rehman, F.U.; Liu, Y.; Zheng, M.; Shi, B. Exosomes Based Strategies for Brain Drug Delivery. Biomaterials 2023, 293, 121949. [Google Scholar] [CrossRef]
- Fu, S.; Wang, Y.; Xia, X.; Zheng, J.C. Exosome Engineering: Current Progress in Cargo Loading and Targeted Delivery. NanoImpact 2020, 20, 100261. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, B.; Wang, H.; Han, M.K.; Zhang, Z.; Viennois, E.; Xu, C.; Merlin, D. Edible Ginger-Derived Nano-Lipids Loaded with Doxorubicin as a Novel Drug-Delivery Approach for Colon Cancer Therapy. Mol. Ther. 2016, 24, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Bahri, F.; Mansoori, M.; Vafaei, S.; Fooladi, S.; Mir, Y.; Mehrabani, M.; Hozhabri, Y.; Nematollahi, M.H.; Iravani, S. A Comprehensive Review on Ginger-Derived Exosome-like Nanoparticles as Feasible Therapeutic Nano-Agents against Diseases. Mater. Adv. 2024, 5, 1846–1867. [Google Scholar] [CrossRef]
- Wang, Q.; Zhuang, X.; Mu, J.; Deng, Z.-B.; Jiang, H.; Zhang, L.; Xiang, X.; Wang, B.; Yan, J.; Miller, D.; et al. Delivery of Therapeutic Agents by Nanoparticles Made of Grapefruit-Derived Lipids. Nat. Commun. 2013, 4, 1867. [Google Scholar] [CrossRef]
- Sergazy, S.; Zhetkenev, S.; Shulgau, Z.; Chulenbayeva, L.; Kamyshanskiy, Y.; Nurgaziyev, M.; Nurgozhina, A.; Mukhanbetzhanova, Z.; Berikkhanova, K.; Gulyayev, A.; et al. Investigating the Suitability of Mare’s Milk-Derived Exosomes as Potential Drug Carriers. Biomolecules 2024, 14, 1247. [Google Scholar] [CrossRef]
- Sauna, Z.E.; Lagassé, H.A.D.; Alexaki, A.; Simhadri, V.L.; Katagiri, N.H.; Jankowski, W.; Kimchi-Sarfaty, C. Recent Advances in (Therapeutic Protein) Drug Development. F1000Res 2017, 6, 113. [Google Scholar] [CrossRef]
- Badawy, A.A.; El-Magd, M.A.; AlSadrah, S.A. Therapeutic Effect of Camel Milk and Its Exosomes on MCF7 Cells In Vitro and In Vivo. Integr. Cancer Ther. 2018, 17, 1235–1246. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Matsumoto, A.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Morishita, M.; Charoenviriyakul, C.; Saji, H.; Takakura, Y. Role of Phosphatidylserine-Derived Negative Surface Charges in the Recognition and Uptake of Intravenously Injected B16BL6-Derived Exosomes by Macrophages. J. Pharm. Sci. 2017, 106, 168–175. [Google Scholar] [CrossRef]
- Suga, K.; Matsui, D.; Watanabe, N.; Okamoto, Y.; Umakoshi, H. Insight into the Exosomal Membrane: From Viewpoints of Membrane Fluidity and Polarity. Langmuir 2021, 37, 11195–11202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ebea, P.; Mutai, E.; Wang, H.; Sukreet, S.; Navazesh, S.; Dogan, H.; Li, W.; Cui, J.; Ji, P.; et al. Small Extracellular Vesicles in Milk Cross the Blood-Brain Barrier in Murine Cerebral Cortex Endothelial Cells and Promote Dendritic Complexity in the Hippocampus and Brain Function in C57BL/6J Mice. Front. Nutr. 2022, 9, 838543. [Google Scholar] [CrossRef]
- Civra, A.; Francese, R.; Donalisio, M.; Tonetto, P.; Coscia, A.; Sottemano, S.; Balestrini, R.; Faccio, A.; Cavallarin, L.; Moro, G.E.; et al. Human Colostrum and Derived Extracellular Vesicles Prevent Infection by Human Rotavirus and Respiratory Syncytial Virus in Vitro. J. Hum. Lact. 2021, 37, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Näslund, T.I.; Paquin-Proulx, D.; Paredes, P.T.; Vallhov, H.; Sandberg, J.K.; Gabrielsson, S. Exosomes from Breast Milk Inhibit HIV-1 Infection of Dendritic Cells and Subsequent Viral Transfer to CD4+ T Cells. Aids 2014, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Yenuganti, V.R.; Afroz, S.; Khan, R.A.; Bharadwaj, C.; Nabariya, D.K.; Nayak, N.; Subbiah, M.; Chintala, K.; Banerjee, S.; Reddanna, P.; et al. Milk Exosomes Elicit a Potent Anti-Viral Activity against Dengue Virus. J. Nanobiotechnol. 2022, 20, 317. [Google Scholar] [CrossRef]
- Kim, H.; Jang, Y.; Kim, E.H.; Jang, H.; Cho, H.; Han, G.; Song, H.K.; Kim, S.H.; Yang, Y. Potential of Colostrum-Derived Exosomes for Promoting Hair Regeneration Through the Transition From Telogen to Anagen Phase. Front. Cell Dev. Biol. 2022, 10, 815205. [Google Scholar] [CrossRef]
- Zhang, H.L.; Qiu, X.X.; Liao, X.H. Dermal Papilla Cells: From Basic Research to Translational Applications. Biology 2024, 13, 842. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Liu, Q.; Zheng, T.; Li, J.; Zhang, T.; Yao, Y.; Liu, Y.; Lin, K.; Liu, T.; Gong, P.; et al. Oral Milk-Derived Extracellular Vesicles Inhibit Osteoclastogenesis and Ameliorate Bone Loss in Ovariectomized Mice by Improving Gut Microbiota. J. Agric. Food Chem. 2024, 72, 4726–4736. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Pieters, B.C.H.; Guimarães, P.B.; Duffles, L.F.; Heredia, J.E.; Silveira, A.L.M.; Oliveira, A.C.C.; Teixeira, M.M.; Ferreira, A.V.M.; Silva, T.A.; et al. Bovine Milk Extracellular Vesicles Are Osteoprotective by Increasing Osteocyte Numbers and Targeting RANKL/OPG System in Experimental Models of Bone Loss. Front. Bioeng. Biotechnol. 2020, 8, 891. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; He, Y.F.; Chen, P.; Liu, K.L.; Shaukat, A. Gut Microbiota as a Target in the Bone Health of Livestock and Poultry: Roles of Short-Chain Fatty Acids. Anim. Dis. 2023, 3, 23. [Google Scholar] [CrossRef]
- Bae, I.S.; Kim, S.H. Milk Exosome-Derived Microrna-2478 Suppresses Melanogenesis through the Akt-Gsk3β Pathway. Cells 2021, 10, 2848. [Google Scholar] [CrossRef]
- Lu, L.; Bai, W.; Wang, M.; Han, C.; Du, H.; Wang, N.; Gao, M.; Li, D.; Dong, F.; Ge, X. Novel Roles of Bovine Milk-Derived Exosomes in Skin Antiaging. J. Cosmet. Dermatol. 2024, 23, 1374–1385. [Google Scholar] [CrossRef]
- An, Q.; van Bel, A.J.; Hückelhoven, R. Do Plant Cells Secrete Exosomes Derived from Multivesicular Bodies? Plant Signal Behav. 2007, 2, 4–7. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants Send Small RNAs in Extracellular Vesicles to Fungal Pathogen to Silence Virulence Genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles as Key Mediators of Plant-Microbe Interactions. Curr. Opin. Plant Biol. 2018, 44, 16–22. [Google Scholar] [CrossRef]
- Regente, M.; Corti-Monzón, G.; Maldonado, A.M.; Pinedo, M.; Jorrín, J.; de la Canal, L. Vesicular Fractions of Sunflower Apoplastic Fluids Are Associated with Potential Exosome Marker Proteins. FEBS Lett. 2009, 583, 3363–3366. [Google Scholar] [CrossRef]
- Regente, M.; Pinedo, M.; Clemente, H.S.; Balliau, T.; Jamet, E.; de la Canal, L. Plant Extracellular Vesicles Are Incorporated by a Fungal Pathogen and Inhibit Its Growth. J. Exp. Bot. 2017, 68, 5485–5495. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular Vesicles Isolated from the Leaf Apoplast Carry Stress-Response Proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, F.A.; Khan, M.I.; Kameli, N.; Alsahafi, E.; Riza, Y.M. Plant-Derived Extracellular Vesicles and Their Exciting Potential as the Future of Next-Generation Drug Delivery. Biomolecules 2023, 13, 839. [Google Scholar] [CrossRef]
- Ju, S.; Mu, J.; Dokland, T.; Zhuang, X.; Wang, Q.; Jiang, H.; Xiang, X.; Deng, Z.; Wang, B.; Zhang, L.; et al. Grape Exosome-like Nanoparticles Induce Intestinal Stem Cells and Protect Mice from DSS-Induced Colitis. Mol. Ther. 2013, 21, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Perut, F.; Roncuzzi, L.; Avnet, S.; Massa, A.; Zini, N.; Sabbadini, S.; Giampieri, F.; Mezzetti, B.; Baldini, N. Strawberry-Derived Exosome-Like Nanoparticles Prevent Oxidative Stress in Human Mesenchymal Stromal Cells. Biomolecules 2021, 11, 87. [Google Scholar] [CrossRef]
- Mun, J.-G.; Song, D.-H.; Kee, J.-Y.; Han, Y. Recent Advances in the Isolation Strategies of Plant-Derived Exosomes and Their Therapeutic Applications. Curr. Issues Mol. Biol. 2025, 47, 144. [Google Scholar] [CrossRef]
- Manca, S.; Upadhyaya, B.; Mutai, E.; Desaulniers, A.T.; Cederberg, R.A.; White, B.R.; Zempleni, J. Milk Exosomes Are Bioavailable and Distinct MicroRNA Cargos Have Unique Tissue Distribution Patterns. Sci. Rep. 2018, 8, 11321. [Google Scholar] [CrossRef]
- Kim, K.U.; Kim, J.; Jang, H.; Dan, K.B.; Kim, B.K.; Ji, Y.W.; Yi, D.Y.; Min, H. Protective Effects of Human Breast Milk-Derived Exosomes on Inflammatory Bowel Disease through Modulation of Immune Cells. Npj Sci. Food 2025, 9, 34. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The Role of Milk-Derived Exosomes in the Treatment of Diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.S.; et al. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Induces Senescence in the Primary Tumor but Accelerates Cancer Metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Wei, S.-Y.; Shelton, S.E.; Chen, Y.-C.; Huang, K. In Vivo Trafficking of Cancer-Derived Exosomes and Their Role in Metastasis. Extracell. Vesicle 2025, 5, 100063. [Google Scholar] [CrossRef]
- Mu, N.; Li, J.; Zeng, L.; You, J.; Li, R.; Qin, A.; Liu, X.; Yan, F.; Zhou, Z. Plant-Derived Exosome-Like Nanovesicles: Current Progress and Prospects. Int. J. Nanomed. 2023, 18, 4987–5009. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Deng, Z.-B.; Mu, J.; Zhang, L.; Yan, J.; Miller, D.; Feng, W.; McClain, C.J.; Zhang, H.-G. Ginger-Derived Nanoparticles Protect against Alcohol-Induced Liver Damage. J. Extracell. Vesicles 2015, 4, 28713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Suharta, S.; Barlian, A.; Hidajah, A.C.; Notobroto, H.B.; Ana, I.D.; Indariani, S.; Wungu, T.D.K.; Wijaya, C.H. Plant-Derived Exosome-like Nanoparticles: A Concise Review on Its Extraction Methods, Content, Bioactivities, and Potential as Functional Food Ingredient. J. Food Sci. 2021, 86, 2838–2850. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Y.; Mu, J.; Egilmez, N.K.; Zhuang, X.; Deng, Z.; Zhang, L.; Yan, J.; Miller, D.; Zhang, H.-G. Grapefruit-Derived Nanovectors Use an Activated Leukocyte Trafficking Pathway to Deliver Therapeutic Agents to Inflammatory Tumor Sites. Cancer Res. 2015, 75, 2520–2529. [Google Scholar] [CrossRef]
- He, J.; Ren, W.; Wang, W.; Han, W.; Jiang, L.; Zhang, D.; Guo, M. Exosomal Targeting and Its Potential Clinical Application. Drug Deliv. Transl. Res. 2022, 12, 2385–2402. [Google Scholar] [CrossRef]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15, 3454. [Google Scholar] [CrossRef]
- Bensalem, J.; Servant, L.; Alfos, S.; Gaudout, D.; Layé, S.; Pallet, V.; Lafenetre, P. Dietary Polyphenol Supplementation Prevents Alterations of Spatial Navigation in Middle-Aged Mice. Front. Behav. Neurosci. 2016, 10, 9. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Etchamendy, N.; Pellay, H.; Amadieu, C.; Gaudout, D.; Dubreuil, S.; Paradis, M.-E.; Pomerleau, S.; Capuron, L.; et al. Polyphenols From Grape and Blueberry Improve Episodic Memory in Healthy Elderly with Lower Level of Memory Performance: A Bicentric Double-Blind, Randomized, Placebo-Controlled Clinical Study. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 996–1007. [Google Scholar] [CrossRef]
- Zhang, L.; He, F.; Gao, L.; Cong, M.; Sun, J.; Xu, J.; Wang, Y.; Hu, Y.; Asghar, S.; Hu, L.; et al. Engineering Exosome-Like Nanovesicles Derived from Asparagus Cochinchinensis Can Inhibit the Proliferation of Hepatocellular Carcinoma Cells with Better Safety Profile. Int. J. Nanomed. 2021, 16, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zu, M.; Gong, H.; Ma, Y.; Sun, J.; Ran, S.; Shi, X.; Zhang, J.; Xiao, B. Tea Leaf-Derived Exosome-like Nanotherapeutics Retard Breast Tumor Growth by pro-Apoptosis and Microbiota Modulation. J. Nanobiotechnol. 2023, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, X.; Luo, Q.; Xu, L.; Chen, F. An Efficient Method to Isolate Lemon Derived Extracellular Vesicles for Gastric Cancer Therapy. J. Nanobiotechnol. 2020, 18, 100. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Miller, D.P.; Kumar, A.; Teng, Y.; Sayed, M.; Mu, J.; Lei, C.; Sriwastva, M.K.; Zhang, L.; Jun, Y.; et al. Plant-Derived Exosomal Nanoparticles Inhibit Pathogenicity of Porphyromonas Gingivalis. iScience 2019, 21, 308–327. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Sayed, M.; Hu, X.; Lei, C.; Kumar, A.; Hutchins, E.; Mu, J.; Deng, Z.; Luo, C.; et al. Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host Microbe 2018, 24, 637. [Google Scholar] [CrossRef]
- Solas, M.; Milagro, F.I.; Ramírez, M.J.; Martínez, J.A. Inflammation and Gut-Brain Axis Link Obesity to Cognitive Dysfunction: Plausible Pharmacological Interventions. Curr. Opin. Pharmacol. 2017, 37, 87–92. [Google Scholar] [CrossRef]
- Sundaram, K.; Mu, J.; Kumar, A.; Behera, J.; Lei, C.; Sriwastva, M.K.; Xu, F.; Dryden, G.W.; Zhang, L.; Chen, S.Y.; et al. Garlic Exosome-like Nanoparticles Reverse High-Fat Diet Induced Obesity via the Gut/Brain Axis. Theranostics 2022, 12, 1220–1246. [Google Scholar] [CrossRef]
- Pang, W.; Zuo, Z.; Sun, W.; Zhang, Z.; Wang, J.; Wang, Y.; Zhang, D. Kidney Bean Derived Exosome-like Nanovesicles Ameliorate High-Fat Diet-Induced Obesity via Reshaping Gut Microbiota. J. Funct. Foods 2024, 113, 105997. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. Biomed. Res. Int. 2017, 2017, 9351597. [Google Scholar] [CrossRef]
- Rajput, M.; Momin, T.; Singh, A.; Banerjee, S.; Villasenor, A.; Sheldon, J.; Paudel, P.; Rajput, R. Determining the Association between Gut Microbiota and Its Metabolites with Higher Intestinal Immunoglobulin A Response. Vet. Anim. Sci. 2022, 19, 100279. [Google Scholar] [CrossRef] [PubMed]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Yang, N.; Nadithe, V. Exosomes as Therapeutic Drug Carriers and Delivery Vehicles across Biological Membranes: Current Perspectives and Future Challenges. Acta Pharm. Sin. B 2016, 6, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hareendran, S.; Loh, Y.P. Function of Exosomes in Neurological Disorders and Brain Tumors. Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 55. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Kang, K.; Ma, R.; Cai, W.; Huang, W.; Paul, C.; Liang, J.; Wang, Y.; Zhao, T.; Kim, H.W.; Xu, M.; et al. Exosomes Secreted from CXCR4 Overexpressing Mesenchymal Stem Cells Promote Cardioprotection via Akt Signaling Pathway Following Myocardial Infarction. Stem Cells Int. 2015, 2015, 659890. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, Y.; Sun, L.; Sun, X.; Zhao, X.; Sun, X.; Qian, H.; Xu, W.; Zhu, W. Exosomes Derived from Akt -Modified Human Umbilical Cord Mesenchymal Stem Cells Improve Cardiac Regeneration and Promote Angiogenesis via Activating Platelet-Derived Growth Factor D. Stem Cells Transl. Med. 2017, 6, 51–59. [Google Scholar] [CrossRef]
- Bang, O.Y.; Kim, E.H. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front. Neurol. 2019, 10, 443392. [Google Scholar] [CrossRef]
- Kamerkar, S.; Lebleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A Phase I Study of Dexosome Immunotherapy in Patients with Advanced Non-Small Cell Lung Cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Kordelas, L.; Rebmann, V.; Ludwig, A.K.; Radtke, S.; Ruesing, J.; Doeppner, T.R.; Epple, M.; Horn, P.A.; Beelen, D.W.; Giebel, B. MSC-Derived Exosomes: A Novel Tool to Treat Therapy-Refractory Graft-versus-Host Disease. Leukemia 2014, 28, 970–973. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhuang, X.; Deng, Z.; Jiang, H.; Mu, J.; Wang, Q.; Xiang, X.; Guo, H.; Zhang, L.; Dryden, G.; et al. Targeted Drug Delivery to Intestinal Macrophages by Bioactive Nanovesicles Released from Grapefruit. Mol. Ther. 2014, 22, 522–534. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface Functionalized Exosomes as Targeted Drug Delivery Vehicles for Cerebral Ischemia Therapy. Biomaterials 2018, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Sergazy, S.; Shulgau, Z.; Kamyshanskiy, Y.; Zhumadilov, Z.; Krivyh, E.; Gulyayev, A.; Aljofan, M. Blueberry and Cranberry Extracts Mitigate CCL4-Induced Liver Damage, Suppressing Liver Fibrosis, Inflammation and Oxidative Stress. Heliyon 2023, 9, e15370. [Google Scholar] [CrossRef]

| Therapeutic Effect | Mechanism of Action | References |
|---|---|---|
| Anti-inflammatory | Stable RNA transport via milk exosomes to immune cells | [1] |
| Targeted drug delivery | Encapsulation of therapeutic agents in exosomes | [13] |
| Anti-inflammatory | Modulation of inflammatory pathways in immune cells | [17] |
| Anticancer | Delivery of doxorubicin and targeting colon cancer cells | [37] |
| Antioxidant, anticancer | Intrinsic bioactive compounds in ginger nanoparticles | [38] |
| Drug delivery | Utilization of natural plant lipids for systemic distribution | [39] |
| Enhanced chemotherapy efficacy | Exosomal encapsulation of paclitaxel for sustained release | [24] |
| Immunomodulation | Expression of TGF-β on vesicle surface | [11] |
| Neurological targeting | Enhanced targeting of exosomes to brain tissues | [10] |
| Enhanced bioavailability | Encapsulation of polyphenols for sustained release | [40] |
| Description of Clinical Trial | Possible Mechanisms of Action | Disease Treated | References |
|---|---|---|---|
| MSC-derived exosomes for acute ischemic stroke | Immunomodulation, reduction in inflammation, and neuroprotection via paracrine signaling | Acute ischemic stroke | [100] |
| Exosome-based delivery of KRAS G12D siRNA (iExosomes) | Targeted gene silencing of KRAS G12D oncogene in pancreatic cancer cells | Pancreatic cancer | [101] |
| Dendritic-cell-derived exosomes pulsed with tumor antigens | Activation of anti-tumor immune responses via antigen presentation | Non-small-cell lung cancer (NSCLC) | [102] |
| MSC-derived exosomes for graft-versus-host disease (GvHD) | Immunosuppressive and anti-inflammatory activity through miRNA delivery | Graft-versus-host disease | [103] |
| Curcumin-loaded exosomes for inflammation control in inflammatory bowel disease (IBD) | Enhanced bioavailability and delivery of anti-inflammatory compounds | Inflammatory bowel disease (IBD) | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergazy, S.; Adekenov, S.; Khabarov, I.; Adekenova, K.; Maikenova, A.; Aljofan, M. Harnessing Mammalian- and Plant-Derived Exosomes for Drug Delivery: A Comparative Review. Int. J. Mol. Sci. 2025, 26, 4857. https://doi.org/10.3390/ijms26104857
Sergazy S, Adekenov S, Khabarov I, Adekenova K, Maikenova A, Aljofan M. Harnessing Mammalian- and Plant-Derived Exosomes for Drug Delivery: A Comparative Review. International Journal of Molecular Sciences. 2025; 26(10):4857. https://doi.org/10.3390/ijms26104857
Chicago/Turabian StyleSergazy, Shynggys, Sergazy Adekenov, Ilya Khabarov, Kymbat Adekenova, Assiya Maikenova, and Mohamad Aljofan. 2025. "Harnessing Mammalian- and Plant-Derived Exosomes for Drug Delivery: A Comparative Review" International Journal of Molecular Sciences 26, no. 10: 4857. https://doi.org/10.3390/ijms26104857
APA StyleSergazy, S., Adekenov, S., Khabarov, I., Adekenova, K., Maikenova, A., & Aljofan, M. (2025). Harnessing Mammalian- and Plant-Derived Exosomes for Drug Delivery: A Comparative Review. International Journal of Molecular Sciences, 26(10), 4857. https://doi.org/10.3390/ijms26104857







