Ketone Bodies in the Regulation of Myocardial Perfusion in Cardiovascular Disease: Metabolic and Vasodilatory Effects
Abstract
1. Introduction
2. Methodology
3. Physiological Regulation of Myocardial Blood Flow in the Healthy Heart
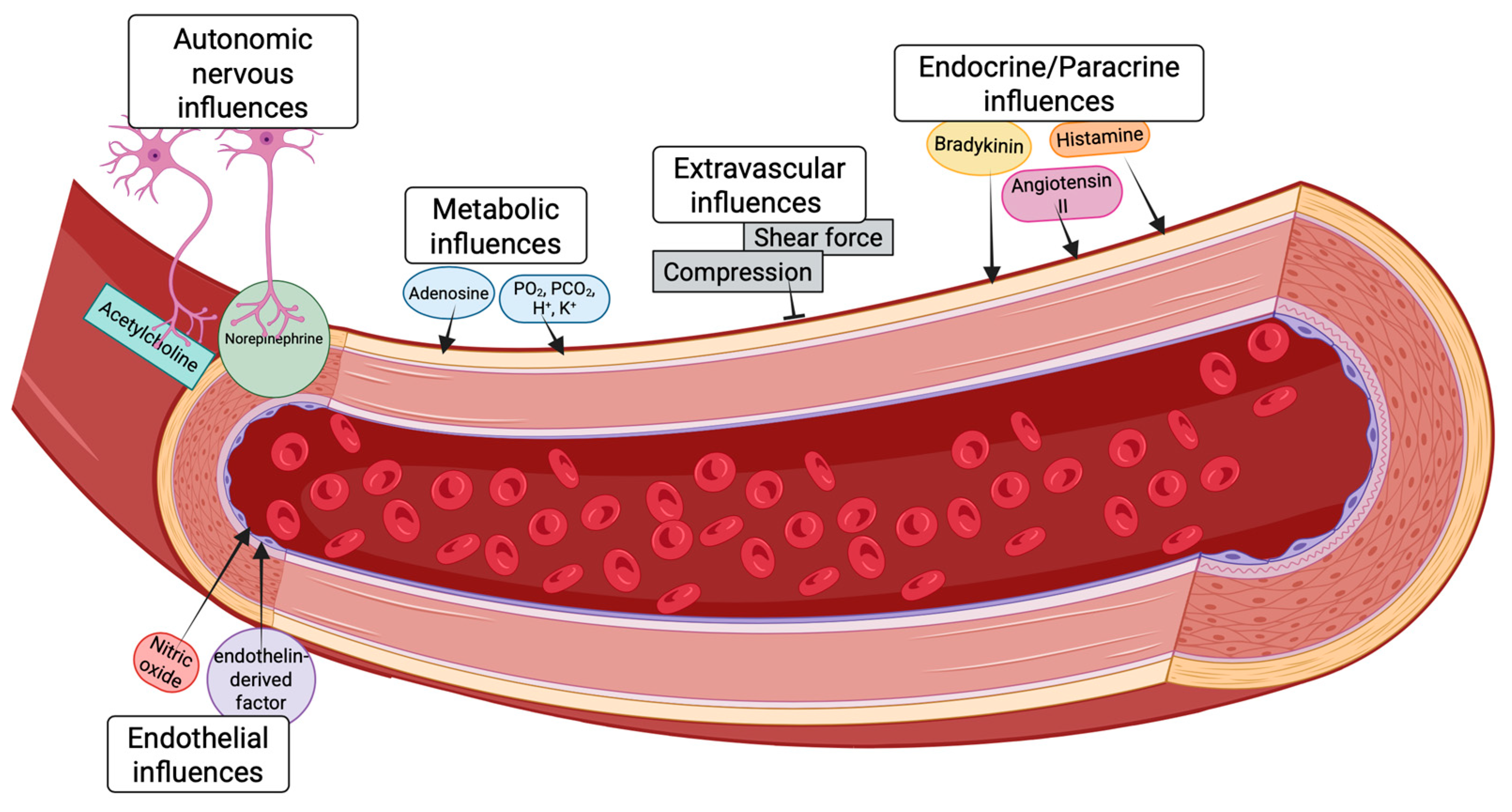
3.1. Autoregulation
3.2. Metabolic Mechanisms of Autoregulatory Behavior
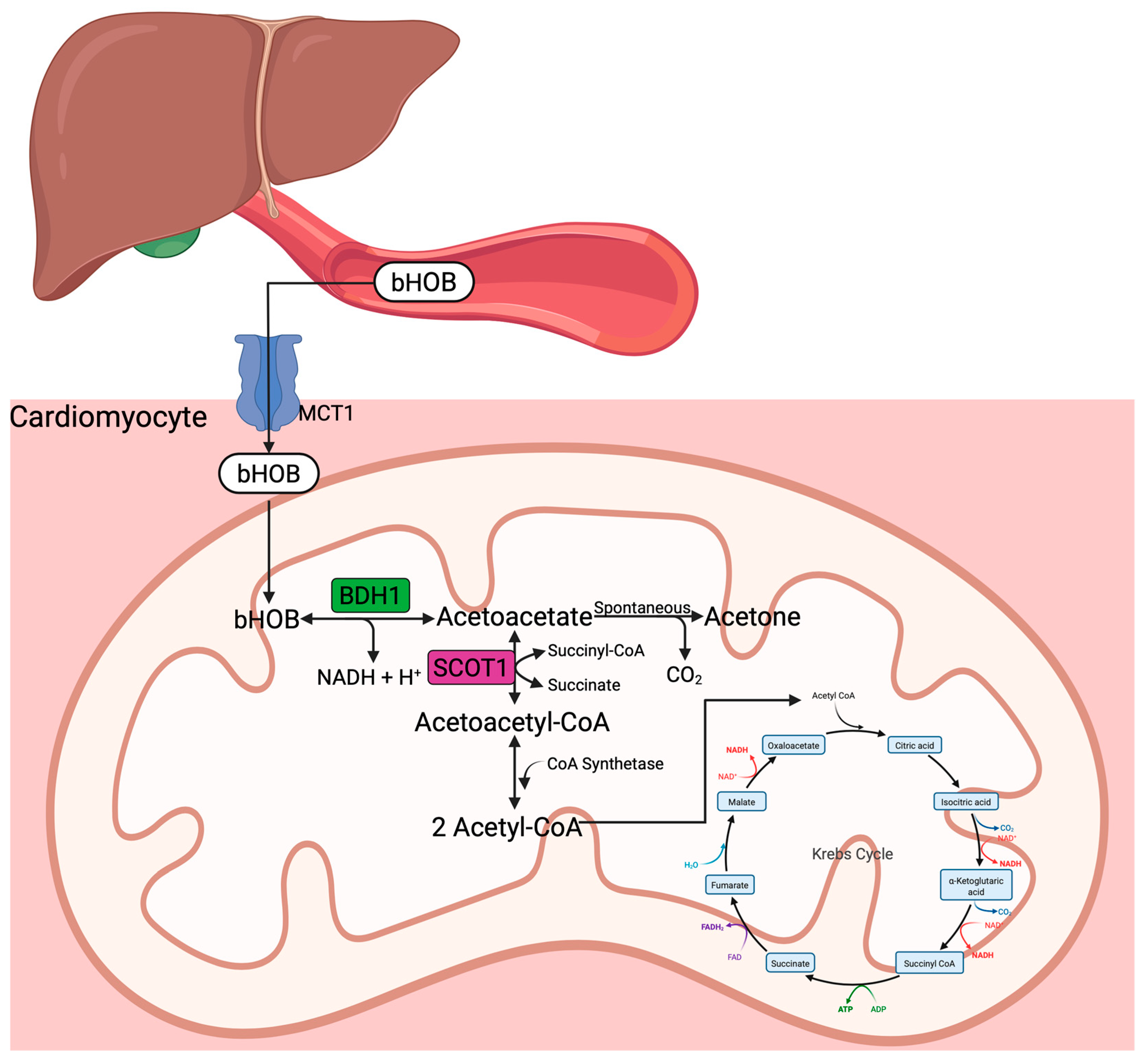
3.3. Ketone Bodies and Ketogenesis
4. Influence of Ketone Bodies on Myocardial Perfusion
4.1. Clinical Findings
| Author | Subjects (N) | Ketone Body | Outcome |
|---|---|---|---|
| Gormsen et al. [8] 2017 | 8 human subjects | Na-beta-hydroxybutyrate infusion (390 min) infusion vs. saline | Changes observed in hyperketonemia arm: -Myocardial glucose uptake reduction by half (304 ± 97 nmol/g/min [saline] vs. 156 ± 62 nmol/g/min [ketone], p < 0.01) -Increased heart rate by ~25% -Increase myocardial blood flow by ~75% |
| Nielsen et al. [9] 2019 | 10 HFrEF and 10 healthy subjects | 3-h bOHB or placebo infusion: sodium-bOHB at a 7.5% concentration; glucose (20% solution, 60 mM KCl); low-dose insulinemic euglycemic clamp (0.3 IE insulin kg−1 h−1); randomized, single-blinded crossover study | Changes observed from before to after bOHB infusion: -MBF increased in both study groups during bOHB infusion, but slightly more in age-matched volunteers -SV increased in proportion to MVO2 -CO and HR increased -MEE was unchanged -MAP and SVR decreased |
| Solis-Herrera et al. [49] 2023 | 12 subjects with T2DM and HF (EF < 50%) | (I) 6-h bOHB infusion: Prime = 0.4 mg/kg/min for 20 min followed by constant rate = 0.2 mg/kg/min (II) 6 h bOHB infusion w/HCO3 control: Prime = 1.5 mg/kg/min for 20 min followed by constant rate = 0.75 mg/kg/min (III) 3-h bOHB infusion: Prime = 4.0 mg/kg/min for 20 min followed by constant rate = 2.0 mg/kg/min | (I) Plasma bOHB: 0.7 ± 0.3 (II) Plasma bOHB: 1.6 ± 0.2 -no change in MBF (1.23 ± 0.09 vs. 1.18 ± 0.11 mL/min, p = 0.76) MBF significantly increased in six subjects (“responders” 1.05 ± 0.13 to 1.38 ± 0.17, p = 0.003) -no change in MBF (1.23 ± 0.09 vs. 1.18 ± 0.11 mL/min, p = 0.76) MBF significantly decreased in six subjects (“non-responders” 1.44 ± 0.07 to 0.95 ± 0.08, p = 0.007) -CO, EF, SV increased (III) Plasma bOHB: 3.2 ± 0.2 mmol/L -CO, EF, SV increased |
| Svart et al. [51] 2018 | 9 human subjects | D,L-3-hydroxybutyric acid (75 g bOHB/L over four hours at a rate of 0.22 g/kg/h) vs. isotonic saline | At bOHB concentration of 5.5 ± 0.4 mmol/L: -Cerebral glucose utilization decreased by 14% -Cerebral blood flow increased 30% |
| Fioretto et al. [50] 1987 | 11 healthy human subjects and 11 patients with IDDM | D,L-3-hydroxybutyric acid infusion (40 μmol/kg/min and 30 μmol/kg/min for 180 min) | 40 μmol/kg/min group: -Healthy—increased RPF (from 588 ± 78 to 706 ± 129 mL/min/1.73 m2) -IDDM—increased RPF (from 671 ± 101 to 781 ± 99 mL/min/1.73 m2) 30 μmol/kg/min increased RPF to a lesser extent than the 40 μmol/kg/min dose |
| Homilius et al. [36] 2023 | Male Sprague Dawley rats | Na-beta-hydroxybutyrate infusion (390 min) | In vivo bOHB [2–4 mM]: -increased CO (by 28.3 ± 7.8%), SV (by 22.4 ± 6.0%), and LVEF (by 13.3 ± 4.6%) -decreased SVR (by 30.6 ± 11.2%) Ex vivo bOHB [10 mM]: -increase coronary perfusion (by 20.2 ± 9.5%] |
| Gopalasingam et al. [52] 2024 | Danish Landrace × Yorkshire pigs | 3-h infusion of D, L, or racemic mixture of b-OHB vs. isovolumic control | -D/L-bOHB and L-bOHB increased CO by 2.7 L/min -D-bOHB increased CO nonsignificantly -D/L-bOHB and L-bOHB reduced arterial elastance (afterload) -end-systolic elastance (contractility) decreased in L-bOHB -EDV (preload) decreased in D/L-bOHB -isolated coronary arteries, D- and L-bOHB dilated coronary arteries equally at concentrations 3 mmol/L |
4.2. Preclinical Findings
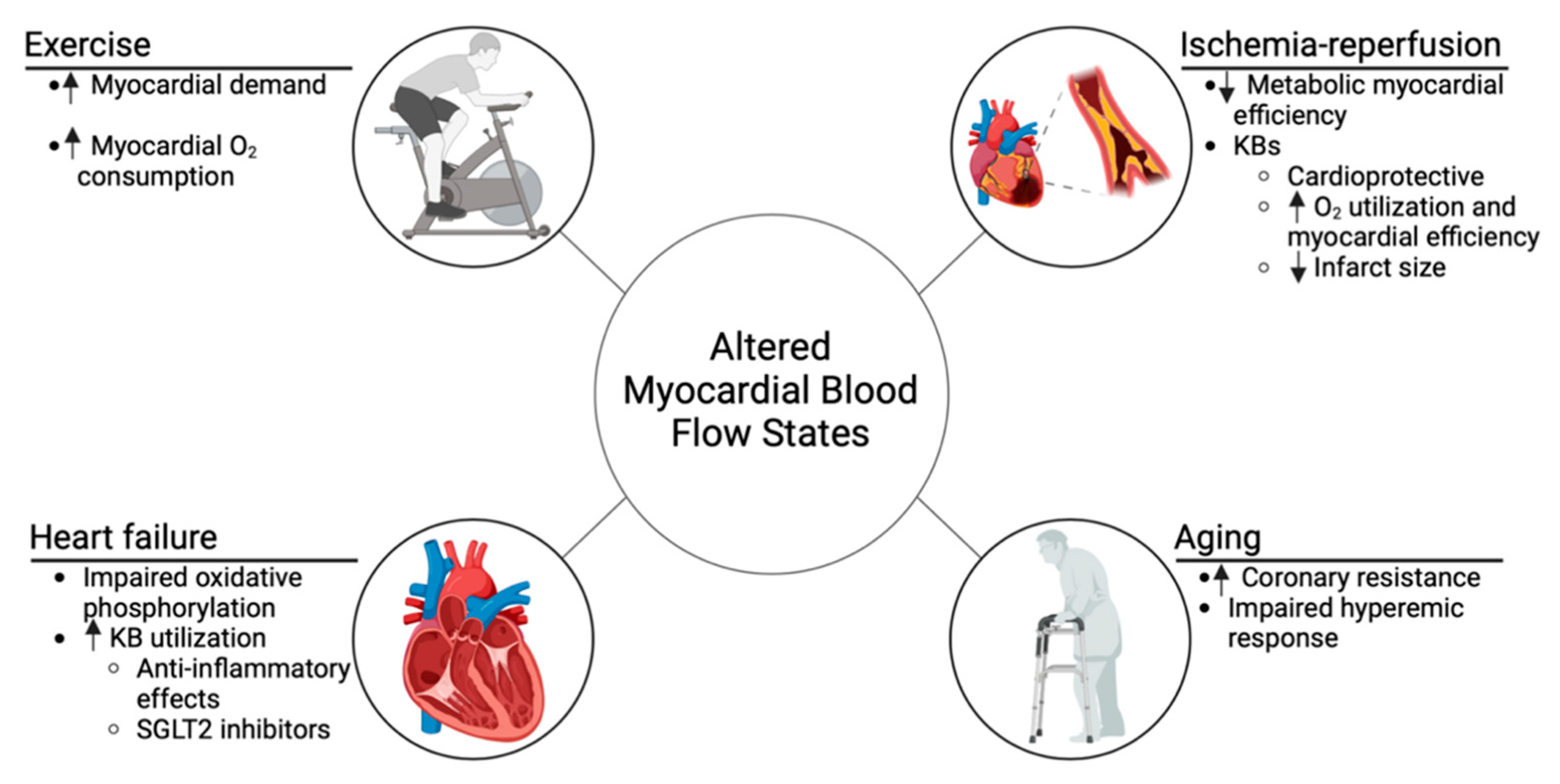
5. Impact of Altered Myocardial Blood Flow on Ketone Body Utilization
5.1. Ischemia–Reperfusion
5.2. Heart Failure
| Study | Aim | Methodology | Outcome |
|---|---|---|---|
| Monzo et al. [64] (2021) | Assessed the maximal KB utilization capacity in HFrEF patients compared to controls, with a focus on the effect of oral KE administration. | 19 HFrEF patients and 9 controls underwent arterial and coronary sinus sampling to measure substrate and oxygen extraction. In a separate experiment, 11 HFrEF patients and 6 controls were given 25 g of oral KE, with measurements taken 80 min post-administration to assess ketone utilization. | bOHB levels increased 12.9-fold after KE administration. HFrEF patients showed higher fractional extraction (52%) compared to controls (39%, p = 0.035). bOHB fractional extraction correlated with bOHB delivery (r = 0.90), LV mass (r = 0.56), LV diameter (r = 0.65), and inversely with LVEF (r = −0.59), all p < 0.05. |
| Hansen et al. [70] (2024) | Assessed the effects of 14-day KE treatment on resting and exercise hemodynamics in HFrEF patients. | 24 patients with HFrEF participated in a randomized, double-blind, crossover design. Each patient received 14 days of KE treatment or an isocaloric comparator, with a 14-day washout period. Hemodynamic and echocardiographic assessments were performed after each treatment phase. | KE treatment increased resting cardiac output by 0.3 L/min (5.2 vs. 5.0 L/min) and reduced pulmonary capillary wedge pressure by 2 mmHg (8 vs. 11 mmHg). LVEF improved by 3% (37% vs. 34%), and NT-proBNP levels decreased by 18% (98 ng/L). |
| Sramko et al. [66] (2022) | Investigated the feasibility, safety, and acute hemodynamic effects of BHB administration in HFrEF patients receiving inotropic support. | 8 patients with decompensated heart failure on inotropic support received 75 g of oral bOHB over 24 h in 3 h intervals. Hemodynamics were measured using Swan–Ganz catheterization and serum bOHB concentrations were assessed before and after administration. | bOHB increased cardiac index by 39% within 3 h (from 2.5 ± 0.5 to 3.4 ± 0.8 L/min/m2, p = 0.003), with a peak increase of 52% (1.2 ± 0.4 L/min/m2) observed after 5 h. Mild ketosis was induced (1.8 ± 0.6 mmol/L), with no significant complications reported. |
| Kotha et al. [68] (2024) | Evaluated the effects of short-term exogenous KE on cardiac energetics, function, and myocardial lipid content in patients with T2DM, HFrEF, and healthy volunteers. | 87 participants (33 T2DM, 29 HFrEF, and 25 healthy volunteers) received 2 weeks of daily KE supplementation (30 g/day). Cardiac function and energetics were assessed via blood tests, MRI, and spectroscopy. | T2D patients showed an increase in the phosphocreatine/ATP ratio from 1.6 to 1.9 (p = 0.01), with no significant changes in HFrEF or healthy participants. No improvements were noted in LVEF, GLS, myocardial blood flow, or lipid content across all groups. |
| Hansen et al. [65] (2023) | Evaluated the hemodynamic effects of a single dose of exogenous KE in patients with cardiogenic shock. | 12 patients with cardiogenic shock were randomized in a double-blind, crossover design to receive an enteral bolus of KE or isocaloric placebo. Hemodynamics were assessed over 3 h with pulmonary artery catheterization, echocardiography, and blood tests. | KE increased circulating bOHB to 2.9 ± 0.3 mmol/L (p < 0.001), improved cardiac output by AUC of relative change of 61 ± 22 L (p = 0.044), and increased LVEF by 4% (p = 0.005). KE also reduced right (p = 0.048) and left (p = 0.017) ventricular filling pressures and enhanced forearm perfusion by 3% (p = 0.026). |
| Solis-Herrera et al. [67] (2022) | Investigated the mechanism behind SGLT2 inhibitors by examining the effects of elevated ketone levels on left ventricular function and myocardial glucose uptake in patients with T2DM and HFrEF. | 36 patients with T2DM and HFrEF (LVEF < 45%) were divided into three groups. Each group received increasing doses of bOHB or NaHCO3 (control), with cardiac MRI and PET used to assess cardiac function and metabolism. | Plasma ketone levels increased to 1.0, 1.3, and 2.5 mmol/L in Groups I, II, and III, respectively. Higher ketone levels improved cardiac output, ejection fraction, and stroke volume, with Group III showing the greatest improvement in LVEF (p < 0.001). Myocardial glucose uptake was unchanged, suggesting ketones provided an additional fuel source without altering glucose metabolism. |
5.3. Aging
5.4. Exercise
5.5. Unresolved Questions
6. Strengths and Limitations
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| bOHB | beta-hydroxybutyrate |
| FFA | Free fatty acid |
| HFpEF | Heart failure with preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| KB | Ketone bodies |
| LVEF | Left ventricular ejection fraction |
| MCT | Monocarboxylate transporters |
| MBF | Myocardial blood flow |
| MRI | Magnetic resonance imaging |
| MVO2 | Myocardial volume oxygen consumption |
| NLRP3 | NOD-like receptor protein 3 |
| PET | Positron emission tomography |
| RPP | Rate–product pressure |
| SGLT2 | Sodium–glucose cotransporter 2 |
| T2DM | Type 2 diabetes mellitus |
| VSM | Vascular smooth muscle |
References
- Heusch, G. Heart Rate in the Pathophysiology of Coronary Blood Flow and Myocardial Ischaemia: Benefit from Selective Bradycardic Agents. Br. J. Pharmacol. 2008, 153, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Asakura, M.; Hibi, K.; Okada, K.; Shimizu, W.; Takano, H.; Suwa, S.; Fujii, K.; Okumura, Y.; Mano, T.; et al. Evolocumab for Prevention of Microvascular Dysfunction in Patients Undergoing Percutaneous Coronary Intervention: The Randomised, Open-Label EVOCATION Trial. Available online: https://eurointervention.pcronline.com/article/evolocumab-for-prevention-of-microvascular-dysfunction-in-patients-undergoing-percutaneous-coronary-intervention-the-randomised-open-label-evocation-trial (accessed on 14 November 2024).
- Ma, T.K.; Kam, K.K.; Yan, B.P.; Lam, Y.-Y. Renin–Angiotensin–Aldosterone System Blockade for Cardiovascular Diseases: Current Status. Br. J. Pharmacol. 2010, 160, 1273. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Bajaj, N.; Gupta, A.; Sun, Y.-P.; Divakaran, S.; Bibbo, C.; Hainer, J.; Taqueti, V.; Dorbala, S.; Blankstein, R.; et al. Coronary Microvascular Dysfunction, Left Ventricular Remodeling, and Clinical Outcomes in Aortic Stenosis. J. Nucl. Cardiol. 2021, 28, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Njoroge, J.N.; Teerlink, J.R. Pathophysiology and Therapeutic Approaches to Acute Decompensated Heart Failure. Circ. Res. 2021, 128, 1468–1486. [Google Scholar] [CrossRef]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar] [CrossRef]
- Bugger, H.; Byrne, N.J.; Abel, E.D. Animal Models of Dysregulated Cardiac Metabolism. Circ. Res. 2022, 130, 1965–1993. [Google Scholar] [CrossRef]
- Gormsen, L.C.; Svart, M.; Thomsen, H.H.; Søndergaard, E.; Vendelbo, M.H.; Christensen, N.; Tolbod, L.P.; Harms, H.J.; Nielsen, R.; Wiggers, H.; et al. Ketone Body Infusion with 3-Hydroxybutyrate Reduces Myocardial Glucose Uptake and Increases Blood Flow in Humans: A Positron Emission Tomography Study. J. Am. Heart Assoc. 2017, 6, e005066. [Google Scholar] [CrossRef]
- Nielsen, R.; Møller, N.; Gormsen, L.C.; Tolbod, L.P.; Hansson, N.H.; Sorensen, J.; Harms, H.J.; Frøkiær, J.; Eiskjaer, H.; Jespersen, N.R.; et al. Cardiovascular Effects of Treatment with the Ketone Body 3-Hydroxybutyrate in Chronic Heart Failure Patients. Circulation 2019, 139, 2129–2141. [Google Scholar] [CrossRef]
- Cox, P.J.; Kirk, T.; Ashmore, T.; Willerton, K.; Evans, R.; Smith, A.; Murray, A.J.; Stubbs, B.; West, J.; McLure, S.W.; et al. Nutritional Ketosis Alters Fuel Preference and Thereby Endurance Performance in Athletes. Cell Metab. 2016, 24, 256–268. [Google Scholar] [CrossRef]
- Deussen, A.; Ohanyan, V.; Jannasch, A.; Yin, L.; Chilian, W. Mechanisms of Metabolic Coronary Flow Regulation. J. Mol. Cell Cardiol. 2012, 52, 794–801. [Google Scholar] [CrossRef]
- Laughlin, M.H.; Tomanek, R.J. Myocardial Capillarity and Maximal Capillary Diffusion Capacity in Exercise-Trained Dogs. J. Appl. Physiol. 1987, 63, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Gorman, M.W.; Feigl, E.O. Matching Coronary Blood Flow to Myocardial Oxygen Consumption. J. Appl. Physiol. 2004, 97, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Bechsgaard, D.F.; Hove, J.D.; Suhrs, H.E.; Bové, K.B.; Shahriari, P.; Gustafsson, I.; Prescott, E. Women with Coronary Microvascular Dysfunction and No Obstructive Coronary Artery Disease Have Reduced Exercise Capacity. Int. J. Cardiol. 2019, 293, 1–9. [Google Scholar] [CrossRef]
- Ross, A.J.; Gao, Z.; Pollock, J.P.; Leuenberger, U.A.; Sinoway, L.I.; Muller, M.D. β-Adrenergic Receptor Blockade Impairs Coronary Exercise Hyperemia in Young Men but Not Older Men. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H1497–H1503. [Google Scholar] [CrossRef]
- Yildiz, M.; Ashokprabhu, N.; Shewale, A.; Pico, M.; Henry, T.D.; Quesada, O. Myocardial Infarction with Non-Obstructive Coronary Arteries (MINOCA). Front. Cardiovasc. Med. 2022, 9, 1032436. [Google Scholar] [CrossRef]
- Uren, N.G.; Camici, P.G.; Melin, J.A.; Bol, A.; de Bruyne, B.; Radvan, J.; Olivotto, I.; Rosen, S.D.; Impallomeni, M.; Wijns, W. Effect of Aging on Myocardial Perfusion Reserve. J. Nucl. Med. 1995, 36, 2032–2036. [Google Scholar]
- Hamburg, N.M.; McMackin, C.J.; Huang, A.L.; Shenouda, S.M.; Widlansky, M.E.; Schulz, E.; Gokce, N.; Ruderman, N.B.; Keaney, J.F., Jr.; Vita, J.A. Physical Inactivity Rapidly Induces Insulin Resistance and Microvascular Dysfunction in Healthy Volunteers. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2650. [Google Scholar] [CrossRef]
- Mosher, P.; Ross, J.; Mcfate, P.A.; Shaw, R.F. Control of Coronary Blood Flow by an Autoregulatory Mechanism. Circ. Res. 1964, 14, 250–259. [Google Scholar] [CrossRef]
- Johnson, N.P.; Gould, K.L.; De Bruyne, B. Autoregulation of Coronary Blood Supply in Response to Demand: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 2335–2345. [Google Scholar] [CrossRef]
- Smith, T.P.; Canty, J.M. Modulation of Coronary Autoregulatory Responses by Nitric Oxide. Evidence for Flow-Dependent Resistance Adjustments in Conscious Dogs. Circ. Res. 1993, 73, 232–240. [Google Scholar] [CrossRef]
- Gorman, M.W.; Tune, J.D.; Richmond, K.N.; Feigl, E.O. Quantitative Analysis of Feedforward Sympathetic Coronary Vasodilation in Exercising Dogs. J. Appl. Physiol. 2000, 89, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Berwick, Z.C.; Moberly, S.P.; Kohr, M.C.; Morrical, E.B.; Kurian, M.M.; Dick, G.M.; Tune, J.D. Contribution of Voltage-Dependent K+ and Ca2+ Channels to Coronary Pressure-Flow Autoregulation. Basic. Res. Cardiol. 2012, 107, 264. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Warne, C.M.; Essajee, S.I.; Tucker, S.M.; Figueroa, C.A.; Dick, G.M.; Beard, D.A. Unraveling the Gordian Knot of Coronary Pressure-Flow Autoregulation. J. Mol. Cell Cardiol. 2024, 190, 82–91. [Google Scholar] [CrossRef]
- Warne, C.M.; Essajee, S.I.; Tucker, S.M.; Figueroa, C.A.; Beard, D.A.; Dick, G.M.; Tune, J.D. Oxygen-Sensing Pathways below Autoregulatory Threshold Act to Sustain Myocardial Oxygen Delivery during Reductions in Perfusion Pressure. Basic. Res. Cardiol. 2023, 118, 12. [Google Scholar] [CrossRef]
- Kiel, A.M.; Goodwill, A.G.; Baker, H.E.; Dick, G.M.; Tune, J.D. Local Metabolic Hypothesis Is Not Sufficient to Explain Coronary Autoregulatory Behavior. Basic. Res. Cardiol. 2018, 113, 33. [Google Scholar] [CrossRef]
- Duncker, D.J.; Bache, R.J. Regulation of Coronary Blood Flow During Exercise. Physiol. Rev. 2008, 88, 1009–1086. [Google Scholar] [CrossRef]
- Kahles, H.; Hellige, G.; Hunneman, D.H.; Mezger, V.A.; Bretschneider, H.J. Influence of Myocardial Substrate Utilization on the Oxygen Consumption of the Heart. Clin. Cardiol. 1982, 5, 286–293. [Google Scholar] [CrossRef]
- Fulghum, K.; Hill, B.G. Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front. Cardiovasc. Med. 2018, 5, 127. [Google Scholar] [CrossRef]
- Moreira, J.B.N.; Wohlwend, M.; Wisløff, U. Exercise and Cardiac Health: Physiological and Molecular Insights. Nat. Metab. 2020, 2, 829–839. [Google Scholar] [CrossRef]
- Starnes, J.W.; Wilson, D.F.; Erecińska, M. Substrate Dependence of Metabolic State and Coronary Flow in Perfused Rat Heart. Am. J. Physiol. 1985, 249, H799–H806. [Google Scholar] [CrossRef]
- Rogers, W.J.; Russell, R.O.; McDaniel, H.G.; Rackley, C.E. Acute Effects of Glucose-Insulin-Potassium Infusion on Myocardial Substrates, Coronary Blood Flow and Oxygen Consumption in Man. Am. J. Cardiol. 1977, 40, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Dwenger, M.M.; Raph, S.M.; Reyzer, M.L.; Lisa Manier, M.; Riggs, D.W.; Wohl, Z.B.; Ohanyan, V.; Mack, G.; Pucci, T.; Moore, J.B.; et al. Pyridine Nucleotide Redox Potential in Coronary Smooth Muscle Couples Myocardial Blood Flow to Cardiac Metabolism. Nat. Commun. 2022, 13, 2051. [Google Scholar] [CrossRef] [PubMed]
- Ohanyan, V.; Raph, S.M.; Dwenger, M.M.; Hu, X.; Pucci, T.; Mack, G.; Moore, J.B.; Chilian, W.M.; Bhatnagar, A.; Nystoriak, M.A. Myocardial Blood Flow Control by Oxygen Sensing Vascular Kvβ Proteins. Circ. Res. 2021, 128, 738–751. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Hems, R.; Krebs, H.A. Utilization of Energy-Providing Substrates in the Isolated Working Rat Heart. Biochem. J. 1980, 186, 701–711. [Google Scholar] [CrossRef]
- Homilius, C.; Seefeldt, J.M.; Axelsen, J.S.; Pedersen, T.M.; Sørensen, T.M.; Nielsen, R.; Wiggers, H.; Hansen, J.; Matchkov, V.V.; Bøtker, H.E.; et al. Ketone Body 3-Hydroxybutyrate Elevates Cardiac Output through Peripheral Vasorelaxation and Enhanced Cardiac Contractility. Basic. Res. Cardiol. 2023, 118, 37. [Google Scholar] [CrossRef]
- Horton, J.L.; Davidson, M.T.; Kurishima, C.; Vega, R.B.; Powers, J.C.; Matsuura, T.R.; Petucci, C.; Lewandowski, E.D.; Crawford, P.A.; Muoio, D.M.; et al. The Failing Heart Utilizes 3-Hydroxybutyrate as a Metabolic Stress Defense. JCI Insight 2019, 4, e124079. [Google Scholar] [CrossRef]
- Gouwens, K.R.; Nong, Y.; Chen, N.; Schulman-Geltzer, E.B.; Collins, H.E.; Hill, B.G.; Nystoriak, M.A. Myocardial Hyperemia via Cardiomyocyte Catabolism of β-Hydroxybutyrate. Arterioscler. Thromb. Vasc. Biol. 2025, 45, 341–343. [Google Scholar] [CrossRef]
- Kim, D.K.; Heineman, F.W.; Balaban, R.S. Effects of Beta-Hydroxybutyrate on Oxidative Metabolism and Phosphorylation Potential in Canine Heart in Vivo. Am. J. Physiol. 1991, 260, H1767–H1773. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Karwi, Q.G.; Ho, K.L.; Pherwani, S.; Ketema, E.B. Ketone Metabolism in the Failing Heart. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2020, 1865, 158813. [Google Scholar] [CrossRef]
- Bedi, K.C.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Boedtkjer, E.; Matchkov, V.V.; Boedtkjer, D.M.B.; Aalkjaer, C. Negative News: Cl- and HCO3- in the Vascular Wall. Physiology 2016, 31, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Husted, A.S.; Trauelsen, M.; Rudenko, O.; Hjorth, S.A.; Schwartz, T.W. GPCR-Mediated Signaling of Metabolites. Cell Metab. 2017, 25, 777–796. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H.; Wolfe, R.R. Influence of Beta-Hydroxybutyrate Infusion on Glucose and Free Fatty Acid Metabolism in Dogs. Am. J. Physiol. 1984, 247, E756–E764. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Kashiwaya, Y.; Keon, C.A.; Tsuchiya, N.; King, M.T.; Radda, G.K.; Chance, B.; Clarke, K.; Veech, R.L. Insulin, Ketone Bodies, and Mitochondrial Energy Transduction. FASEB J. 1995, 9, 651–658. [Google Scholar] [CrossRef]
- Ho, K.L.; Karwi, Q.G.; Wagg, C.; Zhang, L.; Vo, K.; Altamimi, T.; Uddin, G.M.; Ussher, J.R.; Lopaschuk, G.D. Ketones Can Become the Major Fuel Source for the Heart but Do Not Increase Cardiac Efficiency. Cardiovasc. Res. 2021, 117, 1178–1187. [Google Scholar] [CrossRef]
- Taegtmeyer, H. On the Inability of Ketone Bodies to Serve as the Only Energy Providing Substrate for Rat Heart at Physiological Work Load. Basic. Res. Cardiol. 1983, 78, 435–450. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Qin, Y.; Honka, H.; Acosta, F.M.; Moody, A.; Chavez, A.; Triplitt, C.L.; Clarke, G.D.; Cersosimo, E.; Deforonzo, R.A. 99-LB: Myocardial Blood Flow in Association with Baseline Ejection Fraction after Elevation in Plasma Ketones in Patients with T2DM and Heart Failure. Diabetes 2023, 72, 99-LB. [Google Scholar] [CrossRef]
- Fioretto, P.; Trevisan, R.; Velussi, M.; Cernigoi, A.; De Riva, C.; Bressan, M.; Doria, A.; Pauletto, N.; Angeli, P.; De Donà, C. Glomerular Filtration Rate Is Increased in Man by the Infusion of Both D,L-3-Hydroxybutyric Acid and Sodium D,L-3-Hydroxybutyrate. J. Clin. Endocrinol. Metab. 1987, 65, 331–338. [Google Scholar] [CrossRef]
- Svart, M.; Gormsen, L.C.; Hansen, J.; Zeidler, D.; Gejl, M.; Vang, K.; Aanerud, J.; Moeller, N. Regional Cerebral Effects of Ketone Body Infusion with 3-Hydroxybutyrate in Humans: Reduced Glucose Uptake, Unchanged Oxygen Consumption and Increased Blood Flow by Positron Emission Tomography. A Randomized, Controlled Trial. PLoS ONE 2018, 13, e0190556. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Moeslund, N.; Christensen, K.H.; Berg-Hansen, K.; Seefeldt, J.; Homilius, C.; Nielsen, E.N.; Dollerup, M.R.; Alstrup Olsen, A.K.; Johannsen, M.; et al. Enantiomer-Specific Cardiovascular Effects of the Ketone Body 3-Hydroxybutyrate. JAHA 2024, 13, e033628. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.G.; Chakraborty, S.; Singh, G.; Yeoh, B.S.; Schreckenberger, Z.J.; Singh, A.; Mell, B.; Bearss, N.R.; Yang, T.; Cheng, X.; et al. Ketone Body β-Hydroxybutyrate Is an Autophagy-Dependent Vasodilator. JCI Insight 2021, 6, e149037. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, J.S.; Keung, W.; Wang, W.; Ussher, J.R.; Lopaschuk, G.D. Targeting Fatty Acid and Carbohydrate Oxidation—A Novel Therapeutic Intervention in the Ischemic and Failing Heart. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2011, 1813, 1333–1350. [Google Scholar] [CrossRef]
- Matsuura, T.R.; Puchalska, P.; Crawford, P.A.; Kelly, D.P. Ketones and the Heart: Metabolic Principles and Therapeutic Implications. Circ. Res. 2023, 132, 882–898. [Google Scholar] [CrossRef]
- de Koning, M.-S.L.Y.; Westenbrink, B.D.; Assa, S.; Garcia, E.; Connelly, M.A.; van Veldhuisen, D.J.; Dullaart, R.P.F.; Lipsic, E.; van der Harst, P. Association of Circulating Ketone Bodies with Functional Outcomes After ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 78, 1421–1432. [Google Scholar] [CrossRef]
- Byrne, N.J.; Soni, S.; Takahara, S.; Ferdaoussi, M.; Al Batran, R.; Darwesh, A.M.; Levasseur, J.L.; Beker, D.; Vos, D.Y.; Schmidt, M.A.; et al. Chronically Elevating Circulating Ketones Can Reduce Cardiac Inflammation and Blunt the Development of Heart Failure. Circ. Heart Fail. 2020, 13, e006573. [Google Scholar] [CrossRef]
- Selvaraj, S.; Hu, R.; Vidula, M.K.; Dugyala, S.; Tierney, A.; Ky, B.; Margulies, K.B.; Shah, S.H.; Kelly, D.P.; Bravo, P.E. Acute Echocardiographic Effects of Exogenous Ketone Administration in Healthy Participants. J. Am. Soc. Echocardiogr. 2022, 35, 305–311. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Am. Coll. Cardiol. 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Dyck, J.R.B. Ketones and the Cardiovascular System. Nat. Cardiovasc. Res. 2023, 2, 425–437. [Google Scholar] [CrossRef]
- Oyetoro, R.O.; Conners, K.M.; Joo, J.; Turecamo, S.; Sampson, M.; Wolska, A.; Remaley, A.T.; Otvos, J.D.; Connelly, M.A.; Larson, N.B.; et al. Circulating Ketone Bodies and Mortality in Heart Failure: A Community Cohort Study. Front. Cardiovasc. Med. 2024, 11, 1293901. [Google Scholar] [CrossRef]
- Voros, G.; Ector, J.; Garweg, C.; Droogne, W.; Van Cleemput, J.; Peersman, N.; Vermeersch, P.; Janssens, S. Increased Cardiac Uptake of Ketone Bodies and Free Fatty Acids in Human Heart Failure and Hypertrophic Left Ventricular Remodeling. Circ. Heart Fail. 2018, 11, e004953. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Rasool, S.; Devi, S.; Talha, M.; Waqar, F.; Nasir, M.; Khan, M.R.; Ibne Ali Jaffari, S.M.; Haider, A.; Shah, S.U.; et al. Exploring the Cardiovascular Benefits of Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors: Expanding Horizons Beyond Diabetes Management. Cureus 2023, 15, e46243. [Google Scholar] [CrossRef] [PubMed]
- Monzo, L.; Sedlacek, K.; Hromanikova, K.; Tomanova, L.; Borlaug, B.A.; Jabor, A.; Kautzner, J.; Melenovsky, V. Myocardial Ketone Body Utilization in Patients with Heart Failure: The Impact of Oral Ketone Ester. Metabolism 2021, 115, 154452. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Christensen, K.H.; Gopalasingam, N.; Nielsen, R.; Eiskjær, H.; Møller, N.; Birkelund, T.; Christensen, S.; Wiggers, H. Beneficial Effects of Ketone Ester in Patients with Cardiogenic Shock: A randomized, controlled, double-blind trial. JACC Heart Fail. 2023, 11, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Sramko, M.; Melenovsky, V.; Kleissner, M.; Benak, A.; Holek, M.; Pazdernik, M.; Kautzner, J. Acute Hemodynamic Effect of Ketone Bodies in Patients with Decompensated Heart Failure. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, zuac041.073. [Google Scholar] [CrossRef]
- Solis-Herrera, C.; Qin, Y.; Acosta, F.; Honka, H.; Moody, A.; Brune, S.; Clarke, G.; Triplitt, C.; Cersosimo, E.; DeFronzo, R. #1315139: Cardiometabolic Effects of Elevated Plasma Ketones in Patients with Heart Failure and T2DM. Endocr. Pract. 2022, 28, S3. [Google Scholar] [CrossRef]
- Kotha, S.; Procter, H.; Giannoudi, M.; Plein, S.; Xue, H.; Valkovič, L.; Kellman, P.; Greenwood, J.; Clarke, K.; Levelt, E. 138 Effects of Exogenous Ketone Supplementation on Cardiac Energetics, Steatosis, Function, and Perfusion in Type 2 Diabetes, Heart Failure, and Healthy Participants—A Single Center, Open Labelled Clinical Study. Heart 2024, 110, A145–A147. [Google Scholar] [CrossRef]
- Gopalasingam, N.; Berg-Hansen, K.; Christensen, K.H.; Ladefoged, B.T.; Poulsen, S.H.; Andersen, M.J.; Borlaug, B.A.; Nielsen, R.; Møller, N.; Wiggers, H. Randomized Crossover Trial of 2-Week Ketone Ester Treatment in Patients with Type 2 Diabetes and Heart Failure with Preserved Ejection Fraction. Circulation 2024, 150, 1570–1583. [Google Scholar] [CrossRef]
- Berg-Hansen, K.; Gopalasingam, N.; Christensen, K.H.; Ladefoged, B.; Andersen, M.J.; Poulsen, S.H.; Borlaug, B.A.; Nielsen, R.; Møller, N.; Wiggers, H. Cardiovascular Effects of Oral Ketone Ester Treatment in Patients with Heart Failure with Reduced Ejection Fraction: A Randomized, Controlled, Double-Blind Trial. Circulation 2024, 149, 1474–1489. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Sun, Z. Aging, Arterial Stiffness and Hypertension. Hypertension 2015, 65, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Stehouwer, C.D.A.; Henry, R.M.A.; Ferreira, I. Arterial Stiffness in Diabetes and the Metabolic Syndrome: A Pathway to Cardiovascular Disease. Diabetologia 2008, 51, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Czernin, J.; Müller, P.; Chan, S.; Brunken, R.C.; Porenta, G.; Krivokapich, J.; Chen, K.; Chan, A.; Phelps, M.E.; Schelbert, H.R. Influence of Age and Hemodynamics on Myocardial Blood Flow and Flow Reserve. Circulation 1993, 88, 62–69. [Google Scholar] [CrossRef]
- Senneff, M.J.; Geltman, E.M.; Bergmann, S.R. Noninvasive Delineation of the Effects of Moderate Aging on Myocardial Perfusion. J. Nucl. Med. 1991, 32, 2037–2042. [Google Scholar] [PubMed]
- Brown, L.A.E.; Gulsin, G.S.; Onciul, S.C.; Broadbent, D.A.; Yeo, J.L.; Wood, A.L.; Saunderson, C.E.D.; Das, A.; Jex, N.; Chowdhary, A.; et al. Sex- and Age-Specific Normal Values for Automated Quantitative Pixel-Wise Myocardial Perfusion Cardiovascular Magnetic Resonance. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 426–434. [Google Scholar] [CrossRef]
- Kates, A.M.; Herrero, P.; Dence, C.; Soto, P.; Srinivasan, M.; Delano, D.G.; Ehsani, A.; Gropler, R.J. Impact of Aging on Substrate Metabolism by the Human Heart. J. Am. Coll. Cardiol. 2003, 41, 293–299. [Google Scholar] [CrossRef]
- Xie, S.; Xu, S.-C.; Deng, W.; Tang, Q. Metabolic Landscape in Cardiac Aging: Insights into Molecular Biology and Therapeutic Implications. Signal Transduct. Target. Ther. 2023, 8, 114. [Google Scholar] [CrossRef]
- Abu-Erreish, G.M.; Neely, J.R.; Whitmer, J.T.; Whitman, V.; Sanadi, D.R. Fatty Acid Oxidation by Isolated Perfused Working Hearts of Aged Rats. Am. J. Physiol. 1977, 232, E258–E262. [Google Scholar] [CrossRef]
- Hyyti, O.M.; Ledee, D.; Ning, X.-H.; Ge, M.; Portman, M.A. Aging Impairs Myocardial Fatty Acid and Ketone Oxidation and Modifies Cardiac Functional and Metabolic Responses to Insulin in Mice. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H868–H875. [Google Scholar] [CrossRef]
- Lombardo, T.A.; Rose, L.; Taeschler, M.; Tuluy, S.; Bing, R.J. The Effect of Exercise on Coronary Blood Flow, Myocardial Oxygen Consumption and Cardiac Efficiency in Man. Circulation 1953, 7, 71–78. [Google Scholar] [CrossRef]
- von Restorff, W.; Höfling, B.; Holtz, J.; Bassenge, E. Effect of Increased Blood Fluidity through Hemodilution on Coronary Circulation at Rest and during Exercise in Dogs. Pflug. Arch. 1975, 357, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Duncker, D.J.; Zhang, J.; Bache, R.J. ATP-Sensitive K+ Channels, Adenosine, and Nitric Oxide-Mediated Mechanisms Account for Coronary Vasodilation during Exercise. Circ. Res. 1998, 82, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Tune, J.D.; Richmond, K.N.; Gorman, M.W.; Feigl, E.O. KATP + Channels, Nitric Oxide, and Adenosine Are Not Required for Local Metabolic Coronary Vasodilation. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H868–H875. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]
- Evans, M.; McClure, T.S.; Koutnik, A.P.; Egan, B. Exogenous Ketone Supplements in Athletic Contexts: Past, Present, and Future. Sports Med. 2022, 52, 25–67. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aromiwura, A.A.; Gouwens, K.R.; Nguyen, D.C.; Sztukowska, M.; Didelot, L.; Kalra, D.K. Ketone Bodies in the Regulation of Myocardial Perfusion in Cardiovascular Disease: Metabolic and Vasodilatory Effects. Int. J. Mol. Sci. 2025, 26, 4856. https://doi.org/10.3390/ijms26104856
Aromiwura AA, Gouwens KR, Nguyen DC, Sztukowska M, Didelot L, Kalra DK. Ketone Bodies in the Regulation of Myocardial Perfusion in Cardiovascular Disease: Metabolic and Vasodilatory Effects. International Journal of Molecular Sciences. 2025; 26(10):4856. https://doi.org/10.3390/ijms26104856
Chicago/Turabian StyleAromiwura, Afolasayo A., Kara R. Gouwens, Daniel C. Nguyen, Maryta Sztukowska, Luanne Didelot, and Dinesh K. Kalra. 2025. "Ketone Bodies in the Regulation of Myocardial Perfusion in Cardiovascular Disease: Metabolic and Vasodilatory Effects" International Journal of Molecular Sciences 26, no. 10: 4856. https://doi.org/10.3390/ijms26104856
APA StyleAromiwura, A. A., Gouwens, K. R., Nguyen, D. C., Sztukowska, M., Didelot, L., & Kalra, D. K. (2025). Ketone Bodies in the Regulation of Myocardial Perfusion in Cardiovascular Disease: Metabolic and Vasodilatory Effects. International Journal of Molecular Sciences, 26(10), 4856. https://doi.org/10.3390/ijms26104856







