Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes
Abstract
1. Introduction
2. Results
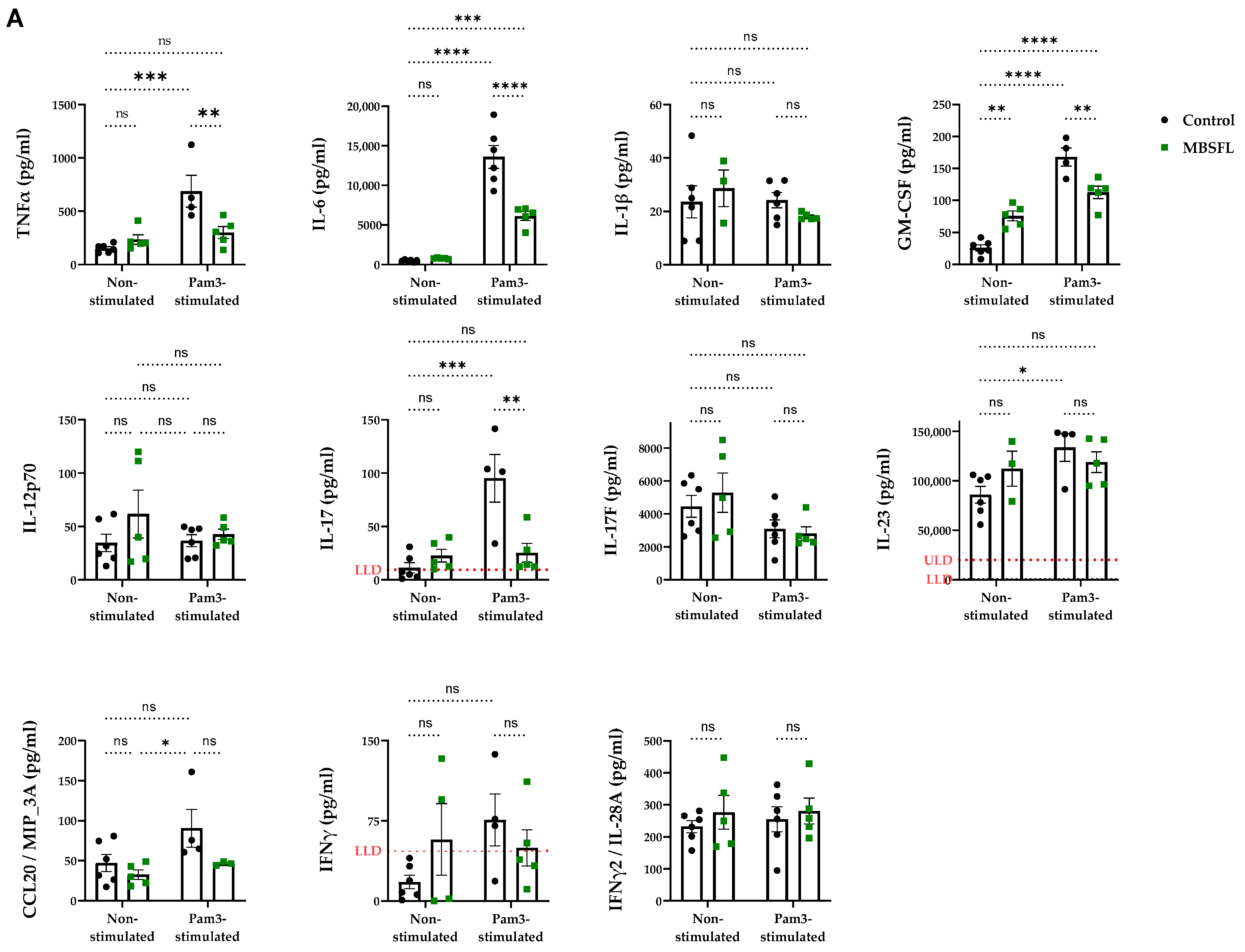
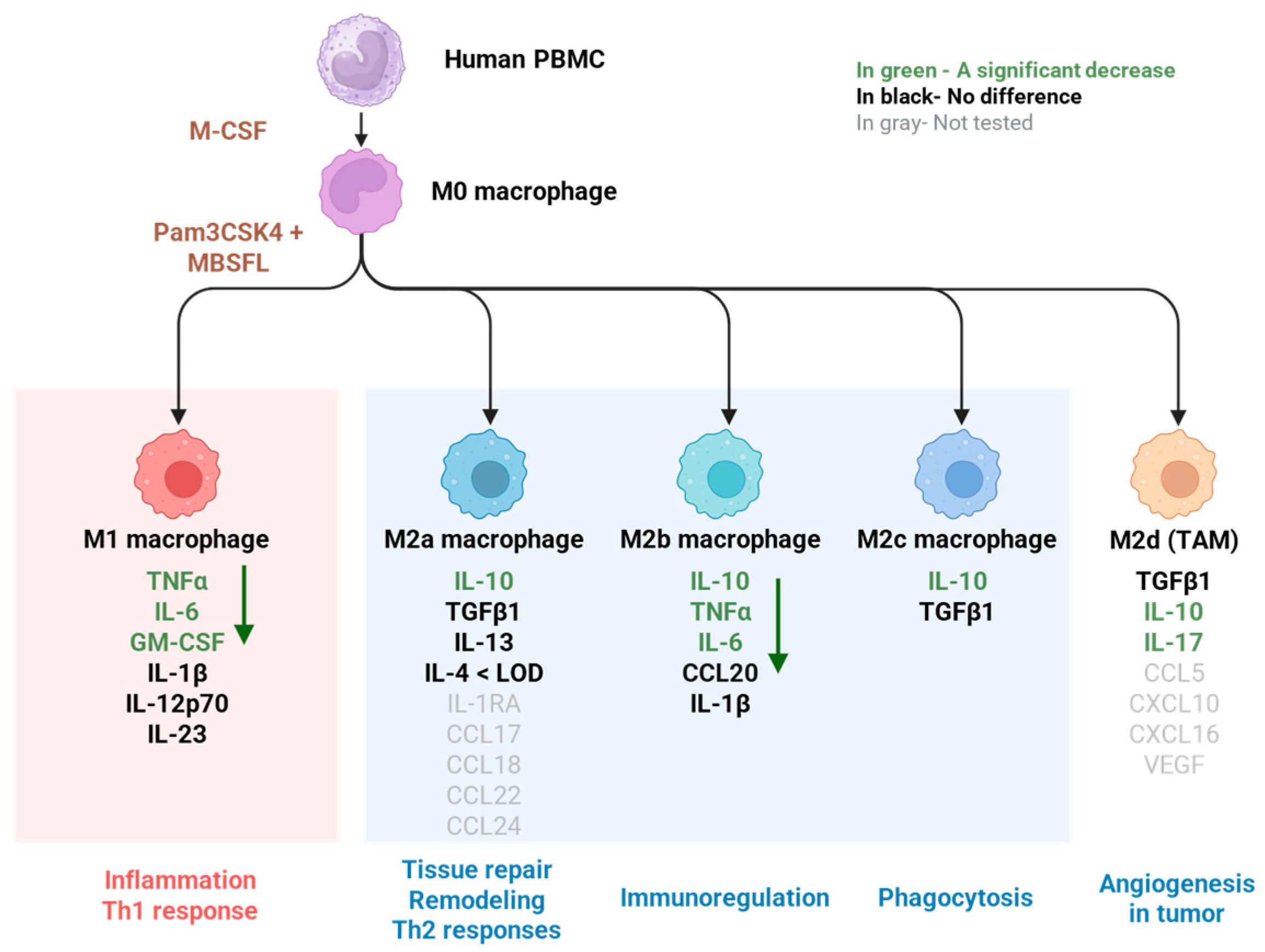
2.1. Saponified Black Soldier Fly Larvae Oil (MBSFL) Suppresses M1-Associated Pro-Inflammatory Cytokine Secretion Without Altering M2-Associated Cytokines
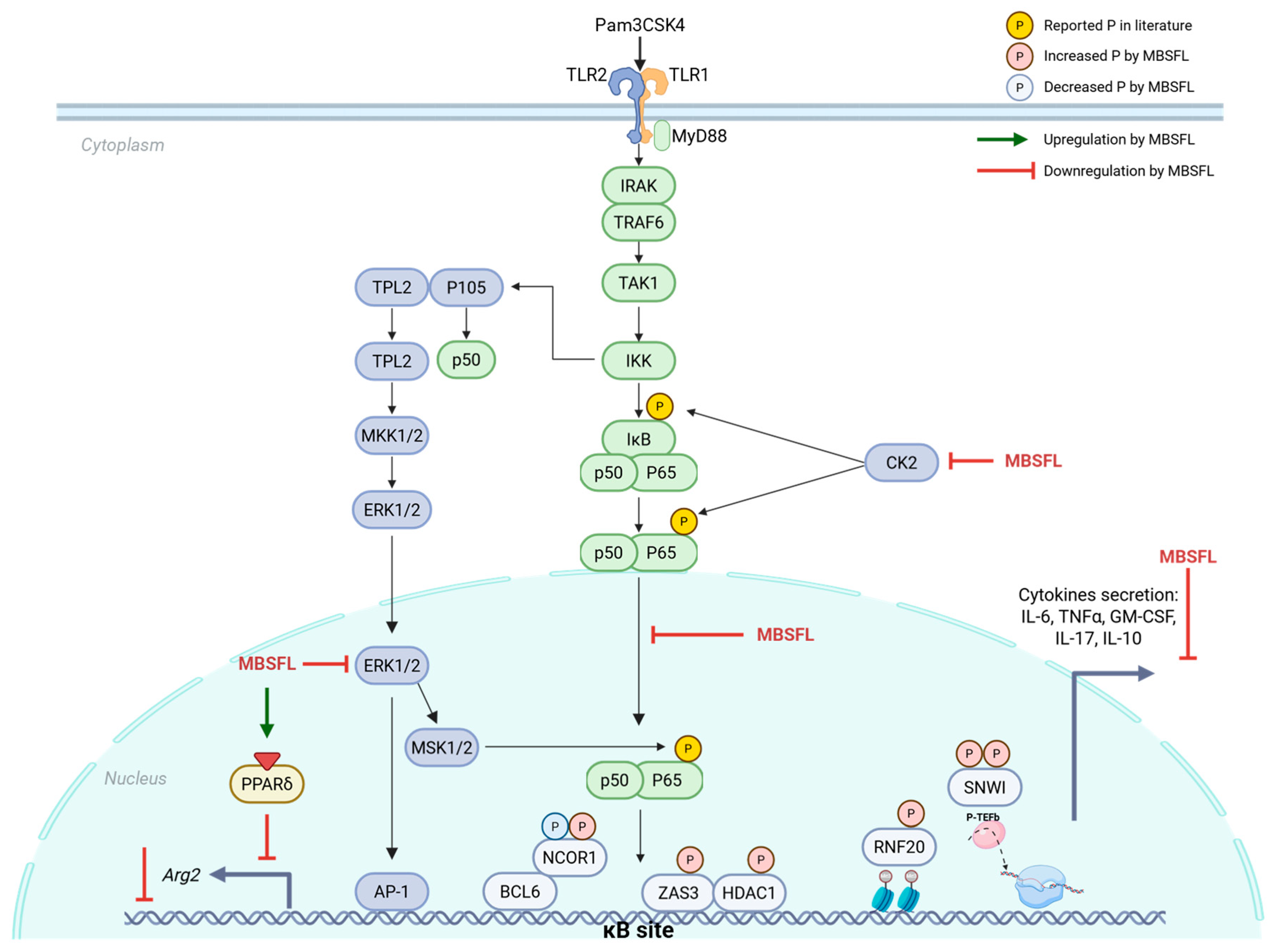
2.2. Phosphoproteomic Analysis Unveils a Role for MBSFL in Chromatin Dynamics to Inhibit Inflammation
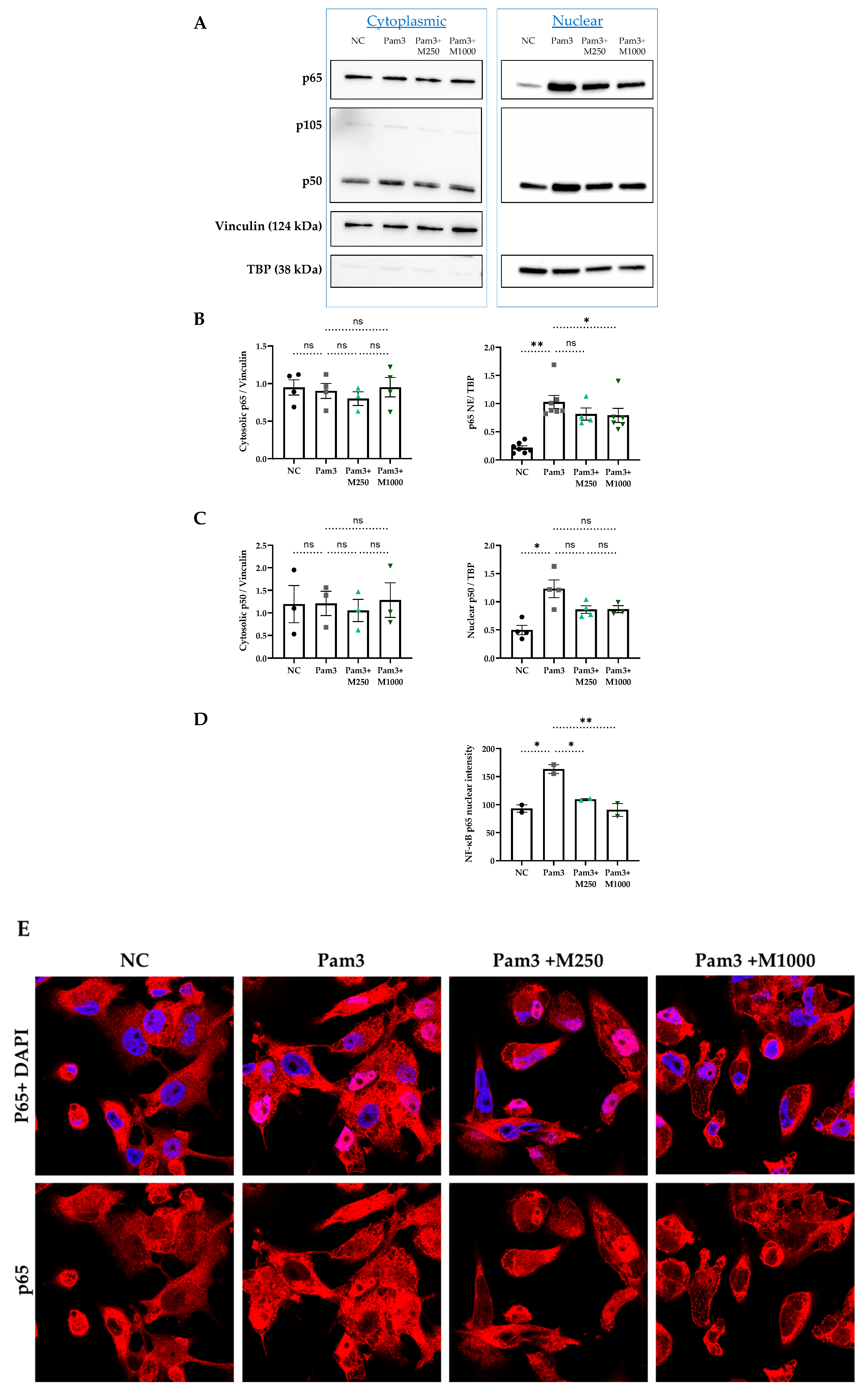
2.3. MBSFL Modulates Anti-Inflammatory Responses Through Suppression of Nuclear Factor Kappa B (NF-κB) p65 Nuclear Translocation
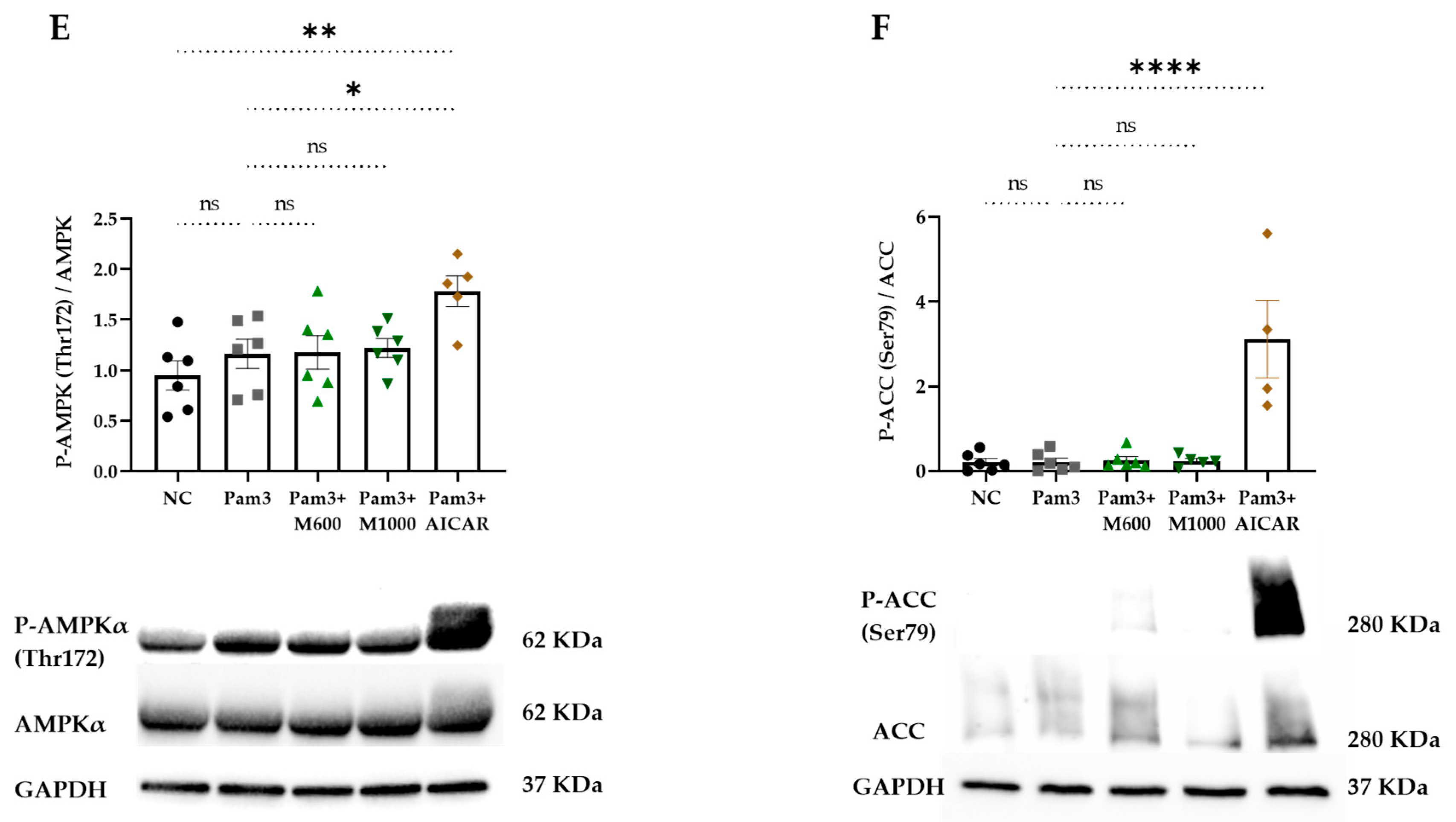
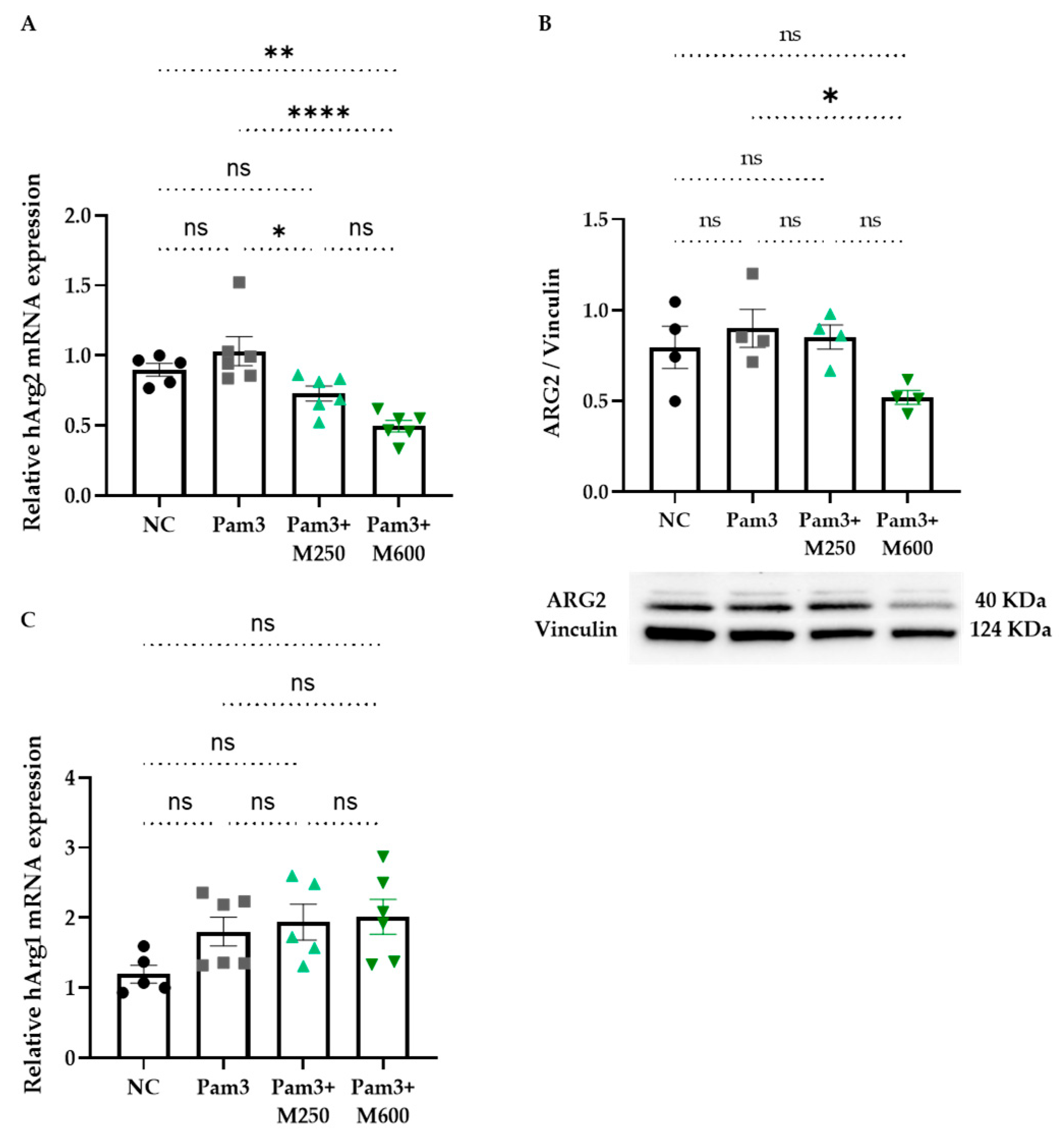
2.4. Role of MBSFL in Immunometabolism via Activation of Peroxisome Proliferator-Activated Receptor (PPAR)-δ
3. Discussion
4. Materials and Methods
4.1. Saponified BSFL Oil (MBSFL)
4.2. Cell Culture
4.3. Cell Treatment
4.4. Cell Viability Assay
4.5. Multiplexed Sandwich ELISA-Based Quantitative Array
4.6. Phosphoproteomic Analysis
4.7. Immunofluorescence Staining and Confocal Microscopy
4.8. Protein Extraction and Quantification
4.9. Western Blot Analysis
4.10. Human PPARs Reporter Assay System
4.11. Real-Time PCR (qPCR)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl CoA Carboxylase |
| AICAR | 5-Aminoimidazole-4-carboxamide Ribonucleotide |
| AMPK | AMP-Activated Protein Kinase |
| ARG | Arginase |
| BCL6 | B-Cell Lymphoma 6 |
| BSA | Bovine Serum Albumin |
| BSFL | Black Soldier Fly Larvae |
| CDK2 | Cyclin-Dependent Kinase 2 |
| CITI | Collaborative Institutional Training Initiative |
| CK2 | Casein Kinase II |
| CSM | Compound-Screening Medium |
| DiHOMEs | Di-Hydroxy-Octadecadienoic Acids |
| DMSO | Dimethyl Sulfoxide |
| EC50 | Half Maximal Effective Concentration |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| ERK | Extracellular Signal-Regulated Kinases |
| FDRs | Peptide-Level False Discovery Rates |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase. |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| h | Hour |
| HBSS | Hanks′ Balanced Salt Solution |
| HDAC1 | Histone Deacetylase 1 |
| IKK | Inhibitor κB Kinase |
| IL | Interleukin |
| iNOS | Inducible Nitric Oxide Synthase |
| IκB | Inhibitor κB |
| KSEA | Kinase Substrate Enrichment Analysis |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| LLD | Lower Limit of Detection |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-Activated Protein Kinase |
| MBSFL | Saponified BSFL Oil |
| M-CSF | Macrophage Colony-Stimulating Factor |
| min | Minutes |
| MSK1 | Mitogen- and Stress-activated Kinase 1 |
| MTT | Thiazolyl Blue Tetrazolium Bromide (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| MyD88 | Myeloid Differentiation Primary Response 88 |
| NCOR1 | Nuclear Receptor Corepressor 1 |
| NF-κB | Nuclear Factor Kappa B |
| NIK | NF-κB-inducing Kinase |
| Pam3 | Pam3CSK4 (Pam3CysSerLys4) |
| PAMPs | Pathogen-Associated Molecular Patterns |
| PANTHER | Protein ANalysis THrough Evolutionary Relationships |
| PBMCs | Peripheral Blood Mononuclear Cells |
| PBS | Phosphate-Buffered Saline |
| PKA | Protein Kinase A |
| PLK1 | Polo-Like Kinase 1 |
| PMA | Phorbol 12-Myristate-13-Acetate |
| PPARs | Peroxisome Proliferator-Activated Receptors |
| PPRE | Peroxisome Proliferator Response Element |
| PVDF | Polyvinylidene Fluoride |
| qPCR | Real-time PCR |
| RIPA | Radioimmunoprecipitation Assay |
| RLU | Relative Light Units |
| RNF20 | Ring Finger Protein 20 |
| ROS | Reactive Oxygen Species |
| RPMI 1640 | Roswell Park Memorial Institute 1640 |
| SDS-PAGE | Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis |
| Ser | Serine |
| TAK1 | TGF-beta Activated Kinase 1 |
| TBP | TATA-binding protein |
| TGFβ1 | Transforming Growth Factor Beta 1 |
| Thr | Threonine |
| TLR | Toll-like Receptor |
| TNFα | Tumor Necrosis Factor Alpha |
| ULD | Upper Limit of Detection |
| 13S-HOTrE | 3(S)-Hydroxyoctadecatrienoic acid |
References
- Mariod, A.A. Insect Oil as a Source of Nutraceuticals; Academic Press: New York, NY, USA, 2004. [Google Scholar]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.B.; Kim, D.H.; Jeong, S.B.; Lee, J.W.; Kim, T.H.; Lee, H.G.; Lee, K.W. Black Soldier Fly Larvae Oil as an Alternative Fat Source in Broiler Nutrition. Poult. Sci. 2020, 99, 3133–3143. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Ferrocino, I.; Gasco, L.; Schiavone, A.; Trocino, A.; Xiccato, G.; Barroeta, A.C.; Maione, S.; Soglia, D.; Biasato, I.; et al. Antimicrobial Effects of Black Soldier Fly and Yellow Mealworm Fats and Their Impact on Gut Microbiota of Growing Rabbits. Animals 2020, 10, 1292. [Google Scholar] [CrossRef] [PubMed]
- Zabulionė, A.; Šalaševičienė, A.; Makštutienė, N.; Šarkinas, A. Exploring the Antimicrobial Potential and Stability of Black Soldier Fly (Hermentia illucens) Larvae Fat for Enhanced Food Shelf-Life. Gels 2023, 9, 793. [Google Scholar] [CrossRef]
- Richter, H.; Gover, O.; Schwartz, B. Anti-Inflammatory Activity of Black Soldier Fly Oil Associated with Modulation of TLR Signaling: A Metabolomic Approach. Int. J. Mol. Sci. 2023, 24, 10634. [Google Scholar] [CrossRef]
- Verma, S.; De Jesus, P.; Chanda, S.K.; Verma, I.M. SNW1, a Novel Transcriptional Regulator of the NF-kB Pathway. Mol. Cell. Biol. 2019, 39, e00415-18. [Google Scholar] [CrossRef]
- Dorrington, M.G.; Fraser, I.D.C. NF-ΚB Signaling in Macrophages: Dynamics, Crosstalk, and Signal Integration. Front. Immunol. 2019, 10, 705. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-ΚB and IκB Proteins: Implications in Cancer and Inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, Non-Canonical and Atypical Pathways of Nuclear Factor Kb Activation in Preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef]
- Bhatt, D.; Ghosh, S. Regulation of the NF-ΚB-Mediated Transcription of Inflammatory Genes. Front. Immunol. 2014, 5, 71. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The Complexity of NF-ΚB Signaling in Inflammation and Cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.C.; Ley, S.C. Mitogen-Activated Protein Kinases in Innate Immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 Macrophages and Their Overlaps–Myth or Reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Aksoylar, H.I.; Yu, J.; Snyder, N.W.; Worth, A.J.; Iyer, S.S.; Wang, J.; Ben-Sahra, I.; Byles, V.; Polynne-Stapornkul, T.; et al. Akt-MTORC1 Signaling Regulates Acly to Integrate Metabolic Input to Control of Macrophage Activation. Elife 2016, 5, e11612. [Google Scholar] [CrossRef]
- Wang, S.; Liu, R.; Yu, Q.; Dong, L.; Bi, Y.; Liu, G. Metabolic Reprogramming of Macrophages during Infections and Cancer. Cancer Lett. 2019, 452, 14–22. [Google Scholar] [CrossRef]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. [Google Scholar] [CrossRef]
- Adhikary, T.; Wortmann, A.; Schumann, T.; Finkernagel, F.; Lieber, S.; Roth, K.; Toth, P.M.; Diederich, W.E.; Nist, A.; Stiewe, T.; et al. The Transcriptional PPARβ/δ Network in Human Macrophages Defines a Unique Agonist-Induced Activation State. Nucleic Acids Res. 2015, 43, 5033–5051. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.F.U.H.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.G.; Rao, L.; Duo, Y. Macrophages in Immunoregulation and Therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Toobian, D.; Ghosh, P.; Katkar, G.D. Parsing the Role of PPARs in Macrophage Processes. Front. Immunol. 2021, 12, 783780. [Google Scholar] [CrossRef]
- Yu, L.; Gao, Y.; Aaron, N.; Qiang, L. A Glimpse of the Connection between PPARγ and Macrophage. Front Pharmacol 2023, 14, 1254317. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding Light on the Mechanisms and Opportunities in Cardiovascular Diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Vogelpoel, L.T.C.; Hansen, I.S.; Rispens, T.; Muller, F.J.M.; van Capel, T.M.M.; Turina, M.C.; Vos, J.B.; Baeten, D.L.P.; Kapsenberg, M.L.; de Jong, E.C.; et al. Fc Gamma Receptor-TLR Cross-Talk Elicits pro-Inflammatory Cytokine Production by Human M2 Macrophages. Nat. Commun. 2014, 5, 5444. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.F.; Kelsey, I.; Forbes, H.; Miller-Jensen, K. Single-Cell Secretion Analysis Reveals a Dual Role for IL-10 in Restraining and Resolving the TLR4-Induced Inflammatory Response. Cell Rep. 2021, 36, 109728. [Google Scholar] [CrossRef]
- Funderburg, N.T.; Jadlowsky, J.K.; Lederman, M.M.; Feng, Z.; Weinberg, A.; Sieg, S.F. The Toll-like Receptor 1/2 Agonists Pam3CSK4 and Human β-Defensin-3 Differentially Induce Interleukin-10 and Nuclear Factor-ΚB Signalling Patterns in Human Monocytes. Immunology 2011, 134, 151–160. [Google Scholar] [CrossRef]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte Differentiation and Macrophage Polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Hong, J.W.; Wu, L.C. ZAS3 Represses NFκB-Dependent Transcription by Direct Competition for DNA Binding. BMB Rep. 2010, 43, 807–812. [Google Scholar] [CrossRef]
- Geiger, M.A.; Guillaumon, A.T.; Paneni, F.; Matter, C.M.; Stein, S. Role of the Nuclear Receptor Corepressor 1 (NCOR1) in Atherosclerosis and Associated Immunometabolic Diseases. Front. Immunol. 2020, 11, 569358. [Google Scholar] [CrossRef]
- Barish, G.D.; Yu, R.T.; Karunasiri, M.S.; Becerra, D.; Kim, J.; Tseng, T.W.; Tai, L.J.; Leblanc, M.; Diehl, C.; Cerchietti, L.; et al. The Bcl6-SMRT/NCoR Cistrome Represses Inflammation to Attenuate Atherosclerosis. Cell Metab. 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Zhong, H.; May, M.J.; Jimi, E.; Ghosh, S. The Phosphorylation Status of Nuclear NF-ΚB Determines Its Association with CBP/P300 or HDAC-1. Mol. Cell 2002, 9, 625–636. [Google Scholar] [CrossRef]
- Tarcic, O.; Pateras, I.S.; Cooks, T.; Shema, E.; Kanterman, J.; Ashkenazi, H.; Boocholez, H.; Hubert, A.; Rotkopf, R.; Baniyash, M.; et al. RNF20 Links Histone H2B Ubiquitylation with Inflammation and Inflammation-Associated Cancer. Cell Rep. 2016, 14, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Luc, Y.; Martinez, J.; Bi, Y.; Lian, G.; Wang, T.; Milasta, S.; Wang, J.; Yang, M.; Liu, G.; et al. Proinflammatory Signal Suppresses Proliferation and Shifts Macrophage Metabolism from Myc-Dependent to HIF1α-Dependent. Proc. Natl. Acad. Sci. USA 2016, 113, 1564–1569. [Google Scholar] [CrossRef]
- Daniel, B.; Belk, J.A.; Meier, S.L.; Chen, A.Y.; Sandor, K.; Czimmerer, Z.; Varga, Z.; Bene, K.; Buquicchio, F.A.; Qi, Y.; et al. Macrophage Inflammatory and Regenerative Response Periodicity Is Programmed by Cell Cycle and Chromatin State. Mol. Cell 2023, 83, 121–138.e7. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D.; Felzien, L.K.; Betts, J.C.; Leung, K.; Beach, D.H.; Nabel, G.J. Regulation of NF-ΚB by Cyclin-Dependent Kinases Associated with the P300 Coactivator. Science (1979) 1997, 275, 523–527. [Google Scholar] [CrossRef]
- Schevzov, G.; Kee, A.J.; Wang, B.; Sequeira, V.B.; Hook, J.; Coombes, J.D.; Lucas, C.A.; Stehn, J.R.; Musgrove, E.A.; Cretu, A.; et al. Regulation of Cell Proliferation by ERK and Signal-Dependent Nuclear Translocation of ERK Is Dependent on Tm5NM1-Containing Actin Filaments. Mol. Biol. Cell 2015, 26, 2475–2490. [Google Scholar] [CrossRef]
- Tian, Y.; Yang, C.; Yao, Q.; Qian, L.; Liu, J.; Xie, X.; Ma, W.; Nie, X.; Lai, B.; Xiao, L.; et al. Procyanidin B2 Activates PPARγ to Induce M2 Polarization in Mouse Macrophages. Front. Immunol. 2019, 10, 1895. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Y.; Nelin, L.D. Extracellular Signal-Regulated Kinase Mediates Expression of Arginase II but Not Inducible Nitric-Oxide Synthase in Lipopolysaccharide-Stimulated Macrophages. J. Biol. Chem. 2015, 290, 2099–2111. [Google Scholar] [CrossRef]
- Planavila, A.; Rodríguez-Calvo, R.; Jové, M.; Michalik, L.; Wahli, W.; Laguna, J.C.; Vázquez-Carrera, M. Peroxisome Proliferator-Activated Receptor β/δ Activation Inhibits Hypertrophy in Neonatal Rat Cardiomyocytes. Cardiovasc. Res. 2005, 65, 832–841. [Google Scholar] [CrossRef]
- Rodríguez-Calvo, R.; Serrano, L.; Coll, T.; Moullan, N.; Sánchez, R.M.; Merlos, M.; Palomer, X.; Laguna, J.C.; Michalik, L.; Wahli, W.; et al. Activation of Peroxisome Proliferator–Activated Receptor β/δ Inhibits Lipopolysaccharide-Induced Cytokine Production in Adipocytes by Lowering Nuclear Factor-ΚB Activity via Extracellular Signal–Related Kinase 1/2. Diabetes 2008, 57, 2149–2157. [Google Scholar] [CrossRef]
- Mottis, A.; Mouchiroud, L.; Auwerx, J. Emerging Roles of the Corepressors NCoR1 and SMRT in Homeostasis. Genes Dev. 2013, 27, 819–835. [Google Scholar] [CrossRef]
- Richter, H.; Steinberg, S.; Baron, Y.; Inbart, A. Modified Black Soldier Fly Larvae Oil with Modified Lauric Acid for Treatment Against Biofilm Formation and Microorganism Growth. WO2020234884A1, 26 November 2020. [Google Scholar]
- Muñiz-Buenrostro, A.; Arce-Mendoza, A.Y.; Montes-Zapata, E.I.; CalderónMeléndez, R.C.; Montelongo-Rodríguez, M.J. Simplified Neutrophil Isolation Protocol. Int. J. Immunol. Immunother. 2020, 7, 041. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Casado, P.; Rodriguez-Prados, J.-C.; Cosulich, S.C.; Guichard, S.; Vanhaesebroeck, B.; Joel, S.; Cutillas, P.R. Kinase-Substrate Enrichment Analysis Provides Insights into the Heterogeneity of Signaling Pathway Activation in Leukemia Cells. Sci. Signal. 2013, 6, rs6. [Google Scholar] [CrossRef] [PubMed]
- Wiredja, D.D.; Koyutürk, M.; Chance, M.R. The KSEA App: A Web-Based Tool for Kinase Activity Inference from Quantitative Phosphoproteomics. Bioinformatics 2017, 33, 3489–3491. [Google Scholar] [CrossRef]
- Dowling, J.K.; Afzal, R.; Gearing, L.J.; Cervantes-Silva, M.P.; Annett, S.; Davis, G.M.; De Santi, C.; Assmann, N.; Dettmer, K.; Gough, D.J.; et al. Mitochondrial Arginase-2 Is Essential for IL-10 Metabolic Reprogramming of Inflammatory Macrophages. Nat. Commun. 2021, 12, 1460. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, H.I.; Gover, O.; Hamburg, A.; Bendalak, K.; Ziv, T.; Schwartz, B. Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes. Int. J. Mol. Sci. 2025, 26, 4855. https://doi.org/10.3390/ijms26104855
Richter HI, Gover O, Hamburg A, Bendalak K, Ziv T, Schwartz B. Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes. International Journal of Molecular Sciences. 2025; 26(10):4855. https://doi.org/10.3390/ijms26104855
Chicago/Turabian StyleRichter, Hadas Inbart, Ofer Gover, Amit Hamburg, Keren Bendalak, Tamar Ziv, and Betty Schwartz. 2025. "Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes" International Journal of Molecular Sciences 26, no. 10: 4855. https://doi.org/10.3390/ijms26104855
APA StyleRichter, H. I., Gover, O., Hamburg, A., Bendalak, K., Ziv, T., & Schwartz, B. (2025). Impact of Black Soldier Fly Larvae Oil on Immunometabolic Processes. International Journal of Molecular Sciences, 26(10), 4855. https://doi.org/10.3390/ijms26104855





