TSPO Ligand 2-Cl-MGV-1 Mitigates Traumatic Brain Injury (TBI) in a Mouse Model
Abstract
1. Introduction
2. Results
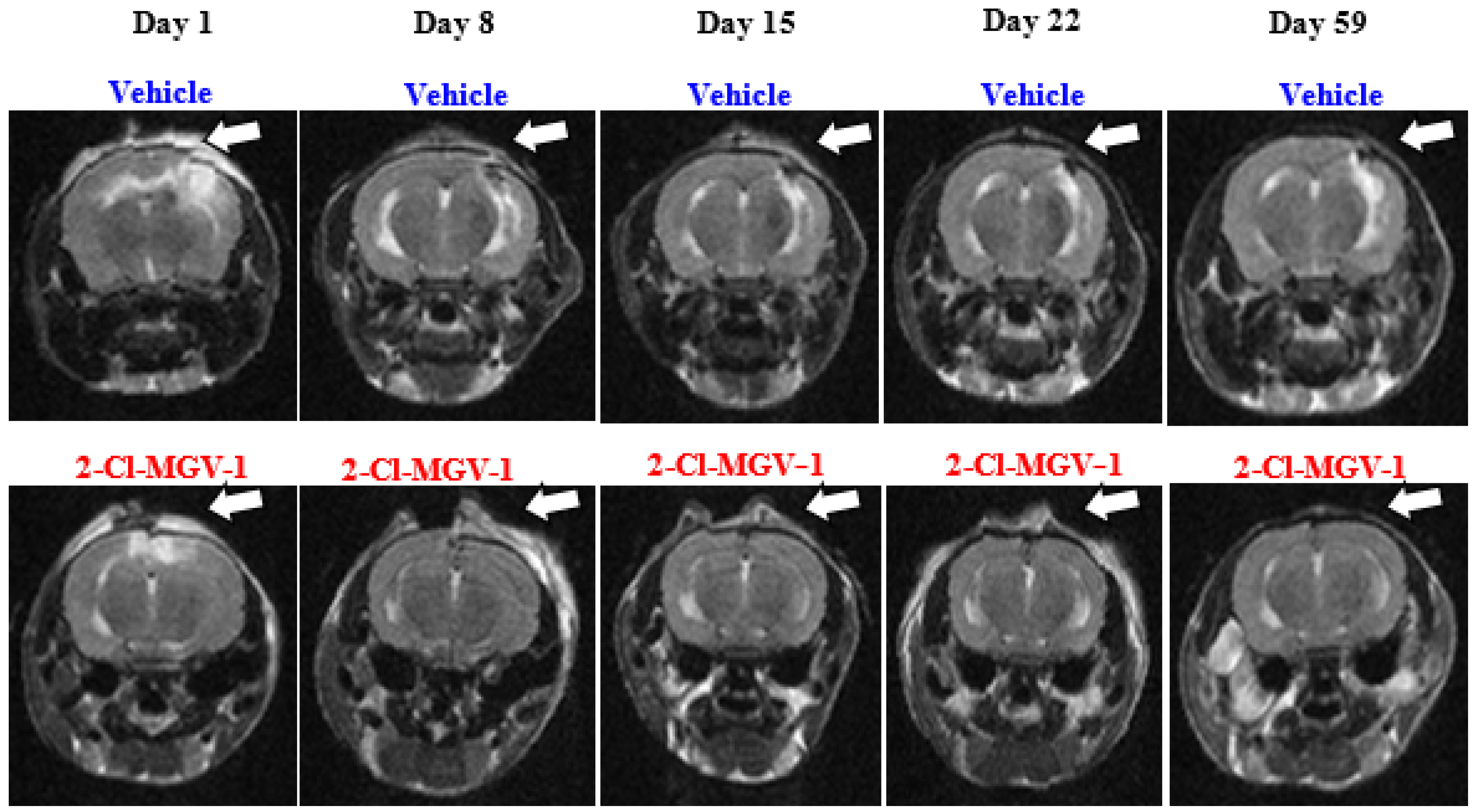
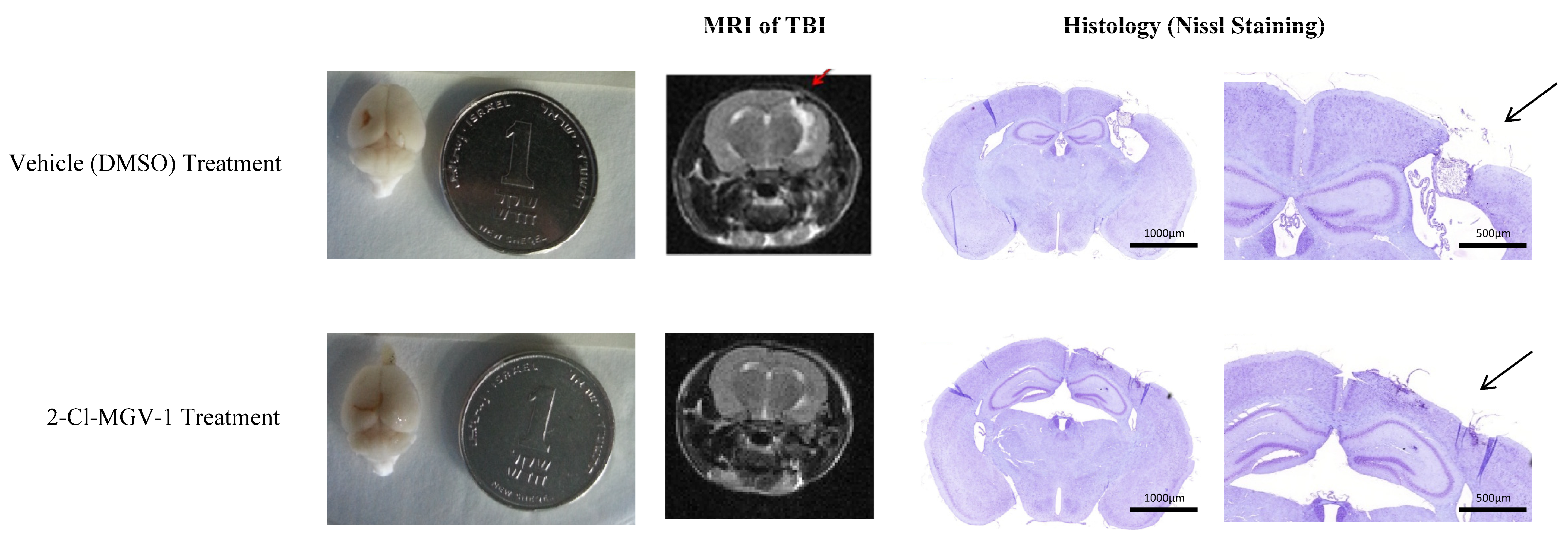
2.1. Experiment 1
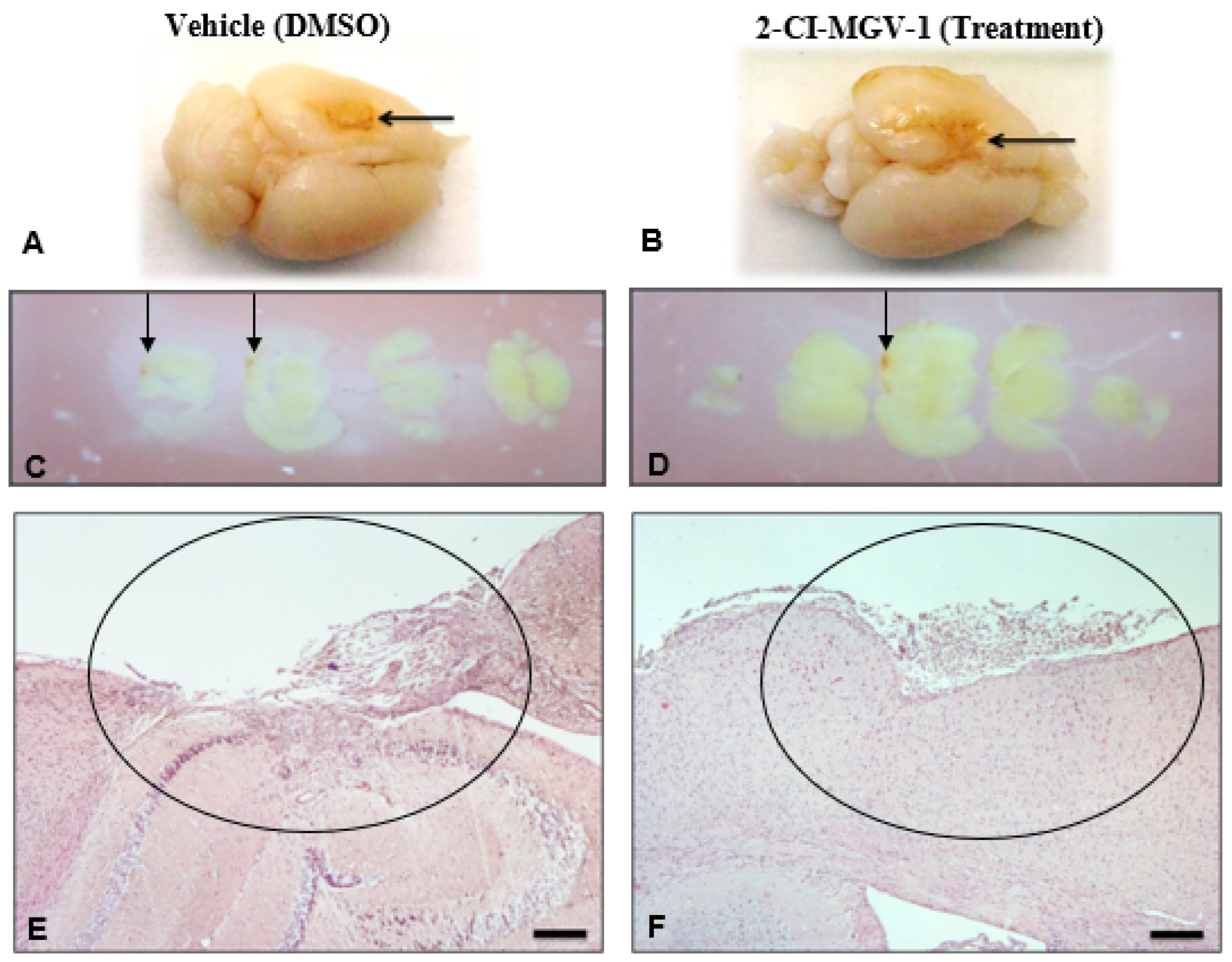
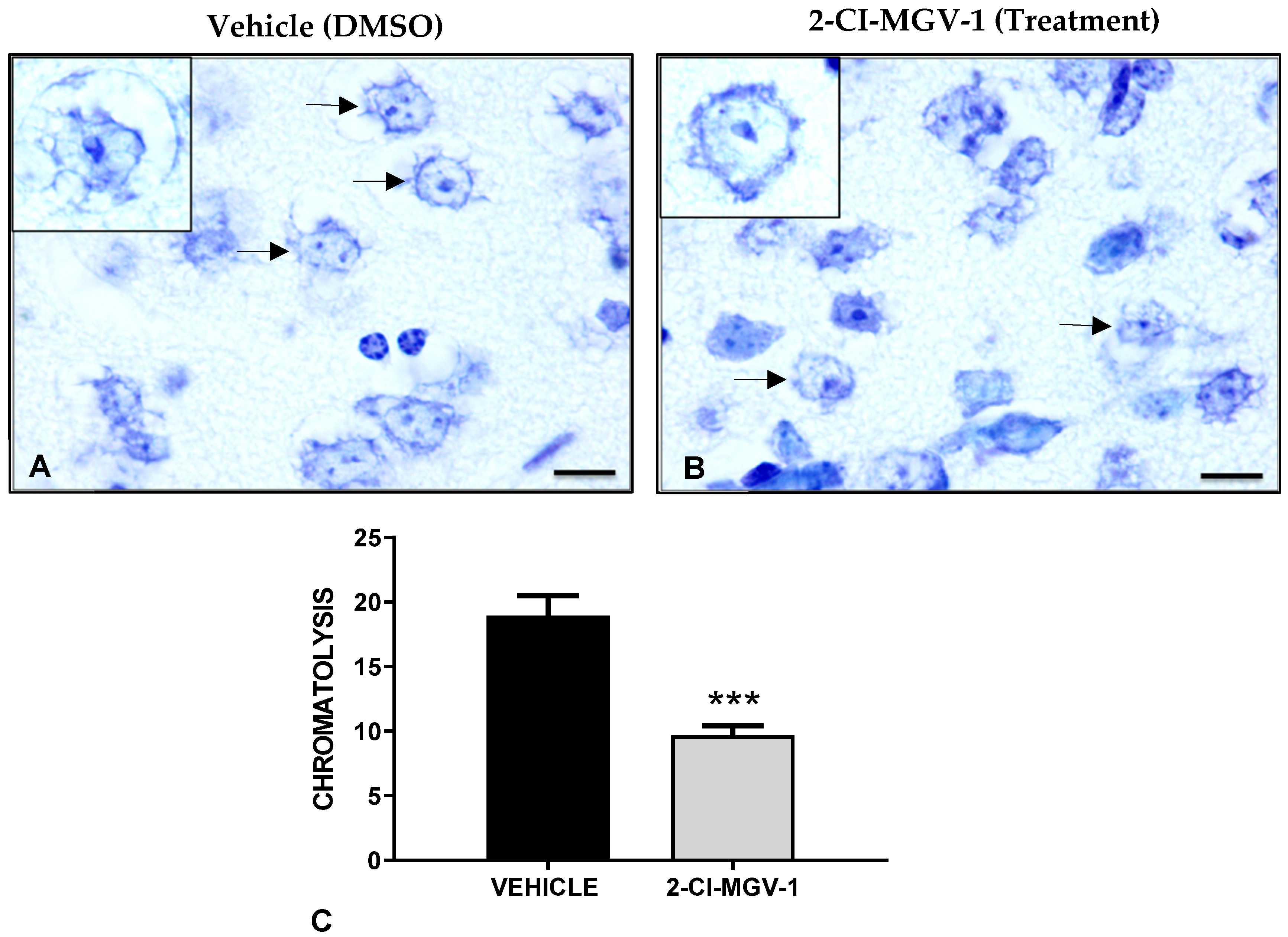
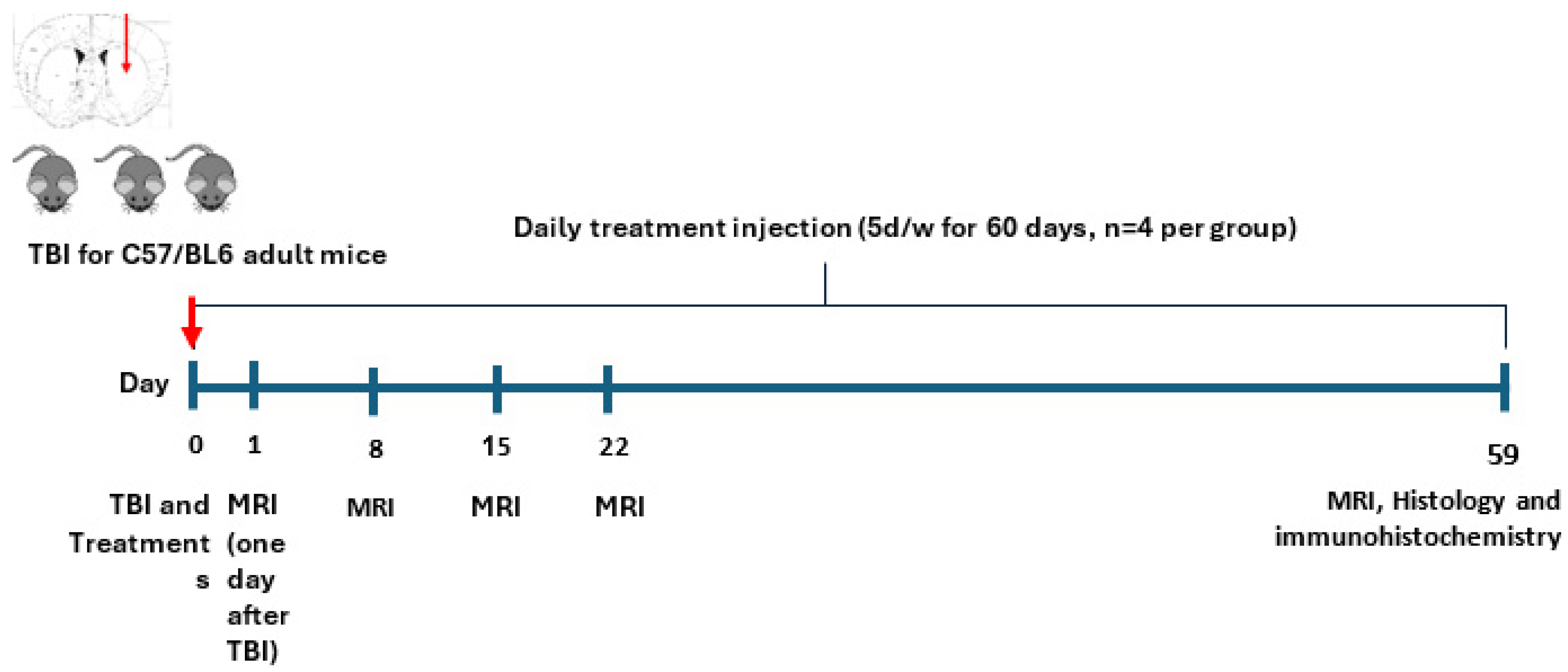
2.2. Experiment 2
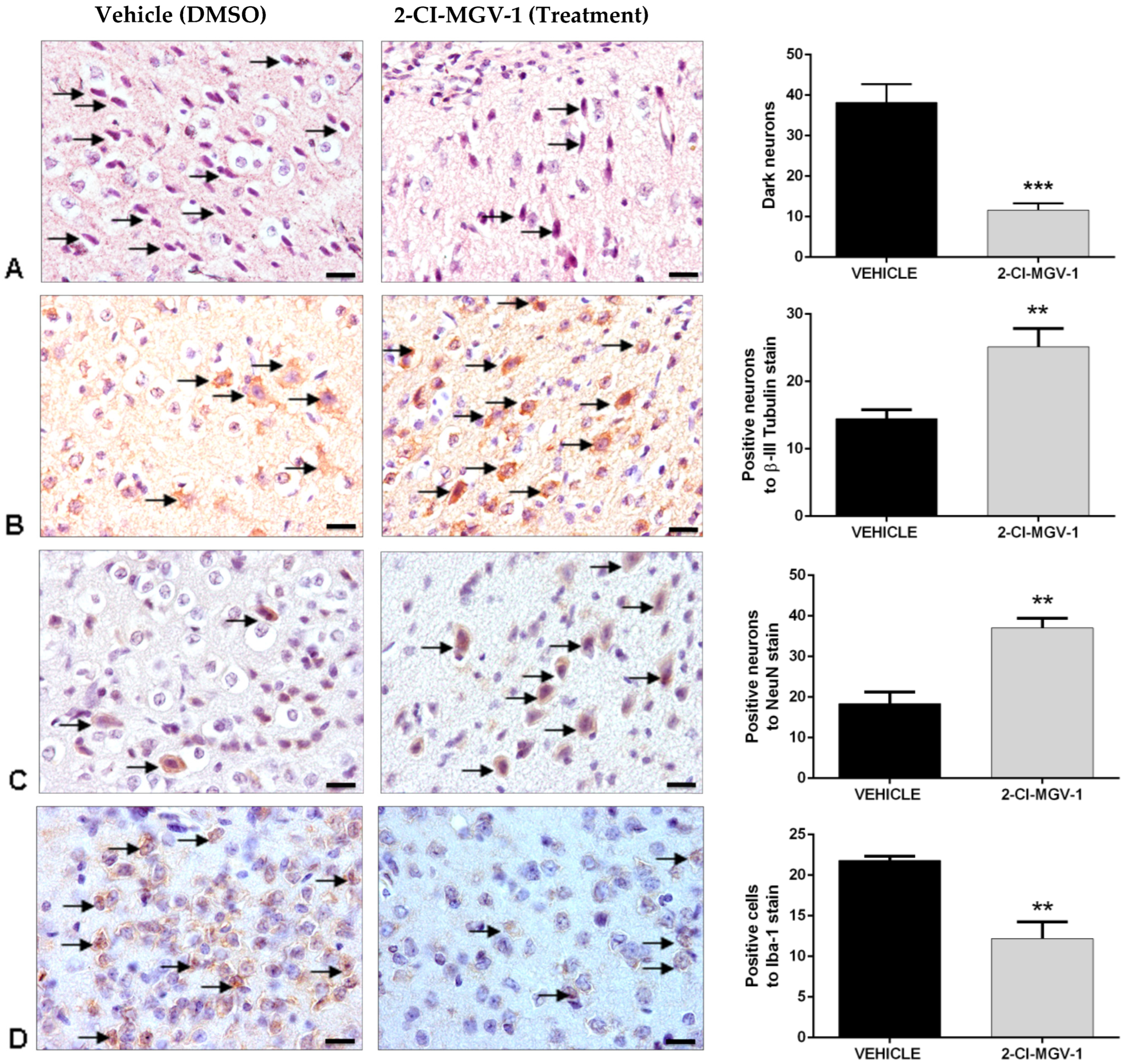
Neurohistological Results, Experiment 2, Young Mice (~20 gr)
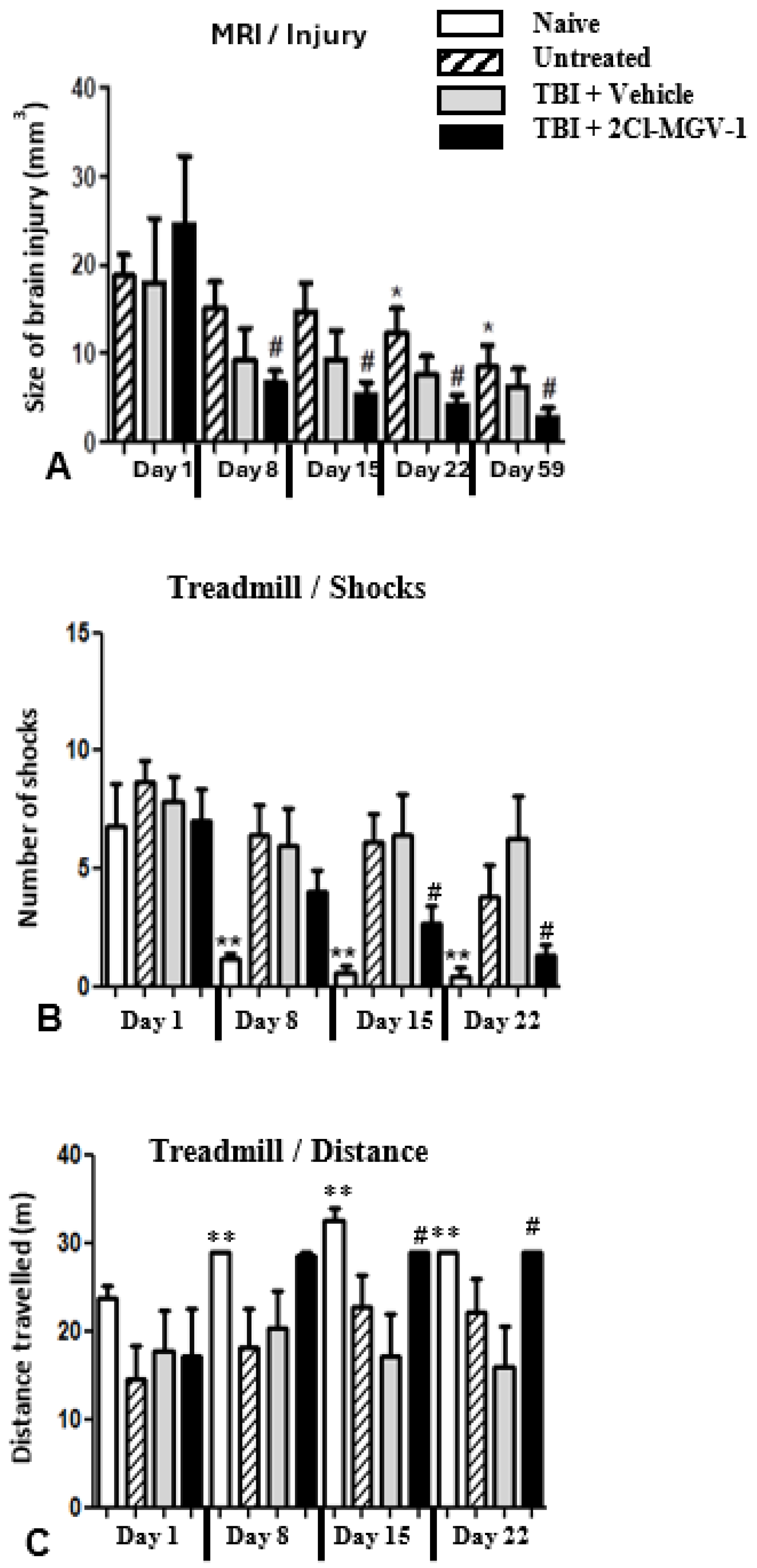
2.3. Experiment 3
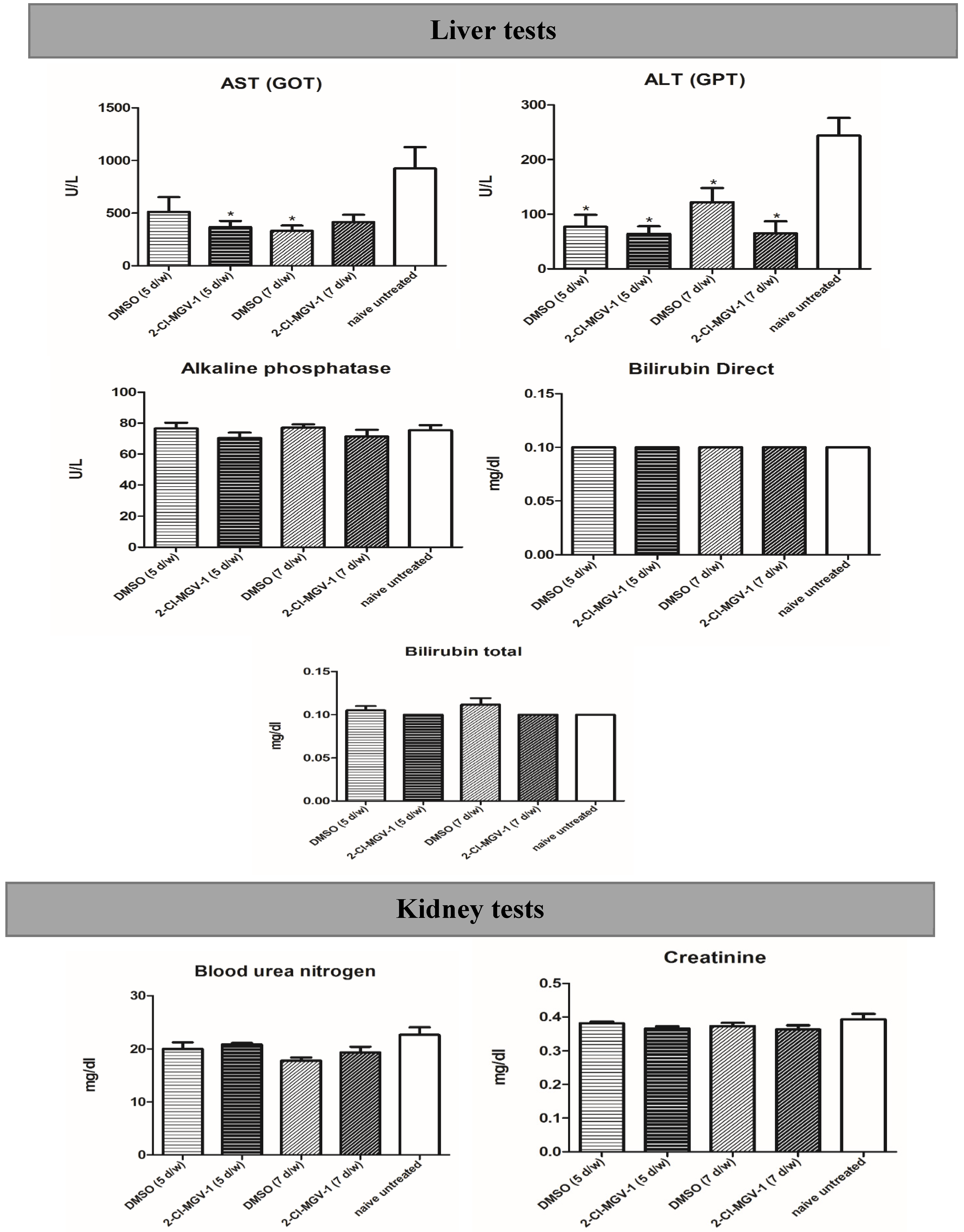
2.4. Experiment 4
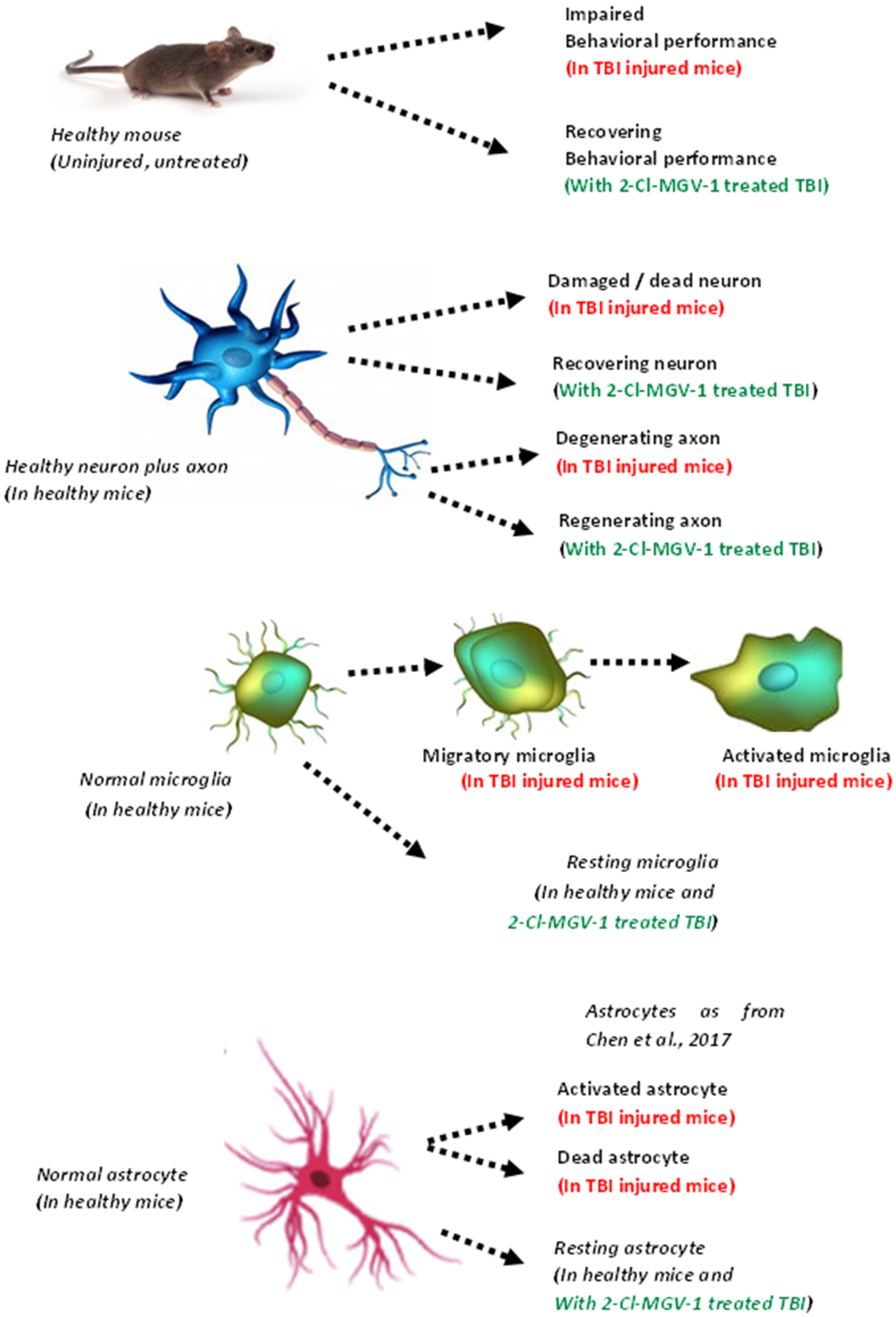
3. Discussion
3.1. Comparison of 2-Cl-MGV-1 Efficacy with Other TSPO Ligands
3.2. Other Neurological Disorders Where 2-Cl-MGV-1 May Be Beneficial
4. Materials and Methods
4.1. Mouse Description
4.2. Study Design
4.3. Tool Description
Weight Drop Apparatus for Induction of TBI
4.4. Magnetic Resonance Imaging (MRI)
4.4.1. Calculation of the MRI Signal
4.4.2. Treadmill
- The number of shocks an animal received (where the higher the number of shocks, the more severe the motor impairment)
- The maximal running distance (where the longer the distance, the less the motor impairment).
4.5. Treatment
4.6. Sacrifice of Mice and Fixation of Brains
4.7. Experiments on Traumatic Brain Injury and Safety of 2-Cl-MGV-1 Ligand
4.7.1. Experiment 1 (Preliminary)
Primary Goal: To Test Feasibility
Histological Evaluation of Brain Damage in Experiment 1
4.7.2. Experiment 2
Histological Evaluation of Brain Damage in Experiment 2
Preparation of Brain Sections for Immunohistochemical Analyses in Experiment 2
Microscopy and Image Quantification in Experiment 2
4.7.3. Experiment 3
Primary Goals: Assessment of the Protective Effect of 2-Cl-MGV-1 Treatment on TBI Behavioral Outcomes
Statistical Analysis
4.7.4. Experiment 4
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quatman-Yates, C.C.; Hunter-Giordano, A.; Shimamura, K.K.; Landel, R.; Alsalaheen, B.A.; Hanke, T.A.; McCulloch, K.L. Physical Therapy Evaluation and Treatment After Concussion/Mild Traumatic Brain Injury. J. Orthop. Sports Phys. Ther. 2020, 50, Cpg1–Cpg73. [Google Scholar] [CrossRef] [PubMed]
- Quatman-Yates, C.; Cupp, A.; Gunsch, C.; Haley, T.; Vaculik, S.; Kujawa, D. Physical Rehabilitation Interventions for Post-mTBI Symptoms Lasting Greater Than 2 Weeks: Systematic Review. Phys. Ther. 2016, 96, 1753–1763. [Google Scholar] [CrossRef]
- Coronado, V.G.; Xu, L.; Basavaraju, S.V.; McGuire, L.C.; Wald, M.M.; Faul, M.D.; Guzman, B.R.; Hemphill, J.D. Surveillance for traumatic brain injury-related deaths: United States, 1997–2007. MMWR Surveill. Summ. 2011, 60, 1–32. [Google Scholar] [PubMed]
- Breunig, J.J.; Guillot-Sestier, M.V.; Town, T. Brain injury, neuroinflammation and Alzheimer’s disease. Front. Aging Neurosci. 2013, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Diagnostic and Therapeutic Potential of TSPO Studies Regarding Neurodegenerative Diseases, Psychiatric Disorders, Alcohol Use Disorders, Traumatic Brain Injury, and Stroke: An Update. Cells 2020, 9, 870. [Google Scholar] [CrossRef]
- Monga, S.; Nagler, R.; Amara, R.; Weizman, A.; Gavish, M. Inhibitory Effects of the Two Novel TSPO Ligands 2-Cl-MGV-1 and MGV-1 on LPS-induced Microglial Activation. Cells 2019, 8, 486. [Google Scholar] [CrossRef]
- Darkazalli, A.; Vied, C.; Badger, C.D.; Levenson, C.W. Human Mesenchymal Stem Cell Treatment Normalizes Cortical Gene Expression after Traumatic Brain Injury. J. Neurotrauma 2017, 34, 204–212. [Google Scholar] [CrossRef]
- Rupprecht, R.; Papadopoulos, V.; Rammes, G.; Baghai, T.C.; Fan, J.; Akula, N.; Groyer, G.; Adams, D.; Schumacher, M. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2010, 9, 971–988. [Google Scholar] [CrossRef]
- Caballero, B.; Veenman, L.; Bode, J.; Leschiner, S.; Gavish, M. Concentration-dependent bimodal effect of specific 18 kDa translocator protein (TSPO) ligands on cell death processes induced by ammonium chloride: Potential implications for neuropathological effects due to hyperammonemia. CNS Neurol. Disord. Drug Targets 2014, 13, 574–592. [Google Scholar] [CrossRef]
- Vainshtein, A.; Veenman, L.; Shterenberg, A.; Singh, S.; Masarwa, A.; Dutta, B.; Island, B.; Tsoglin, E.; Levin, E.; Leschiner, S.; et al. Quinazoline-based tricyclic compounds that regulate programmed cell death, induce neuronal differentiation, and are curative in animal models for excitotoxicity and hereditary brain disease. Cell Death Discov. 2015, 1, 15027. [Google Scholar] [CrossRef]
- Simon-O’Brien, E.; Gauthier, D.; Riban, V.; Verleye, M. Etifoxine improves sensorimotor deficits and reduces glial activation, neuronal degeneration, and neuroinflammation in a rat model of traumatic brain injury. J. Neuroinflamm. 2016, 13, 203. [Google Scholar] [CrossRef]
- Shehadeh, M.; Palzur, E.; Apel, L.; Soustiel, J.F. Reduction of Traumatic Brain Damage by Tspo Ligand Etifoxine. Int. J. Mol. Sci. 2019, 20, 2639. [Google Scholar] [CrossRef] [PubMed]
- Soustiel, J.F.; Palzur, E.; Vlodavsky, E.; Veenman, L.; Gavish, M. The effect of oxygenation level on cerebral post-traumatic apoptotsis is modulated by the 18-kDa translocator protein (also known as peripheral-type benzodiazepine receptor) in a rat model of cortical contusion. Neuropathol. Appl. Neurobiol. 2008, 34, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Veenman, L.; Singh, S.; Ouyang, F.; Liang, J.; Huang, W.; Marek, I.; Zeng, J.; Gavish, M. 2-Cl-MGV-1 Ameliorates Apoptosis in the Thalamus and Hippocampus and Cognitive Deficits After Cortical Infarct in Rats. Stroke 2017, 48, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
- Unterrainer, M.; Mahler, C.; Vomacka, L.; Lindner, S.; Havla, J.; Brendel, M.; Böning, G.; Ertl-Wagner, B.; Kümpfel, T.; Milenkovic, V.M.; et al. TSPO PET with [(18)F]GE-180 sensitively detects focal neuroinflammation in patients with relapsing-remitting multiple sclerosis. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1423–1431. [Google Scholar] [CrossRef]
- Veenman, L.; Bode, J.; Gaitner, M.; Caballero, B.; Pe’er, Y.; Zeno, S.; Kietz, S.; Kugler, W.; Lakomek, M.; Gavish, M. Effects of 18-kDa translocator protein knockdown on gene expression of glutamate receptors, transporters, and metabolism, and on cell viability affected by glutamate. Pharmacogenet. Genom. 2012, 22, 606–619. [Google Scholar] [CrossRef]
- Yasin, N.; Veenman, L.; Singh, S.; Azrad, M.; Bode, J.; Vainshtein, A.; Caballero, B.; Marek, I.; Gavish, M. Classical and Novel TSPO Ligands for the Mitochondrial TSPO Can Modulate Nuclear Gene Expression: Implications for Mitochondrial Retrograde Signaling. Int. J. Mol. Sci. 2017, 18, 786. [Google Scholar] [CrossRef]
- Khalin, I.; Jamari, N.L.; Razak, N.B.; Hasain, Z.B.; Nor, M.A.; Zainudin, M.H.; Omar, A.B.; Alyautdin, R. A mouse model of weight-drop closed head injury: Emphasis on cognitive and neurological deficiency. Neural Regen. Res. 2016, 11, 630–635. [Google Scholar] [CrossRef]
- Lecca, D.; Bader, M.; Tweedie, D.; Hoffman, A.F.; Jung, Y.J.; Hsueh, S.C.; Hoffer, B.J.; Becker, R.E.; Pick, C.G.; Lupica, C.R.; et al. (-)-Phenserine and the prevention of pre-programmed cell death and neuroinflammation in mild traumatic brain injury and Alzheimer’s disease challenged mice. Neurobiol. Dis. 2019, 130, 104528. [Google Scholar] [CrossRef]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential Beneficial Actions of Fucoidan in Brain and Liver Injury, Disease, and Intoxication-Potential Implication of Sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef]
- Veenman, L. Raloxifene as Treatment for Various Types of Brain Injuries and Neurodegenerative Diseases: A Good Start. Int. J. Mol. Sci. 2020, 21, 7586. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xia, Y.; Meiler, J.; Ferguson-Miller, S. Characterization and modeling of the oligomeric state and ligand binding behavior of purified translocator protein 18 kDa from Rhodobacter sphaeroides. Biochemistry 2013, 52, 5884–5899. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef] [PubMed]
- Bandiwadekar, A.; Jose, J.; Khayatkashani, M.; Habtemariam, S.; Khayat Kashani, H.R.; Nabavi, S.M. Emerging Novel Approaches for the Enhanced Delivery of Natural Products for the Management of Neurodegenerative Diseases. J. Mol. Neurosci. 2022, 72, 653–676. [Google Scholar] [CrossRef]
- Azrad, M.; Zeineh, N.; Weizman, A.; Veenman, L.; Gavish, M. The TSPO Ligands 2-Cl-MGV-1, MGV-1, and PK11195 Differentially Suppress the Inflammatory Response of BV-2 Microglial Cell to LPS. Int. J. Mol. Sci. 2019, 20, 594. [Google Scholar] [CrossRef]
- Veenman, L.; Alten, J.; Linnemannstöns, K.; Shandalov, Y.; Zeno, S.; Lakomek, M.; Gavish, M.; Kugler, W. Potential involvement of F0F1-ATP(synth)ase and reactive oxygen species in apoptosis induction by the antineoplastic agent erucylphosphohomocholine in glioblastoma cell lines: A mechanism for induction of apoptosis via the 18 kDa mitochondrial translocator protein. Apoptosis 2010, 15, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Zeno, S.; Zaaroor, M.; Leschiner, S.; Veenman, L.; Gavish, M. CoCl(2) induces apoptosis via the 18 kDa translocator protein in U118MG human glioblastoma cells. Biochemistry 2009, 48, 4652–4661. [Google Scholar] [CrossRef]
- Veenman, L.; Vainshtein, A.; Gavish, M. TSPO as a target for treatments of diseases, including neuropathological disorders. Cell Death Dis. 2015, 6, e1911. [Google Scholar] [CrossRef]
- Flierl, M.A.; Stahel, P.F.; Beauchamp, K.M.; Morgan, S.J.; Smith, W.R.; Shohami, E. Mouse closed head injury model induced by a weight-drop device. Nat. Protoc. 2009, 4, 1328–1337. [Google Scholar] [CrossRef]
- Akçay, G. Weight Drop Models in Traumatic Brain Injury. Middle Black Sea J. Health Sci. 2023, 9, 375–384. [Google Scholar] [CrossRef]
- Navarro, A.; Tolivia, J.; Valle, E.D. Congo Red Method for Demonstrating Amyloid in Paraffin Sections. J. Histotechnol. 1999, 22, 305–308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasin, N.; Veenman, L.; Caballero, B.; Zeineh, N.; Gonzalez-Blanco, L.; Weizman, A.; Gavish, M. TSPO Ligand 2-Cl-MGV-1 Mitigates Traumatic Brain Injury (TBI) in a Mouse Model. Int. J. Mol. Sci. 2025, 26, 4854. https://doi.org/10.3390/ijms26104854
Yasin N, Veenman L, Caballero B, Zeineh N, Gonzalez-Blanco L, Weizman A, Gavish M. TSPO Ligand 2-Cl-MGV-1 Mitigates Traumatic Brain Injury (TBI) in a Mouse Model. International Journal of Molecular Sciences. 2025; 26(10):4854. https://doi.org/10.3390/ijms26104854
Chicago/Turabian StyleYasin, Nasra, Leo Veenman, Beatriz Caballero, Nidal Zeineh, Laura Gonzalez-Blanco, Abraham Weizman, and Moshe Gavish. 2025. "TSPO Ligand 2-Cl-MGV-1 Mitigates Traumatic Brain Injury (TBI) in a Mouse Model" International Journal of Molecular Sciences 26, no. 10: 4854. https://doi.org/10.3390/ijms26104854
APA StyleYasin, N., Veenman, L., Caballero, B., Zeineh, N., Gonzalez-Blanco, L., Weizman, A., & Gavish, M. (2025). TSPO Ligand 2-Cl-MGV-1 Mitigates Traumatic Brain Injury (TBI) in a Mouse Model. International Journal of Molecular Sciences, 26(10), 4854. https://doi.org/10.3390/ijms26104854









