Icariin Ameliorates Cyclophosphamide-Induced Renal Encephalopathy by Modulating the NF-κB and Keap1-Nrf2 Signaling Pathways
Abstract
1. Introduction
2. Results
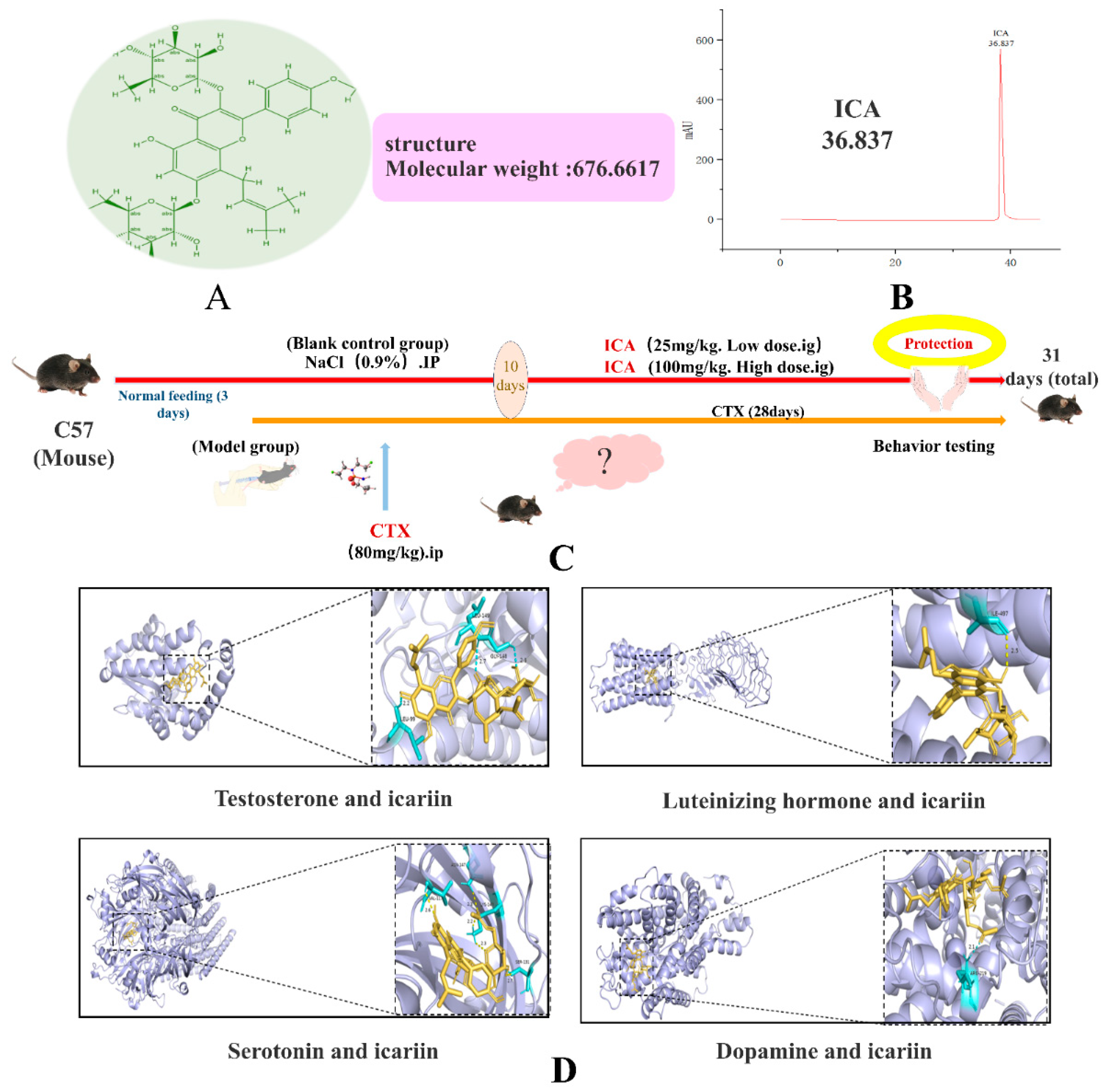
2.1. Molecular Docking Results
2.2. In Vivo Results
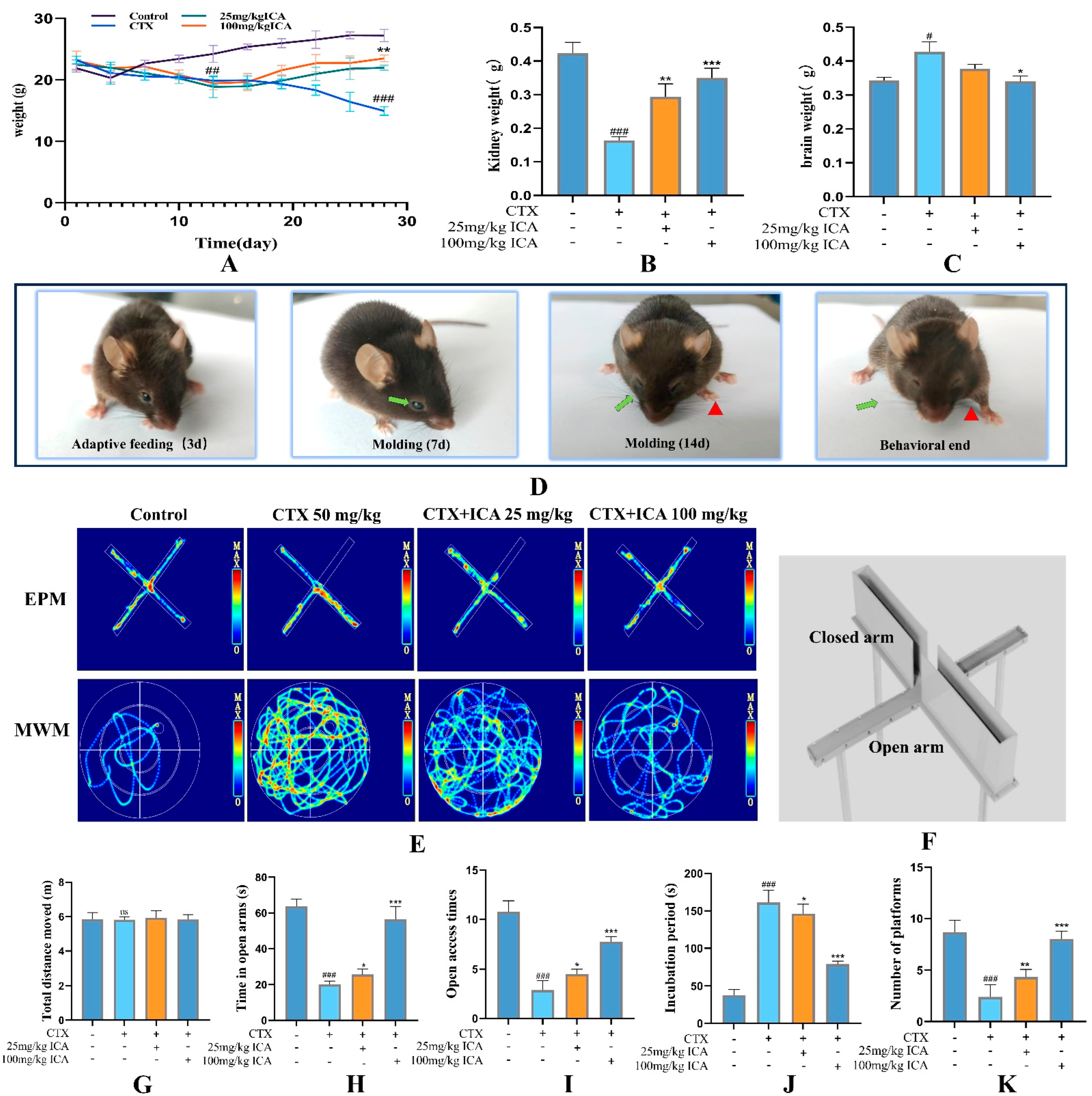
2.2.1. ICA Alleviates CTX-Induced Spatial Learning Memory in RE Mice
2.2.2. Serum, Brain, and Kidney Tissue-Related Indicator Tests
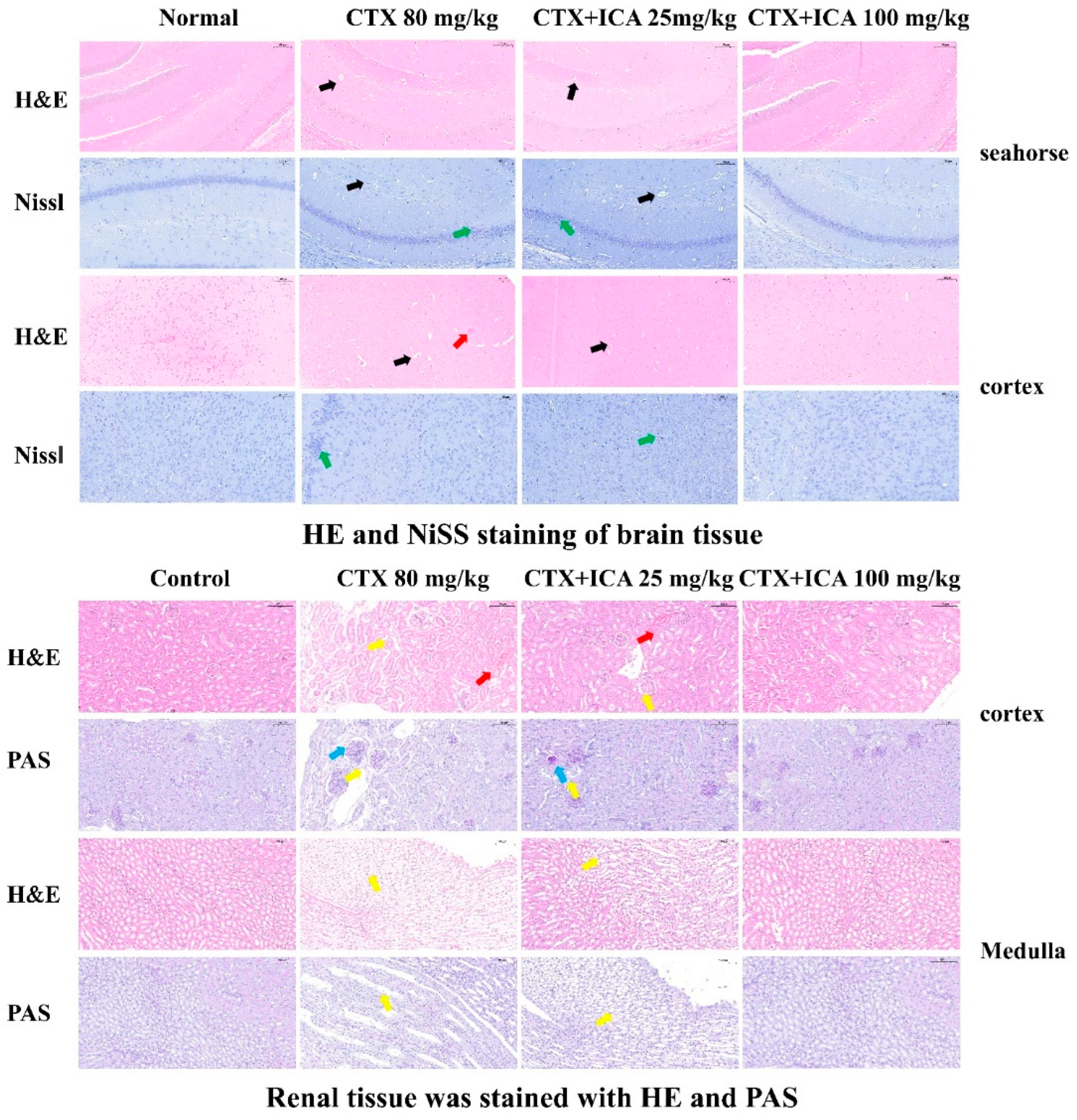
2.2.3. H&E, PAS, and Nissl Stain Results
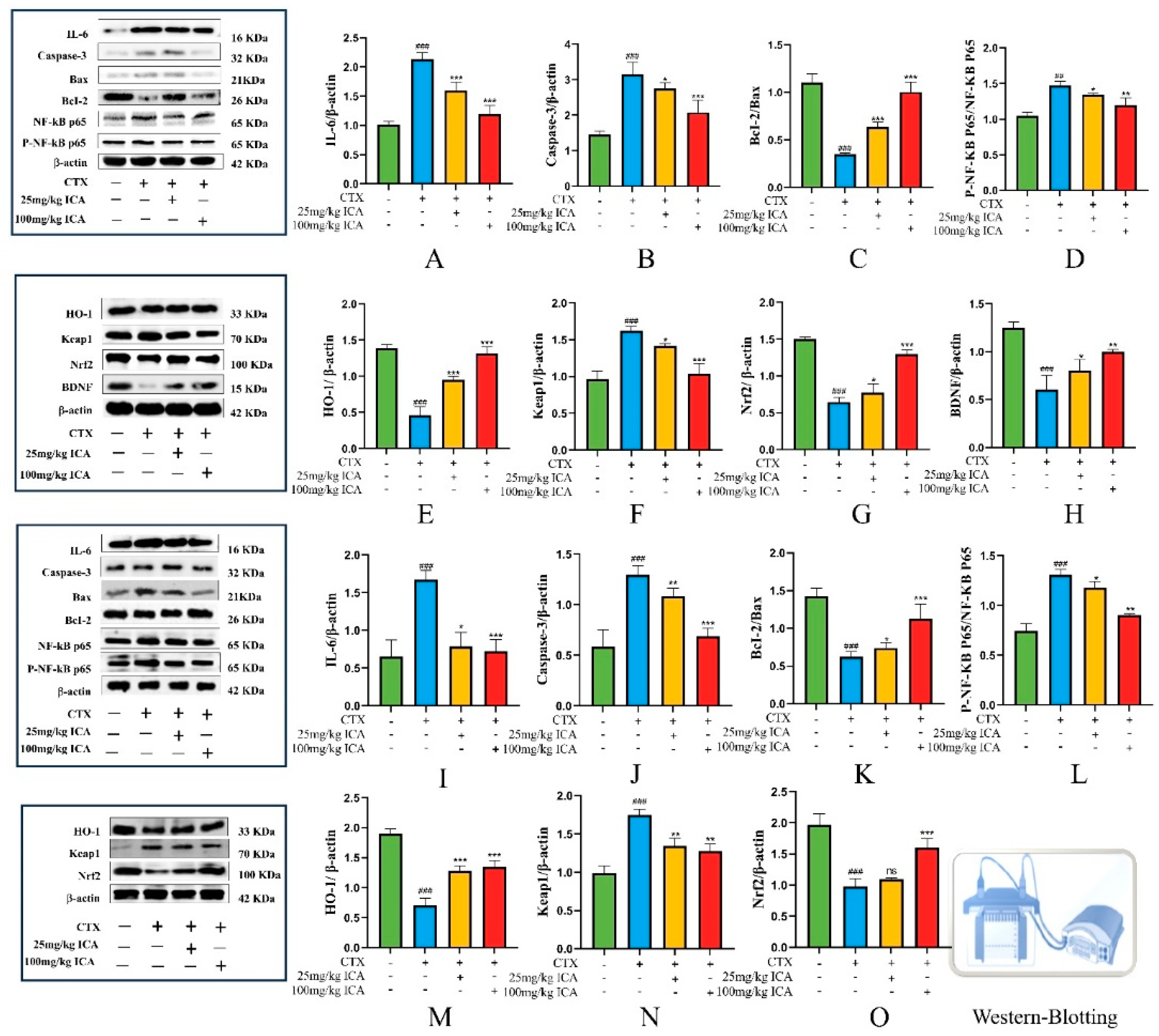
2.2.4. Western Protein Blotting Results for Brain and Kidney Tissues
2.3. In Vitro Results
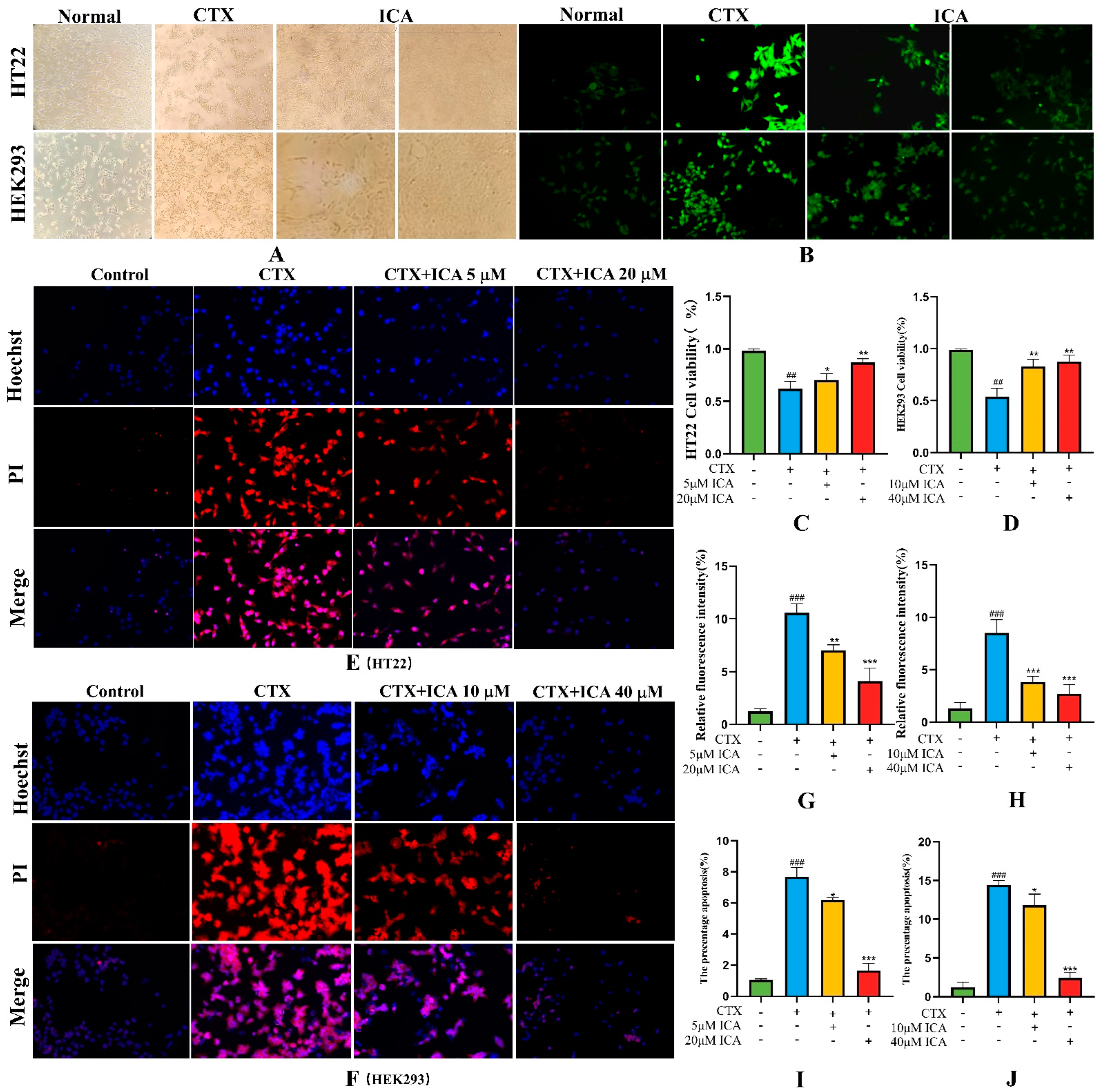
2.3.1. Effect of ICA on CTX Injury to HT22 and HEK293 Cell Viability
2.3.2. Intracellular ROS Detection
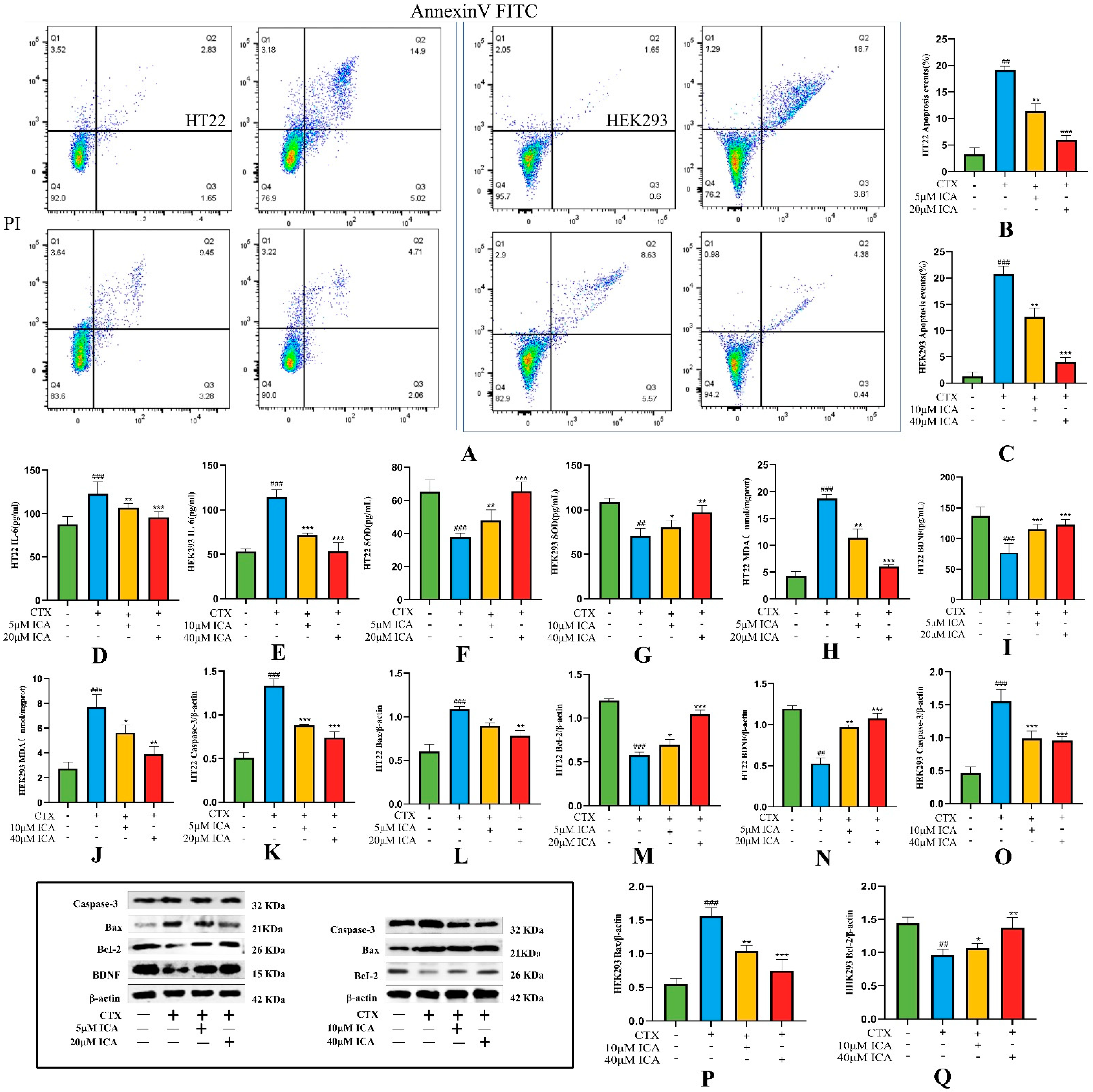
2.3.3. Effect of ICA on CTX-Induced Apoptosis
2.3.4. Detection of Biochemical Indicators
2.3.5. Western Protein Blotting Assay for Apoptosis-Related Proteins Caspase-3, Bax, Bcl-2, and BDNF Expression Levels
3. Discussion
4. Methods and Materials
4.1. Molecular Docking
4.2. Drugs and Reagents
4.3. Instrumentation
4.4. In Vivo Experiments
4.4.1. Grouping and Processing of Animals
4.4.2. Behavioral Tests
- 1.
- Elevated plus maze, EPM
- 2.
- Morris water maze, MWM
- 3.
- Radial arm maze test, RMT
- 4.
- Testing serum and brain and kidney tissue (biochemical indicators)
- 5.
- Histopathological observations
- 6.
- Western blotting
4.5. In Vitro Experiments
4.5.1. Cell Recovery and Culture
4.5.2. Screening for Safe Drug Concentrations
4.5.3. Detection of Cell Viability by CCK-8 Assay
4.5.4. ROS Detection
4.5.5. Hoechst 33342/PI, Annexin V-FITC/PI Staining, and Flow-Through Detection of Apoptosis
4.5.6. Detection of Biochemical Indicators
4.5.7. Cell Western Blotting
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICA | Icariin |
| CTX | cyclophosphamide |
| ROS | reactive oxygen species |
| BDNF | brain-derived neurotrophic factor |
| MDA | malondialdehyde |
| IL-6 | Interleukin-6 |
| NO | nitric oxide |
| SOD | superoxide dismutase |
| DMEM | Dulbecco’s modified eagle medium |
| Cre | creatinine |
| UA | uric acid |
| BUN | urea nitrogen |
| Nissl | Nissant staining |
| PAS | periodic acid–Schiff |
| HE | hematoxylin–eosin |
| FSH | follicle-stimulating hormone |
| LH | luteinizing hormone |
| T | testosterone |
| DA | dopamine |
| 5-HT | serotonin |
| AD | Alzheimer’s disease |
References
- Salama, A.; Elgohary, R.; Amin, M.M.; Elwahab, S.A. Immunomodulatory effect of protocatechuic acid on cyclophosphamide induced brain injury in rat: Modulation of inflammosomes NLRP3 and SIRT1. Eur. J. Pharmacol. 2022, 932, 175217. [Google Scholar] [CrossRef] [PubMed]
- Krukowski, K.; Chou, A.; Jopson, T.; Zhu, P.J.; Costa-Mattioli, M.; Walter, P.; Rosi, S. Abstract # 2056 Inhibition of the integrated stress response reverses cognitive deficits after traumatic brain injury. Brain Behav. Immun. 2019, 76, e5. [Google Scholar] [CrossRef]
- Alam, M.F.; Ajeibi, A.O.; Safhi, M.H.; Alabdly, A.J.A.; Alshahrani, S.; Rashid, H.; Qadri, M.; Jali, A.M.; Alqahtani, S.; Nomier, Y.; et al. Therapeutic Potential of Capsaicin against Cyclophosphamide-Induced Liver Damage. J. Clin. Med. 2023, 12, 911. [Google Scholar] [CrossRef]
- Peres, T.; Aeppli, S.; Fischer, S.; Gysel, K.; Rothermundt, C. Metronomic cyclophosphamide for bone marrow carcinomatosis in metastatic castration-resistant prostate cancer. J. Cancer Res. Clin. Oncol. 2024, 150, 84. [Google Scholar] [CrossRef] [PubMed]
- Cousin, S.; Toulmonde, M.; Kind, M.; Guegan, J.-P.; Bessede, A.; Cantarel, C.; Bellera, C.; Italiano, A. Phase 2 trial of intravenous oncolytic virus JX-594 combined with low-dose cyclophosphamide in patients with advanced breast cancer. Exp. Hematol. Oncol. 2022, 11, 104. [Google Scholar] [CrossRef]
- Webb, E.R.; Moreno-Vicente, J.; Easton, A.; Lanati, S.; Taylor, M.; James, S.; Williams, E.L.; English, V.; Penfold, C.; Beers, S.A.; et al. Cyclophosphamide depletes tumor infiltrating T regulatory cells and combined with anti-PD-1 therapy improves survival in murine neuroblastoma. iScience 2022, 25, 104995. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, X.; Hou, X.; Wei, H.; Zhai, J.; Xia, T.; Gong, X.; Gao, S.; Feng, G.; Tao, X.; et al. Wuzhi capsule regulates chloroacetaldehyde pharmacokinetics behaviour and alleviates high-dose cyclophosphamide-induced nephrotoxicity and neurotoxicity in rats. Basic Clin. Pharmacol. Toxicol. 2019, 125, 142–151. [Google Scholar] [CrossRef]
- Rabie, O.; El-Nashar, H.A.; George, M.Y.; Majrashi, T.A.; Al-Warhi, T.; Hassan, F.E.; Eldehna, W.M.; Mostafa, N.M. Phytochemical profiling and neuroprotective activity of Callistemon subulatus leaves against cyclophosphamide-induced chemobrain. Biomed. Pharmacother. 2023, 167, 115596. [Google Scholar] [CrossRef]
- Kshirsagar, S.R.; Gondake, S.P.; Shinde, S.I.; Hait, M. Probing a lead inhibitor from the Thymus vulgaris against the Alzheimer protein: Human beta secretase. ES Mater. Manuf. 2023, 20, 832. [Google Scholar] [CrossRef]
- Fani, F.; Hosseinimehr, S.J.; Zargari, M.; Mirzaei, M.; Malekshah, A.K.; Amiri, F.T. Piperine mitigates oxidative stress, inflammation, and apoptosis in the testicular damage induced by cyclophosphamide in mice. J. Biochem. Mol. Toxicol. 2024, 38, e23696. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Kow, A.S.F.; Yusof, R.; Tham, C.L.; Ho, Y.-C.; Lee, M.T. Menopause-Associated Depression: Impact of Oxidative Stress and Neuroinflammation on the Central Nervous System—A Review. Biomedicines 2024, 12, 184. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Wang, F.; Wang, L. Sex-Dimorphic Kidney-Brain Connectivity Map of Mice. Neurosci. Bull. 2024, 40, 1445–1457. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Wilson, L.E.; Greiner, M.A.; Pritchard, J.E.; Zhang, T.; Kaye, D.R.; Cohen, H.J.; Becher, R.D.; Maerz, L.L.; Dinan, M.A. Evaluation of mild cognitive impairment and dementia in patients with metastatic renal cell carcinoma. J. Geriatr. Oncol. 2022, 13, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Khezri, M.R.; Ghasemnejad-Berenji, M. Icariin: A Potential Neuroprotective Agent in Alzheimer’s Disease and Parkinson’s Disease. Neurochem. Res. 2022, 47, 2954–2962. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Hua, X.; Chen, D.; Huang, C.; Chen, Z. An outline for the pharmacological effect of icariin in the nervous system. Eur. J. Pharmacol. 2018, 842, 20–32. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Li, J.-R.; Wang, Y.-Y.; Lin, S.-Y.; Ou, Y.-C.; Lin, C.-J.; Wang, J.-D.; Liao, S.-L.; Chen, C.-J. p-Cresol Sulfate Caused Behavior Disorders and Neurodegeneration in Mice with Unilateral Nephrectomy Involving Oxidative Stress and Neuroinflammation. Int. J. Mol. Sci. 2020, 21, 6687. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, S.; Min, P.; Li, H.; Zhao, F.; Liu, M.; Jin, W.; Wang, L.; Zhao, J.; Jia, L. Protective effect of Inonotus obliquus polysaccharide on mice infected with Neospora caninum. Int. J. Biol. Macromol. 2024, 261, 129906. [Google Scholar] [CrossRef]
- de Abreu, I.C.M.E.; de Albuquerque, R.C.M.F.; Brandão, A.B.P.; Barssotti, L.; de Souza, L.B.; Ferreira, F.G.; de Oliveira, L.C.G.; Yokota, R.; Sparvoli, L.G.; Dias, D.d.S.; et al. Saccharomyces boulardii exerts renoprotection by modulating oxidative stress, renin angiotensin system and uropathogenic microbiota in a murine model of diabetes. Life Sci. 2022, 301, 120616. [Google Scholar] [CrossRef]
- Singh, A.D.; Chawda, M.; A Kulkarni, Y. Vasant Kusumakar Rasa Ameliorates Diabetic Encephalopathy by Reducing Oxidative Stress and Neuroinflammation and Improving Neurotransmitter Levels in Experimental Animals. Cureus 2024, 6, e75905. [Google Scholar] [CrossRef]
- Dinh, P.; Tran, C.; Dinh, T.; Ha, H.-A.; Utegenova, A.; Ali, A.; Alamri, A. Identification and assessment of hub genes and miRNAs coregulatory associated with immune infiltrations and drug interactions in latent tuberculosis based on MicroarrayData analysis, molecular docking, and dynamic simulation. Biochem. Biophys. Rep. 2025, 41, 101952. [Google Scholar] [CrossRef] [PubMed]
- Czerska, M.; Mikołajewska, K.; Zieliński, M.; Gromadzińska, J.; Wąsowicz, W. Today’s oxidative stress markers. Med. Pr. 2015, 66, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ugoya, S.O.; Tu, J. Bench to Bedside of Neural Stem Cell in Traumatic Brain Injury. Stem Cells Int. 2012, 2012, 141624. [Google Scholar] [CrossRef]
- Chuang, J.-Y.; Kao, T.-J.; Lin, S.-H.; Wu, A.-C.; Lee, P.-T.; Su, T.-P.; Yeh, S.-H.; Lee, Y.-C.; Wu, C.-C.; Chang, W.-C. Specificity protein 1-zinc finger protein 179 pathway is involved in the attenuation of oxidative stress following brain injury. Redox Biol. 2017, 11, 135–143. [Google Scholar] [CrossRef]
- Zeller, M.V.; Dias, D.M.; Sebastio, A.M.; Valente, C.A. NLRP3 Inflammasome: A Starring Role in Amyloid-β- and Tau-Driven Pathological Events in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 83, 939–961. [Google Scholar] [CrossRef]
- Gan, W.-J.; Gao, C.-L.; Zhang, W.-Q.; Gu, J.-L.; Zhao, T.-T.; Guo, H.-L.; Zhou, H.; Xu, Y.; Yu, L.-L.; Li, L.-F.; et al. Kuwanon G protects HT22 cells from advanced glycation end product-induced damage. Exp. Ther. Med. 2021, 21, 425. [Google Scholar] [CrossRef]
- Shi, L.-Y.; Zhang, L.; Li, H.; Liu, T.-L.; Lai, J.-C.; Wu, Z.-B.; Qin, J. Protective effects of curcumin on acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Pharmacol. Rep. 2018, 70, 1040–1046. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, G.; He, J.; Yang, Q.; Li, D.; Li, J.; Zhang, F. Icariin attenuates neuroinflammation and exerts dopamine neuroprotection via an Nrf2-dependent manner. J. Neuroinflamm. 2019, 16, 92. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; O’Connor, J.C. Brain-derived neurotrophic factor and inflammation in depression: Pathogenic partners in crime? World J. Psychiatry 2022, 12, 77–97. [Google Scholar] [CrossRef]
- Dabrowska, S.; Andrzejewska, A.; Kozlowska, H.; Strzemecki, D.; Janowski, M.; Lukomska, B. Neuroinflammation evoked by brain injury in a rat model of lacunar infarct. Exp. Neurol. 2020, 336, 113531. [Google Scholar] [CrossRef]
- Liu, X.; Gu, J.; Wang, C.; Peng, M.; Zhou, J.; Fei, X.; Zhong, Z.; Li, B. Ginsenoside Rg3 attenuates neuroinflammation and hippocampal neuronal damage after traumatic brain injury in mice by inactivating the NF-kB pathway via SIRT1 activation. Cell Cycle 2024, 23, 662–681. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Clair, D.K.S.; Butterfield, D. Dysregulation of cytokine mediated chemotherapy induced cognitive impairment. Pharmacol. Res. 2017, 117, 267–273. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Zhang, W.-D.; Li, N.; Du, Z.-R.; Zhang, M.; Chen, S.; Chen, W.-F. IGF-1 receptor is involved in the regulatory effects of icariin and icaritin in astrocytes under basal conditions and after an inflammatory challenge. Eur. J. Pharmacol. 2021, 906, 174269. [Google Scholar] [CrossRef] [PubMed]
- Su, X.D.; Li, W.; Ma, J.Y.; Kim, Y.H. Chemical constituents from Epimedium koreanum Nakai and their chemotaxonomic significance. Nat. Prod. Res. 2017, 32, 2347–2351. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Mengxia, W.; Xiaoxia, Y.; Qian, Z.; Yingqi, G.; Yingxiu, C.; Linyang, S.; Junhua, Y.; Lixia, L.; Li, L. Neuroprotective Mechanism of Icariin on Hypoxic Ischemic Brain Damage in Neonatal Mice. Oxidative Med. Cell. Longev. 2022, 2022, 1330928. [Google Scholar]
- Tang, C.; Liu, X.; Zhu, H.; Lu, Q. Antagonizing effect of icaritin on apoptosis and injury of hippocampal neurocytes induced by amyloid beta via GR/BDNF signaling pathway. J. Recept. Signal Transduct. Res. 2020, 40, 550–559. [Google Scholar] [CrossRef]
- Conroy, A.L.; Datta, D.; Hoffmann, A.; Wassmer, S.C. The kidney–brain pathogenic axis in severe falciparum malaria. Trends Parasitol. 2023, 39, 191–199. [Google Scholar] [CrossRef]
- Ma, J.; Wang, F.; Yang, J.; Dong, Y.; Su, G.; Zhang, K.; Pan, X.; Ma, P.; Zhou, T.; Wu, C. Xiaochaihutang attenuates depressive/anxiety-like behaviors of social isolation-reared mice by regulating monoaminergic system, neurogenesis and BDNF expression. J. Ethnopharmacol. 2017, 208, 94–104. [Google Scholar] [CrossRef]
- Cao, L.-H.; Qiao, J.-Y.; Huang, H.-Y.; Fang, X.-Y.; Zhang, R.; Miao, M.-S.; Li, X.-M. PI3K–AKT Signaling Activation and Icariin: The Potential Effects on the Perimenopausal Depression-Like Rat Model. Molecules 2019, 24, 3700. [Google Scholar] [CrossRef] [PubMed]
- Kondaurova, E.M.; Bazovkina, D.V.; Naumenko, V.S. 5-HT1A/5-HT7 receptor interplay: Chronic activation of 5-HT7 receptors decreases the functional activity of 5-HT1A receptor and its content in the mouse brain. Mol. Biol. 2017, 51, 136–142. [Google Scholar] [CrossRef]
- Bokhary, T.; Refaat, B.; Bakr, E.-S.; Baz, S.; Rajab, B.; Gadalla, H.; El-Boshy, M. Salvadora persica extract attenuates cyclophosphamide-induced hepatorenal damage by modulating oxidative stress, inflammation and apoptosis in rats. J. Integr. Med. 2022, 20, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Heckler, C.E.; Thompson, B.D.; Gross, R.A.; Opanashuk, L.A.; Cory-Slechta, D.A. A clinically relevant dose of cyclophosphamide chemotherapy impairs memory performance on the delayed spatial alternation task that is sustained over time as mice age. NeuroToxicology 2016, 56, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Morid, O.F.; Menze, E.T.; Tadros, M.G.; George, M.Y. L-carnitine Modulates Cognitive Impairment Induced by Doxorubicin and Cyclophosphamide in Rats; Insights to Oxidative Stress, Inflammation, Synaptic Plasticity, Liver/brain, and Kidney/brain Axes. J. Neuroimmune Pharmacol. 2023, 18, 310–326. [Google Scholar] [CrossRef]
- Song, L.-J.; Han, Q.-X.; Ding, Z.-B.; Liu, K.; Zhang, X.-X.; Guo, M.-F.; Ma, D.; Wang, Q.; Xiao, B.-G.; Ma, C.-G. Icariin ameliorates the cuprizone-induced demyelination associated with antioxidation and anti-inflammation. Inflammopharmacology 2024, 32, 809–823. [Google Scholar] [CrossRef]
- Verma, A.; Aggarwal, K.; Agrawal, R.; Pradhan, K.; Goyal, A. Molecular mechanisms regulating the pharmacological actions of icariin with special focus on PI3K-AKT and Nrf-2 signaling pathways. Mol. Biol. Rep. 2022, 49, 9023–9032. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, S.; Jiang, H.; Liu, S.; Kong, F. DIA proteomics analysis reveals the mechanism of folic acid-induced acute kidney injury and the effects of icariin. Chem. Interactions 2024, 390, 110878. [Google Scholar] [CrossRef]
- Wang, M.; Rong, Y.; Luo, L. Neuroprotective effects of icariin in neonatal hypoxia-ischemic brain damage via its anti-apoptotic property. Child’s Nerv. Syst. 2020, 37, 39–46. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, J.; Yang, Y.; Zhao, D.; Wang, C.; Zhong, P.; Jia, J.; Dang, W.; Lu, Q.; Zhang, C.; et al. Icaritin production from Epimedium folium extract by a one-pot enzymatic cascade of a multifunctional glycosidase and rhamnosidase. Int. J. Biol. Macromol. 2024, 283, 137784. [Google Scholar] [CrossRef]
- Rui, L.L.; Gautam, S.; Xing, Z.; Liu, L.C.; Yan, H.; Qun, L.; Xu, R.B.; Ru, T.F. The neuroprotective effects of icariin on ageing, various neurological, neuropsychiatric disorders, and brain injury induced by radiation exposure. Aging 2022, 14, 1562. [Google Scholar]
- El-Baz, F.K.; Salama, A.; Ali, S.I.; Elgohary, R. Lutein isolated from Scenedesmus obliquus microalga boosts immunity against cyclophosphamide-induced brain injury in rats. Sci. Rep. 2022, 12, 22601. [Google Scholar] [CrossRef]
- Grygorczuk, S.; Parczewski, M.; Świerzbińska, R.; Czupryna, P.; Moniuszko, A.; Dunaj, J.; Kondrusik, M.; Pancewicz, S. The increased concentration of macrophage migration inhibitory factor in serum and cerebrospinal fluid of patients with tick-borne encephalitis. J. Neuroinflammation 2017, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Tongzhou, Q.; Ling, G.; Xing, W.; Guiqiang, Z.; Liyuan, L.; Zhaowen, Z.; Guirong, D. Repetitive transcranial magnetic stimulation ameliorates cognitive deficits in mice with radiation-induced brain injury by attenuating microglial pyroptosis and promoting neurogenesis via BDNF pathway. Cell Commun. Signal. 2024, 22, 216. [Google Scholar]
- Kristanti, A.N.; Aminaha, N.S.; Siswanto, I.; Wardana, A.P.; Abdjan, M.I.; Khoirunisak, A.R.; Noviana, E. Synthesis, Pharmacokinetic, Molecular Docking, and Molecular Dynamics Simulation of 2-Styrylchromone Derivatives as Potential Inhibitor of Human Kinesin Eg5. Eng. Sci. 2024, 30, 1168. [Google Scholar] [CrossRef]
- Corey, D.A.; Rymut, S.M.; Kelley, T.J. Alleviation of depression-like behavior in a cystic fibrosis mouse model by Hdac6 depletion. Sci. Rep. 2020, 10, 16278. [Google Scholar] [CrossRef]
- Seo, M.K.; Jeong, S.; Seog, D.-H.; Lee, J.-H.; Lee, Y.; McIntyre, R.S.; Park, S.W.; Lee, J.G. Effects of liraglutide on depressive behavior in a mouse depression model and cognition in the probe trial of Morris water maze test. J. Affect. Disord. 2022, 324, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Yang, Z.; Li, J.; Zheng, C.; Zhang, F. Divide-and-Attention Network for HE-Stained Pathological Image Classification. Biology 2022, 11, 982. [Google Scholar] [CrossRef]
- Tao, Y.; Shen, W.; Zhou, H.; Li, Z.; Pi, T.; Wu, H.; Shi, H.; Huang, F.; Wu, X. Sex differences in a corticosterone-induced depression model in mice: Behavioral, neurochemical, and molecular insights. Brain Res. 2023, 1823, 148678. [Google Scholar] [CrossRef]
- Hong, W.; Luan, Y.; Ma, Y.; Zhang, B.; Xiong, Y. Transcriptome analysis provides insights into high fat diet-induced kidney injury and moderate intensity continuous training-mediated protective effects. Heliyon 2024, 10, e27157. [Google Scholar] [CrossRef]
- Zhao, K.; Qi, L.; Li, Q.; Wang, Y.; Qian, C.; Shi, Z. Self-absorbing multilayer skin-like composite with Phyllostachys nigra polysaccharides promotes wound healing. Adv. Compos. Hybrid Mater. 2024, 7, 225. [Google Scholar] [CrossRef]
- Abdjan, M.I.; Rosyda, M.; Aminah, N.S.; Kristanti, A.N.; Siswanto, I.; Shehzad, W.; Siddiqui, H.; Wardana, A.P.; Indriani, I.; Saputra, M.A.; et al. Synthesis of Ester Derivatives of Catechin Isolated from Uncaria gambir and their Anticancer Activity. Eng. Sci. 2023, 27, 997. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Ding, K.; Zeng, B.; Li, B.; Zhou, J.; Li, J.; Wang, J.; Su, X.; Sun, R. Yanghe decoction inhibits inflammation-induced lung metastasis of colorectal cancer. J. Ethnopharmacol. 2024, 340, 119257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-l.; Lei, B.-x.; Wu, G.-y.; Wang, Y.-y.; Huang, Q.-h. Protective effects of berberine against β-amyloid-induced neurotoxicity in HT22 cells via the Nrf2/HO-1 pathway. Bioorg. Chem. 2023, 133, 106210. [Google Scholar] [CrossRef]


| Group | n | Dose | Error of Working Memory | Reference Memory Error |
|---|---|---|---|---|
| Control group | 12 | —— | 2.11 ± 0.23 | 3.42 ± 0.11 |
| CTX | 12 | 80 mg/kg | 7.56 ± 0.33 ### | 10.44 ± 0.31 ### |
| ICA | 12 | 25 mg/kg | 5.04 ± 0.29 ** | 7.11 ± 0.26 *** |
| ICA | 12 | 100 mg/kg | 3.48 ± 0.11 *** | 5.11 ± 0.22 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Kan, H.; Tang, Y.; Tian, L.; Guo, X.; Chen, W.; Geng, J.; Zong, Y.; Bi, Y.; He, Z. Icariin Ameliorates Cyclophosphamide-Induced Renal Encephalopathy by Modulating the NF-κB and Keap1-Nrf2 Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 4838. https://doi.org/10.3390/ijms26104838
Shi M, Kan H, Tang Y, Tian L, Guo X, Chen W, Geng J, Zong Y, Bi Y, He Z. Icariin Ameliorates Cyclophosphamide-Induced Renal Encephalopathy by Modulating the NF-κB and Keap1-Nrf2 Signaling Pathways. International Journal of Molecular Sciences. 2025; 26(10):4838. https://doi.org/10.3390/ijms26104838
Chicago/Turabian StyleShi, Meiling, Hong Kan, Yijia Tang, Lanshi Tian, Xiangjuan Guo, Weijia Chen, Jianan Geng, Ying Zong, Yunfeng Bi, and Zhongmei He. 2025. "Icariin Ameliorates Cyclophosphamide-Induced Renal Encephalopathy by Modulating the NF-κB and Keap1-Nrf2 Signaling Pathways" International Journal of Molecular Sciences 26, no. 10: 4838. https://doi.org/10.3390/ijms26104838
APA StyleShi, M., Kan, H., Tang, Y., Tian, L., Guo, X., Chen, W., Geng, J., Zong, Y., Bi, Y., & He, Z. (2025). Icariin Ameliorates Cyclophosphamide-Induced Renal Encephalopathy by Modulating the NF-κB and Keap1-Nrf2 Signaling Pathways. International Journal of Molecular Sciences, 26(10), 4838. https://doi.org/10.3390/ijms26104838






