A Comprehensive Review of Long Non-Coding RNAs in the Cancer–Immunity Cycle: Mechanisms and Therapeutic Implications
Abstract
1. Introduction
2. Cancer Immunosurveillance and Immunoediting
2.1. Overview of the Cancer–Immunity Cycle and Immune Evasion
2.2. Tumor Antigens: Release and Uptake
2.3. Participation of LncRNA as a Regulator of DAMPs Expression
2.3.1. Calreticulin and CD47 Expression and lncRNAs: Regulating Phagocytosis
2.3.2. CD73 and lncRNAs: Immunosuppressive Mechanisms
2.3.3. HMGB1 and lncRNAs: Immune Activation and Evasion
2.3.4. Immunogenic Cell Death (ICD), ER Stress Responses, and lncRNAs
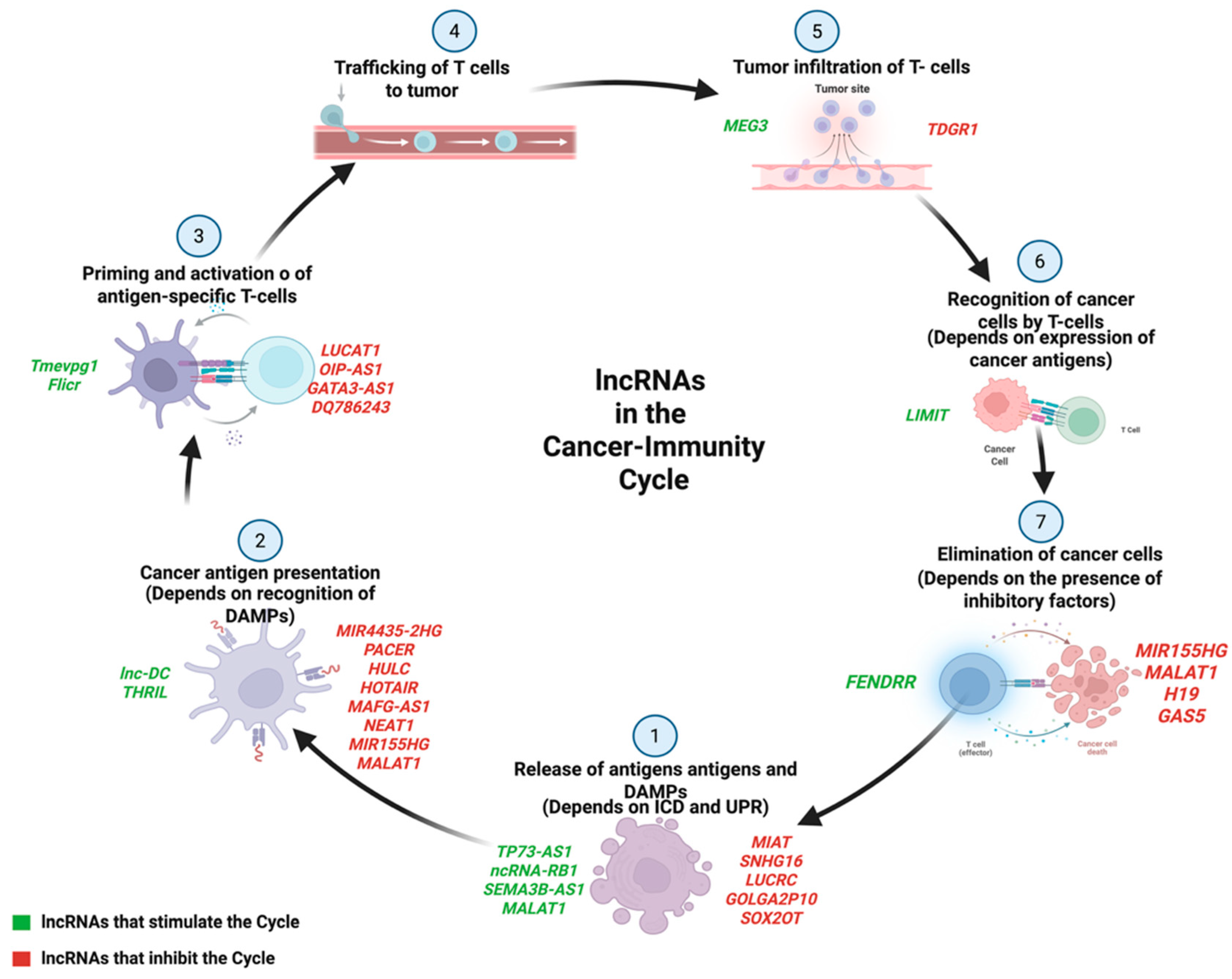
3. Cancer–Immunity Cycle and the Role of lncRNAs
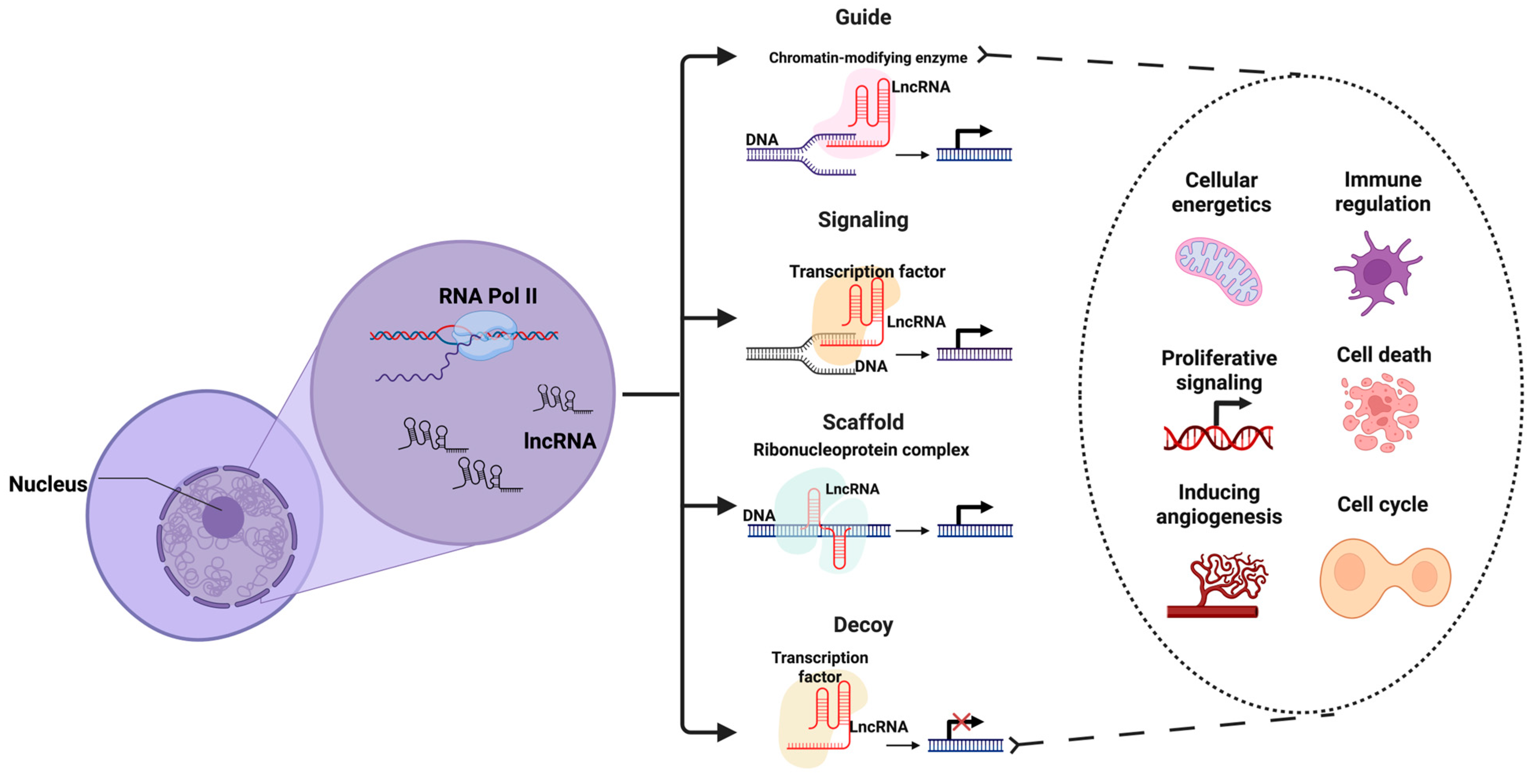
4. LncRNAs in Immune Modulation
4.1. Type I Interferon Signaling and lncRNA-Mediated Immune Regulation
4.2. Micropeptides Encoded by lncRNAs: Emerging Players in Cancer Immunity
5. T Cell Activation and lncRNA-Mediated Impaired T Cell Dysregulation
5.1. IL-2 Signaling and lncRNA Interactions
5.2. CTLA-4 Regulation by lncRNAs
5.3. GAS5: Tumor-Suppressor lncRNA in Immune Regulation
5.4. LncRNA-Mediated Regulation of T Cell Phenotypes
6. LncRNAs in T Cell Trafficking
7. Metabolic Reprogramming and lncRNA-Mediated Immune Escape
8. Extracellular Vesicles and Intercellular Communication
9. Therapeutic Potential of lncRNAs in Cancer Immunotherapy
10. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the Mechanisms and Challenges of Cancer Drug Resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-G.; Pyo, S.-J.; Cui, Y.; Yoon, S.-H.; Nam, J.-W. Tumor Immune Microenvironment lncRNAs. Brief. Bioinform. 2021, 23, bbab504. [Google Scholar] [CrossRef]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic Resistance to Immune Checkpoint Blockade. Nat. Rev. Immunol. 2019, 20, 25–39. [Google Scholar] [CrossRef]
- Emens, L.A.; Romero, P.J.; Anderson, A.C.; Bruno, T.C.; Capitini, C.M.; Collyar, D.; Gulley, J.L.; Hwu, P.; Posey, A.D.; Silk, A.W.; et al. Challenges and Opportunities in Cancer Immunotherapy: A Society for Immunotherapy of Cancer (SITC) Strategic Vision. J. Immunother. Cancer 2024, 12, e009063. [Google Scholar] [CrossRef]
- Xiang, Y.; Liu, X.; Wang, Y.; Zheng, D.; Meng, Q.; Jiang, L.; Yang, S.; Zhang, S.; Zhang, X.; Liu, Y.; et al. Mechanisms of Resistance to Targeted Therapy and Immunotherapy in Non-Small Cell Lung Cancer: Promising Strategies to Overcoming Challenges. Front. Immunol. 2024, 15, 1366260. [Google Scholar] [CrossRef]
- Akhade, V.S.; Pal, D.; Kanduri, C. Long Noncoding RNA: Genome Organization and Mechanism of Action. Adv. Exp. Med. Biol. 2017, 1008, 47–74. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Abdelmohsen, K.; Gorospe, M. Posttranscriptional Gene Regulation by Long Noncoding RNA. J. Mol. Biol. 2013, 425, 3723–3730. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef]
- Cabili, M.N.; Dunagin, M.C.; McClanahan, P.D.; Biaesch, A.; Padovan-Merhar, O.; Regev, A.; Rinn, J.L.; Raj, A. Localization and Abundance Analysis of Human lncRNAs at Single-Cell and Single-Molecule Resolution. Genome Biol. 2015, 16, 20. [Google Scholar] [CrossRef]
- Zhan, D.; Xian, H. Exploring the Regulatory Role of lncRNA in Cancer Immunity. Front. Oncol. 2023, 13, 1191913. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and Cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Burnet, F.M. The Concept of Immunological Surveillance. Prog. Tumor Res. 1970, 13, 1–27. [Google Scholar] [CrossRef]
- Lawrence, H.S. Cellular and Humoral Aspects of the Hypersensitive States: A Symposium Held at the New York Academy of Medicine; P.B. Hoeber: New York, NY, USA, 1959. [Google Scholar]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising Targets for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Manjili, M.H. Revisiting Cancer Immunoediting by Understanding Cancer Immune Complexity. J. Pathol. 2011, 224, 5–9. [Google Scholar] [CrossRef]
- Medrano, R.F.V.; Hunger, A.; Mendonça, S.A.; Barbuto, J.A.M.; Strauss, B.E. Immunomodulatory and Antitumor Effects of Type I Interferons and Their Application in Cancer Therapy. Oncotarget 2017, 8, 71249–71284. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer Immunoediting: From Immunosurveillance to Tumor Escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Galassi, C.; Chan, T.A.; Vitale, I.; Galluzzi, L. The Hallmarks of Cancer Immune Evasion. Cancer Cell 2024, 42, 1825–1863. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the Role of CD4+ T Cells in Cancer Immunotherapy—New Insights into Old Paradigms. Cancer Gene Ther. 2020, 28, 5–17. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The Cancer-Immunity Cycle: Indication, Genotype, and Immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T Cells in Cancer and Cancer Immunotherapy. Br. J. Cancer 2020, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the Immune System in Cancer: From Tumor Initiation to Metastatic Progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, J.; Yu, S.; Zheng, Y.; Duan, M.; Zhao, L.; Wang, Y.; Yang, Z.; Jiang, X. Cellular Senescence and Metabolic Reprogramming: Unraveling the Intricate Crosstalk in the Immunosuppressive Tumor Microenvironment. Cancer Commun. 2024, 44, 929–966. [Google Scholar] [CrossRef]
- Lv, D.; Fei, Y.; Chen, H.; Wang, J.; Han, W.; Cui, B.; Feng, Y.; Zhang, P.; Chen, J. Crosstalk between T Lymphocyte and Extracellular Matrix in Tumor Microenvironment. Front. Immunol. 2024, 15, 1340702. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and Senescence: Two Crucial Dysfunctional States of T Cells in the Tumor Microenvironment. Cell. Mol. Immunol. 2019, 17, 27–35. [Google Scholar] [CrossRef]
- Renner, K.; Singer, K.; Koehl, G.E.; Geissler, E.K.; Peter, K.; Siska, P.J.; Kreutz, M. Metabolic Hallmarks of Tumor and Immune Cells in the Tumor Microenvironment. Front. Immunol. 2017, 8, 248. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Moon, C.Y.; Belabed, M.; Park, M.D.; Mattiuz, R.; Puleston, D.; Merad, M. Dendritic Cell Maturation in Cancer. Nat. Rev. Cancer 2025, 25, 225–248. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, J. Balancing Act: The Complex Role of NK Cells in Immune Regulation. Front. Immunol. 2023, 14, 1275028. [Google Scholar] [CrossRef]
- Park, W.; Wei, S.; Kim, B.-S.; Kim, B.; Bae, S.-J.; Chae, Y.C.; Ryu, D.; Ha, K.-T. Diversity and Complexity of Cell Death: A Historical Review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular Mechanisms of Cell Death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Liu, C.-C.; Leclair, P.; Pedari, F.; Vieira, H.; Monajemi, M.; Sly, L.M.; Reid, G.S.; Lim, C.J. Integrins and ERp57 Coordinate to Regulate Cell Surface Calreticulin in Immunogenic Cell Death. Front. Oncol. 2019, 9, 411. [Google Scholar] [CrossRef] [PubMed]
- Panaretakis, T.; Kepp, O.; Brockmeier, U.; Tesniere, A.; Bjorklund, A.-C.; Chapman, D.C.; Durchschlag, M.; Joza, N.; Pierron, G.; van Endert, P.; et al. Mechanisms of Pre-Apoptotic Calreticulin Exposure in Immunogenic Cell Death. EMBO J. 2009, 28, 578–590. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, L.; Guo, C.; Xin, Q.; Gu, X.; Jiang, C.; Wu, J. “Find Me” and “Eat Me” Signals: Tools to Drive Phagocytic Processes for Modulating Antitumor Immunity. Cancer Commun. 2024, 44, 791–832. [Google Scholar] [CrossRef]
- Chen, X.; Fosco, D.; Kline, D.E.; Kline, J. Calreticulin Promotes Immunity and Type I Interferon-Dependent Survival in Mice with Acute Myeloid Leukemia. OncoImmunology 2017, 6, e1278332. [Google Scholar] [CrossRef]
- Fucikova, J.; Coosemans, A.; Orsulic, S.; Cibula, D.; Vergote, I.; Galluzzi, L.; Spisek, R. Immunological Configuration of Ovarian Carcinoma: Features and Impact on Disease Outcome. J. Immunother. Cancer 2021, 9, e002873. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47–SIRPα Pathway in Cancer Immune Evasion and Potential Therapeutic Implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The Mechanism of HMGB1 Secretion and Release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef]
- Antonioli, L.; Yegutkin, G.G.; Pacher, P.; Blandizzi, C.; Haskó, G. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities. Trends Cancer 2016, 2, 95–109. [Google Scholar] [CrossRef]
- Thibaudin, M.; Chaix, M.; Boidot, R.; Végran, F.; Derangère, V.; Limagne, E.; Berger, H.; Ladoire, S.; Apetoh, L.; Ghiringhelli, F. Human Ectonucleotidase-Expressing CD25highTh17 Cells Accumulate in Breast Cancer Tumors and Exert Immunosuppressive Functions. OncoImmunology 2015, 5, e1055444. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, I.; Mathes, A.; Groth, C.; Karakhanova, S.; Müller, V.; Utikal, J.; Werner, J.; Bazhin, A.V.; Umansky, V. Enhanced Expression of CD39 and CD73 on T Cells in the Regulation of Anti-Tumor Immune Responses. OncoImmunology 2020, 9, 1744946. [Google Scholar] [CrossRef] [PubMed]
- Bystryn, J.C. Release of Tumor-Associated Antigens by Murine Melanoma Cells. J. Immunol. 1976, 116, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Szlasa, W.; Janicka, N.; Sauer, N.; Michel, O.; Nowak, B.; Saczko, J.; Kulbacka, J. Chemotherapy and Physical Therapeutics Modulate Antigens on Cancer Cells. Front. Immunol. 2022, 13, 889950. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, Z.; Shu, S.; Fang, Y.; Twitty, C.; Hilton, T.L.; Aung, S.; Urba, W.J.; Fox, B.A.; Hu, H.-M.; et al. Autophagy-Assisted Antigen Cross-Presentation. OncoImmunology 2012, 1, 976–978. [Google Scholar] [CrossRef]
- Tesniere, A.; Panaretakis, T.; Kepp, O.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Molecular Characteristics of Immunogenic Cancer Cell Death. Cell Death Differ. 2007, 15, 3–12. [Google Scholar] [CrossRef]
- Zhou, J.; Hui, X.; Mao, Y.; Fan, L. Identification of Novel Genes Associated with a Poor Prognosis in Pancreatic Ductal Adenocarcinoma via a Bioinformatics Analysis. Biosci. Rep. 2019, 39, BSR20190625. [Google Scholar] [CrossRef]
- Musahl, A.-S.; Huang, X.; Rusakiewicz, S.; Ntini, E.; Marsico, A.; Kroemer, G.; Kepp, O.; Ørom, U.A. A Long Non-Coding RNA Links Calreticulin-Mediated Immunogenic Cell Removal to RB1 Transcription. Oncogene 2015, 34, 5046–5054. [Google Scholar] [CrossRef]
- Zeinelabdeen, Y.; Abaza, T.; Yasser, M.B.; Elemam, N.M.; Youness, R.A. MIAT LncRNA: A Multifunctional Key Player in Non-Oncological Pathological Conditions. Non-Coding RNA Res. 2024, 9, 447–462. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, S.; Xia, Y.; Hu, R.; Chen, S.; Li, B.; Chen, S.; Luo, X.; Mao, L.; Li, Y.; et al. LncRNA MIAT Sponges miR-149-5p to Inhibit Efferocytosis in Advanced Atherosclerosis Through CD47 Upregulation. Cell Death Dis. 2019, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, F.J.; Asadi, M.H.; Torkzadeh-Mahani, M. MIAT lncRNA Is Overexpressed in Breast Cancer and Its Inhibition Triggers Senescence and G1 Arrest in MCF7 Cell Line. J. Cell. Biochem. 2018, 119, 6470–6481. [Google Scholar] [CrossRef]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res. 2010, 70, 2245–2255. [Google Scholar] [CrossRef]
- Yang, M.; Wei, W. SNHG16: A Novel Long-Non Coding RNA in Human Cancers. OncoTargets Ther. 2019, 12, 11679–11690. [Google Scholar] [CrossRef]
- Ni, C.; Fang, Q.-Q.; Chen, W.-Z.; Jiang, J.-X.; Jiang, Z.; Ye, J.; Zhang, T.; Yang, L.; Meng, F.-B.; Xia, W.-J.; et al. Breast Cancer-Derived Exosomes Transmit lncRNA SNHG16 to Induce CD73+γδ1 Treg Cells. Signal Transduct. Target. Ther. 2020, 5, 41. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, Y.; Lin, A.; Jiang, A.; Zhou, C.; Cheng, Q.; Liu, Z.; Chen, X.; Zhang, J.; Luo, P. Gamma Delta T-Cell-Based Immune Checkpoint Therapy: Attractive Candidate for Antitumor Treatment. Mol. Cancer 2023, 22, 31. [Google Scholar] [CrossRef]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory Cytokines as a Third Signal for T Cell Activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef]
- Huang, G.; Xiang, Z.; Wu, H.; He, Q.; Dou, R.; Yang, C.; Song, J.; Huang, S.; Wang, S.; Xiong, B. The lncRNA SEMA3B-AS1/HMGB1/FBXW7 Axis Mediates the Peritoneal Metastasis of Gastric Cancer by Regulating BGN Protein Ubiquitination. Oxid. Med. Cell. Longev. 2022, 2022, 1–26. [Google Scholar] [CrossRef]
- Wang, T.; Gan, X. Emerging Roles of HMGB1-Related lncRNA: From Molecular Biology to Clinical Application. Am. J. Physiol.-Cell Physiol. 2022, 323, C1149–C1160. [Google Scholar] [CrossRef]
- Chen, R.; Li, W.; Sun, Y.; Duan, Y.; Li, Q.; Zhang, A.; Hu, J.; Wang, Y.; Gao, Y. Comprehensive Analysis of lncRNA and mRNA Expression Profiles in Lung Cancer. Clin. Lab. 2017, 63, 313–320. [Google Scholar] [CrossRef]
- Liang, D.; Tatomer, D.C.; Luo, Z.; Wu, H.; Yang, L.; Chen, L.-L.; Cherry, S.; Wilusz, J.E. The Output of Protein-Coding Genes Shifts to Circular RNAs When the Pre-mRNA Processing Machinery Is Limiting. Mol. Cell 2017, 68, 940–954.e3. [Google Scholar] [CrossRef] [PubMed]
- Martins, I.; Kepp, O.; Schlemmer, F.; Adjemian, S.; Tailler, M.; Shen, S.; Michaud, M.; Menger, L.; Gdoura, A.; Tajeddine, N.; et al. Restoration of the Immunogenicity of Cisplatin-Induced Cancer Cell Death by Endoplasmic Reticulum Stress. Oncogene 2010, 30, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, P.; Li, J.; Wu, F.; Guo, M.; Zhou, E.; Song, S.; Wang, S.; Zhang, S.; Jin, Y. Dihydroartemisinin Restores the Immunogenicity and Enhances the Anticancer Immunosurveillance of Cisplatin by Activating the PERK/eIF2α Pathway. Cell Biosci. 2024, 14, 100. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, Regulation and Functions of the Unfolded Protein Response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Tang, G.-H.; Chen, X.; Ding, J.-C.; Du, J.; Lin, X.-T.; Xia, L.; Lian, J.-B.; Ye, F.; He, X.-S.; Liu, W. LncRNA LUCRC Regulates Colorectal Cancer Cell Growth and Tumorigenesis by Targeting Endoplasmic Reticulum Stress Response. Front. Genet. 2020, 10, 1409. [Google Scholar] [CrossRef]
- Wu, M.-Z.; Fu, T.; Chen, J.-X.; Lin, Y.-Y.; Yang, J.-E.; Zhuang, S.-M. LncRNA GOLGA2P10 Is Induced by PERK/ATF4/CHOP Signaling and Protects Tumor Cells from ER Stress-Induced Apoptosis by Regulating Bcl-2 Family Members. Cell Death Dis. 2020, 11, 276. [Google Scholar] [CrossRef]
- Jiang, X.; Li, D.; Wang, G.; Liu, J.; Su, X.; Yu, W.; Wang, Y.; Zhai, C.; Liu, Y.; Zhao, Z. Thapsigargin Promotes Colorectal Cancer Cell Migration through Upregulation of lncRNA MALAT1. Oncol. Rep. 2020, 43, 1245–1255. [Google Scholar] [CrossRef]
- Ferraro-Peyret, C.; Askarian-Amiri, M.E.; Sarkar, D.; Joseph, W.R.; Hansji, H.; Baguley, B.C.; Leung, E.Y. SOX2OT Long Noncoding RNA Is Regulated by the UPR in Oestrogen Receptor-Positive Breast Cancer. Sci 2021, 3, 26. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Lian, J.; Xu, H. LncRNA Sox2ot Overexpression Serves as a Poor Prognostic Biomarker in Gastric Cancer. Am. J. Transl. Res. 2016, 8, 5035–5043. [Google Scholar]
- Zhan, Y.; Chen, Z.; He, S.; Gong, Y.; He, A.; Li, Y.; Zhang, L.; Zhang, X.; Fang, D.; Li, X.; et al. Long Non-Coding RNA SOX2OT Promotes the Stemness Phenotype of Bladder Cancer Cells by Modulating SOX2. Mol. Cancer 2020, 19, 25. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, J.; Wang, C.; Chi, Y.; Wei, Q.; Fu, Z.; Lian, C.; Huang, Q.; Liao, C.; Yang, Z.; et al. LncRNA SOX2OT Promotes Temozolomide Resistance by Elevating SOX2 Expression via ALKBH5-Mediated Epigenetic Regulation in Glioblastoma. Cell Death Dis. 2020, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.C.; Elsamadicy, A.A.; Snyder, D.J.; et al. Temozolomide Lymphodepletion Enhances CAR Abundance and Correlates with Antitumor Efficacy against Established Glioblastoma. OncoImmunology 2018, 7, e1434464. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ali, A.; Dutta, S.; Banday, S.; Malonia, S.K. Emerging Trends in Immunotherapy for Cancer. Diseases 2022, 10, 60. [Google Scholar] [CrossRef]
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the Efficacy of This Promising Therapeutic in Multiple Cancers. Clin. Sci. 2019, 133, 181–193. [Google Scholar] [CrossRef]
- Denaro, N.; Merlano, M.C.; Lo Nigro, C. Long Noncoding RNAs as Regulators of Cancer Immunity. Mol. Oncol. 2018, 13, 61–73. [Google Scholar] [CrossRef]
- Yu, W.; Wang, H.; He, Q.; Xu, Y.; Wang, X. Long Noncoding RNAs in Cancer-immunity Cycle. J. Cell. Physiol. 2018, 233, 6518–6523. [Google Scholar] [CrossRef]
- Wu, M.; Fu, P.; Qu, L.; Liu, J.; Lin, A. Long Noncoding RNAs, New Critical Regulators in Cancer Immunity. Front. Oncol. 2020, 10, 550987. [Google Scholar] [CrossRef]
- Dutta, A.; Roy, A.; Chatterjee, S. Long Noncoding RNAs in Cancer Immunity: A New Avenue in Drug Discovery. Drug Discov. Today 2021, 26, 264–272. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Xu, Y.; Wu, X.; Zhou, Y.; Mo, J. Immune-related Long Noncoding RNA Signature for Predicting Survival and Immune Checkpoint Blockade in Hepatocellular Carcinoma. J. Cell. Physiol. 2020, 235, 9304–9316. [Google Scholar] [CrossRef]
- Liang, Y.-L.; Zhang, Y.; Tan, X.-R.; Qiao, H.; Ma, J.; Li, Y.-Q.; Liu, N. Insights from Long Noncoding RNAs into Cancer-Immunity Cycle Regulation. J. Cancer Immunol. 2023, 5, 13–28. [Google Scholar] [CrossRef]
- Martinez-Castillo, M.; Elsayed, A.M.; López-Berestein, G.; Amero, P.; Rodríguez-Aguayo, C. An Overview of the Immune Modulatory Properties of Long Non-Coding RNAs and Their Potential Use as Therapeutic Targets in Cancer. Non-Coding RNA 2023, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kryczek, I.; Nam, J.; Li, X.; Li, S.; Li, J.; Wei, S.; Grove, S.; Vatan, L.; Zhou, J.; et al. LIMIT Is an Immunogenic lncRNA in Cancer Immunity and Immunotherapy. Nat. Cell Biol. 2021, 23, 526–537. [Google Scholar] [CrossRef]
- Baek, A.E. Immunogenic Lncs to Cancer Therapy. Sci. Signal. 2023, 16, eadh4085. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.T.; Riillo, C.; Scionti, F.; Grillone, K.; Polerà, N.; Caracciolo, D.; Arbitrio, M.; Tagliaferri, P.; Tassone, P. miRNAs and lncRNAs as Novel Therapeutic Targets to Improve Cancer Immunotherapy. Cancers 2021, 13, 1587. [Google Scholar] [CrossRef]
- Li, H.; Xiong, H.-G.; Xiao, Y.; Yang, Q.-C.; Yang, S.-C.; Tang, H.-C.; Zhang, W.-F.; Sun, Z.-J. Long Non-Coding RNA LINC02195 as a Regulator of MHC I Molecules and Favorable Prognostic Marker for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 615. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, M.; Yu, L.; Hu, Y.; Deng, Y.; Liu, Y.; Xiao, S.; Ding, Y. Long Non-coding RNA lnc-DC in Dendritic Cells Regulates Trophoblast Invasion via p-STAT3-mediated TIMP/MMP Expression. Am. J. Reprod. Immunol. 2020, 83, e13239. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, Y.; Luo, Y. The Role of Long Non-Coding RNAs in the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 851004. [Google Scholar] [CrossRef]
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The Role of Dendritic Cells in Tissue-Specific Autoimmunity. J. Immunol. Res. 2014, 2014, 857143. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-Binding Long Noncoding RNA Lnc-DC Controls Human Dendritic Cell Differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef]
- Basler, M.; Kirk, C.J.; Groettrup, M. The Immunoproteasome in Antigen Processing and Other Immunological Functions. Curr. Opin. Immunol. 2013, 25, 74–80. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Petermann, F.; Pękowska, A.; Johnson, C.A.; Jankovic, D.; Shih, H.-Y.; Jiang, K.; Hudson, W.H.; Brooks, S.R.; Sun, H.-W.; Villarino, A.V.; et al. The Magnitude of IFN-γ Responses Is Fine-Tuned by DNA Architecture and the Non-Coding Transcript of Ifng-As1. Mol. Cell 2019, 75, 1229–1242.e5. [Google Scholar] [CrossRef]
- Li, Z.; Chao, T.-C.; Chang, K.-Y.; Lin, N.; Patil, V.S.; Shimizu, C.; Head, S.R.; Burns, J.C.; Rana, T.M. The Long Noncoding RNA THRIL Regulates TNFα Expression through Its Interaction with hnRNPL. Proc. Natl. Acad. Sci. USA 2013, 111, 1002–1007. [Google Scholar] [CrossRef]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D.; et al. Tumor-Infiltrating DCs Suppress Nucleic Acid–Mediated Innate Immune Responses Through Interactions Between the Receptor TIM-3 and the Alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar] [CrossRef]
- Li, G.; Liang, X.; Lotze, M.T. HMGB1: The Central Cytokine for All Lymphoid Cells. Front. Immunol. 2013, 4, 68. [Google Scholar] [CrossRef]
- Li, S.; Huang, Y.; Huang, Y.; Fu, Y.; Tang, D.; Kang, R.; Zhou, R.; Fan, X. The Long Non-Coding RNA TP73-AS1 Modulates HCC Cell Proliferation through miR-200a-Dependent HMGB1/RAGE Regulation. J. Exp. Clin. Cancer Res. 2017, 36, 51. [Google Scholar] [CrossRef]
- Salek-Ardakani, S.; Schoenberger, S.P. T Cell Exhaustion: A Means or an End? Nat. Immunol. 2013, 14, 531–533. [Google Scholar] [CrossRef]
- Odegaard, J.I.; Chawla, A. Old HDL Learns a New (Anti-Inflammatory) Trick. Nat. Immunol. 2014, 15, 138–139. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen Presentation in Cancer: Insights into Tumour Immunogenicity and Immune Evasion. Nat. Rev. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Chen, R.; Ma, L.; Jiang, C.; Zhang, S. Expression and Potential Role of CCL4 in CD8+T Cells in NSCLC. Clin. Transl. Oncol. 2022, 24, 2420–2431. [Google Scholar] [CrossRef]
- Williford, J.-M.; Ishihara, J.; Ishihara, A.; Mansurov, A.; Hosseinchi, P.; Marchell, T.M.; Potin, L.; Swartz, M.A.; Hubbell, J.A. Recruitment of CD103+ Dendritic Cells via Tumor-Targeted Chemokine Delivery Enhances Efficacy of Checkpoint Inhibitor Immunotherapy. Sci. Adv. 2019, 5, eaay1357. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Gajewski, T.F. Mechanisms of Tumor Cell–Intrinsic Immune Evasion. Annu. Rev. Cancer Biol. 2018, 2, 213–228. [Google Scholar] [CrossRef]
- Qian, H.; Chen, L.; Huang, J.; Wang, X.; Ma, S.; Cui, F.; Luo, L.; Ling, L.; Luo, K.; Zheng, G. The lncRNA MIR4435-2HG Promotes Lung Cancer Progression by Activating β-Catenin Signalling. J. Mol. Med. 2018, 96, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yu, X.; Zhang, Q.; Sun, Z.; He, Y.; Guo, W. MIR4435-2HG: A Newly Proposed lncRNA in Human Cancer. Biomed. Pharmacother. 2022, 150, 112971. [Google Scholar] [CrossRef]
- Vazana-Netzarim, R.; Elmalem, Y.; Sofer, S.; Bruck, H.; Danino, N.; Sarig, U. Distinct HAND2/HAND2-AS1 Expression Levels May Fine-Tune Mesenchymal and Epithelial Cell Plasticity of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2023, 24, 16546. [Google Scholar] [CrossRef]
- Zelenay, S.; van der Veen, A.G.; Böttcher, J.P.; Snelgrove, K.J.; Rogers, N.; Acton, S.E.; Chakravarty, P.; Girotti, M.R.; Marais, R.; Quezada, S.A.; et al. Cyclooxygenase-Dependent Tumor Growth through Evasion of Immunity. Cell 2015, 162, 1257–1270. [Google Scholar] [CrossRef]
- Ghahramani Almanghadim, H.; Karimi, B.; Valizadeh, S.; Ghaedi, K. Biological Functions and Affected Signaling Pathways by Long Non-Coding RNAs in the Immune System. Non-Coding RNA Res. 2025, 10, 70–90. [Google Scholar] [CrossRef]
- Krawczyk, M.; Emerson, B.M. P50-Associated COX-2 Extragenic RNA (PACER) Activates COX-2 Gene Expression by Occluding Repressive NF-κB Complexes. eLife 2014, 3, e01776. [Google Scholar] [CrossRef]
- Desind, S.Z.; Bell, S.K.; Davidson, Z.M.; Lutz, C.S. Long Noncoding RNAs and Their Complex Role in Shaping and Regulating Arachidonic Acid Metabolism: Learning to Love the (Not-really) Junk. WIREs RNA 2023, 15, e1828. [Google Scholar] [CrossRef]
- Li, P.; Ma, X.; Gu, X. LncRNA MAFG-AS1 Is Involved in Human Cancer Progression. Eur. J. Med. Res. 2023, 28, 497. [Google Scholar] [CrossRef]
- Krawczyk, C.M.; Holowka, T.; Sun, J.; Blagih, J.; Amiel, E.; DeBerardinis, R.J.; Cross, J.R.; Jung, E.; Thompson, C.B.; Jones, R.G.; et al. Toll-like Receptor-Induced Changes in Glycolytic Metabolism Regulate Dendritic Cell Activation. Blood 2010, 115, 4742–4749. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, D.-D.; Sang, X.-B.; Wang, L.-L.; Zong, Z.-H.; Sun, K.-X.; Liu, B.-L.; Zhao, Y. The lncRNA HULC Functions as an Oncogene by Targeting ATG7 and ITGB1 in Epithelial Ovarian Carcinoma. Cell Death Dis. 2017, 8, e3118. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wei, C.; Yang, Z.; Chang, W.; Zhang, X. LncRNA HULC Promotes the Progression of Gastric Cancer by Regulating miR-9-5p/MYH9 Axis. Biomed. Pharmacother. 2020, 121, 109607. [Google Scholar] [CrossRef]
- Lu, W.; Wan, X.; Tao, L.; Wan, J. Long Non-Coding RNA HULC Promotes Cervical Cancer Cell Proliferation, Migration and Invasion via miR-218/TPD52 Axis. OncoTargets Ther. 2020, 13, 1109–1118. [Google Scholar] [CrossRef]
- Zheng, P.; Li, H.; Xu, P.; Wang, X.; Shi, Z.; Han, Q.; Li, Z. High lncRNA HULC Expression Is Associated with Poor Prognosis and Promotes Tumor Progression by Regulating Epithelial-Mesenchymal Transition in Prostate Cancer. Arch. Med. Sci. 2018, 14, 679–686. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, Z.; Liu, F.; Su, C.; Zhu, H.; Li, Y.; Tao, R.; Liang, H.; Zhang, B.; Zhang, X. Long Noncoding RNA HULC Promotes Hepatocellular Carcinoma Progression. Aging 2019, 11, 9111–9127. [Google Scholar] [CrossRef]
- Neuss, H.; Huang, X.; Hetfeld, B.K.J.; Deva, R.; Henklein, P.; Nigam, S.; Mall, J.W.; Schwenk, W.; Dubiel, W. The Ubiquitin- and Proteasome-Dependent Degradation of COX-2 Is Regulated by the COP9 Signalosome and Differentially Influenced by Coxibs. J. Mol. Med. 2007, 85, 961–970. [Google Scholar] [CrossRef]
- Hu, W.; Xu, W.; Shi, Y.; Dai, W. lncRNA HOTAIR Upregulates COX-2 Expression to Promote Invasion and Migration of Nasopharyngeal Carcinoma by Interacting with miR-101. Biochem. Biophys. Res. Commun. 2018, 505, 1090–1096. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Zheng, Y.; Jin, X.; Liu, M.; Li, S.; Zhao, Q.; Liu, X.; Wang, Y.; Shi, M.; et al. The Long Noncoding RNA MALAT1 Induces Tolerogenic Dendritic Cells and Regulatory T Cells via miR155/Dendritic Cell-Specific Intercellular Adhesion Molecule-3 Grabbing Nonintegrin/IL10 Axis. Front. Immunol. 2018, 9, 1847. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The lncRNA Neat1 Promotes Activation of Inflammasomes in Macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Chen, C.; Zhang, R.; Wang, K. Pan-Cancer Analysis of Long Non-Coding RNA NEAT1 in Various Cancers. Genes Dis. 2018, 5, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Lou, F.; Sun, Y.; Sun, L.; Cai, X.; Liu, Z.; Zhou, H.; Wang, H.; Wang, Z.; Bai, J.; et al. A Micropeptide Encoded by lncRNA MIR155HG Suppresses Autoimmune Inflammation via Modulating Antigen Presentation. Sci. Adv. 2020, 6, eaaz2059. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, Y.; Cai, J. LncRNA MIR155HG Regulates M1/M2 Macrophage Polarization in Chronic Obstructive Pulmonary Disease. Biomed. Pharmacother. 2019, 117, 109015. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Z.; Chen, Y.; Wang, X.; Tang, N. MIR155HG Is a Prognostic Biomarker and Associated with Immune Infiltration and Immune Checkpoint Molecules Expression in Multiple Cancers. Cancer Med. 2019, 8, 7161–7173. [Google Scholar] [CrossRef]
- Cao, W.; Ramakrishnan, R.; Tuyrin, V.A.; Veglia, F.; Condamine, T.; Amoscato, A.; Mohammadyani, D.; Johnson, J.J.; Min Zhang, L.; Klein-Seetharaman, J.; et al. Oxidized Lipids Block Antigen Cross-Presentation by Dendritic Cells in Cancer. J. Immunol. 2014, 192, 2920–2931. [Google Scholar] [CrossRef]
- Suravajhala, P.; Kogelman, L.J.A.; Mazzoni, G.; Kadarmideen, H.N. Potential Role of lncRNA Cyp2c91–Protein Interactions on Diseases of the Immune System. Front. Genet. 2015, 6, 255. [Google Scholar] [CrossRef]
- Huangfu, N.; Xu, Z.; Zheng, W.; Wang, Y.; Cheng, J.; Chen, X. LncRNA MALAT1 Regulates oxLDL-Induced CD36 Expression via Activating β-Catenin. Biochem. Biophys. Res. Commun. 2018, 495, 2111–2117. [Google Scholar] [CrossRef]
- Chen, L.; Hu, L.; Zhu, X.; Wang, Y.; Li, Q.; Ma, J.; Li, H. MALAT1 Overexpression Attenuates AS by Inhibiting Ox-LDL-Stimulated Dendritic Cell Maturation via miR-155-5p/NFIA Axis. Cell Cycle 2020, 19, 2472–2485. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kinder, M.; Matsushita, H.; Mashayekhi, M.; Dunn, G.P.; Archambault, J.M.; Lee, H.; Arthur, C.D.; White, J.M.; Kalinke, U.; et al. Type I Interferon Is Selectively Required by Dendritic Cells for Immune Rejection of Tumors. J. Exp. Med. 2011, 208, 1989–2003. [Google Scholar] [CrossRef]
- Agarwal, S.; Vierbuchen, T.; Ghosh, S.; Chan, J.; Jiang, Z.; Kandasamy, R.K.; Ricci, E.; Fitzgerald, K.A. The Long Non-Coding RNA LUCAT1 Is a Negative Feedback Regulator of Interferon Responses in Humans. Nat. Commun. 2020, 11, 6348. [Google Scholar] [CrossRef]
- Xing, C.; Sun, S.; Yue, Z.-Q.; Bai, F. Role of lncRNA LUCAT1 in Cancer. Biomed. Pharmacother. 2021, 134, 111158. [Google Scholar] [CrossRef]
- Wang, L.; Xie, Y.; Wang, J.; Zhang, Y.; Liu, S.; Zhan, Y.; Zhao, Y.; Li, J.; Li, P.; Wang, C. Characterization of a Novel LUCAT1/miR-4316/VEGF-A Axis in Metastasis and Glycolysis of Lung Adenocarcinoma. Front. Cell Dev. Biol. 2022, 10, 833579. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Yamada, T.; Wang, R.; Tanimura, K.; Adachi, Y.; Nishiyama, A.; Tanimoto, A.; Takeuchi, S.; Araujo, L.H.; Boroni, M.; et al. AXL Confers Intrinsic Resistance to Osimertinib and Advances the Emergence of Tolerant Cells. Nat. Commun. 2019, 10, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Chava, S.; Verma, S.S.; Mishra, S.; Gorantla, S.; Coulter, D.W.; Byrareddy, S.N.; Batra, S.K.; Gupta, S.C.; Challagundla, K.B. PD-L1, Inflammation, Non-Coding RNAs, and Neuroblastoma: Immuno-Oncology Perspective. Semin. Cancer Biol. 2018, 52, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Wang, X.; Xie, X.; Liao, Y.; Liu, N.; Liu, J.; Miao, N.; Shen, J.; Peng, T. lncRNA DANCR Promotes Tumor Progression and Cancer Stemness Features in Osteosarcoma by Upregulating AXL via miR-33a-5p Inhibition. Cancer Lett. 2017, 405, 46–55. [Google Scholar] [CrossRef]
- Zhang, J.; Du, C.; Zhang, L.; Wang, Y.; Zhang, Y.; Li, J. lncRNA GSEC Promotes the Progression of Triple Negative Breast Cancer (TNBC) by Targeting the miR-202-5p/AXL Axis. OncoTargets Ther. 2021, 14, 2747–2759. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Y.; Hu, W.; Paliouras, A.R.; Zhang, W.; Zhong, L.; Yang, K.; Su, L.; Wang, P.; Li, Y.; et al. Long Non-Coding RNA-Encoded Micropeptides: Functions, Mechanisms and Implications. Cell Death Discov. 2024, 10, 450. [Google Scholar] [CrossRef]
- Barczak, W.; Carr, S.M.; Liu, G.; Munro, S.; Nicastri, A.; Lee, L.N.; Hutchings, C.; Ternette, N.; Klenerman, P.; Kanapin, A.; et al. Long Non-Coding RNA-Derived Peptides Are Immunogenic and Drive a Potent Anti-Tumour Response. Nat. Commun. 2023, 14, 1078. [Google Scholar] [CrossRef]
- Camarena, M.E.; Theunissen, P.; Ruiz, M.; Ruiz-Orera, J.; Calvo-Serra, B.; Castelo, R.; Castro, C.; Sarobe, P.; Fortes, P.; Perera-Bel, J.; et al. Microproteins Encoded by Noncanonical ORFs Are a Major Source of Tumor-Specific Antigens in a Liver Cancer Patient Meta-Cohort. Sci. Adv. 2024, 10, eadn3628. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, J.; Lou, F.; Zhou, H.; Cai, X.; Wang, Z.; Sun, L.; Sun, Y.; Li, X.; Fan, L.; et al. A lncRNA Dleu2-Encoded Peptide Relieves Autoimmunity by Facilitating Smad3-Mediated Treg Induction. EMBO Rep. 2024, 25, 1208–1232. [Google Scholar] [CrossRef]
- Bhatta, A.; Atianand, M.; Jiang, Z.; Crabtree, J.; Blin, J.; Fitzgerald, K.A. A Mitochondrial Micropeptide Is Required for Activation of the Nlrp3 Inflammasome. J. Immunol. 2020, 204, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Cinque, S.; Verheyden, Y.; Adnane, S.; Marino, A.; Katopodi, V.; Demesmaeker, E.; Knezevic, Z.; Hanache, S.; Vendramin, R.; Cuomo, A.; et al. The Assembly of Cancer-Specific Ribosomes by the lncRNA LISRR Suppresses Melanoma Anti-Tumour Immunity. bioRXiv 2023. bioRxiv:2023.01.06.523012. [Google Scholar]
- Wang, W.; Sung, N.; Gilman-Sachs, A.; Kwak-Kim, J. T Helper (Th) Cell Profiles in Pregnancy and Recurrent Pregnancy Losses: Th1/Th2/Th9/Th17/Th22/Tfh Cells. Front. Immunol. 2020, 11, 2025. [Google Scholar] [CrossRef]
- Zagorulya, M.; Spranger, S. Once upon a Prime: DCs Shape Cancer Immunity. Trends Cancer 2023, 9, 172–184. [Google Scholar] [CrossRef]
- Ross, S.H.; Cantrell, D.A. Signaling and Function of Interleukin-2 in T Lymphocytes. Annu. Rev. Immunol. 2018, 36, 411–433. [Google Scholar] [CrossRef]
- Chen, X.; Luo, X.; Wei, Y.; Sun, H.; Dai, L.; Tangzhou, Y.; Jin, H.; Yin, Z. LncRNA H19 Induces Immune Dysregulation of BMMSCs, at Least Partly, by Inhibiting IL-2 Production. Mol. Med. 2021, 27, 61. [Google Scholar] [CrossRef]
- Raveh, E.; Matouk, I.J.; Gilon, M.; Hochberg, A. The H19 Long Non-Coding RNA in Cancer Initiation, Progression and Metastasis—A Proposed Unifying Theory. Mol. Cancer 2015, 14, 184. [Google Scholar] [CrossRef]
- Dehbashi, M.; Hojati, Z.; Motovali-Bashi, M.; Cho, W.C.; Shimosaka, A.; Ganjalikhani-Hakemi, M. Systems Biology Unravels the Relationship of lncRNA OIP5-AS1 with CD25. Gene Rep. 2021, 24, 101223. [Google Scholar] [CrossRef]
- Meng, L.; Yue, X.; Zhou, D.; Li, H. Long Non Coding RNA OIP5-AS1 Promotes Metastasis of Breast Cancer via miR-340-5p/ZEB2 Axis. Oncol. Rep. 2020, 44, 1662–1670. [Google Scholar] [CrossRef]
- Wooten, S.; Smith, K.N. Long Non-coding RNA OIP5-AS1 (Cyrano): A Context-specific Regulator of Normal and Disease Processes. Clin. Transl. Med. 2022, 12, e706. [Google Scholar] [CrossRef]
- Yang, C.; Shu, J.; Li, Y.; Zhao, N.; Liu, X.; Tian, X.; Sun, Z.; Tabish, M.S.; Hong, Y.; Chen, K.; et al. Long Non-Coding RNAs Are Involved in the Crosstalk Between Cancer-Associated Fibroblasts and Tumor Cells. Front. Immunol. 2024, 15, 1469918. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, X.; Wang, X.; Xu, M.; Sheng, W. The Role of Cancer-Associated Fibroblasts in Tumorigenesis of Gastric Cancer. Cell Death Dis. 2022, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gurrapu, S.; Wang, Y.; Bae, S.-Y.; Pandey, P.R.; Chen, H.; Mondal, J.; Han, H.; Wu, C.-J.; Karaiskos, S.; et al. LncRNA Malat1 Suppresses Pyroptosis and T Cell-Mediated Killing of Incipient Metastatic Cells. Nat. Cancer 2024, 5, 262–282. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, Y.; Lyu, H.; Xu, X.; Wu, Q.; Liu, N.; Yin, Q.; Li, J.; Sheng, X. Long Noncoding RNA GAS5, Which Acts as a Tumor Suppressor via microRNA 21, Regulates Cisplatin Resistance Expression in Cervical Cancer. Int. J. Gynecol. Cancer 2017, 27, 1096–1108. [Google Scholar] [CrossRef]
- Yang, X.; Xie, Z.; Lei, X.; Gan, R. Long Non-Coding RNA GAS5 in Human Cancer. Oncol. Lett. 2020, 20, 2587–2594. [Google Scholar] [CrossRef]
- Gupta, S.; Panda, P.K.; Luo, W.; Hashimoto, R.F.; Ahuja, R. Network Analysis Reveals That the Tumor Suppressor lncRNA GAS5 Acts as a Double-Edged Sword in Response to DNA Damage in Gastric Cancer. Sci. Rep. 2022, 12, 18312. [Google Scholar] [CrossRef]
- Fang, P.; Xiang, L.; Chen, W.; Li, S.; Huang, S.; Li, J.; Zhuge, L.; Jin, L.; Feng, W.; Chen, Y.; et al. LncRNA GAS5 Enhanced the Killing Effect of NK Cell on Liver Cancer through Regulating miR-544/RUNX3. Innate Immun. 2019, 25, 99–109. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Hu, X.; Jin, F.; Gao, Z.; Yin, L.; Qin, J.; Yin, F.; Li, C.; Wang, Y. IDO1 Impairs NK Cell Cytotoxicity by Decreasing NKG2D/NKG2DLs via Promoting miR-18a. Mol. Immunol. 2018, 103, 144–155. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hedge, V.L.; Kirkham, L.; Farzaneh, F.; Williams, G.T. Growth Arrest in Human T-Cells Is Controlled by the Non-Coding RNA Growth-Arrest-Specific Transcript 5 (GAS5). J. Cell Sci. 2008, 121, 939–946. [Google Scholar] [CrossRef]
- Mourtada-Maarabouni, M.; Hasan, A.M.; Farzaneh, F.; Williams, G.T. Inhibition of Human T-Cell Proliferation by Mammalian Target of Rapamycin (mTOR) Antagonists Requires Noncoding RNA Growth-Arrest-Specific Transcript 5 (GAS5). Mol. Pharmacol. 2010, 78, 19–28. [Google Scholar] [CrossRef]
- Liu, Q.; Deng, Y.; Li, C.; Xie, H.; Liu, Q.; Ming, S.; Wu, D.; Luo, F. LncRNA GAS5 Suppresses CD4+ T Cell Activation by Upregulating E4BP4 via Inhibiting miR-92a-3p in Systemic Lupus Erythematosus. Immunol. Lett. 2020, 227, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, X.; Wang, Y.; Zhao, Y. Exosomal Long Non-Coding RNA GAS5 Suppresses Th1 Differentiation and Promotes Th2 Differentiation via Downregulating EZH2 and T-Bet in Allergic Rhinitis. Mol. Immunol. 2020, 118, 30–39. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, L.; Zhan, J.; Zhan, H.; Qiu, C. LncRNA GAS5 Expression in Non-Small Cell Lung Cancer Tissues and Its Correlation with Ki67 and EGFR. Am. J. Transl. Res. 2021, 13, 4900–4907. [Google Scholar]
- Qi, Y.; Cui, Q.; Zhang, W.; Yao, R.; Xu, D.; Zhang, F. Long Non-Coding RNA GAS5 Targeting microRNA-21 to Suppress the Invasion and Epithelial-Mesenchymal Transition of Uveal Melanoma. Cancer Manag. Res. 2020, 12, 12259–12267. [Google Scholar] [CrossRef]
- Nguyen, L.N.T.; Nguyen, L.N.; Zhao, J.; Schank, M.; Dang, X.; Cao, D.; Khanal, S.; Chand Thakuri, B.K.; Lu, Z.; Zhang, J.; et al. Long Non-Coding RNA GAS5 Regulates T Cell Functions via miR21-Mediated Signaling in People Living With HIV. Front. Immunol. 2021, 12, 601298. [Google Scholar] [CrossRef]
- Kureshi, C.T.; Dougan, S.K. Cytokines in Cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef]
- Gibbons, H.R.; Shaginurova, G.; Kim, L.C.; Chapman, N.; Spurlock, C.F.; Aune, T.M. Divergent lncRNA GATA3-AS1 Regulates GATA3 Transcription in T-Helper 2 Cells. Front. Immunol. 2018, 9, 2512. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, N.; Song, P.; Fu, Y.; Ren, Y.; Li, Z.; Wang, J. LncRNA GATA3-AS1 Facilitates Tumour Progression and Immune Escape in Triple-negative Breast Cancer through Destabilization of GATA3 but Stabilization of PD-L1. Cell Prolif. 2020, 53, e12855. [Google Scholar] [CrossRef]
- Wan, Y.Y. GATA3: A Master of Many Trades in Immune Regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef]
- Zemmour, D.; Pratama, A.; Loughhead, S.M.; Mathis, D.; Benoist, C. Flicr, a Long Noncoding RNA, Modulates Foxp3 Expression and Autoimmunity. Proc. Natl. Acad. Sci. USA 2017, 114, E3472–E3480. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Huang, M.L.; Xu, A.T.; Zhao, D.; Ran, Z.H.; Shen, J. LncRNA DQ786243 Affects Treg Related CREB and Foxp3 Expression in Crohn’s Disease. J. Biomed. Sci. 2013, 20, 87. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Silva, M.A.; Li, H.; Zhu, L.; Li, P.; Li, X.; Wang, X.; Gao, J.; Wang, P.; Zhang, Z. Long Noncoding RNA DQ786243 Interacts with miR-506 and Promotes Progression of Ovarian Cancer Through Targeting cAMP Responsive Element Binding Protein 1. J. Cell. Biochem. 2018, 119, 9764–9780. [Google Scholar] [CrossRef] [PubMed]
- Uzan, V.R.M.; van Lengert, A.H.; Boldrini, É.; Penna, V.; Scapulatempo-Neto, C.; Scrideli, C.A.; de Filho, A.P.M.; Cavalcante, C.E.B.; de Oliveira, C.Z.; Lopes, L.F.; et al. High Expression of HULC Is Associated with Poor Prognosis in Osteosarcoma Patients. PLoS ONE 2016, 11, e0156774. [Google Scholar] [CrossRef]
- Wang, X.; Mo, X.; Yang, Z.; Zhao, C. Qntrolling the LncRNA HULC-Tregs-PD-1 Axis Inhibits Immune Escape in the Tumor Microenvironment. Heliyon 2024, 10, e28386. [Google Scholar] [CrossRef]
- Shihabudeen Haider Ali, M.S.; Cheng, X.; Moran, M.; Haemmig, S.; Naldrett, M.J.; Alvarez, S.; Feinberg, M.W.; Sun, X. LncRNA Meg3 Protects Endothelial Function by Regulating the DNA Damage Response. Nucleic Acids Res. 2019, 47, 1505–1522. [Google Scholar] [CrossRef]
- Soghala, S.; Harsiny, K.; Momeni, P.; Hatami, M.; Kholghi Oskooei, V.; Hussen, B.M.; Taheri, M.; Ghafouri-Fard, S. Down-Regulation of LINC-ROR, HOXA-AS2 and MEG3 in Gastric Cancer. Heliyon 2022, 8, e11155. [Google Scholar] [CrossRef]
- Li, F.; Yang, J.; Li, Y.; Tan, Z.; Li, H.; Zhang, N. Long Non-Coding RNA FENDRR Suppresses Cancer-Associated Fibroblasts and Serves as a Prognostic Indicator in Colorectal Cancer. Transl. Oncol. 2023, 36, 101740. [Google Scholar] [CrossRef]
- Zhu, K.P.; Ma, X.L.; Zhang, C.L. LncRNA FENDRR Sensitizes Doxorubicin-Resistance of Osteosarcoma Cells through down-Regulating ABCB1 and ABCC1. Oncotarget 2017, 8, 71881–71893. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, H.; Feng, X.; Li, H.; Qiu, C.; Yi, X.; Tang, H.; Zhang, J. Long Non-Coding RNA FENDRR Acts as a miR-423-5p Sponge to Suppress the Treg-Mediated Immune Escape of Hepatocellular Carcinoma Cells. Mol. Ther.–Nucleic Acids 2019, 17, 516–529. [Google Scholar] [CrossRef]
- Shang, A.; Wang, W.; Gu, C.; Chen, C.; Zeng, B.; Yang, Y.; Ji, P.; Sun, J.; Wu, J.; Lu, W.; et al. Long Non-Coding RNA HOTTIP Enhances IL-6 Expression to Potentiate Immune Escape of Ovarian Cancer Cells by Upregulating the Expression of PD-L1 in Neutrophils. J. Exp. Clin. Cancer Res. 2019, 38, 411. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, L.; Shen, N.; Ning, X.; Wu, D.; Jiang, K.; Huang, X. M1 Macrophage-Derived Exosomes and Their Key Molecule lncRNA HOTTIP Suppress Head and Neck Squamous Cell Carcinoma Progression by Upregulating the TLR5/NF-κB Pathway. Cell Death Dis. 2022, 13, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, X.; Jiang, P. Cytokine and Chemokine Signals of T-Cell Exclusion in Tumors. Front. Immunol. 2020, 11, 594609. [Google Scholar] [CrossRef]
- Huinen, Z.R.; Huijbers, E.J.M.; van Beijnum, J.R.; Nowak-Sliwinska, P.; Griffioen, A.W. Anti-Angiogenic Agents—Overcoming Tumour Endothelial Cell Anergy and Improving Immunotherapy Outcomes. Nat. Rev. Clin. Oncol. 2021, 18, 527–540. [Google Scholar] [CrossRef]
- Huijbers, E.J.M.; Khan, K.A.; Kerbel, R.S.; Griffioen, A.W. Tumors Resurrect an Embryonic Vascular Program to Escape Immunity. Sci. Immunol. 2022, 7, eabm6388. [Google Scholar] [CrossRef]
- Song, X.; Guo, Y.; Song, P.; Duan, D.; Guo, W. Non-Coding RNAs in Regulating Tumor Angiogenesis. Front. Cell Dev. Biol. 2021, 9, 751578. [Google Scholar] [CrossRef]
- Mabeta, P.; Hull, R.; Dlamini, Z. LncRNAs and the Angiogenic Switch in Cancer: Clinical Significance and Therapeutic Opportunities. Genes 2022, 13, 152. [Google Scholar] [CrossRef]
- El-Lateef, A.E.A.; El-Shemi, A.G.A.; Hassanein, R.A.M.; Iqbal, M.S.; Albloshi, S.A. Analysis of Correlation Between LncRNA TDRG1 Expression and Its Prognosis in Cervical Carcinoma Tissues. Appl. Biochem. Biotechnol. 2023, 196, 1079–1088. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, X.L.; Huang, H.G.; Li, Y.F.; Li, Z.G. LncRNA TDRG1 Promotes the Aggressiveness of Gastric Carcinoma through Regulating miR-873-5p/HDGF Axis. Biomed. Pharmacother. 2020, 121, 109425. [Google Scholar] [CrossRef]
- Hu, X.; Mu, Y.; Wang, J.; Zhao, Y. LncRNA TDRG1 Promotes the Metastasis of NSCLC Cell through Regulating miR-873-5p/ZEB1 Axis. J. Cell. Biochem. 2019, 122, 969–982. [Google Scholar] [CrossRef]
- Gong, Q.; Dong, W.; Fan, Y.; Chen, F.; Bian, X.; Xu, X.; Qian, T.; Yu, P. LncRNA TDRG1-Mediated Overexpression of VEGF Aggravated Retinal Microvascular Endothelial Cell Dysfunction in Diabetic Retinopathy. Front. Pharmacol. 2020, 10, 1703. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.; Sun, K.; Liu, Y.; Guan, X.; Zong, Z.; Zhao, Y. LncRNA TDRG1 Enhances Tumorigenicity in Endometrial Carcinoma by Binding and Targeting VEGF-A Protein. Biochim. Biophys. Acta BBA–Mol. Basis Dis. 2018, 1864, 3013–3021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Qin, H.; Leng, Y.; Li, X.; Zhang, L.; Bai, D.; Meng, Y.; Wang, J. LncRNA MEG3 Overexpression Inhibits the Development of Diabetic Retinopathy by Regulating TGF-β1 and VEGF. Exp. Ther. Med. 2018, 16, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Tang, J.; Chen, Y.; Deng, L.; Ji, J.; Xie, Y.; Wang, K.; Jia, W.; Chu, W.-M.; Sun, B. The Long Noncoding RNA Lnc-EGFR Stimulates T-Regulatory Cells Differentiation Thus Promoting Hepatocellular Carcinoma Immune Evasion. Nat. Commun. 2017, 8, 15129. [Google Scholar] [CrossRef]
- Chen, Z.; Guan, D.; Zhu, Q.; Wang, Z.; Han, F.; Zhou, W. Biological Roles and Pathogenic Mechanisms of LncRNA MIR4435-2HG in Cancer: A Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 8864–8881. [Google Scholar] [CrossRef]
- Collier, S.P.; Henderson, M.A.; Tossberg, J.T.; Aune, T.M. Regulation of the Th1 Genomic Locus from Ifng through Tmevpg1 by T-Bet. J. Immunol. 2014, 193, 3959–3965. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Benito-Lopez, J.J.; Marroquin-Muciño, M.; Perez-Medina, M.; Chavez-Dominguez, R.; Aguilar-Cazares, D.; Galicia-Velasco, M.; Lopez-Gonzalez, J.S. Partners in Crime: The Feedback Loop between Metabolic Reprogramming and Immune Checkpoints in the Tumor Microenvironment. Front. Oncol. 2023, 12, 1101503. [Google Scholar] [CrossRef]
- Beltrán-Anaya, F.O.; Cedro-Tanda, A.; Hidalgo-Miranda, A.; Romero-Cordoba, S.L. Insights into the Regulatory Role of Non-Coding RNAs in Cancer Metabolism. Front. Physiol. 2016, 7, 342. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Marsh-Wakefield, F.; Ferguson, A.L.; Liu, K.; Santhakumar, C.; McCaughan, G.; Palendira, U. Approaches to Spatially Resolving the Tumour Immune Microenvironment of Hepatocellular Carcinoma. Ther. Adv. Med. Oncol. 2022, 14, 17588359221113270. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Schietinger, A. CD8+ T Cell Differentiation and Dysfunction in Cancer. Nat. Rev. Immunol. 2021, 22, 209–223. [Google Scholar] [CrossRef]
- Andersen, M.H. Tumor Microenvironment Antigens. Semin. Immunopathol. 2022, 45, 253–264. [Google Scholar] [CrossRef]
- Schmidtmann, M.; D’Souza-Schorey, C. Extracellular Vesicles: Biological Packages That Modulate Tumor Cell Invasion. Cancers 2023, 15, 5617. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Arner, E.N.; Rathmell, J.C. Metabolic Programming and Immune Suppression in the Tumor Microenvironment. Cancer Cell 2023, 41, 421–433. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, e2403199. [Google Scholar] [CrossRef]
- Takahashi, K.; Yan, I.K.; Kogure, T.; Haga, H.; Patel, T. Extracellular Vesicle-mediated Transfer of Long Non-coding RNA ROR Modulates Chemosensitivity in Human Hepatocellular Cancer. FEBS Open Bio 2014, 4, 458–467. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L.; Li, J.; Du, Y.; Wang, J.; Liu, J. Effects of Long Noncoding RNA (Linc-VLDLR) Existing in Extracellular Vesicles on the Occurrence and Multidrug Resistance of Esophageal Cancer Cells. Pathol. Res. Pract. 2019, 215, 470–477. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Zhou, Z.-Y.; Dong, X.; Guo, L.; Zhang, K.-J. LncRNA GNAS-AS1 Facilitates ER+ Breast Cancer Cells Progression by Promoting M2 Macrophage Polarization via Regulating miR-433-3p/GATA3 Axis. Biosci. Rep. 2020, 40, BSR20200626. [Google Scholar] [CrossRef]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-Associated Fibroblasts Promote the Stemness and Chemoresistance of Colorectal Cancer by Transferring Exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Concepcion, J.; Uprety, D.; Adjei, A.A. Challenges in the Use of Targeted Therapies in Non-Small Cell Lung Cancer. Cancer Res. Treat. 2022, 54, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.B.; Zhou, H.; Simion, V.; Feinberg, M.W. Long Noncoding RNAs as Therapeutic Targets. Adv. Exp. Med. Biol. 2022, 1363, 161–175. [Google Scholar] [CrossRef]
- Saw, P.E.; Song, E. Current Development of RNA Delivery Systems. In RNA Therapeutics in Human Diseases; Springer Nature Singapore: Singapore, 2025; pp. 591–616. ISBN 978-981-96-3040-0. [Google Scholar]
| LncRNA | Effect on Immune Cells | Reference |
|---|---|---|
| MIAT | Overexpresses CD47, blocking macrophage-mediated phagocytosis | [52] |
| SNHG16 | Inhibits CD4 and CD8 T cell activity | [56] |
| SEMA3B-AS1 | Reduces inflammatory cytokines | [59] |
| ncRNA-RB1 | Overexpresses CRT, promoting antigen presentation | [85] |
| Lnc-DC | Stimulates DC differentiation and T cell activation | [90] |
| TP73-AS1 | Overexpresses HMGB1 | [97] |
| IFNG-AS1 | Increases IFN-γ expression | [98] |
| lnc-THRIL | Regulates TNF-α secretion | [99] |
| Effect on Immune Cells | Effect on Cancer Cells | Reference | |
|---|---|---|---|
| LURCR | - | Promotes tumorigenesis. | [66] |
| GOLGA2P10 | - | Inhibits cell death. | [67] |
| SOX2OT | - | Induces tumor resistance. | [69] |
| PACER, HULC, HOTAIR, MAFG-AS1 | Regulate COX-2 expression. | - | [109,111,113,119] |
| HULC, lnc-EGFR | Promote Tregs differentiation. | - | [113,194] |
| HOTAIRM1, ZEB2-AS1 | Reduce DC differentiation. | Promote proliferation, progression, migration, and apoptosis inhibition of cancer cells. | [119,150] |
| NEAT1 | Increases inflammatory cytokines and co-stimulatory molecules. | Correlated with proliferation, metastasis, survival, and drug resistance. | [122] |
| MIR155HG | Promotes antigen presentation. | Increases PD-1, PD-L1, and CTLA-4 in hypoxic conditions. | [123] |
| MALAT1 | Increases HMGB1 and reduces inflammatory cytokines. | Induces tolerogenic dendritic cells and promotes tumor growth. | [128,129] |
| LUCAT1 | Reduces Type I interferon. | Promotes tumor progression. | [131] |
| H19 | Reduces IL-2 production. | Promotes resistance in cancer cells. | [147] |
| OIP5-AS1 | Reduces CD25 expression. | Promotes cancer development and progression. | [150] |
| GATA3-AS1 | Favors Th2 differentiation. | - | [169] |
| DQ786243 | Induces Tregs by stimulating FOXP3 expression. | - | [172] |
| FENDRR | Promotes pro-inflammatory cytokine production. | - | [178] |
| MEG3 | Promotes Tregs differentiation, blocks Th17 cell differentiation. | - | [193] |
| MIR4435-2HG | - | Facilitates immune evasion. | [195] |
| Tmevpg1 | Favors Th1 differentiation. | - | [196] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Medina, M.; Benito-Lopez, J.J.; Aguilar-Cazares, D.; Lopez-Gonzalez, J.S. A Comprehensive Review of Long Non-Coding RNAs in the Cancer–Immunity Cycle: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 4821. https://doi.org/10.3390/ijms26104821
Perez-Medina M, Benito-Lopez JJ, Aguilar-Cazares D, Lopez-Gonzalez JS. A Comprehensive Review of Long Non-Coding RNAs in the Cancer–Immunity Cycle: Mechanisms and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(10):4821. https://doi.org/10.3390/ijms26104821
Chicago/Turabian StylePerez-Medina, Mario, Jesus J. Benito-Lopez, Dolores Aguilar-Cazares, and Jose S. Lopez-Gonzalez. 2025. "A Comprehensive Review of Long Non-Coding RNAs in the Cancer–Immunity Cycle: Mechanisms and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 10: 4821. https://doi.org/10.3390/ijms26104821
APA StylePerez-Medina, M., Benito-Lopez, J. J., Aguilar-Cazares, D., & Lopez-Gonzalez, J. S. (2025). A Comprehensive Review of Long Non-Coding RNAs in the Cancer–Immunity Cycle: Mechanisms and Therapeutic Implications. International Journal of Molecular Sciences, 26(10), 4821. https://doi.org/10.3390/ijms26104821







