Recent Advances in Nano-Drug Delivery Strategies for Chalcogen–Based Therapeutic Agents in Cancer Phototherapy
Abstract
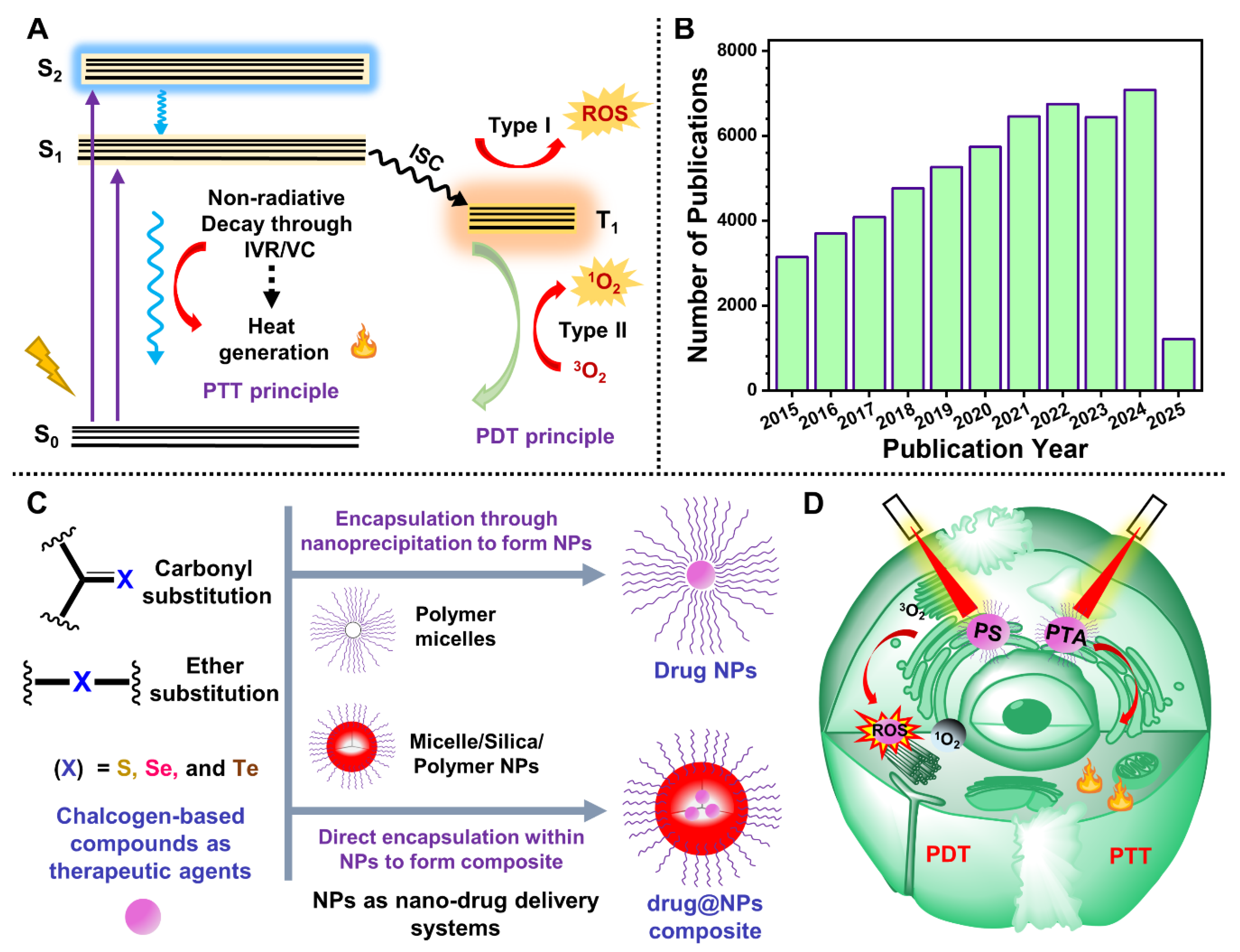
1. Introduction
- Sustainable synthesis: Chalcogen incorporation facilitates more efficient, economically viable synthetic strategies with high product yield and reproducibility, essential for scalable biomedical applications.
- Enhanced optical absorption: The substitution of heavier chalcogens extends the visible to NIR (VIS–NIR) absorption by lowering the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy gap and enhancing the intramolecular charge transfer (ICT) character.
- Improved ROS generation: The presence of heavier chalcogens enhances the spin-orbit coupling (SOC) constant due to the heavy–atom effect and narrows the singlet to triplet energy gap (ΔEST) to facilitate ISC, leading to high triplet harvesting and ROS production, which is crucial for PDT.
- Elevated PCE: Chalcogen substitution triggers ICT and other nonradiative processes, resulting in increased PCE, a fundamental property for effective PTT.
- Biocompatibility: Chalcogen–containing compounds or TAs demonstrate superior in vivo biocompatibility and lower dark cytotoxicity, thereby improving the biological window for synergistic therapy.
2. Advances in Nano Delivery Strategies for Sulfur–Containing TAs in Phototherapy
2.1. Thiocarbonyl–Based PSs in Phototherapy
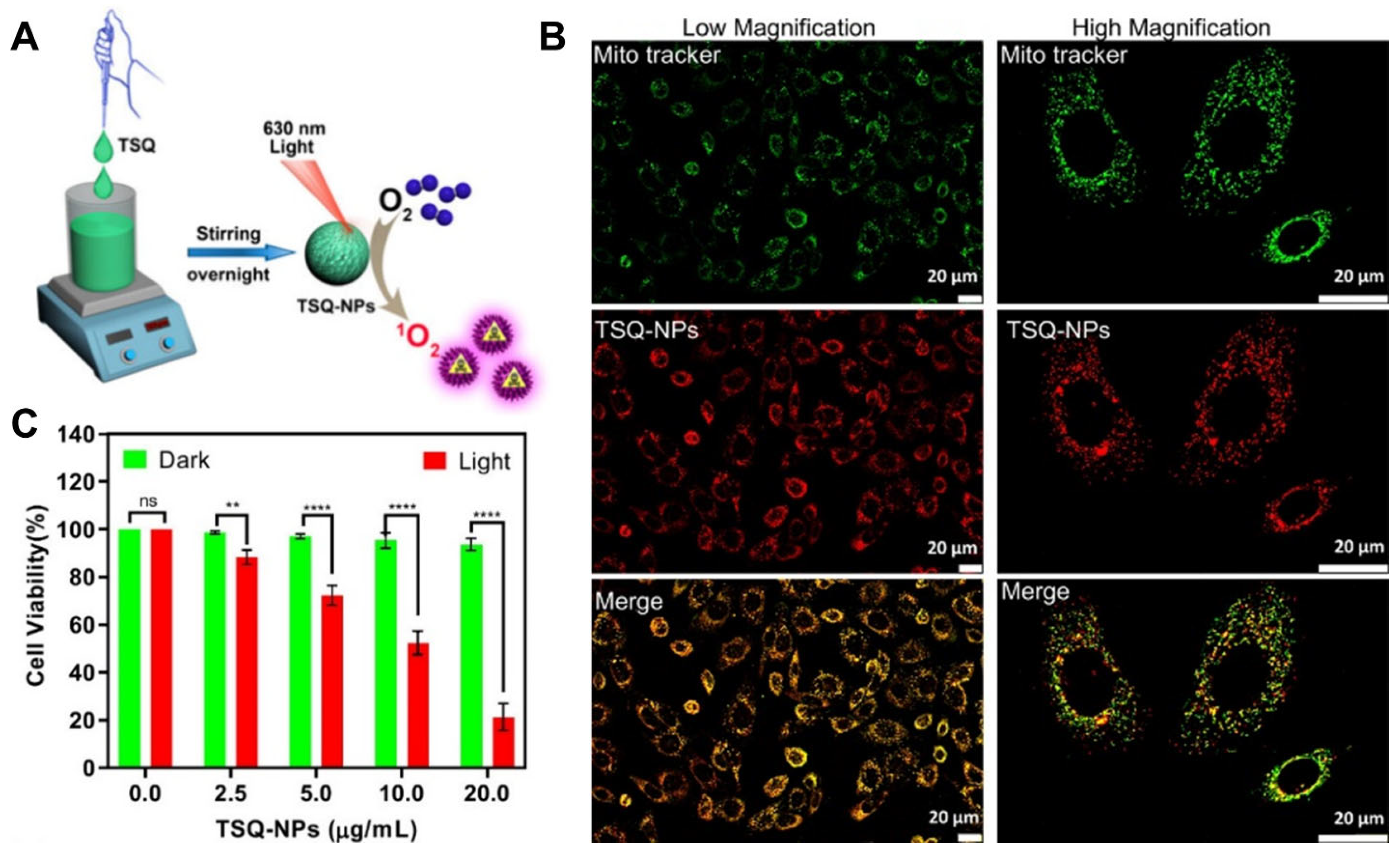
2.1.1. Northiosquaraine–Based PSs in PDT
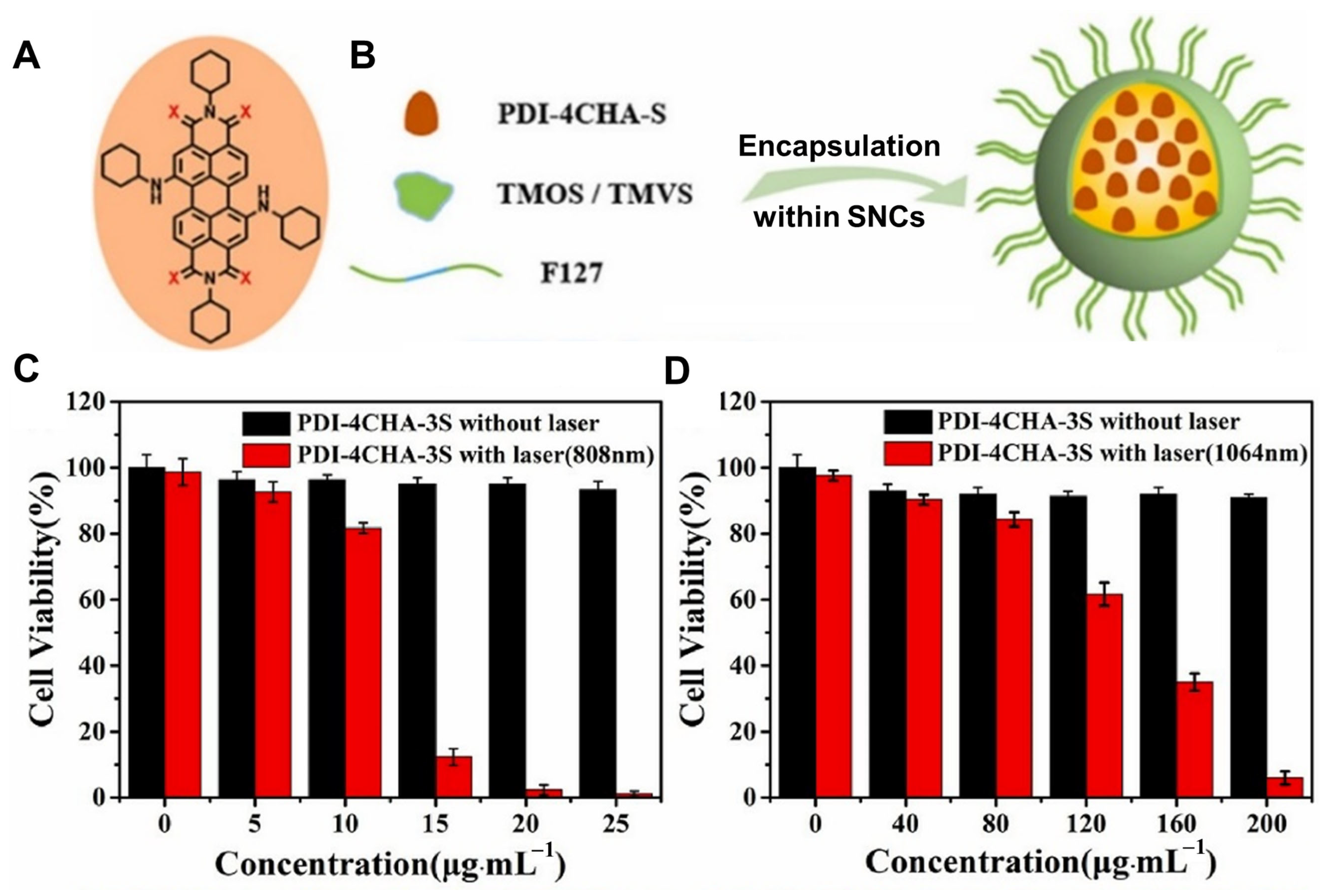
2.1.2. Thione–Derived Perylene Di–Imide–Based PTAs in PTT
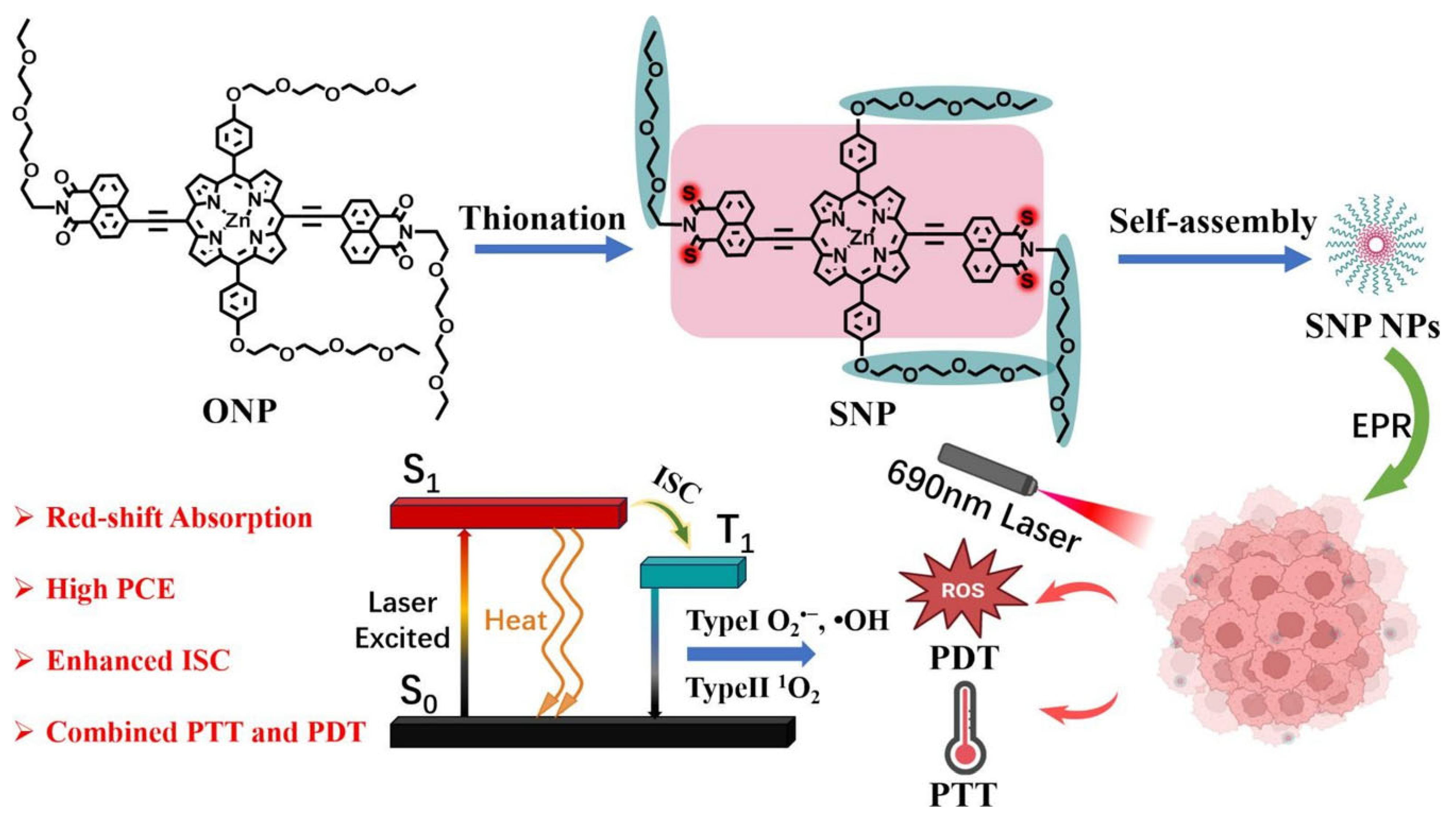
2.1.3. Thionated Porphyrin–Based TAs in Synergistic PDT/PTT
2.2. Thiophene–Fused PSs/PTAs in Phototherapy
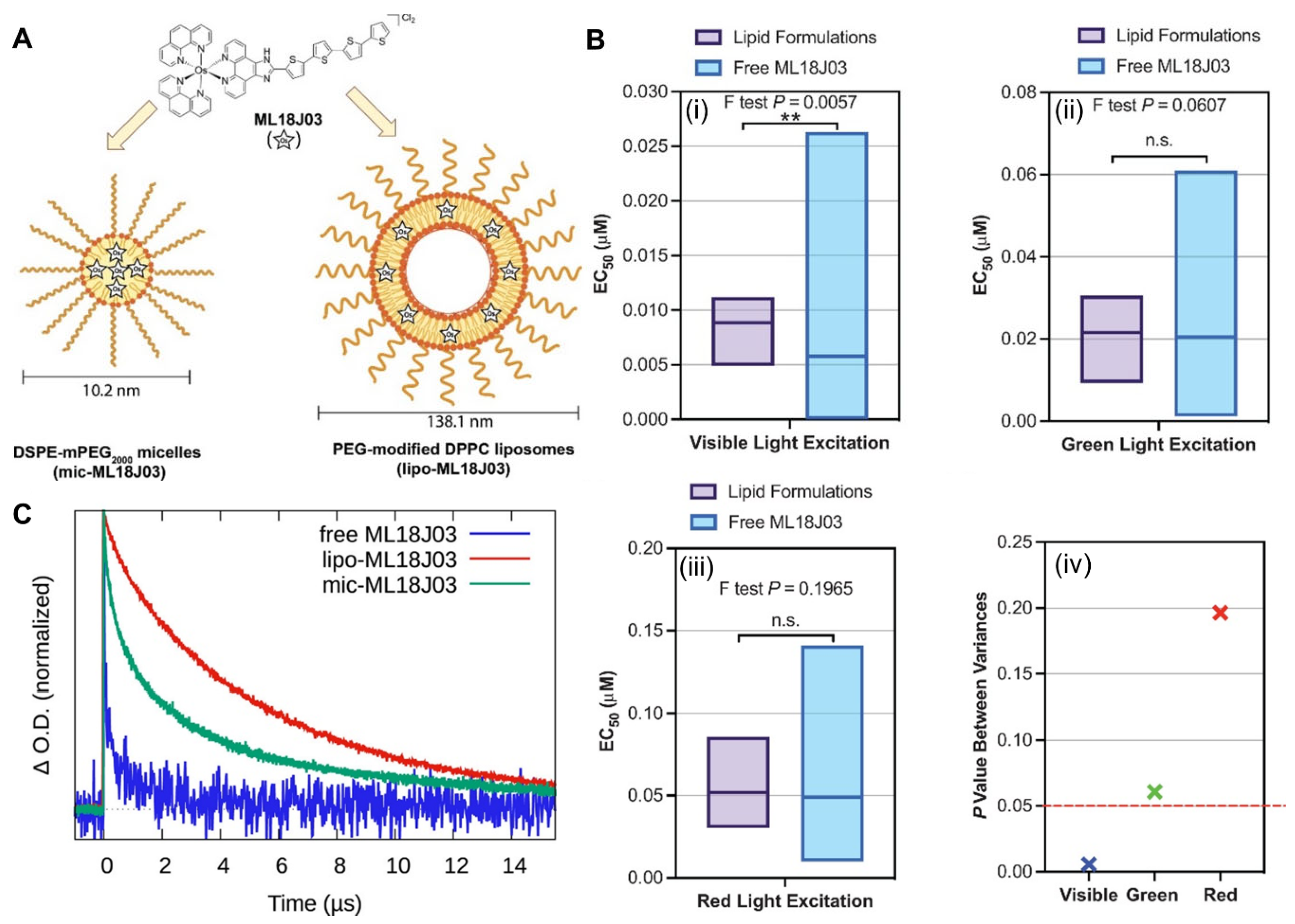
2.2.1. Thiophene–Fused Osmium–Based PSs in PDT
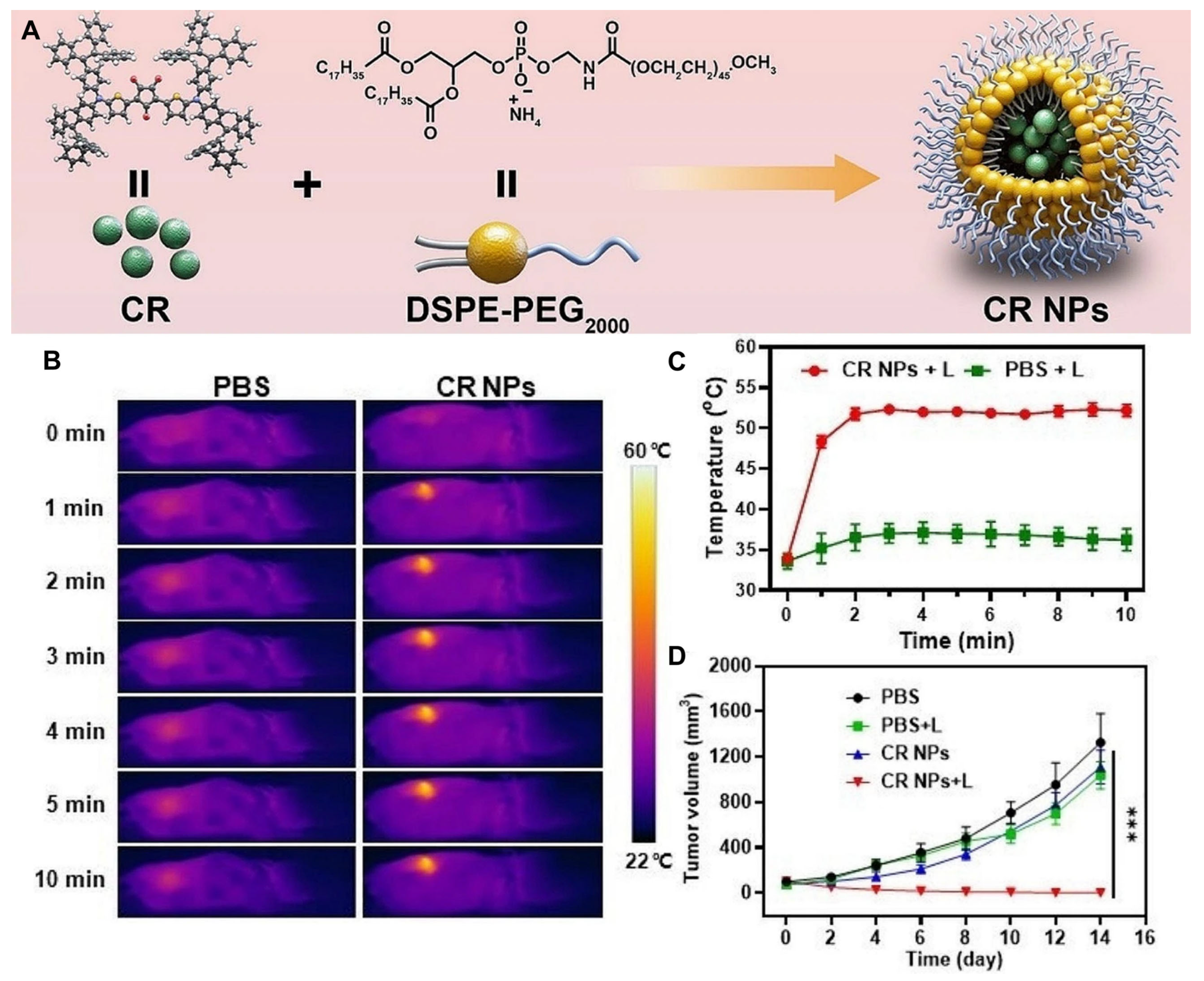
2.2.2. Thiophene–Fused Croconaine Dye–Based PTAs in PTT
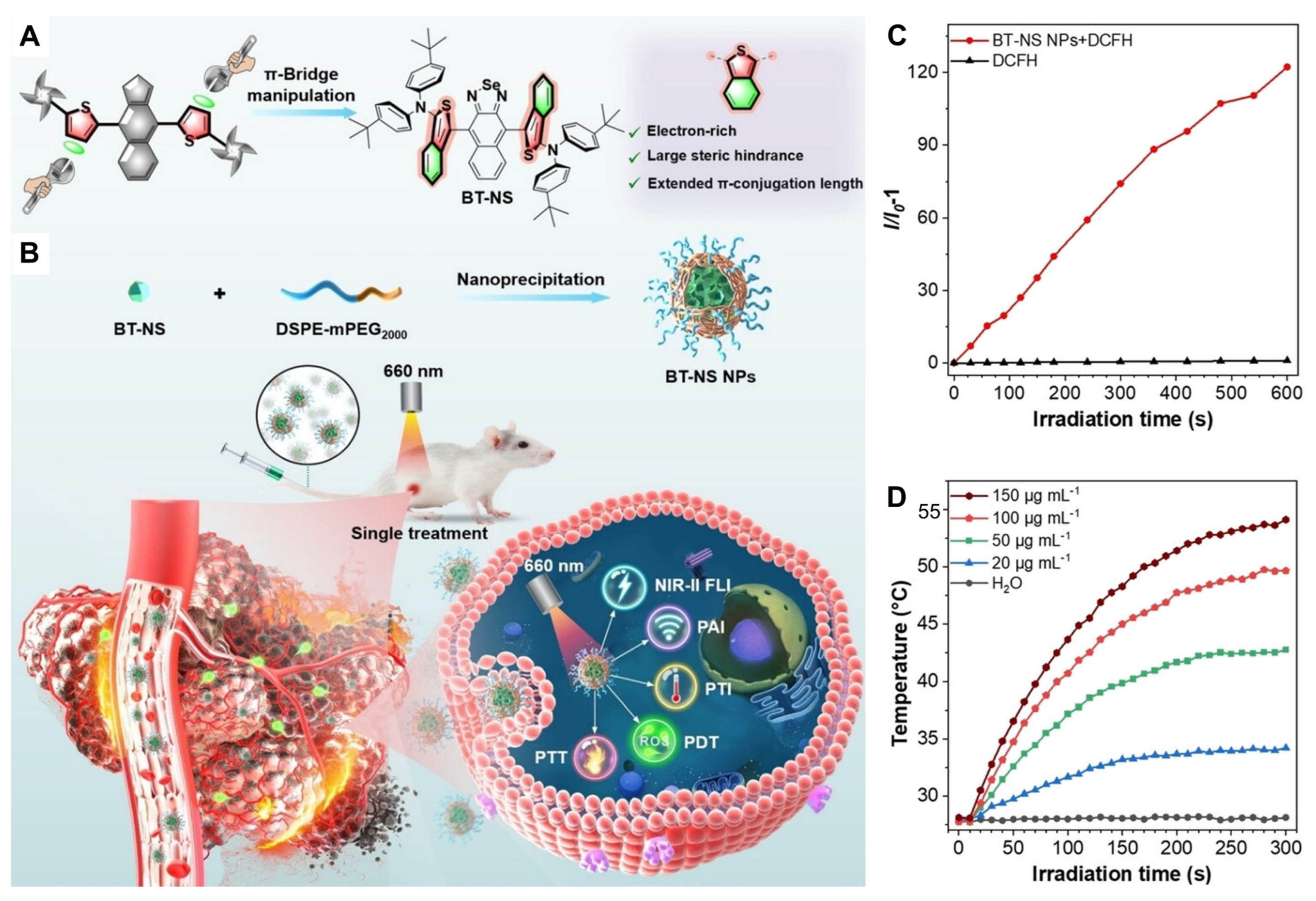
2.2.3. Thiophene π–Bridge Manipulation–Based TAs in Multimodal Synergistic PDT/PTT
3. Advances in Nano Delivery Strategies for Selenium–Containing TAs in Phototherapy
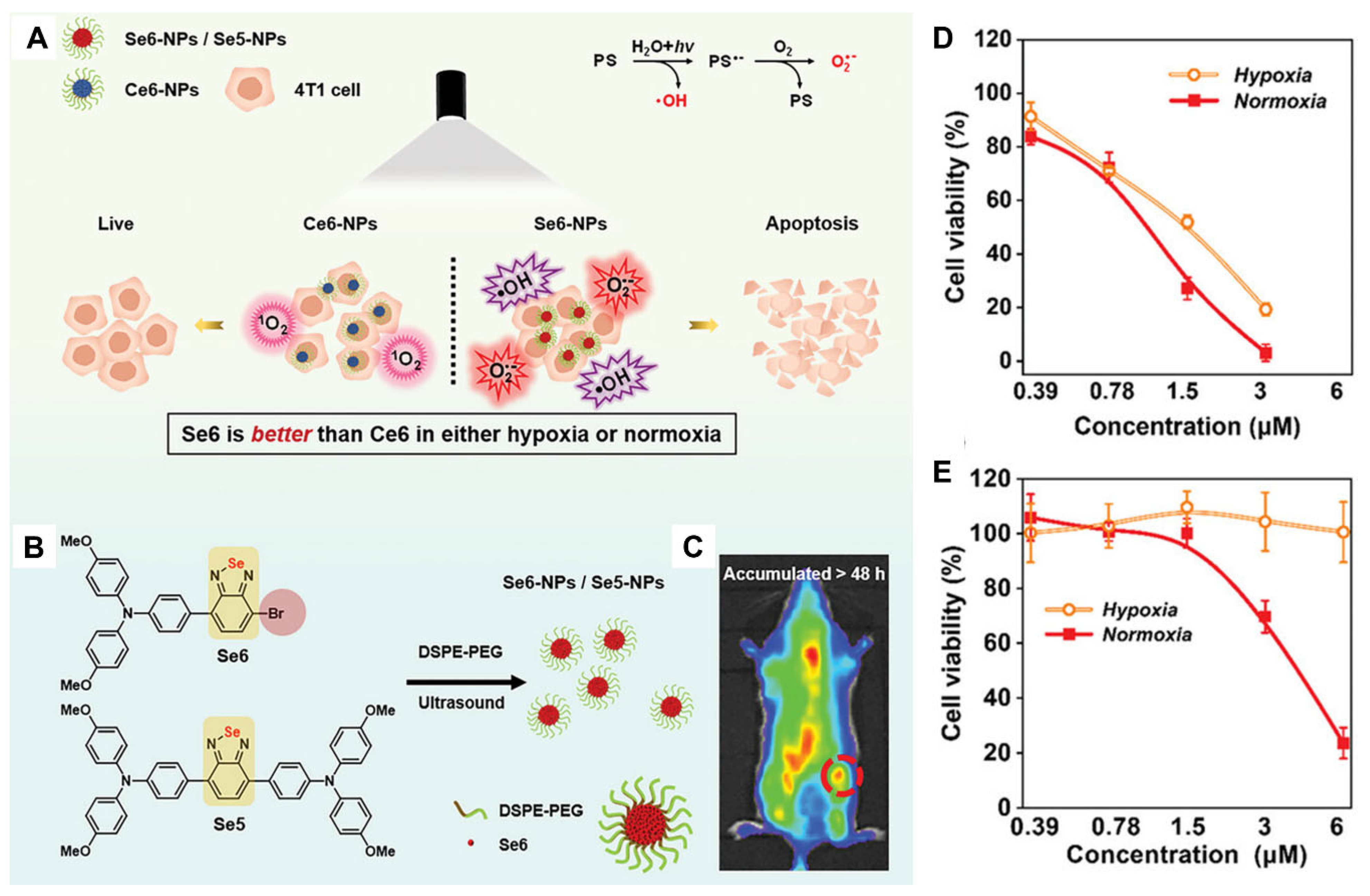
3.1. Benzoselenadiazole–Containing PSs in Type–I PDT
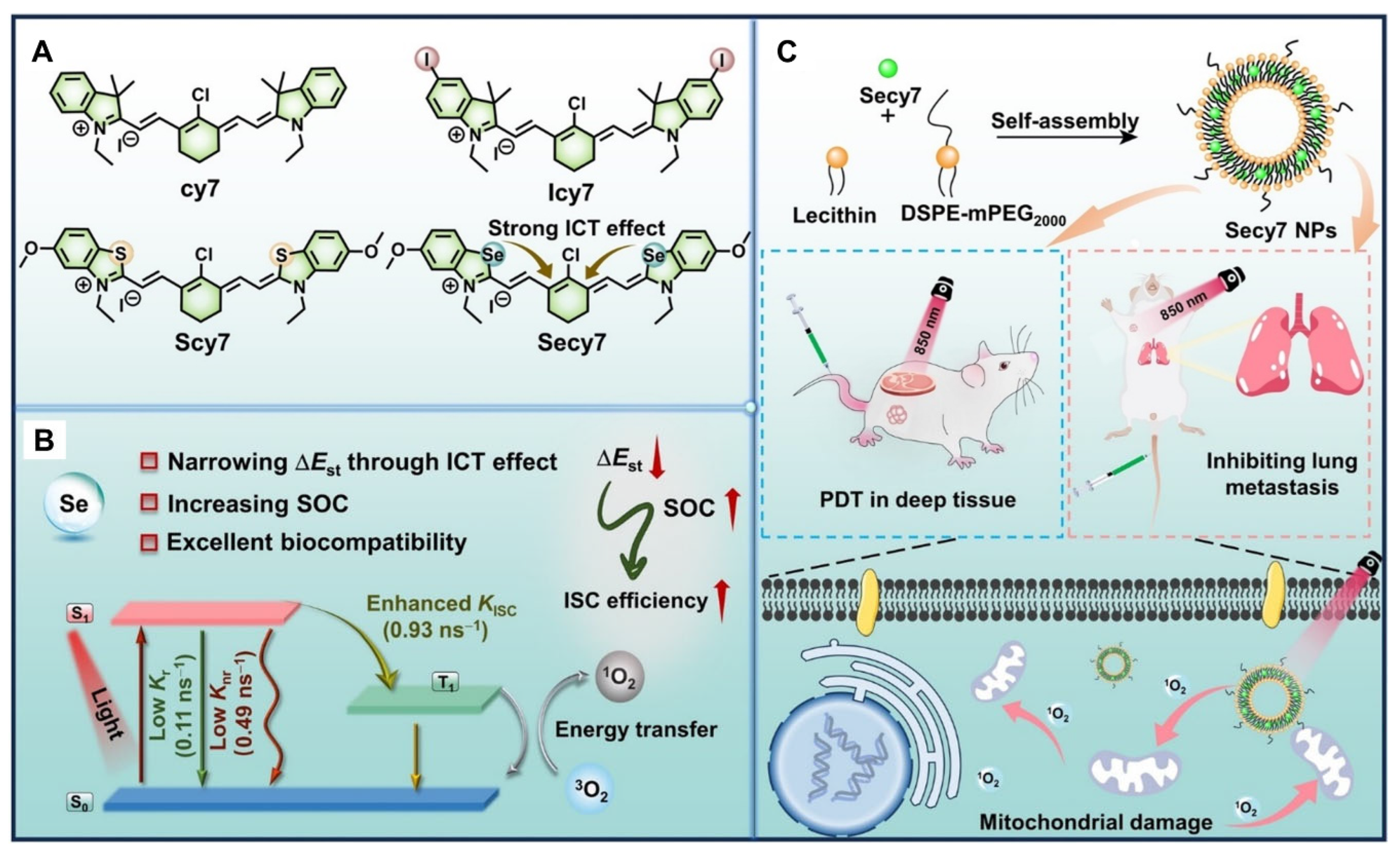
3.2. Heptamethine Cyanine PSs in NIR PDT
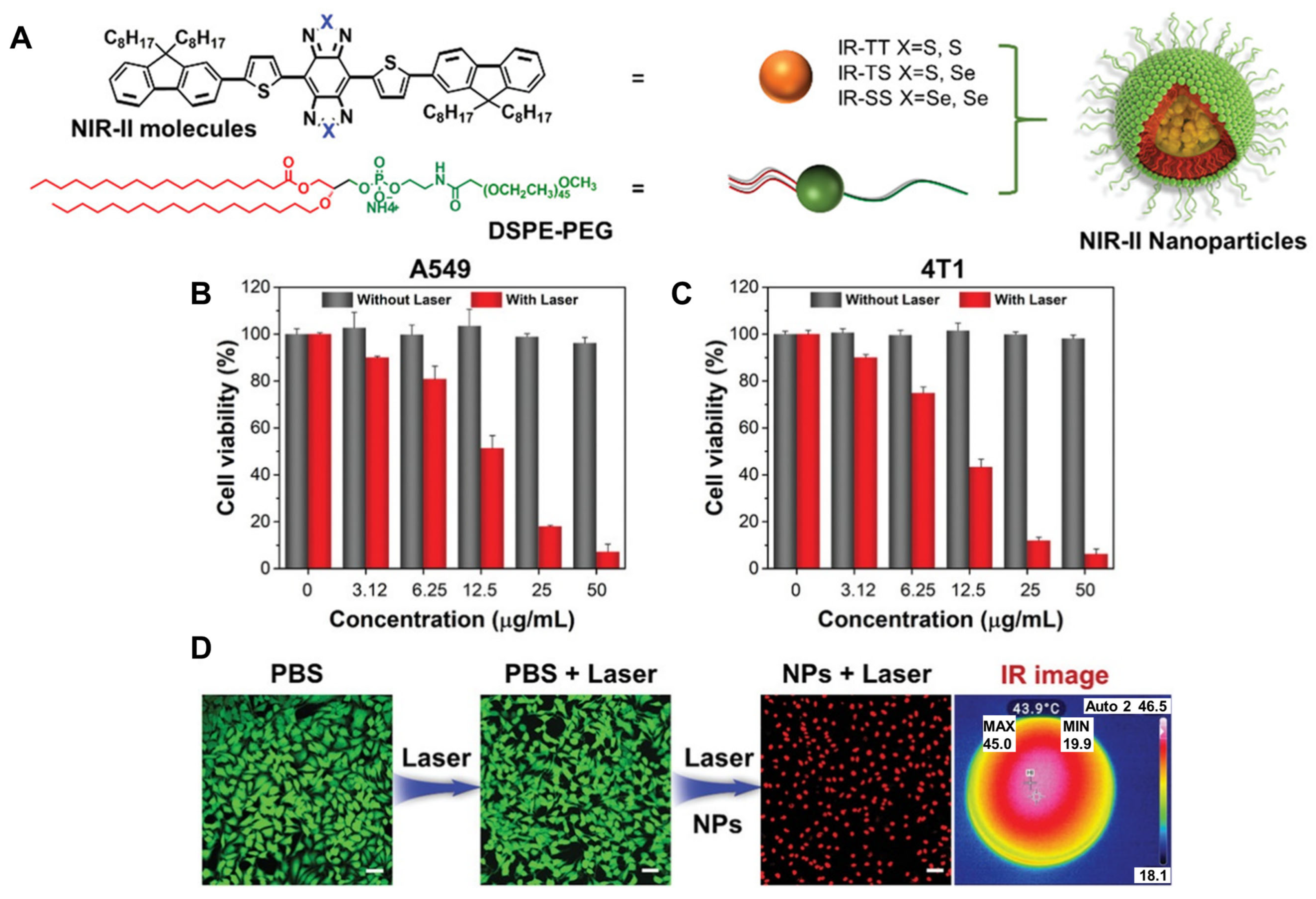
3.3. Conjugated Small Molecule–Based PTAs in NIR–II PTT
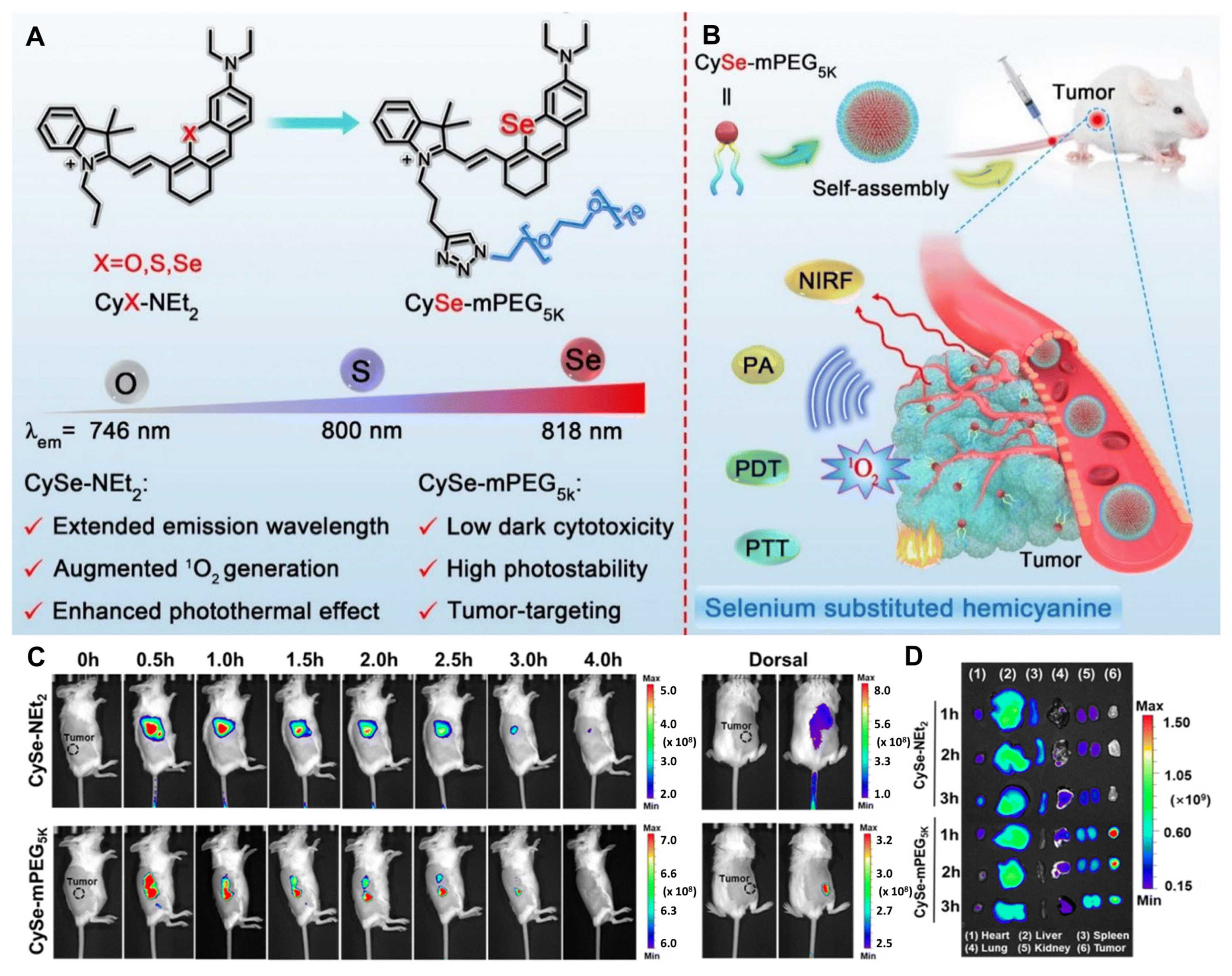
3.4. Single–Atom Engineered Hemicyanine–Based TAs in Imaging–Guided Synergistic PDT/PTT
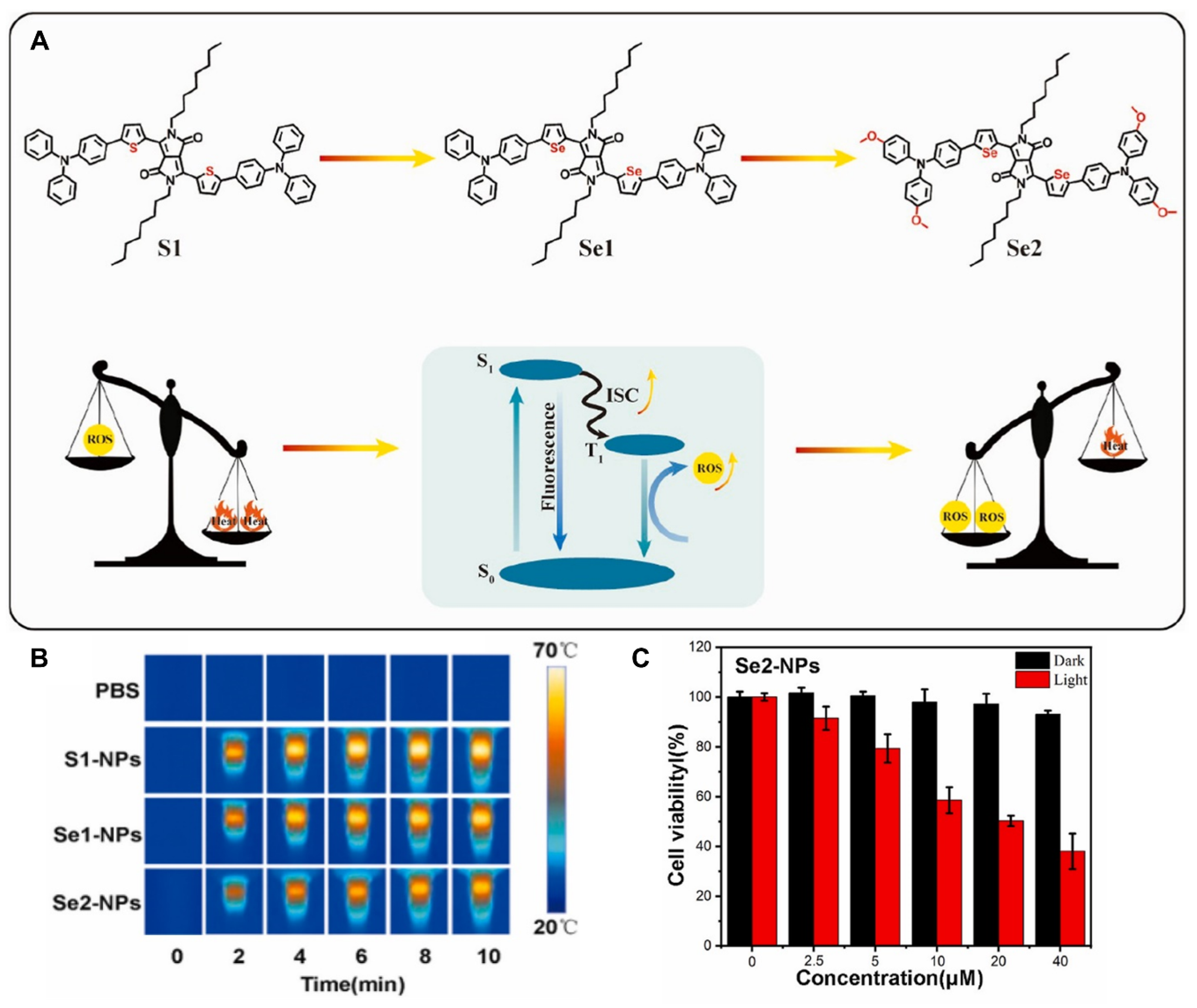
3.5. Selenophene–Fused Diketopyrrolopyrrole–Based TAs in Synergistic PDT/PTT
4. Advances in Nano Delivery Strategies for Tellurium–Containing TAs in Phototherapy
4.1. Chalcogenopyrylium–Fused Croconaine–Based PTAs in PTT
4.2. Poly–Pyrrole Tellurium–Based Polymer NPs as TAs in Synergistic PDT/PTT
4.3. Chalcogen–Modified Iodinated BODIPY PSs/PTAs in NIR Phototherapy
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AIEgens | Aggregation–induced emission luminogens |
| D-A | Donor–acceptor |
| DPBF | 1,3-diphenylisobenzofuran |
| DLS | Dynamic light scattering |
| HAFPSs | Heavy–atom free photosensitizers |
| HOMO | Highest occupied molecular orbitals |
| ICT | Intramolecular charge transfer |
| ISC | Inter–system crossing |
| LUMO | Lowest unoccupied molecular orbitals |
| NIR | Near infrared |
| NPs | Nanoparticles |
| PAI | Photoacoustic imaging |
| PBS | Phosphate buffer solution |
| PCE | Photothermal conversion efficiency |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PSs | Photosensitizers |
| PTAs | Photothermal agents |
| PTT | Photothermal therapy |
| ROS | Reactive oxygen species |
| 1O2 | Singlet oxygen |
| SEM | Scanning electron microscopy |
| SOC | Spin–orbit coupling |
| ΔEST | Singlet–triplet energy gap |
| TD-DFT | Time–dependent density functional theory |
| TEM | Transmission electron microscopy |
| VIS | Visible |
| VIS-NIR | Visible to NIR |
References
- Murray, S.G.; Hartley, F.R. Coordination chemistry of thioethers, selenoethers, and telluroethers in transition-metal complexes. Chem. Rev. 1981, 81, 365–414. [Google Scholar] [CrossRef]
- Ma, Y.-Z.; Yu, L.; Zhou, Q.; Fu, W. Dinuclear ytterbium(iii) benzamidinate complexes with bridging s32−, se22− and te22− ligands: Synthesis, structure and magnetic properties. Dalton Trans. 2024, 53, 8118–8123. [Google Scholar] [CrossRef]
- Kaur, M.; Rob, A.; Caton-Williams, J.; Huang, Z. Biochemistry of nucleic acids functionalized with sulfur, selenium, and tellurium: Roles of the single-atom substitution. In Biochalcogen Chemistry: The Biological Chemistry of Sulfur, Selenium, and Tellurium; American Chemical Society: Washington, DC, USA, 2013; Volume 1152, pp. 89–126. [Google Scholar]
- Yang, S.; Zhao, G.; Gao, Y.; Sun, Y.; Zhang, G.; Fan, X.; Li, Y.; Li, Y. In-solution direct oxidative coupling for the integration of sulfur/selenium into DNA-encoded chemical libraries. Chem. Sci. 2022, 13, 2604–2613. [Google Scholar] [CrossRef]
- Chen, X.; Li, B. How nature incorporates sulfur and selenium into bioactive natural products. Curr. Opin. Chem. Biol. 2023, 76, 102377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ding, X.; Chen, X.; Ni, Z.; He, T.; Qiu, H.; Li, C.-Z.; Zhang, Q. Deciphering the effect of chalcogen-incorporated heterocycles in nonfused electron acceptors for organic photovoltaics. Dyes Pigm. 2022, 207, 110680. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Jiang, X.; An, J.; Dai, J.; Ying, Y.; Li, R.; Wang, W.; Liu, L.; Wu, H. Chalcogen-based fluorescent probes for metal ion detection: Principles, applications, and design strategies. Coord. Chem. Rev. 2024, 513, 215896. [Google Scholar] [CrossRef]
- Baez-Castro, A.; Peñuelas, C.A.; Soto-Rojo, R.; Soto-Acosta, S.; Delgado-Montiel, T.; Luque-Román, M.; Ruelas-Ávila, M.E.; Glossman-Mitnik, D.; Baldenebro-López, J. Computational analysis of chalcogen-enhanced triphenylamine–based d-π-a sensitizers for DSSCS: A DFT study. Struct. Chem. 2025. [Google Scholar] [CrossRef]
- Chand, A.; Sahoo, D.K.; Rana, A.; Jena, S.; Biswal, H.S. The prodigious hydrogen bonds with sulfur and selenium in molecular assemblies, structural biology, and functional materials. Acc. Chem. Res. 2020, 53, 1580–1592. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Gurbanov, A.V.; Aliyeva, V.A.; Guedes da Silva, M.F.C.; Resnati, G.; Pombeiro, A.J.L. Chalcogen bonding in coordination chemistry. Coord. Chem. Rev. 2022, 464, 214556. [Google Scholar] [CrossRef]
- Jena, S.; Tulsiyan, K.D.; Rana, A.; Choudhury, S.S.; Biswal, H.S. Non-conventional hydrogen bonding and aromaticity: A systematic study on model nucleobases and their solvated clusters. ChemPhysChem 2020, 21, 1826–1835. [Google Scholar] [CrossRef]
- Saez Talens, V.; Davis, J.; Wu, C.-H.; Wen, Z.; Lauria, F.; Gupta, K.B.S.S.; Rudge, R.; Boraghi, M.; Hagemeijer, A.; Trinh, T.T.; et al. Thiosquaramide-based supramolecular polymers: Aromaticity gain in a switched mode of self-assembly. J. Am. Chem. Soc. 2020, 142, 19907–19916. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Routray, C.; Dutta, J.; Biswal, H.S. Hydrogen bonding directed reversal of 13c nmr chemical shielding. Angew. Chem. Int. Ed. 2022, 61, e202207521. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, J.; Mazzone, G.; Russo, N.; Bertini, L. Photophysical properties of s, se and te-substituted deoxyguanosines: Insight into their ability to act as chemotherapeutic agents. J. Chem. Inf. Model. 2017, 57, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Tulsiyan, K.D.; Kar, R.K.; Kisan, H.K.; Biswal, H.S. Doubling förster resonance energy transfer efficiency in proteins with extrinsic thioamide probes: Implications for thiomodified nucleobases. Chem. Eur. J. 2021, 27, 4373–4383. [Google Scholar] [CrossRef]
- Takeda, Y. Modulating the photophysical properties of twisted donor–acceptor–donor π-conjugated molecules: Effect of heteroatoms, molecular conformation, and molecular topology. Acc. Chem. Res. 2024, 57, 2219–2232. [Google Scholar] [CrossRef]
- Jena, S.; Tulsiyan, K.D.; Sahoo, R.R.; Rout, S.; Sahu, A.K.; Biswal, H.S. Critical assessment of selenourea as an efficient small molecule fluorescence quenching probe to monitor protein dynamics. Chem. Sci. 2023, 14, 14200–14210. [Google Scholar] [CrossRef]
- Henriquez-Figuereo, A.; Morán-Serradilla, C.; Angulo-Elizari, E.; Sanmartín, C.; Plano, D. Small molecules containing chalcogen elements (s, se, te) as new warhead to fight neglected tropical diseases. Eur. J. Med. Chem. 2023, 246, 115002. [Google Scholar] [CrossRef]
- Domínguez-Álvarez, E.; Rácz, B.; Marć, M.A.; Nasim, M.J.; Szemerédi, N.; Viktorová, J.; Jacob, C.; Spengler, G. Selenium and tellurium in the development of novel small molecules and nanoparticles as cancer multidrug resistance reversal agents. Drug Resist. Updat. 2022, 63, 100844. [Google Scholar] [CrossRef]
- Senthamil, C.; Hemalatha, J.; Nandhabala, S.; Nivetha, A.; Sakthivel, C.; Prabha, I. Multifunctionalized metal chalcogenides and their roles in catalysis and biomedical applications. ChemistrySelect 2022, 7, e202203394. [Google Scholar] [CrossRef]
- Dansereau, S.J.; Sheng, J. Heavy chalcogen properties of sulfur and selenium enhance nucleic acid-based therapeutics. Biomolecules 2025, 15, 218. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Marć, M.A.; Domínguez-Álvarez, E.; Sanmartín, C. Organoselenium compounds as novel adjuvants of chemotherapy drugs—A promising approach to fight cancer drug resistance. Molecules 2019, 24, 336. [Google Scholar] [CrossRef]
- Morán-Serradilla, C.; Plano, D.; Sharma, A.K.; Sanmartín, C. Following the trace of cyclodextrins on the selenium and tellurium odyssey. Int. J. Mol. Sci. 2024, 25, 7799. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Deng, Q.; Zhang, Y.; Li, X.; Wen, G.; Cui, X.; Wan, Y.; Huang, Y.; Chen, J.; Liu, Z.; et al. Rational design of conjugated small molecules for superior photothermal theranostics in the NIR-II biowindow. Adv. Mater. 2020, 32, 2001146. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Tan, H.; Peng, Q.; Chen, H.; Ma, H.; Wang, L.; Peng, A.; Shi, Q.; Cai, X.; Huang, H. Achieving efficient NIR-II type-I photosensitizers for photodynamic/photothermal therapy upon regulating chalcogen elements. Adv. Mater. 2022, 34, 2108146. [Google Scholar] [CrossRef]
- Liu, H.; Li, H.; Li, W.; Zhang, J.; Ye, J.; Liao, S.; Li, Y.; Yin, S. Chalcogen modification: One-step strategy for tuning the photophysical properties and nir phototherapy of iodinated bodipy. Mater. Chem. Front. 2024, 8, 3308–3320. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Yang, G.; Huang, G.; Dai, H.; Yu, J.; Liu, N. Chalcogen atom-modulated croconaine for efficient nir-ii photothermal theranostics. ACS Appl. Mater. Interfaces 2024, 16, 12332–12338. [Google Scholar] [CrossRef]
- Jena, S.; Mohanty, P.; Dash, L.; Biswal, H.S. Expanding the horizons of phototherapy: A review on the superior performance of chalcogen-based photosensitizers and photothermal agents for cancer therapy. ChemPhotoChem 2025, 2400415. [Google Scholar] [CrossRef]
- Saleeby, C.W. The advance of heliotherapy. Nature 1922, 109, 663. [Google Scholar] [CrossRef]
- McDonagh, A.F. Phototherapy: From ancient Egypt to the new millennium. J. Perinatol. 2001, 21, S7–S12. [Google Scholar] [CrossRef]
- Grzybowski, A.; Sak, J.; Pawlikowski, J. A brief report on the history of phototherapy. Clin. Dermatol. 2016, 34, 532–537. [Google Scholar] [CrossRef]
- Zanolli, M. The modern paradigm of phototherapy. Clin. Dermatol. 2003, 21, 398–406. [Google Scholar] [CrossRef]
- Grundmann, S.A.; Beissert, S. Modern aspects of phototherapy for atopic dermatitis. J. Allergy 2012, 2012, 121797. [Google Scholar] [CrossRef] [PubMed]
- Hönigsmann, H. History of phototherapy in dermatology. Photochem. Photobiol. Sci. 2013, 12, 16–21. [Google Scholar] [CrossRef]
- Roelandts, R. A new light on Niels Finsen, a century after his Nobel Prize. Photodermatol. Photoimmunol. Photomed. 2005, 21, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bule, M.L.; Naharro-Rodríguez, J.; Bacci, S.; Fernández-Guarino, M. Unlocking the power of light on the skin: A comprehensive review on photobiomodulation. Int. J. Mol. Sci. 2024, 25, 4483. [Google Scholar] [CrossRef]
- Lo, P.-C.; Ng, D.K.P.; Pandey, R.K.; Zimcik, P. Photodynamic therapy: An innovative and versatile treatment modality triggered by light. ChemPlusChem 2023, 88, e202300159. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, K.; Kang, M.; Yan, D.; Niu, N.; Yan, S.; Sun, P.; Zhang, L.; Sun, L.; Wang, D.; et al. A new era of cancer phototherapy: Mechanisms and applications. Chem. Soc. Rev. 2024, 53, 12014–12042. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Pathak, R.; Samanta, S.; Sen Sarma, N. A comprehensive analysis of photothermal therapy (PTT) and photodynamic therapy (PDT) for the treatment of cancer. Process Biochem. 2025, 148, 17–31. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy–mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Ji, W.; Wang, J.; Wang, N.; Wu, W.; Wu, Q.; Hou, X.; Hu, W.; Li, L. Near infrared photothermal conversion materials: Mechanism, preparation, and photothermal cancer therapy applications. J. Mater. Chem. B 2021, 9, 7909–7926. [Google Scholar] [CrossRef]
- Jena, S.; Mohanty, P.; Rout Rout, S.; Kumar Pati, S.; Biswal, H.S. Thio and seleno-psoralens as efficient triplet harvesting photosensitizers for photodynamic therapy. Chem. Eur. J. 2024, 30, e202400733. [Google Scholar] [CrossRef]
- Mohanty, P.; Sarang, S.; Rout, S.; Biswal, H.S. Thio and seleno derivatives of angelicin as efficient triplet harvesting photosensitizers: Implications in photodynamic therapy. ChemPhysChem 2024, 25, e202400636. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.d.S.; Costa, É.d.O.; Duarte, T.G.; Charret, T.S.; Castiglione, R.C.; Simões, R.L.; Pascoal, V.D.B.; Döring, T.H.; da Silva, F.d.C.; Ferreira, V.F.; et al. New chalcogen-functionalized naphthoquinones: Design, synthesis, and evaluation, in vitro and in silico, against squamous cell carcinoma. ACS Omega 2024, 9, 21948–21963. [Google Scholar] [CrossRef] [PubMed]
- Sonego, J.M.; de Diego, S.I.; Szajnman, S.H.; Gallo-Rodriguez, C.; Rodriguez, J.B. Organoselenium compounds: Chemistry and applications in organic synthesis. Chem. Eur. J. 2023, 29, e202300030. [Google Scholar] [CrossRef]
- Hoover, G.C.; Seferos, D.S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019, 10, 9182–9188. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Rheima, A.M.; Kadhim, M.M.; Ahmed, N.N.; Mohammed, S.H.; Abbas, F.H.; Abed, Z.T.; Mahdi, Z.M.; Abbas, Z.S.; Hachim, S.K.; et al. An overview of nanoparticles in drug delivery: Properties and applications. S. Afr. J. Chem. Eng. 2023, 46, 233–270. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Bhandari, M.; Nguyen, S.; Yazdani, M.; Utheim, T.P.; Hagesaether, E. The therapeutic benefits of nanoencapsulation in drug delivery to the anterior segment of the eye: A systematic review. Front. Pharmacol. 2022, 13, 903519. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, S.; Wang, J.; Chen, Q. A review on polymer and lipid-based nanocarriers and its application to nano-pharmaceutical and food-based systems. Front. Nutr. 2021, 8, 783831. [Google Scholar] [CrossRef]
- Kong, C.; Chen, X. Combined photodynamic and photothermal therapy and immunotherapy for cancer treatment: A review. Int. J. Nanomed. 2022, 17, 6427–6446. [Google Scholar] [CrossRef] [PubMed]
- Mishchenko, T.; Balalaeva, I.; Gorokhova, A.; Vedunova, M.; Krysko, D.V. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis. 2022, 13, 455. [Google Scholar] [CrossRef]
- Urazaliyeva, A.; Kanabekova, P.; Beisenbayev, A.; Kulsharova, G.; Atabaev, T.; Kim, S.; Lim, C.-K. All organic nanomedicine for PDT–PTT combination therapy of cancer cells in hypoxia. Sci. Rep. 2024, 14, 17507. [Google Scholar] [CrossRef]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and photothermal therapies: Synergy opportunities for nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Choi, K.Y. Advances in nanomaterial-mediated photothermal cancer therapies: Toward clinical applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Li, G.; Wang, C.; Jin, B.; Sun, T.; Sun, K.; Wang, S.; Fan, Z. Advances in smart nanotechnology-supported photodynamic therapy for cancer. Cell Death Discov. 2024, 10, 466. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.-A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2023, 23, 471–507. [Google Scholar] [CrossRef]
- Rauchfuss, T. Under sulfur’s spell. Nat. Chem. 2011, 3, 648. [Google Scholar] [CrossRef]
- Scott, K.A.; Njardarson, J.T. Analysis of us FDA-approved drugs containing sulfur atoms. Top. Curr. Chem 2018, 376, 5. [Google Scholar] [CrossRef]
- Minghao, F.; Bingqing, T.; Steven, H.L.; Xuefeng, J. Sulfur containing scaffolds in drugs: Synthesis and application in medicinal chemistry. Curr. Top. Med. Chem. 2016, 16, 1200–1216. [Google Scholar]
- Nguyen, V.-N.; Yan, Y.; Zhao, J.; Yoon, J. Heavy-atom-free photosensitizers: From molecular design to applications in the photodynamic therapy of cancer. Acc. Chem. Res. 2021, 54, 207–220. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, X.; Chen, X.; Zhao, J. Heavy atom-free triplet photosensitizers: Molecular structure design, photophysical properties and application in photodynamic therapy. Molecules 2023, 28, 2170. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Rodríguez, L.A.; Crespo-Hernández, C.E. Thionated organic compounds as emerging heavy-atom-free photodynamic therapy agents. Chem. Sci. 2020, 11, 11113–11123. [Google Scholar] [CrossRef]
- Pollum, M.; Jockusch, S.; Crespo-Hernández, C.E. 2,4-dithiothymine as a potent uva chemotherapeutic agent. J. Am. Chem. Soc. 2014, 136, 17930–17933. [Google Scholar] [CrossRef]
- Jena, S.; Tulsiyan, K.D.; Kumari, A.; Das, R.; Biswal, H.S. Thiolumazines as heavy-atom-free photosensitizers for applications in daylight photodynamic therapy: Insights from ultrafast excited-state dynamics. J. Phys. Chem. B 2022, 126, 6083–6094. [Google Scholar] [CrossRef]
- Sriwastav, S.; Sarkar, A.R.; Datta, A.; Jana, N.R.; Malik, S. Northiosquaraine-based nanoparticles for red-light-induced photodynamic therapy via singlet oxygen generation. ACS Appl. Nano Mater. 2024, 7, 21674–21682. [Google Scholar] [CrossRef]
- An, F.; Zhao, Y.; Li, H.; Meng, J.; Jiao, L.; Zhang, Z.; Li, X.; Sun, X. Intramolecular charge transfer versus intersystem crossing: The way toward super-high photothermal efficiency by thionation. Dyes Pigm. 2023, 217, 111411. [Google Scholar] [CrossRef]
- Tong, S.; Cheng, Y.; Liu, H.; Pang, Y.; Lin, X.; Hu, Z.; Wu, F. Thionation of conjugated porphyrin with enhanced photodynamic and photothermal effects for cancer therapy. J. Photochem. Photobiol. A Chem. 2025, 459, 116085. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Jin, X.; Deng, Q.; Yin, Z.; Tong, S.; Qing, W.; Huang, Y. Regioisomer-manipulating thio-perylenediimide nanoagents for photothermal/photodynamic theranostics. J. Mater. Chem. B 2020, 8, 5535–5544. [Google Scholar] [CrossRef]
- Fam, K.T.; Collot, M.; Klymchenko, A.S. Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chem. Sci. 2020, 11, 8240–8248. [Google Scholar] [CrossRef]
- Mei, A.; He, X.; Lei, D.; Wang, L.; Wang, W.; Shao, J.; Shen, Q.; Jiang, F.; Dong, X. Squaraine-based nir dyes for phototheranostics. Coord. Chem. Rev. 2025, 527, 216419. [Google Scholar] [CrossRef]
- Kharchenko, O.; Hryniuk, A.; Krupka, O.; Hudhomme, P. Synthesis of thionated perylenediimides: State of the art and first investigations of an alternative to lawesson’s reagent. Molecules 2024, 29, 2538. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, Q. Recent advances in perylene diimides (PDI)-based small molecules used for emission and photothermal conversion. ChemPhotoChem 2024, 8, e202300213. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Chou, Y.-T.; Su, B.-K.; Wu, C.-c.; Wang, C.-H.; Chang, K.-H.; Ho, J.-a.A.; Chou, P.-T. Comprehensive thione-derived perylene diimides and their bio-conjugation for simultaneous imaging, tracking, and targeted photodynamic therapy. J. Am. Chem. Soc. 2022, 144, 17249–17260. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Liu, N.S.; He, B.; Wong, S.Y.; Chen, Z.-K.; Li, X.; Wang, J. Facile synthesis of hybrid silica nanocapsules by interfacial templating condensation and their application in fluorescence imaging. Chem. Commun. 2009, 6240–6242. [Google Scholar] [CrossRef] [PubMed]
- Kharchenko, O.; Gouju, J.; Verdu, I.; Bastiat, G.; Hudhomme, P.; Passirani, C.; Saulnier, P.; Krupka, O. Heavy-atom-free photosensitizer-loaded lipid nanocapsules for photodynamic therapy. ACS Appl. Bio Mater. 2025, 8, 3086–3095. [Google Scholar] [CrossRef]
- Huang, H.; Song, W.; Rieffel, J.; Lovell, J.F. Emerging applications of porphyrins in photomedicine. Front. Phys. 2015, 3, 23. [Google Scholar] [CrossRef]
- Akbar, A.; Khan, S.; Chatterjee, T.; Ghosh, M. Unleashing the power of porphyrin photosensitizers: Illuminating breakthroughs in photodynamic therapy. J. Photochem. Photobiol. B 2023, 248, 112796. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Tian, Z.; Liu, X.; Guo, Y.; He, J.; Wang, Z.; Zhou, T.; Liu, Y. Porphyrin-based nanoparticles: A promising phototherapy platform. ChemPlusChem 2022, 87, e202200156. [Google Scholar] [CrossRef]
- Sang-aroon, W.; Alberto, M.E.; Toscano, M.; Russo, N. Chalcogen atom effect on the intersystem crossing kinetic constant of oxygen- and sulfur disubstituted heteroporphyrins. J. Comput. Chem. 2024, 45, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, J.J. Thiophene stability in photodynamic therapy: A mathematical model approach. Int. J. Mol. Sci. 2024, 25, 2528. [Google Scholar] [CrossRef] [PubMed]
- Fuse, S.; Takizawa, M.; Sato, S.; Okazaki, S.; Nakamura, H. Elucidating the mode of action for thiophene-based organic D-π-A sensitizers for use in photodynamic therapy. Bioorg. Med. Chem. 2019, 27, 315–321. [Google Scholar] [CrossRef]
- Liu, Q.; Tian, J.; Tian, Y.; Sun, Q.; Sun, D.; Liu, D.; Wang, F.; Xu, H.; Ying, G.; Wang, J.; et al. Corrigendum to ‘thiophene donor for NIR-II fluorescence imaging guided photothermal/photodynamic/chemo combination therapy’. Acta Biomater. 2022, 142, 432. [Google Scholar] [CrossRef] [PubMed]
- Pang, E.; Huang, R.; Zhao, S.; Yang, K.; Li, B.; Tan, Q.; Tan, S.; Lan, M.; Wang, B.; Song, X. A water-soluble thiophene-croconaine dye with a high molar extinction coefficient for nir fluorescence imaging-guided synergistic photothermal/photodynamic therapy of cancer. J. Mater. Chem. B. 2022, 10, 9848–9854. [Google Scholar] [CrossRef]
- Roque, J.A.; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the barrier: An osmium photosensitizer with unprecedented hypoxic phototoxicity for real world photodynamic therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef]
- Roque, J.A., III; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Bradner, E.; Shi, G.; von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Os(II) oligothienyl complexes as a hypoxia-active photosensitizer class for photodynamic therapy. Inorg. Chem. 2020, 59, 16341–16360. [Google Scholar] [CrossRef]
- Cole, H.D.; Eroy, M.; Roque Iii, J.A.; Shi, G.; Guirguis, M.; Fakhry, J.; Cameron, C.G.; Obaid, G.; McFarland, S.A. Establishing a robust and reliable response from a potent osmium-based photosensitizer via lipid nanoformulation. Photochem. Photobiol. 2023, 99, 751–760. [Google Scholar] [CrossRef]
- Lei, S.; Zhang, Y.; Blum, N.T.; Huang, P.; Lin, J. Recent advances in croconaine dyes for bioimaging and theranostics. Bioconjugate Chem. 2020, 31, 2072–2084. [Google Scholar] [CrossRef]
- Kataria, S.; Sareen, D. Revisiting croconaine dyes: A glimpse into their applications in bioimaging, therapy and metal detection. J. Anal. Chem. 2024, 79, 15–28. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, H.; Zhang, X.; Ding, Y.; Zou, Y.; Wang, J.; Zhao, S.-C.; Li, Z. Croconaine-based NIR-II fluorescence imaging-guided tumor photothermal therapy induces long-term antitumor immune memory. J. Nanobiotechnol. 2024, 22, 481. [Google Scholar] [CrossRef]
- Gui, Y.; Wang, Y.; Wang, D.; Qin, Y.; Song, G.; Yan, D.; Tang, B.Z.; Wang, D. Thiophene π-bridge manipulation of NIR-II aiegens for multimodal tumor phototheranostics. Angew. Chem. Int. Ed. 2024, 63, e202318609. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why nature chose selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef]
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules 2010, 15, 7292–7312. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, C.; Plano, D.; Sharma, A.K.; Palop, J.A. Selenium compounds, apoptosis and other types of cell death: An overview for cancer therapy. Int. J. Mol. Sci. 2012, 13, 9649–9672. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Boylan, M.; Selvam, A.; Spallholz, J.E.; Björnstedt, M. Redox-active selenium compounds—From toxicity and cell death to cancer treatment. Nutrients 2015, 7, 3536–3556. [Google Scholar] [CrossRef]
- Qin, W.; Zhang, P.; Li, H.; Lam, J.W.Y.; Cai, Y.; Kwok, R.T.K.; Qian, J.; Zheng, W.; Tang, B.Z. Ultrabright red aiegens for two-photon vascular imaging with high resolution and deep penetration. Chem. Sci. 2018, 9, 2705–2710. [Google Scholar] [CrossRef]
- Dash, B.P.; Hamilton, I.; Tate, D.J.; Crossley, D.L.; Kim, J.-S.; Ingleson, M.J.; Turner, M.L. Benzoselenadiazole and benzotriazole directed electrophilic C–H borylation of conjugated donor–acceptor materials. J. Mater. Chem. C 2019, 7, 718–724. [Google Scholar] [CrossRef]
- Wang, H.; Qin, T.; Wang, W.; Zhou, X.; Lin, F.; Liang, G.; Yang, Z.; Chi, Z.; Tang, B.Z. Selenium-containing type-i organic photosensitizers with dual reactive oxygen species of superoxide and hydroxyl radicals as switch-hitter for photodynamic therapy. Adv. Sci. 2023, 10, 2301902. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Huang, W.; Liu, S.; Zhang, H.; Zhang, X.; Peng, X. Design strategies and applications of cyanine dyes in phototherapy. Chem. Soc. Rev. 2025, 54, 341–366. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, H.; Liu, X.; Zou, Y.; Sun, J.; Liu, M.; Jia, S.; Liu, N.; Li, Y.; Wang, Q. Heptamethine cyanine–based application for cancer theranostics. Front. Pharmacol. 2022, 12, 764654. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, S.; Ma, X.; Lv, C.; Gu, H.; Cao, J.; Du, J.; Sun, W.; Fan, J.; Peng, X. Near–infrared heptamethine cyanine photosensitizers with efficient singlet oxygen generation for anticancer photodynamic therapy. Angew. Chem. Int. Ed. 2024, 63, e202411802. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Gao, S.; Dai, C.; Chen, Y.; Shi, J. A two-dimensional biodegradable niobium carbide (MXENE) for photothermal tumor eradication in NIR-I and NIR-II biowindows. J. Am. Chem. Soc. 2017, 139, 16235–16247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, J.; Zhen, X.; Xie, C.; Pu, K. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: A comparative study. Adv. Mater. 2018, 30, 1705980. [Google Scholar] [CrossRef]
- Li, H.; Kim, H.; Xu, F.; Han, J.; Yao, Q.; Wang, J.; Pu, K.; Peng, X.; Yoon, J. Activity-based NIR fluorescent probes based on the versatile hemicyanine scaffold: Design strategy, biomedical applications, and outlook. Chem. Soc. Rev. 2022, 51, 1795–1835. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Y.; Ding, W.; Xu, F.; Liu, Z.; Li, Y.; Wu, Y.; Li, S.; He, W.; Guo, Z. Single-atom engineering of Hemicyanine and its amphiphilic derivative for optimized near infrared phototheranostics. Chem. Sci. 2023, 14, 1234–1243. [Google Scholar] [CrossRef]
- Wang, L.; Lai, B.; Ran, X.; Tang, H.; Cao, D. Recent advances of diketopyrrolopyrrole derivatives in cancer therapy and imaging applications. Molecules 2023, 28, 4097. [Google Scholar] [CrossRef]
- Shi, P.; Lei, L.; Chu, M.; Pang, Z.; Xu, Z.; Sun, Y.; Fang, L. Optimizing phototherapy efficiency via dynamic adjusting the contributions of photodynamic and photothermal therapy of diketopyrrolopyrrole-based compounds. Dyes Pigm. 2024, 224, 112049. [Google Scholar] [CrossRef]
- Prejanò, M.; Alberto, M.E.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Sulphur- and selenium-for-oxygen replacement as a strategy to obtain dual Type I/Type II photosensitizers for photodynamic therapy. Molecules 2023, 28, 3153. [Google Scholar] [CrossRef]
- Alberto, M.E.; De Simone, B.C.; Marino, T.; Toscano, M.; Russo, N. Chalcogen effects in the photophysical properties of dimethylamino-1,8-naphthalimide dyes revealed by DFT investigation. J. Phys. Chem. A 2022, 126, 5167–5172. [Google Scholar] [CrossRef]
- Alberto, M.E.; Prejanò, M.; Marino, T.; De Simone, B.C.; Toscano, M.; Russo, N. Heavy atom effect through chalcogen substitution in red Nile dye: A theoretical investigation. Theor. Chem. Acc. 2023, 142, 101. [Google Scholar] [CrossRef]
- Sadekov, I.D.; Maksimenko, A.A.; Nivorozhkin, V.L. Organic derivatives of monocoordinated tellurium. Russ. Chem. Rev. 1998, 67, 193–208. [Google Scholar] [CrossRef]
- Ba, L.A.; Döring, M.; Jamier, V.; Jacob, C. Tellurium: An element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. [Google Scholar] [CrossRef]
- Chivers, T.; Laitinen, R.S. Tellurium: A maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Detty, M.R.; Merkel, P.B.; Hilf, R.; Gibson, S.L.; Powers, S.K. Chalcogenapyrylium dyes as photochemotherapeutic agents. 2. Tumor uptake, mitochondrial targeting, and singlet-oxygen-induced inhibition of cytochrome c oxidase. J. Med. Chem. 1990, 33, 1108–1116. [Google Scholar] [CrossRef]
- Leonard, K.A.; Nelen, M.I.; Simard, T.P.; Davies, S.R.; Gollnick, S.O.; Oseroff, A.R.; Gibson, S.L.; Hilf, R.; Chen, L.B.; Detty, M.R. Synthesis and evaluation of chalcogenopyrylium dyes as potential sensitizers for the photodynamic therapy of cancer. J. Med. Chem. 1999, 42, 3953–3964. [Google Scholar] [CrossRef]
- Li, J.; Pu, K. Development of organic semiconducting materials for deep-tissue optical imaging, phototherapy and photoactivation. Chem. Soc. Rev. 2019, 48, 38–71. [Google Scholar] [CrossRef]
- Feng, G.; Fang, Y.; Liu, J.; Geng, J.; Ding, D.; Liu, B. Multifunctional conjugated polymer nanoparticles for image-guided photodynamic and photothermal therapy. Small 2017, 13, 1602807. [Google Scholar] [CrossRef]
- Yang, T.; Liu, L.; Deng, Y.; Guo, Z.; Zhang, G.; Ge, Z.; Ke, H.; Chen, H. Ultrastable near-infrared conjugated-polymer nanoparticles for dually photoactive tumor inhibition. Adv. Mater. 2017, 29, 1700487. [Google Scholar] [CrossRef]
- Cai, Y.; Liang, P.; Tang, Q.; Yang, X.; Si, W.; Huang, W.; Zhang, Q.; Dong, X. Diketopyrrolopyrrole–triphenylamine organic nanoparticles as multifunctional reagents for photoacoustic imaging-guided photodynamic/photothermal synergistic tumor therapy. ACS Nano 2017, 11, 1054–1063. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Sheng, X.; Wang, Y.; Xu, H. Ultrathin polypyrrole nanosheets via space-confined synthesis for efficient photothermal therapy in the second near-infrared window. Nano Lett. 2018, 18, 2217–2225. [Google Scholar] [CrossRef]
- Zha, Z.; Yue, X.; Ren, Q.; Dai, Z. Uniform polypyrrole nanoparticles with high photothermal conversion efficiency for photothermal ablation of cancer cells. Adv. Mater. 2013, 25, 777–782. [Google Scholar] [CrossRef]
- Wen, K.; Xu, X.; Chen, J.; Lv, L.; Wu, L.; Hu, Y.; Wu, X.; Liu, G.; Peng, A.; Huang, H. Triplet tellurophene-based semiconducting polymer nanoparticles for near-infrared-mediated cancer theranostics. ACS Appl. Mater. Interfaces 2019, 11, 17884–17893. [Google Scholar] [CrossRef]
- Wen, K.; Wu, L.; Wu, X.; Lu, Y.; Duan, T.; Ma, H.; Peng, A.; Shi, Q.; Huang, H. Precisely tuning photothermal and photodynamic effects of polymeric nanoparticles by controlled copolymerization. Angew. Chem. Int. Ed. 2020, 59, 12756–12761. [Google Scholar] [CrossRef] [PubMed]
- Bumagina, N.A.; Antina, E.V.; Ksenofontov, A.A.; Antina, L.A.; Kalyagin, A.A.; Berezin, M.B. Basic structural modifications for improving the practical properties of bodipy. Coord. Chem. Rev. 2022, 469, 214684. [Google Scholar] [CrossRef]
- Hu, X.; Fang, Z.; Zhu, C.; Yang, Y.; Yang, Z.; Huang, W. Crucial breakthrough of bodipy-based nir-ii fluorescent emitters for advanced biomedical theranostics. Adv. Funct. Mater. 2024, 34, 2401325. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, T.; Dong, X.; Mou, X. Bodipy based nanomedicine for cancer imaging and phototherapy. Colloids Interface Sci. Commun. 2025, 64, 100816. [Google Scholar] [CrossRef]
- Gaviria-Soteras, L.; Sharma, A.K.; Sanmartín, C.; Plano, D. Recent insights into bioactive dichalcogen derivatives: From small molecules to complex materials. Int. J. Mol. Sci. 2025, 26, 2436. [Google Scholar] [CrossRef]
- Caeran Bueno, D.; Meinerz, D.F.; Allebrandt, J.; Waczuk, E.P.; dos Santos, D.B.; Mariano, D.O.C.; Rocha, J.B.T. Cytotoxicity and genotoxicity evaluation of organochalcogens in human leucocytes: A comparative study between ebselen, diphenyl diselenide, and diphenyl ditelluride. BioMed Res. Int. 2013, 2013, 537279. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological activity of selenium and its impact on human health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef]
- Letavayová, L.; Vlčková, V.; Brozmanová, J. Selenium: From cancer prevention to DNA damage. Toxicology 2006, 227, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Spallholz, J.E. Toxicity of selenium compounds and nano-selenium particles. Gen. Appl. Syst. Toxicol. 2011. [Google Scholar] [CrossRef]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and impact of selenium on human health, a review. Molecules 2025, 30, 50. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.W.; Haider, S.I.; Solangi, A.R.; Memon, A.F. Toxicity of tellurium and its compounds. Phys. Sci. Rev. 2023, 8, 4375–4390. [Google Scholar] [CrossRef]
- Sári, D.; Ferroudj, A.; Semsey, D.; El-Ramady, H.; Brevik, E.C.; Prokisch, J. Tellurium and nano-tellurium: Medicine or poison? Nanomaterials 2024, 14, 670. [Google Scholar] [CrossRef]
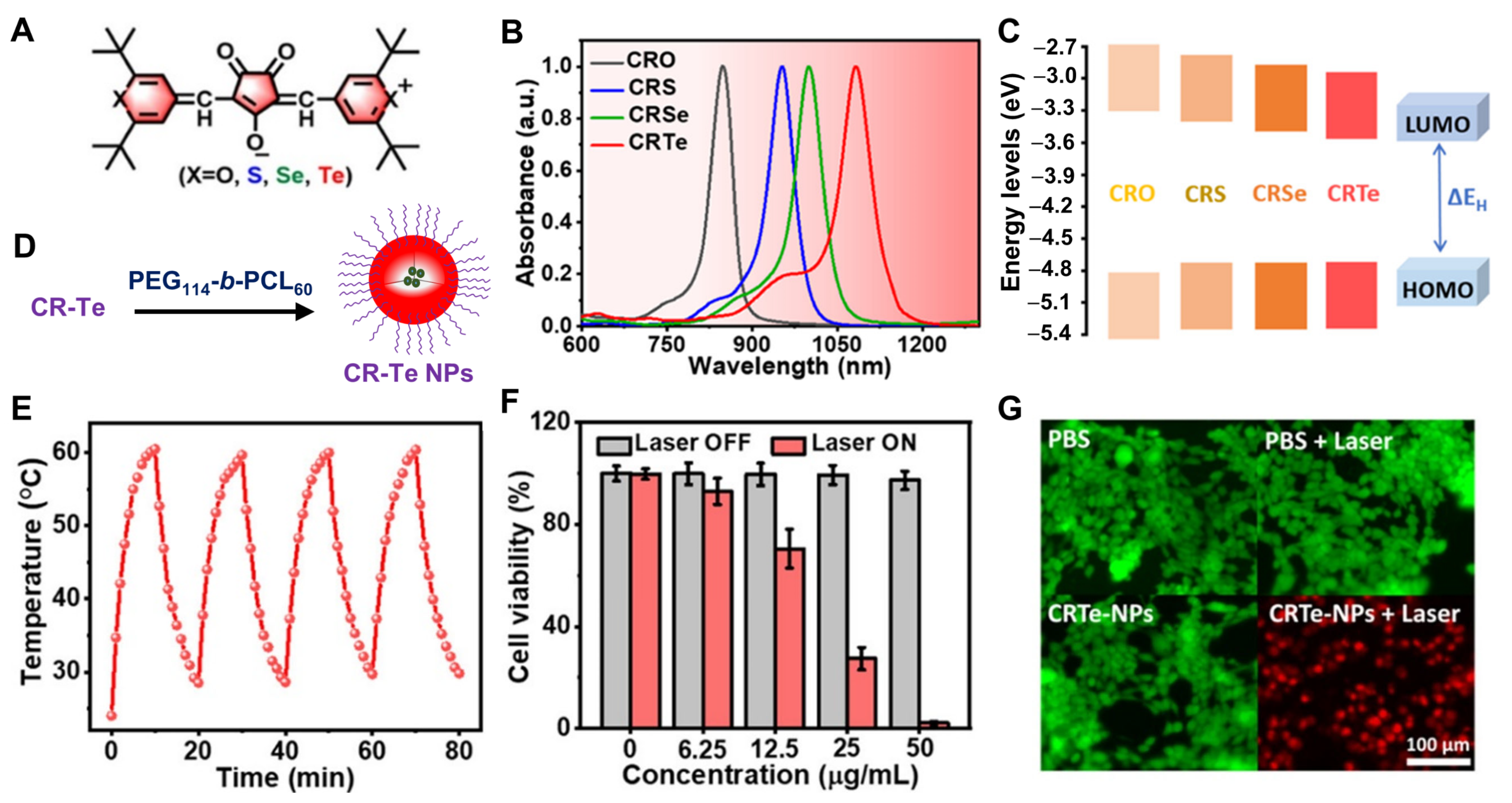

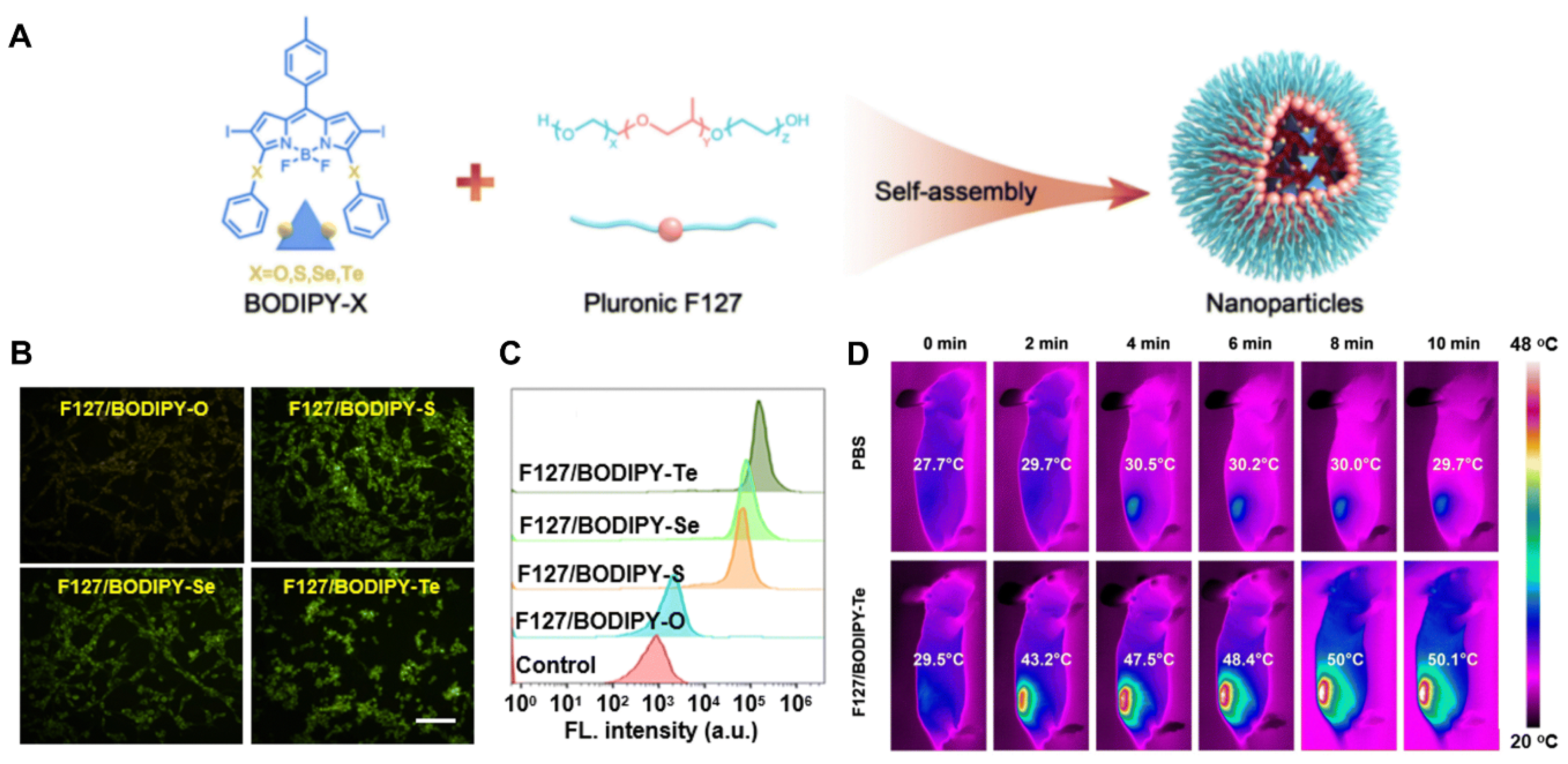
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jena, S.; Douhal, A. Recent Advances in Nano-Drug Delivery Strategies for Chalcogen–Based Therapeutic Agents in Cancer Phototherapy. Int. J. Mol. Sci. 2025, 26, 4819. https://doi.org/10.3390/ijms26104819
Jena S, Douhal A. Recent Advances in Nano-Drug Delivery Strategies for Chalcogen–Based Therapeutic Agents in Cancer Phototherapy. International Journal of Molecular Sciences. 2025; 26(10):4819. https://doi.org/10.3390/ijms26104819
Chicago/Turabian StyleJena, Subhrakant, and Abderrazzak Douhal. 2025. "Recent Advances in Nano-Drug Delivery Strategies for Chalcogen–Based Therapeutic Agents in Cancer Phototherapy" International Journal of Molecular Sciences 26, no. 10: 4819. https://doi.org/10.3390/ijms26104819
APA StyleJena, S., & Douhal, A. (2025). Recent Advances in Nano-Drug Delivery Strategies for Chalcogen–Based Therapeutic Agents in Cancer Phototherapy. International Journal of Molecular Sciences, 26(10), 4819. https://doi.org/10.3390/ijms26104819







