Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans
Abstract
1. Introduction
2. Supplementation of ω-3 Polyunsaturated Fatty Acids for Cardiovascular Risk Reduction: Evidence from Randomized Controlled Trials Shows Effects on Plasma ApoB
3. Dietary Fatty Acids Affect Hepatic Synthesis and Plasma Levels of Triglycerides and ApoB: Results from Controlled-Feeding Studies
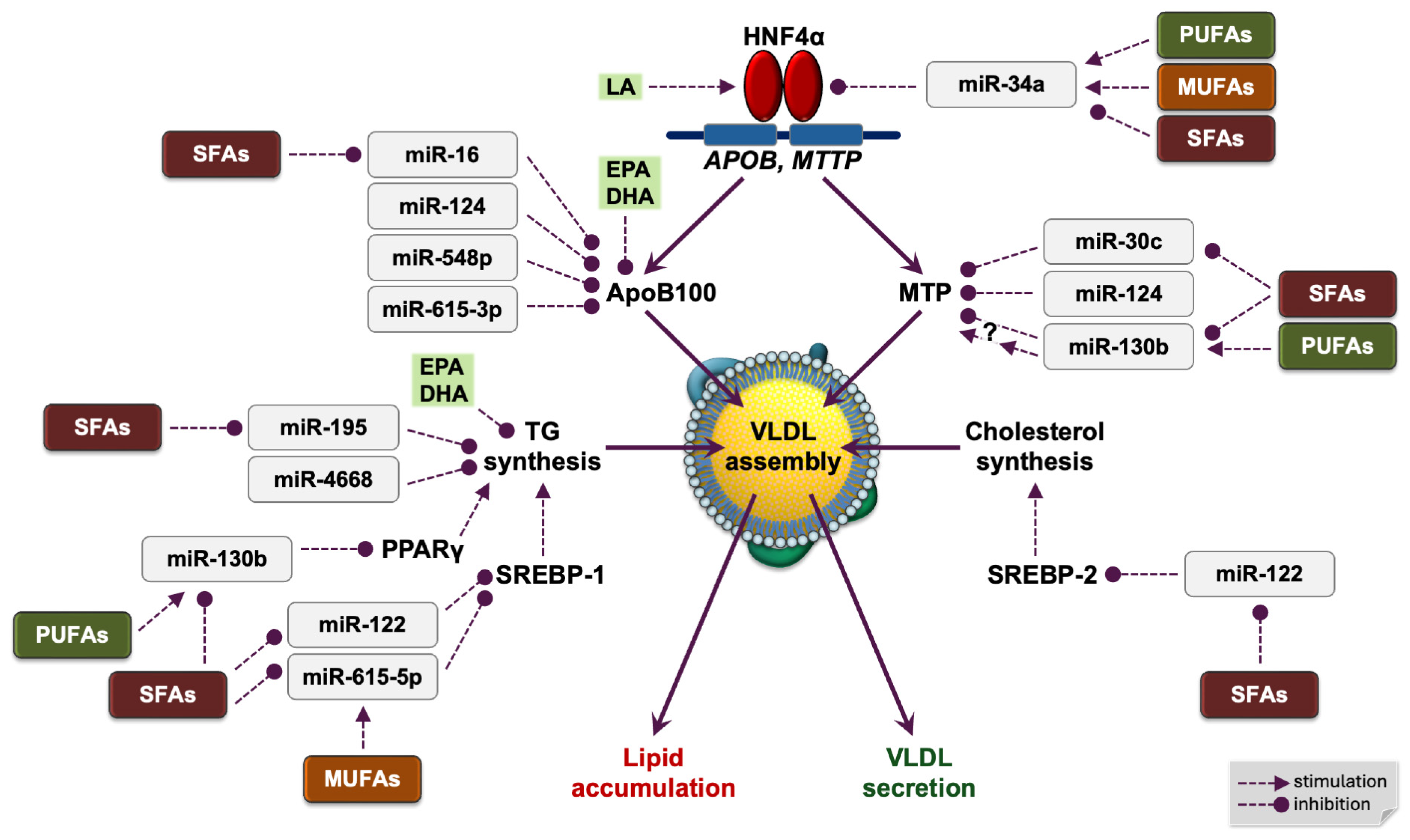
4. Dietary Fatty Acids and MicroRNAs Regulate Hepatic APOB Gene Transcription
4.1. HNF4α Activates APOB Transcription in the Liver
4.2. An Essential Dietary Fatty Acid Is a Structural Cofactor of Human HNF4α
4.3. Human miR-34a and Fatty Acids Regulate HNF4α in the Liver
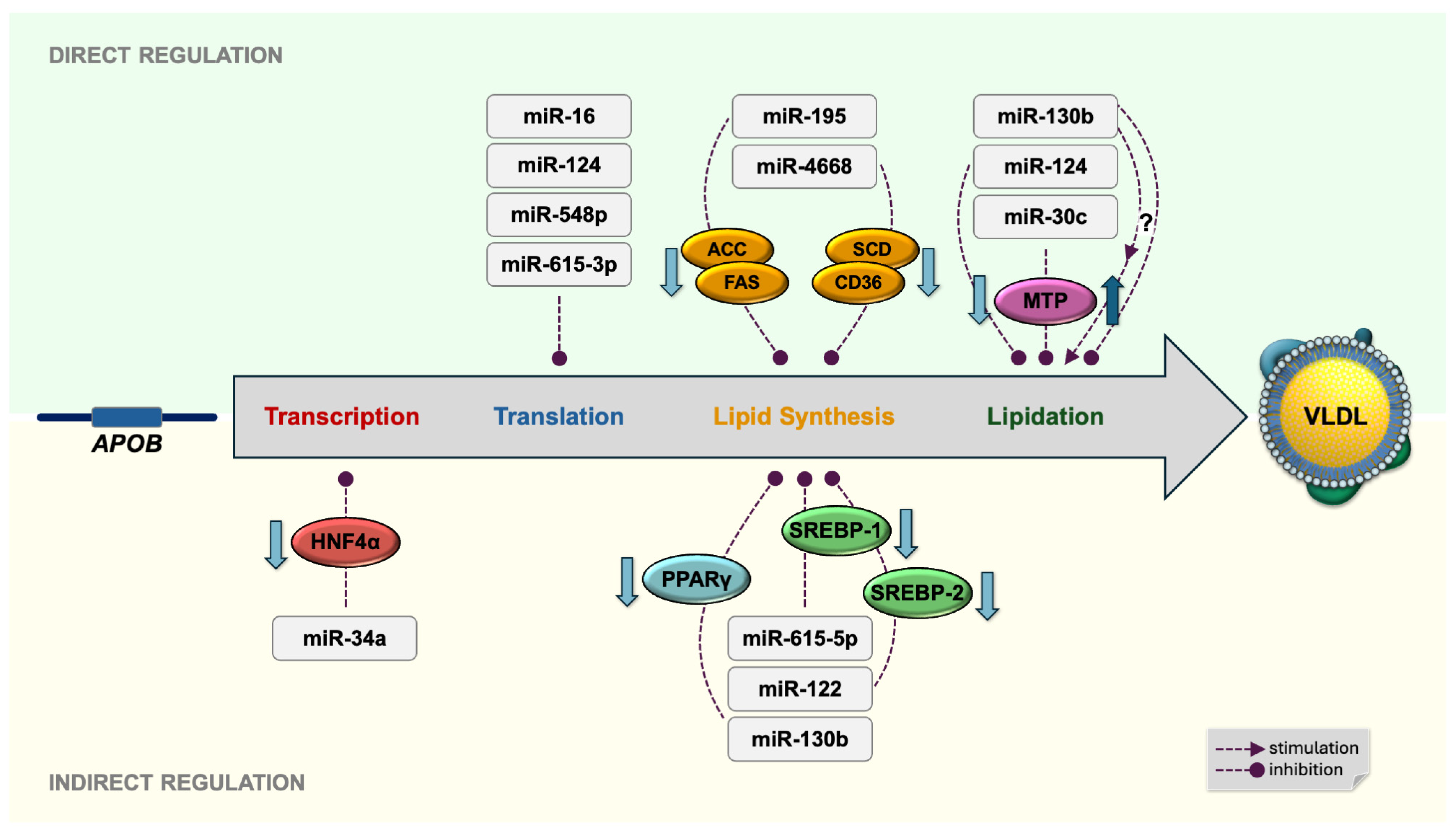
5. Posttranscriptional Regulation of Hepatic ApoB Expression by Dietary Fatty Acids and MicroRNAs
5.1. miR-16
5.2. miR-124
5.3. miR-548p
5.4. miR-615-3p
6. Fatty Acids Can Regulate the Intracellular Degradation of Hepatic ApoB Protein
7. Dietary Fatty Acids and MicroRNAs Modulate Hepatic Lipid Synthesis
7.1. Suppression of De Novo Lipogenesis by ω-3 PUFA
7.2. miR-195
7.3. miR-4668
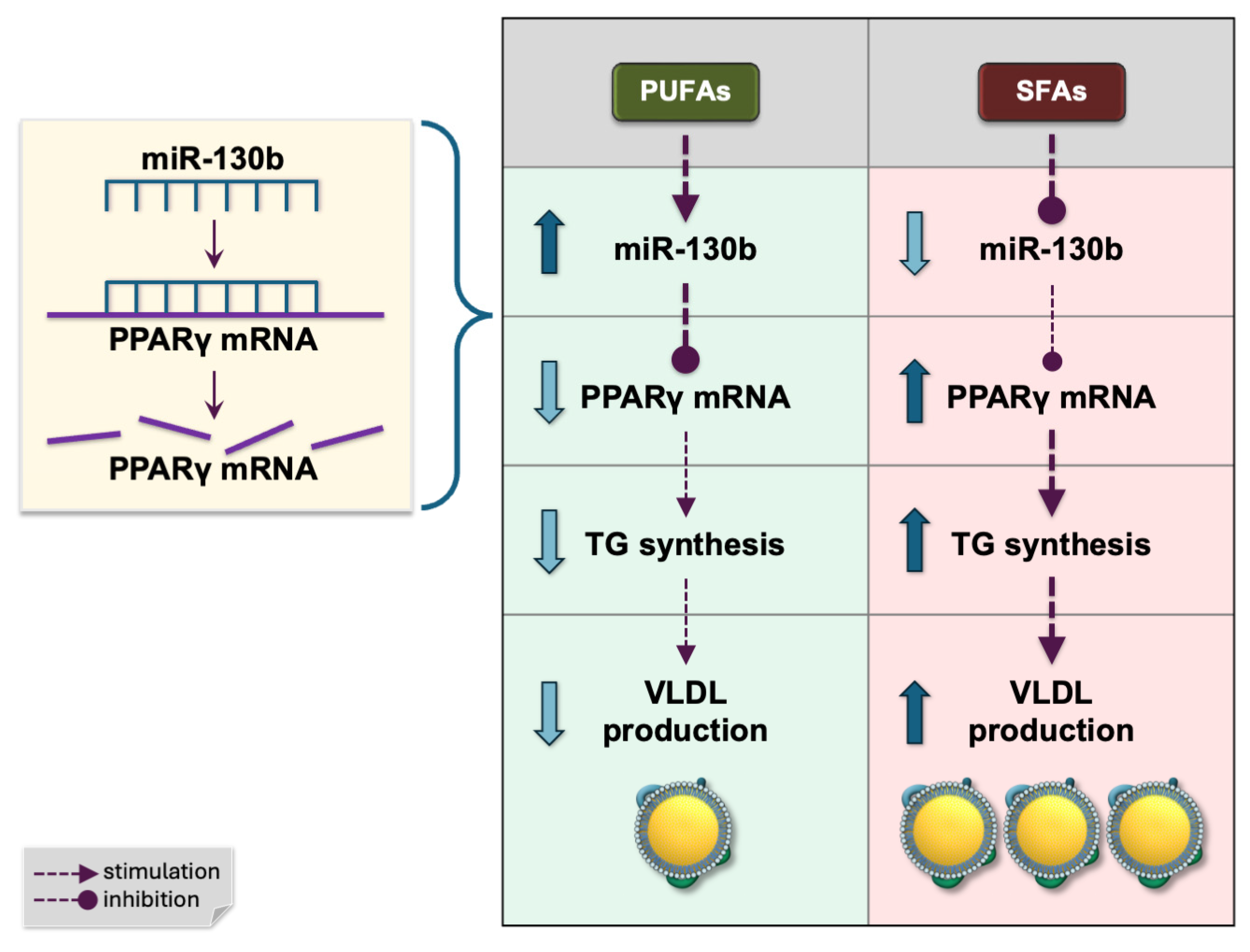
7.4. miR-130b
7.5. miR-122
7.6. miR-615-5p
8. Lipidation of ApoB—MicroRNAs and Dietary Fatty Acids Regulating Hepatic MTP Expression
8.1. miR-30c
8.2. miR-124
8.3. miR-130b
9. Final Remarks
9.1. Limitations
9.2. Species Differences in Hepatic ApoB-Containing Lipoprotein Synthesis: Humans vs. Rodent Models
9.3. Future Research Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blackhart, B.D.; Ludwig, E.M.; Pierotti, V.R.; Caiati, L.; Onasch, M.A.; Wallis, S.C.; Powell, L.; Pease, R.; Knott, T.J.; Chu, M.L.; et al. Structure of the human apolipoprotein B gene. J. Biol. Chem. 1986, 261, 15364–15367. [Google Scholar] [CrossRef] [PubMed]
- Gene. National Library of Medicine, National Center for Biotechnology Information. Available online: https://www.ncbi.nlm.nih.gov/gene/ (accessed on 3 February 2025).
- Wang, A.B.; Liu, D.P.; Liang, C.C. Regulation of human apolipoprotein B gene expression at multiple levels. Exp. Cell Res. 2003, 290, 1–12. [Google Scholar] [CrossRef]
- Johs, A.; Hammel, M.; Waldner, I.; May, R.P.; Laggner, P.; Prassl, R. Modular structure of solubilized human apolipoprotein B-100. Low resolution model revealed by small angle neutron scattering. J. Biol. Chem. 2006, 281, 19732–19739. [Google Scholar] [CrossRef]
- Chen, S.H.; Habib, G.; Yang, C.Y.; Gu, Z.W.; Lee, B.R.; Weng, S.A.; Silberman, S.R.; Cai, S.J.; Deslypere, J.P.; Rosseneu, M.; et al. Apolipoprotein B-48 is the product of a messenger RNA with an organ-specific in-frame stop codon. Science 1987, 238, 363–366. [Google Scholar] [CrossRef]
- Uniprot. Apolipoprotein B-100. Available online: https://www.uniprot.org/uniprotkb/P04114/entry (accessed on 26 February 2025).
- Nakajima, K.; Nakajima, Y.; Takeichi, S.; Fujita, M.Q. ApoB-100 carrying lipoprotein, but not apoB-48, is the major subset of proatherogenic remnant-like lipoprotein particles detected in plasma of sudden cardiac death cases. Atherosclerosis 2007, 194, 473–482. [Google Scholar] [CrossRef]
- Morze, J.; Melloni, G.E.; Rynkiewicz, A.; Gruchala, M.; Guasch-Ferre, M.; Ruff, C.T.; Hu, F.B.; Sabatine, M.S.; Marston, N.A. The relative importance of particle count, type and size of ApoB-containing lipoproteins in risk of myocardial infarction. Eur. Heart J. 2022, 43, 2295. [Google Scholar] [CrossRef]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef]
- Zuber, V.; Gill, D.; Ala-Korpela, M.; Langenberg, C.; Butterworth, A.; Bottolo, L.; Burgess, S. High-throughput multivariable Mendelian randomization analysis prioritizes apolipoprotein B as key lipid risk factor for coronary artery disease. Int. J. Epidemiol. 2021, 50, 893–901. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Watanabe, T.; Ando, K.; Daidoji, H.; Otaki, Y.; Sugawara, S.; Matsui, M.; Ikeno, E.; Hirono, O.; Miyawaki, H.; Yashiro, Y.; et al. CHERRY study investigators. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J. Cardiol. 2017, 70, 537–544. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of High-Dose Omega-3 Fatty Acids vs. Corn Oil on Major Adverse Cardiovascular Events in Patients at High Cardiovascular Risk: The STRENGTH Randomized Clinical Trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Bays, H.E.; Ballantyne, C.M.; Kastelein, J.J.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am. J. Cardiol. 2011, 108, 682–690. [Google Scholar] [CrossRef]
- Ballantyne, C.M.; Bays, H.E.; Kastelein, J.J.; Stein, E.; Isaacsohn, J.L.; Braeckman, R.A.; Soni, P.N. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am. J. Cardiol. 2012, 110, 984–992. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; MacFadyen, J.; Glynn, R.J.; Jiao, L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Tardif, J.C.; et al. Effects of Randomized Treatment With Icosapent Ethyl and a Mineral Oil Comparator on Interleukin-1β, Interleukin-6, C-Reactive Protein, Oxidized Low-Density Lipoprotein Cholesterol, Homocysteine, Lipoprotein(a), and Lipoprotein-Associated Phospholipase A2: A REDUCE-IT Biomarker Substudy. Circulation 2022, 146, 372–379. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture, Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 27 February 2025).
- Parry, S.A.; Rosqvist, F.; Mozes, F.E.; Cornfield, T.; Hutchinson, M.; Piche, M.E.; Hülsmeier, A.J.; Hornemann, T.; Dyson, P.; Hodson, L. Intrahepatic Fat and Postprandial Glycemia Increase After Consumption of a Diet Enriched in Saturated Fat Compared With Free Sugars. Diabetes Care 2020, 43, 1134–1141. [Google Scholar] [CrossRef]
- Gill, J.M.; Brown, J.C.; Caslake, M.J.; Wright, D.M.; Cooney, J.; Bedford, D.; Hughes, D.A.; Stanley, J.C.; Packard, C.J. Effects of dietary monounsaturated fatty acids on lipoprotein concentrations, compositions, and subfraction distributions and on VLDL apolipoprotein B kinetics: Dose-dependent effects on LDL. Am. J. Clin. Nutr. 2003, 78, 47–56. [Google Scholar] [CrossRef]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Maki, K.C.; Lawless, A.L.; Kelley, K.M.; Kaden, V.N.; Geiger, C.J.; Palacios, O.M.; Dicklin, M.R. Corn oil intake favorably impacts lipoprotein cholesterol, apolipoprotein and lipoprotein particle levels compared with extra-virgin olive oil. Eur. J. Clin. Nutr. 2017, 71, 33–38. [Google Scholar] [CrossRef]
- Chan, D.C.; Watts, G.F.; Mori, T.A.; Barrett, P.H.; Redgrave, T.G.; Beilin, L.J. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am. J. Clin. Nutr. 2003, 77, 300–307. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.P.; Tremblay, A.J.; Lépine, M.C.; Lemelin, V.; Lamarche, B.; Couture, P. Substitution of dietary ω-6 polyunsaturated fatty acids for saturated fatty acids decreases LDL apolipoprotein B-100 production rate in men with dyslipidemia associated with insulin resistance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 26–34. [Google Scholar] [CrossRef]
- García-Montero, C.; Fraile-Martínez, O.; Gómez-Lahoz, A.M.; Pekarek, L.; Castellanos, A.J.; Noguerales-Fraguas, F.; Coca, S.; Guijarro, L.G.; García-Honduvilla, N.; Asúnsolo, A.; et al. Nutritional Components in Western Diet Versus Mediterranean Diet at the Gut Microbiota–Immune System Interplay. Implications for Health and Disease. Nutrients 2021, 13, 699. [Google Scholar] [CrossRef]
- Metzger, S.; Halaas, J.L.; Breslow, J.L.; Sladek, F.M. Orphan receptor HNF-4 and bZip protein C/EBP alpha bind to overlapping regions of the apolipoprotein B gene promoter and synergistically activate transcription. J. Biol. Chem. 1993, 268, 16831–16838. [Google Scholar] [CrossRef]
- Burris, T.P.; de Vera, I.M.S.; Cote, I.; Flaveny, C.A.; Wanninayake, U.S.; Chatterjee, A.; Walker, J.K.; Steinauer, N.; Zhang, J.; Coons, L.A.; et al. International Union of Basic and Clinical Pharmacology CXIII: Nuclear Receptor Superfamily-Update 2023. Pharmacol. Rev. 2023, 75, 1233–1318. [Google Scholar] [CrossRef]
- Ko, H.L.; Zhuo, Z.; Ren, E.C. HNF4α Combinatorial Isoform Heterodimers Activate Distinct Gene Targets that Differ from Their Corresponding Homodimers. Cell Rep. 2019, 26, 2549–2557. [Google Scholar] [CrossRef]
- Yuan, X.; Ta, T.C.; Lin, M.; Evans, J.R.; Dong, Y.; Bolotin, E.; Sherman, M.A.; Forman, B.M.; Sladek, F.M. Identification of an Endogenous Ligand Bound to a Native Orphan Nuclear Receptor. PLoS ONE 2009, 4, e5609. [Google Scholar] [CrossRef]
- Chandra, V.; Huang, P.; Potluri, N.; Wu, D.; Kim, Y.; Rastinejad, F. Multidomain integration in the structure of the HNF-4α nuclear receptor complex. Nature 2013, 495, 394–398. [Google Scholar] [CrossRef]
- Xu, Y.; Zalzala, M.; Xu, J.; Li, Y.; Yin, L.; Zhang, Y. A Metabolic Stress-Inducible miR-34a-HNF4α Pathway Regulates Lipid and Lipoprotein Metabolism. Nat. Commun. 2015, 6, 7466. [Google Scholar] [CrossRef]
- Rishik, S.; Hirsch, P.; Grandke, F.; Fehlmann, T.; Keller, A. miRNATissueAtlas 2025: An Update to the Uniformly Processed and Annotated Human and Mouse Non-Coding RNA Tissue Atlas. Nucleic Acids Res. 2025, 53, D129–D137. [Google Scholar] [CrossRef]
- Latorre, J.; Ortega, F.J.; Liñares-Pose, L.; Moreno-Navarrete, J.M.; Lluch, A.; Comas, F.; Oliveras-Cañellas, N.; Ricart, W.; Höring, M.; Zhou, Y.; et al. Compounds that modulate AMPK activity and hepatic steatosis impact the biosynthesis of microRNAs required to maintain lipid homeostasis in hepatocytes. EBioMedicine 2020, 53, 102697. [Google Scholar] [CrossRef]
- Quintanilha, B.J.; Pinto Ferreira, L.R.; Ferreira, F.M.; Neto, E.C.; Sampaio, G.R.; Rogero, M.M. Circulating plasma microRNAs dysregulation and metabolic endotoxemia induced by a high-fat high-saturated diet. Clin. Nutr. 2020, 39, 554–562. [Google Scholar] [CrossRef]
- Mahjoubin-Tehran, M.; Aghaee-Bakhtiari, S.H.; Sahebkar, A.; Butler, A.E.; Oskuee, R.K.; Jalili, A. In silico and in vitro analysis of microRNAs with therapeutic potential in atherosclerosis. Sci. Rep. 2022, 12, 20334. [Google Scholar] [CrossRef]
- Keirns, B.H.; Sciarrillo, C.M.; Poindexter, K.L.; Emerson, S.R. Daily Triglyceride Kinetics When Consuming a Realistic Western Diet in at-Risk Individuals across the Metabolic Spectrum: A Case Study. Obesities 2021, 1, 107–112. [Google Scholar] [CrossRef]
- Zhou, L.; Hussain, M.M. Human MicroRNA-548p Decreases Hepatic Apolipoprotein B Secretion and Lipid Synthesis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 786–793. [Google Scholar] [CrossRef]
- Ansari, A.; Yadav, P.K.; Valmiki, S.; Laine, A.; Rimbert, A.; Islam, S.; Osman, I.; Najafi-Shoushtari, S.H.; Hussain, M.M. MicroRNA-615-3p decreases apo B expression in human liver cells. J. Lipid. Res. 2024, 65, 100659. [Google Scholar] [CrossRef]
- Fisher, E.A.; Pan, M.; Chen, X.; Wu, X.; Wang, H.; Jamil, H.; Sparks, J.D.; Williams, K.J. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J. Biol. Chem. 2001, 276, 27855–27863. [Google Scholar] [CrossRef] [PubMed]
- Kumari, D.; Fisher, E.A.; Brodsky, J.L. Hsp40s play distinct roles during the initial stages of apolipoprotein B biogenesis. Mol. Biol. Cell 2022, 33, ar15. [Google Scholar] [CrossRef]
- Green, C.J.; Pramfalk, C.; Charlton, C.A.; Gunn, P.J.; Cornfield, T.; Pavlides, M.; Karpe, F.; Hodson, L. Hepatic de novo lipogenesis is suppressed and fat oxidation is increased by omega-3 fatty acids at the expense of glucose metabolism. BMJ Open Diabetes Res. Care 2020, 8, e000871. [Google Scholar] [CrossRef]
- Nanthirudjanar, T.; Furumoto, H.; Hirata, T.; Sugawara, T. Oxidized eicosapentaenoic acids more potently reduce LXRα-induced cellular triacylglycerol via suppression of SREBP-1c, PGC-1β and GPA than its intact form. Lipids Health Dis. 2013, 12, 73. [Google Scholar] [CrossRef]
- Guo, J.; Fang, W.; Sun, L.; Lu, Y.; Dou, L.; Huang, X.; Tang, W.; Yu, L.; Li, J. Ultraconserved element uc.372 drives hepatic lipid accumulation by suppressing miR-195/miR4668 maturation. Nat. Commun. 2018, 9, 612. [Google Scholar] [CrossRef]
- Seal, R.L.; Braschi, B.; Gray, K.; Jones, T.E.M.; Tweedie, S.; Haim-Vilmovsky, L.; Bruford, E.A. Genenames.org: The HGNC resources in 2023. Nucleic Acids Res. 2023, 51, D1003–D1009. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Cui, S.; Yu, S.; Huang, H.Y.; Lin, Y.C.; Huang, Y.; Zhang, B.; Xiao, J.; Zuo, H.; Wang, J.; Li, Z.; et al. miRTarBase 2025: Updates to the collection of experimentally validated microRNA-target interactions. Nucleic Acids Res. 2025, 53, D147–D156. [Google Scholar] [CrossRef]
- Alonso-Villa, E.; Mangas, A.; Bonet, F.; Campuzano, Ó.; Quezada-Feijoo, M.; Ramos, M.; García-Padilla, C.; Franco, D.; Toro, R. The Protective Role of miR-130b-3p Against Palmitate-Induced Lipotoxicity in Cardiomyocytes Through PPARγ Pathway. Int. J. Mol. Sci. 2024, 25, 12161. [Google Scholar] [CrossRef]
- Berthier, A.; Johanns, M.; Zummo, F.P.; Lefebvre, P.; Staels, B. PPARs in liver physiology. Biochim. Biophys. Acta Mol. Basis. Dis. 2021, 1867, 166097. [Google Scholar] [CrossRef]
- Monroy-Ramirez, H.C.; Galicia-Moreno, M.; Sandoval-Rodriguez, A.; Meza-Rios, A.; Santos, A.; Armendariz-Borunda, J. PPARs as Metabolic Sensors and Therapeutic Targets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 8298. [Google Scholar] [CrossRef]
- Maruyama, H.; Kiyono, S.; Kondo, T.; Sekimoto, T.; Yokosuka, O. Palmitate-induced Regulation of PPARγ via PGC1α: A Mechanism for Lipid Accumulation in the Liver in Nonalcoholic Fatty Liver Disease. Int. J. Med. Sci. 2016, 13, 169–178. [Google Scholar] [CrossRef]
- Lee, S.M.; Muratalla, J.; Karimi, S.; Diaz-Ruiz, A.; Frutos, M.D.; Guzman, G.; Ramos-Molina, B.; Cordoba-Chacon, J. Hepatocyte PPARγ contributes to the progression of non-alcoholic steatohepatitis in male and female obese mice. Cell. Mol. Life Sci. 2023, 80, 39. [Google Scholar] [CrossRef]
- Qian, G.; Morral, N. Role of non-coding RNAs on liver metabolism and NAFLD pathogenesis. Hum. Mol. Genet. 2022, 31, R4–R21. [Google Scholar] [CrossRef]
- Li, Z.Y.; Xi, Y.; Zhu, W.N.; Zeng, C.; Zhang, Z.Q.; Guo, Z.C.; Hao, D.L.; Liu, G.; Feng, L.; Chen, H.Z.; et al. Positive regulation of hepatic miR-122 expression by HNF4α. J. Hepatol. 2011, 55, 602–611. [Google Scholar] [CrossRef]
- Cheung, O.; Puri, P.; Eicken, C.; Contos, M.J.; Mirshahi, F.; Maher, J.W.; Kellum, J.M.; Min, H.; Luketic, V.A.; Sanyal, A.J. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology 2008, 48, 1810–1820. [Google Scholar] [CrossRef]
- Ferré, P.; Phan, F.; Foufelle, F. SREBP-1c and lipogenesis in the liver: An update. Biochem. J. 2021, 478, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Latorre, J.; Moreno-Navarrete, J.M.; Mercader, J.M.; Sabater, M.; Rovira, Ò.; Gironès, J.; Ricart, W.; Fernández-Real, J.M.; Ortega, F.J. Decreased lipid metabolism but increased FA biosynthesis are coupled with changes in liver microRNAs in obese subjects with NAFLD. Int. J. Obes. 2017, 41, 620–630. [Google Scholar] [CrossRef]
- El Sobky, S.A.; Aboud, N.K.; El Assaly, N.M.; Fawzy, I.O.; El-Ekiaby, N.; Abdelaziz, A.I. Regulation of lipid droplet (LD) formation in hepatocytes via regulation of SREBP1c by non-coding RNAs. Front. Med. 2022, 9, 903856. [Google Scholar] [CrossRef]
- Lopez, S.; Bermudez, B.; Montserrat-de la Paz, S.; Abia, R.; Muriana, F.J.G. A microRNA expression signature of the postprandial state in response to a high-saturated-fat challenge. J. Nutr. Biochem. 2018, 57, 45–55. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Pathway Summary for Pathway R-HSA-8866423, VLDL Assembly, Source: Reactome. Available online: https://pubchem.ncbi.nlm.nih.gov/pathway/Reactome:R-HSA-8866423 (accessed on 27 February 2025).
- Iqbal, J.; Walsh, M.T.; Hammad, S.M.; Cuchel, M.; Tarugi, P.; Hegele, R.A.; Davidson, N.O.; Rader, D.J.; Klein, R.L.; Hussain, M.M. Microsomal triglyceride transfer protein transfers and determines plasma concentrations of ceramide and sphingomyelin but not glycosylceramide. J. Biol. Chem. 2015, 290, 25863–25875. [Google Scholar] [CrossRef]
- Sheena, V.; Hertz, R.; Nousbeck, J.; Berman, I.; Magenheim, J.; Bar-Tana, J. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4α. J. Lipid Res. 2005, 46, 328–341. [Google Scholar] [CrossRef]
- Jeiran, K.; Gordon, S.M.; Sviridov, D.O.; Aponte, A.M.; Haymond, A.; Piszczek, G.; Lucero, D.; Neufeld, E.B.; Vaisman, I.I.; Liotta, L.; et al. A New Structural Model of Apolipoprotein B100 Based on Computational Modeling and Cross Linking. Int. J. Mol. Sci. 2022, 23, 11480. [Google Scholar] [CrossRef]
- OMIM. 200100 Abetalipoproteinemia; Online Mendelian Inheritance in Man, McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University: Baltimore, MD, USA, 2025; Available online: https://omim.org/entry/200100 (accessed on 25 February 2025).
- Soh, J.; Hussain, M.M. Supplementary site interactions are critical for the regulation of microsomal triglyceride transfer protein by microRNA-30c. Nutr. Metab. 2013, 10, 56. [Google Scholar] [CrossRef]
- Yadav, P.K.; Haruehanroengra, P.; Irani, S.; Wang, T.; Ansari, A.; Sheng, J.; Hussain, M.M. Novel efficacious microRNA-30c analogs reduce apolipoprotein B secretion in human hepatoma and primary hepatocyte cells. J. Biol. Chem. 2022, 298, 101813. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhu, M.; Liu, X.; Chen, X.; Yuan, Y.; Li, L.; Liu, J.; Lu, Y.; Cheng, J.; Chen, Y. Oleic acid ameliorates palmitic acid induced hepatocellular lipotoxicity by inhibition of ER stress and pyroptosis. Nutr. Metab. 2020, 17, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jazii, F.R.; Haghighi, M.M.; Alvares, D.; Liu, L.; Khosraviani, N.; Adeli, K. miR-130b is a Potent Stimulator of Hepatic Very-Low-Density Lipoprotein Assembly and Secretion via Marked Induction of Microsomal Triglyceride Transfer Protein. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E262–E275. [Google Scholar] [CrossRef]
- Ortega, F.J.; Cardona-Alvarado, M.I.; Mercader, J.M.; Moreno-Navarrete, J.M.; Moreno, M.; Sabater, M.; Fuentes-Batllevell, N.; Ramírez-Chávez, E.; Ricart, W.; Molina-Torres, J.; et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J. Nutr. Biochem. 2015, 26, 1095–1101. [Google Scholar] [CrossRef]
- Mazidi, M.; Webb, R.J.; George, E.S.; Shekoohi, N.; Lovegrove, J.A.; Davies, I.G. Nutrient patterns are associated with discordant apoB and LDL: A population-based analysis. Br. J. Nutr. 2022, 128, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.W.; Ooi, E.M.; Watts, G.F.; Chan, D.C.; Barrett, P.H. Genetic determinants of apolipoprotein B-100 kinetics. Curr. Opin. Lipidol. 2010, 21, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.J.; Green, C.J.; Pramfalk, C.; Hodson, L. In vitro cellular models of human hepatic fatty acid metabolism: Differences between Huh7 and HepG2 cell lines in human and fetal bovine culturing serum. Physiol. Rep. 2017, 5, e13532. [Google Scholar] [CrossRef] [PubMed]
- Huggett, Z.J.; Smith, A.; De Vivo, N.; Gomez, D.; Jethwa, P.; Brameld, J.M.; Bennett, A.; Salter, A.M. A Comparison of Primary Human Hepatocytes and Hepatoma Cell Lines to Model the Effects of Fatty Acids, Fructose and Glucose on Liver Cell Lipid Accumulation. Nutrients 2023, 15, 40. [Google Scholar] [CrossRef]
- Davidson, N.O.; Shelness, G.S. Apolipoprotein B: mRNA editing, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 2000, 20, 169–193. [Google Scholar] [CrossRef]
- Wang, Y.; Robinson, P.S.; Coorens, T.H.H.; Moore, L.; Lee-Six, H.; Noorani, A.; Sanders, M.A.; Jung, H.; Katainen, R.; Heuschkel, R.; et al. APOBEC mutagenesis is a common process in normal human small intestine. Nat. Genet. 2023, 55, 246–254. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chen, X.W. The biogenesis and transport of triglyceride-rich lipoproteins. Trends Endocrinol. Metab. 2025, 36, 262–277. [Google Scholar] [CrossRef]
- Fernández-Tussy, P.; Ruz-Maldonado, I.; Fernández-Hernando, C. MicroRNAs and Circular RNAs in Lipoprotein Metabolism. Curr. Atheroscler. Rep. 2021, 23, 33. [Google Scholar] [CrossRef]
- Hendriks, D.; Artegiani, B.; Margaritis, T.; Zoutendijk, I.; Chuva de Sousa Lopes, S.; Clevers, H. Mapping of mitogen and metabolic sensitivity in organoids defines requirements for human hepatocyte growth. Nat. Commun. 2024, 15, 4034. [Google Scholar] [CrossRef] [PubMed]
- Pramfalk, C.; Jakobsson, T.; Verzijl, C.R.C.; Minniti, M.E.; Obensa, C.; Ripamonti, F.; Olin, M.; Pedrelli, M.; Eriksson, M.; Parini, P. Generation of new hepatocyte-like in vitro models better resembling human lipid metabolism. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158659. [Google Scholar] [CrossRef] [PubMed]
| Trial | Duration | Intervention | Control | Outcomes vs. Baseline | Outcomes vs. Control | ||
|---|---|---|---|---|---|---|---|
| ApoB [%] | TGs [%] | ApoB [%] | TGs [%] | ||||
| MARINE [14] | 12 weeks | EPA 4 g/day (n = 76) | MO (n = 75) | −3.8 | –26.6 | −8.5 * | −33.1 ** |
| ANCHOR [15] | 12 weeks | EPA 4 g/day + statin (n = 226) | MO + statin (n = 227) | −2.2 | −17.5 | −9.3 ** | −21.5 ** |
| CHERRY [12] | 6–8 months | EPA 1.8 g/day + pitavastatin (n = 97) | pitavastatin (n = 96) | −9.3 ** | 2.9 | 0.6 | 4.4 |
| REDUCE-IT [11] | 4.9 years | EPA 4 g/day + statin (n = 4089) | MO + statin (n = 4090) | −2.5 | −21.6 ** | −6.7 ** | −14.1 ** |
| STRENGTH [13] | 12 months | EPA + DHA 4 g/day + statin (n = 6539) | CO + statin (n = 6539) | −2.0 | −19.0 ** | −1.0 | −18.1 ** |
| Fatty Acid | Type | Dietary Source |
|---|---|---|
| palmitic (C16:0) | SFA | palm oil, butter, beef tallow |
| stearic (C18:0) | SFA | butter, beef tallow, lard |
| palmitoleic (C16:1 ω-7) | MUFA | fish oils |
| oleic (C18:1 ω-9) | MUFA | olive oil, canola oil, olives |
| linoleic (C18:2 ω-6) | PUFA | safflower oil, corn oil, soybean oil, cottonseed oil |
| α-linolenic (C18:3 ω-3) | PUFA | flaxseed oil, perilla oil, canola oil, soybean oil, chia seeds, walnuts |
| arachidonic (C20:4 ω-6) | PUFA | meats |
| eicosapentaenoic (C20:5 ω-3) | PUFA | oily fish, fish oils, shellfish |
| docosahexaenoic (C22:6 ω-3) | PUFA | oily fish, fish oils, shellfish |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karbowska, J.; Kochan, Z. Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans. Int. J. Mol. Sci. 2025, 26, 4817. https://doi.org/10.3390/ijms26104817
Karbowska J, Kochan Z. Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans. International Journal of Molecular Sciences. 2025; 26(10):4817. https://doi.org/10.3390/ijms26104817
Chicago/Turabian StyleKarbowska, Joanna, and Zdzislaw Kochan. 2025. "Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans" International Journal of Molecular Sciences 26, no. 10: 4817. https://doi.org/10.3390/ijms26104817
APA StyleKarbowska, J., & Kochan, Z. (2025). Crosstalk Between Dietary Fatty Acids and MicroRNAs in the Regulation of Hepatic ApoB-Containing Lipoprotein Synthesis in Humans. International Journal of Molecular Sciences, 26(10), 4817. https://doi.org/10.3390/ijms26104817







