Bisphenol A in the Urine: Association with Urinary Creatinine, Impaired Kidney Function, Use of Plastic Food and Beverage Storage Products but Not with Serum Anti-Müllerian Hormone in Ovarian Malignancies
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Population Compared to Healthy Controls
2.2. Urine Levels of BPA in Patients with Epithelial Ovarian Cancer and Epithelial Borderline Ovarian Tumors Compared to Healthy Controls
2.3. Associations Between Urinary BPA and Kidney Function in Women
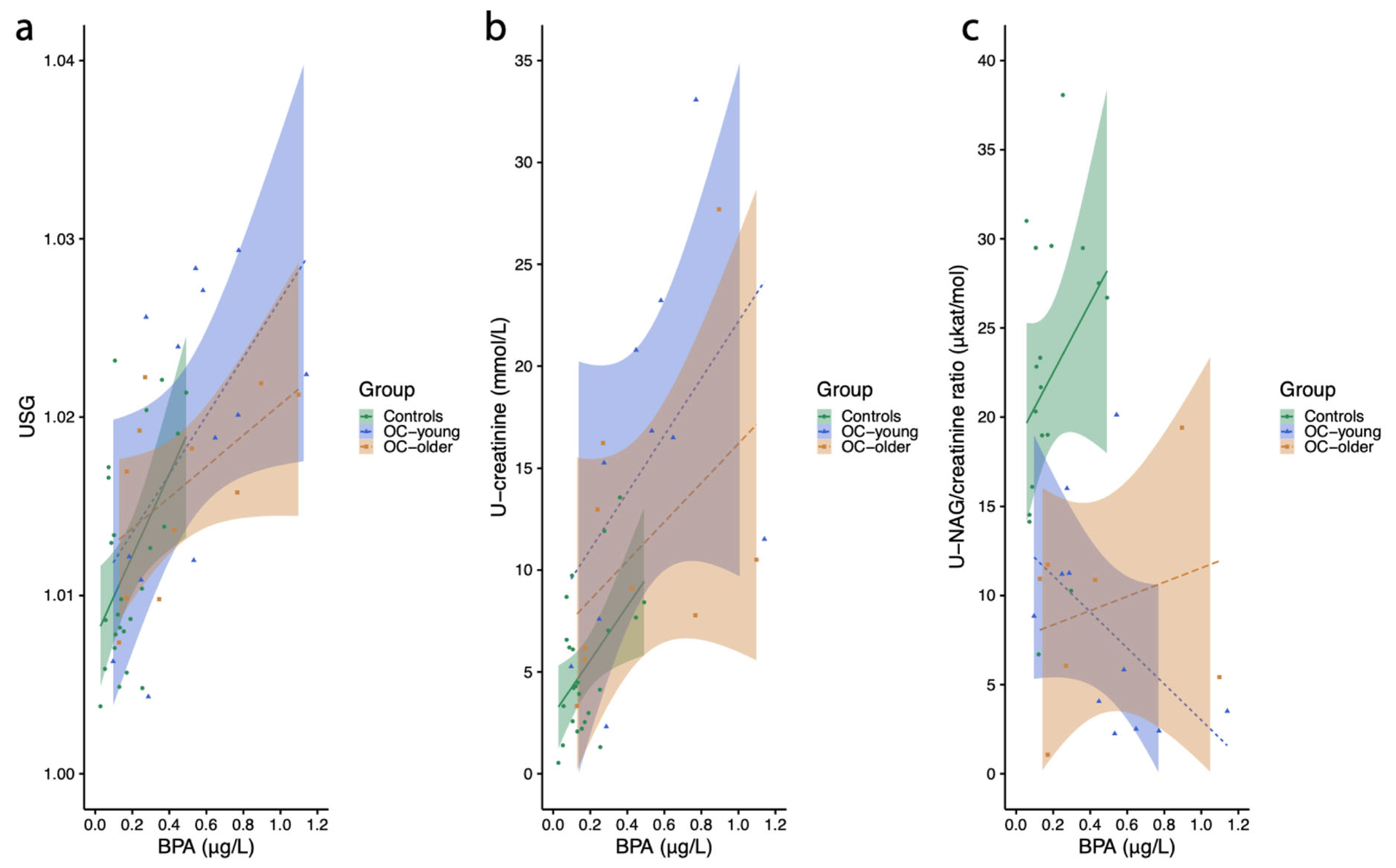
2.3.1. Urine Specific Gravity
2.3.2. Urinary Creatinine Concentration
2.3.3. NAG (N-acetyl-beta-d-glucosamidase)/Creatinine Ratio
2.3.4. Glomerular Filtration Rate (eGFR) Estimation
2.4. Associations Between Urinary BPA, Platelets, and Triglycerides in Blood of Women
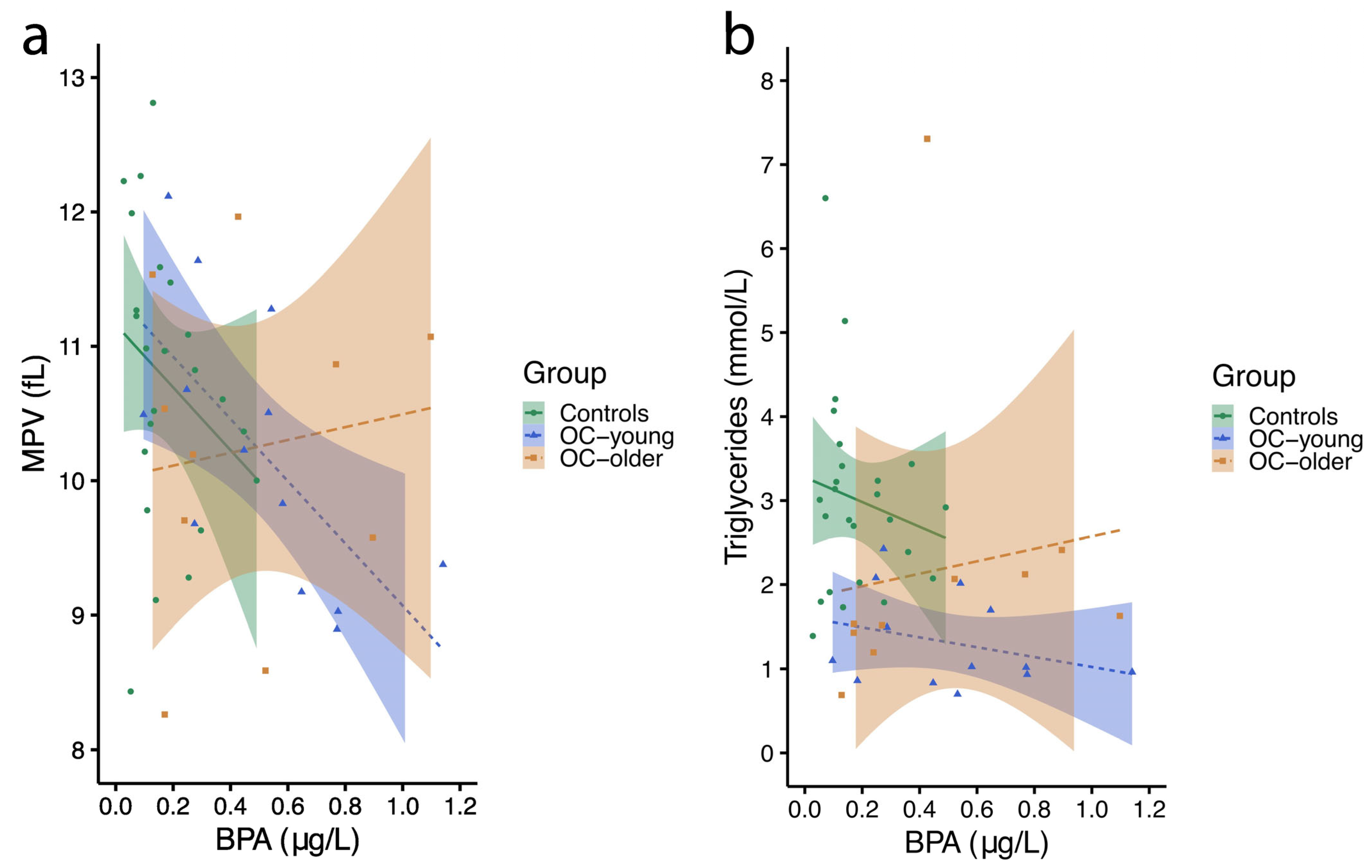
2.4.1. Mean Platelet Volume
2.4.2. Blood Triglycerides
2.5. Serum AMH and Urinary BPA Levels in Women
2.6. Lifestyle Habits in Women with Ovarian Cancer Compared to Controls
2.7. Lifestyle Habits of Women Associated with BPA Concentrations in Their Urine
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Sampling
4.3. Routine Diagnostic Biochemical Analyses of Biological Samples
4.4. BPA Analysis
4.5. Serum AMH Analysis for Ovarian Reserve Estimation
4.6. Questionnaire for the Evaluation of Lifestyle and Habits
4.7. Study Power Test
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance. |
| BMI | Body mass index. |
| BOT | Borderline ovarian tumor. |
| BPA | Bisphenol A. |
| BPAF | Bisphenol AF. |
| BPF | Bisphenol F. |
| BPZ | Bisphenol Z. |
| CAT | Catalase |
| EBOT | Epithelial borderline ovarian tumors. |
| ECHA | The European Chemical Agency. |
| EDC | Endocrine-disrupting chemical. |
| EDTA | Ethylenediaminetetraacetic acid. |
| EMT | Epithelial–mesenchymal transition. |
| EOC | Epithelial ovarian cancer. |
| ER | Estrogen receptor. |
| FOXO3 | Forkhead box O3. |
| GC-MS/MS | Gas chromatography tandem mass spectrometry. |
| eGFR | Glomerual filtration rate estimation. |
| GM | Geometrical median. |
| GPX1 | Gluthatione peroxidase 1. |
| HBM4EU | European Humam Biomonitoring Initiative. |
| HBM-GVs | Human biomonitoring guidance values. |
| IPCS | International Programme on Chemical Safety. |
| IUPAC | International Union of Pure and Applied Chemistry. |
| KIKKB | Institute of Clinical Chemistry and Biochemistry. |
| LOQ | Limit of quantification. |
| MDRD | Modification of Diet in Renal Disease. |
| MMPs | Matrix metalloproteinases. |
| MPV | Mean platelet volume. |
| MSC | Member State Committee. |
| NAG | N-acetyl-beta-D-glucosamidase. |
| NEOC | Non-epithelial ovarian cancer. |
| OC | Ovarian cancer. |
| PCOS | Polycystic ovary syndrome. |
| PVC | Polyvinyl chloride. |
| RACK1 | Receptor for Activated C Kinase 1. |
| ROS | Reactive oxygen species. |
| SOD1 | Superoxide dismutase 1. |
| SOD2 | Superoxide dismutase 2. |
| SVHC | Substances of very high concern. |
| TME | Tumour microenvironment. |
| USG | Urinary specific gravity. |
| MDRD | Modification of Diet in Renal Disease. |
| WHO | World Helath Organization. |
References
- Mikołajewska, K.; Stragierowicz, J.; Gromadzińska, J. Bisphenol A—Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Geens, T.; Aerts, D.; Berthot, C.; Bourguignon, J.P.; Goeyens, L.; Lecomte, P.; Maghuin-Rogister, G.; Pironnet, A.M.; Pussemier, L.; Scippo, M.L.; et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012, 50, 3725–3740. [Google Scholar] [CrossRef]
- den Braver-Sewradj, S.P.; van Spronsen, R.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef]
- Abraham, A.; Chakraborty, P. A review on sources and health impacts of bisphenol A. Rev. Environ. Health 2020, 35, 201–210. [Google Scholar] [CrossRef]
- Snoj Tratnik, J.; Kosjek, T.; Heath, E.; Mazej, D.; Ćehić, S.; Karakitsios, S.P.; Sarigiannis, D.A.; Horvat, M. Urinary bisphenol A in children, mothers and fathers from Slovenia: Overall results and determinants of exposure. Environ. Res. 2019, 168, 32–40. [Google Scholar] [CrossRef]
- Becher, R.; Wellendorf, H.; Sakhi, A.K.; Samuelsen, J.T.; Thomsen, C.; Bølling, A.K.; Kopperud, H.M. Presence and leaching of bisphenol a (BPA) from dental materials. Acta Biomater. Odontol. Scand. 2018, 4, 56–62. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Limosani, R.V.; Oliviero, C.; Saeed, S.; Iulini, M.; Passoni, F.C.; Racchi, M.; Corsini, E. Endocrine Disrupting Toxicity of Bisphenol A and Its Analogs: Implications in the Neuro-Immune Milieu. J. Xenobiot. 2025, 15, 13. [Google Scholar] [CrossRef]
- Kamaludin, R.; Rasdi, Z.; Othman, M.H.D.; Sheikh Abdul Kadir, S.H. Human metabolic effects of BPA and the application of a hybrid photocatalytic membrane for BPA contaminated water. Sustain. Environ. Res. 2024, 34, 15. [Google Scholar] [CrossRef]
- Sendra, M.; Moreno-Garrido, I.; Blasco, J. Single and multispecies microalgae toxicological tests assessing the impact of several BPA analogues used by industry. Environ. Pollut. 2023, 333, 122073. [Google Scholar] [CrossRef]
- Shafei, A.; Ramzy, M.M.; Hegazy, A.I.; Husseny, A.K.; El-Hadary, U.G.; Taha, M.M.; Mosa, A.A. The molecular mechanisms of action of the endocrine disrupting chemical bisphenol A in the development of cancer. Gene 2018, 647, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.F.; Tariq, T.; Fatima, B.; Sahar, A.; Tariq, F.; Munir, S.; Khan, S.; Nawaz Ranjha, M.M.A.; Sameen, A.; Zeng, X.A.; et al. An insight into bisphenol A, food exposure and its adverse effects on health: A review. Front. Nutr. 2022, 9, 1047827. [Google Scholar] [CrossRef] [PubMed]
- Zalko, D.; Jacques, C.; Duplan, H.; Bruel, S.; Perdu, E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere 2011, 82, 424–430. [Google Scholar] [CrossRef]
- Gerona, R.R.; Pan, J.; Zota, A.R.; Schwartz, J.M.; Friesen, M.; Taylor, J.A.; Hunt, P.A.; Woodruff, T.J. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: A cross-sectional study. Environ. Health 2016, 15, 50. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Nelson, J.W.; Scammell, M.K.; Hatch, E.E.; Webster, T.F. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: A cross-sectional study within NHANES 2003–2006. Environ. Health 2012, 11, 10. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of phenolic endocrine disrupting chemicals (EDCs) from maternal blood plasma and amniotic fluid in Indian population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, B.; Yang, R.; Wu, Y.; Zhao, Y.; Li, C.; Zhang, J.; Xing, Y.; Shao, B. Bisphenol Analogues and Their Chlorinated Derivatives in Breast Milk in China: Occurrence and Exposure Assessment. J. Agric. Food Chem. 2021, 69, 1391–1397. [Google Scholar] [CrossRef]
- Gould, J.C.; Leonard, L.S.; Maness, S.C.; Wagner, B.L.; Conner, K.; Zacharewski, T.; Safe, S.; McDonnell, D.P.; Gaido, K.W. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol. Cell Endocrinol. 1998, 142, 203–214. [Google Scholar] [CrossRef]
- Hafezi, S.A.; Abdel-Rahman, W.M. The Endocrine Disruptor Bisphenol A (BPA) Exerts a Wide Range of Effects in Carcinogenesis and Response to Therapy. Curr. Mol. Pharmacol. 2019, 12, 230–238. [Google Scholar] [CrossRef]
- Fenichel, P.; Chevalier, N.; Brucker-Davis, F. Bisphenol A: An endocrine and metabolic disruptor. Ann. Endocrinol. 2013, 74, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Pallottini, V.; Marino, M. Molecular Mechanisms of Action of BPA. Dose Response 2015, 13, 1559325815610582. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Magdalena, P.; Ropero, A.B.; Soriano, S.; García-Arévalo, M.; Ripoll, C.; Fuentes, E.; Quesada, I.; Nadal, Á. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Mol. Cell Endocrinol. 2012, 355, 201–207. [Google Scholar] [CrossRef]
- Liu, X.; Sakai, H.; Nishigori, M.; Suyama, K.; Nawaji, T.; Ikeda, S.; Nishigouchi, M.; Okada, H.; Matsushima, A.; Nose, T.; et al. Receptor-binding affinities of bisphenol A and its next-generation analogs for human nuclear receptors. Toxicol. Appl. Pharmacol. 2019, 377, 114610. [Google Scholar] [CrossRef]
- Pathak, R.K.; Kim, J.M. Structural insight into the mechanisms and interacting features of endocrine disruptor Bisphenol A and its analogs with human estrogen-related receptor gamma. Environ. Pollut. 2024, 345, 123549. [Google Scholar] [CrossRef]
- Sun, H.; Xu, L.C.; Chen, J.F.; Song, L.; Wang, X.R. Effect of bisphenol A, tetrachlorobisphenol A and pentachlorophenol on the transcriptional activities of androgen receptor-mediated reporter gene. Food Chem. Toxicol. 2006, 44, 1916–1921. [Google Scholar] [CrossRef]
- Buoso, E.; Kenda, M.; Masi, M.; Linciano, P.; Galbiati, V.; Racchi, M.; Dolenc, M.S.; Corsini, E. Effects of Bisphenols on RACK1 Expression and Their Immunological Implications in THP-1 Cells. Front. Pharmacol. 2021, 12, 743991. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Long, A.; Chiappini, C.; Travelli, C.; Govoni, S.; Racchi, M. Ribosomes as a nexus between translation and cancer progression: Focus on ribosomal Receptor for Activated C Kinase 1 (RACK1) in breast cancer. Br. J. Pharmacol. 2022, 179, 2813–2828. [Google Scholar] [CrossRef]
- Corsini, E.; Buoso, E.; Galbiati, V.; Racchi, M. Role of Protein Kinase C in Immune Cell Activation and Its Implication Chemical-Induced Immunotoxicity. Adv. Exp. Med. Biol. 2021, 1275, 151–163. [Google Scholar] [CrossRef]
- Buoso, E.; Masi, M.; Racchi, M.; Corsini, E. Endocrine-Disrupting Chemicals’ (EDCs) Effects on Tumour Microenvironment and Cancer Progression: Emerging Contribution of RACK1. Int. J. Mol. Sci. 2020, 21, 9229. [Google Scholar] [CrossRef]
- Masi, M.; Racchi, M.; Travelli, C.; Corsini, E.; Buoso, E. Molecular Characterization of Membrane Steroid Receptors in Hormone-Sensitive Cancers. Cells 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Lambré, C.; Barat Baviera, J.M.; Bolognesi, C.; Chesson, A.; Cocconcelli, P.S.; Crebelli, R.; Gott, D.M.; Grob, K.; Lampi, E.; Mengelers, M.; et al. Re-evaluation of the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2023, 21, e06857. [Google Scholar] [CrossRef]
- Pahović, P.; Iulini, M.; Maddalon, A.; Galbiati, V.; Buoso, E.; Dolenc, M.S.; Corsini, E. In Vitro Effects of Bisphenol Analogs on Immune Cells Activation and Th Differentiation. Endocr. Metab. Immune Disord. Drug Targets 2023, 23, 1750–1761. [Google Scholar] [CrossRef]
- Sharpe, R.M.; Skakkebaek, N.E. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet 1993, 341, 1392–1395. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-de-Toro, M.; Markey, C.M.; Wadia, P.R.; Luque, E.H.; Rubin, B.S.; Sonnenschein, C.; Soto, A.M. Perinatal exposure to bisphenol-A alters peripubertal mammary gland development in mice. Endocrinology 2005, 146, 4138–4147. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Jørgensen, N.; Carlsen, E.; Petersen, P.M.; Giwercman, A.; Andersen, A.G.; Jensen, T.K.; Andersson, A.M.; Müller, J. Germ cell cancer and disorders of spermatogenesis: An environmental connection? Apmis 1998, 106, 3–11; discussion 12. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Costa, E.M.; Spritzer, P.M.; Hohl, A.; Bachega, T.A. Effects of endocrine disruptors in the development of the female reproductive tract. Arq. Bras. Endocrinol. Metabol. 2014, 58, 153–161. [Google Scholar] [CrossRef]
- Czubacka, E.; Wielgomas, B.; Klimowska, A.; Radwan, M.; Radwan, P.; Karwacka, A.; Kałużny, P.; Jurewicz, J. Urinary Bisphenol A Concentrations and Parameters of Ovarian Reserve among Women from a Fertility Clinic. Int. J. Environ. Res. Public Health 2021, 18, 8041. [Google Scholar] [CrossRef]
- Patrick, L. Thyroid disruption: Mechanism and clinical implications in human health. Altern. Med. Rev. 2009, 14, 326–346. [Google Scholar]
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 254, 109275. [Google Scholar] [CrossRef] [PubMed]
- Heindel, J.J.; Blumberg, B.; Cave, M.; Machtinger, R.; Mantovani, A.; Mendez, M.A.; Nadal, A.; Palanza, P.; Panzica, G.; Sargis, R.; et al. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017, 68, 3–33. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef]
- Murray, T.J.; Maffini, M.V.; Ucci, A.A.; Sonnenschein, C.; Soto, A.M. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod. Toxicol. 2007, 23, 383–390. [Google Scholar] [CrossRef]
- Wetherill, Y.B.; Hess-Wilson, J.K.; Comstock, C.E.; Shah, S.A.; Buncher, C.R.; Sallans, L.; Limbach, P.A.; Schwemberger, S.; Babcock, G.F.; Knudsen, K.E. Bisphenol A facilitates bypass of androgen ablation therapy in prostate cancer. Mol. Cancer Ther. 2006, 5, 3181–3190. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Tian, Y.; Xu, P.; Yue, H.; Sang, N. Bisphenol B and bisphenol AF exposure enhances uterine diseases risks in mouse. Environ. Int. 2023, 173, 107858. [Google Scholar] [CrossRef]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef]
- Stanculeanu, D.L.; Mosoiu, D.; Mihnea, A.; Simion, L. Actualities in Ovarian Cancer in the Perspective of 2015 (ASCO and ECCO). Chirurgia 2016, 111, 9–11. [Google Scholar]
- Trinidad, C.V.; Tetlow, A.L.; Bantis, L.E.; Godwin, A.K. Reducing Ovarian Cancer Mortality Through Early Detection: Approaches Using Circulating Biomarkers. Cancer Prev. Res. 2020, 13, 241–252. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef]
- Matz, M.; Coleman, M.P.; Sant, M.; Chirlaque, M.D.; Visser, O.; Gore, M.; Allemani, C. The histology of ovarian cancer: Worldwide distribution and implications for international survival comparisons (CONCORD-2). Gynecol. Oncol. 2017, 144, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Samtani, R.; Sharma, N.; Garg, D. Effects of Endocrine-Disrupting Chemicals and Epigenetic Modifications in Ovarian Cancer: A Review. Reprod. Sci. 2018, 25, 7–18. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef]
- Pan, S.Y.; Ugnat, A.M.; Mao, Y.; Wen, S.W.; Johnson, K.C. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1521–1527. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, K.Y.; An, B.S.; Choi, J.H.; Jeung, E.B.; Leung, P.C.; Choi, K.C. Cell growth of ovarian cancer cells is stimulated by xenoestrogens through an estrogen-dependent pathway, but their stimulation of cell growth appears not to be involved in the activation of the mitogen-activated protein kinases ERK-1 and p38. J. Reprod. Dev. 2009, 55, 23–29. [Google Scholar] [CrossRef]
- Ptak, A.; Wróbel, A.; Gregoraszczuk, E.L. Effect of bisphenol-A on the expression of selected genes involved in cell cycle and apoptosis in the OVCAR-3 cell line. Toxicol. Lett. 2011, 202, 30–35. [Google Scholar] [CrossRef]
- Ptak, A.; Rak-Mardyła, A.; Gregoraszczuk, E.L. Cooperation of bisphenol A and leptin in inhibition of caspase-3 expression and activity in OVCAR-3 ovarian cancer cells. Toxicol. In Vitro 2013, 27, 1937–1943. [Google Scholar] [CrossRef]
- Ptak, A.; Hoffmann, M.; Gruca, I.; Barć, J. Bisphenol A induce ovarian cancer cell migration via the MAPK and PI3K/Akt signalling pathways. Toxicol. Lett. 2014, 229, 357–365. [Google Scholar] [CrossRef]
- Ptak, A.; Gregoraszczuk, E.L. Effects of bisphenol A and 17β-estradiol on vascular endothelial growth factor A and its receptor expression in the non-cancer and cancer ovarian cell lines. Cell Biol. Toxicol. 2015, 31, 187–197. [Google Scholar] [CrossRef]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod. Toxicol. 2007, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Dumitrascu, M.C.; Mares, C.; Petca, R.C.; Sandru, F.; Popescu, R.I.; Mehedintu, C.; Petca, A. Carcinogenic effects of bisphenol A in breast and ovarian cancers. Oncol. Lett. 2020, 20, 282. [Google Scholar] [CrossRef] [PubMed]
- Ptak, A.; Gregoraszczuk, E.L. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol. Lett. 2012, 210, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Bredhult, C.; Bäcklin, B.M.; Olovsson, M. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod. Toxicol. 2007, 23, 550–559. [Google Scholar] [CrossRef]
- Hall, J.M.; Korach, K.S. Endocrine disrupting chemicals promote the growth of ovarian cancer cells via the ER-CXCL12-CXCR4 signaling axis. Mol. Carcinog. 2013, 52, 715–725. [Google Scholar] [CrossRef]
- Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 2010, 177, 1053–1064. [Google Scholar] [CrossRef]
- Kenny, H.A.; Lengyel, E. MMP-2 functions as an early response protein in ovarian cancer metastasis. Cell Cycle 2009, 8, 683–688. [Google Scholar] [CrossRef]
- Symowicz, J.; Adley, B.P.; Gleason, K.J.; Johnson, J.J.; Ghosh, S.; Fishman, D.A.; Hudson, L.G.; Stack, M.S. Engagement of collagen-binding integrins promotes matrix metalloproteinase-9-dependent E-cadherin ectodomain shedding in ovarian carcinoma cells. Cancer Res. 2007, 67, 2030–2039. [Google Scholar] [CrossRef]
- Rajaura, S.; Bhardwaj, N.; Singh, A.; Babu, R.; Gupta, N.; Ahmed, M.Z. Bisphenol A-induced oxidative stress increases the production of ovarian cancer stem cells in mice. Reprod. Toxicol. 2024, 130, 108724. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Rajaura, S.; Singh, A.; Rambabu; Nivedita; Ahmed, M.Z. Bisphenol-A-induced ovarian cancer: Changes in epithelial diversity, apoptosis, antioxidant and anti-inflammatory mechanisms. Reprod. Toxicol. 2025, 135, 108909. [Google Scholar] [CrossRef]
- Covaci, A.; Den Hond, E.; Geens, T.; Govarts, E.; Koppen, G.; Frederiksen, H.; Knudsen, L.E.; Mørck, T.A.; Gutleb, A.C.; Guignard, C.; et al. Urinary BPA measurements in children and mothers from six European member states: Overall results and determinants of exposure. Environ. Res. 2015, 141, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Gagné, M.; Nong, A.; Aylward, L.L.; Hays, S.M. Biomonitoring Equivalents for bisphenol A (BPA). Regul. Toxicol. Pharmacol. 2010, 58, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Chahoud, I.; Heindel, J.J.; Padmanabhan, V.; Paumgartten, F.J.; Schoenfelder, G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012, 17, 407–434. [Google Scholar] [CrossRef]
- Suwannarin, N.; Nishihama, Y.; Isobe, T.; Nakayama, S.F. Urinary concentrations of environmental phenol among pregnant women in the Japan Environment and Children’s Study. Environ. Int. 2024, 183, 108373. [Google Scholar] [CrossRef]
- Ougier, E.; Zeman, F.; Antignac, J.P.; Rousselle, C.; Lange, R.; Kolossa-Gehring, M.; Apel, P. Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ. Int. 2021, 154, 106563. [Google Scholar] [CrossRef]
- CDC. National Report on Human Exposure to Environmental Chemicals; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2017. [Google Scholar]
- Ortiz, A.; Sanchez-Niño, M.D.; Sanz, A.B. The meaning of urinary creatinine concentration. Kidney Int. 2011, 79, 791. [Google Scholar] [CrossRef]
- Huang, Q.; Fei, X.; Zhan, H.; Gong, J.; Zhou, J.; Zhang, Y.; Ye, X.; Song, Y.; Ma, J.; Wu, X. Urinary N-acetyl-β-d-glucosaminidase-creatine ratio is a valuable predictor for advanced diabetic kidney disease. J. Clin. Lab. Anal. 2023, 37, e24769. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, Y.H.; Lee, S.G.; Kang, E.S.; Cha, B.S.; Lee, B.W. The renal tubular damage marker urinary N-acetyl-β-D-glucosaminidase may be more closely associated with early detection of atherosclerosis than the glomerular damage marker albuminuria in patients with type 2 diabetes. Cardiovasc. Diabetol. 2017, 16, 16. [Google Scholar] [CrossRef]
- Delanaye, P.; Schaeffner, E.; Ebert, N.; Cavalier, E.; Mariat, C.; Krzesinski, J.-M.; Moranne, O. Normal reference values for glomerular filtration rate: What do we really know? Nephrol. Dial. Transplant. 2012, 27, 2664–2672. [Google Scholar] [CrossRef]
- Tarwater, K. Estimated glomerular filtration rate explained. MO Med. 2011, 108, 29–32. [Google Scholar]
- Shen, Y.; Liu, T.; Shi, Y.; Zhuang, F.; Lu, J.; Zhu, Q.; Ding, F. Bisphenol A analogs in patients with chronic kidney disease and dialysis therapy. Ecotoxicol. Environ. Saf. 2019, 185, 109684. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, J.P.; Choi, K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005-2016). Sci. Total Environ. 2021, 791, 148343. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Park, J.Y.; Kim, S.; An, J.N.; Lee, J.; Park, H.; Jung, S.K.; Kim, S.Y.; Lee, J.P.; Choi, K. Association of exposure to phthalates and environmental phenolics with markers of kidney function: Korean National Environmental Health Survey (KoNEHS) 2015–2017. Environ. Int. 2020, 143, 105877. [Google Scholar] [CrossRef]
- Malits, J.; Attina, T.M.; Karthikraj, R.; Kannan, K.; Naidu, M.; Furth, S.; Warady, B.A.; Vento, S.; Trachtman, H.; Trasande, L. Renal Function and exposure to Bisphenol A and phthalates in children with Chronic Kidney Disease. Environ. Res. 2018, 167, 575–582. [Google Scholar] [CrossRef]
- Jacobson, M.H.; Wu, Y.; Liu, M.; Attina, T.M.; Naidu, M.; Karthikraj, R.; Kannan, K.; Warady, B.A.; Furth, S.; Vento, S.; et al. Serially assessed bisphenol A and phthalate exposure and association with kidney function in children with chronic kidney disease in the US and Canada: A longitudinal cohort study. PLoS Med. 2020, 17, e1003384. [Google Scholar] [CrossRef]
- You, L.; Zhu, X.; Shrubsole, M.J.; Fan, H.; Chen, J.; Dong, J.; Hao, C.M.; Dai, Q. Renal function, bisphenol A, and alkylphenols: Results from the National Health and Nutrition Examination Survey (NHANES 2003–2006). Environ. Health Perspect. 2011, 119, 527–533. [Google Scholar] [CrossRef]
- Jenne, C.N.; Urrutia, R.; Kubes, P. Platelets: Bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol. 2013, 35, 254–261. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Mikhailidis, D.P.; Kitas, G.D. Mean platelet volume: A link between thrombosis and inflammation? Curr. Pharm. Des. 2011, 17, 47–58. [Google Scholar] [CrossRef]
- Li, C.; Cao, M.; Qi, T.; Ye, X.; Ma, L.; Pan, W.; Luo, J.; Chen, P.; Liu, J.; Zhou, J. The association of bisphenol A exposure with premature ovarian insufficiency: A case-control study. Climacteric 2021, 24, 95–100. [Google Scholar] [CrossRef]
- Stavridis, K.; Triantafyllidou, O.; Pisimisi, M.; Vlahos, N. Bisphenol-A and Female Fertility: An Update of Existing Epidemiological Studies. J. Clin. Med. 2022, 11, 7227. [Google Scholar] [CrossRef]
- Pomeroy, D.E.; Tooley, K.L.; Probert, B.; Wilson, A.; Kemps, E. A Systematic Review of the Effect of Dietary Supplements on Cognitive Performance in Healthy Young Adults and Military Personnel. Nutrients 2020, 12, 545. [Google Scholar] [CrossRef] [PubMed]
- IARC monographs on the evaluation of carcinogenic risks to humans. Volume 97. 1,3-butadiene, ethylene oxide and vinyl halides (vinyl fluoride, vinyl chloride and vinyl bromide). IARC Monogr. Eval. Carcinog. Risks Hum. 2008, 97, 3–471.
- Ward, E.; Boffetta, P.; Andersen, A.; Colin, D.; Comba, P.; Deddens, J.A.; De Santis, M.; Engholm, G.; Hagmar, L.; Langard, S.; et al. Update of the follow-up of mortality and cancer incidence among European workers employed in the vinyl chloride industry. Epidemiology 2001, 12, 710–718. [Google Scholar] [CrossRef]
- Solouki, S.; Fazeli, M. Efficiency of Multispecies Probiotic Supplements in Bioremoval of Bisphenol A: An In Vitro Study. Appl. Food Biotechnol. 2018, 5, 37–45. [Google Scholar] [CrossRef]
- Howdeshell, K.L.; Peterman, P.H.; Judy, B.M.; Taylor, J.A.; Orazio, C.E.; Ruhlen, R.L.; Vom Saal, F.S.; Welshons, W.V. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 2003, 111, 1180–1187. [Google Scholar] [CrossRef]
- Le, H.H.; Carlson, E.M.; Chua, J.P.; Belcher, S.M. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol. Lett. 2008, 176, 149–156. [Google Scholar] [CrossRef]
- Wilson, N.K.; Chuang, J.C.; Morgan, M.K.; Lordo, R.A.; Sheldon, L.S. An observational study of the potential exposures of preschool children to pentachlorophenol, bisphenol-A, and nonylphenol at home and daycare. Environ. Res. 2007, 103, 9–20. [Google Scholar] [CrossRef]
- Stahlhut, R.W.; Welshons, W.V.; Swan, S.H. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009, 117, 784–789. [Google Scholar] [CrossRef]
- Völkel, W.; Colnot, T.; Csanády, G.A.; Filser, J.G.; Dekant, W. Metabolism and kinetics of bisphenol a in humans at low doses following oral administration. Chem. Res. Toxicol. 2002, 15, 1281–1287. [Google Scholar] [CrossRef]
- Völkel, W.; Bittner, N.; Dekant, W. Quantitation of bisphenol A and bisphenol A glucuronide in biological samples by high performance liquid chromatography-tandem mass spectrometry. Drug Metab. Dispos. 2005, 33, 1748–1757. [Google Scholar] [CrossRef]
- Sambasivan, S. Epithelial ovarian cancer: Review article. Cancer Treat. Res. Commun. 2022, 33, 100629. [Google Scholar] [CrossRef] [PubMed]
- Lalrinpuii, E.; Bhageerathy, P.S.; Sebastian, A.; Jeyaseelan, L.; VinothaThomas; Thomas, A.; Chandy, R.; Peedicayil, A. Ovarian Cancer in Young Women. Indian. J. Surg. Oncol. 2017, 8, 540–547. [Google Scholar] [CrossRef]
- Koch, H.M.; Calafat, A.M. Human body burdens of chemicals used in plastic manufacture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2063–2078. [Google Scholar] [CrossRef]
- Arakawa, C.; Fujimaki, K.; Yoshinaga, J.; Imai, H.; Serizawa, S.; Shiraishi, H. Daily urinary excretion of bisphenol A. Environ. Health Prev. Med. 2004, 9, 22–26. [Google Scholar] [CrossRef]
- Mahalingaiah, S.; Meeker, J.D.; Pearson, K.R.; Calafat, A.M.; Ye, X.; Petrozza, J.; Hauser, R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ. Health Perspect. 2008, 116, 173–178. [Google Scholar] [CrossRef]
- Ye, X.; Wong, L.Y.; Bishop, A.M.; Calafat, A.M. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ. Health Perspect. 2011, 119, 983–988. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, J.; Wu, Y. Tumor metabolism rewiring in epithelial ovarian cancer. J. Ovarian Res. 2023, 16, 108. [Google Scholar] [CrossRef]
- Donadio, C.; Lucchesi, A.; Ardini, M.; Cosio, S.; Gadducci, A. Renal impairment in patients with ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 106, 198–202. [Google Scholar] [CrossRef]
- Runkel, A.A.; Snoj-Tratnik, J.; Mazej, D.; Horvat, M. Urinary phthalate concentrations in the slovenian population: An attempt to exposure assessment of family units. Environ. Res. 2020, 186, 109548. [Google Scholar] [CrossRef]
- Tkalec, Ž.; Kosjek, T.; Snoj Tratnik, J.; Stajnko, A.; Runkel, A.A.; Sykiotou, M.; Mazej, D.; Horvat, M. Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: Urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 2021, 146, 106172. [Google Scholar] [CrossRef]
| Category | Control Group * (n = 26) | EOC/EBOT Group 1 ** (n = 13) | EOC/EBOT Group 2 *** (n = 11) | p-Value | |
|---|---|---|---|---|---|
| N | 26 | 13 | 11 | ||
| Age (years) | Median | 34 | 36 | 61 | p < 0.001 (study group 2 vs. control group or study group 1) |
| Min–Max | 23–39 | 16–49 | 50–72 | ||
| Body mass index (kg/m2) | Median | 24.5 | 24 | 30.5 | p = 0.002 (study group 2 vs. control group) p = 0.003 (study group 2 vs. study group 1) |
| Min–Max | 19.3–35.4 | 18.6–35.9 | 24.0–38.9 |
| Population | N | ≥0.10 (µg/L) | Min–Max | GM | 95% CI |
|---|---|---|---|---|---|
| Total BPA (µg/L) | |||||
| Control group | 26 | 77 a | 0.10–0.49 | 0.14 | 0.11–0.19 |
| EOC/EBOT group 1 | 13 | 92 b | 0.10–1.14 | 0.42 | 0.28–0.63 |
| EOC/EBOT group 2 | 11 | 100 c | 0.13–1.09 | 0.36 | 0.22–0.59 |
| Population | N | ≥0.10 (µg/L) | Min–max | GM | 95% CI |
| USG-adjusted BPA (µg/L) | |||||
| Control group | 26 | 77 a | 0.10–0.61 | 0.17 | 0.13–0.22 |
| EOC/EBOT group 1 | 13 | 92 b | 0.19–1.28 | 0.47 | 0.34–0.65 |
| EOC/EBOT group 2 | 11 | 100 c | 0.16–0.84 | 0.38 | 0.26–0.57 |
| Category | Healthy Control Group of Women | EOC/EBOT Group of Women | p-Value | |
|---|---|---|---|---|
| Age (years) | Median | 32.5 | 42.3 | <0.001 |
| Smoking (%) | Yes | 24.2 | 4.8 | 0.043 |
| No | 75.8 | 95.2 | ||
| No | 19.7 | 28.6 | ||
| Cups of coffee per day (%) | 1 | 36.2 | 25.0 | 0.016 |
| 1.5 | 19.1 | 12.5 | ||
| 2 | 31.9 | 25.0 | ||
| 2.5 | 4.3 | 6.3 | ||
| 3 | 8.5 | 18.8 | ||
| 4 | 0 | 6.3 | ||
| 10 | 0 | 6.3 | ||
| Consumption of folic acid in pregnancy (%) | Yes | 11.6 | 69.2 | <0.001 |
| No | 88.4 | 30.8 | ||
| Consumption of dietary supplements | Yes | 53.5 | 15.4 | 0.015 |
| No | 46.5 | 84.6 | ||
| Use of any water purification system | Water filter | 6.8 | 35.7 | <0.001 |
| Water softener | 5.1 | 21.4 | ||
| No | 86.4 | 42.9 | ||
| Consumption of food from PVC containers before sampling | 24 h | 17.5 | 30.0 | 0.004 |
| 2 days ago | 10.0 | 50.0 | ||
| >2 days ago | 72.5 | 20 | ||
| Consumption of food from PVC bag/film before sampling | 24 h | 20.0 | 50.0 | 0.012 |
| 2 days ago | 17.5 | 30.0 | ||
| >2 days ago | 62.5 | 20 | ||
| No | 50.0 | 42.1 | ||
| Drinking from plastic sport bottle before sampling | 24 h | 14.0 | 37.5 | 0.004 |
| 2 days ago | 2.3 | 37.5 | ||
| >2 days ago | 83.8 | 25.0 | ||
| Multi-apartment home | 23.3 | 25.0 | ||
| Apartment block | 30.0 | 50.0 | ||
| No | 96.7 | 100 |
| Total BPA in Urine (µg/L) | Mean/Coeff. | Standard Deviation | p-Value | |
|---|---|---|---|---|
| All women (healthy control group + EOC/EBOT group of women) | ||||
| Intake of dietary supplements (n = 54) | Yes (n = 24) No (n = 30) | 0.21 0.35 | 0.16 0.28 | 0.032 |
| Habit of refilling single-use plastic bottle (n = 78) | Yes (n = 42) No (n = 36) | 0.32 0.21 | 0.27 0.14 | 0.048 |
| Time interval between the last intake of the food from PVC containers to urine sampling (n = 48) | −0.327 | 0.023 | ||
| Healthy control group | ||||
| Habit of refilling single-use plastic bottle (n = 78) | Yes (n = 31) No (n = 28) | 0.27 0.17 | 0.21 0.12 | 0.051 (*) |
| Number of amalgam dental fillings (n = 28) | 0.400 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sladič, M.; Smrkolj, Š.; Kavšek, G.; Imamovic-Kumalic, S.; Verdenik, I.; Virant-Klun, I. Bisphenol A in the Urine: Association with Urinary Creatinine, Impaired Kidney Function, Use of Plastic Food and Beverage Storage Products but Not with Serum Anti-Müllerian Hormone in Ovarian Malignancies. Int. J. Mol. Sci. 2025, 26, 4811. https://doi.org/10.3390/ijms26104811
Sladič M, Smrkolj Š, Kavšek G, Imamovic-Kumalic S, Verdenik I, Virant-Klun I. Bisphenol A in the Urine: Association with Urinary Creatinine, Impaired Kidney Function, Use of Plastic Food and Beverage Storage Products but Not with Serum Anti-Müllerian Hormone in Ovarian Malignancies. International Journal of Molecular Sciences. 2025; 26(10):4811. https://doi.org/10.3390/ijms26104811
Chicago/Turabian StyleSladič, Mateja, Špela Smrkolj, Gorazd Kavšek, Senka Imamovic-Kumalic, Ivan Verdenik, and Irma Virant-Klun. 2025. "Bisphenol A in the Urine: Association with Urinary Creatinine, Impaired Kidney Function, Use of Plastic Food and Beverage Storage Products but Not with Serum Anti-Müllerian Hormone in Ovarian Malignancies" International Journal of Molecular Sciences 26, no. 10: 4811. https://doi.org/10.3390/ijms26104811
APA StyleSladič, M., Smrkolj, Š., Kavšek, G., Imamovic-Kumalic, S., Verdenik, I., & Virant-Klun, I. (2025). Bisphenol A in the Urine: Association with Urinary Creatinine, Impaired Kidney Function, Use of Plastic Food and Beverage Storage Products but Not with Serum Anti-Müllerian Hormone in Ovarian Malignancies. International Journal of Molecular Sciences, 26(10), 4811. https://doi.org/10.3390/ijms26104811





