Photopolymerization of Styrene–Naphthalenediimide Monomer: Formation of Pattern and Electrochromism
Abstract
:1. Introduction
2. Results and Discussion
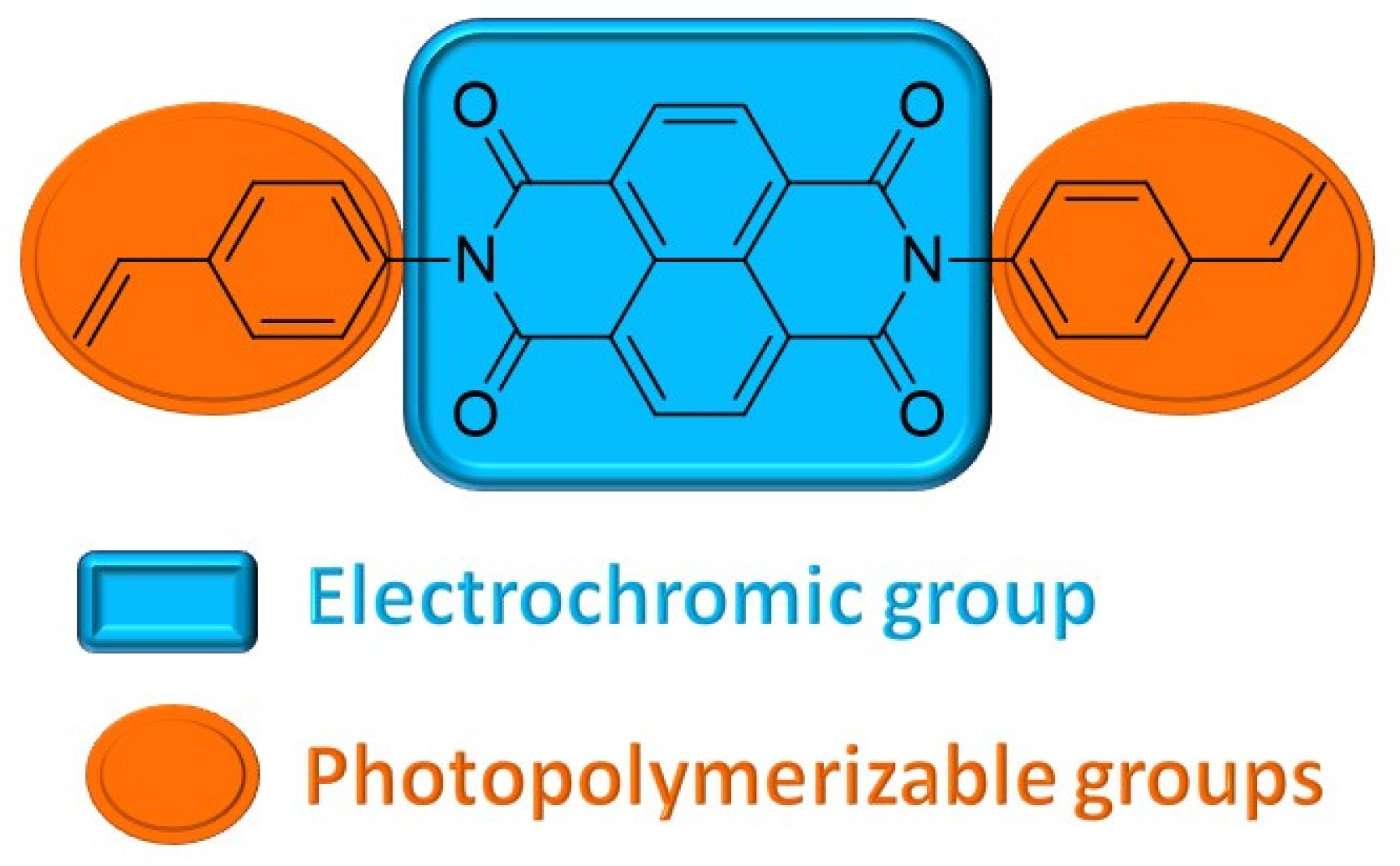
2.1. Synthesis of the Monomer
2.2. Photopolymerization
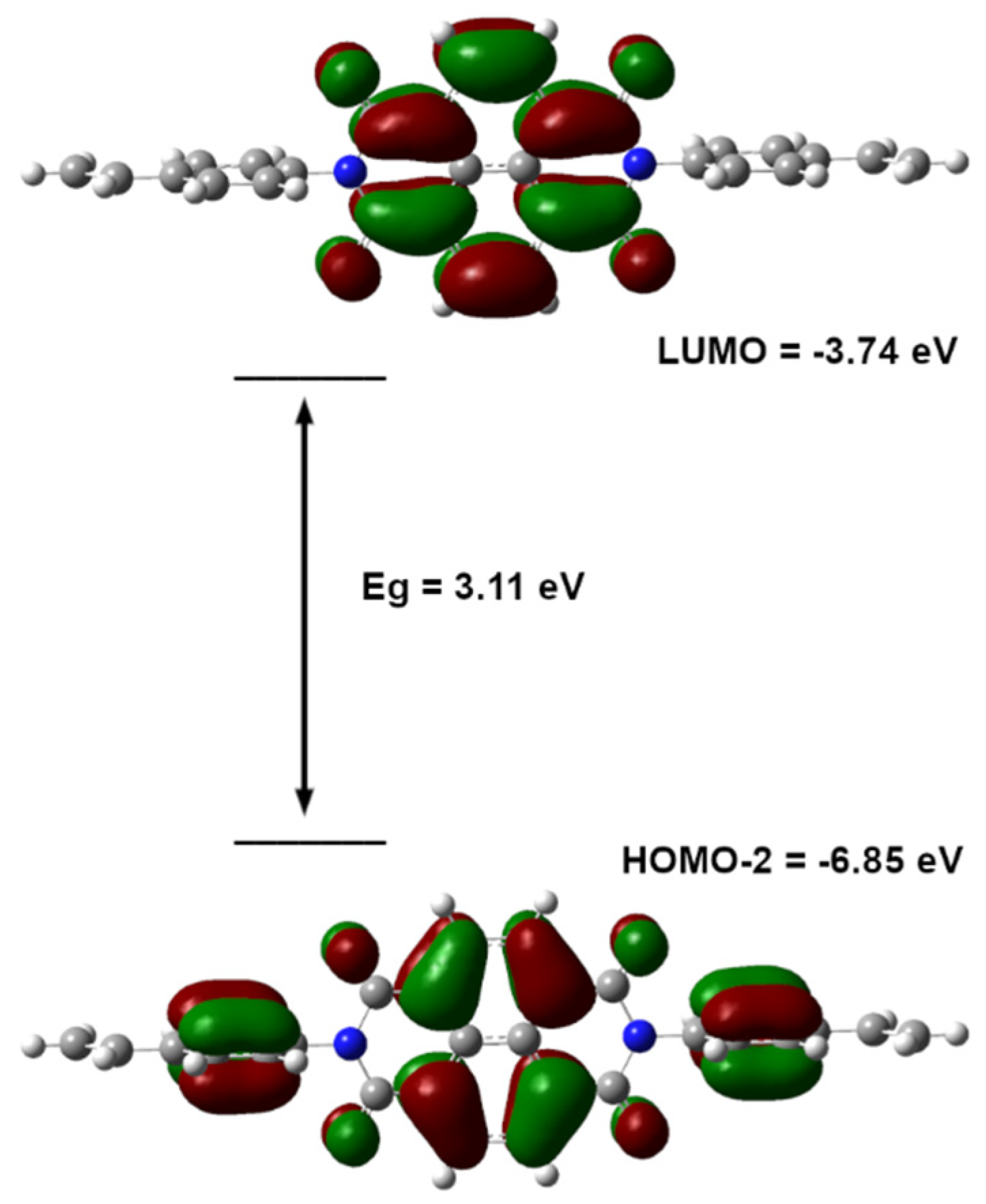
2.3. DFT Calculations
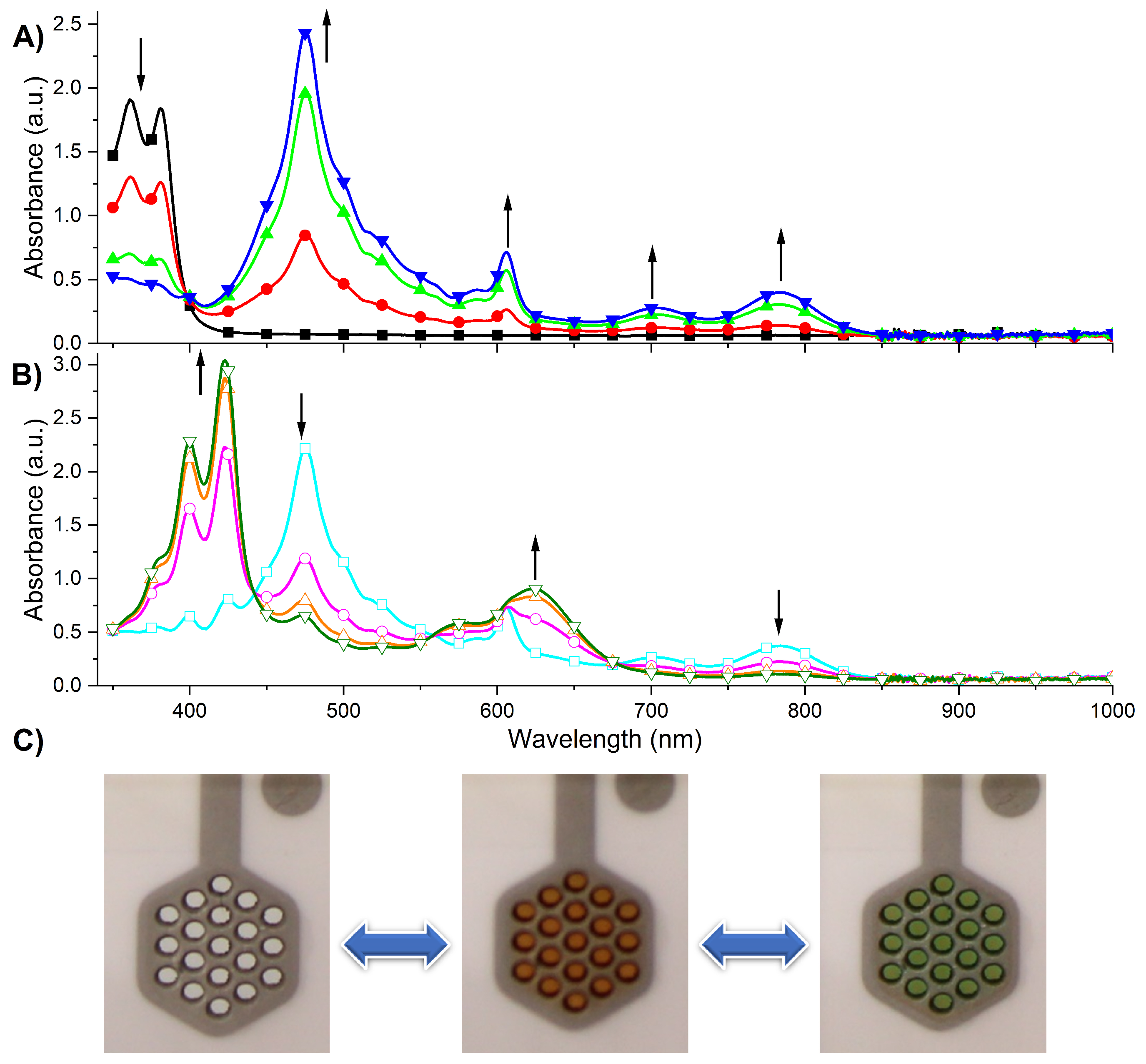
2.4. Electrochemistry and UV-Vis Spectroelectrochemistry
2.5. Electrochromic Device
3. Materials and Methods
3.1. Synthesis of Monomer
3.2. Photopolymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Electrochromic Smart Materials; Xu, J.W., Chua, M.H., Shah, K.W., Eds.; Smart Materials Series; Royal Society of Chemistry: Cambridge, UK, 2019; ISBN 978-1-78801-143-3. [Google Scholar]
- Banasz, R.; Kubicki, M.; Wałȩsa-Chorab, M. Yellow-to-brown and yellow-to-green electrochromic devices based on complexes of transition metal ions with a triphenylamine-based ligand. Dalt. Trans. 2020, 49, 15041–15053. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Muras, K.; Filiatrault, H.L.; Skene, W.G. Suitability of alkyne donor-π-donor-π-donor scaffolds for electrofluorochromic and electrochromic use. J. Mater. Chem. C 2022, 10, 3691–3703. [Google Scholar] [CrossRef]
- Muras, K.; Kubicki, M.; Wałęsa-Chorab, M. Benzochalcodiazole-based donor-acceptor-donor non-symmetric small molecules as dual-functioning electrochromic and electrofluorochromic materials. Dye. Pigment. 2023, 212, 111098. [Google Scholar] [CrossRef]
- Li, M.; Yassin, O.A.; Baczkowski, M.L.; Zhang, X.; Daniels, R.; Deshmukh, A.A.; Zhu, Y.; Otley, M.T.; Sotzing, G.A. Colorless to black electrochromic devices using subtractive color mixing of two electrochromes: A conjugated polymer with a small organic molecule. Org. Electron. 2020, 84, 105748. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsai, C.-Y.; Li, L.-J.; Liaw, D.-J. Colorless-to-colorful switching electrochromic polyimides with very high contrast ratio. Nat. Commun. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yang, H.; Zhong, C.; Rajan, K.; Sagar, R.U.R.; Qi, X.; Deng, Y.; Jiang, H.; Liu, P.; Liang, T. Colorless-to-black electrochromic devices based on ambipolar electrochromic system consisting of cross-linked poly(4-vinyltriphenylamine) and tungsten trioxide with high optical contrast in visible and near-infrared regions. Chem. Eng. J. 2021, 404, 126402. [Google Scholar] [CrossRef]
- Koo, J.; Amoli, V.; Kim, S.Y.; Lee, C.; Kim, J.; Park, S.-M.; Kim, J.; Ahn, J.M.; Jung, K.J.; Kim, D.H. Low-power, deformable, dynamic multicolor electrochromic skin. Nano Energy 2020, 78, 105199. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, X.; Jiang, S.; Wu, S.; Zhao, Y.; Zhang, L.; Song, L.; Huang, Y. Cephalopods’ skin-inspired design of nanoscale electronic transport layers for adaptive electrochromic tuning. Adv. Sci. 2024, 11, 2405444. [Google Scholar] [CrossRef]
- Wu, S.; Sun, H.; Duan, M.; Mao, H.; Wu, Y.; Zhao, H.; Lin, B. Applications of thermochromic and electrochromic smart windows: Materials to buildings. Cell Rep. Phys. Sci. 2023, 4, 101370. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Yeo, L.P.; Ong, A.J.; Zhiwei, W.; Mandler, D.; Magdassi, S.; Tok, A.I.Y. Electrochromic smart glass coating on functional nano-frameworks for effective building energy conservation. Mater. Today Energy 2020, 18, 100496. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, K.-W.; In, Y.R.; Tang, X.; Kim, P.; Quy, V.H.V.; Kim, Y.M.; Lee, J.; Choi, C.; Jung, C.; et al. Multicolor, dual-image, printed electrochromic displays based on tandem configuration. Chem. Eng. J. 2022, 429, 132319. [Google Scholar] [CrossRef]
- Zhang, W.; Li, H.; Yu, W.W.; Elezzabi, A.Y. Transparent inorganic multicolour displays enabled by zinc-based electrochromic devices. Light Sci. Appl. 2020, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhu, H.; Zhuang, J.; Lu, Y.; Chen, Z.; Guo, W. Recent advance in electrochromic materials and devices for display applications. Chempluschem 2024, 89, e202300770. [Google Scholar] [CrossRef]
- Gu, C.; Jia, A.-B.; Zhang, Y.-M.; Zhang, S.X.-A. Emerging electrochromic materials and devices for future displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef]
- Mecerreyes, D.; Marcilla, R.; Ochoteco, E.; Grande, H.; Pomposo, J.A.; Vergaz, R.; Sánchez Pena, J.M. A simplified all-polymer flexible electrochromic device. Electrochim. Acta 2004, 49, 3555–3559. [Google Scholar] [CrossRef]
- Rozman, M.; Pakseresht, A.; Galusek, D.; Duran, A.; Castro, Y. Durable multicolor electrochromic fibers based on metal oxidecoated steel tapes. Electrochim. Acta 2024, 476, 143382. [Google Scholar] [CrossRef]
- Sheng, M.; Wang, W.; Li, L.; Zhang, L.; Fu, S. All-in-one wearable electronics design: Smart electrochromic liquid-crystal-clad fibers without external electrodes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127535. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Wu, M.; Zhi, C.; Meng, J.; Zhang, L. Multicolor electrochromic fabric with a simple structure of PEDOT:PSS/DMSO. Dye. Pigment. 2023, 219, 111642. [Google Scholar] [CrossRef]
- Faceira, B.; Teulé-Gay, L.; Le Hébel, J.; Labrugère-Sarroste, C.; Ibalot, F.; Huang, H.; Huang, Y.; Dong, C.; Salvetat, J.; Maglione, M.; et al. Origin of the memory effect in electrochromic sputtered WO3 films: Composition, structure, or morphology? Adv. Mater. Interfaces 2023, 10, 2300549. [Google Scholar] [CrossRef]
- Wu, W.; Wu, L.; Ma, H.; Wu, L.; Wang, H.; Fang, H. Electrochromic devices constructed with water-in-salt electrolyte enabling energy-saving and prolonged optical memory effect. Chem. Eng. J. 2022, 446, 137122. [Google Scholar] [CrossRef]
- Ito, T.; Okazaki, S. Pushing the limits of lithography. Nature 2000, 406, 1027–1031. [Google Scholar] [CrossRef]
- Zou, C.; Chang, C.; Sun, D.; Böhringer, K.F.; Lin, L.Y. Photolithographic patterning of perovskite thin films for multicolor display applications. Nano Lett. 2020, 20, 3710–3717. [Google Scholar] [CrossRef]
- Jensen, J.; Hösel, M.; Dyer, A.L.; Krebs, F.C. Development and manufacture of polymer-based electrochromic devices. Adv. Funct. Mater. 2015, 25, 2073–2090. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Hösel, M.; Krebs, F.C. Roll-to-Roll fabrication of large area functional organic materials. J. Polym. Sci. Part. B Polym. Phys. 2013, 51, 16–34. [Google Scholar] [CrossRef]
- Lin, C.-K.; Zhao, Q.; Zhang, Y.; Cestellos-Blanco, S.; Kong, Q.; Lai, M.; Kang, J.; Yang, P. Two-step patterning of scalable all-inorganic halide perovskite arrays. ACS Nano 2020, 14, 3500–3508. [Google Scholar] [CrossRef]
- Wałȩsa-Chorab, M.; Skene, W.G. Visible-to-NIR electrochromic device prepared from a thermally polymerizable electroactive organic monomer. ACS Appl. Mater. Interfaces 2017, 9, 21524–21531. [Google Scholar] [CrossRef] [PubMed]
- Berkowski, K.L.; Plunkett, K.N.; Yu, Q.; Moore, J.S. Introduction to photolithography: Preparation of microscale polymer silhouettes. J. Chem. Educ. 2005, 82, 1365. [Google Scholar] [CrossRef]
- Kim, S.; Yeo, J.; Kim, S.J.; Park, S.; Cho, K.G.; Paeng, K.; Lee, K.H.; Kim, M. Photopatternable and self-healable ionogels for organic thin-film transistors. Org. Electron. 2023, 122, 106895. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Investigation of an electroactive immobilized azomethine for potential electrochromic use. Sol. Energy Mater. Sol. Cells 2019, 200, 109977. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Hu, Y.; Han, L.; Qu, Q.; Liu, D.; Guo, J.; Zeng, Z.; Bai, H.; Kwok, R.T.K.; et al. Catalyst-free spontaneous polymerization with 100% atom economy: Facile synthesis of photoresponsive polysulfonates with multifunctionalities. JACS Au 2021, 1, 344–353. [Google Scholar] [CrossRef]
- Banasz, R.; Wałęsa-Chorab, M. Photolithographic patterning of viologens containing styrene groups. RSC Adv. 2023, 13, 16206–16210. [Google Scholar] [CrossRef] [PubMed]
- Khuong, K.S.; Jones, W.H.; Pryor, W.A.; Houk, K.N. The mechanism of the self-initiated thermal polymerization of styrene. Theoretical solution of a classic problem. J. Am. Chem. Soc. 2005, 127, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- D’Acunzo, F.; Capitani, D.; Masci, G.; Cherubini, C.; Ursini, O. Polymerization, grafting and adsorption in the presence of inorganic substrates: Thermal polymerization of styrene with untreated and γ-irradiated silica gel as a case study. Polymer 2013, 54, 6695–6701. [Google Scholar] [CrossRef]
- Wałȩsa-Chorab, M.; Skene, W.G. Engaging the reversible bonds of an immobilized styreno-thiophene film. Cryst. Growth Des. 2020, 20, 5688–5697. [Google Scholar] [CrossRef]
- Whitfield, R.; Anastasaki, A.; Jones, G.R.; Haddleton, D.M. Cu(0)-RDRP of styrene: Balancing initiator efficiency and dispersity. Polym. Chem. 2018, 9, 4395–4403. [Google Scholar] [CrossRef]
- Napierała, S.; Wałęsa-Chorab, M. On-substrate postsynthetic metal ion exchange as a tool for tuning electrochromic properties of materials. Eur. Polym. J. 2020, 140, 110052. [Google Scholar] [CrossRef]
- Napierała, S.; Muras, K.; Dutkiewicz, G.; Wałęsa-Chorab, M. Reductive electropolymerization and electrochromism of iron(II) complex with styrene-based ligand. Materials 2021, 14, 4831. [Google Scholar] [CrossRef]
- Cui, B.B.; Nie, H.J.; Yao, C.J.; Shao, J.Y.; Wu, S.H.; Zhong, Y.W. Reductive electropolymerization of bis-tridentate ruthenium complexes with 5,5′′-divinyl-4′-tolyl-2,2′:6′, 2′′-terpyridine. Dalt. Trans. 2013, 42, 14125–14133. [Google Scholar] [CrossRef]
- Shao, J.Y.; Yao, C.J.; Cui, B.B.; Gong, Z.L.; Zhong, Y.W. Electropolymerized films of redox-active ruthenium complexes for multistate near-infrared electrochromism, ion sensing, and information storage. Chin. Chem. Lett. 2016, 27, 1105–1114. [Google Scholar] [CrossRef]
- Al Kobaisi, M.; Bhosale, S.V.; Latham, K.; Raynor, A.M.; Bhosale, S.V. Functional naphthalene diimides: Synthesis, properties, and applications. Chem. Rev. 2016, 116, 11685–11796. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Al Kobaisi, M.; Jadhav, R.W.; Morajkar, P.P.; Jones, L.A.; George, S. Naphthalene diimides: Perspectives and promise. Chem. Soc. Rev. 2021, 50, 9845–9998. [Google Scholar] [CrossRef]
- Nad, S.; Paul, B.; Malik, S. Effect of substituents of naphthalene diimide derivatives on electrochromic behaviours observed in proto-type devices. J. Electroanal. Chem. 2023, 946, 117729. [Google Scholar] [CrossRef]
- Halder, S.; Roy, S.; Chakraborty, C. Multicolored and durable electrochromism in water soluble naphthalene and perylene based diimides. Sol. Energy Mater. Sol. Cells 2022, 234, 111429. [Google Scholar] [CrossRef]
- Murray, N.R.; McCabe, T.J.D.; Reid, M.; Draper, E.R. Non-contact computer vision enables analysis of the dynamic performance of naphthalene diimide electrochromic films. J. Mater. Chem. C 2024, 12, 12483–12490. [Google Scholar] [CrossRef]
- Nad, S.; Jana, R.; Datta, A.; Malik, S. Fully organic electroactive monomers for electrochromic behaviors having high coloration efficiency and long cycle stability towards flexible Solid-State electrochromic device. J. Electroanal. Chem. 2022, 918, 116484. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Lang, F.; Liu, Y.; Xu, L.; Xi, X.-J.; Li, Y.; Pang, J.; Zhou, H.-C.; Bu, X.-H. Efficiently regulating the electrochromic behavior of naphthalene-diimide-based zirconium-organic frameworks through linker installation. Nat. Commun. 2025, 16, 1405. [Google Scholar] [CrossRef]
- AlKaabi, K.; Wade, C.R.; Dincă, M. Transparent-to-dark electrochromic behavior in naphthalene-diimide-based mesoporous MOF-74 analogs. Chem 2016, 1, 264–272. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, Y.-Z. Electroactive and ambipolar electrochromic polyimides from arylene diimides with triphenylamine N -substituents. Dye. Pigment. 2017, 144, 173–183. [Google Scholar] [CrossRef]
- Sharma, S.U.; Chang, Y.-L.; Chaganti, S.V.; More, Y.W.; Lee, J.-T. Cross-linked naphthalene diimide-based polymer as a cathode material for high-performance organic batteries. ACS Appl. Energy Mater. 2022, 5, 7550–7558. [Google Scholar] [CrossRef]
- Gawande, M.B.; Shelke, S.N.; Zboril, R.; Varma, R.S. Microwave-assisted chemistry: Synthetic applications for rapid assembly of nanomaterials and organics. Acc. Chem. Res. 2014, 47, 1338–1348. [Google Scholar] [CrossRef]
- Ohtsuka, N.; Nakano, M.; Nakagawa, S.; Shahiduzzaman, M.; Karakawa, M.; Taima, T.; Minoura, M. Naphthalene diimide-incorporated helical thienoacene: A helical molecule with high electron mobility, good solubility, and thermally stable solid phase. Chem. Commun. 2020, 56, 12343–12346. [Google Scholar] [CrossRef] [PubMed]
- Kar, H.; Molla, M.R.; Ghosh, S. Two-component gelation and morphology-dependent conductivity of a naphthalene-diimide (NDI) π-system by orthogonal hydrogen bonding. Chem. Commun. 2013, 49, 4220–4222. [Google Scholar] [CrossRef]
- Ponnuswamy, N.; Pantoş, G.D.; Smulders, M.M.J.; Sanders, J.K.M. Thermodynamics of supramolecular naphthalenediimide nanotube formation: The influence of solvents, side chains, and guest templates. J. Am. Chem. Soc. 2012, 134, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Mucci, V.; Vallo, C. Efficiency of 2,2-dimethoxy-2-phenylacetophenone for the photopolymerization of methacrylate monomers in thick sections. J. Appl. Polym. Sci. 2012, 123, 418–425. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Svirskaite, L.M.; Mandati, S.; Spalatu, N.; Malinauskiene, V.; Karazhanov, S.; Getautis, V.; Malinauskas, T. Asymmetric NDI electron transporting SAM materials for application in photovoltaic devices. Synth. Met. 2022, 291, 117214. [Google Scholar] [CrossRef]
- Gabriel de Assis, R.; de Oliveira Junior, M.; Escote, M.T.; Pinheiro, C.B.; Acuña, J.J.S.; Ferreira, F.F.; Sucupira Pedroza, L.; Brochsztain, S.; de Queiroz, T.B. Preparation and characterization of a disilylated naphthalenediimide molecular crystal: Perspectives for organosilica mesoporous materials. ACS Appl. Eng. Mater. 2024, 2, 1976–1986. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Hoffmann, M.; Rychlewski, J. Effects of Substituting a OH Group by a F Atom in d-Glucose. Ab Initio and DFT Analysis. J. Am. Chem. Soc. 2001, 123, 2308–2316. [Google Scholar] [CrossRef]
- Muńko, M.; Ciesielska, K.; Hoffmann, M.; Pluskota-Karwatka, D. Structural characterisation and pH-dependent preference of pyrrole cross-link isoforms from reactions of oxoenal with cysteine and lysine side chains as model systems. Amino Acids 2023, 55, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Zaranek, M.; Grzelak, M.; Pawluć, P.; Hoffmann, M. Mechanism of silylation of vinyl arenes by hydrodisiloxanes driven by stoichiometric amounts of sodium triethylborohydride—A combined DFT and experimental study. Int. J. Mol. Sci. 2023, 24, 4924. [Google Scholar] [CrossRef] [PubMed]
- Zaranek, M.; Nowicki, M.; Andruszak, P.; Hoffmann, M.; Pawluć, P. Hydrogermylation initiated by trialkylborohydrides: A living anionic mechanism. Chem. Commun. 2022, 58, 13979–13982. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Kalita, A.; Upadhyaya, S.; Sen Sarma, N. Salicylic acid appended naphthalene diimide organic linkers: A systematic investigation towards electronic aspects. ChemistrySelect 2020, 5, 12672–12678. [Google Scholar] [CrossRef]
- Jung, J.; Selerowicz, A.; Maczugowska, P.; Halagan, K.; Rybakiewicz-Sekita, R.; Zagorska, M.; Stefaniuk-Grams, A. Electron transport in naphthalene diimide derivatives. Materials 2021, 14, 4026. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14334–14356. [Google Scholar] [CrossRef]
- Dnaya, F.; Soubane, D.; Bouhassoune, M.; Bellioua, M.; Laaziz, Y.; Nafidi, A.; Ozaki, T. A holistic understanding of optical properties in amorphous H-terminated Si-nanostructures: Combining TD-DFT with AIMD. Results Opt. 2024, 16, 100694. [Google Scholar] [CrossRef]
- Lyakurwa, M.; Numbury, S.B. DFT and TD-DFT study of Optical and Electronic Properties of new donor–acceptor–donor (D–A–D′) monomers for polymer solar cells. Oxf. Open Mater. Sci. 2023, 3, itad003. [Google Scholar] [CrossRef]
- Essam, Z.M.; Ozmen, G.E.; Setiawan, D.; Hamid, R.R.; Abd El-Aal, R.M.; Aneja, R.; Hamelberg, D.; Henary, M. Donor acceptor fluorophores: Synthesis, optical properties, TD-DFT and cytotoxicity studies. Org. Biomol. Chem. 2021, 19, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.C.S.; Taveira, R.J.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Optical band gaps of organic semiconductor materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Song, Q.; Li, F.; Wang, Z.; Zhang, X. A supramolecular strategy for tuning the energy level of naphthalenediimide: Promoted formation of radical anions with extraordinary stability. Chem. Sci. 2015, 6, 3342–3346. [Google Scholar] [CrossRef]
- Ghule, N.V.; Bhosale, R.S.; Bhosale, S.V.; Srikanth, T.; Rao, N.V.S.; Bhosale, S.V. Synthesis and liquid crystalline properties of unsymmetrically substituted naphthalenediimides with a polar headgroup: Effect of amide hydrogen bonding and alkyl chain length. ChemistryOpen 2018, 7, 61–67. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Jani, C.H.; Langford, S.J. Chemistry of naphthalene diimides. Chem. Soc. Rev. 2008, 37, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, L.; Lancia, F.; Steen, J.D.; Browne, W.R. Reversible charge trapping in bis-carbazole-diimide redox polymers with complete luminescence quenching enabling nondestructive read-out by resonance Raman spectroscopy. J. Phys. Chem. C 2017, 121, 14688–14702. [Google Scholar] [CrossRef]
- Nowacki, M.; Wałęsa-Chorab, M. Influence of temperature on electrochemical and electrochromic properties of naphthalenediimide-triphenylamine-based polymer. Prog. Org. Coat. 2023, 182, 107691. [Google Scholar] [CrossRef]
- Bulloch, R.H.; Kerszulis, J.A.; Dyer, A.L.; Reynolds, J.R. An electrochromic painter’s palette: Color mixing via solution co-processing. ACS Appl. Mater. Interfaces 2015, 7, 1406–1412. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Mandler, D.; Lee, P.S. High switching speed and coloration efficiency of titanium-doped vanadium oxide thin film electrochromic devices. J. Mater. Chem. C 2013, 1, 7380. [Google Scholar] [CrossRef]
- Chen, Y.; Bi, Z.; Li, X.; Xu, X.; Zhang, S.; Hu, X. High-coloration efficiency electrochromic device based on novel porous TiO2@prussian blue core-shell nanostructures. Electrochim. Acta 2017, 224, 534–540. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Extending the Color Retention of an Electrochromic Device by Immobilizing Color Switching and Ion-Storage Complementary Layers. Electron. Mater. 2020, 1, 40–53. [Google Scholar] [CrossRef]
- Wu, X.; Wang, K.; Lin, J.; Yan, D.; Guo, Z.; Zhan, H. A thin film of naphthalenediimide-based metal-organic framework with electrochromic properties. J. Colloid. Interface Sci. 2021, 594, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sefer, E.; Baycan Koyuncu, F. Naphthalenediimide bridged D-A polymers: Design, synthesis and electrochromic properties. Electrochim. Acta 2014, 143, 106–113. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]




| Compound | Switching Times | ΔT% | Coloration Efficiency [cm2/C] | Reference | |
|---|---|---|---|---|---|
| tc | tb | ||||
| NDI-based polymer | 18 s | 6 s | 26% | 238 | This work |
| Zn-NDI MOF | 3 s | 91 s | 21% | 117 | [85] |
| Disodium salt of N,N′-bis(4-benzosulfonic acid)NDI | 16.5 s | 15.8 s | 56% | 260 | [44] |
| Thiophene–NDI donor-acceptor polymer | - | - | 23% | 82 | [86] |
| Zr-NDI MOF | 49 s | 35 s | 39.7% | 158 | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacki, M.; Hoffmann, M.; Wałęsa-Chorab, M. Photopolymerization of Styrene–Naphthalenediimide Monomer: Formation of Pattern and Electrochromism. Int. J. Mol. Sci. 2025, 26, 4807. https://doi.org/10.3390/ijms26104807
Nowacki M, Hoffmann M, Wałęsa-Chorab M. Photopolymerization of Styrene–Naphthalenediimide Monomer: Formation of Pattern and Electrochromism. International Journal of Molecular Sciences. 2025; 26(10):4807. https://doi.org/10.3390/ijms26104807
Chicago/Turabian StyleNowacki, Marcin, Marcin Hoffmann, and Monika Wałęsa-Chorab. 2025. "Photopolymerization of Styrene–Naphthalenediimide Monomer: Formation of Pattern and Electrochromism" International Journal of Molecular Sciences 26, no. 10: 4807. https://doi.org/10.3390/ijms26104807
APA StyleNowacki, M., Hoffmann, M., & Wałęsa-Chorab, M. (2025). Photopolymerization of Styrene–Naphthalenediimide Monomer: Formation of Pattern and Electrochromism. International Journal of Molecular Sciences, 26(10), 4807. https://doi.org/10.3390/ijms26104807







