Preconditioning with Rapamycin Improves Therapeutic Potential of Placenta-Derived Mesenchymal Stem Cells in Mouse Model of Hematopoietic Acute Radiation Syndrome
Abstract
1. Introduction
2. Results
2.1. PD-MSCs Identification and Differentiation by ISCT Criteria
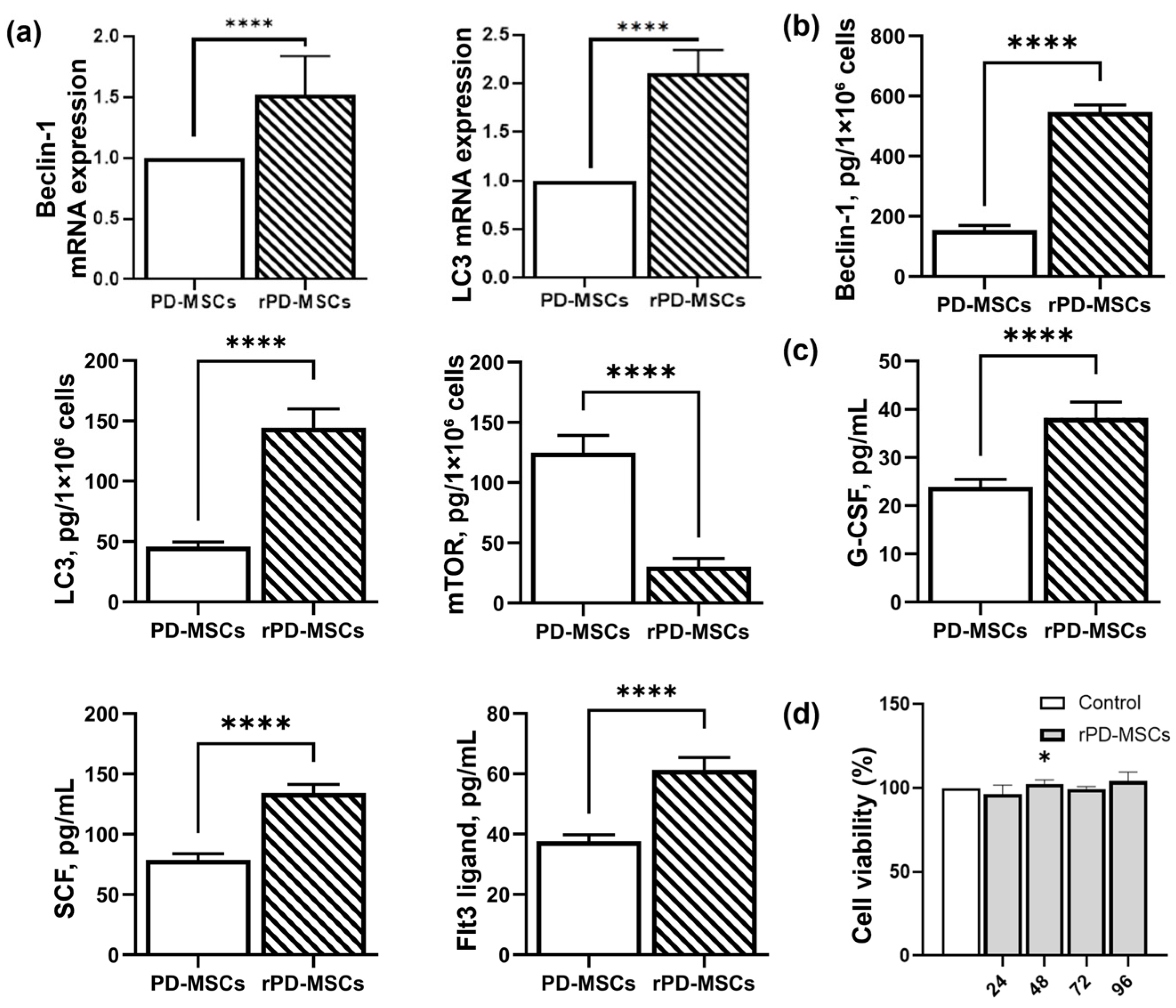
2.2. Rapamycin-Dependent Modulation of Autophagy and Hematopoietic Cytokines Secretion in PD-MSCs
2.3. Rapamycin-Mediated Regulation of Hematopoietic Cytokines (G-CSF, SCF, Flt3LG) Secretion in a Mouse Model of h-ARS
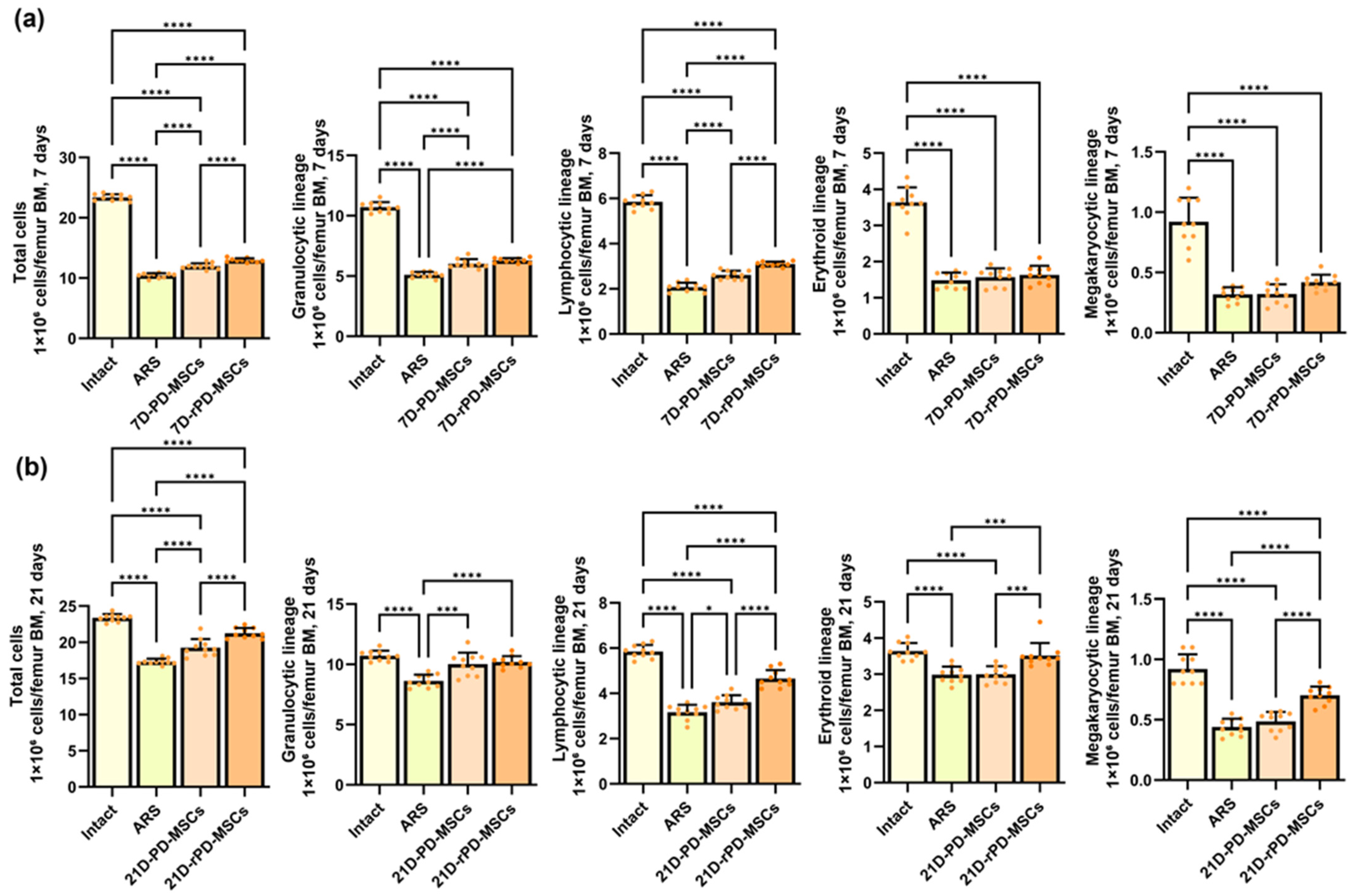
2.4. Rapamycin-Preconditioned PD-MSCs Enhance Hematopoietic Recovery, Restore Bone Marrow Cellularity in a Mouse Model of h-ARS
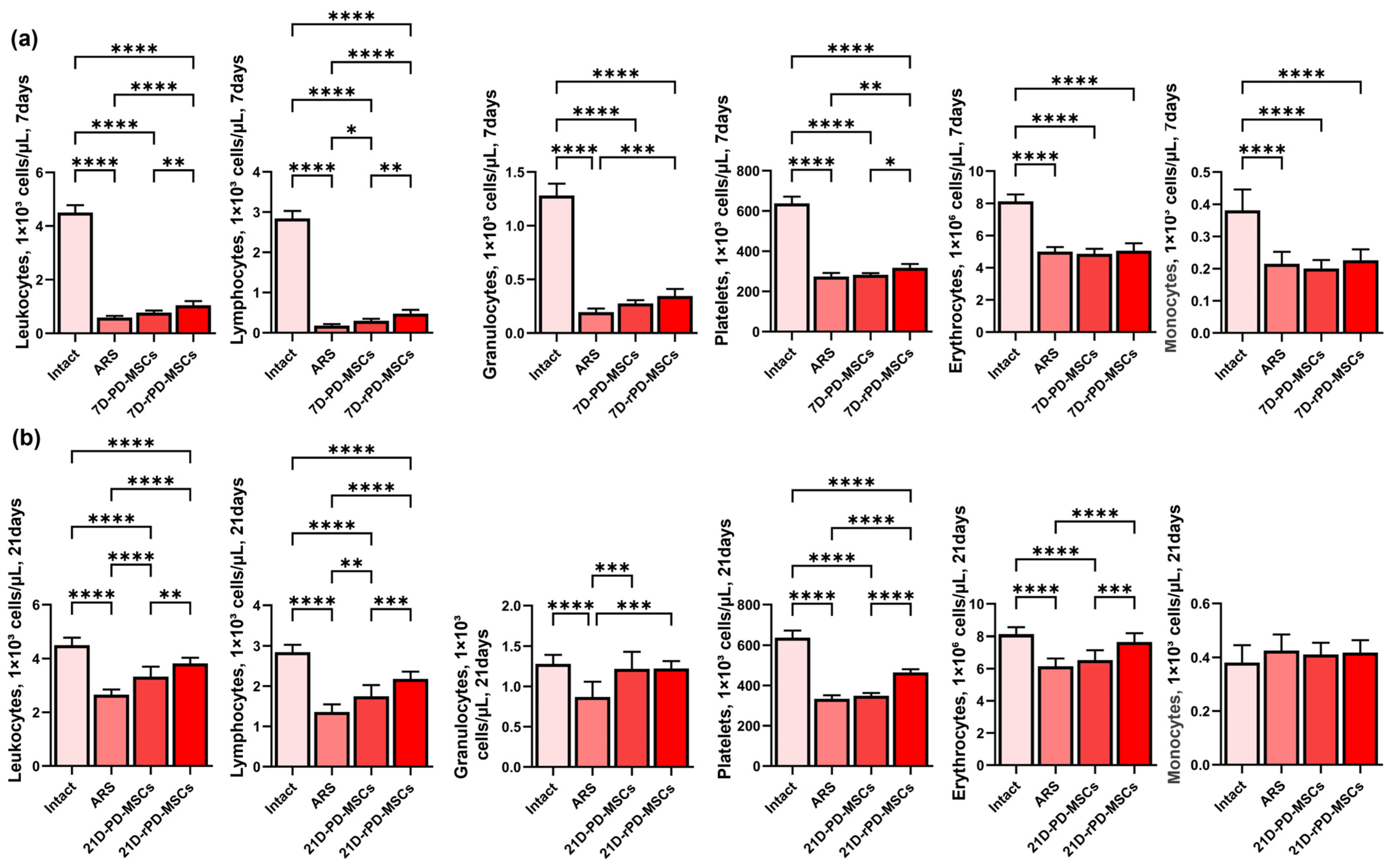
2.5. Rapamycin-Preconditioned PD-MSCs Significantly Increase Peripheral Blood Cell Counts Compared to Non-Preconditioned Cells in Irradiated Mice
3. Discussion
4. Materials and Methods
4.1. Isolation and Culture of PD-MSCs
4.2. Identification of PD-MSCs by ISCT Criteria
4.3. Preconditioning of PD-MSCs
4.4. Cell Viability Assay
4.5. RT-qPCR Analysis of Gene Expression
4.6. ELISA Quantification of Autophagy-Related Proteins and Hematopoietic Cytokines in Cell Lysates, Culture Media, and BM
4.7. Animal Care and Experimental Groups
4.8. Cytological Evaluation of Bone Marrow and Peripheral Blood Counts
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α-SMA | Alpha-Smooth Muscle Actin |
| ARS | Acute radiation syndrome |
| BM | Bone marrow |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| FABP-4 | Fatty Acid-Binding Protein 4 |
| FGF-2 | Fibroblast Growth Factor-2 |
| Flt3LG | FMS-related Tyrosine Kinase 3 Ligand |
| G-CSF | Granulocyte Colony-Stimulating Factor |
| IDO | Indoleamine 2,3-Dioxygenase |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-15 | Interleukin-15 |
| ISCT | International Society for Cell & Gene Therapy |
| LPS | Lipopolysaccharide |
| MAP1LC3β | Microtubule-Associated Protein 1 Light Chain 3 Beta |
| mTOR | Mechanistic Target of Rapamycin |
| PD-MSCs | Placenta-Derived Mesenchymal stem cells |
| SCF | Stem Cell Factor |
| SDF-1 | Stromal Cell-Derived Factor-1 |
| TGF-β | Transforming Growth Factor-Beta |
References
- Chinnadurai, R.; Forsberg, M.H.; Kink, J.A.; Hematti, P.; Capitini, C.M. Use of MSCs and MSC-Educated Macrophages to Mitigate Hematopoietic Acute Radiation Syndrome. Curr. Stem Cell Rep. 2020, 6, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Rybkina, V.L.; Bannikova, M.V.; Adamova, G.V.; Dörr, H.; Scherthan, H.; Azizova, T.V. Immunological Markers of Chronic Occupational Radiation Exposure. Health Phys. 2018, 115, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Grammaticos, P.; Giannoula, E.; Fountos, G.P. Acute Radiation Syndrome and Chronic Radiation Syndrome. Hell. J. Nucl. Med. 2013, 16, 56–59. [Google Scholar] [PubMed]
- Gorbunov, N.V.; Sharma, P. Protracted Oxidative Alterations in the Mechanism of Hematopoietic Acute Radiation Syndrome. Antioxidants 2015, 4, 134–152. [Google Scholar] [CrossRef]
- Plett, P.A.; Pelus, L.M.; Orschell, C.M. Establishing a Murine Model of the Hematopoietic Acute Radiation Syndrome. Methods Mol. Biol. 2023, 2567, 251–262. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Eaton, E.B.; Varney, T.R. Mesenchymal Stem Cell Therapy for Acute Radiation Syndrome: Innovative Medical Approaches in Military Medicine. Mil. Med. Res. 2015, 2, 1–9. [Google Scholar] [CrossRef]
- Bandekar, M.; Maurya, D.K.; Sharma, D.; Sandur, S.K. Preclinical Studies and Clinical Prospects of Wharton’s Jelly-Derived MSC for Treatment of Acute Radiation Syndrome. Curr. Stem Cell Rep. 2021, 7, 85–94. [Google Scholar] [CrossRef]
- Rezvani, M. Therapeutic Potential of Mesenchymal Stromal Cells and Extracellular Vesicles in the Treatment of Radiation Lesions—A Review. Cells 2021, 10, 427. [Google Scholar] [CrossRef]
- Friedenstein, A.J.; Petrakova, K.V.; Kurolesova, A.I.; Frolova, G.P. Heterotopic of Bone Marrow. Analysis of Precursor Cells for Osteogenic and Hematopoietic Tissues. Transplantation 1968, 6, 230–247. [Google Scholar] [CrossRef]
- Lou, S.; Duan, Y.; Nie, H.; Cui, X.; Du, J.; Yao, Y. Mesenchymal Stem Cells: Biological Characteristics and Application in Disease Therapy. Biochimie 2021, 185, 9–21. [Google Scholar] [CrossRef]
- Ripoll, C.B.; Bunnell, B.A. Comparative Characterization of Mesenchymal Stem Cells from EGFP Transgenic and Non-Transgenic Mice. BMC Cell Biol. 2009, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- de la Torre, P.; Flores, A.I. Current Status and Future Prospects of Perinatal Stem Cells. Genes 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Beeravolu, N.; McKee, C.; Alamri, A.; Mikhael, S.; Brown, C.; Perez-Cruet, M.; Chaudhry, G.R. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J. Vis. Exp. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Gao, Y.; Chi, Y.; Chen, Y.; Wang, W.; Li, H.; Zheng, W.; Zhu, P.; An, J.; Duan, Y.; Sun, T.; et al. Multi-Omics Analysis of Human Mesenchymal Stem Cells Shows Cell Aging That Alters Immunomodulatory Activity through the Downregulation of PD-L1. Nat. Commun. 2023, 14, 4273. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.K.; Thiede, M.A.; Mosca, J.D.; Moorman, M.; Gerson, S.L. Phenotypic and Functional Comparison of Cultures of Marrow-Derived Mesenchymal Stem Cells (MSCs) and Stromal Cells. J. Cell. Physiol. 1998, 176, 57–66. [Google Scholar] [CrossRef]
- Mourcin, F.; Grenier, N.; Mayol, J.-F.; Lataillade, J.-J.; Sotto, J.-J.; Hérodin, F.; Drouet, M. Mesenchymal Stem Cells Support Expansion of in Vitro Irradiated CD34(+) Cells in the Presence of SCF, FLT3 Ligand, TPO and IL3: Potential Application to Autologous Cell Therapy in Accidentally Irradiated Victims. Radiat. Res. 2005, 164, 1–9. [Google Scholar] [CrossRef]
- Budgude, P.; Kale, V.; Vaidya, A. Mesenchymal Stromal Cell-Derived Extracellular Vesicles as Cell-Free Biologics for the Ex Vivo Expansion of Hematopoietic Stem Cells. Cell Biol. Int. 2020, 44, 1078–1102. [Google Scholar] [CrossRef]
- Lange, C.; Brunswig-Spickenheier, B.; Reimer, R.; Zustin, J. Mesenchymal Stromal Cells Protect from Consequences of HSCT-Transplantation Preparatory Irradiation: Insights into Possible Mechanisms. Cell. Ther. Transplant. 2016, 5, 50–59. [Google Scholar] [CrossRef]
- Shim, S.; Lee, S.B.; Lee, J.G.; Jang, W.S.; Lee, S.J.; Park, S.; Lee, S.S. Mitigating Effects of HUCB-MSCs on the Hematopoietic Syndrome Resulting from Total Body Irradiation. Exp. Hematol. 2013, 41, 346–353.e2. [Google Scholar] [CrossRef]
- Hu, K.X.; Sun, Q.Y.; Guo, M.; Ai, H.S. The Radiation Protection and Therapy Effects of Mesenchymal Stem Cells in Mice with Acute Radiation Injury. Br. J. Radiol. 2010, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lange, C.; Brunswig-Spickenheier, B.; Cappallo-Obermann, H.; Eggert, K.; Gehling, U.M.; Rudolph, C.; Schlegelberger, B.; Cornils, K.; Zustin, J.; Spiess, A.N.; et al. Radiation Rescue: Mesenchymal Stromal Cells Protect from Lethal Irradiation. PLoS ONE 2011, 6, e14486. [Google Scholar] [CrossRef]
- Forsberg, M.H.; Kink, J.A.; Thickens, A.S.; Lewis, B.M.; Childs, C.J.; Hematti, P.; Capitini, C.M. Exosomes from Primed MSCs Can Educate Monocytes as a Cellular Therapy for Hematopoietic Acute Radiation Syndrome. Stem Cell Res. Ther. 2021, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Y.; Wang, J.; Hou, L.; Li, W.; An, H. CXCR4-Overexpressing Umbilical Cord Mesenchymal Stem Cells Enhance Protection against Radiation-Induced Lung Injury. Stem Cells Int. 2019, 2019, 2457082. [Google Scholar] [CrossRef] [PubMed]
- Kernagis, D.N.; Balcer-Kubiczek, E.; Bazyar, S.; Orschell, C.M.; Jackson, I.L. Medical Countermeasures for the Hematopoietic-Subsyndrome of Acute Radiation Syndrome in Space. Life Sci. Sp. Res. 2022, 35, 36–43. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. Radiation Countermeasures for Hematopoietic Acute Radiation Syndrome: Growth Factors, Cytokines and Beyond. Int. J. Radiat. Biol. 2021, 97, 1526–1547. [Google Scholar] [CrossRef]
- Gaberman, E.; Pinzur, L.; Levdansky, L.; Tsirlin, M.; Netzer, N.; Aberman, Z.; Gorodetsky, R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS ONE 2013, 8, 7–13. [Google Scholar] [CrossRef]
- Guo, M.; Dong, Z.; Qiao, J.; Yu, C.; Sun, Q.; Hu, K.; Liu, G.; Wei, L.; Yao, B.; Man, Q.; et al. Severe Acute Radiation Syndrome: Treatment of a Lethally 60Co-Source Irradiated Accident Victim in China with HLA-Mismatched Peripheral Blood Stem Cell Transplantation and Mesenchymal Stem Cells. J. Radiat. Res. 2014, 55, 205–209. [Google Scholar] [CrossRef]
- Fouillard, L.; Francois, S.; Bouchet, S.; Bensidhoum, M.; Elm’selmi, A.; Chapel, A. Innovative Cell Therapy in the Treatment of Serious Adverse Events Related to Both Chemo-Radiotherapy Protocol and Acute Myeloid Leukemia Syndrome: The Infusion of Mesenchymal Stem Cells Post-Treatment Reduces Hematopoietic Toxicity and Promotes Hematopo. Curr. Pharm. Biotechnol. 2013, 14, 842–848. [Google Scholar] [CrossRef]
- Qian, L.; Cen, J. Hematopoietic Stem Cells and Mesenchymal Stromal Cells in Acute Radiation Syndrome. Oxid. Med. Cell. Longev. 2020, 2020, 8340756. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, E.A.; Tee, A.R. MTOR and Autophagy: A Dynamic Relationship Governed by Nutrients and Energy. Semin. Cell Dev. Biol. 2014, 36, 121–129. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, Y.-J.; Wang, H.; Dong, Q.-T.; Wang, T.-J.; Qian, H.-Y.; Xu, H. Autophagy Activation: A Novel Mechanism of Atorvastatin to Protect Mesenchymal Stem Cells from Hypoxia and Serum Deprivation via AMP-Activated Protein Kinase/Mammalian Target of Rapamycin Pathway. Stem Cells Dev. 2012, 21, 1321–1332. [Google Scholar] [CrossRef]
- Wang, B.; Lin, Y.; Hu, Y.; Shan, W.; Liu, S.; Xu, Y.; Zhang, H.; Cai, S.; Yu, X.; Cai, Z.; et al. MTOR Inhibition Improves the Immunomodulatory Properties of Human Bone Marrow Mesenchymal Stem Cells by Inducing COX-2 and PGE2. Stem Cell Res. Ther. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Awadalla, A.; Hussein, A.M.; El-far, Y.M.; El-senduny, F.F.; Barakat, N.; Hamam, E.T.; Abdeen, H.M.; El-sherbiny, M.; Serria, M.S.; Sarhan, A.A. Rapamycin Improves Adipose-Derived Mesenchymal Stem Cells (ADMSCs) Renoprotective Effect against Cisplatin-Induced Acute Nephrotoxicity in Rats by Inhibiting the MTOR/AKT Signaling Pathway. Biomedicines 2022, 10, 1295. [Google Scholar] [CrossRef]
- Kim, K.; Moon, S.; Park, M.; Kim, B.; Kim, E.; Lee, S.; Lee, E. Optimization of Adipose Tissue-Derived Mesenchymal Stem Cells by Rapamycin in a Murine Model of Acute Graft-versus-Host Disease. Stem Cell Res. Ther. 2015, 6, 202. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Y.-L.; Wang, H.-J.; Wu, J.-H.; Tan, Y.-Z. Rapamycin-Preactivated Autophagy Enhances Survival and Differentiation of Mesenchymal Stem Cells After Transplantation into Infarcted Myocardium. Stem Cell Rev. Rep. 2020, 16, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.M.; Harrison, M.A.A.; Sullivan, B.N.; Al-Ghadban, S.; Aleman, S.J.; Vinluan, A.T.; Monaco, E.R.; Donato, U.M.; Pursell, I.A.; Bunnell, B.A. Short-Term Rapamycin Preconditioning Diminishes Therapeutic Efficacy of Human Adipose-Derived Stem Cells in a Murine Model of Multiple Sclerosis. Cells 2020, 9, 2218. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; John, L.; Baldomero, H.; Roth, J.; Tichelli, A.; Nissen, C.; Lyman, S.D.; Wodnar-Filipowicz, A. FLT-3 Ligand Provides Hematopoietic Protection from Total Body Irradiation in Rabbits. Blood 1998, 92, 765–769. [Google Scholar] [CrossRef]
- Gao, L.; Cen, S.; Wang, P.; Xie, Z.; Liu, Z.; Deng, W.; Su, H.; Wu, X.; Wang, S.; Li, J.; et al. Autophagy Improves the Immunosuppression of CD4+ T Cells by Mesenchymal Stem Cells Through Transforming Growth Factor- β 1. Stem Cells Transl. Med. 2016, 5, 1496–1505. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, O.N.; Borozdina, N.A.; Sadovnikova, E.S.; Pakhomova, I.A.; Rudenko, P.A.; Korolkova, Y.V.; Kozlov, S.A.; Dyachenko, I.A. Comparative Study of the Aftereffect of CO2 Inhalation or Tiletamine–Zolazepam–Xylazine Anesthesia on Laboratory Outbred Rats and Mice. Biomedicines 2022, 10, 512. [Google Scholar] [CrossRef] [PubMed]


| Genes | Forward | Reverse |
|---|---|---|
| GAPDH | 5′-TGTCCGTCGTGGATCTGAC-3′ | 5′-CCTGCTTCACCACCTTCTTG-3′ |
| MAP1LC3B | 5′-CCCCACCAAGATCCCAGT-3′ | 5′-CGCTCATGTTCACGTGGT-3′ |
| BECN1 | 5′-AGGATGGTGTCTCTCGAAGATT-3′ | 5′-GATCAGAGTGAAGCTATTAGCACTTTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Slautin, V.; Ivanov, V.; Bugakov, A.; Chernysheva, A.; Gavrilov, I.; Maklakova, I.; Bazarnyi, V.; Grebnev, D.; Kovtun, O. Preconditioning with Rapamycin Improves Therapeutic Potential of Placenta-Derived Mesenchymal Stem Cells in Mouse Model of Hematopoietic Acute Radiation Syndrome. Int. J. Mol. Sci. 2025, 26, 4804. https://doi.org/10.3390/ijms26104804
Slautin V, Ivanov V, Bugakov A, Chernysheva A, Gavrilov I, Maklakova I, Bazarnyi V, Grebnev D, Kovtun O. Preconditioning with Rapamycin Improves Therapeutic Potential of Placenta-Derived Mesenchymal Stem Cells in Mouse Model of Hematopoietic Acute Radiation Syndrome. International Journal of Molecular Sciences. 2025; 26(10):4804. https://doi.org/10.3390/ijms26104804
Chicago/Turabian StyleSlautin, Vasilii, Vladislav Ivanov, Alexandr Bugakov, Anna Chernysheva, Ilya Gavrilov, Irina Maklakova, Vladimir Bazarnyi, Dmitry Grebnev, and Olga Kovtun. 2025. "Preconditioning with Rapamycin Improves Therapeutic Potential of Placenta-Derived Mesenchymal Stem Cells in Mouse Model of Hematopoietic Acute Radiation Syndrome" International Journal of Molecular Sciences 26, no. 10: 4804. https://doi.org/10.3390/ijms26104804
APA StyleSlautin, V., Ivanov, V., Bugakov, A., Chernysheva, A., Gavrilov, I., Maklakova, I., Bazarnyi, V., Grebnev, D., & Kovtun, O. (2025). Preconditioning with Rapamycin Improves Therapeutic Potential of Placenta-Derived Mesenchymal Stem Cells in Mouse Model of Hematopoietic Acute Radiation Syndrome. International Journal of Molecular Sciences, 26(10), 4804. https://doi.org/10.3390/ijms26104804








