Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients
Abstract
1. Introduction
2. Results
2.1. Copper and Zinc Content in Saliva in Breast Cancer
2.2. Indicators of Antioxidant System Activity, Hormonal, Cytokine Status, and Content of Free Amino Acids in Saliva in Breast Cancer and Fibroadenomas
2.3. Antioxidant System Activity, Hormonal, Cytokine Status, and Free Amino Acid Content in Saliva in Breast Cancer Depending on Tumor Phenotype
3. Discussion
3.1. Relationships Between Salivary Copper and Zinc, Cellular Damage and Immune Response Activation
3.2. Relationship Between Androgen Hormones and Copper and Zinc Metabolism
3.3. The Influence of Copper and Zinc on the Processes of Lipid Peroxidation and Enzymatic Activity of the Antioxidant System
3.4. Changes in the Content of Free Cys, Met, His, Arg Due to Disruption of Copper and Zinc Homeostasis
3.5. The Combination of Mutations in the BRCA1 Gene and Pathologically Altered Levels of Zinc and Copper as a Potential Risk for the Development of Breast Cancer
4. Materials and Methods
4.1. Study Design
4.2. Collection, Storage, and Pre-Treatment of Saliva Samples
4.3. Determination of Copper and Zinc Content in Saliva
4.4. Determination of Cytokines, Hormones, C-Reactive Protein, and 8-OH-Deoxyguanosine by ELISA
4.5. Determination of Free Amino Acids in Saliva
4.6. Determination of the Biochemical Composition of Saliva
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Yu, P.; Chan, W.N.; Xie, F.; Zhang, Y.; Liang, L.; Leung, K.T.; Lo, K.W.; Yu, J.; Tse, G.M.K.; et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct. Target. Ther. 2024, 9, 6. [Google Scholar] [PubMed]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, J.; Liu, C.; Luo, Y.; Chen, J.; Fu, Y.; Xu, Y.; Wu, H.; Li, X.; Wang, H. Relationships Between Biological Heavy Metals and Breast Cancer: A Systematic Review and Meta-Analysis. Front. Nutr. 2022, 9, 838762. [Google Scholar] [CrossRef]
- Bendellaa, M.; Lelièvre, P.; Coll, J.L.; Sancey, L.; Deniaud, A.; Busser, B. Roles of zinc in cancers: From altered metabolism to therapeutic applications. Int. J. Cancer 2024, 154, 7–20. [Google Scholar] [CrossRef]
- Yao, G.; Wang, Z.; Xie, R.; Zhanghuang, C.; Yan, B. Trace element zinc metabolism and its relation to tumors. Front Endocrinol 2024, 15, 1457943. [Google Scholar] [CrossRef]
- Li, Y.; Ou, Y.; Fan, K.; Liu, G. Salivary diagnostics: Opportunities and challenges. Theranostics 2024, 14, 6969–6990. [Google Scholar] [CrossRef]
- Li, K.; Lin, Y.; Luo, Y.; Xiong, X.; Wang, L.; Durante, K.; Li, J.; Zhou, F.; Guo, Y.; Chen, S.; et al. A signature of saliva-derived exosomal small RNAs as predicting biomarker for esophageal carcinoma: A multicenter prospective study. Mol. Cancer 2022, 21, 21. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Wei, F.; Rao, S.L.; Kim, J.; Shin, H.; Cheng, J.; Tu, M.; Wong, D.T.W.; Kim, Y. Clinical validity of saliva and novel technology for cancer detection. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 49–59. [Google Scholar] [CrossRef]
- Nonaka, T.; Wong, D.T.W. Saliva Diagnostics. Annu. Rev. Anal. Chem. 2022, 15, 107–121. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting copper and cancer: From transition metal signalling to metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, C.M.; Chang, C.J. Copper signaling in the brain and beyond. J. Biol. Chem. 2018, 293, 4628–4635. [Google Scholar] [CrossRef] [PubMed]
- Ogra, Y.; Tejima, A.; Hatakeyama, N.; Shiraiwa, M.; Wu, S.; Ishikawa, T.; Yawata, A.; Anan, Y.; Suzuki, N. Changes in intracellular copper concentration and copper-regulating gene expression after PC12 differentiation into neurons. Sci. Rep. 2016, 6, 33007. [Google Scholar] [CrossRef]
- Müller, S.; Versini, A.; Sindikubwabo, F.; Belthier, G.; Niyomchon, S.; Pannequin, J.; Grimaud, L.; Cañeque, T.; Rodriguez, R. Metformin reveals a mitochondrial copper addiction of mesenchymal cancer cells. PLoS ONE 2018, 13, e0206764. [Google Scholar]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Solier, S.; Müller, S.; Cañeque, T.; Versini, A.; Mansart, A.; Sindikubwabo, F.; Baron, L.; Emam, L.; Gestraud, P.; Pantoș, G.D.; et al. A druggable copper-signalling pathway that drives inflammation. Nature 2023, 617, 386–394. [Google Scholar] [CrossRef]
- Perera, N.C.N.; Godahewa, G.I.; Lee, J. Copper-zinc-superoxide dismutase (CuZnSOD), an antioxidant gene from seahorse (Hippocampus abdominalis); molecular cloning, sequence characterization, antioxidant activity and potential peroxidation function of its recombinant protein. Fish Shellfish Immunol. 2016, 57, 386–399. [Google Scholar] [CrossRef]
- Kapper, C.; Oppelt, P.; Ganhör, C.; Gyunesh, A.A.; Arbeithuber, B.; Stelzl, P.; Rezk-Füreder, M. Minerals and the Menstrual Cycle: Impacts on Ovulation and Endometrial Health. Nutrients 2024, 16, 1008. [Google Scholar] [CrossRef]
- Maio, N.; Polticelli, F.; De Francesco, G.; Rizzo, G.; Bonaccorsi di Patti, M.C.; Musci, G. Role of external loops of human ceruloplasmin in copper loading by ATP7B and Ccc2p. J. Biol. Chem. 2010, 285, 20507–20513. [Google Scholar] [CrossRef]
- Linder, M.C. Apoceruloplasmin: Abundance, detection, formation, and metabolism. Biomedicines 2021, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Aurélia, P.; Joël, P.; France, W. Direct Determination of Non-Ceruloplasmin-Bound Copper in Plasma. In Clinical and Translational Perspectives on WILSON DISEASE; Kerkar, N., Roberts, E.A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 22, pp. 249–255. [Google Scholar]
- Arredondo, M.; Muñoz, P.; Mura, C.V.; Nùñez, M.T. DMT1, a physiologically relevant apical Cu1+ transporter of intestinal cells. Am. J. Physiol. Cell Physiol. 2003, 284, C1525–C1530. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Ivy, K.; Kaplan, J.H. Acquisition of dietary copper: A role for anion transporters in intestinal apical copper uptake. Am. J. Physiol. Cell Physiol. 2011, 300, C588–C599. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Maryon, E.B.; Kaplan, J.H. Human copper transporter hCTR1 mediates basolateral uptake of copper into enterocytes: Implications for copper homeostasis. J. Biol. Chem. 2007, 282, 26471–26480. [Google Scholar] [CrossRef]
- Wyman, S.; Simpson, R.J.; McKie, A.T.; Sharp, P.A. Dcytb (Cybrd1) functions as both a ferric and a cupric reductase in vitro. FEBS Lett. 2008, 582, 1901–1906. [Google Scholar] [CrossRef]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef]
- Sailer, J.; Nagel, J.; Akdogan, B.; Jauch, A.T.; Engler, J.; Knolle, P.A.; Zischka, H. Deadly excess copper. Redox Biol. 2024, 75, 103256. [Google Scholar] [CrossRef]
- Kar, S.; Sen, S.; Maji, S.; Saraf, D.; Ruturaj Paul, R.; Dutt, S.; Mondal, B.; Rodriguez-Boulan, E.; Schreiner, R.; Sengupta, D.; et al. Copper(II) import and reduction are dependent on His-Met clusters in the extracellular amino terminus of human copper transporter-1. J. Biol. Chem. 2022, 298, 101631. [Google Scholar] [CrossRef]
- Hatori, Y.; Lutsenko, S. The Role of Copper Chaperone Atox1 in Coupling Redox Homeostasis to Intracellular Copper Distribution. Antioxidants 2016, 5, 25. [Google Scholar] [CrossRef]
- Horn, N.; Wittung-Stafshede, P. ATP7A-Regulated Enzyme Metalation and Trafficking in the Menkes Disease Puzzle. Biomedicines 2021, 9, 391. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper homeostasis and cuproptosis in health and disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Shalygin, S.P.; Postnova, T.V.; Kosenok, V.K. Potential Diagnostic Significance of Salivary Copper Determination in Breast Cancer Patients: A Pilot Study. Biol. Trace Elem. Res. 2022, 200, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Rink, L.; Gabriel, P. Zinc and the immune system. Proc. Nutr. Soc. 2000, 59, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Lapa, B.S.; Jorge, J.; Alves, R.; Carreira, I.M.; Sarmento-Ribeiro, A.B.; Gonçalves, A.C. Zinc Prevents DNA Damage in Normal Cells but Shows Genotoxic and Cytotoxic Effects in Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2022, 23, 2567. [Google Scholar] [CrossRef]
- Griguolo, G.; Pascual, T.; Dieci, M.V.; Guarneri, V.; Prat, A. Interaction of host immunity with HER2-targeted treatment and tumor heterogeneity in HER2-positive breast cancer. J. Immunother. Cancer 2019, 7, 90. [Google Scholar] [CrossRef]
- Dyachenko, E.I.; Bel’skaya, L.V. Salivary Transmembrane Mucins of the MUC1 Family (CA 15-3, CA27.29, MCA) in Breast Cancer: The Effect of Human Epidermal Growth Factor Receptor 2 (HER2). Cancers 2024, 16, 3461. [Google Scholar] [CrossRef]
- Dyachenko, E.I.; Bel’skaya, L.V. Transmembrane Amino Acid Transporters in Shaping the Metabolic Profile of Breast Cancer Cell Lines: The Focus on Molecular Biological Subtype. Curr. Issues Mol. Biol. 2025, 47, 4. [Google Scholar] [CrossRef]
- Song, S.; Zhang, M.; Xie, P.; Wang, S.; Wang, Y. Comprehensive analysis of cuproptosis-related genes and tumor microenvironment infiltration characterization in breast cancer. Front. Immunol. 2022, 13, 978909. [Google Scholar] [CrossRef]
- Hirano, T. Repair system of 7,8-dihydro-8-oxoguanine as a defense line against carcinogenesis. J. Radiat. Res. 2008, 49, 329–440. [Google Scholar] [CrossRef]
- Sova, H.; Jukkola-Vuorinen, A.; Puistola, U.; Kauppila, S.; Karihtala, P. 8-Hydroxydeoxyguanosine: A new potential independent prognostic factor in breast cancer. Br. J. Cancer 2010, 102, 1018–1023. [Google Scholar] [CrossRef]
- Herzberg, M.; Lusky, A.; Blonder, J.; Frenkel, Y. The effect of estrogen replacement therapy on zinc in serum and urine. Obs. Gynecol. 1996, 87, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, J.H.; Hong, S.H.; Lee, J.Y.; Cherny, R.A.; Bush, A.I.; Palmiter, R.D.; Koh, J.Y. Estrogen decreases zinc transporter 3 expression and synaptic vesicle zinc levels in mouse brain. J. Biol. Chem. 2004, 279, 8602–8607. [Google Scholar] [CrossRef] [PubMed]
- Amtage, F.; Birnbaum, D.; Reinhard, T.; Niesen, W.D.; Weiller, C.; Mader, I.; Meyer, P.T.; Rijntjes, M. Estrogen intake and copper depositions: Implications for Alzheimer’s disease? Case Rep. Neurol. 2014, 6, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Orlin, A.; Orlin, S.E.; Makar, G.A.; Bunya, V.Y. Presumed corneal copper deposition and oral contraceptive use. Cornea 2010, 29, 476–478. [Google Scholar] [CrossRef]
- Van den Berghe, P.V.; Klomp, L.W. New developments in the regulation of intestinal copper absorption. Nutr. Rev. 2009, 67, 658–672. [Google Scholar] [CrossRef]
- Hardman, B.; Michalczyk, A.; Greenough, M.; Camakaris, J.; Mercer, J.F.; Ackland, M.L. Hormonal regulation of the Menkes and Wilson copper-transporting ATPases in human placental Jeg-3 cells. Biochem. J. 2007, 402, 241–250. [Google Scholar] [CrossRef]
- Prema, K.; Ramalakshmi, B.A.; Babu, S. Serum copper and zinc in hormonal contraceptive users. Fertil Steril. 1980, 33, 267–271. [Google Scholar] [CrossRef]
- Bonaventura, L.M.; Cleary, R.E.; Young, P.C. The effect of copper in vivo on specific progesterone binding by human endometrial cytosol. Fertil. Steril. 1979, 32, 531–535. [Google Scholar] [CrossRef]
- Shimizu, K.; Nishikawa, T.; Nozaki, M.; Oshima, K. Effects of an intrauterine copper device on serum copper, endometrial histology, and ovarian, hepatic, and renal functions in the Japanese monkey (Macaca fuscata fuscata). J. Med. Primatol. 1991, 20, 277–283. [Google Scholar] [CrossRef]
- Kamal, E.M.; Hafez, A.M. Effect of copper intrauterine device vs. injectable contraceptive on serum hormone levels and cell mitotic activity in endometrium. Middle East Fertil. Soc. J. 2013, 18, 142–146. [Google Scholar] [CrossRef]
- Guleria, K.; Agarwal, N.; Mishra, K.; Gulati, R.; Mehendiratta, A. Evaluation of endometrial steroid receptors and cell mitotic activity in women using copper intrauterine device: Can Cu-T prevent endometrial cancer? J. Obs. Gynaecol. Res. 2004, 30, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, G.G.; Van Noorden, S.; Balato, A.; Cimini, V. Copper/Zinc Superoxide Dismutase in Human Skin: Current Knowledge. Front. Med. 2020, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Ghernaout, D. Advanced Oxidation Phenomena in Electrocoagulation Process: A Myth or a Reality? Desalination Water Treat. 2013, 51, 7536–7554. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Guerra-Rodríguez, S.; Rodríguez, E.; Singh, D.N.; Rodríguez-Chueca, J. Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review. Water 2018, 10, 1828. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Fujii, J.; Homma, T.; Osaki, T. Superoxide Radicals in the Execution of Cell Death. Antioxidants 2022, 11, 501. [Google Scholar] [CrossRef]
- Chevriau, J.; De Palma, G.Z.; Jozefkowicz, C.; Vitali, V.; Canessa, F.A.; Ayub, N.; Soto, G.; Bienert, G.P.; Zeida, A.; Alleva, K. Permeation mechanisms of hydrogen peroxide and water through Plasma Membrane Intrinsic Protein aquaporins. Biochem. J. 2024, 481, 1329–1347. [Google Scholar] [CrossRef]
- Onyango, A.N. Endogenous Generation of Singlet Oxygen and Ozone in Human and Animal Tissues: Mechanisms, Biological Significance, and Influence of Dietary Components. Oxid. Med. Cell. Longev. 2016, 2016, 2398573. [Google Scholar] [CrossRef]
- Prado, F.M.; Oliveira, M.C.; Miyamoto, S.; Martinez, G.R.; Medeiros, M.H.; Ronsein, G.E.; Di Mascio, P. Thymine hydroperoxide as a potential source of singlet molecular oxygen in DNA. Free Radic. Biol. Med. 2009, 47, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Kalita, J.; Kumar, V.; Misra, U.K.; Bora, H.K. Movement Disorder in Copper Toxicity Rat Model: Role of Inflammation and Apoptosis in the Corpus Striatum. Neurotox. Res. 2020, 37, 904–912. [Google Scholar] [CrossRef]
- Robinett, N.G.; Peterson, R.L.; Culotta, V.C. Eukaryotic copper-only superoxide dismutases (SODs): A new class of SOD enzymes and SOD-like protein domains. J. Biol. Chem. 2018, 293, 4636–4643. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Sandoval, J.M.; Fattaccioli, A.; Dejeans, N.; Garbe, J.C.; Dieu, M.; Verrax, J.; Renard, P.; Huang, P.; Calderon, P.B. Chromatin remodeling regulates catalase expression during cancer cells adaptation to chronic oxidative stress. Free Radic. Biol. Med. 2016, 99, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Zamocky, M.; Sandoval, J.M.; Verrax, J.; Calderon, P.B. Regulation of catalase expression in healthy and cancerous cells. Free Radic. Biol. Med. 2015, 87, 84–97. [Google Scholar] [CrossRef]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef]
- Vázquez-Meza, H.; Vilchis-Landeros, M.M.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Cellular Compartmentalization, Glutathione Transport and Its Relevance in Some Pathologies. Antioxidants 2023, 12, 834. [Google Scholar] [CrossRef]
- Akaydın, S.Y.; Salihoğlu, E.M.; Güngör, D.G.; Karanlık, H.; Demokan, S. Correlation Between Gamma-Glutamyl Transferase Activity and Glutathione Levels in Molecular Subgroups of Breast Cancer. Eur. J. Breast Health 2019, 16, 72–76. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Marceau, F.; Farci, S.; Ouchane, S.; Chauvat, F. The glutathione system: A journey from cyanobacteria to higher eukaryotes. Antioxidants 2023, 12, 199. [Google Scholar] [CrossRef]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.R.; Falco, M.; et al. Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients. Nutrients 2024, 16, 1000. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Trapani, D.; Viale, G.; Criscitiello, C.; Curigliano, G. Practical classification of triple-negative breast cancer: Intratumoral heterogeneity, mechanisms of drug resistance, and novel therapies. NPJ Breast Cancer 2020, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Asleh, K.; Riaz, N.; Nielsen, T.O. Heterogeneity of triple negative breast cancer: Current advances in subtyping and treatment implications. J. Exp. Clin. Cancer Res. 2022, 41, 265. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Liu, Q.; Kong, X.; Wang, X.; Wang, Z.; Wang, J.; Fang, Y. A Systematic Study on Zinc-Related Metabolism in Breast Cancer. Nutrients 2023, 15, 1703. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Jiang, M. Copper-related genes predict prognosis and characteristics of breast cancer. Front. Immunol. 2023, 14, 1145080. [Google Scholar] [CrossRef]
- Abdelmagid, S.A.; Rickard, J.A.; McDonald, W.J.; Thomas, L.N.; Too, C.K. CAT-1-mediated arginine uptake and regulation of nitric oxide synthases for the survival of human breast cancer cell lines. J. Cell. Biochem. 2011, 112, 1084–1092. [Google Scholar] [CrossRef]
- Greene, J.M.; Feugang, J.M.; Pfeiffer, K.E.; Stokes, J.V.; Bowers, S.D.; Ryan, P.L. L-arginine enhances cell proliferation and reduces apoptosis in human endometrial RL95-2 cells. Reprod. Biol. Endocrinol. 2013, 11, 15. [Google Scholar] [CrossRef]
- Haase, H.; Maret, W. Partial oxidation and oxidative polymerization of metallothionein. Electrophoresis 2008, 29, 4169–4176. [Google Scholar] [CrossRef]
- Yang, R.; Roshani, D.; Gao, B.; Li, P.; Shang, N. Metallothionein: A Comprehensive Review of Its Classification, Structure, Biological Functions, and Applications. Antioxidants 2024, 13, 825. [Google Scholar] [CrossRef]
- Babu, C.S.; Lee, Y.M.; Dudev, T.; Lim, C. Modeling Zn(2)(+) release from metallothionein. J. Phys. Chem. A. 2014, 118, 9244–9252. [Google Scholar] [CrossRef]
- Ashina, K.; Tsubosaka, Y.; Nakamura, T.; Omori, K.; Kobayashi, K.; Hori, M.; Ozaki, H.; Murata, T. Histamine Induces Vascular Hyperpermeability by Increasing Blood Flow and Endothelial Barrier Disruption In Vivo. PLoS ONE 2015, 10, e0132367. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, K.; Ku, W.; Wang, D.; Wake, H.; Qiao, H.; Teshigawara, K.; Nishibori, M. Histamine induced high mobility group box-1 release from vascular endothelial cells through H1 receptor. Front. Immunol. 2022, 13, 930683. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.; Korolnek, T.; Lee, C.J.; Coyne, H.J., 3rd; Winge, D.R.; Kim, B.E.; Petris, M.J. An extracellular histidine-containing motif in the zinc transporter ZIP4 plays a role in zinc sensing and zinc-induced endocytosis in mammalian cells. J. Biol. Chem. 2019, 294, 2815–2826. [Google Scholar] [CrossRef]
- Nishida, S.; Mizuno, T.; Obata, H. Involvement of histidine-rich domain of ZIP family transporter TjZNT1 in metal ion specificity. Plant Physiol. Biochem. 2008, 46, 601–606. [Google Scholar] [CrossRef]
- Kanarek, N.; Keys, H.R.; Cantor, J.R.; Lewis, C.A.; Chan, S.H.; Kunchok, T.; Abu-Remaileh, M.; Freinkman, E.; Schweitzer, L.D.; Sabatini, D.M. Histidine catabolism is a major determinant of methotrexate sensitivity. Nature 2018, 559, 632–636. [Google Scholar] [CrossRef]
- Chik, F.; Machnes, Z.; Szyf, M. Synergistic anti-breast cancer effect of a combined treatment with the methyl donor S-adenosyl methionine and the DNA methylation inhibitor 5-aza-2’-deoxycytidine. Carcinogenesis 2014, 35, 138–144. [Google Scholar] [CrossRef]
- Bonifácio, V.D.B.; Pereira, S.A.; Serpa, J.; Vicente, J.B. Cysteine metabolic circuitries: Druggable targets in cancer. Br. J. Cancer 2021, 124, 862–879. [Google Scholar] [CrossRef]
- Ghergurovich, J.M.; Xu, X.; Wang, J.Z.; Yang, L.; Ryseck, R.P.; Wang, L.; Rabinowitz, J.D. Methionine synthase supports tumour tetrahydrofolate pools. Nat. Metab. 2021, 3, 1512–1520. [Google Scholar] [CrossRef]
- Matuszczak, M.; Kiljańczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Cybulski, C.; Dębniak, T.; Gronwald, J.; et al. Antioxidant Properties of Zinc and Copper—Blood Zinc-to Copper-Ratio as a Marker of Cancer Risk BRCA1 Mutation Carriers. Antioxidants 2024, 13, 841. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different Roles in a Common Pathway of Genome Protection. Nat. Rev. Cancer 2012, 12, 68–78. [Google Scholar] [CrossRef]
- Hartman, A.-R.; Ford, J.M. BRCA1 and P53: Compensatory Roles in DNA Repair. J. Mol. Med. 2003, 81, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-X. BRCA1: Cell Cycle Checkpoint, Genetic Instability, DNA Damage Response and Cancer Evolution. Nucleic. Acids Res. 2006, 34, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, W.; Tomimatsu, N.; Yu, C.H.; Ji, J.-H.; Alejo, S.; Witus, S.R.; Alimbetov, D.; Fitzgerald, O.; Wu, B.; et al. Crucial Roles of the BRCA1-BARD1 E3 Ubiquitin Ligase Activity in Homology-Directed DNA Repair. Mol. Cell 2023, 83, 3679–3691.e8. [Google Scholar] [CrossRef] [PubMed]
- Matuszczak, M.; Kiljańczyk, A.; Marciniak, W.; Derkacz, R.; Stempa, K.; Baszuk, P.; Bryśkiewicz, M.; Sun, P.; Cheriyan, A.; Cybulski, C.; et al. Zinc and Its Antioxidant Properties: The Potential Use of Blood Zinc Levels as a Marker of Cancer Risk in BRCA1 Mutation Carriers. Antioxidants 2024, 13, 609. [Google Scholar] [CrossRef]
- Arun, B.; Couch, F.J.; Abraham, J.; Tung, N.; Fasching, P.A. BRCA-mutated breast cancer: The unmet need, challenges and therapeutic benefits of genetic testing. Br. J. Cancer 2024, 131, 1400–1414. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Kosenok, V.K.; Massard, G. Endogenous Intoxication and Saliva Lipid Peroxidation in Patients with Lung Cancer. Diagnostics 2016, 6, 39. [Google Scholar] [CrossRef]
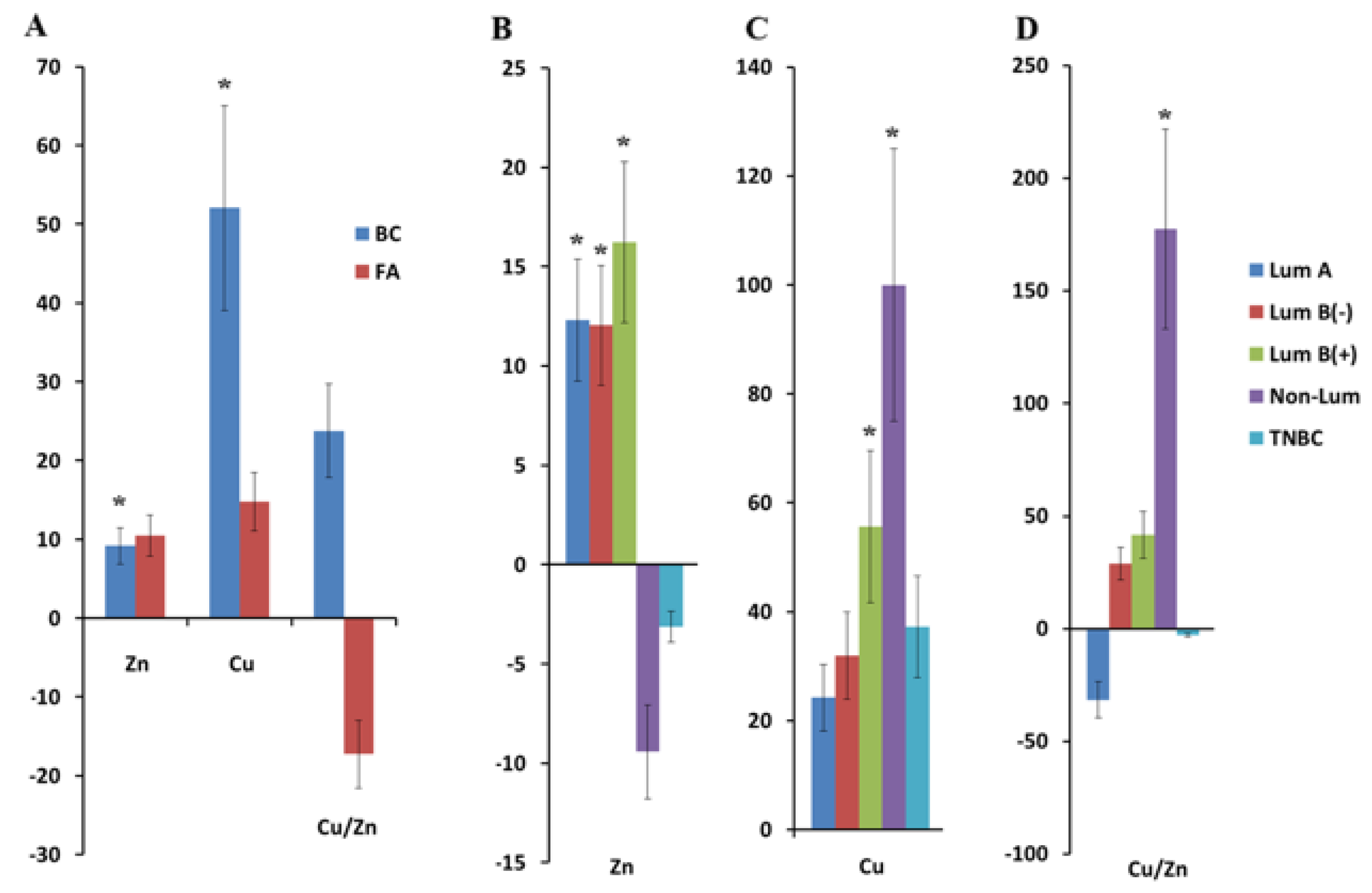
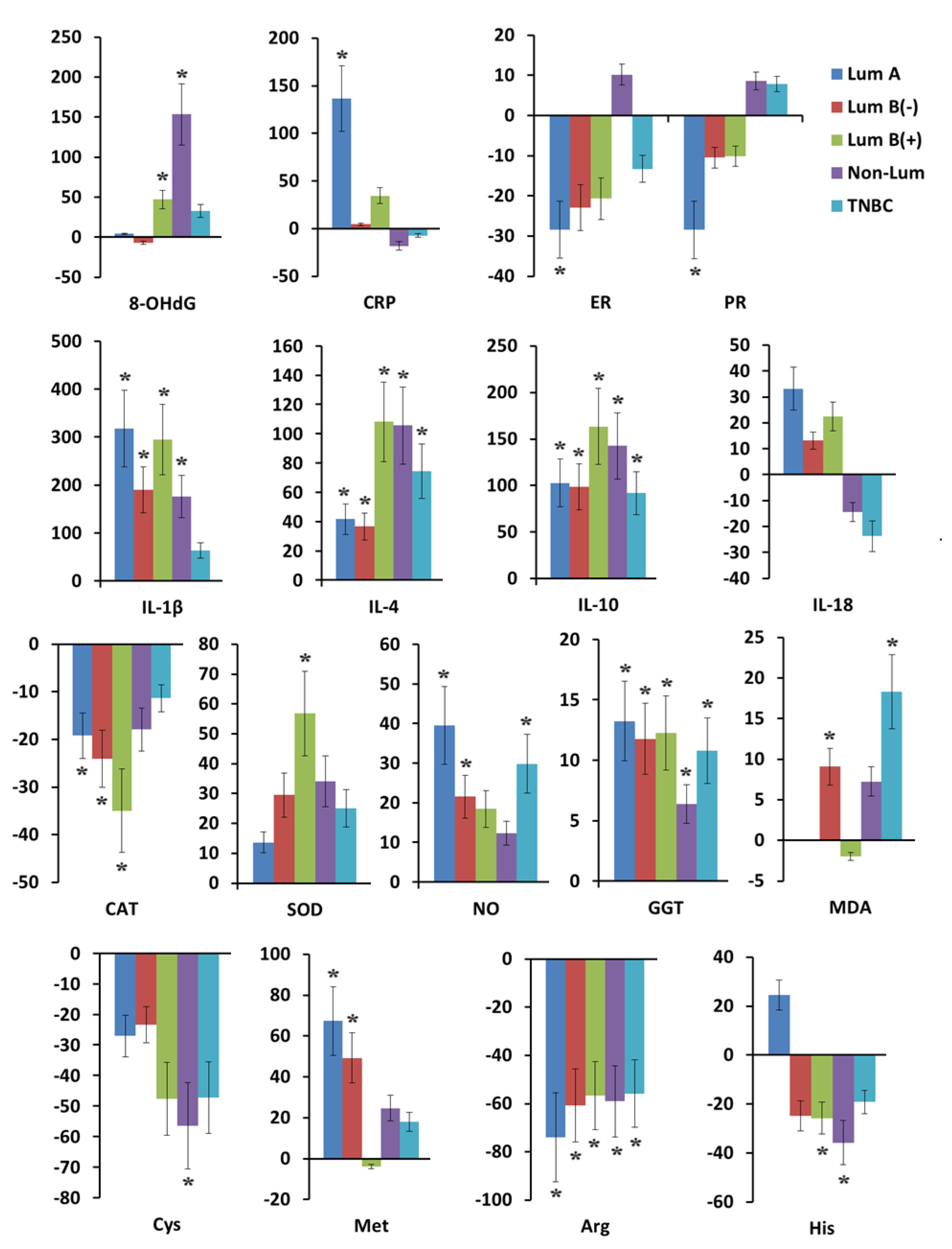
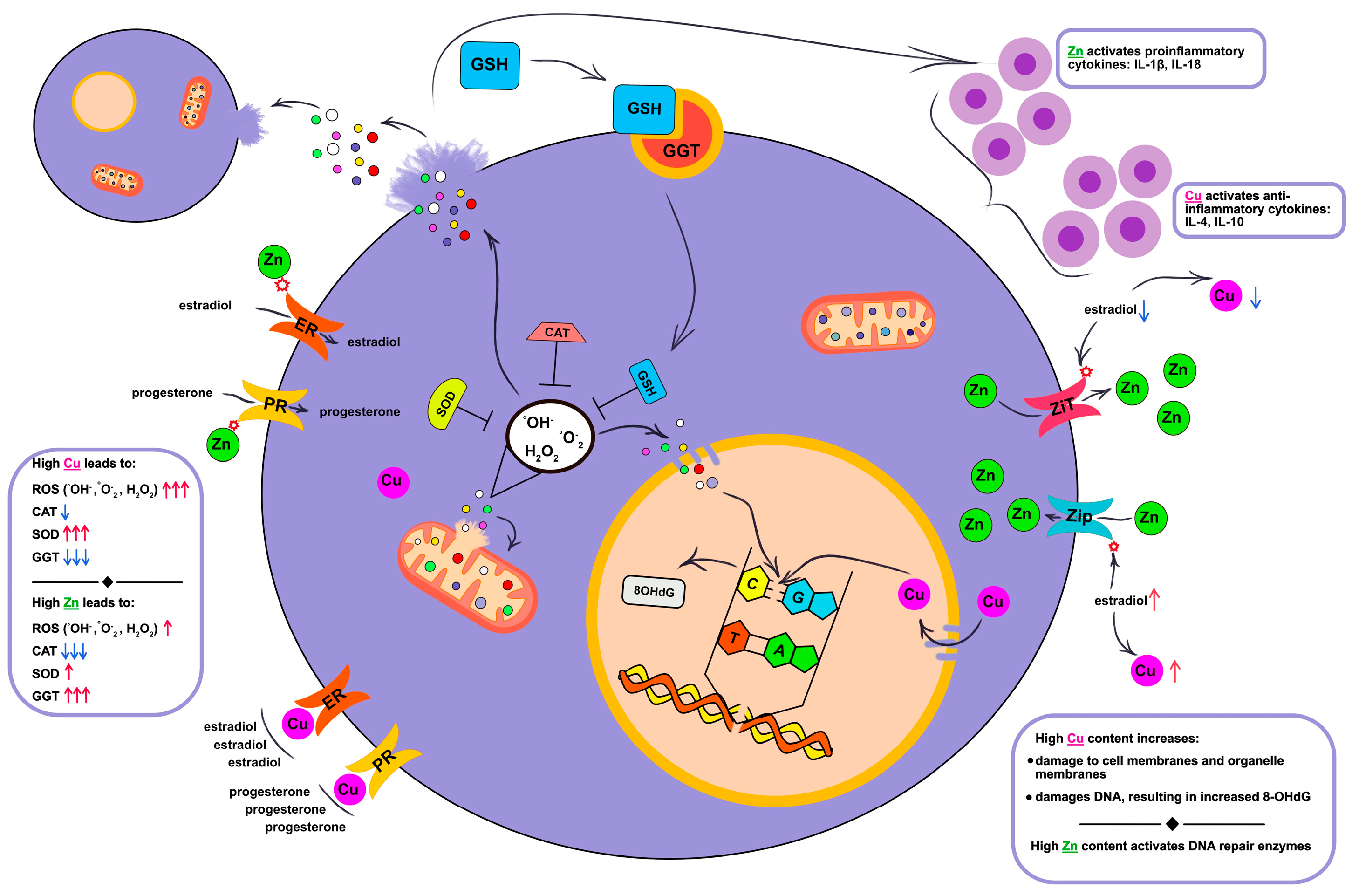
| Indicators | Breast Cancer, n = 230 | Fibroadenomas, n = 92 | Healthy Controls, n = 59 | Kruskal–Wallis Test; p-Value |
|---|---|---|---|---|
| Age, years | 60.2 [50.1; 66.5] | 52.0 [45.0; 62.0] | 53.7 [40.6; 60.3] | 5.324; 0.0741 |
| 8-OHdG, pg/mL | 235.5 [156.3; 636.2] | 299.2 [249.5; 347.1] | 188.4 [89.1; 309.1] | 4.866; 0.0926 |
| CRP, mU/mL | 0.176 [0.122; 0.312] | - | 0.153 [0.118; 0.212] | 0.2528 |
| Cytokines | ||||
| IL-1β, pg/mL | 124.8 [30.84; 305.4] | 144.5 [37.00; 310.1] | 37.01 [11.78; 106.1] | 8.796; 0.0123 * |
| IL-4, pg/mL | 2.49 [1.75; 4.04] | 2.79 [1.74; 4.88] | 1.61 [1.03; 2.96] | 18.12; 0.0001 * |
| IL-10, pg/mL | 4.70 [3.30; 7.15] | 4.97 [3.78; 6.38] | 2.25 [1.68; 3.48] | 44.73; 0.0000 * |
| IL-18, pg/mL | 67.05 [31.13; 132.9] | 62.50 [40.90; 134.3] | 63.86 [22.50; 141.8] | 0.3925; 0.8218 |
| Hormones | ||||
| Estradiol, nmol/L | 3.01 [2.55; 3.72] | - | 3.62 [2.63; 5.20] | 0.2076 |
| Progesterone, nmol/L | 2.47 [2.08; 2.99] | - | 2.67 [2.34; 3.08] | 0.4281 |
| Activity of the antioxidant system | ||||
| Catalase, nkat/L | 3.77 [2.56; 5.94] | 3.64 [2.48; 5.21] | 4.58 [3.32; 5.79] | 18.46; 0.0001 * |
| SOD, c.u. | 73.7 [34.2; 142.1] | 63.2 [34.2; 115.8] | 57.9 [31.6; 113.2] | 7.223; 0.0270 * |
| NO, μmol/L | 28.3 [18.3; 41.9] | 32.7 [18.4; 55.8] | 22.8 [13.2; 36.8] | 35.74; 0.0000 * |
| MDA, μmol/L | 6.92 [5.56; 8.80] | 7.01 [5.90; 8.97] | 6.50 [5.73; 7.95] | 14.60; 0.0007 * |
| GGT, U/L | 23.3 [20.0; 26.5] | 21.5 [18.9; 24.4] | 20.4 [17.4; 22.4] | 50.92; 0.0000 * |
| Amino acids | ||||
| Cys, nmol/L | 1.33 [0.59; 2.63] | 1.58 [1.10; 2.68] | 2.18 [1.04; 3.57] | 3.604; 0.1649 |
| Met, nmol/L | 4.97 [3.38; 7.12] | 6.61 [3.28; 10.35] | 4.16 [1.85; 5.30] | 7.837; 0.0199 * |
| His, nmol/L | 19.44 [12.76; 30.00] | 20.31 [15.67; 36.75] | 24.85 [21.25; 29.24] | 4.582; 0.1012 |
| Arg, nmol/L | 10.22 [5.95; 21.23] | 13.78 [6.83; 21.91] | 25.23 [20.72; 38.10] | 14.55; 0.0007 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyachenko, E.I.; Sarf, E.A.; Bel’skaya, L.V. Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients. Int. J. Mol. Sci. 2025, 26, 4784. https://doi.org/10.3390/ijms26104784
Dyachenko EI, Sarf EA, Bel’skaya LV. Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients. International Journal of Molecular Sciences. 2025; 26(10):4784. https://doi.org/10.3390/ijms26104784
Chicago/Turabian StyleDyachenko, Elena I., Elena A. Sarf, and Lyudmila V. Bel’skaya. 2025. "Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients" International Journal of Molecular Sciences 26, no. 10: 4784. https://doi.org/10.3390/ijms26104784
APA StyleDyachenko, E. I., Sarf, E. A., & Bel’skaya, L. V. (2025). Salivary Zinc and Copper Levels Are Differentially Associated with ROS Levels in Breast Cancer Patients. International Journal of Molecular Sciences, 26(10), 4784. https://doi.org/10.3390/ijms26104784






