Advances in Genome Editing Through Haploid Induction Systems
Abstract
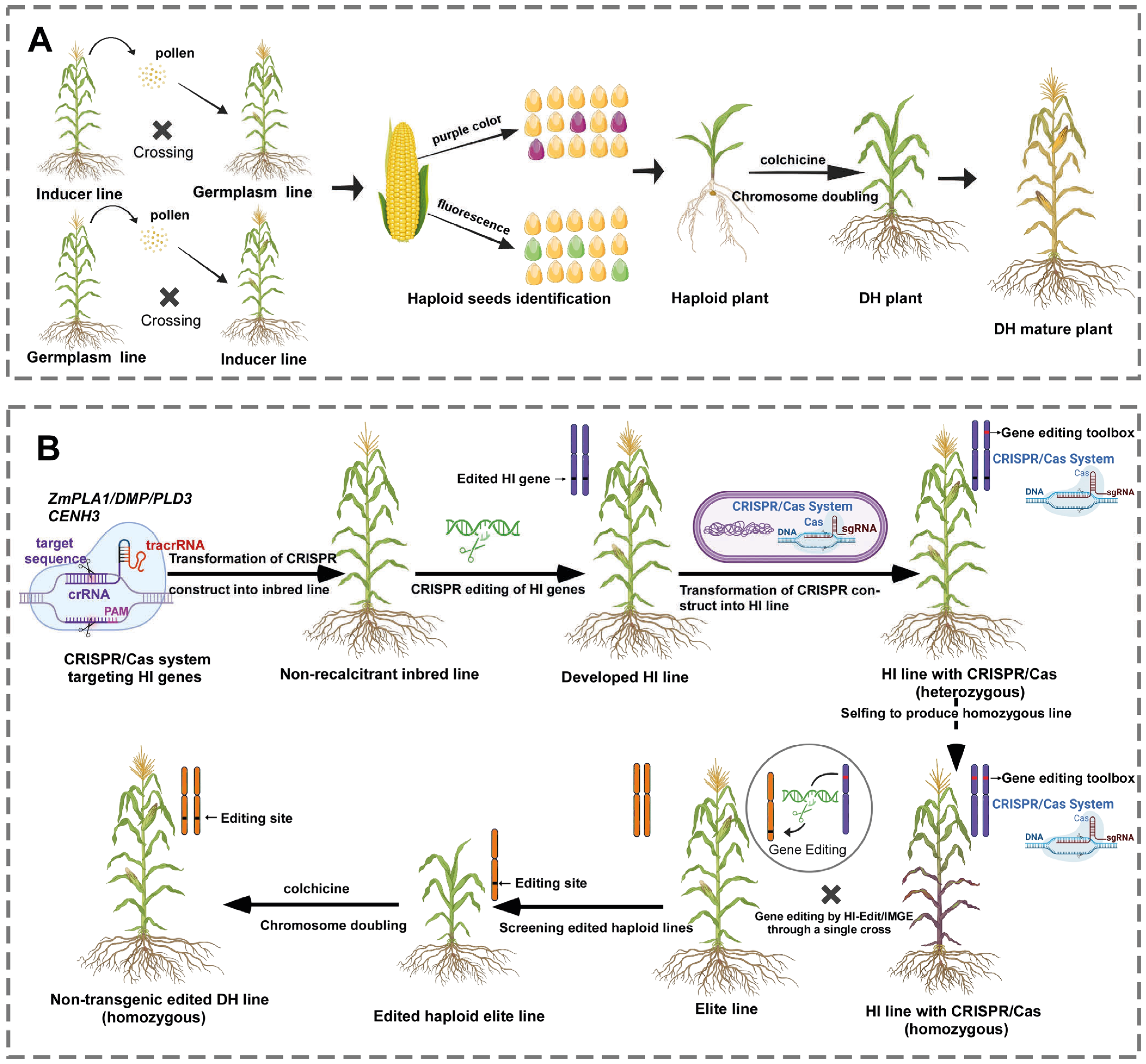
1. Introduction
2. Haploid Induction Systems in Crop Breeding
3. Haploid Genes Discovery and Genome Editing Applications for Haploid Inducer Line Development
| Gene | Species | HIR (%) | Haploid Induction Type | Reference | Gene Editing Tool |
|---|---|---|---|---|---|
| ZmPLA1/NLD/MTL | Oryza sativa | 2.8–12.0% | Maternal | Lv et al., 2023 [29] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Zea mays | 4.7–11.0% | Maternal | Chaikam et al., 2019 [32] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Zea mays | 4.0–12.5% | Maternal | Kelliher et al., 2017 [46] | TALEN |
| ZmPLA1/NLD/MTL | Zea mays | 1.9–6.7% | Maternal | Liu et al., 2017 [48] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Oryza sativa | 2.0–6.0% | Maternal | Yao et al., 2018 [52] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Triticum aestivum | 5.9–15.7% | Maternal | Liu et al., 2019 [53] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Triticum aestivum | 10.0–31.6% | Maternal | Liu et al., 2020 [54] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Setaria italica | 2.0–3.0% | Maternal | Cheng et al., 2021 [55] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Triticum aestivum | 10.2–15.2% | Maternal | Tang et al., 2023 [56] | CRISPR/Cas9 |
| ZmPLA1/NLD/MTL | Saccharum officinarum | 0.6–1.0% | Maternal | Guo et al., 2024 [57] | CRISPR/Cas9 |
| DMP | Zea mays | 0.1–0.3% | Maternal | Zhong et al., 2019 [58] | CRISPR/Cas9 |
| DMP | Arabidopsis thaliana | 1.0–4.0% | Maternal | Zhong et al., 2020 [59] | CRISPR/Cas9 |
| DMP | Medicago | 0.3–0.8% | Maternal | Wang et al., 2021 [60] | CRISPR/Cas9 |
| DMP | Solanum lycopersicum | 0.5–3.7% | Maternal | Zhong et al., 2021 [61] | CRISPR/Cas9 |
| DMP | Nicotiana tabacum | 1.5–1.8% | Maternal | Zhang et al., 2022 [62] | CRISPR/Cas9 |
| DMP | Brassica napus | 1.0–4.4% | Maternal | Li et al., 2022 [63] | CRISPR/Cas9 |
| DMP | Brassica napus | 1.5–2.4% | Maternal | Zhong et al., 2022 [64] | CRISPR/Cas9 |
| DMP | Brassica oleracea | 0.4–2.4% | Maternal | Zhao et al., 2022 [65] | CRISPR/Cas9 |
| DMP | Citrullus lanatus | 0.5–1.1% | Maternal | Chen et al., 2023 [66] | CRISPR/Cas9 |
| DMP | Cucumis sativus | 0.1–0.4% | Maternal | Yin et al., 2024 [67] | CRISPR/Cas9 |
| ZmPOD65 | Zea mays | 0.9–7.7% | Maternal | Jiang et al., 2022 [51] | CRISPR/Cas9 |
| ZmPLD3 | Zea mays | 0.9–1.0% | Maternal | Li et al., 2021 [68] | CRISPR/Cas9 |
| ZmBBM2 | Zea mays | 0.4–3.6% | Maternal | Qi et al., 2023 [71] | CRISPRa |
| pPLAIIγ | Arabidopsis thaliana | 1.0–1.1% | Maternal | Jang et al., 2023 [73] | CRISPR/Cas9 |
| ECS | Oryza sativa | 3.1–3.6% | Maternal | Zhang et al., 2023 [74] | CRISPR/Cas9 |
| CENH3 | Arabidopsis thaliana | 8.0–25.7% | Paternal | Kuppu et al., 2020 [81] | CRISPR/Cas9 |
| CENH3 | Daucus carota | 0.2–1.1% | Paternal | Dunemann et al., 2019 [82] | CRISPR/Cas9 |
| CENH3 | Triticum aestivum | 7.0–8.0% | Paternal | Lv et al., 2020 [83] | CRISPR/Cas9 |
| CENH3 | Arabidopsis thaliana | 13.6–28.6% | Paternal | Han et al., 2024 [84] | CRISPR/Cas9 |
| CENH3 | Arabidopsis thaliana | 1.6–24.8% | Maternal | Han et al., 2024 [84] | CRISPR/Cas9 |
| CENH3 | Brassica oleracea | 0.5–1.1% | Paternal | Han et al., 2024 [84] | CRISPR/Cas9 |
4. Integration of Haploid Induction Systems with Gene Editing Tools
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Correction Statement
References
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, K.; Chamas, S.; Unkel, K.; Demidov, D.; Lermontova, I.; Dresselhaus, T.; Kumlehn, J.; Dunemann, F.; Houben, A. State-of-the-art and novel developments of in vivo haploid technologies. Theor. Appl. Genet. 2019, 132, 593–605. [Google Scholar] [CrossRef]
- Peng, T.; Sun, X.; Mumm, R.H. Optimized breeding strategies for multiple trait integration: I. Minimizing linkage drag in single event introgression. Mol. Breed. 2014, 33, 89–104. [Google Scholar] [CrossRef]
- Weyen, J. Applications of doubled haploids in plant breeding and applied research. In Doubled Haploid Technology: Volume 1: General Topics, Alliaceae, Cereals; Humana: New York, NY, USA, 2021; pp. 23–39. [Google Scholar]
- Ren, J.; Wu, P.; Trampe, B.; Tian, X.; Lübberstedt, T.; Chen, S. Novel technologies in doubled haploid line development. Plant Biotechnol. J. 2017, 15, 1361–1370. [Google Scholar] [CrossRef]
- Forster, B.P.; Heberle-Bors, E.; Kasha, K.J.; Touraev, A. The resurgence of haploids in higher plants. Trends Plant Sci. 2007, 12, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ghalagi, C. In Vitro Production of Doubled Haploids from KRH-4 Rice Hybrid and Development of Strategies to Identify Hploids at Early Developmental Stages. Ph.D. Thesis, University of Agricultural Sciences, GKVK, Bangalore, India, 2022. [Google Scholar]
- Al-Ashkar, I.; Al-Doss, A.; Ullah, N. Accelerating crop improvement through speed breeding. In Climate-Resilient Agriculture, Vol 1: Crop Responses and Agroecological Perspectives; Springer International Publishing: Cham, Switzerland, 2023; pp. 821–847. [Google Scholar]
- Sun, L.; Lai, M.; Ghouri, F.; Nawaz, M.A.; Ali, F.; Baloch, F.S.; Nadeem, M.A.; Aasim, M.; Shahid, M.Q. Modern plant breeding techniques in crop improvement and genetic diversity: From molecular markers and gene editing to artificial intelligence—A critical review. Plants 2024, 13, 2676. [Google Scholar] [CrossRef]
- Carroll, D. Genome engineering with targetable nucleases. Annu. Rev. Biochem. 2014, 83, 409–439. [Google Scholar] [CrossRef]
- Wyman, C.; Kanaar, R. DNA double-strand break repair: All’s well that ends well. Annu. Rev. Genet. 2006, 40, 363–383. [Google Scholar] [CrossRef]
- Cohen-Tannoudji, M.; Robine, S.; Choulika, A.; Pinto, D.; El Marjou, F.; Babinet, C.; Louvard, D.; Jaisser, F. I-Sce I-induced gene replacement at a natural locus in embryonic stem cells. Mol. Cell. Biol. 1998, 18, 1444–1448. [Google Scholar] [CrossRef]
- Bibikova, M.; Golic, M.; Golic, K.G.; Carroll, D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 2002, 161, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Joung, J.K.; Sander, J.D. TALENs: A widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 2013, 14, 49–55. [Google Scholar] [CrossRef]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Norville, J.E.; Aach, J.E.; McCormack, M.; Zhang, D.; Bush, J.; Church, G.M.; Sheen, J. Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 2013, 31, 688–691. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, B.; Ding, W.; Liu, X.; Yang, D.-L.; Wei, P.; Cao, F.; Zhu, S.; Zhang, F.; Mao, Y.; et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013, 23, 1229–1232. [Google Scholar] [CrossRef]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Khatodia, S.; Bhatotia, K.; Passricha, N.; Khurana, S.M.P.; Tuteja, N. The CRISPR/Cas genome-editing tool: Application in improvement of crops. Front. Plant Sci. 2016, 7, 506. [Google Scholar] [CrossRef]
- Wang, Y.; Zafar, N.; Ali, Q.; Manghwar, H.; Wang, G.; Yu, L.; Ding, X.; Ding, F.; Hong, N.; Wang, G.; et al. CRISPR/Cas genome editing technologies for plant improvement against biotic and abiotic stresses: Advances, limitations, and future perspectives. Cells 2022, 11, 3928. [Google Scholar] [CrossRef]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef]
- Das, D.; Singha, D.L.; Paswan, R.R.; Chowdhury, N.; Sharma, M.; Reddy, P.S.; Chikkaputtaiah, C. Recent advancements in CRISPR/Cas technology for accelerated crop improvement. Planta 2022, 255, 109. [Google Scholar] [CrossRef]
- Son, S.; Park, S.R. Challenges facing CRISPR/Cas9-based genome editing in plants. Front. Plant Sci. 2022, 13, 902413. [Google Scholar] [CrossRef] [PubMed]
- Bate, N.J.; Dardick, C.D.; de Maagd, R.A.; Williams, R.W. Opportunities and challenges applying gene editing to specialty crops. Vitr. Cell. Dev. Biol.-Plant 2021, 57, 709–719. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y. Technological breakthroughs in generating transgene-free and genetically stable CRISPR-edited plants. Abiotech 2020, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.M.; Gilles, L.M.; Pyott, D.E.; Martinant, J.P.; Rogowsky, P.M.; Widiez, T. Puzzling out plant reproduction by haploid induction for innovations in plant breeding. Nat. Plants 2020, 6, 610–619. [Google Scholar] [CrossRef]
- Lv, J.; Kelliher, T. Recent advances in engineering of in vivo haploid induction systems. In Plant Genome Engineering: Methods and Protocols; Humana: New York, NY, USA, 2023; pp. 365–383. [Google Scholar]
- Blakeslee, A.F.; Belling, J.; Farnham, M.E.; Bergner, A.D. A haploid mutant in the jimson weed, “Datura stramonium”. Science 1922, 55, 646–647. [Google Scholar] [CrossRef]
- Guha, S.; Maheshwari, S.C. In vitro production of embryos from anthers of Datura. Nature 1964, 204, 497. [Google Scholar] [CrossRef]
- Chaikam, V.; Molenaar, W.; Melchinger, A.E.; Boddupalli, P.M. Doubled haploid technology for line development in maize: Technical advances and prospects. Theor. Appl. Genet. 2019, 132, 3227–3243. [Google Scholar] [CrossRef]
- Ferrie, A.M.; Möllers, C. Haploids and doubled haploids in Brassica spp. for genetic and genomic research. Plant Cell Tissue Organ Cult. 2011, 104, 375–386. [Google Scholar] [CrossRef]
- Eliby, S.; Bekkuzhina, S.; Kishchenko, O.; Iskakova, G.; Kylyshbayeva, G.; Jatayev, S.; Soole, K.; Langridge, P.; Borisjuk, N.; Shavrukov, Y. Developments and prospects for doubled haploid wheat. Biotechnol. Adv. 2022, 60, 108007. [Google Scholar] [CrossRef]
- Gilles, L.M.; Martinant, J.P.; Rogowsky, P.M.; Widiez, T. Haploid induction in plants. Curr. Biol. 2017, 27, R1095–R1097. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.K.; Goswami, R. Haploid production in higher plant. Int. J. Chem. Biol. Sci 2014, 1, 26–45. [Google Scholar]
- Niazian, M.; Shariatpanahi, M.E. In vitro-based doubled haploid production: Recent improvements. Euphytica 2020, 216, 69. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, M.; Konzak, C. Improving green plant production via isolated microspore culture in bread wheat (Triticum aestivum L.). Plant Cell Rep. 2002, 20, 821–824. [Google Scholar] [CrossRef]
- Maluszynski, M.; Kasha, K.J.; Szarejko, I. Published doubled haploid protocols in plant species. In Doubled Haploid Production in Crop Plants: A Manual; Springer: Dordrecht, The Netherlands, 2003; pp. 309–335. [Google Scholar]
- Segui-Simarro, J.M.; Moreno, J.B.; Fernández, M.G.; Mir, R. Doubled haploid technology. Methods Mol. Bsiol. 2021, 2287, 50. [Google Scholar]
- Laurie, D.A.; Bennett, M.D. The production of haploid wheat plants from wheat x maize crosses. Theor. Appl. Genet. 1988, 76, 393–397. [Google Scholar] [CrossRef]
- Clausen, R.E.; Mann, M.C. Inheritance in Nicotiana tabacum: V. The occurrence of haploid plants in interspecific progenies. Proc. Natl. Acad. Sci. USA 1924, 10, 121–124. [Google Scholar] [CrossRef]
- Ishii, T.; Karimi-Ashtiyani, R.; Houben, A. Haploidization via chromosome elimination: Means and mechanisms. Annu. Rev. Plant Biol. 2016, 67, 421–438. [Google Scholar] [CrossRef]
- Guan, X.; Peng, J.; Fu, D. Technology for production of wheat doubled haploid via maize pollen induction—Updated review. Agronomy 2024, 14, 375. [Google Scholar] [CrossRef]
- Sharma, H.C. Embryo rescue following wide crosses. In Plant Cell Culture Protocols; Humana: New York, NY, USA, 1999; pp. 293–307. [Google Scholar]
- Kelliher, T.; Starr, D.; Richbourg, L.; Chintamanani, S.; Delzer, B.; Nuccio, M.L.; Green, J.; Chen, Z.; McCuiston, J.; Wang, W.; et al. MATRILINEAL, a sperm-specific phospholipase, triggers maize haploid induction. Nature 2017, 542, 105–109. [Google Scholar] [CrossRef]
- Coe, E.H., Jr. A line of maize with high haploid frequency. Am. Nat. 1959, 93, 381–382. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Meng, D.; Zhong, Y.; Chen, C.; Dong, X.; Xu, X.; Chen, B.; Li, W.; Li, L.; et al. A 4-bp insertion at ZmPLA1 encoding a putative phospholipase A generates haploid induction in maize. Mol. Plant 2017, 10, 520–522. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Liu, C.; Chen, S.; Jin, W. Haploid induction and its application in maize breeding. Mol. Breed. 2021, 41, 20. [Google Scholar] [CrossRef] [PubMed]
- Gilles, L.M.; Khaled, A.; Laffaire, J.; Chaignon, S.; Gendrot, G.; Laplaige, J.; Bergès, H.; Beydon, G.; Bayle, V.; Barret, P.; et al. Loss of pollen-specific phospholipase NOT LIKE DAD triggers gynogenesis in maize. EMBO J. 2017, 36, 707–717. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, J.; Li, R.; Yan, S.; Chen, W.; Guo, L.; Qin, G.; Wang, P.; Luo, C.; Huang, W.; et al. A reactive oxygen species burst causes haploid induction in maize. Mol. Plant 2022, 15, 943–955. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, Y.; Liu, C.; Liu, Y.; Wang, Y.; Liang, D.; Liu, J.; Sahoo, G.; Kelliher, T. OsMATL mutation induces haploid seed formation in indica rice. Nat. Plants 2018, 4, 530–533. [Google Scholar] [CrossRef]
- Liu, C.; Zhong, Y.; Qi, X.; Chen, M.; Liu, Z.; Chen, C.; Tian, X.; Li, J.; Jiao, Y.; Wang, D.; et al. Extension of the in vivo haploid induction system from diploid maize to hexaploid wheat. Plant Biotechnol. J. 2019, 18, 316. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Jia, Z.; Gong, Q.; Lin, Z.; Du, L.; Pei, X.; Ye, X. Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. J. Exp. Bot. 2020, 71, 1337–1349. [Google Scholar] [CrossRef]
- Cheng, Z.; Sun, Y.; Yang, S.; Zhi, H.; Yin, T.; Ma, X.; Zhang, H.; Diao, X.; Guo, Y.; Li, X.; et al. Establishing in planta haploid inducer line by edited SiMTL in foxtail millet (Setaria italica). Plant Biotechnol. J. 2021, 19, 1089. [Google Scholar] [CrossRef]
- Tang, H.; Qiu, Y.; Wang, W.; Yu, M.; Chang, Y.; Han, Z.; Du, L.; Lin, Z.; Wang, K.; Ye, X. Development of a haploid inducer by editing HvMTL in barley. J. Genet. Genom. Yi Chuan Xue Bao 2023, 50, 366–369. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, S.; Chen, S.; Zhang, Y.; Wang, W.; Deng, Z.; Liu, Z.; Yu, Z.; Xu, H.; Guo, J.; et al. In vivo induction of sugarcane (Saccharum spp.) haploids by genome editing. Plant Physiol. 2024, 196, 731–734. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, C.; Qi, X.; Jiao, Y.; Wang, D.; Wang, Y.; Liu, Z.; Chen, C.; Chen, B.; Tian, X.; et al. Mutation of ZmDMP enhances haploid induction in maize. Nat. Plants 2019, 5, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, B.; Li, M.; Wang, D.; Jiao, Y.; Qi, X.; Wang, M.; Liu, Z.; Chen, C.; Wang, Y.; et al. A DMP-triggered in vivo maternal haploid induction system in the dicotyledonous Arabidopsis. Nat. Plants 2020, 6, 466–472. [Google Scholar] [CrossRef]
- Wang, N.; Xia, X.; Jiang, T.; Li, L.; Zhang, P.; Niu, L.; Cheng, H.; Wang, K.; Lin, H. In planta haploid induction by genome editing of DMP in the model legume Medicago truncatula. Plant Biotechnol. J. 2021, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Chen, B.; Wang, D.; Zhu, X.; Li, M.; Zhang, J.; Chen, M.; Wang, M.; Riksen, T.; Liu, J.; et al. In vivo maternal haploid induction in tomato. Plant Biotechnol. J. 2022, 20, 250–252. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Zhang, J.; Jia, M.; Cao, L.; Yu, J.; Zhao, D. Haploid induction in allotetraploid tobacco using DMPs mutation. Planta 2022, 255, 98. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, D.; Xiao, Q.; Wang, H.; Wen, J.; Tu, J.; Shen, J.; Fu, T.; Yi, B. An in planta haploid induction system in Brassica napus. J. Integr. Plant Biol. 2022, 64, 1140–1144. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, Y.; Chen, B.; Liu, J.; Wang, D.; Li, M.; Qi, X.; Liu, C.; Boutilier, K.; Chen, S. Establishment of a dmp based maternal haploid induction system for polyploid Brassica napus and Nicotiana tabacum. J. Integr. Plant Biol. 2022, 64, 1281–1294. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, K.; Liu, Y.; Zhang, N.; Yang, L.; Zhang, Y.; Wang, Y.; Ji, J.; Fang, Z.; Han, F.; et al. In vivo maternal haploid induction based on genome editing of DMP in Brassica oleracea. Plant Biotechnol. J. 2022, 20, 2242. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Ai, G.; Chen, J.; Guo, D.; Zhu, Z.; Zhu, X.; Tian, S.; Wang, J.; Liu, M.; et al. Creation of a watermelon haploid inducer line via ClDMP3-mediated single fertilization of the central cell. Hortic. Res. 2023, 10, uhad081. [Google Scholar] [CrossRef]
- Yin, S.; Li, S.; Sun, L.; Shi, K.; Fan, S.; Liu, X.; Ren, H. Mutating the maternal haploid inducer gene CsDMP in cucumber produces haploids in planta. Plant Physiol. 2024, 194, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Z.; Yue, Y.; Zhao, H.; Fei, X.; E., L.; Liu, C.; Chen, S.; Lai, J.; Song, W. Loss-of-function alleles of ZmPLD3 cause haploid induction in maize. Nat. Plants 2021, 7, 1579–1588. [Google Scholar] [CrossRef]
- Conner, J.A.; Mookkan, M.; Huo, H.; Chae, K.; Ozias-Akins, P. A parthenogenesis gene of apomict origin elicits embryo formation from unfertilized eggs in a sexual plant. Proc. Natl. Acad. Sci. USA 2015, 112, 11205–11210. [Google Scholar] [CrossRef]
- Chahal, L.S.; Conner, J.A.; Ozias-Akins, P. Phylogenetically distant BABY BOOM genes from Setaria italica induce parthenogenesis in rice. Front. Plant Sci. 2022, 13, 863908. [Google Scholar] [CrossRef]
- Qi, X.; Gao, H.; Lv, R.; Mao, W.; Zhu, J.; Liu, C.; Mao, L.; Li, X.; Xie, C. CRISPR/dCas-mediated gene activation toolkit development and its application for parthenogenesis induction in maize. Plant Commun. 2023, 4, 100449. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.M.; Calhau, A.R.; Fierlej, Y.; Martinant, J.P.; Rogowsky, P.M.; Gilles, L.M.; Widiez, T. In planta haploid induction by kokopelli mutants. Plant Physiol. 2023, 193, 182–185. [Google Scholar] [CrossRef]
- Jang, J.H.; Seo, H.S.; Widiez, T.; Lee, O.R. Loss-of-function of gynoecium-expressed phospholipase pPLAIIγ triggers maternal haploid induction in Arabidopsis. New Phytol. 2023, 238, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, C.; Li, S.; Zhang, B.; Luo, P.; Peng, X.; Zhao, P.; Dresselhaus, T.; Sun, M.-X. A female in vivo haploid-induction system via mutagenesis of egg cell-specific peptidases. Mol. Plant 2023, 16, 471–480. [Google Scholar] [CrossRef]
- Kindiger, B.; Hamann, S. Generation of haploids in maize: A modification of the indeterminate gametophyte (ig) system. Crop Sci. 1993, 33, 342–344. [Google Scholar] [CrossRef]
- Ravi, M.; Chan, S.W. Haploid plants produced by centromere-mediated genome elimination. Nature 2010, 464, 615–618. [Google Scholar] [CrossRef]
- Wang, S.; Jin, W.; Wang, K. Centromere histone H3-and phospholipase-mediated haploid induction in plants. Plant Methods 2019, 15, 42. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Wang, W.; McCuiston, J.; Zhong, H.; Nuccio, M.L.; Martin, B. Maternal haploids are preferentially induced by CENH3-tailswap transgenic complementation in maize. Front. Plant Sci. 2016, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Tan, E.H.; West, A.; Franklin, F.C.H.; Comai, L.; Chan, S.W. Naturally occurring differences in CENH3 affect chromosome segregation in zygotic mitosis of hybrids. PLoS Genet. 2015, 11, e1004970. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Ashtiyani, R.; Ishii, T.; Niessen, M.; Stein, N.; Heckmann, S.; Gurushidze, M.; Banaei-Moghaddam, A.M.; Fuchs, J.; Schubert, V.; Koch, K.; et al. Point mutation impairs centromeric CENH3 loading and induces haploid plants. Proc. Natl. Acad. Sci. USA 2015, 112, 11211–11216. [Google Scholar] [CrossRef]
- Kuppu, S.; Ron, M.; Marimuthu, M.P.; Li, G.; Huddleson, A.; Siddeek, M.H.; Terry, J.; Buchner, R.; Shabek, N.; Comai, L.; et al. A variety of changes, including CRISPR/Cas9-mediated deletions, in CENH3 lead to haploid induction on outcrossing. Plant Biotechnol. J. 2020, 18, 2068–2080. [Google Scholar] [CrossRef] [PubMed]
- Dunemann, F.; Unkel, K.; Sprink, T. Using CRISPR/Cas9 to produce haploid inducers of carrot through targeted mutations of centromeric histone H3 (CENH3). Acta Hortic. 2019, 1264, 211–220. [Google Scholar] [CrossRef]
- Lv, J.; Yu, K.; Wei, J.; Gui, H.; Liu, C.; Liang, D.; Wang, Y.; Zhou, H.; Carlin, R.; Rich, R.; et al. Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nat. Biotechnol. 2020, 38, 1397–1401. [Google Scholar] [CrossRef]
- Han, F.; Zhang, X.; Liu, Y.; Liu, Y.; Zhao, H.; Li, Z. One-step creation of CMS lines using a BoCENH3-based haploid induction system in Brassica crop. Nat. Plants 2024, 10, 581–586. [Google Scholar] [CrossRef]
- Meng, D.; Luo, H.; Dong, Z.; Huang, W.; Liu, F.; Li, F.; Chen, S.; Yu, H.; Jin, W. Overexpression of modified CENH3 in Maize Stock6-derived inducer lines can effectively improve maternal haploid induction rates. Front. Plant Sci. 2022, 13, 892055. [Google Scholar] [CrossRef]
- Impens, L.; Jacobs, T.B.; Nelissen, H.; Inzé, D.; Pauwels, L. Mini-review: Transgenerational CRISPR/Cas9 gene editing in plants. Front. Genome Ed. 2022, 4, 825042. [Google Scholar] [CrossRef]
- Bhowmik, P.; Ellison, E.; Polley, B.; Bollina, V.; Kulkarni, M.; Ghanbarnia, K.; Song, H.; Gao, C.; Voytas, D.F.; Kagale, S. Targeted mutagenesis in wheat microspores using CRISPR/Cas9. Sci. Rep. 2018, 8, 6502. [Google Scholar] [CrossRef]
- Bilichak, A.; Sastry-Dent, L.; Sriram, S.; Simpson, M.; Samuel, P.; Webb, S.; Jiang, F.; Eudes, F. Genome editing in wheat microspores and haploid embryos mediated by delivery of ZFN proteins and cell-penetrating peptide complexes. Plant Biotechnol. J. 2020, 18, 1307–1316. [Google Scholar] [CrossRef]
- Kelliher, T.; Starr, D.; Su, X.; Tang, G.; Chen, Z.; Carter, J.; Wittich, P.E.; Dong, S.; Green, J.; Burch, E.; et al. One-step genome editing of elite crop germplasm during haploid induction. Nat. Biotechnol. 2019, 37, 287–292. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, L.; Zhao, B.; Zhao, Y.; Xie, Y.; Zheng, Z.; Li, Y.; Sun, J.; Wang, H. Development of a haploid-inducer mediated genome editing system for accelerating maize breeding. Mol. Plant 2019, 12, 597–602. [Google Scholar] [CrossRef]
- Rai, A.; Dubey, K.; Han, S.S. CENH3 mediated haploid induction: Application and future perspectives in crop plants. Hortic. Environ. Biotechnol. 2023, 64, 1055–1069. [Google Scholar] [CrossRef]
- Budhagatapalli, N.; Halbach, T.; Hiekel, S.; Büchner, H.; Müller, A.E.; Kumlehn, J. Site-directed mutagenesis in bread and durum wheat via pollination by cas9/guide RNA-transgenic maize used as haploidy inducer. Plant Biotechnol. J. 2020, 18, 2376. [Google Scholar] [CrossRef]
- Li, C.; Sang, S.; Sun, M.; Yang, J.; Shi, Y.; Hu, X.; Li, Y.; Hao, M.; Chu, W.; Zhang, H.; et al. Direct modification of multiple gene homoeologs in Brassica oleracea and Brassica napus using doubled haploid inducer-mediated genome-editing system. Plant Biotechnol. J. 2021, 19, 1889. [Google Scholar] [CrossRef]
- Ye, H.; Louden, M.; Reinders, J.A. A novel in vivo genome editing doubled haploid system for Zea mays L. Nat. Plants 2024, 10, 1493–1501. [Google Scholar] [CrossRef]
- Ahmadi, B.; Ebrahimzadeh, H. In vitro androgenesis: Spontaneous vs. artificial genome doubling and characterization of regenerants. Plant Cell Rep. 2020, 39, 299–316. [Google Scholar] [CrossRef]
- Gu, X.; Liu, L.; Zhang, H. Transgene-free genome editing in plants. Front. Genome Ed. 2021, 3, 805317. [Google Scholar] [CrossRef]
- Ceasar, S.A.; Ignacimuthu, S. CRISPR/Cas genome editing in plants: Dawn of Agrobacterium transformation for recalcitrant and transgene-free plants for future crop breeding. Plant Physiol. Biochem. 2023, 196, 724–730. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by His Majesty the King in Right of Canada as represented by the National Research Council Canada. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, H.; Gao, P.; Yang, C.; Quilichini, T.D.; Kochian, L.V.; Datla, R.; Xiang, D. Advances in Genome Editing Through Haploid Induction Systems. Int. J. Mol. Sci. 2025, 26, 4779. https://doi.org/10.3390/ijms26104779
Sheng H, Gao P, Yang C, Quilichini TD, Kochian LV, Datla R, Xiang D. Advances in Genome Editing Through Haploid Induction Systems. International Journal of Molecular Sciences. 2025; 26(10):4779. https://doi.org/10.3390/ijms26104779
Chicago/Turabian StyleSheng, Huajin, Peng Gao, Changye Yang, Teagen D. Quilichini, Leon V. Kochian, Raju Datla, and Daoquan Xiang. 2025. "Advances in Genome Editing Through Haploid Induction Systems" International Journal of Molecular Sciences 26, no. 10: 4779. https://doi.org/10.3390/ijms26104779
APA StyleSheng, H., Gao, P., Yang, C., Quilichini, T. D., Kochian, L. V., Datla, R., & Xiang, D. (2025). Advances in Genome Editing Through Haploid Induction Systems. International Journal of Molecular Sciences, 26(10), 4779. https://doi.org/10.3390/ijms26104779







