Effect of Metal Additives on the Structure, Morphology, and Adsorption Characteristics of the Composites: Silicon Monoxide/Phenol–Formaldehyde-Derived Carbon
Abstract
1. Introduction
2. Results and Discussion
2.1. Thermal Decomposition of Polymeric Composites in N2 Atmosphere
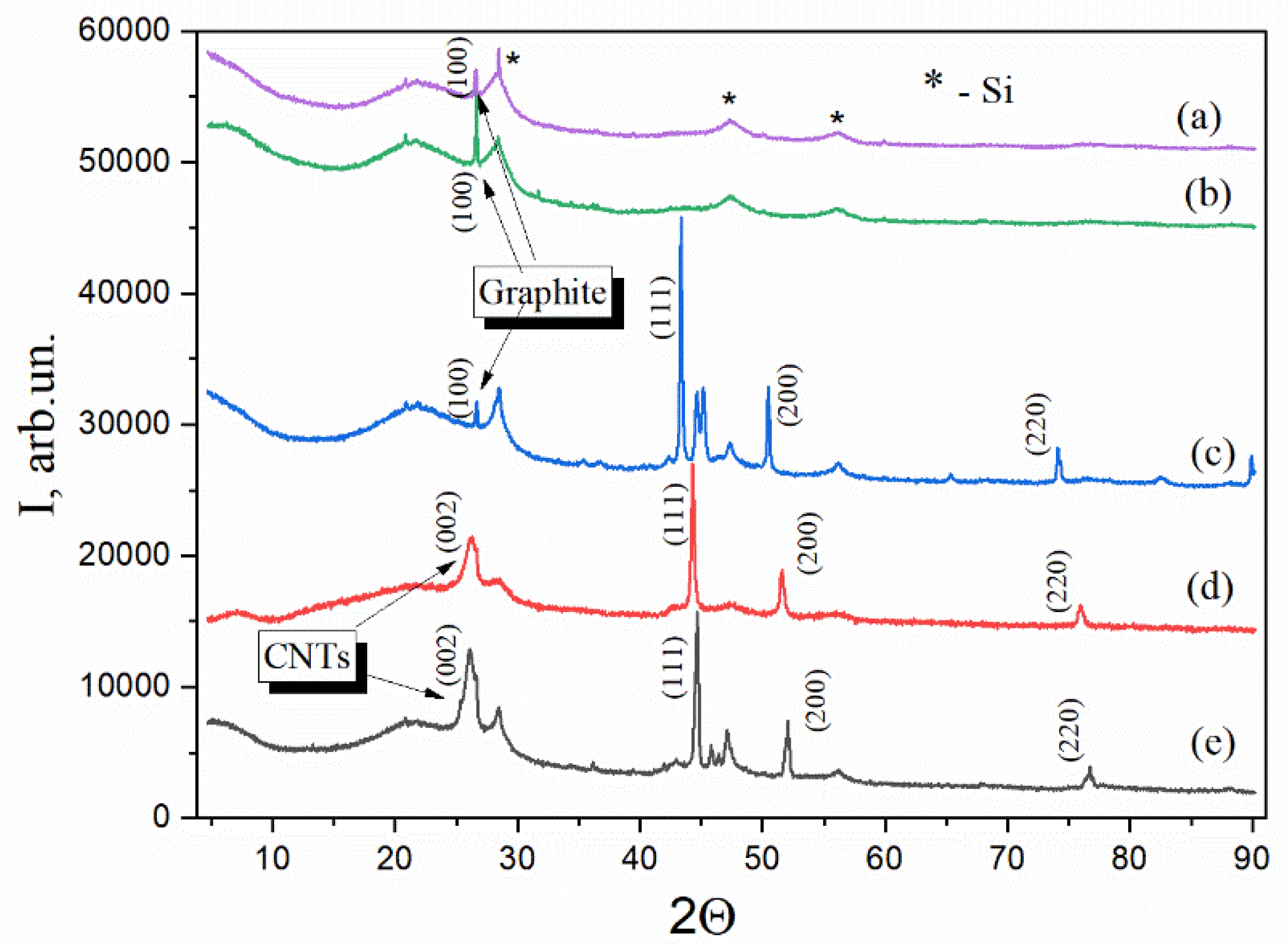
2.2. Structural Evolution of Nanocomposites Revealed by XRD
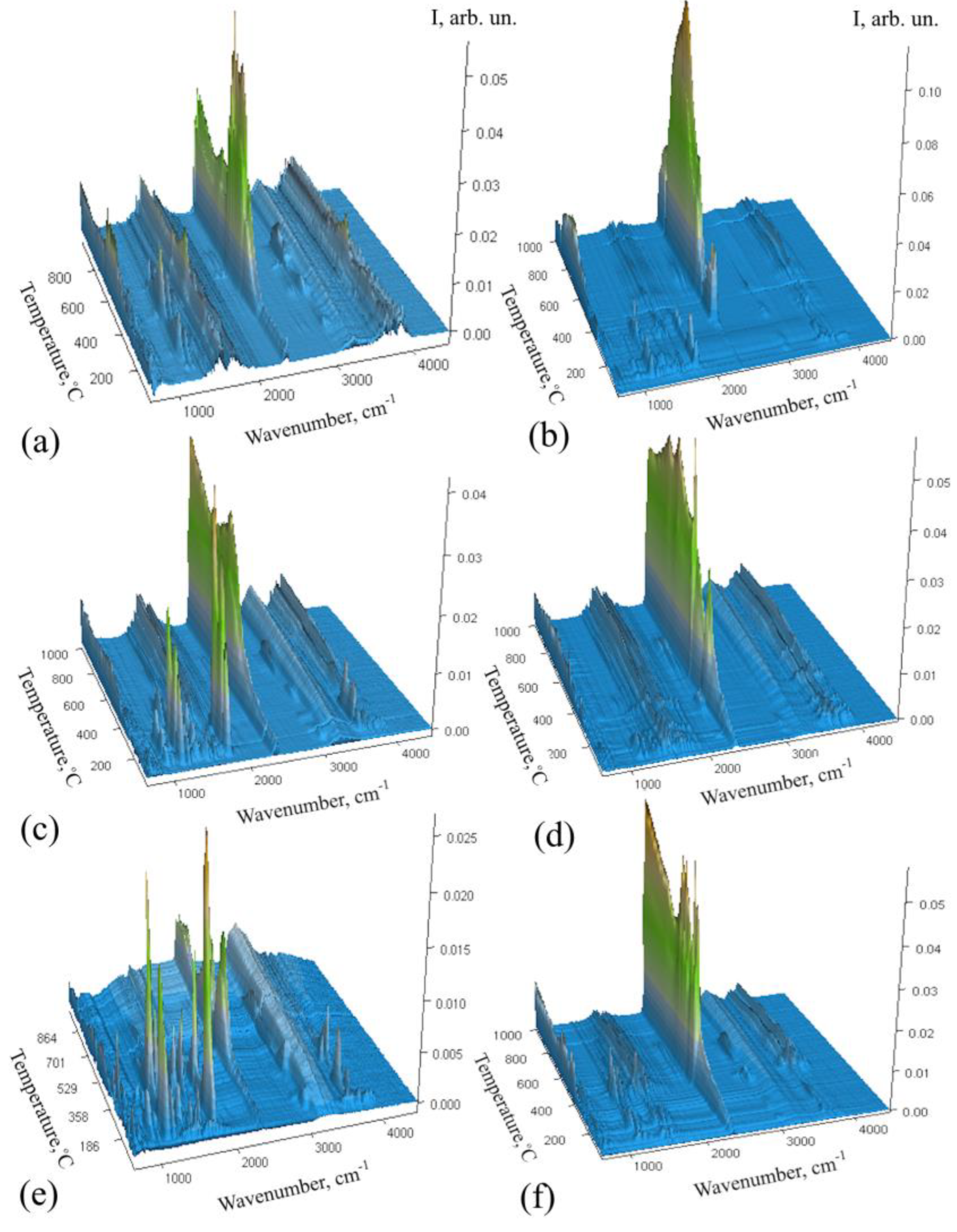
2.3. Characterization of Carbon Nanostructures via Raman Spectroscopy
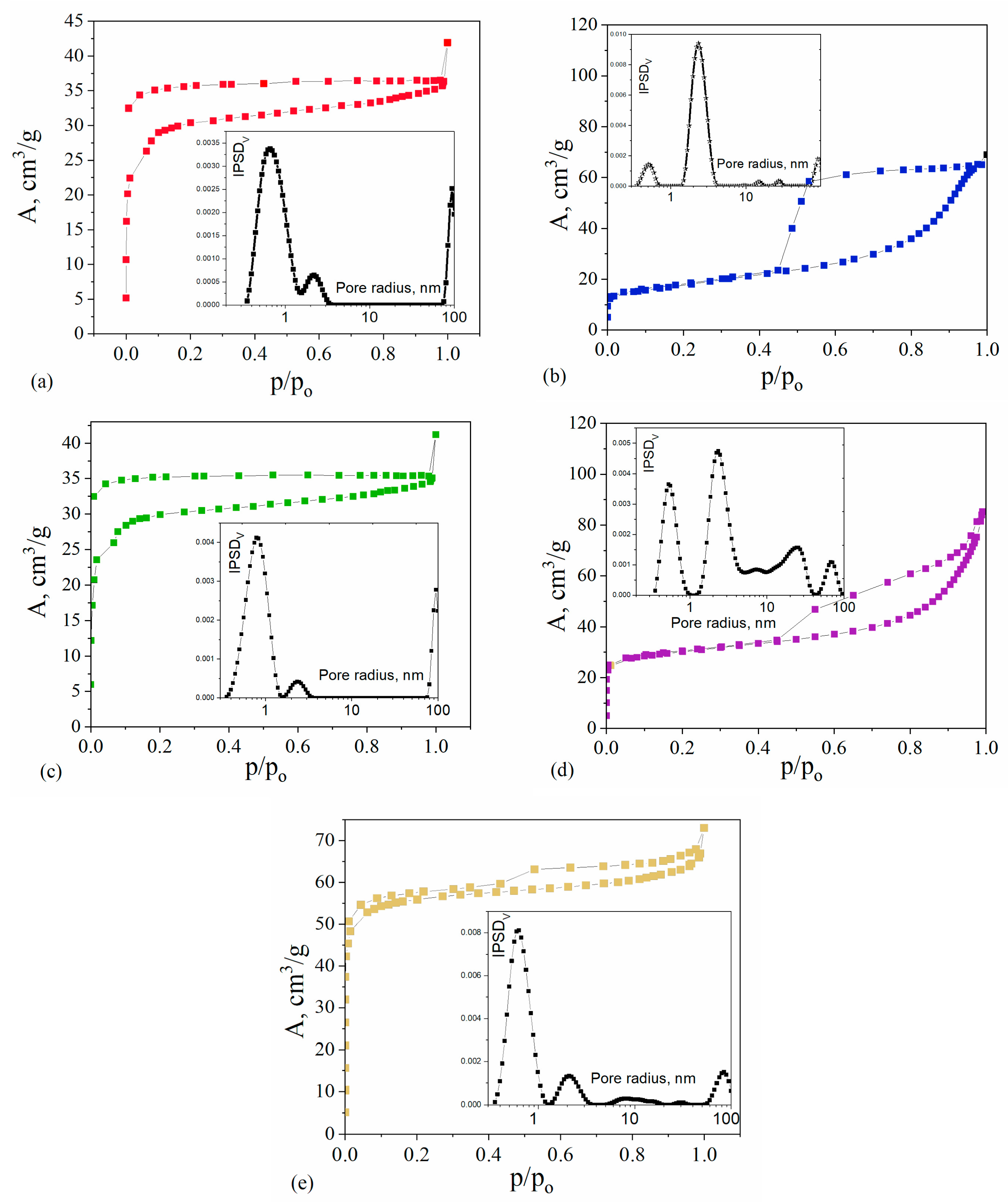
2.4. Textural Properties
2.5. SEM Analysis of Surface Texture and Microstructure
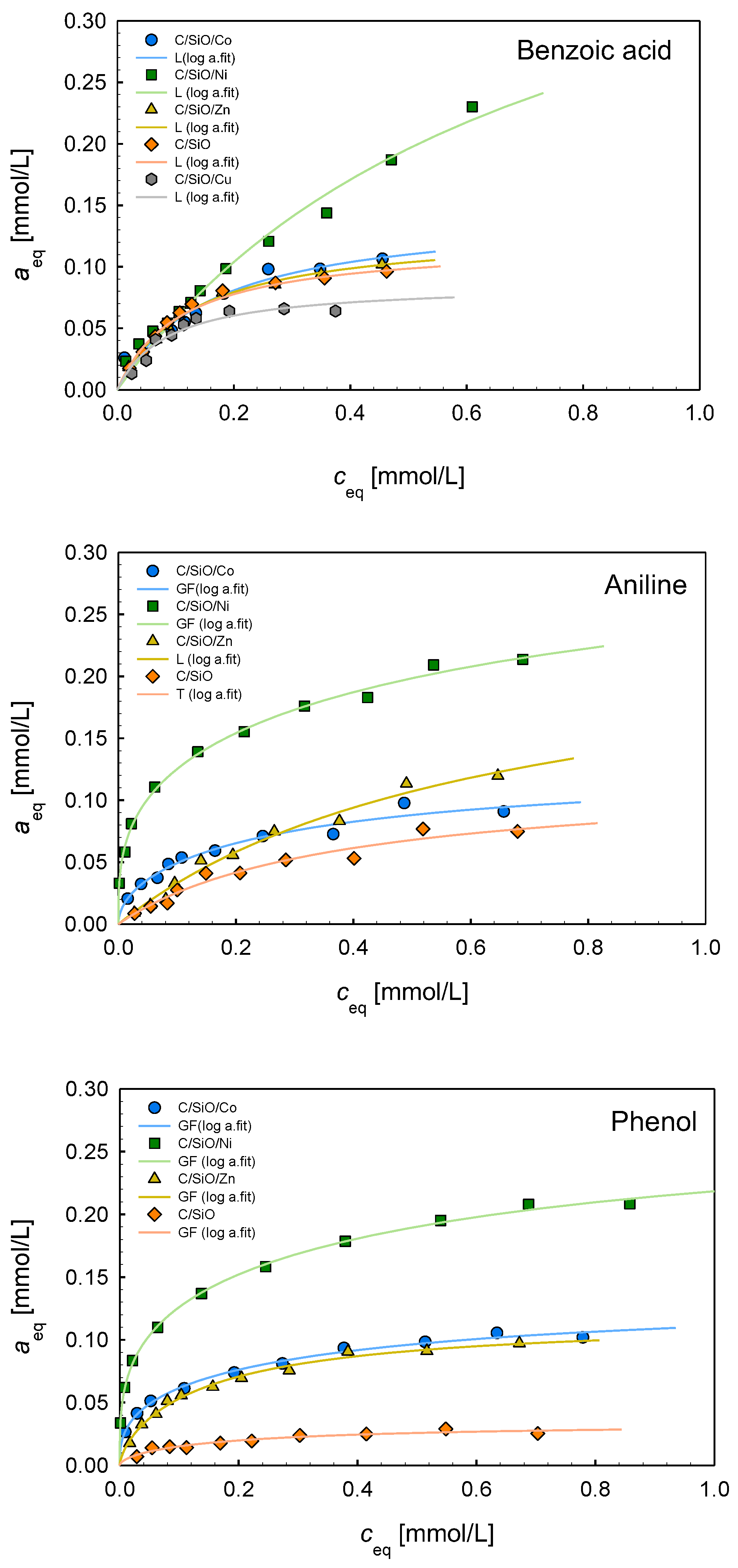
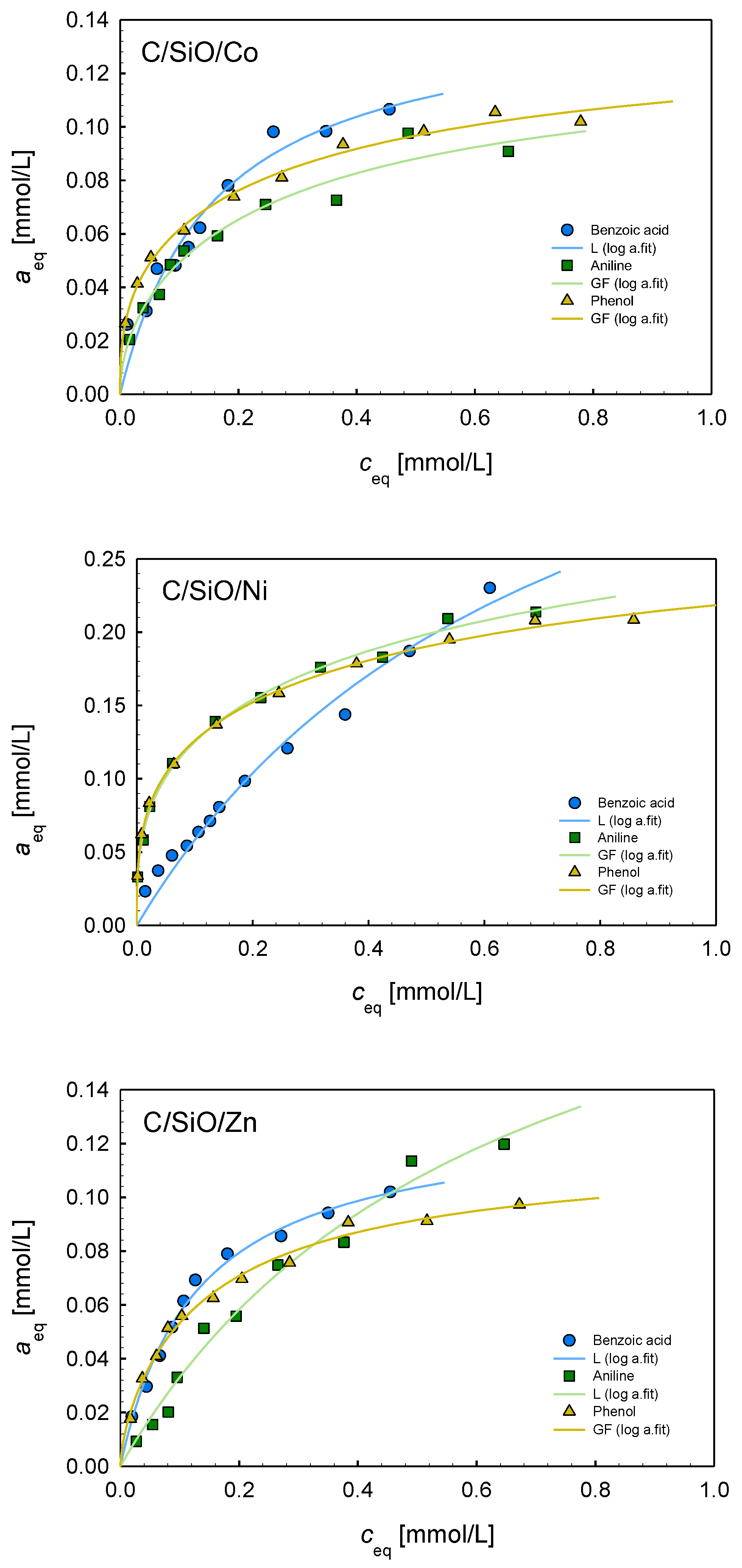
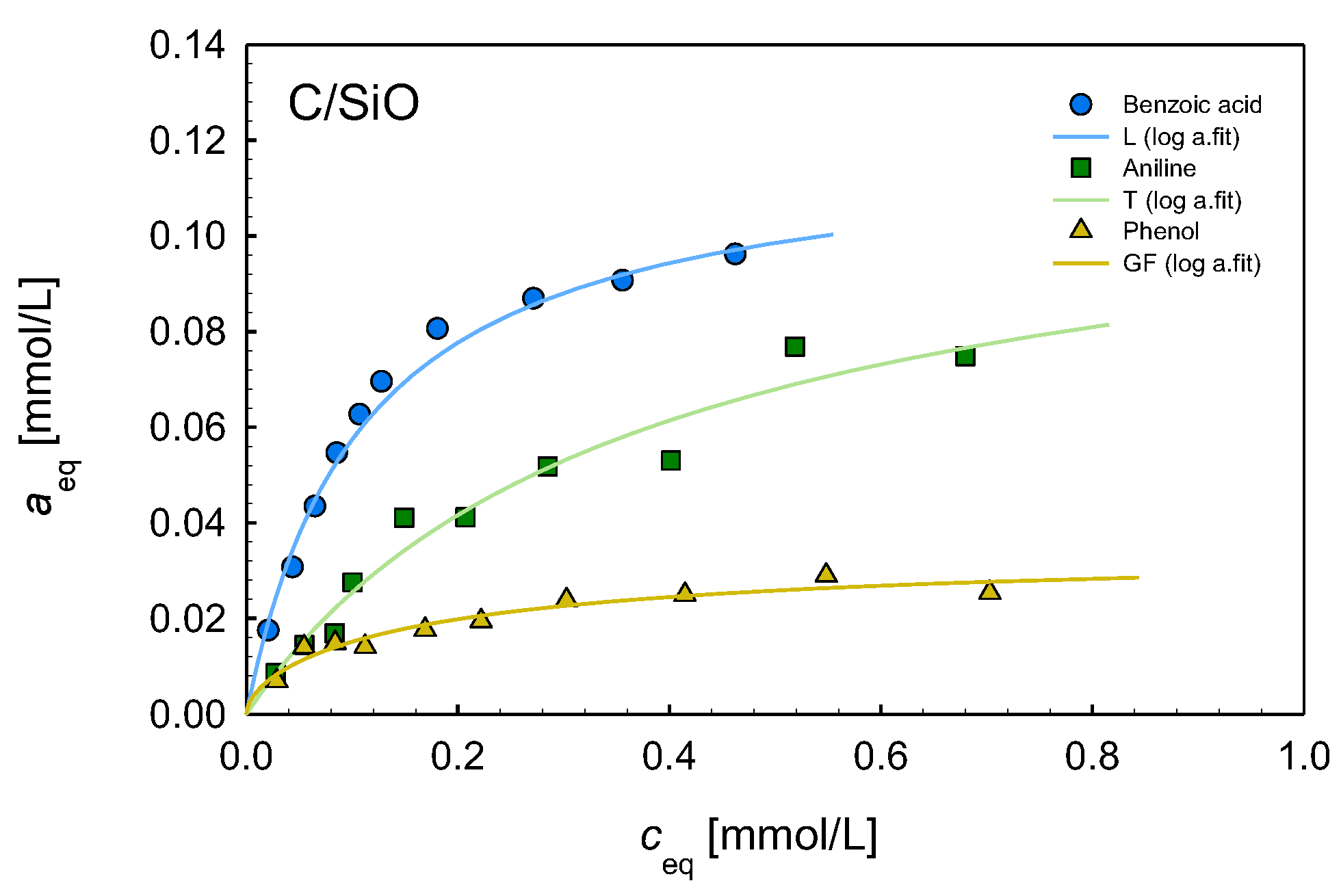
2.6. Adsorption Properties
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Nanocomposites
3.3. Characterization Methods
3.4. Adsorption from Solutions
- when the heterogeneity parameters take the values m = n ϵ (0,1), we obtain the Langmuir–Freundlich (LF) isotherm equation;
- when the heterogeneity parameters take the values n = 1 and m ϵ (0,1), we obtain the Generalized Freundlich (GF) isotherm equation;
- when the heterogeneity parameters take the values m = 1 and n ϵ (0,1), we obtain the Tóth (T) isotherm equation;
- when the heterogeneity parameters take the values m = n = 1, we obtain the Langmuir (L) isotherm equation.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saxena, V. Water Quality, Air Pollution, and Climate Change: Investigating the Environmental Impacts of Industrialization and Urbanization. Water Air Soil. Pollut. 2025, 236, 73. [Google Scholar] [CrossRef]
- Ahmed, J.; Thakur, A.; Goyal, A. Industrial Wastewater and Its Toxic Effects. In Biological Treatment of Industrial Wastewater; The Royal Society of Chemistry: Cambridge, UK, 2021; pp. 1–14. [Google Scholar]
- Shumbula, P.; Maswanganyi, C.; Shumbula, N. Type, Sources, Methods and Treatment of Organic Pollutants in Wastewater; InTechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Al-Khalid, T.; Surkatti, R.; El-Naas, M.H. Organic Contaminants in Industrial Wastewater: Prospects of Waste Management by Integrated Approaches. In Combined Application of Physico-Chemical & Microbiological Processes for Industrial Effluent Treatment Plant; Springer: Singapore, 2020; pp. 205–235. [Google Scholar]
- Iyiola, A.O.; Ipinmoroti, M.O.; Akingba, O.O.; Ewutanure, J.S.; Setufe, S.B.; Bilikoni, J.; Ofori-Boateng, E.; Wangboje, O.M. Organic Chemical Pollutants Within Water Systems and Sustainable Management Strategies. In Water Crises and Sustainable Management in the Global South; Springer Nature: Singapore, 2024; pp. 211–251. [Google Scholar]
- Jani, Y. Adsorption: A Cost-Effective Wastewater Treatment Technology for Removal of Conventional and Emerging Organic Contaminants; Springer: Cham, Switzerland, 2022; pp. 17–33. [Google Scholar]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Jiang, X.; Liu, X.; Zhou, W.; Garba, Z.N.; Lawan, I.; Wang, L.; Yuan, Z. Adsorption of Organic Dyes from Wastewater by Metal-Doped Porous Carbon Materials. J. Clean. Prod. 2021, 284, 124773. [Google Scholar] [CrossRef]
- Kumar, P. Synthesis Methods for Carbon-Based Materials. In Handbook on Synthesis Strategies for Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 367–420. [Google Scholar]
- Devi, M.; Rawat, S.; Sharma, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxf. Open Mater. Sci. 2020, 1, itab014. [Google Scholar] [CrossRef]
- Manawi, Y.M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M.A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11, 822. [Google Scholar] [CrossRef]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of Organic and Inorganic Pollutants Removal by Biochar and Biochar-Based Composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Galaburda, M.; Bosacka, A.; Sternik, D.; Bogatyrov, V.; Oranska, O.; Gun’ko, V.; Deryło-Marczewska, A. Development, Synthesis and Characterization of Tannin/Bentonite-Derived Biochar for Water and Wastewater Treatment from Methylene Blue. Water 2022, 14, 2407. [Google Scholar] [CrossRef]
- Galaburda, M.; Bosacka, A.; Sternik, D.; Oranska, O.; Borysenko, M.; Gun’ko, V.; Derylo-Marczewska, A. Physicochemical and Sorption Characteristics of Carbon Biochars Based on Lignin and Industrial Waste Magnetic Iron Dust. Water 2023, 15, 189. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Bogatyrov, V.M.; Oranska, O.I.; Galaburda, M.V.; Polshin, E.V.; Urubkov, I.V.; Leboda, R.; Skubiszewska-Zięba, J.; Charmas, B. Effect of Nanosilica on Characteristics of Carbonizates of Phenol-Formaldehyde Resin—Fe(Acac)3. Appl. Surf. Sci. 2013, 264, 707–712. [Google Scholar] [CrossRef]
- Galaburda, M.V.; Bogatyrov, V.M.; Tomaszewski, W.; Oranska, O.I.; Borysenko, M.V.; Skubiszewska-Zięba, J.; Gun’ko, V.M. Adsorption/Desorption of Explosives on Ni-, Co-, and NiCo-Carbon Composites: Application in Solid Phase Extraction. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 950–958. [Google Scholar] [CrossRef]
- Xu, L.; Zhong, Q.; Dong, Q.; Zhang, L.; Fang, Z. Co-Production of Phenolic Oil and CaO/Char Deoxidation Catalyst via Catalytic Fast Pyrolysis of Phenol-Formaldehyde Resin with Ca(OH)2. J. Anal. Appl. Pyrolysis 2019, 142, 104663. [Google Scholar] [CrossRef]
- Gałko, G.; Sajdak, M. Trends for the Thermal Degradation of Polymeric Materials: Analysis of Available Techniques, Issues, and Opportunities. Appl. Sci. 2022, 12, 9138. [Google Scholar] [CrossRef]
- Hunter, R.D.; Ramírez-Rico, J.; Schnepp, Z. Iron-Catalyzed Graphitization for the Synthesis of Nanostructured Graphitic Carbons. J. Mater. Chem. A Mater. 2022, 10, 4489–4516. [Google Scholar] [CrossRef]
- Galaburda, M.; Skubiszewska-Zięba, J.; Gun’ko, V.; Urubkov, I.; Bogatyrov, V.; Oranska, O. Synthesis and Characterization of Carbon Composites Containing Fe, Co, Ni Nanoparticles. J. Therm. Anal. Calorim. 2015, 122, 553–561. [Google Scholar] [CrossRef]
- Yakovenko, O.S.; Matzui, L.Y.; Vovchenko, L.L.; Bogatyrov, V.M.; Galaburda, M.V.; Bodnaruk, A.V.; Kalita, V.M.; Syvolozhskyi, O.A.; Vilchinskyi, V.V. Electric Properties of Ni-C and Co-C Core–Shell Nanoparticles in Polymer Matrix. Mol. Cryst. Liq. Cryst. 2021, 718, 132–141. [Google Scholar] [CrossRef]
- Galaburda, M.; Kovalska, E.; Hogan, B.T.; Baldycheva, A.; Nikolenko, A.; Dovbeshko, G.I.; Oranska, O.I.; Bogatyrov, V.M. Mechanochemical Synthesis of Carbon-Stabilized Cu/C, Co/C and Ni/C Nanocomposites with Prolonged Resistance to Oxidation. Sci. Rep. 2019, 9, 17435. [Google Scholar] [CrossRef]
- Keller, T.M.; Laskoski, M.; Qadri, S.B. Ferrocene Catalyzed Carbon Nanotube Formation in Carbonaceous Solid. J. Phys. Chem. C 2007, 111, 2514–2519. [Google Scholar] [CrossRef]
- Díez, N.; Sevilla, M.; Fuertes, A.B. Synthesis Strategies of Templated Porous Carbons beyond the Silica Nanocasting Technique. Carbon N. Y 2021, 178, 451–476. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, B.; Guan, D.; Dong, X. Mesoporous Activated Carbon Spheres Derived from Resorcinol-Formaldehyde Resin with High Performance for Supercapacitors. J. Solid. State Electrochem. 2015, 19, 1783–1791. [Google Scholar] [CrossRef]
- Teng, H.; Wang, S.-C. Influence of Oxidation on the Preparation of Porous Carbons from Phenol−Formaldehyde Resins with KOH Activation. Ind. Eng. Chem. Res. 2000, 39, 673–678. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Chen, S. Catalytic Effects of Different Metal Salts on Cotton Waste Textiles by Pyrolysis: Pyrolysis Behavior and Properties of Activated Carbon. Textiles 2025, 5, 4. [Google Scholar] [CrossRef]
- Pego, M.; Carvalho, J.; Guedes, D. Surface Modifications of Activated Carbon and Its Impact on Application. Surf. Rev. Lett. 2019, 26, 1830006. [Google Scholar] [CrossRef]
- Rehman, A.; Park, M.; Park, S.-J. Current Progress on the Surface Chemical Modification of Carbonaceous Materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef]
- Mojoudi, N.; Mirghaffari, N.; Soleimani, M.; Shariatmadari, H.; Belver, C.; Bedia, J. Phenol Adsorption on High Microporous Activated Carbons Prepared from Oily Sludge: Equilibrium, Kinetic and Thermodynamic Studies. Sci. Rep. 2019, 9, 19352. [Google Scholar] [CrossRef] [PubMed]
- Pardo, B.; Ferrer, N.; Sempere, J.; Gonzalez-Olmos, R. A Key Parameter on the Adsorption of Diluted Aniline Solutions with Activated Carbons: The Surface Oxygen Content. Chemosphere 2016, 162, 181–188. [Google Scholar] [CrossRef]
- Qin, H.; Xiao, R.; Zhang, R.; Chen, J. Efficient Adsorption of Benzoic Acid from Aqueous Solution by Nitrogen-Containing Activated Carbon. Water Sci. Technol. 2018, 2017, 686–694. [Google Scholar] [CrossRef]
- Li, S.; De Silva, T.; Arsano, I.; Gallaba, D.; Karunanithy, R.; Wasala, M.; Zhang, X.; Sivakumar, P.; Migone, A.; Tsige, M.; et al. High Adsorption of Benzoic Acid on Single Walled Carbon Nanotube Bundles. Sci. Rep. 2020, 10, 10013. [Google Scholar] [CrossRef]
- Obaid, A.Y.; Alyoubi, A.O.; Samarkandy, A.A.; Al-Thabaiti, S.A.; Al-Juaid, S.S.; El-Bellihi, A.A.; Deifallah, E.-H.M. Kinetics of Thermal Decomposition of Copper(II) Acetate Monohydrate. J. Therm. Anal. Calorim. 2000, 61, 985–994. [Google Scholar] [CrossRef]
- Bouajila, J.; Raffin, G.; Alamercery, S.; Waton, H.; Sanglar, C.; Grenier-Loustalot, M.F. Phenolic Resins (IV). Thermal Degradation of Crosslinked Resins in Controlled Atmospheres. Polym. Polym. Compos. 2003, 11, 345–357. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Wu, S.; Yuan, Z.; Hu, Z.; Wu, R.; Liu, Q. The Pyrolysis Mechanism of Phenol Formaldehyde Resin. Polym. Degrad. Stab. 2012, 97, 1527–1533. [Google Scholar] [CrossRef]
- Wang, D.; Ding, J.; Wang, B.; Zhuang, Y.; Huang, Z. Synthesis and Thermal Degradation Study of Polyhedral Oligomeric Silsesquioxane (POSS) Modified Phenolic Resin. Polymers 2021, 13, 1182. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A.; Ibeh, C.C. Phenol-Formaldehyde Resins. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 13–40. [Google Scholar]
- Ahamad, T.; Alshehri, S.M. Thermal Degradation and Evolved Gas Analysis of Epoxy (DGEBA)/Novolac Resin Blends (ENB) during Pyrolysis and Combustion. J. Therm. Anal. Calorim. 2013, 111, 445–451. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Nanda, S.; Dalai, A.K. Carbon Nanotubes: A Review of Synthesis Methods and Applications. Reactions 2024, 5, 429–451. [Google Scholar] [CrossRef]
- Grabda, M.; Oleszek-Kudlak, S.; Shibata, E.; Nakamura, T. Vaporization of Zinc during Thermal Treatment of ZnO with Tetrabromobisphenol A (TBBPA). J. Hazard. Mater. 2011, 187, 473–479. [Google Scholar] [CrossRef]
- Wang, L.; Xue, G.; Ye, T.; Li, J.; Liu, C.; Liu, J.; Ma, P. Zn-Modified Biochar Preparation from Solvent Free in-Situ Pyrolysis and Its Removal of Methylene Blue. Diam. Relat. Mater. 2023, 140, 110438. [Google Scholar] [CrossRef]
- Jorio, A.; Cançado, L.G. Disorder and Defects in Two-Dimensional Materials Probed by Raman Spectroscopy. In Raman Spectroscopy of Two-Dimensional Materials; Tan, P.-H., Ed.; Springer: Singapore, 2019; Volume 276, pp. 99–110. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Liu, J.; Wu, L.; Hu, Y.; Zhang, Q.; Liu, Y.; Shu, S.; Li, Z.; Xu, J.; Zhang, J. Synthesis and Carbonization of Nickel-Modified Thermoplastic Phenolic Resin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 423, 012080. [Google Scholar] [CrossRef]
- Galaburda, M.V.; Bogatyrov, V.M.; Skubiszewska-ZiĿba, J.; Oranska, O.I.; Sternik, D.; Gunko, V.M. Synthesis and Structural Features of Resorcinol-Formaldehyde Resin Chars Containing Nickel Nanoparticles. Appl. Surf. Sci. 2016, 360, 722–730. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, L.; Zhang, L.; Tang, D.-M.; Ma, R.; Wang, Y.; Zhang, B.; Ding, F.; Liu, C.; Cheng, H.-M. Precise Identification of the Active Phase of Cobalt Catalyst for Carbon Nanotube Growth by In Situ Transmission Electron Microscopy. ACS Nano 2020, 14, 16823–16831. [Google Scholar] [CrossRef]
- Jang, L.-W.; Shim, J.; Son, D.I.; Cho, H.; Zhang, L.; Zhang, J.; Menghini, M.; Locquet, J.-P.; Seo, J.W. Simultaneous Growth of Three-Dimensional Carbon Nanotubes and Ultrathin Graphite Networks on Copper. Sci. Rep. 2019, 9, 12344. [Google Scholar] [CrossRef]
- Mahmoodinia, M.; Farooq, H.; Røe, T.; Svenum, I.-H.; Venvik, H.J. Effect of Copper Catalyst Content and Zinc Promoter on Carbon Formation in the Direct Synthesis of Methylchlorosilanes. Ind. Eng. Chem. Res. 2023, 62, 21579–21589. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Jaroniec, M. A New Isotherm Equation for Single-Solute Adsorption from Dilute Solutions on Energetically Heterogeneous Solids. Monatshefte Fur Chem. Chem. Mon. 1983, 114, 711–715. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Gun’ko, V.M. Composite Materials: Textural Characteristics. Appl. Surf. Sci. 2014, 307, 444–454. [Google Scholar] [CrossRef]
- Gun’ko, V.M.; Mikhalovsky, S.V. Evaluation of Slitlike Porosity of Carbon Adsorbents. Carbon N. Y. 2004, 42, 843–849. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Swiatkowski, A.; Grajek, H.; Biniak, S.; Witkiewicz, Z. Changes in the Surface Chemistry and Adsorptive Properties of Active Carbon Previously Oxidized and Heat-Treated at Various Temperatures. III. Studies of the Adsorption of Organic Solutes from Aqueous Solutions. Adsorpt. Sci. Technol. 2005, 23, 867–879. [Google Scholar] [CrossRef]
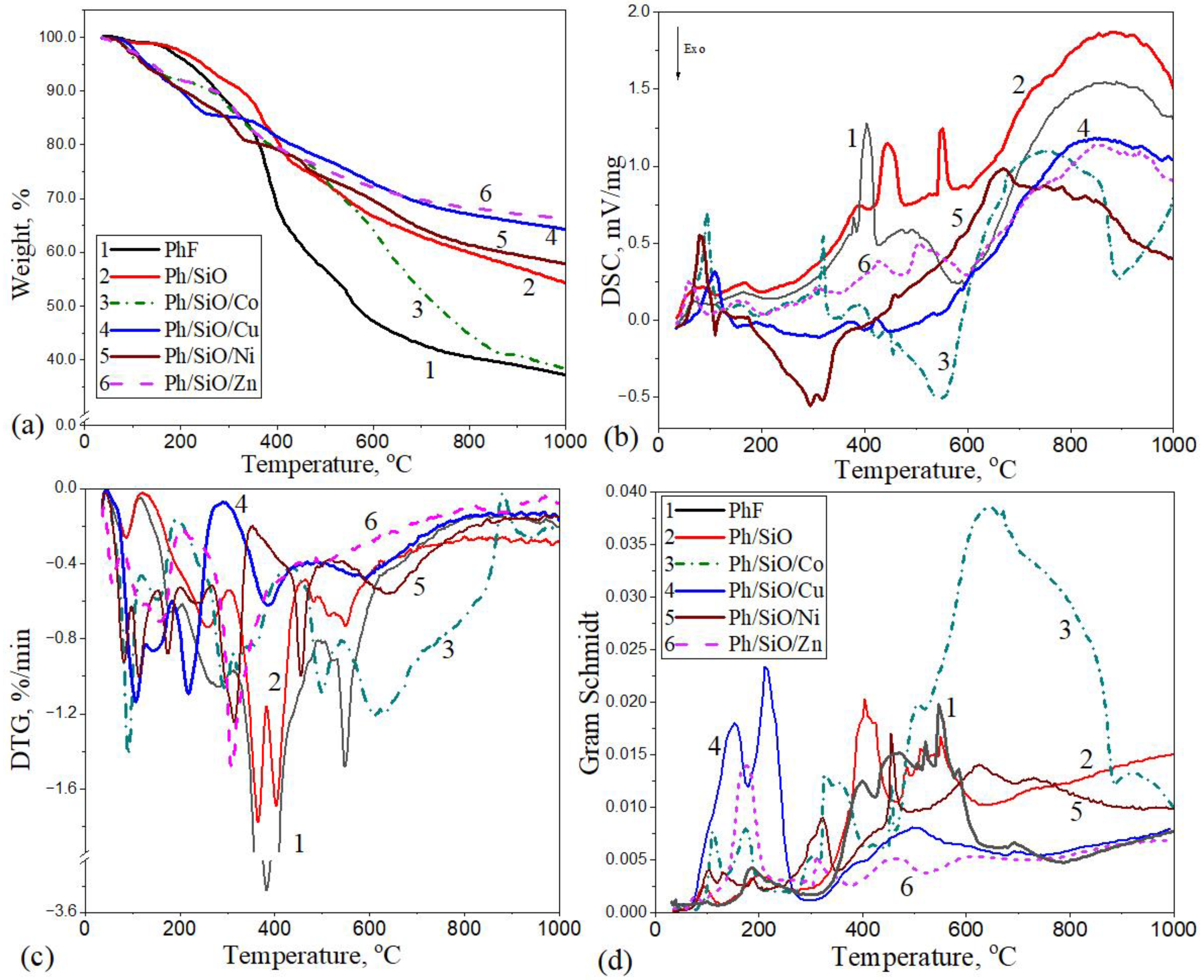


| Sample | Temperature Range °C | Mass Loss % | Δmtotal % | TmaxDTG °C | TmaxDSC; (Endo↑; Exo↓) °C |
|---|---|---|---|---|---|
| PFR | 36–116 | 1.02 | 62.87 | 85.9 | 87.4↑ |
| 116–205 | 3.22 | 183.2 | 168.7↑ | ||
| 205–315 | 9.60 | 283.5 | |||
| 315–495 | 29.05 | 383.5 | 393.2↑; 445.8↑ | ||
| 495–626 | 11.34 | 548.1 | 555.4↑ | ||
| 626–1000 | 8.64 | - | 878.7↑ | ||
| PFR/SiO | 36–120 | 0.96 | 45.54 | 86.9 | 70.3↑ |
| 120–306 | 7.69 | 258 | 166↑ | ||
| 306–384 | 8.33 | 364.9 | 373.7↑; 381.7↑ | ||
| 384–462 | 7.84 | 402.8 | 406.8↑ | ||
| 462–1000 | 20.72 | 483.5; 516.6; 548.6 | 473.3↑; 867↑ | ||
| PFR/SiO/Co | 36–120 | 4.69 | 61.47 | 91.7 | 95.1↑ |
| 120–192 | 3.06 | 152.9 | 157.9↑ | ||
| 192–312 | 6.31 | 295.6 | 301↑; 320.9↑ | ||
| 312–440 | 8.28 | 321 | 385.9↑ | ||
| 440–542 | 7.86 | 499.5 | 456↑ | ||
| 542–881 | 28.72 | 609.6; 633.2 | 547.1↑ | ||
| 881–1000 | 2.55 | 949.3 | 761.5↑ | ||
| PFR/SiO/Cu | 36–130 | 4.71 | 35.64 | 106.6 | 110.9↑ |
| 130–185 | 4.13 | 144.5 | |||
| 185–293 | 5.76 | 218.1 | 189↑; 218.1↑ | ||
| 293–491 | 7.65 | 386.2 | 381.4↑; 422.5↑ | ||
| 491–1000 | 13.39 | 590.6 | 857.8↑ | ||
| PFR/SiO/Ni | 36–98 | 2.77 | 41.98 | 82.3 | 82.3↑ |
| 98–152 | 3.80 | 114.1 | 124.9↑ | ||
| 152–202 | 3.08 | 174.8 | |||
| 202–268 | 3.58 | 233.4 | |||
| 268–352 | 6.23 | 314.8 | 294.5↑; 318.9↑ | ||
| 352–514 | 7.08 | 455.5 | 458↑ | ||
| 514–1000 | 15.44 | 633.6 | 667.8↑ | ||
| PFR/SiO/Zn | 36–80 | 1.71 | 33.49 | 57.1 | 58.8↑ |
| 80–204 | 6.21 | 120.5;151.8 | 150.7↑; 165.7↑ | ||
| 204–471 | 15.36 | 309.4 | 309↑; 426.7↑ | ||
| 471–821 | 8.53 | 499.4 | 505↑ | ||
| 821–1000 | 1.68 | 888.7 | 847.5↑ |
| Sample | D | G | 2D | FWHM D | FWHM G | IG/D (Area Integral) |
|---|---|---|---|---|---|---|
| C/SiO/Co | 1308.41 | 1594.3 | 2617 | 69.1 | 62.2 | (0.97) |
| C/SiO/Cu | 1309.71 | 1593.1 | 119.6 | 63.5 | (0.59) | |
| C/SiO/Ni | 1305.51 | 1590.2 | 2610 | 101.1 | 63.2 | (0.85) |
| C/SiO/Zn | 1299 | 1592.2 | 131 | 70.2 | (0.43) | |
| C/SiO | 1302 | 1592 | 121.4 | 67.7 | (0.45) |
| Sample | SBET m2/g | Smicro m2/g | Smeso m2/g | Smacro m2/g | Vp cm3/g | Vmicro cm3/g | Vmeso cm3/g | Vmacro cm3/g | Vmicro/Vp | Vmeso/Vp |
|---|---|---|---|---|---|---|---|---|---|---|
| C/SiO/Co | 61 | 20.5 | 40.3 | 0.1 | 0.108 | 0.010 | 0.090 | 0.008 | 0.090 | 0.834 |
| C/SiO/Cu | 95 | 86.6 | 8.4 | 0.1 | 0.063 | 0.045 | 0.009 | 0.009 | 0.721 | 0.139 |
| C/SiO/Ni | 103 | 71.4 | 31.1 | 0.4 | 0.132 | 0.032 | 0.086 | 0.014 | 0.246 | 0.651 |
| C/SiO/Zn | 189 | 179.7 | 9.0 | 0.2 | 0.112 | 0.085 | 0.017 | 0.010 | 0.755 | 0.154 |
| C/SiO | 105 | 96.6 | 8.7 | 0.1 | 0.063 | 0.045 | 0.010 | 0.008 | 0.710 | 0.157 |
| Element Composition (wt%) | |||||
|---|---|---|---|---|---|
| Sample | C | O | Si | Metal | Total |
| C/SiO/Co | 74.5 | 7.78 | 9.15 | 8.57 | 100 |
| C/SiO/Cu | 81.93 | 3.17 | 6.93 | 7.97 | 100 |
| C/SiO/Ni | 73.89 | 8.13 | 9.15 | 8.83 | 100 |
| C/SiO/Zn | 88.65 | 5.13 | 6.22 | - | 100 |
| C/SiO | 79.27 | 11.50 | 9.23 | - | 100 |
| Composite | Isotherm Type | am | m | n | log K | R2 | SD(a) |
|---|---|---|---|---|---|---|---|
| Benzoic acid | |||||||
| C/SiO/Co | L | 0.14 | 1 | 1 | 0.79 | 0.941 | 0.007 |
| C/SiO/Ni | L | 0.45 | 1 | 1 | 0.14 | 0.979 | 0.009 |
| C/SiO/Zn | L | 0.13 | 1 | 1 | 0.89 | 0.989 | 0.003 |
| C/SiO | L | 0.12 | 1 | 1 | 0.96 | 0.977 | 0.005 |
| C/SiO/Cu | L | 0.08 | 1 | 1 | 1.06 | 0.928 | 0.005 |
| Aniline | |||||||
| C/SiO/Co | GF | 0.12 | 0.51 | 1 | 0.24 | 0.963 | 0.005 |
| C/SiO/Ni | GF | 0.33 | 0.32 | 1 | −0.32 | 0.996 | 0.004 |
| C/SiO/Zn | L | 0.19 | 1 | 1 | 0.20 | 0.982 | 0.006 |
| C/SiO | T | 0.11 | 1 | 0.95 | 0.44 | 0.958 | 0.005 |
| Phenol | |||||||
| C/SiO/Co | GF | 0.13 | 0.36 | 1 | 0.07 | 0.992 | 0.002 |
| C/SiO/Ni | GF | 0.28 | 0.29 | 1 | −0.17 | 0.998 | 0.003 |
| C/SiO/Zn | GF | 0.12 | 0.65 | 1 | 0.63 | 0.990 | 0.003 |
| C/SiO | GF | 0.03 | 0.56 | 1 | 0.48 | 0.931 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galaburda, M.; Chrzanowska, A.; Sternik, D.; Zienkiewicz-Strzalka, M.; Derylo-Marczewska, A. Effect of Metal Additives on the Structure, Morphology, and Adsorption Characteristics of the Composites: Silicon Monoxide/Phenol–Formaldehyde-Derived Carbon. Int. J. Mol. Sci. 2025, 26, 4770. https://doi.org/10.3390/ijms26104770
Galaburda M, Chrzanowska A, Sternik D, Zienkiewicz-Strzalka M, Derylo-Marczewska A. Effect of Metal Additives on the Structure, Morphology, and Adsorption Characteristics of the Composites: Silicon Monoxide/Phenol–Formaldehyde-Derived Carbon. International Journal of Molecular Sciences. 2025; 26(10):4770. https://doi.org/10.3390/ijms26104770
Chicago/Turabian StyleGalaburda, Mariia, Agnieszka Chrzanowska, Dariusz Sternik, Malgorzata Zienkiewicz-Strzalka, and Anna Derylo-Marczewska. 2025. "Effect of Metal Additives on the Structure, Morphology, and Adsorption Characteristics of the Composites: Silicon Monoxide/Phenol–Formaldehyde-Derived Carbon" International Journal of Molecular Sciences 26, no. 10: 4770. https://doi.org/10.3390/ijms26104770
APA StyleGalaburda, M., Chrzanowska, A., Sternik, D., Zienkiewicz-Strzalka, M., & Derylo-Marczewska, A. (2025). Effect of Metal Additives on the Structure, Morphology, and Adsorption Characteristics of the Composites: Silicon Monoxide/Phenol–Formaldehyde-Derived Carbon. International Journal of Molecular Sciences, 26(10), 4770. https://doi.org/10.3390/ijms26104770










