Adsorption of Pb2+ and Cd2+ from Aqueous Solutions by Porous Carbon Foam Derived from Biomass Phenolic Resin
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Two Carbon Foams
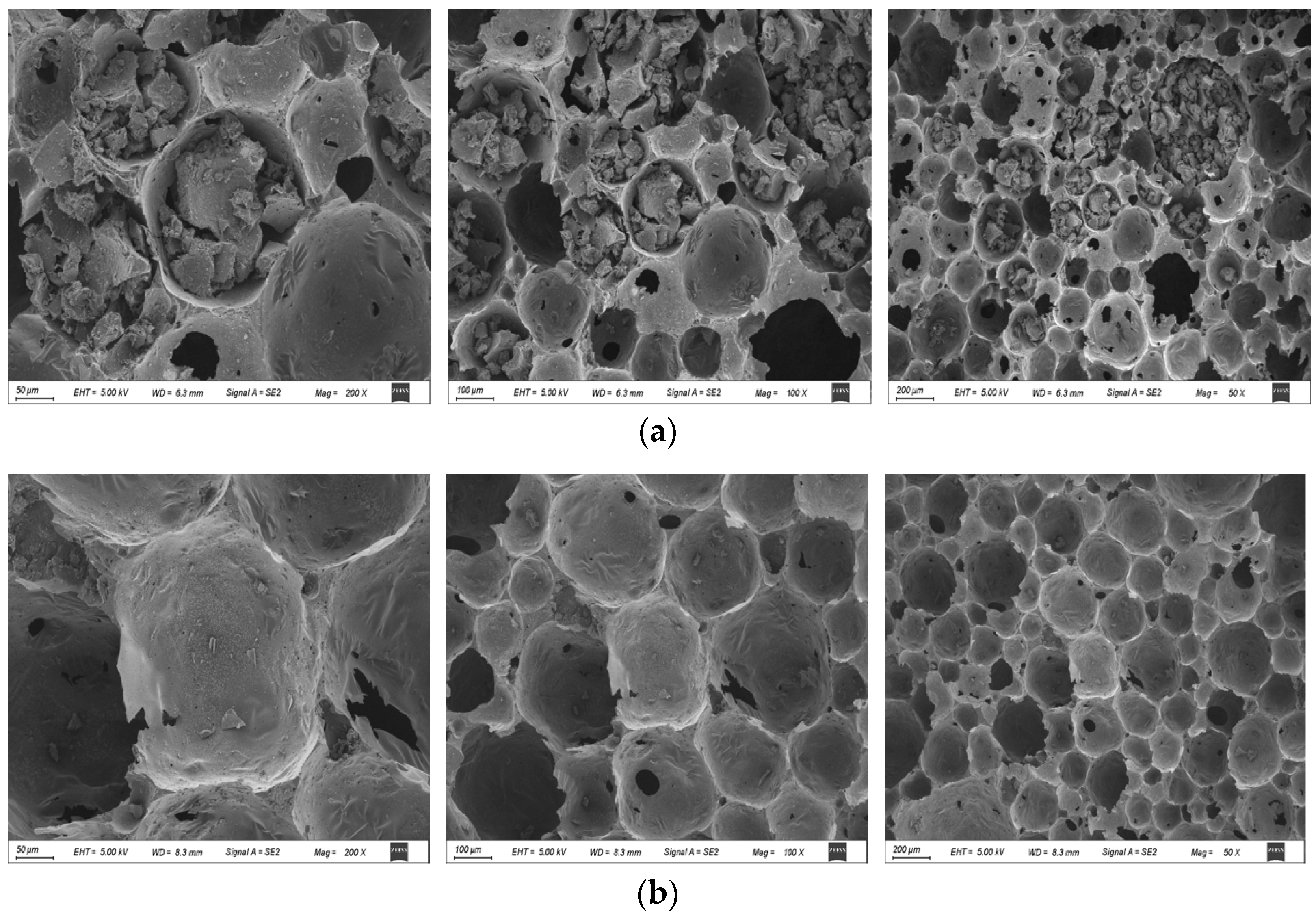
2.1.1. SEM Images of U-CF and M-CF
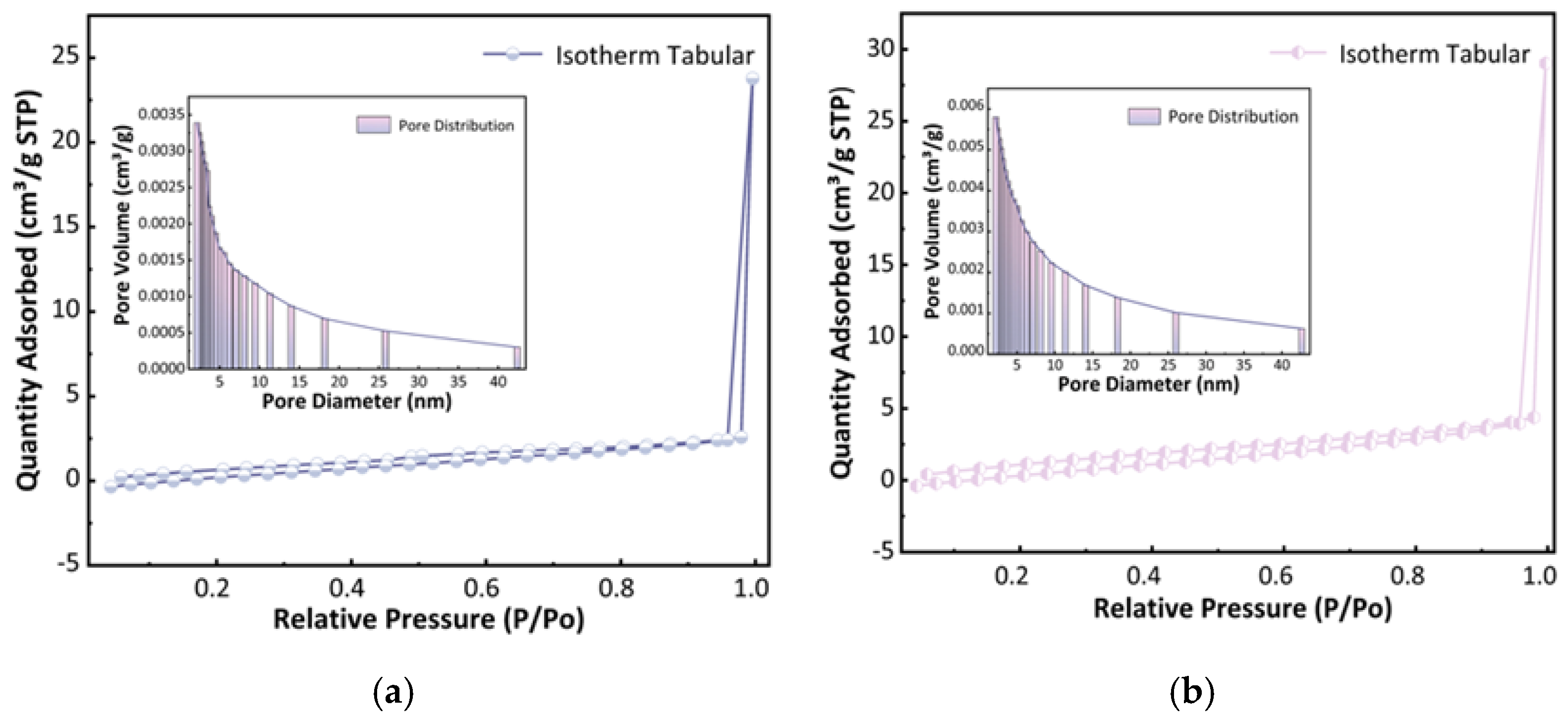
2.1.2. Porosity Structure Analysis of U-CF and M-CF
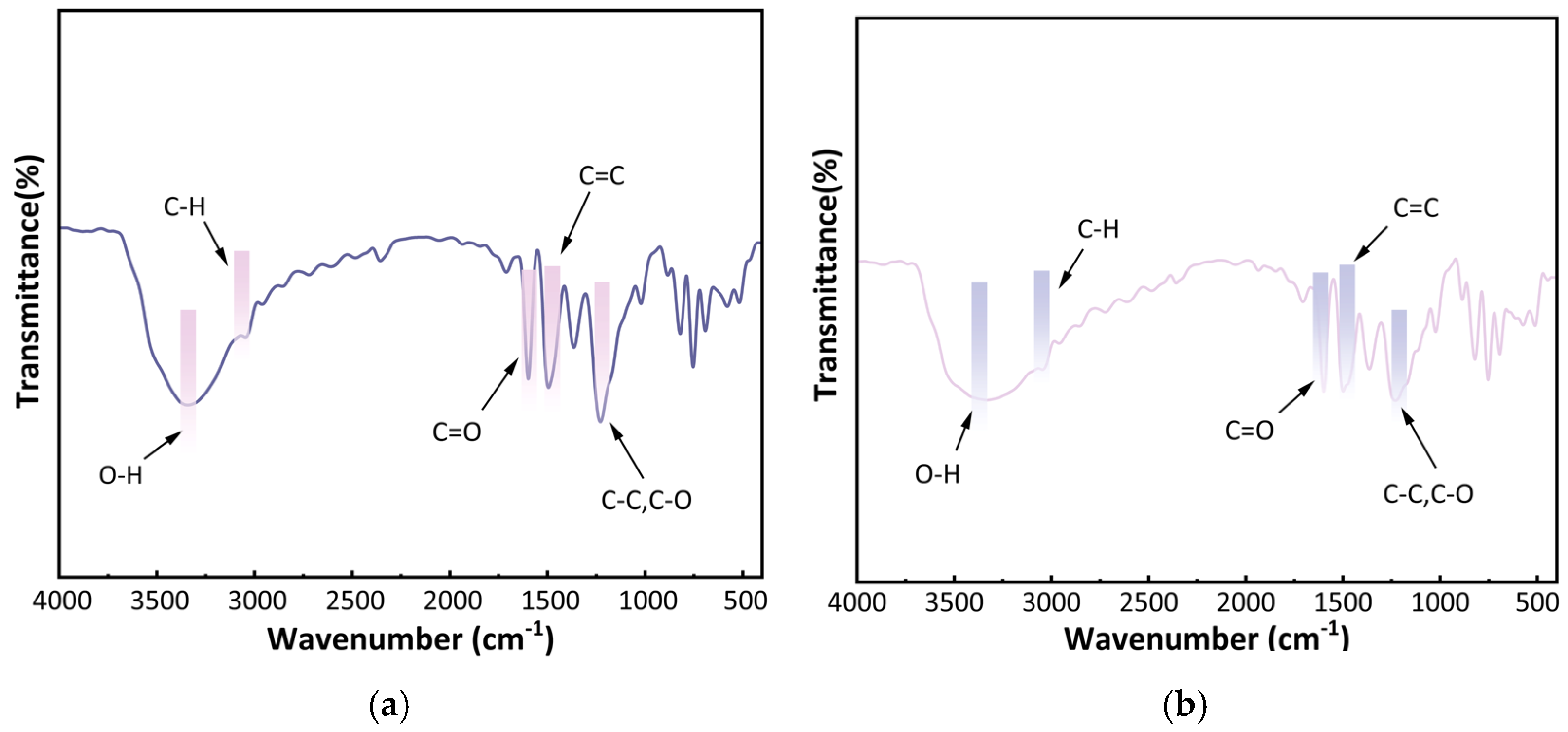
2.1.3. Infrared Spectral Analysis of Carbon Foam
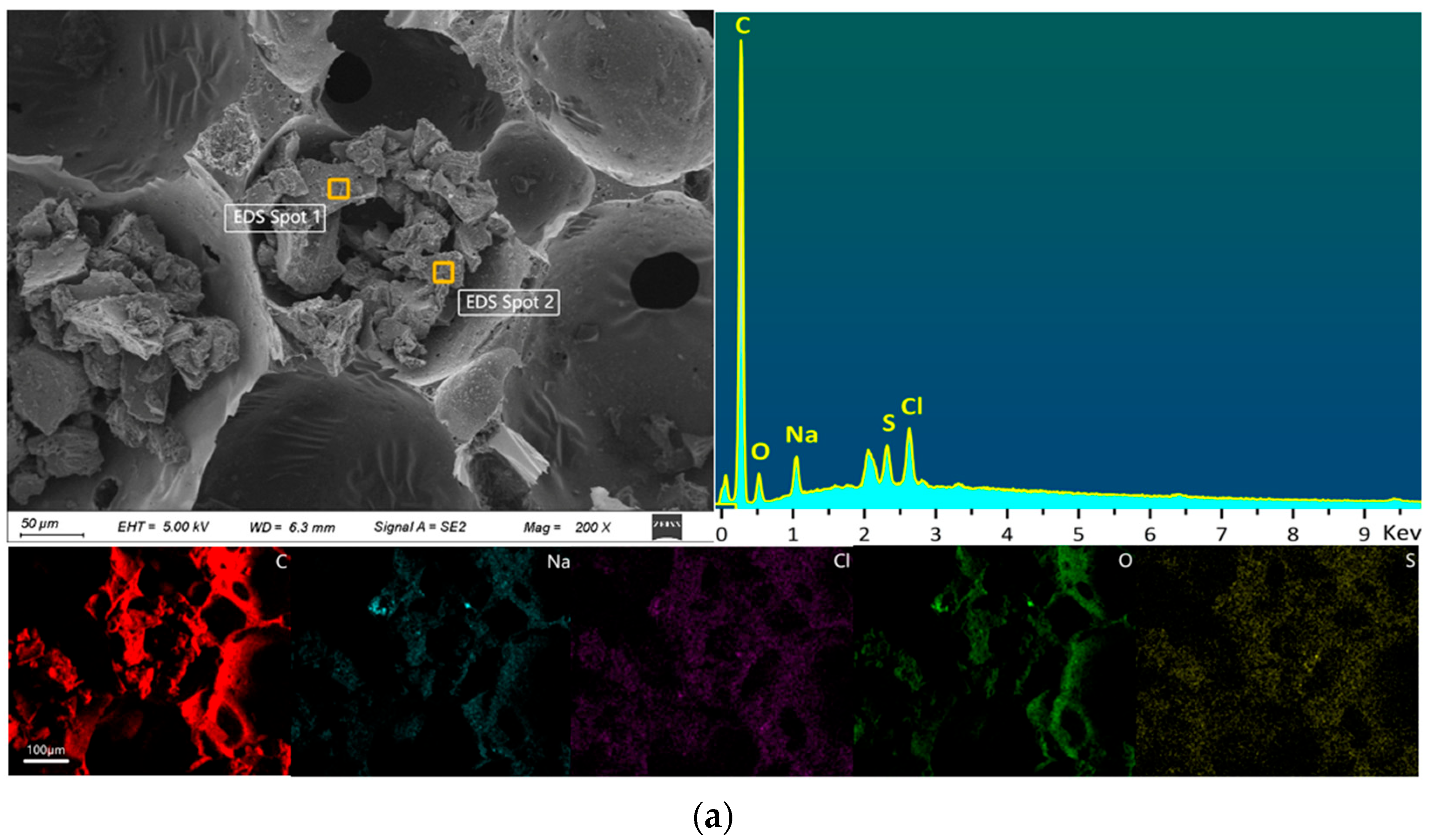
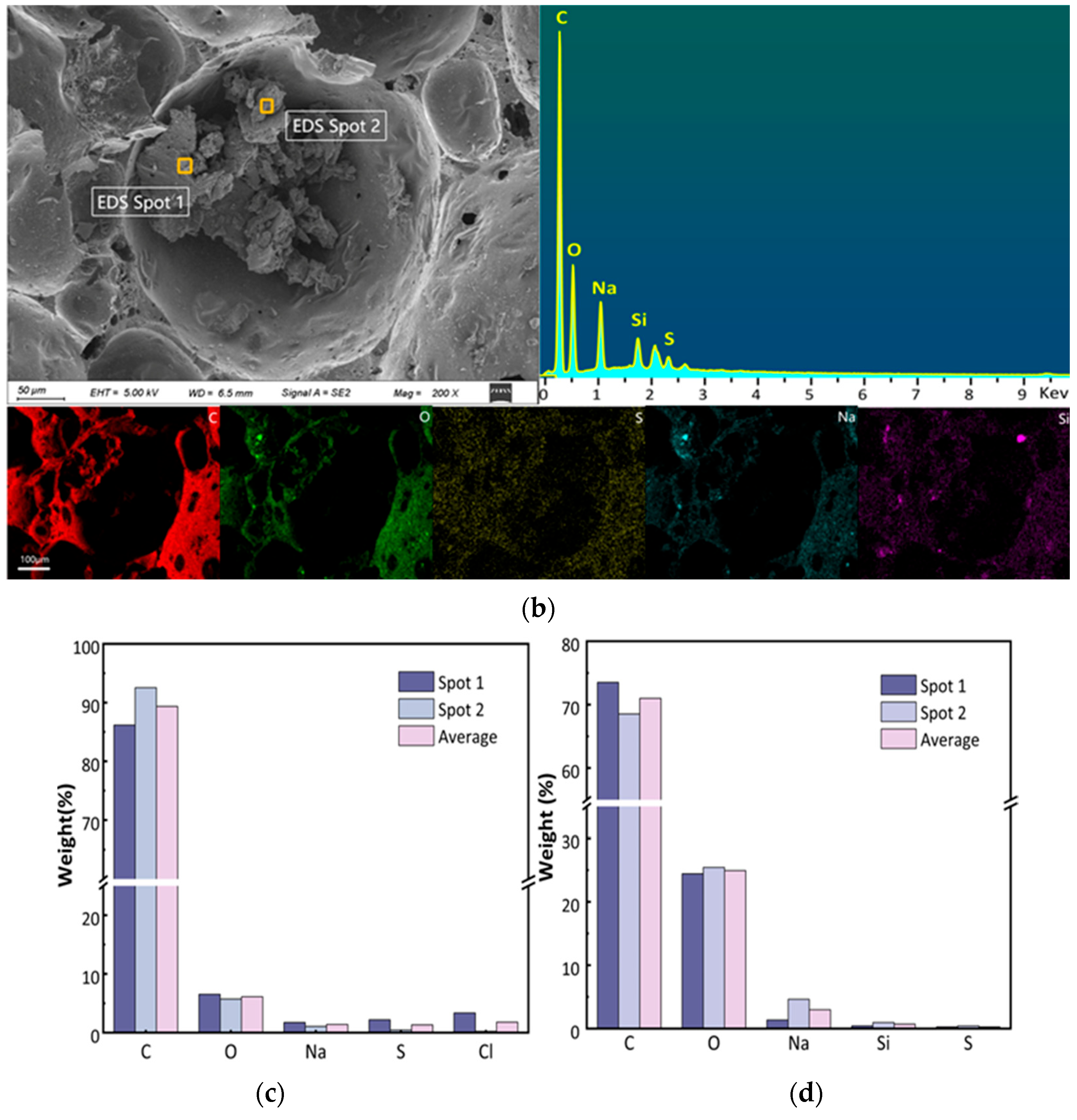
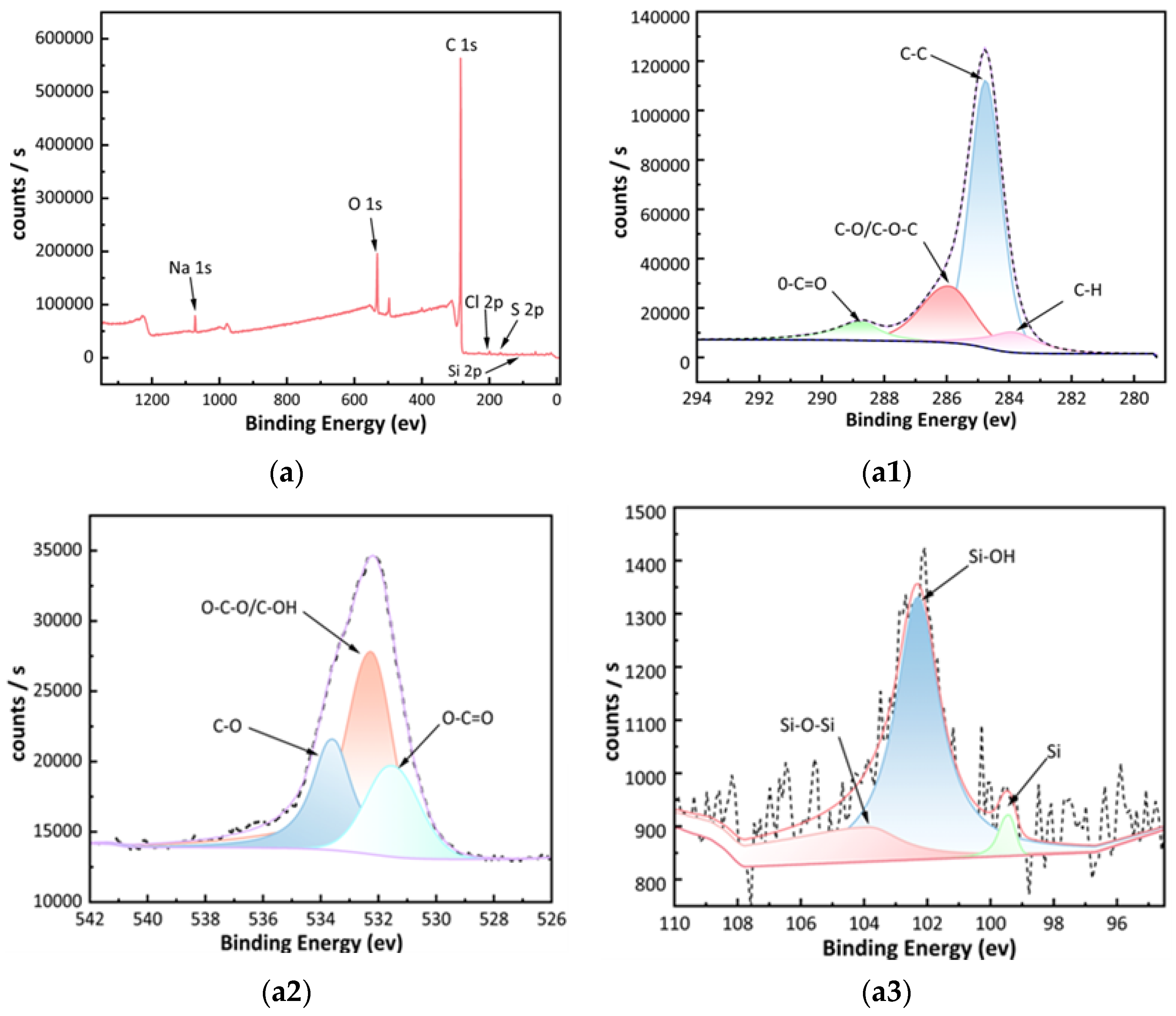
2.1.4. SEM-EDS Analysis
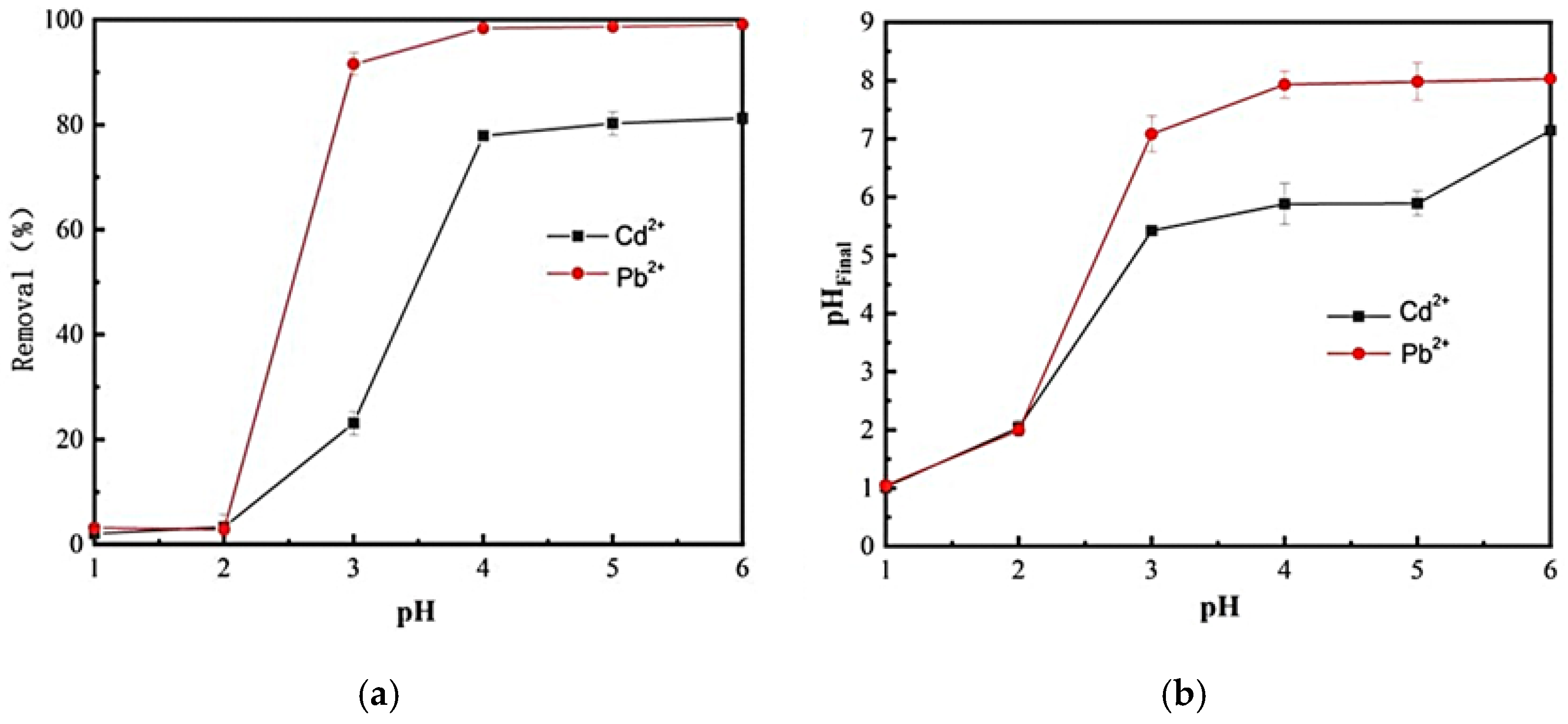
2.2. Effect of pH
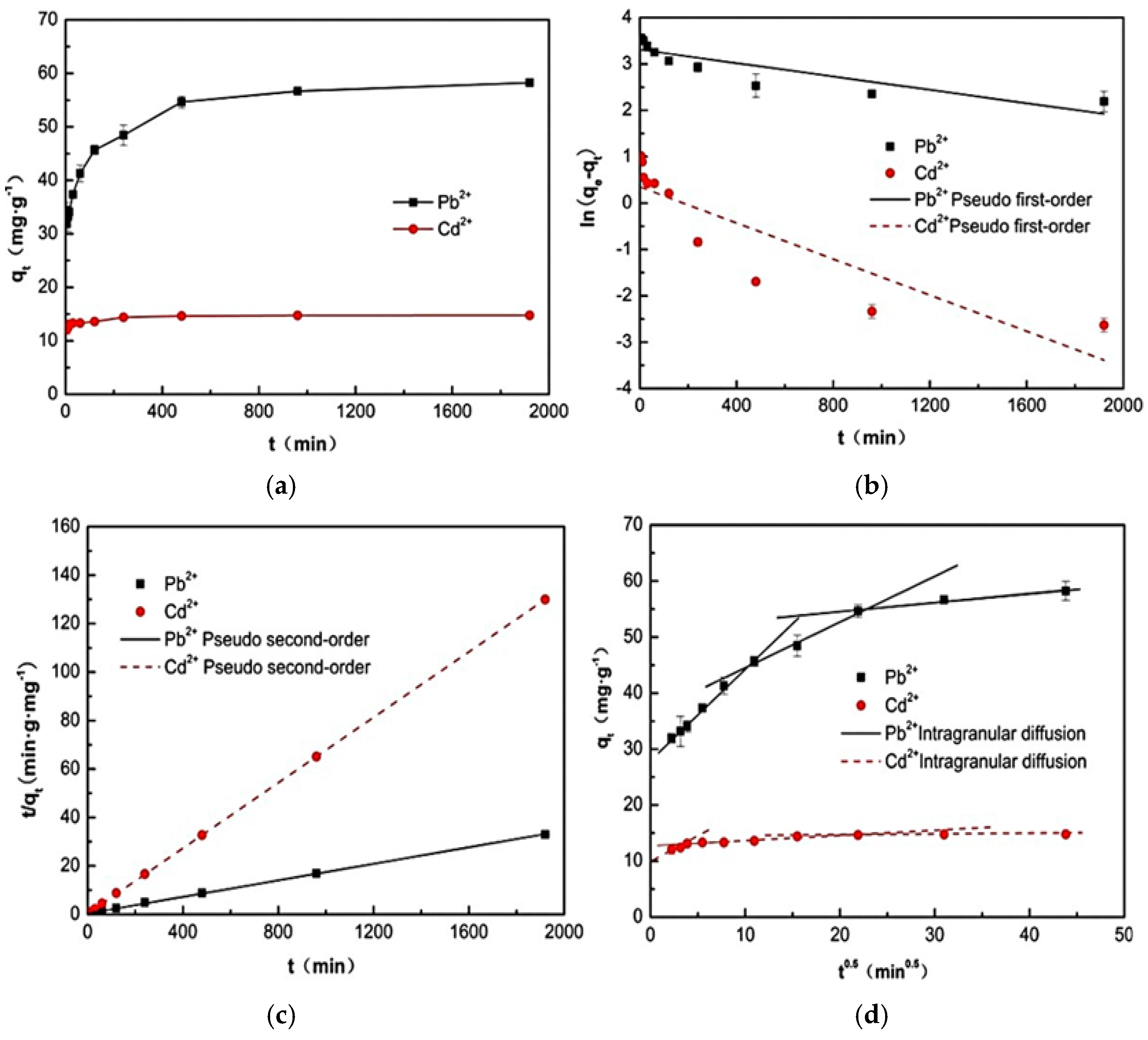
2.3. Kinetic Analysis
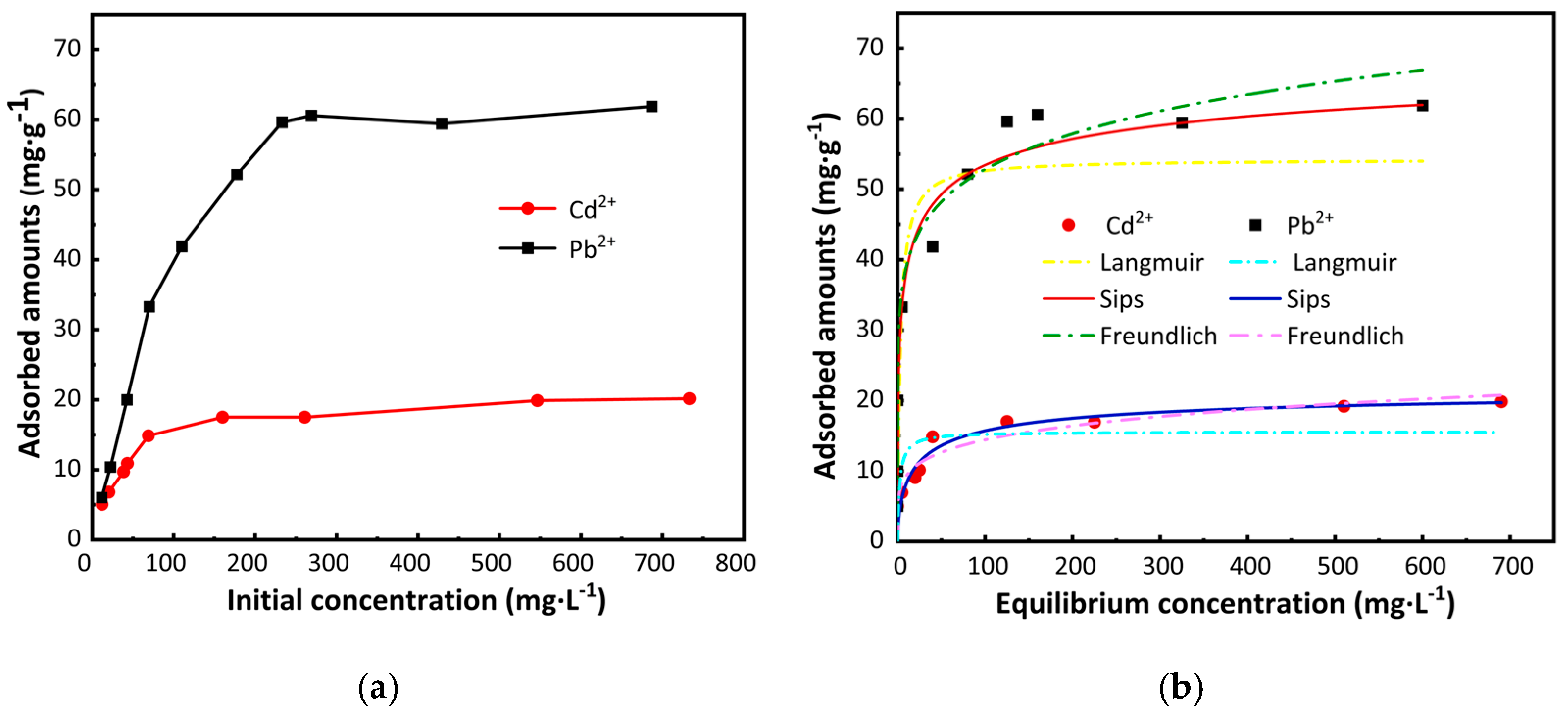
2.4. Adsorption Isotherm Analysis
2.5. Adsorption Mechanism Analysis
3. Materials and Methods
3.1. Materials
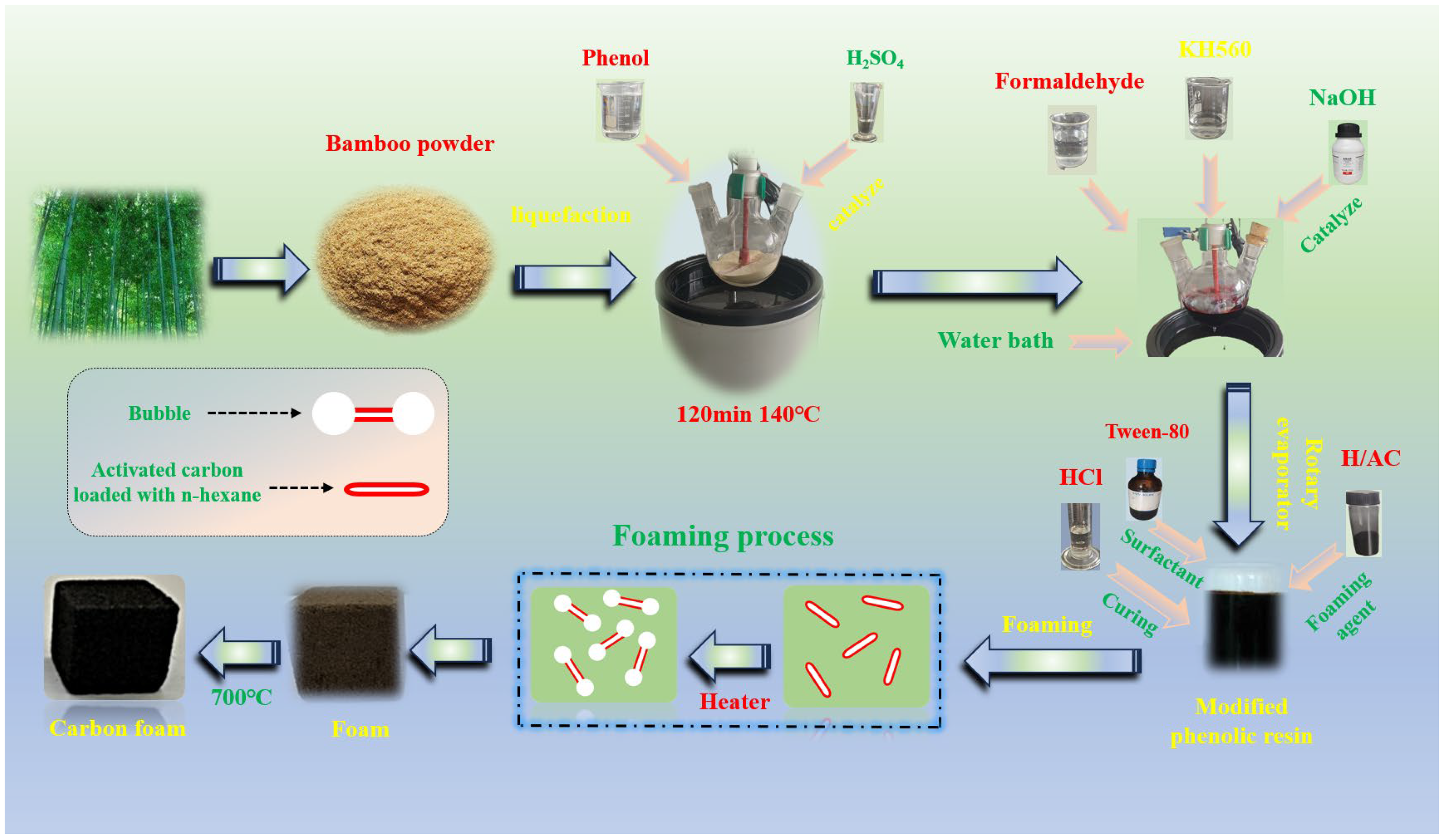
3.2. Preparation of U-CF and M-CF
3.2.1. Preparation of Unmodified Phenolic Resin Foam
3.2.2. Preparation of Modified Phenolic Resin Foam
3.2.3. Preparation of Foaming Agent and Carbonization of Resin Foam
3.3. Adsorption Experiments and Analytical Methods
3.4. Characterization Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal-organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Mishra, S.; Bharagava, R.N.; More, N.; Yadav, A.; Zainith, S.; Mani, S.; Chowdhary, P. Heavy metal contamination: An alarming threat to environment and human health. In Environmental Biotechnology: For Sustainable Future; Springer: Singapore, 2019; pp. 103–125. [Google Scholar]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A. Impact of heavy metals on the environment and human health: Novel therapeutic insights to counter the toxicity. J. King Saud. Univ.–Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Panneerselvam, B.; Priya K, S. Phytoremediation potential of water hyacinth in heavy metal removal in chromium and lead contaminated water. Int. J. Environ. Anal. Chem. 2023, 103, 3081–3096. [Google Scholar] [CrossRef]
- Orooji, N.; Mohammadi, M.J.; Takdastan, A.; Feizi, R.; Kargari, A. Investigation of the efficiency of removing turbidity, aluminum, and total organic compounds by using poly-aluminum chloride and chitosan from the water of Karoon River, Iran. Desalination Water Treat. 2020, 200, 82–89. [Google Scholar] [CrossRef]
- Song, C.; Zhang, C.; Zhang, S.; Lin, H.; Kim, Y.; Ramakrishnan, M.; Du, Y.; Zhang, Y.; Zheng, H.; Barceló, D. Thermochemical liquefaction of agricultural and forestry wastes into biofuels and chemicals from circular economy perspectives. Sci. Total Environ. 2020, 749, 141972. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, Q.; Li, M.; Zhao, H.; Jin, S.; Huang, Y.; Fan, J.; Chen, J. Analysis of factors influencing pore structure development of agricultural and forestry waste-derived activated carbon for adsorption application in gas and liquid phases: A review. J. Environ. Chem. Eng. 2021, 9, 105905. [Google Scholar] [CrossRef]
- Karić, N.; Maia, A.S.; Teodorović, A.; Atanasova, N.; Langergraber, G.; Crini, G.; Ribeiro, A.R.; Đolić, M. Bio-waste valorisation: Agricultural wastes as biosorbents for removal of (in) organic pollutants in wastewater treatment. Chem. Eng. J. Adv. 2022, 9, 100239. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Luo, J.; Jiao, L.; Wu, W.; Fang, G.; Dai, H. Lignocellulosic nanofibrils produced using wheat straw and their pulping solid residue: From agricultural waste to cellulose nanomaterials. Waste Manag. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Klass, D.L. Biomass for renewable energy and fuels. Encycl. Energy 2004, 1, 193–212. [Google Scholar]
- Yu, S.; Zhang, W.; Dong, X.; Wang, F.; Yang, W.; Liu, C.; Chen, D. A review on recent advances of biochar from agricultural and forestry wastes: Preparation, modification and applications in wastewater treatment. J. Environ. Chem. Eng. 2024, 12, 111638. [Google Scholar] [CrossRef]
- Shanmugam, H.; Raghavan, V.; Rajagopal, R.; Goyette, B.; Lyu, L.; Zhou, S.; An, C. Evaluating Sustainable Practices for Managing Residue Derived from Wheat Straw. Bioengineering 2024, 11, 554. [Google Scholar] [CrossRef] [PubMed]
- Whalen, J.; Xu, C.C.; Shen, F.; Kumar, A.; Eklund, M.; Yan, J. Sustainable biofuel production from forestry, agricultural and waste biomass feedstocks. Appl. Energy 2017, 198, 281–283. [Google Scholar] [CrossRef]
- Faria, D.L.; Gonçalves, F.G.; Maffioletti, F.D.; Scatolino, M.V.; Soriano, J.; de Paula Protásio, T.; Lopez, Y.M.; Paes, J.B.; Mendes, L.M.; Junior, J.B.G. Particleboards based on agricultural and agroforestry wastes glued with vegetal polyurethane adhesive: An efficient and eco–friendly alternative. Ind. Crops Prod. 2024, 214, 118540. [Google Scholar] [CrossRef]
- Kukwa, D.T.; Chetty, M.; Anekwe, I.; Adedeji, J. Conversion of Agricultural and Forest Wastes into Water Purifying Materials. In Agricultural and Kitchen Waste; CRC Press: Boca Raton, FL, USA, 2022; pp. 63–81. [Google Scholar]
- Zhou, C.; Wang, Y. Recent progress in the conversion of biomass wastes into functional materials for value–added applications. Sci. Technol. Adv. Mater. 2020, 21, 787–804. [Google Scholar] [CrossRef]
- Li, H.; Yuan, Z.; Zhang, Y.; Chang, C.; Xu, C. Synthesis of biobased polyurethane foams from agricultural and forestry wastes. In Reactive and Functional Polymers Volume One: Biopolymers, Polyesters, Polyurethanes, Resins and Silicones; Springer: Cham, Switzerland, 2020; pp. 137–156. [Google Scholar]
- Ding, Z.; Awasthi, S.K.; Kumar, M.; Kumar, V.; Dregulo, A.M.; Yadav, V.; Sindhu, R.; Binod, P.; Sarsaiya, S.; Pandey, A. A thermo-chemical and biotechnological approaches for bamboo waste recycling and conversion to value added product: Towards a zero-waste biorefinery and circular bioeconomy. Fuel 2023, 333, 126469. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Adeyanju, C.A.; Iwuozor, K.O.; Odeyemi, S.O.; Emenike, E.C.; Ogunniyi, S.; Te-Erebe, D.K. Retort carbonization of bamboo (Bambusa vulgaris) waste for thermal energy recovery. Clean. Technol. Environ. Policy 2023, 25, 937–947. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Taylor, A.; Serwinowski, N.; Parkerson, Z.J.; Confer, M.P.; Kammakakam, I.; Bara, J.E.; Esfahani, A.R.; Mahmoodi, S.N.; Koutahzadeh, N. Sustainable novel bamboo-based membranes for water treatment fabricated by regeneration of bamboo waste fibers. ACS Sustain. Chem. Eng. 2020, 8, 4225–4235. [Google Scholar] [CrossRef]
- Leng, Y.; Dong, S.; Chen, Z.; Sun, Y.; Xu, Q.; Ma, L.; He, X.; Hai, C.; Zhou, Y. Preparation of high-performance anode materials for sodium-ion batteries using bamboo waste: Achieving resource recycling. J. Power Sources 2024, 613, 234826. [Google Scholar] [CrossRef]
- Song, Y.; Lee, Y.G.; Lee, D.-S.; Nguyen, D.-T.; Bae, H.-J. Utilization of bamboo biomass as a biofuels feedstocks: Process optimization with yeast immobilization and the sequential fermentation of glucose and xylose. Fuel 2022, 307, 121892. [Google Scholar] [CrossRef]
- Nagel, B.; Pusz, S.; Trzebicka, B. Review: Tailoring the properties of macroporous carbon foams. J. Mater. Sci. 2014, 49, 1–17. [Google Scholar] [CrossRef]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Wang, R.; Yu, J.; Islam, F.; Tahmasebi, A.; Lee, S.; Chen, Y. State-of-the-art research and applications of carbon foam composite materials as electrodes for high–capacity lithium batteries. Energy Fuels 2020, 34, 7935–7954. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, J.; Tan, J.; Ma, C.; Luo, S.; Li, W.; Liu, S. Multi–walled carbon nanotubes/carbon foam nanocomposites derived from biomass for CO2 capture and supercapacitor applications. Fuel 2021, 305, 121622. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, C.; Song, L.; Du, P.; Yang, Y.; Xiong, J. A facile controllable preparation of highly porous carbon foam and its application in photocatalysis. Mater. Res. Bull. 2020, 122, 110697. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Tian, N.; You, C.; Yang, Y. Foam-structured carbon materials and composites for electromagnetic interference shielding: Design principles and structural evolution. Carbon 2024, 217, 118608. [Google Scholar] [CrossRef]
- Lee, C.-G.; Song, M.-K.; Ryu, J.-C.; Park, C.; Choi, J.-W.; Lee, S.-H. Application of carbon foam for heavy metal removal from industrial plating wastewater and toxicity evaluation of the adsorbent. Chemosphere 2016, 153, 1–9. [Google Scholar] [CrossRef]
- Lee, C.-G.; Jeon, J.-W.; Hwang, M.-J.; Ahn, K.-H.; Park, C.; Choi, J.-W.; Lee, S.-H. Lead and copper removal from aqueous solutions using carbon foam derived from phenol resin. Chemosphere 2015, 130, 59–65. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Lin, Q.; Fang, C. A stiff ZnO/carbon foam composite with second-level macroporous structure filled ZnO particles for heavy metal ions removal. Environ. Res. 2020, 188, 109698. [Google Scholar] [CrossRef]
- Lee, C.-G.; Lee, S.; Park, J.-A.; Park, C.; Lee, S.J.; Kim, S.-B.; An, B.; Yun, S.-T.; Lee, S.-H.; Choi, J.-W. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 2017, 166, 203–211. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.; Zhu, Y.; Liang, W.; Nie, Y.; Shi, W.; Ai, S. Core-shell structural nitrogen–doped carbon foam loaded with nano zero-valent iron for simultaneous remediation of Cd(II) and NAP in water and soil: Kinetics, mechanism, and environmental evaluation. Sci. Total Environ. 2022, 832, 155091. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Li, J.; Xu, J.; Wang, X. Fabrication of Fe/Fe3C@ porous carbon sheets from biomass and their application for simultaneous reduction and adsorption of uranium (VI) from solution. Inorg. Chem. Front. 2014, 1, 641–648. [Google Scholar] [CrossRef]
- Pei, N.; Luo, W.; Huang, Q.; Sun, Y. Adsorption Properties of Fishbone and Fishbone-Derived Biochar for Cadmium in Aqueous Solution. Agronomy 2024, 14, 2717. [Google Scholar] [CrossRef]
- Glosz, K.; Stolarczyk, A.; Jarosz, T. Siloxanes-Versatile Materials for Surface Functionalisation and Graft Copolymers. Int. J. Mol. Sci. 2020, 21, 6387. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Huang, J.; Shao, Y.; Zhang, W.; Dai, C. Mechanical and adsorptive properties of foamed EVA–modified polypropylene/bamboo charcoal composites. Materials 2021, 14, 1524. [Google Scholar] [CrossRef]
- Yang, F.; Sun, L.; Zhang, W.; Zhang, Y. One–pot synthesis of porous carbon foam derived from corn straw: Atrazine adsorption equilibrium and kinetics. Environ. Sci. Nano 2017, 4, 625–635. [Google Scholar] [CrossRef]
- Chen, L.; Fan, Z.; Mao, W.; Dai, C.; Chen, D.; Zhang, X. Analysis of formation mechanisms of sugar–derived dense carbons via hydrogel carbonization method. Nanomaterials 2022, 12, 4090. [Google Scholar] [CrossRef]
- Song, G.; Fan, W.; Zhang, J.; Xue, T.; Shi, Y.; Sun, Y.; Ding, G. Adsorption of anionic dyes from aqueous solutions by a novel CTAB/MXene/carbon nanotube composite: Characterization, experiments, and theoretical analysis. Appl. Surf. Sci. 2024, 661, 160036. [Google Scholar] [CrossRef]
- Stoycheva, I.; Tsyntsarski, B.; Kosateva, A.; Petrova, B.; Dolashka, P. Assessment of the Possibilities for Removal of Ni(II) from Contaminated Water by Activated Carbon foam Derived from Treatment Products of RDF. Processes 2022, 10, 570. [Google Scholar] [CrossRef]
- Yeh, S.-K.; Hsieh, C.-C.; Chang, H.-C.; Yen, C.C.; Chang, Y.-C. Synergistic effect of coupling agents and fiber treatments on mechanical properties and moisture absorption of polypropylene-rice husk composites and their foam. Compos. Part A Appl. Sci. Manuf. 2015, 68, 313–322. [Google Scholar] [CrossRef]
- Li, R.; Fan, G.; Wang, P.; Ouyang, X.; Ma, N.; Wei, H. Effects of silane coupling agent modifications of hollow glass microspheres on syntactic foams with epoxy matrix. Polym. Polym. Compos. 2021, 29, S1191–S1203. [Google Scholar] [CrossRef]
- Mnasri-Ghnimi, S.; Frini-Srasra, N. Removal of heavy metals from aqueous solutions by adsorption using single and mixed pillared clays. Appl. Clay Sci. 2019, 179, 105151. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.H.K.; Lee, S.-M. Three-dimensional porous spinel ferrite as an adsorbent for Pb(II) removal from aqueous solutions. Ind. Eng. Chem. Res. 2013, 52, 15789–15800. [Google Scholar] [CrossRef]
- Tao, H.-C.; Zhang, H.-R.; Li, J.-B.; Ding, W.-Y. Biomass based activated carbon obtained from sludge and sugarcane bagasse for removing lead ion from wastewater. Bioresour. Technol. 2015, 192, 611–617. [Google Scholar] [CrossRef]
- Depci, T. Comparison of activated carbon and iron impregnated activated carbon derived from Gölbaşı lignite to remove cyanide from water. Chem. Eng. J. 2012, 181, 467–478. [Google Scholar] [CrossRef]
- Depci, T.; Kul, A.R.; Önal, Y. Competitive adsorption of lead and zinc from aqueous solution on activated carbon prepared from Van apple pulp: Study in single–and multi–solute systems. Chem. Eng. J. 2012, 200, 224–236. [Google Scholar] [CrossRef]
- Zou, J.; Liu, J.; Yu, Q.; Gao, Y.; Chen, S.; Huang, X.; Hu, D.; Liu, S.; Lu, L. Facile Synthesis of Nitrogen Self-Doped Porous Carbon Derived from Cicada Shell via KOH Activation for Simultaneous Detection and Removal of Cu2+. Molecules 2022, 27, 4516. [Google Scholar] [CrossRef]
- Meng, J.; Feng, X.; Dai, Z.; Liu, X.; Wu, J.; Xu, J. Adsorption characteristics of Cu(II) from aqueous solution onto biochar derived from swine manure. Environ. Sci. Pollut. Res. 2014, 21, 7035–7046. [Google Scholar] [CrossRef]
- Witek-Krowiak, A.; Reddy, D.H.K. Removal of microelemental Cr(III) and Cu(II) by using soybean meal waste-unusual isotherms and insights of binding mechanism. Bioresour. Technol. 2013, 127, 350–357. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, J.; Wang, S.; Zhang, L.; Sun, W. Bimetallic coordination polymer for highly selective removal of Pb(II): Activation energy, isosteric heat of adsorption and adsorption mechanism. Chem. Eng. J. 2021, 425, 131474. [Google Scholar] [CrossRef]
- Gupta, P.L.; Jung, H.; Tiwari, D.; Kong, S.-H.; Lee, S.-M. Insight into the mechanism of Cd(II) and Pb(II) removal by sustainable magnetic biosorbent precursor to Chlorella vulgaris. J. Taiwan Inst. Chem. Eng. 2017, 71, 206–213. [Google Scholar]
- Qiao, W.; Yao, J.; Yang, T.; Ma, C.; Wang, J.; Xiong, K.; Zhang, Y. Bifunctional 3d Ordered Graphene-Based All–Carbon Foam/Polymer Composite Materials for Thermal Management and Electromagnetic Shielding. J. Mater. Sci. 2025, 60, 9602–9615. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Li, S.; Wang, D.; Zheng, Z. Rational design of freestanding and high-performance thick electrode from carbon foam modified with polypyrrole/polydopamine for supercapacitors. Chem. Eng. J. 2022, 447, 137562. [Google Scholar] [CrossRef]
- Niragatti, S.C.R.; Madhukar, S.T.V.; Kim, J.; Yoo, K. In-situ-grown Co-doped Ni-hexacyanoferrate/Ni-foam composites as battery-type electrode materials for aqueous hybrid supercapacitors. J. Alloys Compd. 2022, 918, 165638. [Google Scholar] [CrossRef]
- Deng, W.; Li, T.; Li, H.; Liu, X.; Dang, A.; Liu, Y.; Wu, H. Controllable graphitization degree of carbon foam bulk toward electromagnetic wave attenuation loss behavior. J. Colloid Interface Sci. 2022, 618, 129–140. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.-H. A durable, fluorine-free, and repairable superhydrophobic aluminum surface with hierarchical micro/nanostructures and its application for continuous oil-water separation. J. Membr. Sci. 2021, 618, 118716. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, L.; Zhang, Y.; Zhang, Y.; Li, Z.; Yang, K.; Chen, L. The adsorption characteristics of phosphorus-modified corn stover biochar on lead and cadmium. Agriculture 2024, 14, 1118. [Google Scholar] [CrossRef]
- Lyu, H.; Gong, Y.; Tang, J.; Huang, Y.; Wang, Q. Immobilization of heavy metals in electroplating sludge by biochar and iron sulfide. Environ. Sci. Pollut. Res. 2016, 23, 14472–14488. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qiu, Y.; Xiao, T.; Wang, J.; Chen, Y.; Xu, X.; Kang, Z.; Fan, L.; Yu, H. Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: High performance, selectivity and application. J. Hazard. Mater. 2019, 373, 39–49. [Google Scholar] [CrossRef]
- Li, T.; Li, X.; Shen, C.; Chen, D.; Li, F.; Xu, W.; Wu, X.; Bao, Y. Nano-hydroxyapatite modified tobacco stalk-based biochar for immobilizing Cd(II): Interfacial adsorption behavior and mechanisms. Processes 2024, 12, 1924. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Li, Y.; Liu, Y.; Chen, Y.; Li, H.; Li, L.; Xu, F.; Jiang, H.; Chen, L. Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: Adsorption behavior and mechanisms. Bioresour. Technol. 2021, 321, 124413. [Google Scholar] [CrossRef] [PubMed]

| SBET (m2/g) | PTotal (cm3/g) | Psize (nm) | St (m2/g) | |
|---|---|---|---|---|
| U-CF | 3.35 ± 0.27 | 0.022 ± 0.015 | 5.04 ± 1.28 | 4.30 ± 1.48 |
| M-CF | 4.27 ± 0.11 | 0.032 ± 0.005 | 3.25 ± 0.25 | 5.94 ± 0.32 |
| Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | Phase | kp | I | R2 | |
| Pb2+ | 7.2 × 10−4 | 27.33 | 0.7612 | 7.5 × 10−4 | 58.56 | 0.9994 | 1 | 1.62 | 28.21 | 0.9956 |
| 2 | 0.829 | 36.22 | 0.9855 | |||||||
| 3 | 0.162 | 51.31 | 0.9706 | |||||||
| Cd2+ | 2.0 × 10−3 | 1.42 | 0.7708 | 1.5 × 10−2 | 14.79 | 0.9990 | 1 | 0.606 | 10.66 | 0.9214 |
| 2 | 0.092 | 12.72 | 0.9462 | |||||||
| 3 | 0.005 | 14.57 | 0.8414 | |||||||
| Heavy Metal Ions | Langmuir | Freundlich | Sips |
|---|---|---|---|
| Pb2+ | qm = 54.28, KL = 0.31, R2 = 0.6405 | KF = 28.79, nF = 7.58, R2 = 0.8554 | qm = 61.59, KS = 0.60, ns = 1.83, R2 = 0.9609 |
| Cd2+ | qm = 19.46, KL = 0.05, R2 = 0.8165 | KF = 6.05, nF = 5.30, R2 = 0.8260 | qm = 22.15, KS = 0.14, ns = 1.62, R2 = 0.9597 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ling, J.; Gao, Y.; Wang, R.; Lu, S.; Li, X.; Liu, S.; Liu, J. Adsorption of Pb2+ and Cd2+ from Aqueous Solutions by Porous Carbon Foam Derived from Biomass Phenolic Resin. Int. J. Mol. Sci. 2025, 26, 7302. https://doi.org/10.3390/ijms26157302
Ling J, Gao Y, Wang R, Lu S, Li X, Liu S, Liu J. Adsorption of Pb2+ and Cd2+ from Aqueous Solutions by Porous Carbon Foam Derived from Biomass Phenolic Resin. International Journal of Molecular Sciences. 2025; 26(15):7302. https://doi.org/10.3390/ijms26157302
Chicago/Turabian StyleLing, Jianwei, Yu Gao, Ruiling Wang, Shiyu Lu, Xuemei Li, Shouqing Liu, and Jianxiang Liu. 2025. "Adsorption of Pb2+ and Cd2+ from Aqueous Solutions by Porous Carbon Foam Derived from Biomass Phenolic Resin" International Journal of Molecular Sciences 26, no. 15: 7302. https://doi.org/10.3390/ijms26157302
APA StyleLing, J., Gao, Y., Wang, R., Lu, S., Li, X., Liu, S., & Liu, J. (2025). Adsorption of Pb2+ and Cd2+ from Aqueous Solutions by Porous Carbon Foam Derived from Biomass Phenolic Resin. International Journal of Molecular Sciences, 26(15), 7302. https://doi.org/10.3390/ijms26157302




