Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration
Abstract
1. Introduction
2. Results
2.1. Physicochemical Analysis of AgNPs
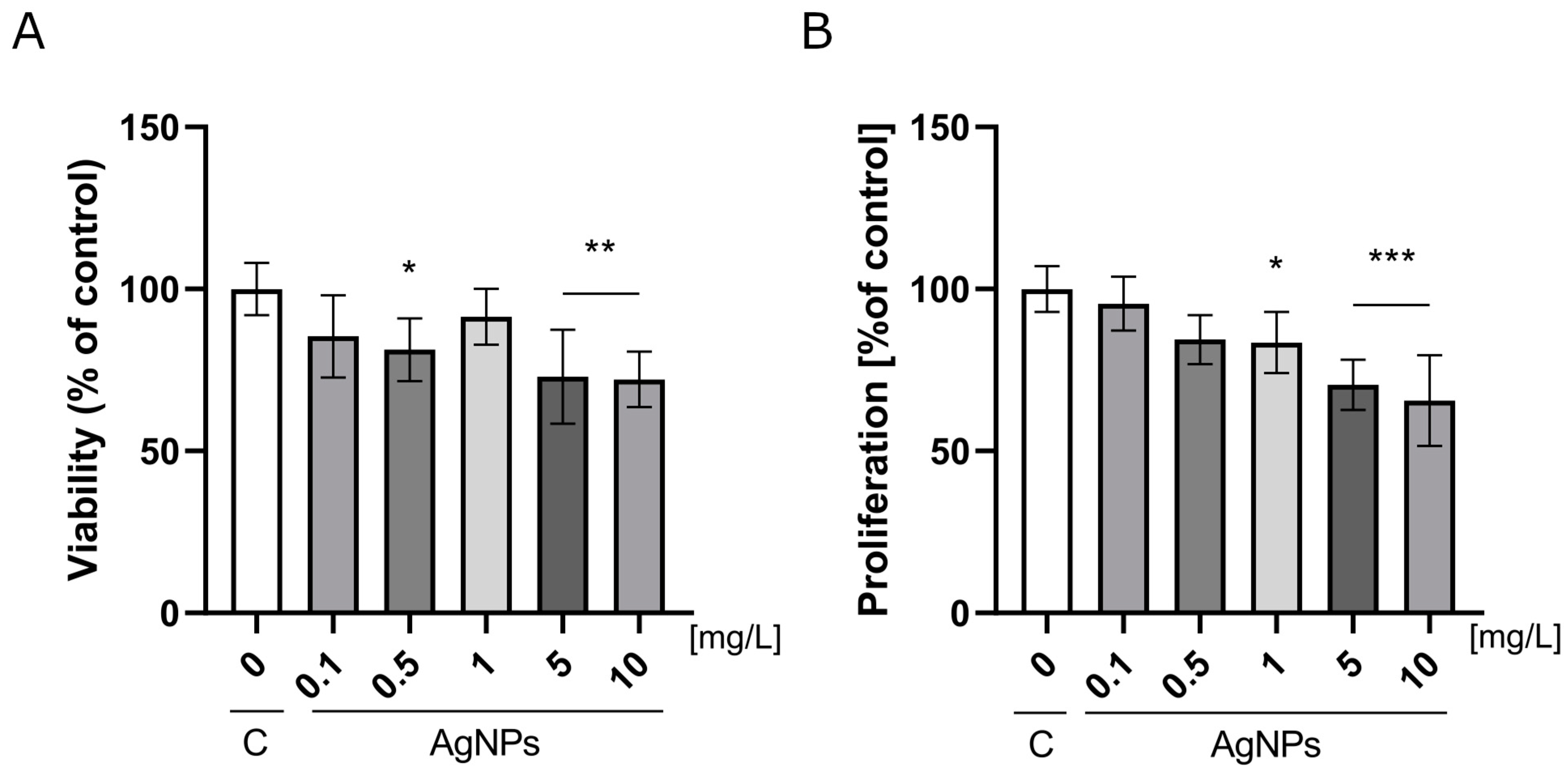
2.2. Analysis of Nanoparticles Toxicity
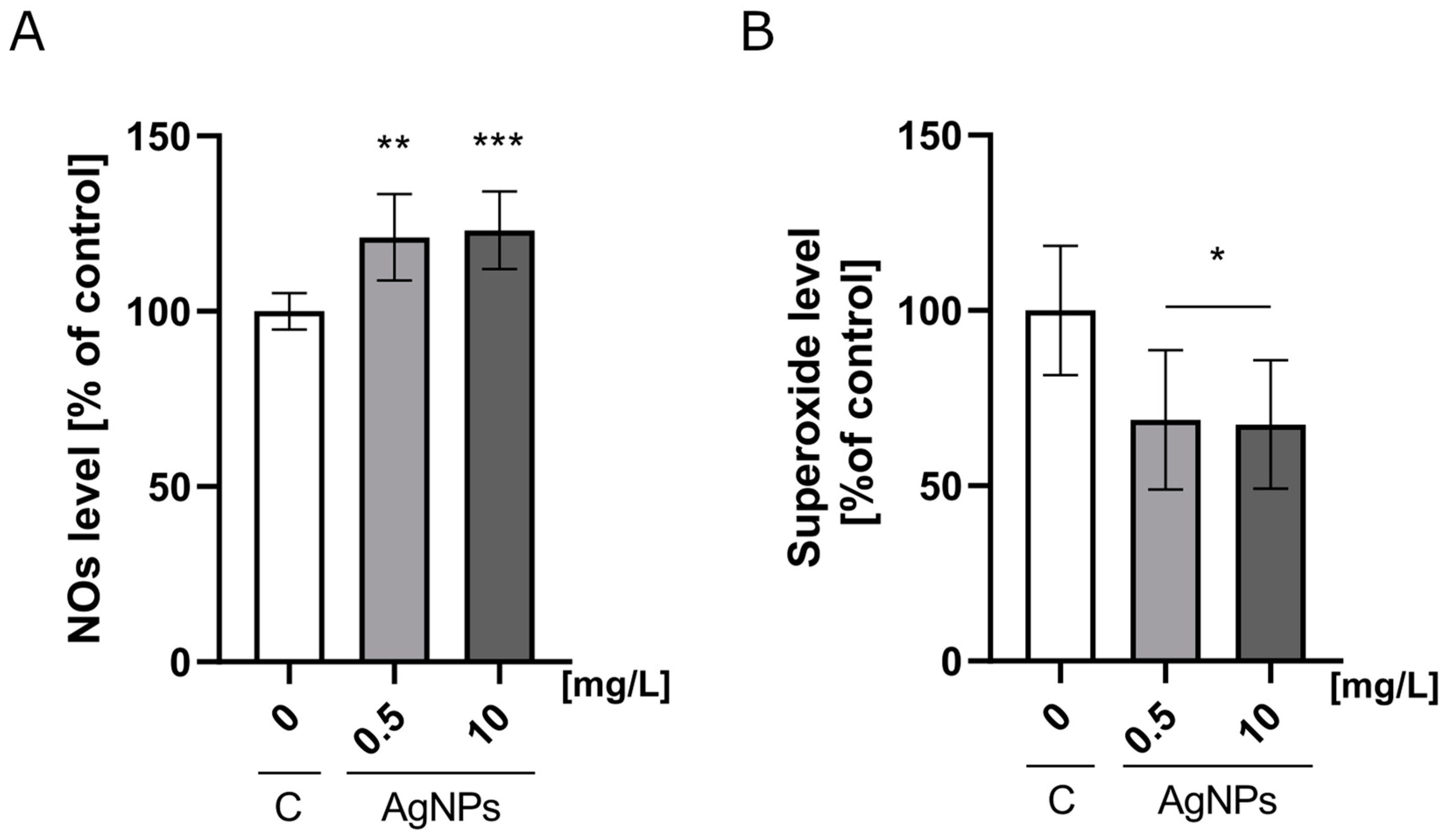
2.3. Nitric Oxide and Mitochondrial Superoxide Production
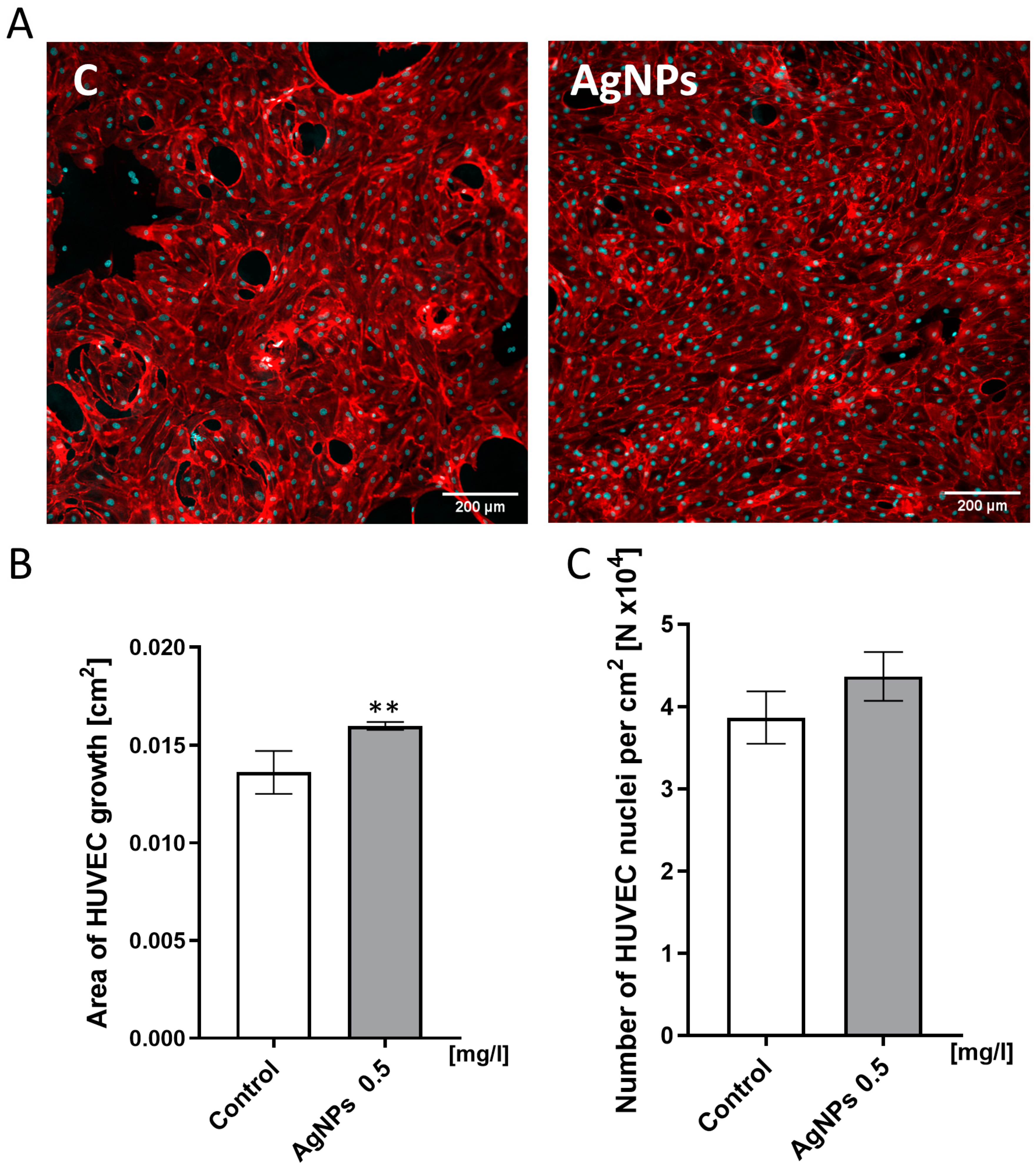
2.4. HUVEC Confluence Level Analysis
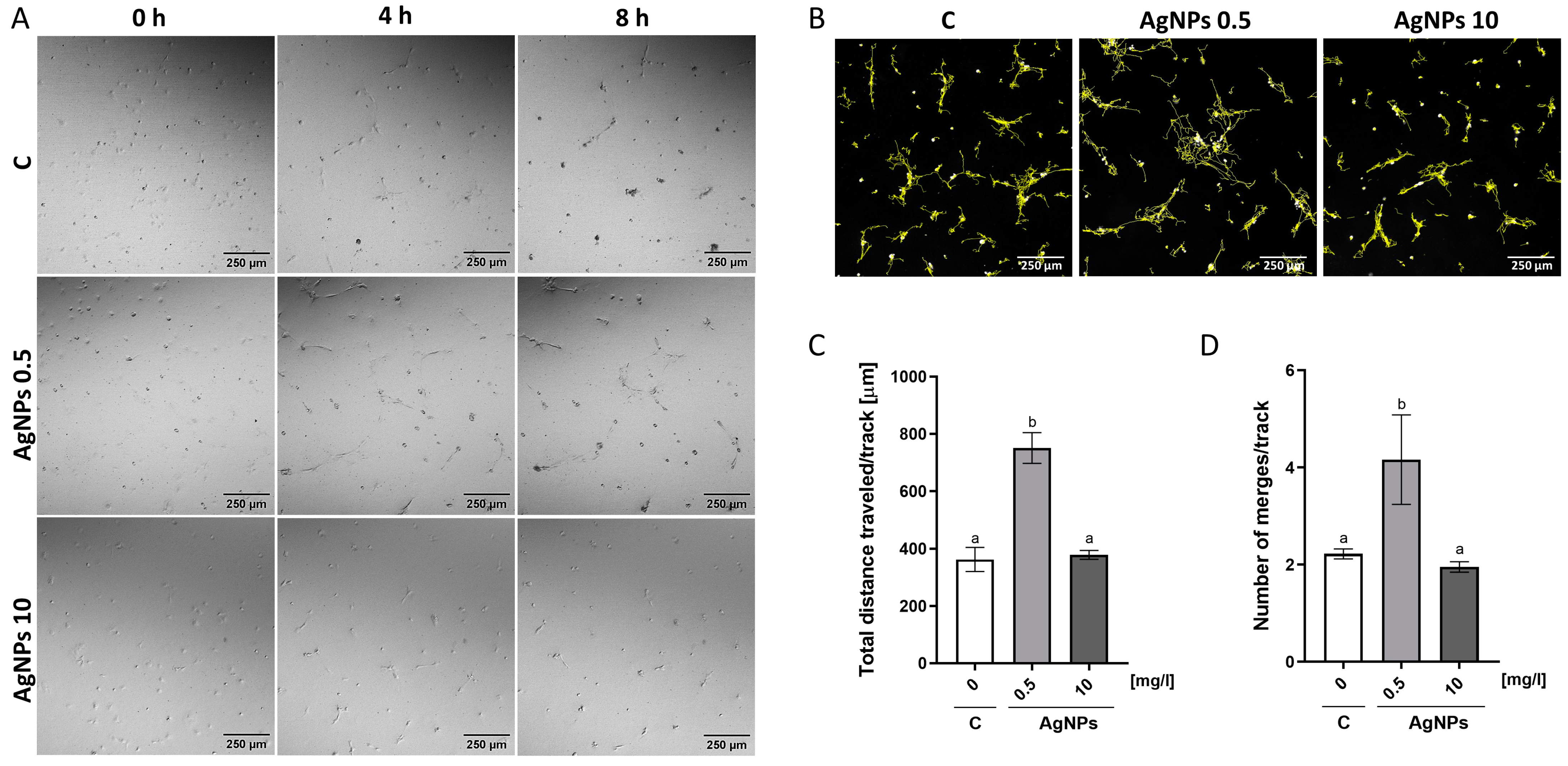
2.5. Migration Analysis
2.6. Antibody Array Analysis
3. Discussion
4. Materials and Methods
4.1. Physicochemical Analysis of AgNPs
4.2. Cell Lines
4.3. Analysis of Nanoparticles Toxicity
4.4. Nitric Oxide and Mitochondrial Superoxide Production
4.5. HUVEC Confluence Level Analysis
4.6. Migration Analysis
4.7. Antibody Array Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | silver nanoparticles |
| bFGF | basic fibroblast growth factor |
| cGMP | cyclic guanosine monophosphate |
| ECM | extracellular matrix |
| EDTA | ethylenediaminetetraacetic acid tetrasodium salt dihydrate |
| FGF | fibroblast growth factor |
| HBSS | Hank’s Balanced Salt Solution |
| HUVEC | human umbilical vein endothelial cells |
| MAPK | mitogen-activated protein kinase |
| NOs | nitric oxide |
| uPAR | urokinase plasminogen activator surface receptor |
| PBS | phosphate-buffered saline |
| PDEs | cGMP-regulated cyclic nucleotide phosphodiesterases |
| PECAM-1 | platelet and endothelial cell adhesion molecule 1 |
| PKGs | cgmp-dependent protein kinases |
| RANTES | regulated on activation, normal T-cell expressed and secreted chemokine |
| RIPA | radio-immunoprecipitation assay |
| ROS | reactive oxygen species |
| SAPKs | stress-activated protein kinases |
| TEM | transmission electron microscopy |
| TGF-β | transforming growth factor beta |
| TIE-2 | angiopoetin-1 receptor |
| VEGF | vascular endothelial growth factor |
| VEGF-D | vascular endothelial growth factor D |
References
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2019, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.P.; Kirsner, R.S. Angiogenesis in Wound Repair: Angiogenic Growth Factors and the Extracellular Matrix. Microsc. Res. Tech. 2003, 60, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Senger, D.R.; Davis, G.E. Angiogenesis. Cold Spring Harb. Perspect. Biol. 2011, 3, a005090. [Google Scholar] [CrossRef]
- Aplin, A.E.; Short, S.M.; Juliano, R.L. Anchorage-Dependent Regulation of the Mitogen-Activated Protein Kinase Cascade by Growth Factors Is Supported by a Variety of Integrin α Chains. J. Biol. Chem. 1999, 274, 31223–31228. [Google Scholar] [CrossRef]
- Assoian, R.K.; Schwartz, M.A. Coordinate Signaling by Integrins and Receptor Tyrosine Kinases in the Regulation of G1 Phase Cell-Cycle Progression. Curr. Opin. Genet. Dev. 2001, 11, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Perruzzi, C.A.; De Fougerolles, A.R.; Koteliansky, V.E.; Whelan, M.C.; Westlin, W.F.; Senger, D.R. Functional Overlap and Cooperativity among Av and B1 Integrin Subfamilies during Skin Angiogenesis. J. Investig. Dermatol. 2003, 120, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Ausprunk, D.H.; Folkman, J. Migration and Proliferation of Endothelial Cells in Preformed and Newly Formed Blood Vessels during Tumor Angiogenesis. Microvasc. Res. 1977, 14, 53–65. [Google Scholar] [CrossRef]
- Senger, D.R.; Perruzzi, C.A.; Streit, M.; Koteliansky, V.E.; De Fougerolles, A.R.; Detmar, M. The A1β1 and A2β1 Integrins Provide Critical Support for Vascular Endothelial Growth Factor Signaling, Endothelial Cell Migration, and Tumor Angiogenesis. Am. J. Pathol. 2002, 160, 195–204. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Garavand, F.; Jafari, S.M. Incorporation of silver nanoparticles into active antimicrobial nanocomposites: Release behavior, analyzing techniques, applications, and safety issues. Adv. Colloid Interface Sci. 2021, 293, 102440. [Google Scholar] [CrossRef]
- Munir, M.U.; Ahmed, A.; Usman, M.; Salman, S. Recent advances in nanotechnology-aided materials in combating microbial resistance and functioning as antibiotics substitutes. Int. J. Nanomed. 2020, 15, 7329–7358. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All that glitters is not silver—A new look at microbiological and medical applications of silver nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, Y.; Huang, Z.; Kou, Y.; Hu, A. Efficacy and safety of nano-silver dressings combined with recombinant human epidermal growth factor for deep second-degree burns: A meta-analysis. Burns 2020, 47, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Farouk, M.M.; El-Molla, A.; Salib, F.A.; Soliman, Y.A.; Shaalan, M. The role of silver nanoparticles in a treatment approach for multidrug-resistant salmonella species isolates. Int. J. Nanomed. 2020, 15, 6993–7011. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; AlSalhi, M.; Siddiqui, M. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Panja, A.; Mishra, A.K.; Dash, M.; Pandey, N.K.; Singh, S.K.; Kumar, B. Silver nanoparticles—A review. Eurasian J. Med. Oncol. 2021, 5, 95–102. [Google Scholar] [CrossRef]
- Morozova, O.V. Silver nanostructures: Limited sensitivity of detection, toxicity and anti-inflammation effects. Int. J. Mol. Sci. 2021, 22, 9928. [Google Scholar] [CrossRef]
- Hung, H.S.; Chang, K.B.; Tang, C.M.; Ku, T.R.; Kung, M.L.; Yu, A.Y.; Shen, C.C.; Yang, Y.C.; Hsieh, H.H.; Hsu, S.H. Anti-Inflammatory Fibronectin-AgNP for Regulation of Biological Performance and Endothelial Differentiation Ability of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 9262, Erratum in Int. J. Mol. Sci. 2022, 23, 14998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.; Shi, J.; Zou, X.; Wang, C.; Yang, Y.; Zhang, H. Silver Nanoparticles Interact with the Cell Membrane and Increase Endothelial Permeability by Promoting VE-Cadherin Internalization. J. Hazard. Mater. 2016, 317, 570–578. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Wu, M.; Guo, H.; Liu, L.; Liu, Y.; Xie, L. Size-Dependent Cellular Uptake and Localization Profiles of Silver Nanoparticles. Int. J. Nanomed. 2019, 14, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
- Gliga, A.R.; Skoglund, S.; Odnevall Wallinder, I.; Fadeel, B.; Karlsson, H.L. Size-Dependent Cytotoxicity of Silver Nanoparticles in Human Lung Cells: The Role of Cellular Uptake, Agglomeration and Ag Release. Part. Fibre Toxicol. 2014, 11, 11. [Google Scholar] [CrossRef]
- Behr, J.-P. The Proton Sponge: A Trick to Enter Cells the Viruses Did Not Exploit. Chimia 1997, 51, 34–36. [Google Scholar] [CrossRef]
- Fahrenkrog, B.; Aebi, U. The Nuclear Pore Complex: Nucleocytoplasmic Transport and Beyond. Nat. Rev. Mol. Cell Biol. 2003, 4, 757–766. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, M.J.; Lee, S.Y.; Oh, S.M.; Chung, K.H. Genotoxic Effects of Silver Nanoparticles Stimulated by Oxidative Stress in Human Normal Bronchial Epithelial (BEAS-2B) Cells. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2011, 726, 129–135. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver Nanoparticles: Evaluation of DNA Damage, Toxicity and Functional Impairment in Human Mesenchymal Stem Cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-Proliferative Activity of Silver Nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Low, G.; Mun, K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.; Kim, J.S.; Choi, I.H. Inflammasome Formation and IL-1β Release by Human Blood Monocytes in Response to Silver Nanoparticles. Biomaterials 2012, 33, 6858–6867. [Google Scholar] [CrossRef]
- Jiang, X.; Foldbjerg, R.; Miclaus, T.; Wang, L.; Singh, R.; Hayashi, Y.; Sutherland, D.; Chen, C.; Autrup, H.; Beer, C. Multi-Platform Genotoxicity Analysis of Silver Nanoparticles in the Model Cell Line CHO-K1. Toxicol. Lett. 2013, 222, 55–63. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y. DNA Damage Response to Different Surface Chemistry of Silver Nanoparticles in Mammalian Cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Chen, Q.; Chen, C. Cytotoxic Potential of Silver Nanoparticles. Yonsei Med. J. 2014, 55, 283–291. [Google Scholar] [CrossRef]
- Alkan, H.; Ciğerci, İ.H.; Ali, M.M.; Hazman, O.; Liman, R.; Colă, F.; Bonciu, E. Cytotoxic and Genotoxic Evaluation of Biosynthesized Silver Nanoparticles Using Moringa Oleifera on MCF-7 and HUVEC Cell Lines. Plants 2022, 11, 1293. [Google Scholar] [CrossRef] [PubMed]
- Sohn, H.Y.; Krotz, F.; Zahler, S.; Gloe, T.; Keller, M.; Theisen, K.; Schiele, T.M.; Klauss, V.; Pohl, U. Crucial Role of Local Peroxynitrite Formation in Neutrophil-Induced Endothelial Cell Activation. Cardiovasc. Res. 2003, 57, 804–815. [Google Scholar] [CrossRef]
- Zuberek, M.; Paciorek, P.; Bartosz, G.; Grzelak, A. Silver Nanoparticles Can Attenuate Nitrative Stress. Redox Biol. 2017, 11, 646–652. [Google Scholar] [CrossRef]
- Shi, J.; Sun, X.; Lin, Y.; Zou, X.; Li, Z.; Liao, Y.; Du, M.; Zhang, H. Endothelial Cell Injury and Dysfunction Induced by Silver Nanoparticles through Oxidative Stress via IKK/NF-ΚB Pathways. Biomaterials 2014, 35, 6657–6666. [Google Scholar] [CrossRef]
- Redolfi-Bristol, D.; Yamamoto, K.; Marin, E.; Zhu, W.; Mazda, O.; Riello, P.; Pezzotti, G. Exploring the Cellular Antioxidant Mechanism against Cytotoxic Silver Nanoparticles: A Raman Spectroscopic Analysis. Nanoscale 2024, 16, 9985–9997. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A.; Gade, A. Silver Nanoparticles as a New Generation of Antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef]
- Gov, N.S. Traction forces during collective cell motion. HFSP J. 2009, 3, 223–227. [Google Scholar] [CrossRef]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Guerra, A.; Belinha, J.; Natal Jorge, R. A preliminary study of endothelial cell migration during angiogenesis using a meshless method approach. Int. J. Numer. Method Biomed. Eng. 2020, 36, e3393. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.J.; Watson, M.G.; Devlin, A.H.; Chaplain, M.A.; McDougall, S.R.; Mitchell, C.A. Dynamics of Angiogenesis During Wound Healing: A Coupled In Vivo and In Silico Study. Microcirculation 2011, 18, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Lee, K.J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic properties of silver nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef]
- Lu, C.; Liu, Y.; Liu, Y.; Kou, G.; Chen, Y.; Wu, X.; Lv, Y.; Cai, J.; Chen, R.; Luo, J.; et al. Silver Nanoparticles Cause Neural and Vascular Disruption by Affecting Key Neuroactive Ligand-Receptor Interaction and VEGF Signaling Pathways. Int. J. Nanomed. 2023, 18, 2693–2706. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Poompradub, S.; Sansanaphongpricha, K.; Jayaraman, S.; Subramani, K.; Sonsudin, F. Increased expression levels of PDGF and VEGF magnify the wound healing potential facilitated by biogenic synthesis of silver nanoparticles. Nano-Struct. Nano-Objects 2024, 39, 101236. [Google Scholar] [CrossRef]
- Rama, P.; Baldelli, A.; Vignesh, A.; Altemimi, A.B.; Lakshmanan, G.; Selvam, R.; Arunagirinathan, N.; Murugesan, K.; Pratap-Singh, A. Antimicrobial, antioxidant, and angiogenic bioactive silver nanoparticles produced using Murraya paniculata (L.) jack leaves. Nanomater. Nanotechnol. 2022, 12, 18479804211056167. [Google Scholar] [CrossRef]
- Mostafavi, S.; Naderi Soorki, M.; Kesmati, M.; Dorostghoal, M.; Andashti, B. Molecular effect of Silver nanoparticles on wound healing activity of Salvia officinalis extract in adult mice. Nanomed. Res. J. 2024, 9, 329–338. [Google Scholar] [CrossRef]
- Arsenopoulou, Z.V.; Taitzoglou, I.A.; Molyvdas, P.A.; Gourgoulianis, K.I.; Hatzoglou, C.; Zarogiannis, S.G. Silver Nanoparticles Alter Cell Adhesion and Proliferation of Sheep Primary Mesothelial Cells. In Vivo 2018, 32, 109–112. [Google Scholar] [CrossRef]
- Goligorsky, M.S.; Budzikowski, A.S.; Tsukahara, H.; Noiri, E. Co-Operation between Endothelin and Nitric Oxide in Promoting Endothelial Cell Migration and Angiogenesis. Clin. Exp. Pharmacol. Physiol. 1999, 26, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Dimmeler, S.; Zeiher, A.M. Nitric Oxide—An Endothelial Cell Survival Factor. Cell Death Differ. 1999, 6, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M.K. Inhibition of Nitric Oxide Synthase Attenuates Blood-Brain Barrier Disruption during Experimental Meningitis. Brain Res. 1996, 720, 75–83. [Google Scholar] [CrossRef]
- Agostinis, C.; Bulla, R.; Tripodo, C.; Gismondi, A.; Stabile, H.; Bossi, F.; Guarnotta, C.; Garlanda, C.; De Seta, F.; Spessotto, P.; et al. An Alternative Role of C1q in Cell Migration and Tissue Remodeling: Contribution to Trophoblast Invasion and Placental Development. J. Immunol. 2010, 185, 4420–4429. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK Signalling Pathway and Tumorigenesis (Review). Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, J.; Silke, J. An Overview of Mammalian P38 Mitogen-Activated Protein Kinases, Central Regulators of Cell Stress and Receptor Signaling. F1000Research 2020, 9, 1–20. [Google Scholar] [CrossRef]
- New, L.; Jiang, Y.; Zhao, M.; Liu, K.; Zhu, W.; Flood, L.J.; Kato, Y.; Parry, G.C.N.; Han, J. PRAK, a Novel Protein Kinase Regulated by the P38 MAP Kinase. EMBO J. 1998, 17, 3372–3384. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Johnson, G.L.; Nakamura, K. The C-Jun Kinase/Stress-Activated Pathway: Regulation, Function and Role in Human Disease. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 1341–1348. [Google Scholar] [CrossRef]
- Faulkner, A.; Purcell, R.; Hibbert, A.; Latham, S.; Thomson, S.; Hall, W.L.; Wheeler-Jones, C.; Bishop-Bailey, D. A thin layer angiogenesis assay: A modified basement matrix assay for assessment of endothelial cell differentiation. BMC Cell Biol. 2014, 15, 41. [Google Scholar] [CrossRef]
- He, J.; Ma, Y.; Niu, X.; Pei, J.; Yan, R.; Xu, F.; Ma, J.; Ma, X.; Jia, S.; Ma, W. Silver nanoparticles induce endothelial cytotoxicity through ROS-mediated mitochondria-lysosome damage and autophagy perturbation: The protective role of N-acetylcysteine. Toxicology 2024, 502, 153734. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, Y.; Zheng, L.; Fan, C.; Luo, F.; Zou, Y.; Li, X.; Zha, Z.G.; Zhang, H.T.; Wang, X. High Self-Supporting Chitosan-Based Hydrogel Ink for In Situ 3D Printed Diabetic Wound Dressing. Adv. Funct. Mater. 2025, 35, 2414625. [Google Scholar] [CrossRef]
- Kang, K.; Lim, D.H.; Choi, I.H.; Kang, T.; Lee, K.; Moon, E.Y.; Yang, Y.; Lee, M.S.; Lim, J.S. Vascular Tube Formation and Angiogenesis Induced by Polyvinylpyrrolidone-Coated Silver Nanoparticles. Toxicol. Lett. 2011, 205, 227–234. [Google Scholar] [CrossRef]
- Saeed, B.A.; Lim, V.; Yusof, N.A.; Khor, K.Z.; Rahman, H.S.; Samad, N.A. Antiangiogenic Properties of Nanoparticles: A Systematic Review. Int. J. Nanomed. 2019, 14, 5135–5146. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast Growth Factors: Biology, Function, and Application for Tissue Regeneration. J. Tissue Eng. 2010, 1, 218142. [Google Scholar] [CrossRef] [PubMed]
- Lertkiatmongkol, P.; Liao, D.; Mei, H.; Hu, Y.; Newman, P.J. Endothelial Functions of Platelet/Endothelial Cell Adhesion Molecule-1 (CD31). Curr. Opin. Hematol. 2016, 23, 253–259. [Google Scholar] [CrossRef]
- Zhai, B.T.; Tian, H.; Sun, J.; Zou, J.B.; Zhang, X.F.; Cheng, J.X.; Shi, Y.J.; Fan, Y.; Guo, D.Y. Urokinase-Type Plasminogen Activator Receptor (UPAR) as a Therapeutic Target in Cancer. J. Transl. Med. 2022, 20, 135. [Google Scholar] [CrossRef]
- Villar, I.C.; Francis, S.; Webb, A.; Hobbs, A.J.; Ahluwalia, A. Novel Aspects of Endothelium-Dependent Regulation of Vascular Tone. Kidney Int. 2006, 70, 840–853. [Google Scholar] [CrossRef]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2011, 10, 4–18. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Tinevez, J.Y.; Perry, N.; Schindelin, J.; Hoopes, G.M.; Reynolds, G.D.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.L.; Eliceiri, K.W. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Thevenaz, P.; Ruttimann, U.E.; Unser, M. A pyramid approach to subpixel registration based on intensity. IEEE Trans. Image Process. 1998, 7, 27–41. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik, B.; Zawadzka, K.; Hotowy, A.; Jóźwiak, M.; Jusińska, K.; Wierzbicki, M. Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration. Int. J. Mol. Sci. 2025, 26, 4761. https://doi.org/10.3390/ijms26104761
Wójcik B, Zawadzka K, Hotowy A, Jóźwiak M, Jusińska K, Wierzbicki M. Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration. International Journal of Molecular Sciences. 2025; 26(10):4761. https://doi.org/10.3390/ijms26104761
Chicago/Turabian StyleWójcik, Barbara, Katarzyna Zawadzka, Anna Hotowy, Maria Jóźwiak, Klaudia Jusińska, and Mateusz Wierzbicki. 2025. "Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration" International Journal of Molecular Sciences 26, no. 10: 4761. https://doi.org/10.3390/ijms26104761
APA StyleWójcik, B., Zawadzka, K., Hotowy, A., Jóźwiak, M., Jusińska, K., & Wierzbicki, M. (2025). Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration. International Journal of Molecular Sciences, 26(10), 4761. https://doi.org/10.3390/ijms26104761







