Construction and Bioengineering of Human Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue
Abstract
1. Introduction
2. Construction of Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue
2.1. Isolation of Follicles from Cryopreserved Ovarian Tissue
2.2. Isolation of Ovarian Stromal Cells
2.3. Matrix
2.3.1. Natural Polymers
2.3.2. Decellularized Extracellular Matrix
2.3.3. Synthetic Polymers
2.4. Additional Bioactive Components
3. Challenges in Bioprosthetic Ovary Construction: Safety and Efficiency
3.1. Safety Concerns in Bioprosthetic Ovary Construction
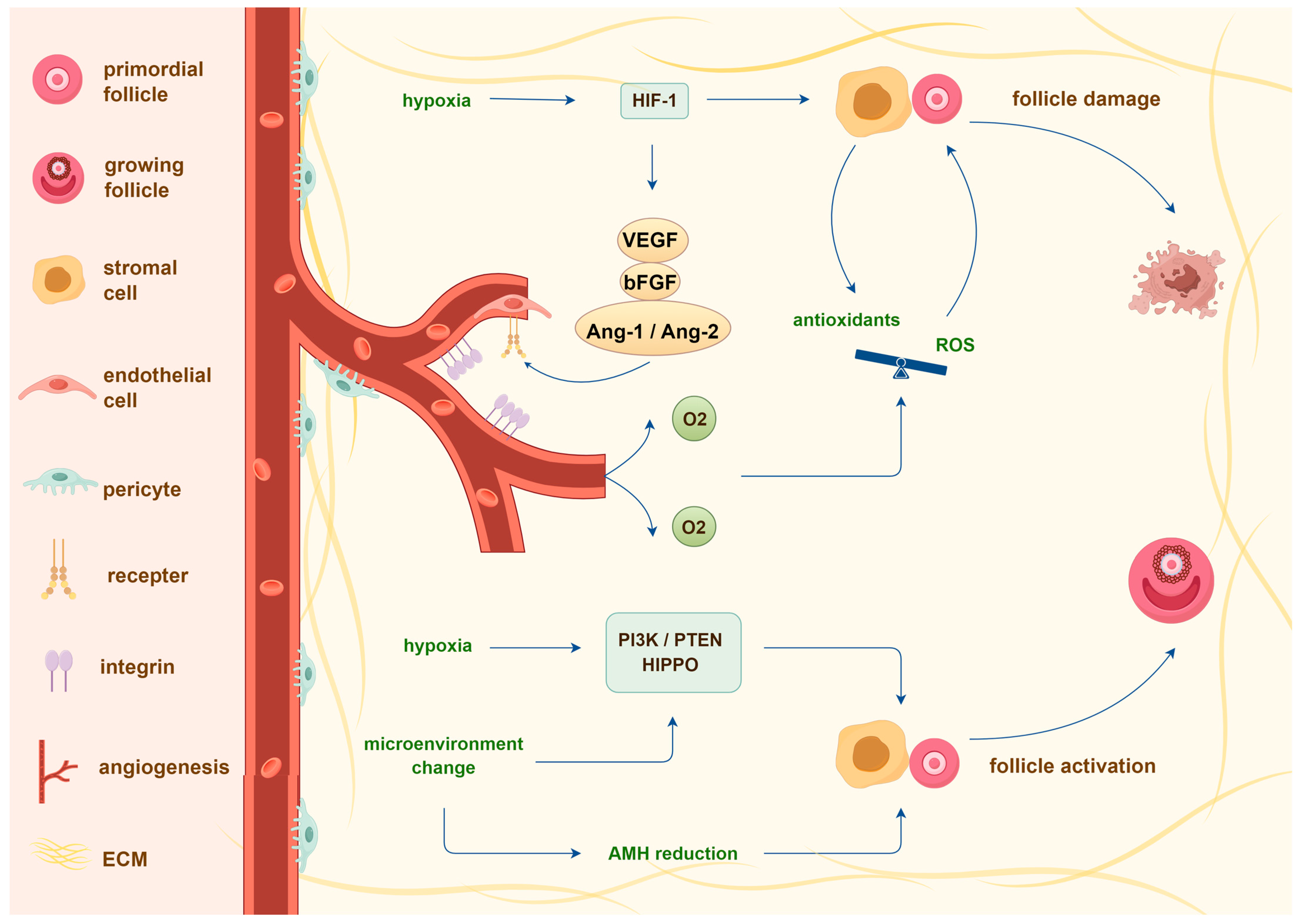
3.2. Transplantation-Induced Follicle Loss and Its Mechanisms
4. Bioengineering of Bioprosthetic Ovaries to Prevent Transplantation-Induced Follicle Loss
4.1. Agents Reducing Transplantation-Induced Follicle Loss
4.1.1. Growth Factors
4.1.2. Hormones
4.1.3. Stem Cells
4.2. Bioengineering of Bioprosthetic Ovary Scaffolds for Targeted Agent Delivery
4.3. Future Directions on Bioprosthetic Ovary Construction
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- The ESHRE Guideline Group on Female Fertility Preservation; Anderson, R.A.; Amant, F.; Braat, D.; D’Angelo, A.; Chuva de Sousa Lopes, S.M.; Demeestere, I.; Dwek, S.; Frith, L.; Lambertini, M.; et al. ESHRE guideline: Female fertility preservation. Hum. Reprod. Open 2020, 2020, hoaa052. [Google Scholar] [CrossRef] [PubMed]
- Donnez, J.; Dolmans, M.-M. Fertility preservation in women. N. Engl. J. Med. 2017, 377, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Donnez, J.; Cacciottola, L. Fertility Preservation: The Challenge of Freezing and Transplanting Ovarian Tissue. Trends Mol. Med. 2021, 27, 777–791. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Demylle, D.; Jadoul, P.; Pirard, C.; Squifflet, J.; Martinez-Madrid, B.; van Langendonckt, A. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet 2004, 364, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, R.; Lotz, L.; Keck, G.; Hoffmann, I.; Mueller, A.; Beckmann, M.W.; van der Ven, H.; Montag, M. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil. Steril. 2012, 97, 387–390. [Google Scholar] [CrossRef]
- Isachenko, V.; Dittrich, R.; Keck, G.; Isachenko, E.; Rahimi, G.; van Der Ven, H.; Montag, M.; Hoffmann, I.; Müller, A.; Distler, W. Cryopreservation of ovarian tissue: Detailed description of methods for transport, freezing and thawing. Geburtshilfe Frauenheilkunde 2012, 72, 927–932. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Falcone, T.; Patrizio, P. Importance of patient selection to analyze in vitro fertilization outcome with transplanted cryopreserved ovarian tissue. Fertil. Steril. 2020, 114, 279–280. [Google Scholar] [CrossRef]
- Shapira, M.; Dolmans, M.-M.; Silber, S.; Meirow, D. Evaluation of ovarian tissue transplantation: Results from three clinical centers. Fertil. Steril. 2020, 114, 388–397. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Marinescu, C.; Saussoy, P.; van Langendonckt, A.; Amorim, C.; Donnez, J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood J. Am. Soc. Hematol. 2010, 116, 2908–2914. [Google Scholar] [CrossRef]
- Rosendahl, M.; Andersen, M.T.; Ralfkiær, E.; Kjeldsen, L.; Andersen, M.K.; Andersen, C.Y. Evidence of residual disease in cryopreserved ovarian cortex from female patients with leukemia. Fertil. Steril. 2010, 94, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.G.; Kimler, B.F.; Smith, B.M.; Woodruff, T.K.; Pavone, M.E.; Duncan, F.E. Ovarian tissue cryopreservation in young females through the Oncofertility Consortium’s National Physicians Cooperative. Future Oncol. 2018, 14, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Masciangelo, R. Risk of transplanting malignant cells in cryopreserved ovarian tissue. Fertil. Preserv. Princ. Pract. 2021, 8, 302. [Google Scholar]
- Fisch, B.; Abir, R. Female fertility preservation: Past, present and future. Reproduction 2018, 156, F11–F27. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Todorov, P.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Wang, M.; Isachenko, V. In vitro activation of cryopreserved ovarian tissue: A single-arm meta-analysis and systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 258, 258–264. [Google Scholar] [CrossRef]
- Accadia, F.G.M. Genetic imprinting: The paradigm of Prader-Willi and Angelman syndromes. Endocr. Involv. Dev. Syndr. 2009, 14, 20–28. [Google Scholar]
- Chen, J.; Todorov, P.; Isachenko, E.; Rahimi, G.; Mallmann, P.; Isachenko, V. Construction and cryopreservation of an artificial ovary in cancer patients as an element of cancer therapy and a promising approach to fertility restoration. Hum. Fertil. 2022, 25, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Shapira, M.; Raanani, H.; Meirow, D. Challenges of fertility preservation in leukemia patients. Minerva Ginecol. 2018, 70, 456–464. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Amorim, C.A. Fertility preservation: Construction and use of artificial ovaries. Reproduction 2019, 158, F15–F25. [Google Scholar] [CrossRef]
- Greve, T.; Clasen-Linde, E.; Andersen, M.T.; Andersen, M.K.; Sørensen, S.D.; Rosendahl, M.; Ralfkiær, E.; Andersen, C.Y. Cryopreserved ovarian cortex from patients with leukemia in complete remission contains no apparent viable malignant cells. Blood J. Am. Soc. Hematol. 2012, 120, 4311–4316. [Google Scholar] [CrossRef]
- Dolmans, M.-M.; Michaux, N.; Camboni, A.; Martinez-Madrid, B.; van Langendonckt, A.; Annarita Nottola, S.; Donnez, J. Evaluation of Liberase, a purified enzyme blend, for the isolation of human primordial and primary ovarian follicles. Hum. Reprod. 2006, 21, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, J.; Camboni, A.; Dath, C.; van Langendonckt, A.; Dolmans, M.-M.; Donnez, J.; Amorim, C.A. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: Protocol for application in a clinical setting. Fertil. Steril. 2011, 96, 379–383.e3. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.-M.; Hobeika, M.; Cernogoraz, A.; Donnez, J.; Amorim, C.A. A modified and tailored human follicle isolation procedure improves follicle recovery and survival. J. Ovarian Res. 2017, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, V.M.; Isachenko, E.; Rappl, G.; Rahimi, G.; Hanstein, B.; Morgenstern, B.; Mallmann, P.; Isachenko, V. Construction of human artificial ovary from cryopreserved ovarian tissue: Appearance of apoptosis and necrosis after enzymatic isolation of follicles. Cryobiology 2018, 84, 10–14. [Google Scholar] [CrossRef]
- Schmidt, V.M.; Isachenko, V.; Rappl, G.; Rahimi, G.; Hanstein, B.; Morgenstern, B.; Mallmann, P.; Isachenko, E. Comparison of the enzymatic efficiency of Liberase TM and tumor dissociation enzyme: Effect on the viability of cells digested from fresh and cryopreserved human ovarian cortex. Reprod. Biol. Endocrinol. 2018, 16, 57. [Google Scholar] [CrossRef]
- Chen, J.; Isachenko, E.; Wang, W.; Du, X.; Wang, M.; Rahimi, G.; Mallmann, P.; Isachenko, V. Optimization of follicle isolation for bioengineering of human artificial ovary. Biopreservation Biobanking 2022, 20, 529–539. [Google Scholar] [CrossRef]
- Lierman, S.; Tilleman, K.; Cornelissen, M.; De Vos, W.H.; Weyers, S.; T’Sjoen, G.; Cuvelier, C.A.; De Sutter, P. Follicles of various maturation stages react differently to enzymatic isolation: A comparison of different isolation protocols. Reprod. Biomed. Online 2015, 30, 181–190. [Google Scholar] [CrossRef]
- Mouloungui, E.; Zver, T.; Roux, C.; Amiot, C. A protocol to isolate and qualify purified human preantral follicles in cases of acute leukemia, for future clinical applications. J. Ovarian Res. 2018, 11, 4. [Google Scholar] [CrossRef]
- Orisaka, M.; Tajima, K.; Mizutani, T.; Miyamoto, K.; Tsang, B.K.; Fukuda, S.; Yoshida, Y.; Kotsuji, F. Granulosa cells promote differentiation of cortical stromal cells into theca cells in the bovine ovary. Biol. Reprod. 2006, 75, 734–740. [Google Scholar] [CrossRef]
- Dath, C.; Dethy, A.; van Langendonckt, A.; van Eyck, A.-S.; Amorim, C.; Luyckx, V.; Donnez, J.; Dolmans, M.-M. Endothelial cells are essential for ovarian stromal tissue restructuring after xenotransplantation of isolated ovarian stromal cells. Hum. Reprod. 2011, 26, 1431–1439. [Google Scholar] [CrossRef]
- Soares, M.; Sahrari, K.; Chiti, M.C.; Amorim, C.; Ambroise, J.; Donnez, J.; Dolmans, M.-M. The best source of isolated stromal cells for the artificial ovary: Medulla or cortex, cryopreserved or fresh? Hum. Reprod. 2015, 30, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Tamadon, A.; Park, K.-H.; Kim, Y.Y.; Kang, B.-C.; Ku, S.-Y. Efficient biomaterials for tissue engineering of female reproductive organs. Tissue Eng. Regen. Med. 2016, 13, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.M.; Lucci, C.M.; Paulini, F.; Donnez, J.; Amorim, C.A. Further insights into the impact of mouse follicle stage on graft outcome in an artificial ovary environment. Mol. Hum. Reprod. 2017, 23, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Shikanov, A. The artificial ovary: Current status and future perspectives. Future Oncol. 2016, 12, 2323–2332. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.; Dolmans, M.-M.; Vanacker, J.; Scalercio, S.R.; Donnez, J.; Amorim, C.A. First step in developing a 3D biodegradable fibrin scaffold for an artificial ovary. J. Ovarian Res. 2013, 6, 83. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, C.; Zhang, X.; He, X.; Liu, Y. Design and application strategies of natural polymer biomaterials in artificial ovaries. Ann. Biomed. Eng. 2023, 51, 461–478. [Google Scholar] [CrossRef]
- Desai, N.; Alex, A.; AbdelHafez, F.; Calabro, A.; Goldfarb, J.; Fleischman, A.; Falcone, T. Three-dimensional in vitro follicle growth: Overview of culture models, biomaterials, design parameters and future directions. Reprod. Biol. Endocrinol. 2010, 8, 119. [Google Scholar] [CrossRef]
- Gougeon, A. Human ovarian follicular development: From activation of resting follicles to preovulatory maturation. Annales D’endocrinologie 2010, 71, 132–143. [Google Scholar] [CrossRef]
- Hummitzsch, K.; Irving-Rodgers, H.F.; Schwartz, J.; Rodgers, R.J. Development of the mammalian ovary and follicles. In The Ovary; Academic Press: London, UK, 2019; pp. 71–82. [Google Scholar]
- Shea, L.D.; Woodruff, T.K.; Shikanov, A. Bioengineering the ovarian follicle microenvironment. Annu. Rev. Biomed. Eng. 2014, 16, 29–52. [Google Scholar] [CrossRef]
- Gosden, R. Restitution of fertility in sterilized mice by transferring primordial ovarian follicles. Hum. Reprod. 1990, 5, 117–122. [Google Scholar] [CrossRef]
- Carroll, J.; Gosden, R.G. Physiology: Transplantation of frozen—Thawed mouse primordial follicles. Hum. Reprod. 1993, 8, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Martinez-Madrid, B.; Gadisseux, E.; Guiot, Y.; Yuan, W.Y.; Torre, A.; Camboni, A.; van Langendonckt, A.; Donnez, J. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction 2007, 134, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, M.-M.; Yuan, W.Y.; Camboni, A.; Torre, A.; van Langendonckt, A.; Martinez-Madrid, B.; Donnez, J. Development of antral follicles after xenografting of isolated small human preantral follicles. Reprod. Biomed. Online 2008, 16, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Barker, T.H. Fibrin-based biomaterials: Modulation of macroscopic properties through rational design at the molecular level. Acta Biomater. 2014, 10, 1502–1514. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.M.; Donnez, J.; Amorim, C.A. Fibrin in reproductive tissue engineering: A review on its application as a biomaterial for fertility preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef]
- Luyckx, V.; Dolmans, M.-M.; Vanacker, J.; Legat, C.; Moya, C.F.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Thomson, K.S.; Dupras, S.K.; Murry, C.E.; Scatena, M.; Regnier, M. Proangiogenic microtemplated fibrin scaffolds containing aprotinin promote improved wound healing responses. Angiogenesis 2014, 17, 195–205. [Google Scholar] [CrossRef][Green Version]
- Park, C.H.; Woo, K.M. Fibrin-based biomaterial applications in tissue engineering and regenerative medicine. In Biomimetic Medical Materials: From Nanotechnology to 3D Bioprinting; Springer: Singapore, 2018; pp. 253–261. [Google Scholar]
- Wang, W.; Pei, C.; Isachenko, E.; Zhou, Y.; Wang, M.; Rahimi, G.; Liu, W.; Mallmann, P.; Isachenko, V. Automatic evaluation for bioengineering of human artificial ovary: A model for fertility preservation for prepubertal female patients with a malignant tumor. Int. J. Mol. Sci. 2022, 23, 12419. [Google Scholar] [CrossRef]
- Smith, R.M.; Shikanov, A.; Kniazeva, E.; Ramadurai, D.; Woodruff, T.K.; Shea, L.D. Fibrin-mediated delivery of an ovarian follicle pool in a mouse model of infertility. Tissue Eng. Part A 2014, 20, 3021–3030. [Google Scholar] [CrossRef]
- Chiti, M.C.; Dolmans, M.-M.; Orellana, R.; Soares, M.; Paulini, F.; Donnez, J.; Amorim, C. Influence of follicle stage on artificial ovary outcome using fibrin as a matrix. Hum. Reprod. 2016, 31, 427–435. [Google Scholar] [CrossRef]
- Kniazeva, E.; Hardy, A.; Boukaidi, S.; Woodruff, T.; Jeruss, J.; Shea, L. Primordial follicle transplantation within designer biomaterial grafts produce live births in a mouse infertility model. Sci. Rep. 2015, 5, 17709. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh, A.; Jahanpeyma, F.; Talebi, A.; Moradi, F.; Hamidieh, A.A.; Eimani, H. Fibrin scaffold incorporating platelet lysate enhance follicle survival and angiogenesis in cryopreserved preantral follicle transplantation. Galen Med. J. 2020, 9, e1558. [Google Scholar] [CrossRef]
- Paulini, F.; Vilela, J.M.; Chiti, M.C.; Donnez, J.; Jadoul, P.; Dolmans, M.-M.; Amorim, C.A. Survival and growth of human preantral follicles after cryopreservation of ovarian tissue, follicle isolation and short-term xenografting. Reprod. Biomed. Online 2016, 33, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.-M.; Mortiaux, L.; Zhuge, F.; Ouni, E.; Shahri, P.A.K.; van Ruymbeke, E.; Champagne, S.-D.; Donnez, J.; Amorim, C.A. A novel fibrin-based artificial ovary prototype resembling human ovarian tissue in terms of architecture and rigidity. J. Assist. Reprod. Genet. 2018, 35, 41–48. [Google Scholar] [CrossRef]
- Guthold, M.; Liu, W.; Sparks, E.; Jawerth, L.; Peng, L.; Falvo, M.; Superfine, R.; Hantgan, R.R.; Lord, S.T. A comparison of the mechanical and structural properties of fibrin fibers with other protein fibers. Cell Biochem. Biophys. 2007, 49, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Vanacker, J.; Amorim, C.A. Alginate: A versatile biomaterial to encapsulate isolated ovarian follicles. Ann. Biomed. Eng. 2017, 45, 1633–1649. [Google Scholar] [CrossRef]
- Xu, M.; Banc, A.; Woodruff, T.K.; Shea, L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009, 103, 378–386. [Google Scholar] [CrossRef]
- Laronda, M.M.; Duncan, F.E.; Hornick, J.E.; Xu, M.; Pahnke, J.E.; Whelan, K.A.; Shea, L.D.; Woodruff, T.K. Alginate encapsulation supports the growth and differentiation of human primordial follicles within ovarian cortical tissue. J. Assist. Reprod. Genet. 2014, 31, 1013–1028. [Google Scholar] [CrossRef]
- Nagashima, J.; Wildt, D.E.; Travis, A.J.; Songsasen, N. Follicular size and stage and gonadotropin concentration affect alginate-encapsulated in vitro growth and survival of pre-and early antral dog follicles. Reprod. Fertil. Dev. 2017, 29, 262–273. [Google Scholar] [CrossRef]
- Vanacker, J.; Luyckx, V.; Dolmans, M.-M.; Des Rieux, A.; Jaeger, J.; van Langendonckt, A.; Donnez, J.; Amorim, C.A. Transplantation of an alginate–matrigel matrix containing isolated ovarian cells: First step in developing a biodegradable scaffold to transplant isolated preantral follicles and ovarian cells. Biomaterials 2012, 33, 6079–6085. [Google Scholar] [CrossRef]
- Vanacker, J.; Dolmans, M.-M.; Luyckx, V.; Donnez, J.; Amorim, C.A. First transplantation of isolated murine follicles in alginate. Regen. Med. 2014, 9, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.D.; Kniazeva, E.; Lee, H.C.; Xiao, S.; Oakes, R.S.; Saito, E.; Jeruss, J.S.; Shikanov, A.; Woodruff, T.K.; Shea, L.D. Retrievable hydrogels for ovarian follicle transplantation and oocyte collection. Biotechnol. Bioeng. 2018, 115, 2075–2086. [Google Scholar] [CrossRef] [PubMed]
- Dadashzadeh, A.; Moghassemi, S.; Shavandi, A.; Amorim, C.A. A review on biomaterials for ovarian tissue engineering. Acta Biomater. 2021, 135, 48–63. [Google Scholar] [CrossRef] [PubMed]
- Kreeger, P.K.; Deck, J.W.; Woodruff, T.K.; Shea, L.D. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials 2006, 27, 714–723. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, H.; Weir, M.D.; Bao, C.; Xu, H.H. Umbilical cord stem cells released from alginate–fibrin microbeads inside macroporous and biofunctionalized calcium phosphate cement for bone regeneration. Acta Biomater. 2012, 8, 2297–2306. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Imani, R.; Moghassemi, S.; Omidfar, K.; Abolfathi, N. Study of hybrid alginate/gelatin hydrogel-incorporated niosomal Aloe vera capable of sustained release of Aloe vera as potential skin wound dressing. Polym. Bull. 2020, 77, 387–403. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef]
- Wu, T.; Gao, Y.; Su, J.; Tang, X.; Chen, Q.; Ma, L.; Zhang, J.; Wu, J.; Wang, S. Three-dimensional bioprinting of artificial ovaries by an extrusion-based method using gelatin-methacryloyl bioink. Climacteric 2022, 25, 170–178. [Google Scholar] [CrossRef]
- Zand, E.; Rajablou, E.; Siadat, S.F.; Beiki, B.; Akbarinejad, V.; Amorim, C.A.; Rezazadeh Valojerdi, M.; Tahaei, L.A.; Fathi, R. Successful 3D culture and transplantation of mouse isolated preantral follicles in hydrogel of bioengineered Wharton’s jelly. PLoS ONE 2023, 18, e0290095. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Hong, H.; Hu, R.; Liu, J.; Liu, C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact. Mater. 2022, 10, 15–31. [Google Scholar] [CrossRef]
- Faulk, D.M.; Johnson, S.A.; Zhang, L.; Badylak, S.F. Role of the extracellular matrix in whole organ engineering. J. Cell. Physiol. 2014, 229, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Fedecostante, M.; Onciu, O.G.; Westphal, K.G.; Masereeuw, R. Towards a bioengineered kidney: Recellularization strategies for decellularized native kidney scaffolds. Int. J. Artif. Organs 2017, 40, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Geerts, S.; Jaramillo, M.; Uygun, B.E. Preparation of decellularized liver scaffolds and recellularized liver grafts. In Decellularized Scaffolds and Organogenesis: Methods and Protocols; Springer: Singapore, 2018; pp. 255–270. [Google Scholar]
- Hodgson, M.J.; Knutson, C.C.; Momtahan, N.; Cook, A.D. Extracellular matrix from whole porcine heart decellularization for cardiac tissue engineering. In Decellularized Scaffolds and Organogenesis: Methods and Protocols; Springer: Singapore, 2018; pp. 95–102. [Google Scholar]
- Oktay, K.; Bedoschi, G.; Pacheco, F.; Turan, V.; Emirdar, V. First pregnancies, live birth, and in vitro fertilization outcomes after transplantation of frozen-banked ovarian tissue with a human extracellular matrix scaffold using robot-assisted minimally invasive surgery. Am. J. Obstet. Gynecol. 2016, 214, 94.e1–94.e9. [Google Scholar] [CrossRef] [PubMed]
- Laronda, M.M.; Jakus, A.E.; Whelan, K.A.; Wertheim, J.A.; Shah, R.N.; Woodruff, T.K. Initiation of puberty in mice following decellularized ovary transplant. Biomaterials 2015, 50, 20–29. [Google Scholar] [CrossRef]
- Pors, S.; Ramløse, M.; Nikiforov, D.; Lundsgaard, K.; Cheng, J.; Andersen, C.Y.; Kristensen, S. Initial steps in reconstruction of the human ovary: Survival of pre-antral stage follicles in a decellularized human ovarian scaffold. Hum. Reprod. 2019, 34, 1523–1535. [Google Scholar] [CrossRef]
- Rieder, E.; Steinacher-Nigisch, A.; Weigel, G. Human immune-cell response towards diverse xenogeneic and allogeneic decellularized biomaterials. Int. J. Surg. 2016, 36, 347–351. [Google Scholar] [CrossRef]
- Padma, A.M.; Alsheikh, A.B.; Song, M.J.; Akouri, R.; Akyürek, L.M.; Oltean, M.; Brännström, M.; Hellström, M. Immune response after allogeneic transplantation of decellularized uterine scaffolds in the rat. Biomed. Mater. 2021, 16, 045021. [Google Scholar] [CrossRef]
- Kim, B.S.; Kim, H.; Gao, G.; Jang, J.; Cho, D.-W. Decellularized extracellular matrix: A step towards the next generation source for bioink manufacturing. Biofabrication 2017, 9, 034104. [Google Scholar] [CrossRef]
- Kim, J.; Perez, A.S.; Claflin, J.; David, A.; Zhou, H.; Shikanov, A. Synthetic hydrogel supports the function and regeneration of artificial ovarian tissue in mice. npj Regen. Med. 2016, 1, 16010. [Google Scholar] [CrossRef]
- Barker, T.H.; Fuller, G.M.; Klinger, M.M.; Feldman, D.; Hagood, J. Modification of fibrinogen with poly(ethylene glycol) and its effects on fibrin clot characteristics. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2001, 56, 529–535. [Google Scholar] [CrossRef]
- Dikovsky, D.; Bianco-Peled, H.; Seliktar, D. Proteolytically Degradable Photo-Polymerized Hydrogels Made From PEG–Fibrinogen Adducts. Adv. Eng. Mater. 2010, 12, B200–B209. [Google Scholar] [CrossRef]
- Dadashzadeh, A.; Moghassemi, S.; Amorim, C. Evaluation of PEGylated fibrin as a three-dimensional biodegradable scaffold for ovarian tissue engineering. Mater. Today Chem. 2021, 22, 100626. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoo, J.J.; Atala, A. Biomaterials and tissue engineering. In Clinical Regenerative Medicine in Urology; Springer: Singapore, 2018; pp. 17–51. [Google Scholar]
- Liu, X.; Wu, K.; Gao, L.; Wang, L.; Shi, X. Biomaterial strategies for the application of reproductive tissue engineering. Bioact. Mater. 2022, 14, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Donnez, J.; Dolmans, M.-M. Ovarian tissue damage after grafting: Systematic review of strategies to improve follicle outcomes. Reprod. Biomed. Online 2021, 43, 351–369. [Google Scholar] [CrossRef]
- Roness, H.; Meirow, D. FERTILITY PRESERVATION: Follicle reserve loss in ovarian tissue transplantation. Reproduction 2019, 158, F35–F44. [Google Scholar] [CrossRef]
- Soares, M.; Sahrari, K.; Amorim, C.A.; Saussoy, P.; Donnez, J.; Dolmans, M.-M. Evaluation of a human ovarian follicle isolation technique to obtain disease-free follicle suspensions before safely grafting to cancer patients. Fertil. Steril. 2015, 104, 672–680.e2. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Saussoy, P.; Maskens, M.; Reul, H.; Amorim, C.A.; Donnez, J.; Dolmans, M.M. Eliminating malignant cells from cryopreserved ovarian tissue is possible in leukaemia patients. Br. J. Haematol. 2017, 178, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.; Saussoy, P.; Sahrari, K.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. Is transplantation of a few leukemic cells inside an artificial ovary able to induce leukemia in an experimental model? J. Assist. Reprod. Genet. 2015, 32, 597–606. [Google Scholar] [CrossRef]
- Barberet, J.; Binquet, C.; Guilleman, M.; Doukani, A.; Choux, C.; Bruno, C.; Bourredjem, A.; Chapusot, C.; Bourc’His, D.; Duffourd, Y. Do assisted reproductive technologies and in vitro embryo culture influence the epigenetic control of imprinted genes and transposable elements in children? Hum. Reprod. 2021, 36, 479–492. [Google Scholar] [CrossRef]
- Barberet, J.; Romain, G.; Binquet, C.; Guilleman, M.; Bruno, C.; Ginod, P.; Chapusot, C.; Choux, C.; Fauque, P. Do frozen embryo transfers modify the epigenetic control of imprinted genes and transposable elements in newborns compared with fresh embryo transfers and natural conceptions? Fertil. Steril. 2021, 116, 1468–1480. [Google Scholar] [CrossRef]
- Ma, Y.; Long, C.; Liu, G.; Bai, H.; Ma, L.; Bai, T.; Zuo, Y.; Li, S. WGBS combined with RNA-seq analysis revealed that Dnmt1 affects the methylation modification and gene expression changes during mouse oocyte vitrification. Theriogenology 2022, 177, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Ge, N.; Huang, B.; Fu, J.; Zhang, Y.; Wang, N.; Xu, Y.; Li, L.; Peng, X.; Zou, Y. Impacts of vitrification on the transcriptome of human ovarian tissue in patients with gynecological cancer. Front. Genet. 2023, 14, 1114650. [Google Scholar] [CrossRef] [PubMed]
- Trapphoff, T.; Dieterle, S. Cryopreservation of Ovarian and Testicular Tissue and the Influence on Epigenetic Pattern. Int. J. Mol. Sci. 2023, 24, 11061. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Testing and interpreting measures of ovarian reserve: A committee opinion. Fertil. Steril. 2015, 103, e9–e17. [Google Scholar] [CrossRef]
- Amorim, C.A.; David, A.; Dolmans, M.-M.; Camboni, A.; Donnez, J.; van Langendonckt, A. Impact of freezing and thawing of human ovarian tissue on follicular growth after long-term xenotransplantation. J. Assist. Reprod. Genet. 2011, 28, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Orth, I.; Isachenko, E.; Mallmann, P.; Peters, D.; Schmidt, T.; Morgenstern, B.; Foth, D.; Hanstein, B.; Rahimi, G. Viability of human ovarian tissue confirmed 5 years after freezing with spontaneous ice-formation by autografting and chorio-allantoic membrane culture. Cryobiology 2013, 66, 233–238. [Google Scholar] [CrossRef]
- Gavish, Z.; Spector, I.; Peer, G.; Schlatt, S.; Wistuba, J.; Roness, H.; Meirow, D. Follicle activation is a significant and immediate cause of follicle loss after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2018, 35, 61–69. [Google Scholar] [CrossRef]
- Martinez-Madrid, B.; Donnez, J.; van Eyck, A.-S.; Veiga-Lopez, A.; Dolmans, M.-M.; van Langendonckt, A. Chick embryo chorioallantoic membrane (CAM) model: A useful tool to study short-term transplantation of cryopreserved human ovarian tissue. Fertil. Steril. 2009, 91, 285–292. [Google Scholar] [CrossRef]
- Van Eyck, A.-S.; Jordan, B.F.; Gallez, B.; Heilier, J.-F.; van Langendonckt, A.; Donnez, J. Electron paramagnetic resonance as a tool to evaluate human ovarian tissue reoxygenation after xenografting. Fertil. Steril. 2009, 92, 374–381. [Google Scholar] [CrossRef]
- Cacciottola, L.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. In vivo characterization of metabolic activity and oxidative stress in grafted human ovarian tissue using microdialysis. Fertil. Steril. 2018, 110, 534–544.e3. [Google Scholar] [CrossRef]
- Takahashi, N.; Davy, P.M.; Gardner, L.H.; Mathews, J.; Yamazaki, Y.; Allsopp, R.C. Hypoxia inducible factor 1 alpha is expressed in germ cells throughout the murine life cycle. PLoS ONE 2016, 11, e0154309. [Google Scholar] [CrossRef]
- Zhou, J.; Li, C.; Yao, W.; Alsiddig, M.; Huo, L.; Liu, H.; Miao, Y.-L. Hypoxia-inducible factor-1α-dependent autophagy plays a role in glycolysis switch in mouse granulosa cells. Biol. Reprod. 2018, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell biology of ischemia/reperfusion injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar]
- Van Eyck, A.-S.; Bouzin, C.; Feron, O.; Romeu, L.; van Langendonckt, A.; Donnez, J.; Dolmans, M.-M. Both host and graft vessels contribute to revascularization of xenografted human ovarian tissue in a murine model. Fertil. Steril. 2010, 93, 1676–1685. [Google Scholar] [CrossRef]
- Gavish, Z.; Peer, G.; Hadassa, R.; Yoram, C.; Meirow, D. Follicle activation and ‘burn-out’contribute to post-transplantation follicle loss in ovarian tissue grafts: The effect of graft thickness. Hum. Reprod. 2014, 29, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Ayuandari, S.; Winkler-Crepaz, K.; Paulitsch, M.; Wagner, C.; Zavadil, C.; Manzl, C.; Ziehr, S.C.; Wildt, L.; Hofer-Tollinger, S. Follicular growth after xenotransplantation of cryopreserved/thawed human ovarian tissue in SCID mice: Dynamics and molecular aspects. J. Assist. Reprod. Genet. 2016, 33, 1585–1593. [Google Scholar] [CrossRef]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.-h.; Kawamura, N.; Tamura, M.; Hashimoto, S. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef]
- Masciangelo, R.; Hossay, C.; Chiti, M.C.; Manavella, D.D.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. Role of the PI3K and Hippo pathways in follicle activation after grafting of human ovarian tissue. J. Assist. Reprod. Genet. 2020, 37, 101–108. [Google Scholar] [CrossRef]
- Gigli, I.; Cushman, R.; Wahl, C.; Fortune, J. Evidence for a role for anti-Müllerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol. Reprod. Dev. 2005, 71, 480–488. [Google Scholar] [CrossRef]
- Meirow, D.; Roness, H.; Kristensen, S.G.; Andersen, C.Y. Optimizing outcomes from ovarian tissue cryopreservation and transplantation; activation versus preservation. Hum. Reprod. 2015, 30, 2453–2456. [Google Scholar] [CrossRef]
- Silber, S. Ovarian tissue cryopreservation and transplantation: Scientific implications. J. Assist. Reprod. Genet. 2016, 33, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Isachenko, V.; Mallmann, P.; Petrunkina, A.M.; Rahimi, G.; Nawroth, F.; Hancke, K.; Felberbaum, R.; Genze, F.; Damjanoski, I.; Isachenko, E. Comparison of in vitro-and chorioallantoic membrane (CAM)-culture systems for cryopreserved medulla-contained human ovarian tissue. PLoS ONE 2012, 7, e32549. [Google Scholar] [CrossRef]
- Isachenko, V.; Todorov, P.; Isachenko, E.; Rahimi, G.; Hanstein, B.; Salama, M.; Mallmann, P.; Tchorbanov, A.; Hardiman, P.; Getreu, N. Cryopreservation and xenografting of human ovarian fragments: Medulla decreases the phosphatidylserine translocation rate. Reprod. Biol. Endocrinol. 2016, 14, 79. [Google Scholar] [CrossRef]
- Shikanov, A.; Zhang, Z.; Xu, M.; Smith, R.M.; Rajan, A.; Woodruff, T.K.; Shea, L.D. Fibrin encapsulation and vascular endothelial growth factor delivery promotes ovarian graft survival in mice. Tissue Eng. Part A 2011, 17, 3095–3104. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-M.; Yan, J.; Li, R.; Li, M.; Yan, L.-Y.; Wang, T.-R.; Zhao, H.-C.; Zhao, Y.; Yu, Y.; Qiao, J. Improvement in the quality of heterotopic allotransplanted mouse ovarian tissues with basic fibroblast growth factor and fibrin hydrogel. Hum. Reprod. 2013, 28, 2784–2793. [Google Scholar] [CrossRef] [PubMed]
- Labied, S.; Delforge, Y.; Munaut, C.; Blacher, S.; Colige, A.; Delcombel, R.; Henry, L.; Fransolet, M.; Jouan, C.; d’Hauterive, S.P. Isoform 111 of vascular endothelial growth factor (VEGF111) improves angiogenesis of ovarian tissue xenotransplantation. Transplantation 2013, 95, 426–433. [Google Scholar] [CrossRef]
- Gao, J.; Huang, Y.; Li, M.; Zhao, H.; Zhao, Y.; Li, R.; Yan, J.; Yu, Y.; Qiao, J. Effect of local basic fibroblast growth factor and vascular endothelial growth factor on subcutaneously allotransplanted ovarian tissue in ovariectomized mice. PLoS ONE 2015, 10, e0134035. [Google Scholar] [CrossRef]
- Kang, B.-J.; Wang, Y.; Zhang, L.; Xiao, Z.; Li, S.-W. bFGF and VEGF improve the quality of vitrified-thawed human ovarian tissues after xenotransplantation to SCID mice. J. Assist. Reprod. Genet. 2016, 33, 281–289. [Google Scholar] [CrossRef]
- Tanaka, A.; Nakamura, H.; Tabata, Y.; Fujimori, Y.; Kumasawa, K.; Kimura, T. Effect of sustained release of basic fibroblast growth factor using biodegradable gelatin hydrogels on frozen-thawed human ovarian tissue in a xenograft model. J. Obstet. Gynaecol. Res. 2018, 44, 1947–1955. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Mehranjani, M.S.; Shariatzadeh, S.M.A.; Eimani, H.; Shahverdi, A. N-acetylcysteine improves function and follicular survival in mice ovarian grafts through inhibition of oxidative stress. Reprod. Biomed. Online 2015, 30, 101–110. [Google Scholar] [CrossRef]
- Olesen, H.Ø.; Pors, S.E.; Jensen, L.B.; Grønning, A.P.; Lemser, C.E.; Nguyen Heimbürger, M.T.H.; Mamsen, L.S.; Getreu, N.; Christensen, S.T.; Andersen, C.Y. N-acetylcysteine protects ovarian follicles from ischemia-reperfusion injury in xenotransplanted human ovarian tissue. Hum. Reprod. 2021, 36, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.S.; Kim, S.K.; Lee, J.; Youm, H.W.; Lee, J.R.; Suh, C.S.; Kim, S.H. Effect of exogenous anti-Müllerian hormone treatment on cryopreserved and transplanted mouse ovaries. Reprod. Sci. 2016, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Park, L.; Bodine, R.; Ginsberg, M.; Zaninovic, N.; Man, O.A.; Schattman, G.; Rosenwaks, Z.; James, D. Engineered endothelium provides angiogenic and paracrine stimulus to grafted human ovarian tissue. Sci. Rep. 2017, 7, 8203. [Google Scholar] [CrossRef] [PubMed]
- Man, L.; Lustgarten Guahmich, N.; Kallinos, E.; Caiazza, B.; Khan, M.; Liu, Z.-Y.; Patel, R.; Torres, C.; Pepin, D.; Yang, H.S. Chronic superphysiologic AMH promotes premature luteinization of antral follicles in human ovarian xenografts. Sci. Adv. 2022, 8, eabi7315. [Google Scholar] [CrossRef]
- Detti, L.; Fletcher, N.M.; Saed, G.M.; Sweatman, T.W.; Uhlmann, R.A.; Pappo, A.; Peregrin-Alvarez, I. Xenotransplantation of pre-pubertal ovarian cortex and prevention of follicle depletion with anti-Müllerian hormone (AMH). J. Assist. Reprod. Genet. 2018, 35, 1831–1841. [Google Scholar] [CrossRef]
- Xia, X.; Yin, T.; Yan, J.; Yan, L.; Jin, C.; Lu, C.; Wang, T.; Zhu, X.; Zhi, X.; Wang, J. Mesenchymal stem cells enhance angiogenesis and follicle survival in human cryopreserved ovarian cortex transplantation. Cell Transplant. 2015, 24, 1999–2010. [Google Scholar] [CrossRef]
- Manavella, D.; Cacciottola, L.; Desmet, C.; Jordan, B.; Donnez, J.; Amorim, C.; Dolmans, M.-M. Adipose tissue-derived stem cells in a fibrin implant enhance neovascularization in a peritoneal grafting site: A potential way to improve ovarian tissue transplantation. Hum. Reprod. 2018, 33, 270–279. [Google Scholar] [CrossRef]
- Manavella, D.; Cacciottola, L.; Payen, V.; Amorim, C.; Donnez, J.; Dolmans, M.-M. Adipose tissue-derived stem cells boost vascularization in grafted ovarian tissue by growth factor secretion and differentiation into endothelial cell lineages. MHR: Basic Sci. Reprod. Med. 2019, 25, 184–193. [Google Scholar] [CrossRef]
- Cacciottola, L.; Nguyen, T.Y.; Chiti, M.C.; Camboni, A.; Amorim, C.A.; Donnez, J.; Dolmans, M.-M. Long-term advantages of ovarian reserve maintenance and follicle development using adipose tissue-derived stem cells in ovarian tissue transplantation. J. Clin. Med. 2020, 9, 2980. [Google Scholar] [CrossRef]
- Cacciottola, L.; Courtoy, G.E.; Nguyen, T.Y.; Hossay, C.; Donnez, J.; Dolmans, M.-M. Adipose tissue–derived stem cells protect the primordial follicle pool from both direct follicle death and abnormal activation after ovarian tissue transplantation. J. Assist. Reprod. Genet. 2021, 38, 151–161. [Google Scholar] [CrossRef]
- Damous, L.L.; Nakamuta, J.S.; de Carvalho, A.E.T.S.; Soares-Jr, J.M.; de Jesus Simões, M.; Krieger, J.E.; Baracat, E.C. Adipose tissue-derived stem cell therapy in rat cryopreserved ovarian grafts. Stem Cell Res. Ther. 2015, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Sarigol-Calamak, E.; Hascicek, C. Tissue scaffolds as a local drug delivery system for bone regeneration. In Cutting-Edge Enabling Technologies for Regenerative Medicine; Springer: Singapore, 2018; pp. 475–493. [Google Scholar]
- Yang, C.; Blum, N.T.; Lin, J.; Qu, J.; Huang, P. Biomaterial scaffold-based local drug delivery systems for cancer immunotherapy. Sci. Bull. 2020, 65, 1489–1504. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Laronda, M.M.; Rashedi, A.S.; Robinson, C.M.; Lee, C.; Jordan, S.W.; Orwig, K.E.; Woodruff, T.K.; Shah, R.N. “Tissue Papers” from organ-specific decellularized extracellular matrices. Adv. Funct. Mater. 2017, 27, 1700992. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Coppeta, J.R.; Rogers, H.B.; Isenberg, B.C.; Zhu, J.; Olalekan, S.A.; McKinnon, K.E.; Dokic, D.; Rashedi, A.S.; Haisenleder, D.J. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat. Commun. 2017, 8, 14584. [Google Scholar] [CrossRef]
- Salama, M.; Woodruff, T.K. From bench to bedside: Current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet. Gynecol. Scand. 2019, 98, 659–664. [Google Scholar] [CrossRef]
- Suhag, D. Regulatory and Ethical Considerations. In Handbook of Biomaterials for Medical Applications, Volume 2: Applications; Springer: Singapore, 2024; pp. 355–372. [Google Scholar]
- De Jongh, D.; Massey, E.K.; Cronin, A.J.; Schermer, M.H.N.; Bunnik, E.M.; Consortium, V. Early-Phase Clinical Trials of Bio-Artificial Organ Technology: A Systematic Review of Ethical Issues. Transpl. Int. 2022, 35, 10751. [Google Scholar] [CrossRef]

| Matrix | Graft Species | Graft Duration | Outcomes | Studies |
|---|---|---|---|---|
| plasma clots | mouse | up to 15 weeks * | offspring | [41] |
| plasma clots | mouse | up to 12 weeks | offspring | [42] |
| plasma clots | human | 7 days | FS (20%); FD | [43] |
| plasma clots | human | 5 months | FS (29%); FD | [44] |
| alginate | mouse | 1 week | FS (35%); FD | [63] |
| alginate | mouse | 1 week | FS (20%); FD | [64] |
| fibrin | mouse | 7 days | FS (32%); FD | [47] |
| fibrin | mouse | 21 days | FS (17%); FD; HP | [51] |
| fibrin with VEGF | mouse | up to 6 months | HP; offspring | [53] |
| fibrin | mouse | 7 days | FS (28%); FD | [52] |
| fibrin | human | 7 days | FS (23%) | [55] |
| fibrin | human | up to 7 days | FS (35%); FD | [23] |
| fibrin with platelet lysate | mouse | 2 weeks | FS (67%); FD | [54] |
| dECM from bovine ovary | mouse | up to 4 weeks | HP | [78] |
| dECM from human ovary | mouse | 3 weeks | FS (21%); FD | [79] |
| dECM from human ovary | human | 3 weeks | FS (25%) | [79] |
| gelatin | mouse | up to 10 weeks * | HP; offspring | [69] |
| PEG-VS | mouse | 60 days | FD; HP | [83] |
| Substance | Graft Species | Administration Routes | Graft Duration | Outcomes | Studies |
|---|---|---|---|---|---|
| VEGF | mouse | encapsulation in fibrin | up to 20 days * | increased FS; offsprings | [119] |
| bFGF | mouse | encapsulated in fibrin | 1 week | increased RV, FS; decreased AP | [120] |
| VEGF | sheep | embedded in collagen | up to 3 weeks | Increased FS | [121] |
| bFGF& VEGF | mouse | encapsulated in fibrin | up to 21 days | increased RV, FS; decreased AP | [122] |
| bFGF | human | tissue culture before grafting | 7 days | increased RV, FS; decreased AP | [123] |
| bFGF | human | encapsulation in gelatin | 6 weeks | increased RV, FS | [124] |
| NAC | mouse | host treatment | 28 days | decreased AP; increased FS | [125] |
| NAC | human | host treatment | 4 weeks | decreased FA; increased RV, FS | [126] |
| AMH | mouse | supplemented during warming | 28 days | decreased AP | [127] |
| AMH | human | co-transplanted with AMH-expressing cells | 2 weeks | decreased FA; increased RV, FS | [128] |
| AMH | human | host treatment | 14 days | decreased AP, FA | [130] |
| AMH | human | co-transplanted with AMH-expressing cells | up to 14 weeks | decreased AP, FA | [129] |
| ASCs | mouse | inject into graft | 30 days | increased AP | [136] |
| BSCs | human | embedded in Matrigel | 21 days | increased RV, FS; decreased AP | [131] |
| ASCs | human | embedded in fibrin | 7 days | increased RV, FS | [132] |
| ASCs | human | embedded in fibrin | 6 months | increased FS | [134] |
| ASCs | human | embedded in fibrin | 10 days | decreased AP, FA | [135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, M.; Todorov, P.; Rahimi, G.; Salama, M.; Woodruff, T.K.; Isachenko, E.; Skala, C.; Isachenko, V. Construction and Bioengineering of Human Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue. Int. J. Mol. Sci. 2025, 26, 5545. https://doi.org/10.3390/ijms26125545
Cao M, Todorov P, Rahimi G, Salama M, Woodruff TK, Isachenko E, Skala C, Isachenko V. Construction and Bioengineering of Human Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue. International Journal of Molecular Sciences. 2025; 26(12):5545. https://doi.org/10.3390/ijms26125545
Chicago/Turabian StyleCao, Mengyang, Plamen Todorov, Gohar Rahimi, Mahmoud Salama, Teresa K. Woodruff, Evgenia Isachenko, Christine Skala, and Volodimir Isachenko. 2025. "Construction and Bioengineering of Human Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue" International Journal of Molecular Sciences 26, no. 12: 5545. https://doi.org/10.3390/ijms26125545
APA StyleCao, M., Todorov, P., Rahimi, G., Salama, M., Woodruff, T. K., Isachenko, E., Skala, C., & Isachenko, V. (2025). Construction and Bioengineering of Human Bioprosthetic Ovaries from Cryopreserved Ovarian Tissue. International Journal of Molecular Sciences, 26(12), 5545. https://doi.org/10.3390/ijms26125545