Iron Mediates Radiation-Induced Glioblastoma Cell Diffusion
Abstract
1. Introduction
2. Results
2.1. Radiation-Induced Iron Accumulation Enhances Glioma Cell Migration
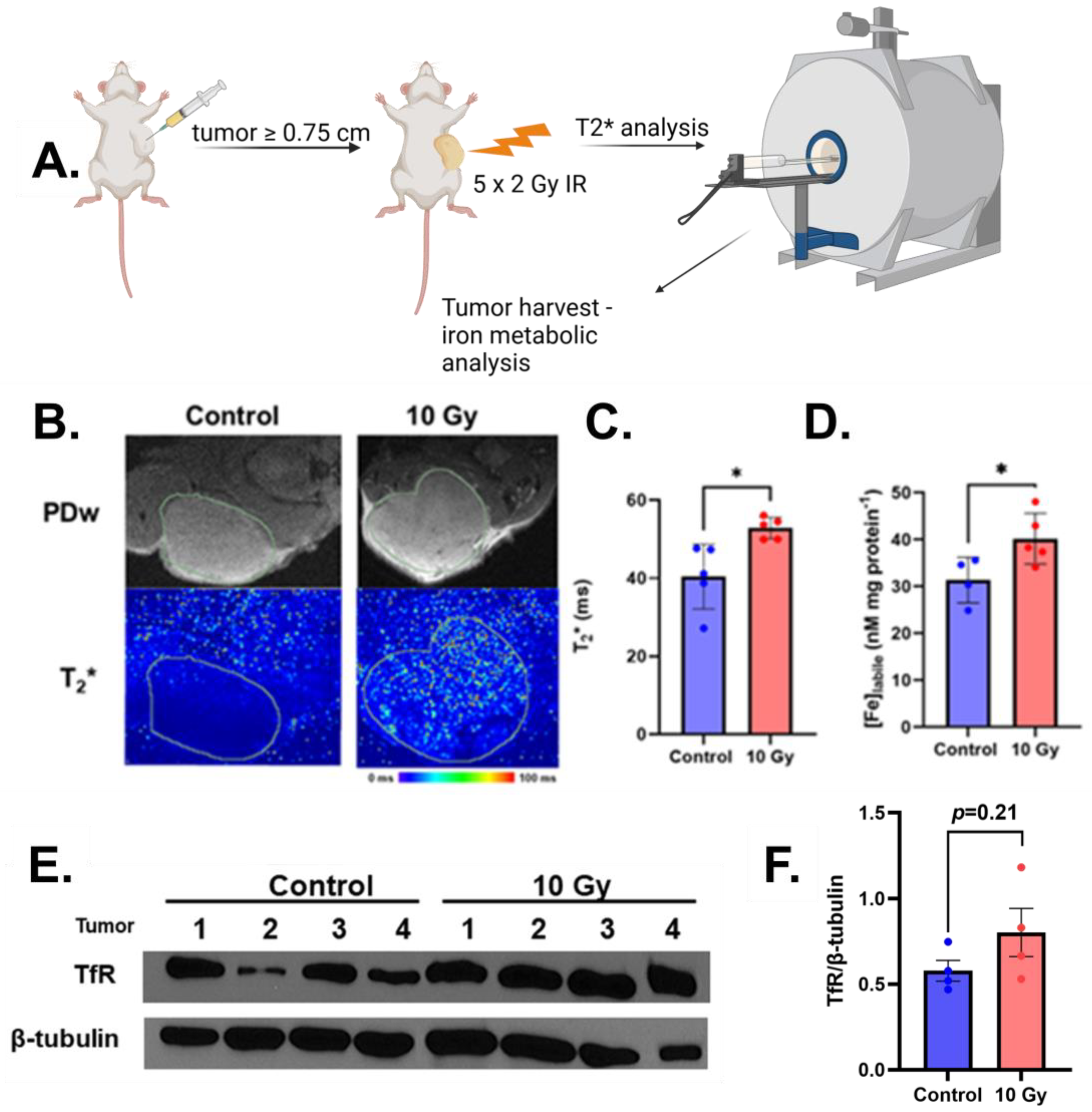
2.2. Radiation Increases MRI Detectable Labile Iron In Vivo
2.3. Ferritin Overexpression Reverses Radiation-Induced Cell Motility
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Colony Formation
4.3. Cell Stiffness
4.4. Live Cell Motility
4.5. Western Blotting
4.6. In Vivo Studies
4.7. MRI Analysis
4.8. Labile Iron Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Barker, C.A.; Chang, M.; Chou, J.F.; Zhang, Z.; Beal, K.; Gutin, P.H.; Iwamoto, F.M. Radiotherapy and concomitant temozolomide may improve survival of elderly patients with glioblastoma. J. Neurooncol. 2012, 109, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Seol, H.J.; Nam, D.H.; Park, C.K.; Kim, I.H.; Kim, T.M.; Kim, J.H.; Cho, Y.H.; Yoon, S.M.; Chang, J.H.; et al. Concurrent Chemoradiotherapy with Temozolomide Followed by Adjuvant Temozolomide for Newly Diagnosed Glioblastoma Patients: A Retrospective Multicenter Observation Study in Korea. Cancer Res. Treat. 2017, 49, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Petronek, M.S.; Spitz, D.R.; Buettner, G.R.; Allen, B.G. Linking Cancer Metabolic Dysfunction and Genetic Instability through the Lens of Iron Metabolism. Cancers 2019, 11, 1077. [Google Scholar] [CrossRef]
- Schonberg, D.L.; Miller, T.E.; Wu, Q.; Flavahan, W.A.; Das, N.K.; Hale, J.S.; Hubert, C.G.; Mack, S.C.; Jarrar, A.M.; Karl, R.T.; et al. Preferential Iron Trafficking Characterizes Glioblastoma Stem-like Cells. Cancer Cell 2015, 28, 441–455. [Google Scholar] [CrossRef]
- Vo, V.T.A.; Kim, S.; Hua, T.N.M.; Oh, J.; Jeong, Y. Iron commensalism of mesenchymal glioblastoma promotes ferroptosis susceptibility upon dopamine treatment. Commun. Biol. 2022, 5, 593. [Google Scholar] [CrossRef]
- Calzolari, A.; Larocca, L.M.; Deaglio, S.; Finisguerra, V.; Boe, A.; Raggi, C.; Ricci-Vitani, L.; Pierconti, F.; Malavasi, F.; De Maria, R.; et al. Transferrin receptor 2 is frequently and highly expressed in glioblastomas. Transl. Oncol. 2010, 3, 123–134. [Google Scholar] [CrossRef]
- Owusu, S.B.; Russell, E.; Ekanayake, A.B.; Tivanski, A.V.; Petronek, M.S. Iron promotes isocitrate dehydrogenase mutant glioma cell motility. Free. Radic. Biol. Med. 2025, 226, 109–116. [Google Scholar] [CrossRef]
- Gupta, K.; Burns, T.C. Radiation-Induced Alterations in the Recurrent Glioblastoma Microenvironment: Therapeutic Implications. Front. Oncol. 2018, 8, 503. [Google Scholar] [CrossRef]
- Owusu, S.B.; Hudik, E.; Férard, C.; Dupré-Crochet, S.; Addison, E.C.D.K.; Preko, K.; Bizouarn, T.; Houée-Levin, C.; Baciou, L. Radiation-induced reactive oxygen species partially assemble neutrophil NADPH oxidase. Free. Radic. Biol. Med. 2021, 164, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Warrington, J.P.; Sonntag, W.E.; Lee, Y.W. Irradiation Alters MMP-2/TIMP-2 System and Collagen Type IV Degradation in Brain. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1559–1566. [Google Scholar] [CrossRef] [PubMed]
- Bhowmik, A.; Khan, R.; Ghosh, M.K. Blood Brain Barrier: A Challenge for Effectual Therapy of Brain Tumors. Biomed Res. Int. 2015, 2015, 320941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Vuckovic, I.; Zhang, S.; Xiong, Y.; Carlson, B.L.; Jacobs, J.; Olson, I.; Petterson, X.-M.; Macura, S.I.; Sarkaria, J.; et al. Radiation Induced Metabolic Alterations Associate With Tumor Aggressiveness and Poor Outcome in Glioblastoma. Front. Oncol. 2020, 10, 535. [Google Scholar] [CrossRef]
- Kruszewski, M. Labile iron pool: The main determinant of cellular response to oxidative stress. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 531, 81–92. [Google Scholar] [CrossRef]
- Ma, S.; Fu, X.; Liu, L.; Liu, Y.; Feng, H.; Jiang, H.; Liu, X.; Liu, R.; Liang, Z.; Li, M.; et al. Iron-Dependent Autophagic Cell Death Induced by Radiation in MDA-MB-231 Breast Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 723801. [Google Scholar] [CrossRef]
- Steinle, M.; Palme, D.; Misovic, M.; Rudner, J.; Dittmann, K.; Lukowski, R.; Ruth, P.; Huber, S.M. Ionizing radiation induces migration of glioblastoma cells by activating BK K+ channels. Radiother. Oncol. 2011, 101, 122–126. [Google Scholar] [CrossRef]
- Wank, M.; Schilling, D.; Reindl, J.; Meyer, B.; Gempt, J.; Motov, S.; Alexander, F.; Wilkens, J.J.; Schlegel, J.; Schmid, T.E.; et al. Evaluation of radiation-related invasion in primary patient-derived glioma cells and validation with established cell lines: Impact of different radiation qualities with differing LET. J. Neurooncol. 2018, 139, 583–590. [Google Scholar] [CrossRef]
- Du, C.; Gao, Z.; Venkatesha, V.A.; Kalen, A.L.; Chaudhuri, L.; Spitz, D.R.; Cullen, J.J.; Oberley, L.W.; Goswami, P.C. Mitochondrial ROS and radiation induced transformation in mouse embryonic fibroblasts. Cancer Biol. Ther. 2009, 8, 1962–1971. [Google Scholar] [CrossRef]
- Petronek, M.S.; Monga, V.; Bodeker, K.L.; Kwofie, M.; Lee, C.Y.; Mapuskar, K.A.; Stolwijk, J.M.; Zaher, A.; Wagner, B.A.; Smith, M.C.; et al. Magnetic Resonance Imaging of Iron Metabolism with T2* Mapping Predicts an Enhanced Clinical Response to Pharmacologic Ascorbate in Patients with GBM. Clin. Cancer Res. 2024, 30, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cushing, C.M.; Petronek, M.S.; Bodeker, K.L.; Vollstedt, S.; Brown, H.A.; Opat, E.; Hollenbeck, N.J.; Shanks, T.; Berg, D.J.; Smith, B.J.; et al. Magnetic resonance imaging (MRI) of pharmacological ascorbate-induced iron redox state as a biomarker in subjects undergoing radio-chemotherapy. Redox Biol. 2021, 38, 101804. [Google Scholar] [CrossRef]
- Petronek, M.S.; Teferi, N.; Lee, C.Y.; Magnotta, V.A.; Allen, B.G. MRI Detection and Therapeutic Enhancement of Ferumoxytol Internalization in Glioblastoma Cells. Nanomaterials 2024, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Bayanbold, K.; Singhania, M.; Fath, M.A.; Searby, C.C.; Stolwijk, J.M.; Henrich, J.B.; Pulliam, C.F.; Schoenfeld, J.D.; Mapuskar, K.A.; Sho, S.; et al. Depletion of Labile Iron Induces Replication Stress and Enhances Responses to Chemoradiation in Non-Small-Cell Lung Cancer. Antioxidants 2023, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Wild-Bode, C.; Weller, M.; Rimner, A.; Dichgans, J.; Wick, W. Sublethal irradiation promotes migration and invasiveness of glioma cells: Implications for radiotherapy of human glioblastoma. Cancer Res. 2001, 61, 2744–2750. [Google Scholar]
- Shankar, A.; Kumar, S.; Iskander, A.; Varma, N.R.; Janic, B.; deCarvalho, A.; Mikkelsen, T.; Frank, J.A.; Ali, M.M.; Knight, R.A.; et al. Subcurative radiation significantly increases cell proliferation, invasion, and migration of primary glioblastoma multiforme in vivo. Chin. J. Cancer 2014, 33, 148–158. [Google Scholar] [CrossRef]
- Wank, M.; Schilling, D.; Schmid, T.E.; Meyer, B.; Gempt, J.; Barz, M.; Schlegel, J.; Liesche, F.; Kessel, K.A.; Wiestler, B.; et al. Human Glioma Migration and Infiltration Properties as a Target for Personalized Radiation Medicine. Cancers 2018, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Saurty-Seerunghen, M.S.; Daubon, T.; Bellenger, L.; Delaunay, V.; Castro, G.; Guyon, J.; Rezk, A.; Fabrega, S.; Idbaih, A.; Almairac, F.; et al. Glioblastoma cell motility depends on enhanced oxidative stress coupled with mobilization of a sulfurtransferase. Cell Death Dis. 2022, 13, 913. [Google Scholar] [CrossRef]
- Bhargav, A.G.; Domino, J.S.; Chamoun, R.; Thomas, S.M. Mechanical Properties in the Glioma Microenvironment: Emerging Insights and Theranostic Opportunities. Front. Oncol. 2022, 11, 805628. [Google Scholar] [CrossRef]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.T.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Katira, P.; Bonnecaze, R.T.; Zaman, M.H. Modeling the Mechanics of Cancer: Effect of Changes in Cellular and Extra-Cellular Mechanical Properties. Front. Oncol. 2013, 3, 145. [Google Scholar] [CrossRef]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef]
- Petronek, M.S.; Bodeker, K.L.; Lee, C.Y.; Teferi, N.; Eschbacher, K.L.; Jones, K.A.; Loeffler, B.T.; Smith, B.J.; Buatti, J.M.; Magnotta, V.A.; et al. Iron-based biomarkers for personalizing pharmacological ascorbate therapy in glioblastoma: Insights from a phase 2 clinical trial. J. Neuro-Oncol. 2024, 166, 493–501. [Google Scholar] [CrossRef]
- Singhania, M.; Zaher, A.; Pulliam, C.F.; Bayanbold, K.; Searby, C.C.; Schoenfeld, J.D.; Mapuskar, K.A.; Fath, M.A.; Allen, B.G.; Spitz, D.R.; et al. Quantitative MRI Evaluation of Ferritin Overexpression in Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2024, 25, 2398. [Google Scholar] [CrossRef] [PubMed]
- Plodinec, M.; Loparic, M.; Monnier, C.A.; Obermann, E.C.; Zanetti-Dallenbach, R.; Oertle, P.; Hyotyla, J.T.; Aebi, U.; Bentires-Alj, M.; Lim, R.Y.H.; et al. The nanomechanical signature of breast cancer. Nat. Nanotech 2012, 7, 757–765. [Google Scholar] [CrossRef]
- Cross, S.E.; Jin, Y.S.; Rao, J.; Gimzewski, J.K. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2007, 2, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Cell Membrane Fluid–Mosaic Structure and Cancer Metastasis. Cancer Res. 2015, 75, 1169–1176. [Google Scholar] [CrossRef]
- Wullkopf, L.; West, A.K.V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer cells’ ability to mechanically adjust to extracellular matrix stiffness correlates with their invasive potential. Mol. Biol. Cell 2018, 29, 2378–2385. [Google Scholar] [CrossRef]
- Yee, P.P.; Wei, Y.; Kim, S.Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.; et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat. Commun. 2020, 11, 5424. [Google Scholar] [CrossRef]
- Keeler, G.; Owusu, S.B.; Zanaty, M.; Petronek, M.S. Mitochondrial Iron Metabolism as a Potential Key Mediator of PD-L1 Thermal Regulation. Cancers 2024, 16, 3736. [Google Scholar] [CrossRef] [PubMed]
- Owusu, S.B.; Zaher, A.; Ahenkorah, S.; Pandya, D.N.; Wadas, T.J.; Petronek, M.S. Gallium Uncouples Iron Metabolism to Enhance Glioblastoma Radiosensitivity. Int. J. Mol. Sci. 2024, 25, 10047. [Google Scholar] [CrossRef] [PubMed]
- Teferi, N.; Ekanayake, A.; Owusu, S.B.; Moninger, T.O.; Sarkaria, J.N.; Tivanski, A.V.; Petronek, M.S. Glutathione peroxidase 4 overexpression induces anomalous subdiffusion and impairs glioblastoma cell growth. J. Biol. Eng. 2024, 18, 72. [Google Scholar] [CrossRef] [PubMed]
- Petronek, M.S.; St-Aubin, J.J.; Lee, C.Y.; Spitz, D.R.; Gillan, E.G.; Allen, B.G.; Magnotta, V.A. Quantum chemical insight into the effects of the local electron environment on T2*-based MRI. Sci. Rep. 2021, 11, 20817. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owusu, S.B.; Ekanayake, A.B.; Tivanski, A.V.; Petronek, M.S. Iron Mediates Radiation-Induced Glioblastoma Cell Diffusion. Int. J. Mol. Sci. 2025, 26, 4755. https://doi.org/10.3390/ijms26104755
Owusu SB, Ekanayake AB, Tivanski AV, Petronek MS. Iron Mediates Radiation-Induced Glioblastoma Cell Diffusion. International Journal of Molecular Sciences. 2025; 26(10):4755. https://doi.org/10.3390/ijms26104755
Chicago/Turabian StyleOwusu, Stephenson Boakye, Akalanka B. Ekanayake, Alexei V. Tivanski, and Michael S. Petronek. 2025. "Iron Mediates Radiation-Induced Glioblastoma Cell Diffusion" International Journal of Molecular Sciences 26, no. 10: 4755. https://doi.org/10.3390/ijms26104755
APA StyleOwusu, S. B., Ekanayake, A. B., Tivanski, A. V., & Petronek, M. S. (2025). Iron Mediates Radiation-Induced Glioblastoma Cell Diffusion. International Journal of Molecular Sciences, 26(10), 4755. https://doi.org/10.3390/ijms26104755




