Comprehensive In Vitro and In Silico Analysis of Antimicrobial and Insecticidal Properties of Essential Oil of Myrtus communis L. from Algeria
Abstract
1. Introduction
2. Results and Discussion
2.1. M. communis Essential Oil Yield
2.2. Composition of M. communis Essential Oil
2.3. Antibacterial Activity
2.4. Antifungal Activity
2.5. Insecticidal Activity
2.5.1. Contact Toxicity
2.5.2. Fumigant Toxicity
2.5.3. Repellency Activity
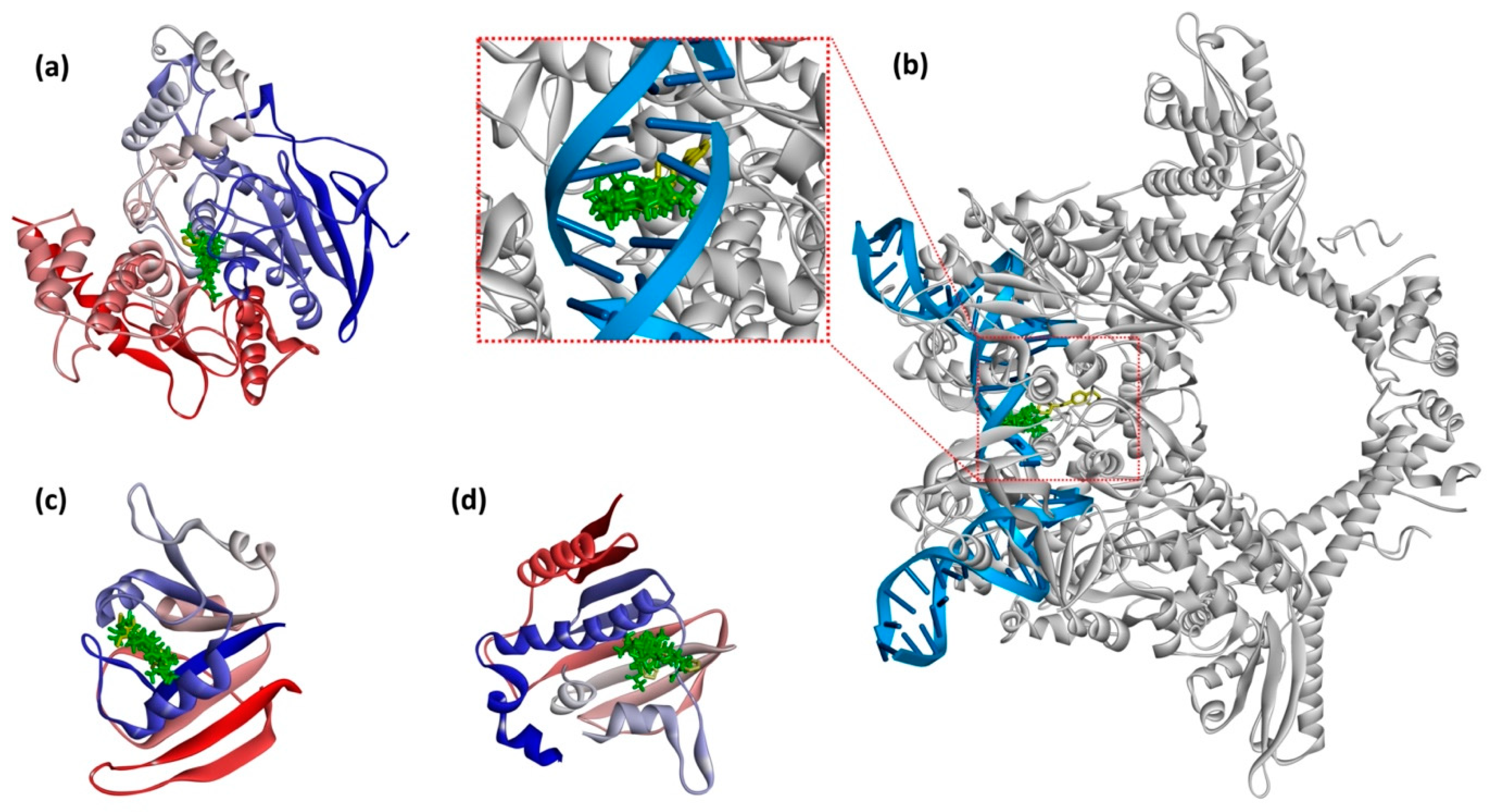
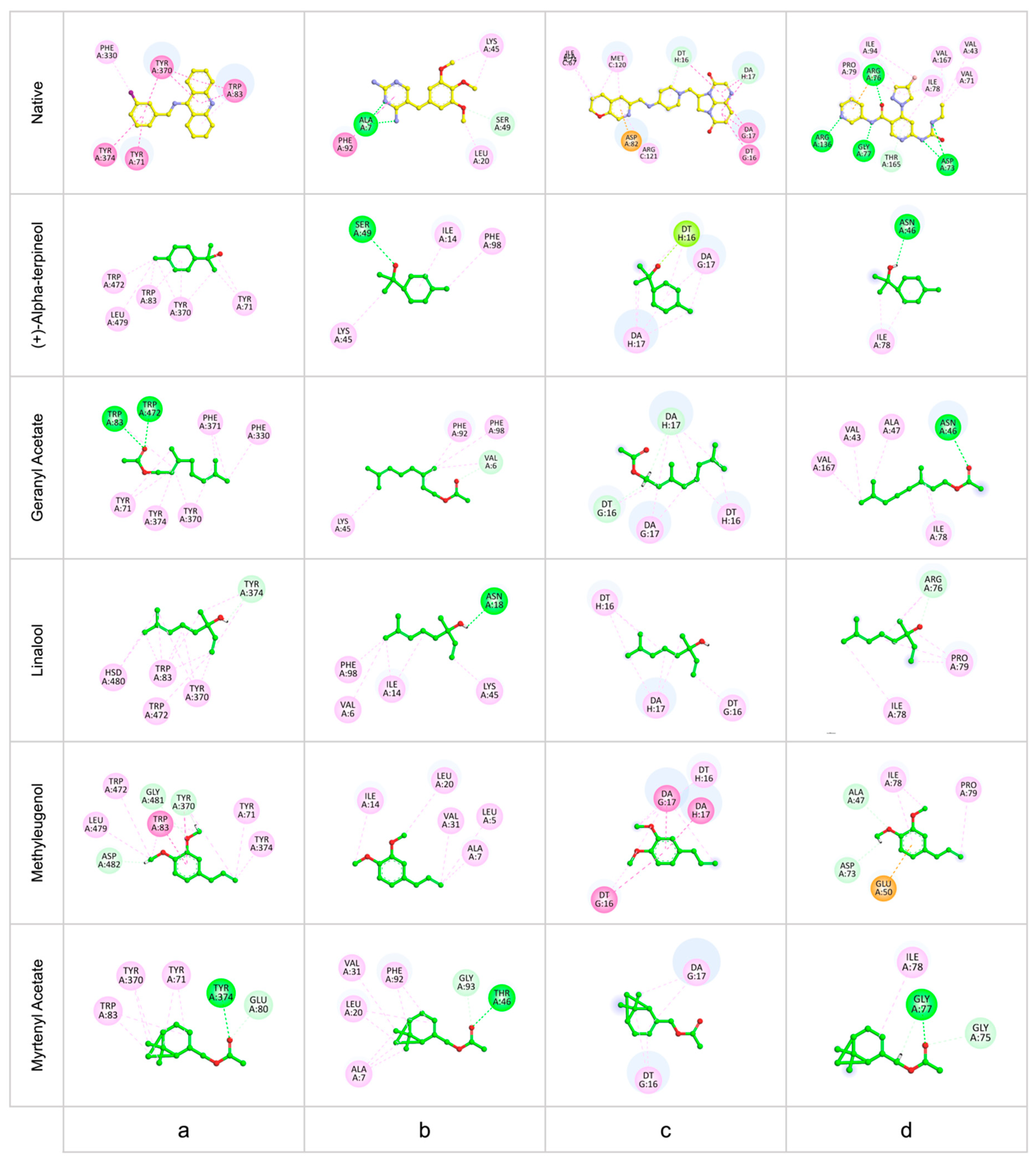
2.5.4. In Silico Investigations
3. Materials and Methods
3.1. Plant Material and Essential Oil Extraction
3.2. GC-MS Analysis
3.3. Antibacterial Activity
3.4. Antifungal Activity
3.5. Insecticidal Activity
3.5.1. Test Insect Culture
3.5.2. Contact Toxicity Assay
3.5.3. Fumigant Toxicity Assay
3.5.4. Repellency Assay
3.6. DFT Calculations
3.7. Molecular Docking
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari, B.; Hadian, J.; Astatkie, T. Applications of essential oils and plant extracts in different industries. Moleculas 2022, 27, 8999. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential oils and their major components: An updated review on antimicrobial activities, mechanism of action and their potential application in the food industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, D.P.; de Assis Oliveira, F.; Arcanjo, D.D.R.; da Fonsêca, D.V.; Duarte, A.B.S.; de Oliveira Barbosa, C.; Ong, T.P.; Brocksom, T.J. Essential Oils: Chemistry and Pharmacological Activities—Part II. Biomedicines 2024, 12, 1185. [Google Scholar] [CrossRef]
- Rahim, N.; Derabli, C.; Bramki, A.; Mahdjoub, S.; Rup-jacques, S.; Barboucha, G.; Hesse, S.; Boulebd, H. Evaluating the multifaceted bioactivity of Syzygium aromaticum essential oil: The central role of eugenol Evaluating the multifaceted bioactivity of Syzygium aromaticum essential oil: The. Turk. J. Biol. Vol. 2025, 49, 102–117. [Google Scholar] [CrossRef] [PubMed]
- Assadpour, E.; Can Karaça, A.; Fasamanesh, M.; Mahdavi, S.A.; Shariat-Alavi, M.; Feng, J.; Kharazmi, M.S.; Rehman, A.; Jafari, S. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 2024, 64, 6477–6497. [Google Scholar] [CrossRef]
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.P.; Gupta, V.; Raghuvanshi, T.S. Essential oils as green promising alternatives to chemical preservatives for agri-food products: New insight into molecular mechanism, toxicity assessment, and safety profile. Food Chem. Toxicol. 2024, 183, 114241. [Google Scholar] [CrossRef]
- Quinn, E.; Ben-Simchon, E.; Gorelick, J.; Oka, Y.; Frenkel, O.; Sionov, E.; Kostyukovsky, M.; Dudai, N.; Shimshoni, J.; Zilkah, S.; et al. Examination of genetic lines of Myrtus communis as potential sources of organic agricultural pest control agents. Heliyon 2024, 10, e35658. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.A.; Jain, V.; Wani, A.R.; Rasool, S.; Nazir, R.; Malik, N.A.; Majid, S.A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Reyes-Ávila, A.; López-Ruiz, R.; Egea González, F.J.; Romero-González, R.; Garrido Frenich, A. Chemistry and development of bioinsecticides for safe and sustainable use. Curr. Opin. Environ. Sci. Health 2024, 41, 100568. [Google Scholar] [CrossRef]
- Ali, K.; Ullah, F.; Khan, N.; Rahman, I.U.; Ullah, S.; Khan, W.; Ali, M.; Uddin, N.; Nisar, M. Ethnobotanical and ecological study of Myrtus communis (L.) in Bajaur agency (FATA) Khyber-Pakhtunkhwa, Pakistan. J. Biodivers. Environ. Sci. 2017, 11, 152–164. [Google Scholar]
- Al-Snafi, A.E.; Teibo, J.O.; Shaheen, H.M.; Akinfe, O.A.; Teibo, T.K.A.; Emieseimokumo, N.; Elfiky, M.M.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; et al. The therapeutic value of Myrtus communis L.: An updated review. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 4579–4600. [Google Scholar] [CrossRef] [PubMed]
- Hennia, A.; Nemmiche, S.; Dandlen, S.; Miguel, M.G. Myrtus communis essential oils: Insecticidal, antioxidant and antimicrobial activities: A review. J. Essent. Oil Res. 2019, 31, 487–545. [Google Scholar] [CrossRef]
- Vega, E.N.; González-Zamorano, L.; Cebadera, E.; Barros, L.; da Silveira, T.F.F.; Vidal-Diez de Ulzurrun, G.; Tardío, J.; Lázaro, A.; Cámara, M.; Fernández-Ruíz, V.; et al. Wild Myrtus communis L. fruit by-product as a promising source of a new natural food colourant: Optimization of the extraction process and chemical characterization. Foods 2025, 14, 520. [Google Scholar] [CrossRef]
- Wannes, W.A.; Mhamdi, B.; Sriti, J.; Jemia, M.B.; Ouchikh, O.; Hamdaoui, G.; Kchouk, M.E.; Marzouk, B. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem. Toxicol. 2010, 48, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Mohamadi, Y.; Lograda, T.; Ramdani, M.; Figueredo, G.; Chalard, P. Chemical composition and antimicrobial activity of Myrtus Communis essential oils from Algeria. Biodiversitas 2021, 22, 933–946. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bugarin, D.; Grbovic, S.; Mitic-Culafic, D.; Vukovic-Gacic, B.; Orcic, D.; Jovin, E.; Couladis, M. Essential oil of Myrtus communis L. As a potential antioxidant and antimutagenic agents. Molecules 2010, 15, 2759–2770. [Google Scholar] [CrossRef]
- Satrani, B.; Farah, A.; Talbi, M. Effet de la distillation fractionnée sur la composition chimique et l’activité antimicrobienne des huiles essentielles du Myrte (Myrtus communis L.) du Maroc. Acta Bot. Gall. 2006, 153, 235–242. [Google Scholar] [CrossRef]
- Mugao, L. Factors influencing yield, chemical composition and efficacy of essential oils. Int. J. Multidiscip. Res. Growth Eval. 2024, 5, 169–178. [Google Scholar] [CrossRef]
- Laftouhi, A.; Eloutassi, N.; Ech-Chihbi, E.; Rais, Z.; Abdellaoui, A.; Taleb, A.; Beniken, M.; Nafidi, H.A.; Salamatullah, A.M.; Bourhia, M.; et al. The impact of environmental stress on the secondary metabolites and the chemical compositions of the essential oils from some medicinal plants used as food supplements. Sustainability 2023, 15, 7842. [Google Scholar] [CrossRef]
- Mkaddem, M.G.; Zrig, A.; Ben Abdallah, M.; Romdhane, M.; Okla, M.K.; Al-Hashimi, A.; Alwase, Y.A.; Hegab, M.Y.; Madany, M.M.Y.; Hassan, A.H.A.; et al. Variation of the chemical composition of essential oils and total phenols content in natural populations of Marrubium vulgare L. Plants 2022, 11, 612. [Google Scholar] [CrossRef]
- Vafadar Shoshtari, Z.; Rahimmalek, M.; Sabzalian, M.R.; Hosseini, H. Essential oil and bioactive compounds variation in myrtle (Myrtus communis L.) as affected by seasonal variation and salt stress. Chem. Biodivers. 2017, 14, e1600365. [Google Scholar] [CrossRef] [PubMed]
- Bradesi, P.; Tomi, F.; Casanova, J.; Costa, J.; Bemardini, A.F. Chemical composition of myrtle leaf essential oil from Corsica (France). J. Essent. Oil Res. 1997, 9, 283–288. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Djebali, K.; Abid, S.; Saada, M.; Ksouri, R. Application of the mixture design for optimum antimicrobial activity: Combined treatment of Syzygium aromaticum, Cinnamomum zeylanicum, Myrtus communis, and Lavandula stoechas essential oils against Escherichia coli. J. Food Process. Preserv. 2019, 43, e14257. [Google Scholar] [CrossRef]
- El Hartiti, H.; El Mostaphi, A.; Barrahi, M.; Ali, A.B.; Chahboun, N.; Amiyare, R.; Zarrouk, A.; Bourkhiss, B.; Ouhssine, M. Chemical composition and antibacterial activity of the essential oil of Myrtus communis leaves. Karbala Int. J. Mod. Sci. 2020, 6, 251–258. [Google Scholar] [CrossRef]
- Ouedrhiri, W.; Mechchate, H.; Moja, S.; Baudino, S.; Saleh, A.; Al Kamaly, O.M.; Grafov, A.; Greche, H. Optimized antibacterial effects in a designed mixture of essential oils of Myrtus communis, Artemisia herba-alba and Thymus serpyllum for wide range of applications. Foods 2022, 11, 132. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical composition, antibiofilm, cytotoxic, and anti-acetylcholinesterase activities of Myrtus communis L. leaves essential oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef]
- Moo, C.L.; Osman, M.A.; Yang, S.K.; Yap, W.S.; Ismail, S.; Lim, S.H.E.; Chong, C.M.; Lai, K.S. Antimicrobial activity and mode of action of 1,8-cineol against carbapenemase-producing Klebsiella pneumoniae. Sci. Rep. 2021, 11, 20824. [Google Scholar] [CrossRef]
- Barhouchi, B.; Menacer, R.; Bouchkioua, S.; Mansour, A.; Belattar, N. Compounds from myrtle flowers as antibacterial agents and SARS-CoV-2 inhibitors: In-vitro and molecular docking studies. Arab. J. Chem. 2023, 16, 104939. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, S.; Deng, Y.; Xu, W.; Wang, Z.; Wang, W.; Lv, R.; Liu, D. Antibacterial activity and mechanisms of α-terpineol against foodborne pathogenic bacteria. Appl. Microbiol. Biotechnol. 2023, 107, 6641–6653. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial activity and mechanism of linalool against Pseudomonas aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef] [PubMed]
- Celuppi, L.C.M.; Capelezzo, A.P.; Cima, L.B.; Zeferino, R.C.F.; Zanetti, M.; Riella, H.G.; Fiori, M.A. Antimicrobial cellulose acetate films by incorporation of geranyl acetate for active food packaging application. Res. Soc. Dev. 2022, 11, e40111125141. [Google Scholar] [CrossRef]
- Joshi, R. Chemical composition, in vitro antimicrobial and antioxidant activities of the essential oils of Ocimum gratissimum, O. sanctum and their major constituents. Indian J. Pharm. Sci. 2013, 75, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Cannas, S.; Molicotti, P.; Ruggeri, M.; Cubeddu, M.; Sanguinetti, M.; Marongiu, B.; Zanetti, S. Antimycotic activity of Myrtus communis L. towards Candida spp. from isolates. J. Infect. Dev. Ctries. 2013, 7, 295–298. [Google Scholar] [CrossRef]
- Bouzabata, A.; Cabral, C.; Gonçalves, M.J.; Cruz, M.T.; Bighelli, A.; Cavaleiro, C.; Casanova, J.; Tomi, F.; Salgueiro, L. Myrtus communis L. as source of a bioactive and safe essential oil. Food Chem. Toxicol. 2015, 75, 166–172. [Google Scholar] [CrossRef]
- Siddique, S.; Perveen, Z.; Nawaz, S.; Shahzad, K.; Ali, Z. Chemical composition and antimicrobial activities of essential oils of six species from family Myrtaceae. J. Essent. Oil-Bear. Plants 2015, 18, 950–956. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Ivanov, M.; Kannan, A.; Stojković, D.S.; Glamočlija, J.; Calhelha, R.C.; Ferreira, I.C.F.R.; Sanglard, D.; Soković, M. Camphor and eucalyptol—Anticandidal spectrum, antivirulence effect, efflux pumps interference and cytotoxicity. Int. J. Mol. Sci. 2021, 22, 483. [Google Scholar] [CrossRef]
- Ghazi Mirsaid, R.; Falahati, M.; Farahyar, S.; Ghasemi, Z.; Roudbary, M.; Mahmoudi, S. In vitro antifungal activity of eucalyptol and its interaction with antifungal drugs against clinical dermatophyte isolates including Trichophyton indotineae. Discov. Public Health 2024, 21, 73. [Google Scholar] [CrossRef]
- Pries, R.; Jeschke, S.; Leichtle, A.; Bruchhage, K.L. Modes of action of 1,8-cineol in infections and inflammation. Metabolites 2023, 13, 751. [Google Scholar] [CrossRef]
- An, P.; Yang, X.; Yu, J.; Qi, J.; Ren, X.; Kong, Q. α-Terpineol and terpene-4-ol, the critical components of tea tree oil, exert antifungal activities in vitro and in vivo against Aspergillus niger in grapes by inducing morphous damage and metabolic changes of fungus. Food Control 2019, 98, 42–53. [Google Scholar] [CrossRef]
- Medeiros, C.I.S.; de Sousa, M.N.A.; Filho, G.G.A.; Freitas, F.O.R.; Uchoa, D.P.L.; Nobre, M.S.C.; Bezerra, A.L.D.; Rolim, L.A.D.M.M.; Morais, A.M.B.; Nogueira, T.B.S.S.; et al. Antifungal activity of linalool against fluconazoleresistant clinical strains of vulvovaginal Candida albicans and its predictive mechanism of action. Braz. J. Med. Biol. Res. 2022, 55, e11831. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A. Pharmacological interactions of essential oil constituents on the viability of micro-organisms. Nat. Prod. Commun. 2010, 5, 1381–1386. [Google Scholar]
- Yezli, A.; Boudjelida, H.; Arroussi, D.E.R. Components and toxicological effects of Myrtus communis L. (Myrtales: Myrtaceae) essential oil against Mosquito culex pipiens L. (Diptera: Culicidae). Appl. Ecol. Environ. Res. 2024, 22, 2149–2164. [Google Scholar] [CrossRef]
- Benddine, H.; Zaid, R.; Babaali, D.; Daoudi-Hacini, S. Biological activity of essential oils of Myrtus communis (Myrtaceae, Family) and Foeniculum vulgare (Apiaceae, Family) on open fields conditions against corn aphids Rhopalosiphum maidis (Fitch, 1856) in western Algeria. J. Saudi Soc. Agric. Sci. 2023, 22, 78–88. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Badawy, M.E.I.; Shawir, M.S.; Mohamed, M.I.E. Chemical composition, fumigant and contact toxicities of essential oils isolated from egyptian plants against the stored grain insects; Sitophilus oryzae L. and Tribolium castaneum (Herbst). Egypt. J. Biol. Pest Control 2015, 25, 639–647. [Google Scholar]
- Firooziyan, S.; Osanloo, M.; Basseri, H.R.; Moosa-Kazemi, S.H.; Mohammadzadeh Hajipirloo, H.; Amani, A.; Sedaghat, M.M. Nanoemulsion of Myrtus communis essential oil and evaluation of its larvicidal activity against Anopheles stephensi. Arab. J. Chem. 2022, 15, 104064. [Google Scholar] [CrossRef]
- Liska, A.; Rozman, V.; Kalinovic, I.; Ivezic, M.; Balicevic, R. Contact and fumigant activity of 1,8-cineole, eugenol and camphor against Tribolium castaneum (Herbst). Julius-Kühn-Archiv 2010, 425, 716–720. [Google Scholar]
- Yildirim, E.; Emsen, B.; Kordali, S. Insecticidal effects of monoterpenes on Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae). J. Appl. Bot. Food Qual. 2013, 86, 198–204. [Google Scholar]
- Khani, A.; Basavand, F. Chemical composition and insecticidal activity of myrtle (Myrtus communis L.) essential oil against two stored-product pests. J. Med. Plants By-Prod. 2012, 2, 83–89. [Google Scholar]
- Senfi, F.; Safaralizadeh, M.H.; Safavi, S.A.; Aramideh, S. Fumigant toxicity of Laurus nobilis and Myrtus communis essential oils on larvae and adults of the Red flour beetle, Tribolium castaneum Herbst (Col.: Tenebrionidae). Arch. Phytopathol. Plant Prot. 2014, 47, 472–476. [Google Scholar] [CrossRef]
- Aouadi, G.; Soltani, A.; Grami, L.K.; Abada, M.B.; Haouel, S.; Boushih, E.; Chaanbi, M.; Elkahoui, S.; Hajlaoui, M.R.; Jemâa, J.M.B.; et al. Chemical investigations on Algerian Mentha rotundifolia and Myrtus communis essential oils and assessment of their insecticidal and antifungal activities. Int. J. Agric. Biol. 2021, 26, 666–680. [Google Scholar]
- Fassbinder, C.; Grodnitzky, J.; Coats, J. Monoterpenoids as possible control agents for Varroa destructor. J. Apic. Res. 2002, 41, 83–88. [Google Scholar] [CrossRef]
- Kheloul, L.; Anton, S.; Bréard, D.; Kellouche, A. Fumigant toxicity of some essential oils and eucalyptol on different life stages of Tribolium confusum (Coleoptera: Tenebrionidae). Bot. Lett. 2023, 170, 3–14. [Google Scholar] [CrossRef]
- Sharma, J.H.; Tiwari, S.N. Fumigant toxicity of alpha-pinene, beta-pinene, eucalyptol, linalool and sabinene against Rice Weevil, Sitophilus oryzae (L.). Pantnagar J. Res. 2022, 19, 50–55. [Google Scholar]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Sakka, M.K.; Mavridis, K.; Papapostolou, K.M.; Riga, M.; Vontas, J.; Athanassiou, C.G. Development, application and evaluation of three novel TaqMan qPCR assays for phosphine resistance monitoring in major stored product pests Tribolium castaneum and Rhyzopertha dominica. Pest Manag. Sci. 2024, 80, 275–281. [Google Scholar] [CrossRef]
- Salehi, T.; Karimi, J.; Hasanshahi, G.; Askarianzadeh, A.; Abbasipour, H.; Garjan, A.S. The effect of essential oils from Laurus nobilis and Myrtus commonis on the adults of mediterranean flour moth, Ephestia kuehniella Zeller (Lep.: Pyralidae). J. Essent. Oil-Bear. Plants 2014, 17, 553–561. [Google Scholar] [CrossRef]
- Tavassoli, M.; Shayeghi, M.; Abai, M.R.; Vatandoost, H.; Khoobdel, M.; Salari, M.; Ghaderi, A.; Rafi, F. Repellency effects of essential oils of myrtle (Myrtus communis), Marigold (Calendula officinalis) compared with DEET against Anopheles stephensi on human volunteers. Iran. J. Arthropod-Borne Dis. 2011, 5, 10–22. [Google Scholar]
- Lackus, N.D.; Lackner, S.; Gershenzon, J.; Unsicker, S.B.; Köllner, T.G. The occurrence and formation of monoterpenes in herbivore-damaged poplar roots. Sci. Rep. 2018, 8, 17936. [Google Scholar] [CrossRef]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Wanna, R.; Bozdoğan, H. Activity of Rosmarinus officinalis (Lamiales: Lamiaceae) essential oil and its main constituent, 1,8-cineole, against Tribolium castaneum (Coleoptera: Tenebrionidae). J. Entomol. Sci. 2024, 60, 86–106. [Google Scholar] [CrossRef]
- Barboucha, G.; Rahim, N.; Boulebd, H.; Bramki, A.; Andolfi, A.; Salvatore, M.M.; Masi, M. Chemical composition, in silico investigations and evaluation of antifungal, antibacterial, insecticidal and repellent activities of Eucalyptus camaldulensis Dehn. leaf essential oil from ALGERIA. Plants 2024, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.F.; Byrne, O. Plant-insect coevolution and inhibition of acetylcholinesterase. J. Chem. Ecol. 1988, 14, 1965–1975. [Google Scholar] [CrossRef]
- Haj Ammar, A.; Zagrouba, F.; Romdhane, M.; Abderrabba, M. Extraction de l’huile essentielle de myrte (Myrtus communis L.) provenant de la Tunisie par hydrodistillation. Acta Hortic. 2010, 241–250. [Google Scholar] [CrossRef]
- Sehout, I.; Boulebd, H.; Boulcina, R.; Nemouchi, S.; Bendjeddou, L.; Bramki, A.; Merazig, H.; Debache, A. Synthesis, crystal structure, Hirshfeld surface analysis, biological evaluation, DFT calculations, and in silico ADME analysis of 4-arylidene pyrazolone derivatives as promising antibacterial agents. J. Mol. Struct. 2021, 1229, 129586. [Google Scholar] [CrossRef]
- Bramki, A.; Frahtia, M.; Jaouani, A.; Dahimat, L.; Kacem Chaouche, N. Extraction and preliminary study of antibacterial compounds of three species of Aspergillus genus. Asia-Pac. J. Mol. Biol. Biotechnol. 2019, 27, 26–34. [Google Scholar] [CrossRef]
- Nemouchi, S.; Sehout, I.; Boulebd, H.; Boulcina, R.; Bramki, A.; Bendjeddou, L.; Benahsene, A.H.; Debache, A.; Nemouchi, S.; Sehout, I.; et al. Facile synthesis, crystal structure, hirshfeld surface analysis, DFT calculation and in vitro antifungal evaluation of 4-arylidene-1H-pyrazol-5(4H)-ones. Org. Prep. Proced. Int. 2023, 55, 421–435. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- McDonald, L.L.; Guy, R.H.; Speirs, R.D. Preliminary evaluation of new candidate materials as toxicants, repellents, and attractants against stored-product insects. In Agricultural Research Service; Agricultural Research Service, United States Department of Agriculture: Washington, DC, USA, 1970; p. 8. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
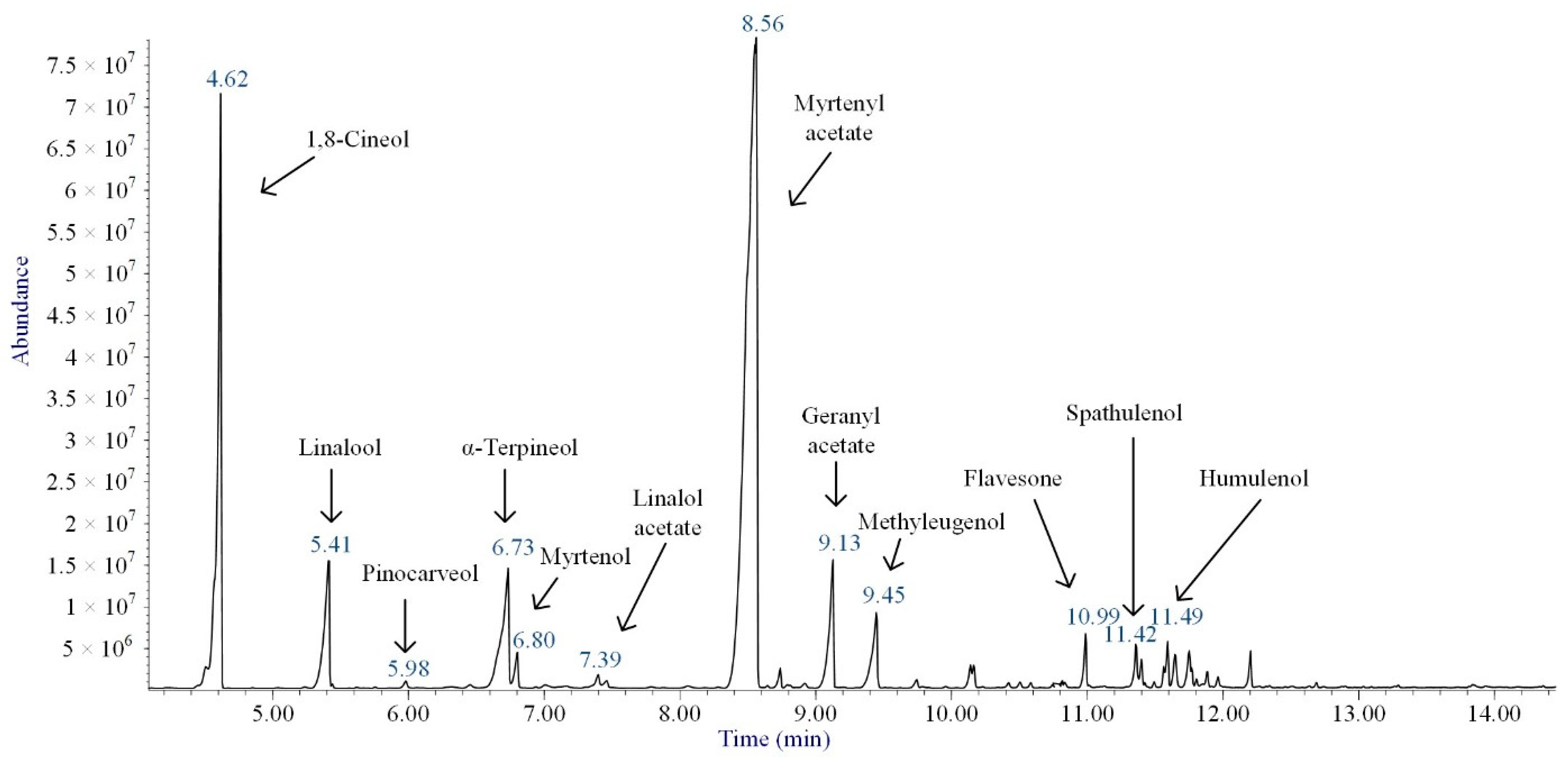

| Compound | Chemical Structure | RIexp | RINIST20 | %ΔRI | Area % |
|---|---|---|---|---|---|
| 1,8-Cineol | 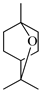 | 1034 | 1032 | 0.19 | 17.83 |
| Linalool |  | 1112 | 1103 | 0.82 | 5.46 |
| Pinocarveol | 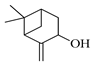 | 1154 | 1139 | 1.32 | 0.20 |
| α-Terpineol | 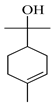 | 1211 | 1194 | 1.42 | 6.83 |
| Myrtenol | 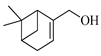 | 1216 | 1202 | 1.16 | 1.01 |
| Linalol acetate | 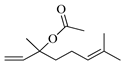 | 1260 | 1258 | 0.16 | 0.29 |
| Myrtenyl acetate | 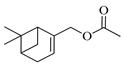 | 1348 | 1335 | 0.97 | 57.58 |
| Geranyl acetate | 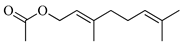 | 1382 | 1384 | −0.14 | 4.57 |
| Methyleugenol | 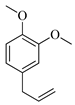 | 1418 | 1412 | 0.42 | 3.08 |
| Flavesone | 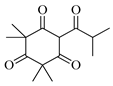 | 1546 | 1546 | 0.00 | 1.16 |
| Spathulenol |  | 1616 | 1620 | −0.25 | 1.14 |
| Humulenol | 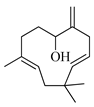 | 1666 | 1650 | 0.97 | 0.86 |
| Diameter of Inhibition Zone in mm | ||||||
|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | P. aeruginosa | S. typhimurium | K. pneumoniae | |
| EO | 13 ± 0.70 bc | 10 ± 1.00 cd | 9 ± 0.70 d | - | 13 ± 1.5 bc | 7.5 ± 0.50 d |
| Gentamicin | 17.5± 0.50 a | 18.75 ± 0.25 a | 11 ± 0.30 cd | 16.5 ± 0.50 ab | 15.5 ± 0.40 ab | 10.5 ± 0.50 cd |
| Diameter of Inhibition Zone in mm | |||||
|---|---|---|---|---|---|
| A. niger | A. fumigatus | Penicillium sp. | C. albicans | F. oxysporum | |
| EO | - | 11 ± 1.0 e | 9 ± 0.60 e | 8 ± 0.6 e | 16.5 ± 0.5 d |
| Nystatin | 24.5 ± 0.25 c | 32.5 ± 0.51 b | 39.5 ± 0.70 a | 22 ± 0.51 c | 33 ± 1.4 b |
| Concentration (μL/Insect) | Mortality (%) | ||||
|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | ||
| 0.020 | 3.33 ± 3.33 d | 10.0 ± 0.0 d | 13.33 ± 3.33 d | 13.33 ± 3.33 e | |
| 0.025 | 26.45 ± 1.13 c | 30.0 ± 0.0 c | 36.65 ± 4.13 c | 43.45 ± 4.22 d | |
| 0.030 | 26.61 ± 3.11 c | 30.0 ± 0.0 c | 46.67 ± 3.21 bc | 53.33 ± 2.33 cd | |
| 0.035 | 50.0 ± 5.77 b | 53.33 ± 3.03 b | 53.33 ± 3.03 b | 60.0 ± 0.0 c | |
| 0.040 | 66.66 ± 2.43 ab | 76.66 ± 1.31 a | 76.66 ± 1.31 a | 76.66 ± 1.31 b | |
| 0.045 | 76.67 ± 3.33 a | 83.33 ± 3.33 a | 83.33 ± 3.33 a | 90.0 ± 0.0 a | |
| One-way ANOVA | F value | 51.83 | 150.73 | 60.30 | 96.85 |
| p-value | <0.005 | <0.005 | <0.005 | <0.005 | |
| Exposure Time (h) | LC50 a (μL/Insect) | LC90 a (μL/Insect) | Slope ± SEM b | Chi-Square (χ2) | df |
|---|---|---|---|---|---|
| 24 | 0.035 (0.033–0.036) | 0.055 (0.051–0.061) | 6.46 ± 0.55 | 9.24 | 4 |
| 48 | 0.033 (0.030–0.036) | 0.052 (0.045–0.070) | 6.32 ± 0.52 | 10.10 | 4 |
| 72 | 0.031 (0.029–0.032) | 0.053 0.048–0.059) | 5.52 ± 0.50 | 5.73 | 4 |
| 96 | 0.029 (0.028–0.031) | 0.049 (0.045–0.054) | 5.81 ± 0.50 | 8.28 | 4 |
| Concentration | 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|
| 100 μL/L air | 0 | 0 | 6.66 ± 3.33 c | 10 ± 0.0 d | |
| 200 μL/L air | 13.33 ± 2.33 c | 66.66 ± 3.33 b | 73.34 ± 3.13 b | 76.16 ± 1.96 c | |
| 300 μL/L air | 43.43 ± 1.86 b | 76.67 ± 2.55 b | 83.21 ± 4.11 b | 86.65 ± 4.33 b | |
| 400 μL/L air | 86.66 ± 3.42 a | 96.33 ± 3.02 a | 100 a | 100 a | |
| 500 μL/L air | 96.70 ± 2.21 a | 100 a | 100 a | 100 a | |
| One-way ANOVA | F value | 207.88 | 245.50 | 223.67 | 315.75 |
| p-value | <0.005 | <0.005 | <0.005 | <0.005 | |
| Exposure Time (h) | LC50 a (µL/Liter Air) | LC90 a (µL/Liter Air) | Slope ± SEM b | Chi-Square (χ2) | df |
|---|---|---|---|---|---|
| 24 | 295.79 (281.35–310.02) | 437.55 (410.81–473.88) | 7.54 ± 0.60 | 4.95 | 3 |
| 48 | 197.73 (110.50–262.01) | 321.67 (244.78–725.51) | 6.06 ± 0.48 | 22.12 | 3 |
| 72 | 172.86 (116.94–221.47) | 288.36 (224.72–495.45) | 5.77 ± 0.44 | 15.22 | 3 |
| 96 | 162.85 (118.18–202.99) | 275.01 (219.12–424.68) | 5.63 ± 0.43 | 11.46 | 3 |
| Concentration | Repellence (%) | Mean Repellence | Repellent Class | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 30 min | 4 h | 6 h | 8h | 12 h | 24 h | ||||
| 2 µL (0.06 mg/cm2) | 86.70 ± 3.10 a | 100 a | 90 ± 5.77 a | 86.66 ± 3.33 b | 80 b | 70 ± 5.77 b | 85.55 ± 1.47 c | V | |
| 4 µL (0.11 mg/cm2) | 96.67 ± 3.33 a | 96.67 ± 3.33 a | 93.33 ± 3.22 a | 93.33 ± 3.22 ab | 90 ± 5.80 ab | 83.33 ± 6.66 ab | 92.22 ± 2.42 bc | V | |
| 6 µL (0.17 mg/cm2) | 100 a | 100 a | 100 a | 96.66 ± 3.33 ab | 96.66 ± 3.33 a | 86.66 ± 3.33 ab | 96.67 ± 0.96 ab | V | |
| 8 µL (0.23 mg/cm2) | 100 a | 100 a | 100 a | 100 a | 100 a | 96.66 ± 3.33 a | 99.44 ± 0.56 a | V | |
| One-way ANOVA | F value | 7.17 | 1.00 | 2.25 | 3.89 | 7.00 | 4.85 | 15.86 | |
| p-value | 0.0123 | 0.441 | 0.160 | 0.055 | 0.013 | 0.033 | 0.01 | ||
| Compound | Docking Binding Energy in kcal/mol | |||
|---|---|---|---|---|
| DNA Gyrase | DHFR | GyraseB | dmAChE | |
| (+)-α-terpineol | −3.22 | −2.92 | −2.76 | −3.10 |
| Geranyl acetate | −3.77 | −3.55 | −3.30 | −3.85 |
| Linalool | −2.95 | −3.09 | −2.56 | −3.04 |
| Methyleugenol | −3.73 | −3.36 | −3.33 | −3.69 |
| Myrtenyl acetate | −2.88 | −3.60 | −3.28 | −3.52 |
| Native ligand | −7.28 | −5.71 | −6.92 | −7.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barboucha, G.; Rahim, N.; Bramki, A.; Boulebd, H.; Andolfi, A.; Boulacheb, K.; Boulacel, A.; Salvatore, M.M.; Masi, M. Comprehensive In Vitro and In Silico Analysis of Antimicrobial and Insecticidal Properties of Essential Oil of Myrtus communis L. from Algeria. Int. J. Mol. Sci. 2025, 26, 4754. https://doi.org/10.3390/ijms26104754
Barboucha G, Rahim N, Bramki A, Boulebd H, Andolfi A, Boulacheb K, Boulacel A, Salvatore MM, Masi M. Comprehensive In Vitro and In Silico Analysis of Antimicrobial and Insecticidal Properties of Essential Oil of Myrtus communis L. from Algeria. International Journal of Molecular Sciences. 2025; 26(10):4754. https://doi.org/10.3390/ijms26104754
Chicago/Turabian StyleBarboucha, Ghozlane, Noureddine Rahim, Amina Bramki, Houssem Boulebd, Anna Andolfi, Khaoula Boulacheb, Amina Boulacel, Maria Michela Salvatore, and Marco Masi. 2025. "Comprehensive In Vitro and In Silico Analysis of Antimicrobial and Insecticidal Properties of Essential Oil of Myrtus communis L. from Algeria" International Journal of Molecular Sciences 26, no. 10: 4754. https://doi.org/10.3390/ijms26104754
APA StyleBarboucha, G., Rahim, N., Bramki, A., Boulebd, H., Andolfi, A., Boulacheb, K., Boulacel, A., Salvatore, M. M., & Masi, M. (2025). Comprehensive In Vitro and In Silico Analysis of Antimicrobial and Insecticidal Properties of Essential Oil of Myrtus communis L. from Algeria. International Journal of Molecular Sciences, 26(10), 4754. https://doi.org/10.3390/ijms26104754












