Systematic Review: Does Exercise Training Influence Ghrelin Levels?
Abstract
1. Introduction
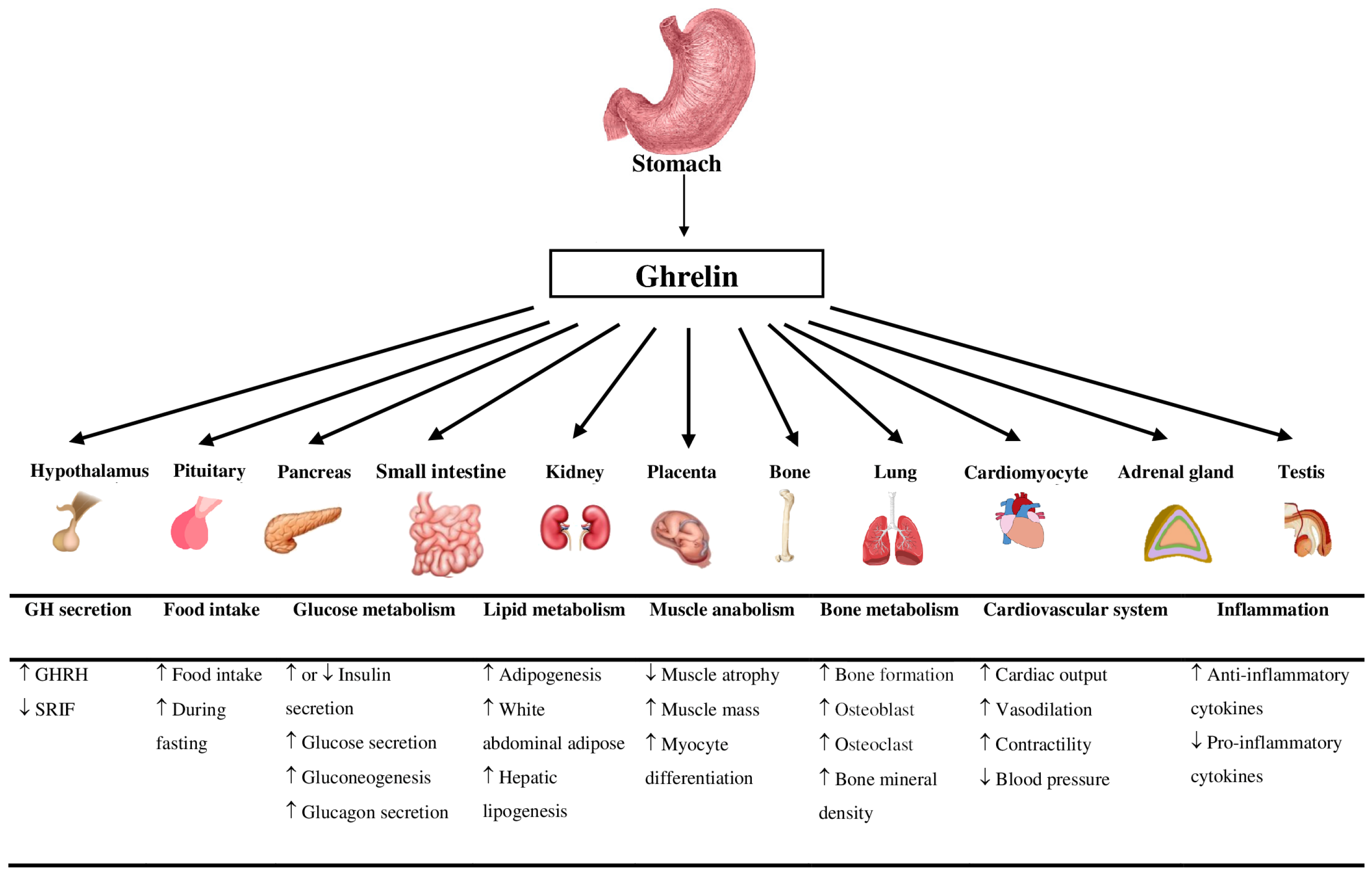
1.1. Ghrelin
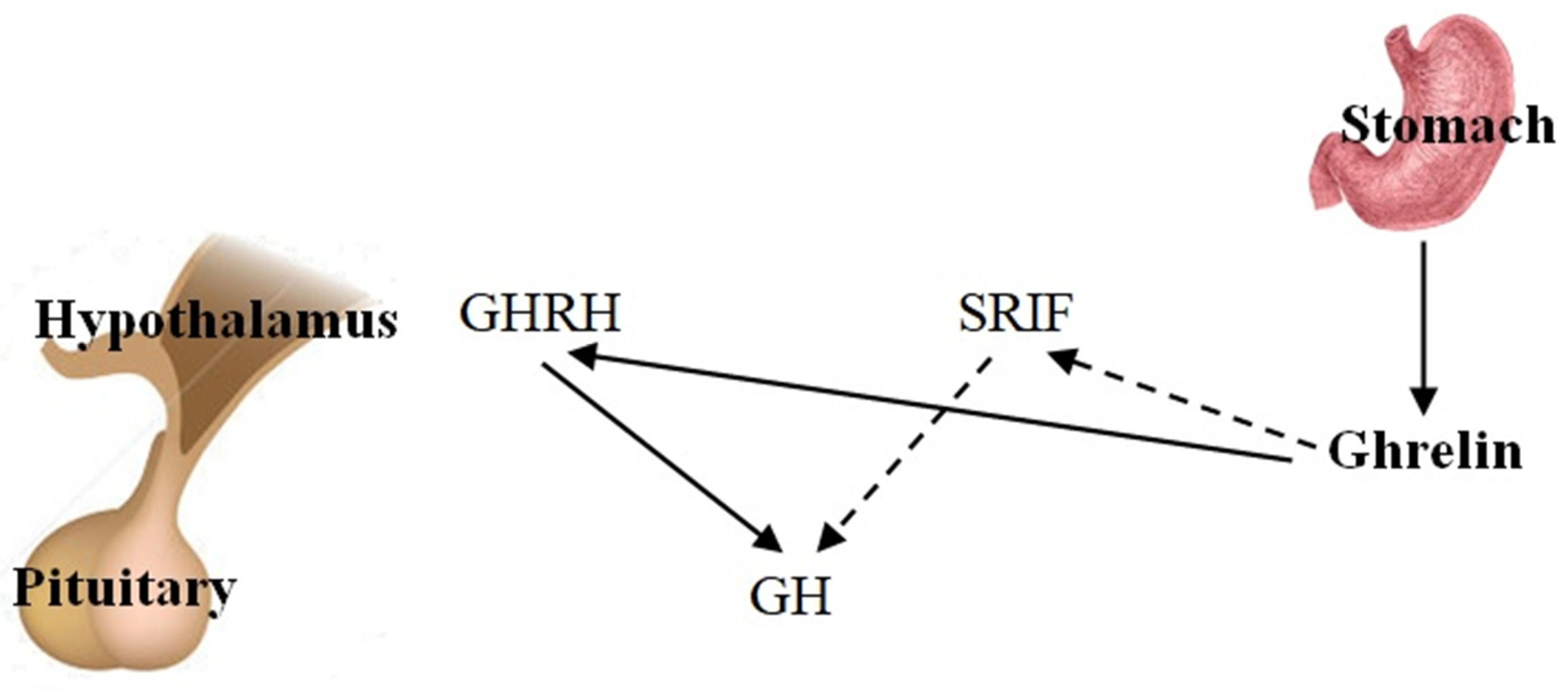
1.2. Ghrelin and Growth Hormone
1.3. Ghrelin and Food Intake
1.4. Ghrelin and Glucose Metabolism
1.5. Ghrelin and Lipid Metabolism
1.6. Ghrelin and Muscle Metabolism
1.7. Ghrelin and Bone Metabolism
1.8. Ghrelin and the Cardiovascular System
1.9. Ghrelin and Inflammation
2. Methods
2.1. Literature Search Strategy and Study Selection
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Selection Process
3.2. Participant Characteristics
3.3. Exercise Characteristics
3.4. Analytical Characteristics
3.5. Response of Ghrelin to Acute and Chronic Exercise Mode
3.5.1. Response to Acute Exercise
Total Ghrelin/Ghrelin
Growth Hormone
3.5.2. Response to Chronic Exercise
Total Ghrelin/Ghrelin
Body Mass, Body Mass Index, and Body Fat
4. Discussion
4.1. The Effect of Acute Exercise on Total Ghrelin
4.2. The Effect of Chronic Exercise on Total Ghrelin
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cifuentes, L.; Acosta, A. Homeostatic regulation of food intake. Clin. Res. Hepatol. Gastroenterol. 2022, 46, 101794. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.; Stengel, A. Control of Food Intake by Gastrointestinal Peptides: Mechanisms of Action and Possible Modulation in the Treatment of Obesity. J. Neurogastroenterol. Motil. 2017, 23, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Jayasena, C.N.; Bloom, S.R. Obesity and appetite control. Exp. Diabetes Res. 2012, 2012, 824305. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Diz-Chaves, Y. Ghrelin, appetite regulation, and food reward: Interaction with chronic stress. Int. J. Pept. 2011, 2011, 898450. [Google Scholar] [CrossRef]
- Pradhan, G.; Samson, S.L.; Sun, Y. Ghrelin: Much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 619–624. [Google Scholar] [CrossRef]
- Williams, D.L.; Cummings, D.E. Regulation of ghrelin in physiologic and pathophysiologic states. J. Nutr. 2005, 135, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.; Gaidhane, S.; Gaidhane, A.M.; Khatib, M.; Simkhada, P.; Gode, D.; Zahiruddin, Q.S. Ghrelin: Ghrelin as a regulatory Peptide in growth hormone secretion. J. Clin. Diagn. Res. 2014, 8, MC13–MC17. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef]
- Alyar, G.; Umudum, F.Z.; Akbaş, N. Changes in ghrelin, GLP-1, and PYY levels after diet and exercise in obese individuals. Rev. Assoc. Med. Bras. 2024, 70, e20230263. [Google Scholar] [CrossRef]
- Liu, H.W.; Cheng, H.C.; Tsai, S.H.; Shao, Y.T. Effects of acute resistance exercise with different loads on appetite, appetite hormones and autonomic nervous system responses in healthy young men. Appetite 2023, 182, 106428. [Google Scholar] [CrossRef]
- Najafi, R.; Heidarianpour, A.; Shokri, E.; Shokri, B. Ameliorative effects of aerobic training in girls with precocious puberty: Role of leptin and ghrelin. Sci. Rep. 2023, 13, 15732. [Google Scholar] [CrossRef]
- Ouerghi, N.; Feki, M.; Bragazzi, N.L.; Knechtle, B.; Hill, L.; Nikolaidis, P.T.; Bouassida, A. Ghrelin Response to Acute and Chronic Exercise: Insights and Implications from a Systematic Review of the Literature. Sports Med. 2021, 51, 2389–2410. [Google Scholar] [CrossRef] [PubMed]
- Carreira, M.C.; Crujeiras, A.B.; Andrade, S.; Monteiro, M.P.; Casanueva, F.F. Ghrelin as a GH-releasing factor. Endocr. Dev. 2013, 25, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Abassi, W.; Ouerghi, N.; Ghouili, H.; Haouami, S.; Bouassida, A. Greater effects of high- compared with moderate-intensity interval training on thyroid hormones in overweight/obese adolescent girls. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20200031. [Google Scholar] [CrossRef]
- Abassi, W.; Ouerghi, N.; Nikolaidis, P.T.; Hill, L.; Racil, G.; Knechtle, B.; Feki, M.; Bouassida, A. Interval Training with Different Intensities in Overweight/Obese Adolescent Females. Int. J. Sports Med. 2022, 43, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Abassi, W.; Ouerghi, N.; Feki, M.; Jebabli, N.; Andrade, M.S.; Bouassida, A.; Sousa, C.V.; Nikolaidis, P.T.; Weiss, K.; Knechtle, B. Effects of moderate-vs. high-intensity interval training on physical fitness, enjoyment, and affective valence in overweight/obese female adolescents: A pre-/post-test study. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3809–3822. [Google Scholar] [CrossRef] [PubMed]
- Abassi, W.; Ouerghi, N.; Hammami, M.B.; Jebabli, N.; Feki, M.; Bouassida, A.; Weiss, K.; Knechtle, B. High-Intensity Interval Training Reduces Liver Enzyme Levels and Improves MASLD-Related Biomarkers in Overweight/Obese Girls. Nutrients 2025, 17, 164. [Google Scholar] [CrossRef]
- Ouerghi, N.; Brini, S.; Zaouali, M.; Feki, M.; Tabka, Z.; Bouassida, A. Ghrelin is not altered after acute exercises at different intensities in overweight middle-aged individuals. Sci. Sports 2019, 34, 149–155. [Google Scholar] [CrossRef]
- Russel, R.D.; Willis, K.S.; Ravussin, E.; Larson-Meyer, E.D. Effects of endurance running and dietary fat on circulating ghrelin and peptide YY. J. Sports Sci. Med. 2009, 8, 574–583. [Google Scholar]
- Jürimäe, J.; Rämson, R.; Mäestu, J.; Purge, P.; Jürimäe, T.; Arciero, P.J.; von Duvillard, S.P. Plasma visfatin and ghrelin response to prolonged sculling in competitive male rowers. Med. Sci. Sports Exerc. 2009, 41, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, R.; Wang, J.; Zheng, X.; Zhao, S.; Zhang, Z.; Yu, W.; Li, S.; Zheng, P. The effect of blood flow restriction exercise on N-lactoylphenylalanine and appetite regulation in obese adults: A cross-design study. Front. Endocrinol. 2023, 14, 1289574. [Google Scholar] [CrossRef] [PubMed]
- Tobin, S.Y.; Cornier, M.A.; White, M.H.; Hild, A.K.; Simonsen, S.E.; Melanson, E.L.; Halliday, T.M. The effects of acute exercise on appetite and energy intake in men and women. Physiol. Behav. 2021, 241, 113562. [Google Scholar] [CrossRef] [PubMed]
- Laursen, T.L.; Zak, R.B.; Shute, R.J.; Heesch, M.W.S.; Dinan, N.E.; Bubak, M.P.; La Salle, D.T.; Slivka, D.R. Leptin, adiponectin, and ghrelin responses to endurance exercise in different ambient conditions. Temperature 2017, 4, 166–175. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Fathi, M.; RashidLamir, A.; Aminian, F. Effects of 8 Weeks Aerobic Training on Plasma Ghrelin Level and Ghrelin Lymphocyte Gene Expression in Elderly Men. Salmand Iran. J. Ageing 2019, 13, 494–505. [Google Scholar] [CrossRef]
- Fico, B.G.; Alkatan, M.; Tanaka, H. No changes in appetite-related hormones following swimming and cycling exercise interventions in adults with obesity. Int. J. Exerc. Sci. 2020, 13, 1819–1825. [Google Scholar] [CrossRef]
- Cho, G.J.; Han, S.W.; Shin, J.H.; Kim, T. Effects of intensive training on menstrual function and certain serum hormones and peptides related to the female reproductive system. Medicine 2017, 96, e6876. [Google Scholar] [CrossRef]
- Liao, J.; Huang, J.; Wang, S.; Xiang, M.; Wang, D.; Deng, H.; Yin, H.; Xu, F.; Hu, M. Effects of exercise and diet intervention on appetite-regulating hormones associated with miRNAs in obese children. Eat. Weight Disord. Stud. Anorexia Bulim. Obes. 2021, 26, 457–465. [Google Scholar] [CrossRef]
- Tremblay, A.; Dutheil, F.; Drapeau, V.; Metz, L.; Lesour, B.; Chapier, R.; Pereira, B.; Verney, J.; Baker, J.S.; Vinet, A.; et al. Long-term effects of high-intensity resistance and endurance exercise on plasma leptin and ghrelin in overweight individuals: The RESOLVE Study. Appl. Physiol. Nutr. Metab. 2019, 44, 1172–1179. [Google Scholar] [CrossRef]
- Ibrahim Abdalla, M.M. Ghrelin—Physiological Functions and Regulation. Eur. Endocrinol. 2015, 11, 90–95. [Google Scholar] [CrossRef]
- Müller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Cao, Y.; Voogd, K.; Steiner, D.F. On the processing of proghrelin to ghrelin. J. Biol. Chem. 2007, 281, 38867–38870. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Nunez-Salces, M.; Li, H.; Feinle-Bisset, C.; Young, R.L.; Page, A.J. The regulation of gastric ghrelin secretion. Acta Physiol. 2021, 231, e13588. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nakamura, Y.; Shiimura, Y.; Ohgusu, H.; Kangawa, K.; Kojima, M. Structure, regulation and function of ghrelin. J. Biochem. 2012, 151, 119–128. [Google Scholar] [CrossRef]
- Hartman, M.L.; Faria, A.C.; Vance, M.L.; Johnson, M.L.; Thorner, M.O.; Veldhuis, J.D. Temporal structure of in vivo growth hormone secretory events in humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1991, 260, E101–E110. [Google Scholar] [CrossRef]
- Thorner, M.O.; Chapman, I.M.; Gaylinn, B.D.; Pezzoli, S.S.; Hartman, M.L. Growth hormone-releasing hormone and growth hormone-releasing peptide as therapeutic agents to enhance growth hormone secretion in disease and aging. In Recent Progress in Hormone Research; Academic Press: New York, NY, USA, 1997; Volume 52, pp. 215–246. [Google Scholar]
- Lanfranco, F.; Motta, G.; Baldi, M.; Gasco, V.; Grottoli, S.; Benso, A.; Broglio, F.; Ghigo, E. Ghrelin and anterior pituitary function. Front. Horm. Res. 2010, 38, 206–211. [Google Scholar] [CrossRef]
- Perchard, R.; Clayton, P.E. Ghrelin and Growth. Endocr. Dev. 2017, 32, 74–86. [Google Scholar] [CrossRef]
- Druce, M.R.; Wren, A.M.; Park, A.J.; Milton, J.E.; Patterson, M.; Frost, G.; Ghatei, M.A.; Small, C.; Bloom, S.R. Ghrelin increases food intake in obese as well as lean subjects. Int. J. Obes. 2005, 29, 1130–1136. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Natalucci, G.; Riedl, S.; Gleiss, A.; Zidek, T.; Frisch, H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur. J. Endocrinol. 2005, 152, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Investig. 2001, 24, RC19–RC21. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Asakawa, A.; Fujimiya, M.; Lee, S.D.; Inui, A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol. Rev. 2009, 61, 430–481. [Google Scholar] [CrossRef]
- Lee, H.M.; Wang, G.; Englander, E.W.; Kojima, M.; Greeley, G.H., Jr. Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: Enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology 2002, 143, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Reimer, M.K.; Pacini, G.; Ahrén, B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology 2003, 144, 916–921. [Google Scholar] [CrossRef]
- Tong, J.; Prigeon, R.L.; Davis, H.W.; Bidlingmaier, M.; Kahn, S.E.; Cummings, D.E.; Tschöp, M.H.; D’Alessio, D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010, 59, 2145–2151. [Google Scholar] [CrossRef]
- Andralojc, K.M.; Mercalli, A.; Nowak, K.W.; Albarello, L.; Calcagno, R.; Luzi, L.; Bonifacio, E.; Doglioni, C.; Piemonti, L. Ghrelin-producing epsilon cells in the developing and adult human pancreas. Diabetologia 2009, 52, 486–493. [Google Scholar] [CrossRef]
- Benitez, C.M.; Goodyer, W.R.; Kim, S.K. Deconstructing pancreas developmental biology. Cold Spring Harb. Perspect. Biol. 2012, 4, a012401. [Google Scholar] [CrossRef]
- Poher, A.L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef]
- Qader, S.S.; Lundquist, I.; Ekelund, M.; Håkanson, R.; Salehi, A. Ghrelin activates neuronal constitutive nitric oxide synthase in pancreatic islet cells while inhibiting insulin release and stimulating glucagon release. Regul. Pept. 2005, 128, 51–56. [Google Scholar] [CrossRef]
- Wiedemann, T.; Bielohuby, M.; Müller, T.D.; Bidlingmaier, M.; Pellegata, N.S. Obesity in MENX rats is accompanied by high circulating levels of ghrelin and improved insulin sensitivity. Diabetes 2016, 65, 406–420. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chuang, J.C.; Sakata, I.; Kohno, D.; Perello, M.; Osborne-Lawrence, S.; Repa, J.J.; Zigman, J.M. Ghrelin directly stimulates glucagon secretion from pancreatic alpha-cells. Mol. Endocrinol. 2011, 25, 1600–1611. [Google Scholar] [CrossRef]
- Yi, C.X.; Heppner, K.M.; Kirchner, H.; Tong, J.; Bielohuby, M.; Gaylinn, B.D.; Müller, T.D.; Bartley, E.; Davis, H.W.; Zhao, Y.; et al. The GOAT-ghrelin system is not essential for hypoglycemia prevention during prolonged calorie restriction. PLoS ONE 2012, 7, e32100. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jiang, H.; Zhang, H.; Smith, R.G. Modification of ghrelin receptor signaling by somatostatin receptor-5 regulates insulin release. Proc. Natl. Acad. Sci. USA 2012, 109, 19003–19008. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Guillén, L.; Barrios, V.; Martos, G.; Chowen, J.A.; Campos-Barros, A.; Argente, J. Effect of oral glucose administration on ghrelin levels in obese children. Eur. J. Endocrinol. 2004, 151, 119–121. [Google Scholar] [CrossRef]
- Martos-Moreno, G.A.; Barrios, V.; Soriano-Guillén, L.; Argente, J. Relationship between adiponectin levels, acylated ghrelin levels, and short-term body mass index changes in children with diabetes mellitus type 1 at diagnosis and after insulin therapy. Eur. J. Endocrinol. 2006, 155, 757–761. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Kamegai, J.; Tamura, H.; Shimizu, T.; Ishii, S.; Sugihara, H.; Wakabayashi, I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001, 50, 2438–2443. [Google Scholar] [CrossRef]
- Notaro, N.M.; Dyck, D.J. Regulation of peripheral tissue substrate metabolism by the gut-derived hormone ghrelin. Metab. Open 2024, 21, 100279. [Google Scholar] [CrossRef]
- Theander-Carrillo, C.; Wiedmer, P.; Cettour-Rose, P.; Nogueiras, R.; Perez-Tilve, D.; Pfluger, P.; Castaneda, T.R.; Muzzin, P.; Schürmann, A.; Szanto, I.; et al. Ghrelin action in the brain controls adipocyte metabolism. J. Clin. Investig. 2006, 116, 1983–1993. [Google Scholar] [CrossRef]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin Induces Adiposity in Rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Hucik, B.; Lovell, A.J.; Hoecht, E.M.; Cervone, D.T.; Mutch, D.M.; Dyck, D.J. Regulation of adipose tissue lipolysis by ghrelin is impaired with high-fat diet feeding and is not restored with exercise. Adipocyte 2021, 10, 338–349. [Google Scholar] [CrossRef]
- Wells, T. Ghrelin—Defender of fat. Prog. Lipid Res. 2009, 48, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.S.; Kotokorpi, P.; Eccles, S.R.; Barnes, S.K.; Tokarczuk, P.F.; Allen, S.K.; Whitworth, H.S.; Guschina, I.A.; Evans, B.A.; Mode, A.; et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol. Endocrinol. 2009, 23, 914–924. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Abbott, C.R.; Dhillo, W.S.; Seal, L.J.; Cohen, M.A.; Batterham, R.L.; Taheri, S.; Stanley, S.A.; Ghatei, M.A.; et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001, 50, 2540–2547. [Google Scholar] [CrossRef]
- Li, Z.; Xu, G.; Qin, Y.; Zhang, C.; Tang, H.; Yin, Y.; Xiang, X.; Li, Y.; Zhao, J.; Mulholland, M.; et al. Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARγ signaling pathway. Proc. Natl. Acad. Sci. USA 2014, 111, 13163–13168. [Google Scholar] [CrossRef]
- Ezquerro, S.; Méndez-Giménez, L.; Becerril, S.; Moncada, R.; Valentí, V.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G.; Rodríguez, A. Acylated and desacyl ghrelin are associated with hepatic lipogenesis, β-oxidation and autophagy: Role in NAFLD amelioration after sleeve gastrectomy in obese rats. Sci. Rep. 2016, 6, 39942. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Mardian, T.; Stephenson, B.; Grammer, E.E.; Stahl, M.E.; Weeldreyer, N.R.; Liu, Z.; Love, K.M.; Kranz, S.; Allen, J.D.; et al. The Impact of Exercise Intensity and Sex on Endogenous Ghrelin Levels and Appetite in Healthy Humans. J. Endocr. Soc. 2024, 8, bvae165. [Google Scholar] [CrossRef]
- Beauloye, V.; Diene, G.; Kuppens, R.; Zech, F.; Winandy, C.; Molinas, C.; Faye, S.; Kieffer, I.; Beckers, D.; Nergårdh, R.; et al. High unacylated ghrelin levels support the concept of anorexia in infants with Prader-Willi syndrome. Orphanet J. Rare Dis. 2016, 11, 56. [Google Scholar] [CrossRef]
- Porporato, P.E.; Filigheddu, N.; Reano, S.; Ferrara, M.; Angelino, E.; Gnocchi, V.F.; Prodam, F.; Ronchi, G.; Fagoonee, S.; Fornaro, M.; et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Investig. 2013, 123, 611–622. [Google Scholar] [CrossRef]
- Balasubramaniam, A.; Joshi, R.; Su, C.; Friend, L.A.; Sheriff, S.; Kagan, R.J.; James, J.H. Ghrelin inhibits skeletal muscle protein breakdown in rats with thermal injury through normalizing elevated expression of E3 ubiquitin ligases MuRF1 and MAFbx. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R893–R901. [Google Scholar] [CrossRef] [PubMed]
- Deboer, M.D.; Zhu, X.; Levasseur, P.R.; Inui, A.; Hu, Z.; Han, G.; Mitch, W.E.; Taylor, J.E.; Halem, H.A.; Dong, J.; et al. Ghrelin treatment of chronic kidney disease: Improvements in lean body mass and cytokine profile. Endocrinology 2008, 149, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Yamaki, A.; Furuya, M.; Inomata, N.; Minamitake, Y.; Ohsuye, K.; Kangawa, K. Ghrelin improves body weight loss and skeletal muscle catabolism associated with angiotensin II-induced cachexia in mice. Regul. Pept. 2012, 178, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Filigheddu, N.; Gnocchi, V.F.; Coscia, M.; Cappelli, M.; Porporato, P.E.; Taulli, R.; Traini, S.; Baldanzi, G.; Chianale, F.; Cutrupi, S.; et al. Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol. Biol. Cell 2007, 18, 986–994. [Google Scholar] [CrossRef]
- Fukushima, N.; Hanada, R.; Teranishi, H.; Fukue, Y.; Tachibana, T.; Ishikawa, H.; Takeda, S.; Takeuchi, Y.; Fukumoto, S.; Kangawa, K.; et al. Ghrelin directly regulates bone formation. J. Bone Miner. Res. 2005, 20, 790–798. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Theocharis, S.; Kouraklis, G. Ghrelin, another factor affecting bone metabolism. Med. Sci. Monit. 2010, 16, RA147–RA162. [Google Scholar]
- van der Velde, M.; Delhanty, P.; van der Eerden, B.; van der Lely, A.J.; van Leeuwen, J. Ghrelin and bone. Vitam. Horm. 2008, 77, 239–258. [Google Scholar] [CrossRef]
- Costa, J.L.; Naot, D.; Lin, J.M.; Watson, M.; Callon, K.E.; Reid, I.R.; Grey, A.B.; Cornish, J. Ghrelin is an Osteoblast Mitogen and Increases Osteoclastic Bone Resorption In Vitro. Int. J. Pept. 2011, 2011, 605193. [Google Scholar] [CrossRef]
- Roelen, B.A.; Dijke, P.T. Controlling mesenchymal stem cell differentiation by TGFBeta family members. J. Orthop. Sci. 2003, 8, 740–748. [Google Scholar] [CrossRef]
- Wells, T.; Houston, P.A. Skeletal growth acceleration with growth hormone secretagogues in transgenic growth retarded rats: Pattern-dependent effects and mechanisms of desensitization. J. Neuroendocrinol. 2001, 13, 496–504. [Google Scholar] [CrossRef]
- Monson, J.P.; Drake, W.M.; Carroll, P.V.; Weaver, J.U.; Rodriguez-Arnao, J.; Savage, M.O. Influence of growth hormone on accretion of bone mass. Horm. Res. 2002, 58 (Suppl. S1), 52–56. [Google Scholar] [CrossRef]
- Delhanty, P.J.; van der Eerden, B.C.; van der Velde, M.; Gauna, C.; Pols, H.A.; Jahr, H.; Chiba, H.; van der Lely, A.J.; van Leeuwen, J.P. Ghrelin and unacylated ghrelin stimulate human osteoblast growth via mitogen-activated protein kinase (MAPK)/phosphoinositide 3-kinase (PI3K) pathways in the absence of GHS-R1a. J. Endocrinol. 2006, 188, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Her, S.J.; Park, S.J.; Kim, D.; Park, K.S.; Lee, H.K.; Han, B.H.; Kim, M.S.; Shin, C.S.; Kim, S.Y. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone 2005, 37, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Maccarinelli, G.; Sibilia, V.; Torsello, A.; Raimondo, F.; Pitto, M.; Giustina, A.; Netti, C.; Cocchi, D. Ghrelin regulates proliferation and differentiation of osteoblastic cells. J. Endocrinol. 2005, 184, 249–256. [Google Scholar] [CrossRef]
- Barre, R.; Beton, N.; Batut, A.; Accabled, F.; Sales de Gauzy, J.; Auriol, F.; Eddiry, S.; Tauber, M.; Laurencin, S.; Salles, J.P.; et al. Ghrelin uses the GHS-R1a/Gi/cAMP pathway and induces differentiation only in mature osteoblasts. This ghrelin pathway is impaired in AIS patients. Biochem. Biophys. Rep. 2020, 24, 100782. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N.; Pedone, C.; Pozzilli, P.; Lauretani, F.; Bandinelli, S.; Ferrucci, L.; Incalzi, R.A. Effect of ghrelin on bone mass density: The InChianti study. Bone 2011, 49, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Makovey, J.; Chen, J.S.; Hayward, C.; Williams, F.M.; Sambrook, P.N. Association between serum cholesterol and bone mineral density. Bone 2009, 44, 208–213. [Google Scholar] [CrossRef]
- Pérez-Castrillón, J.L.; Justo, I.; Sanz, A.; San Miguel, A.; Mazón, M.A.; Abad, L.; Vega, G.; Dueñas, A. Ghrelin and bone mass in postmenopausal hypertensive women. Ann. Nutr. Metab. 2007, 51, 223–227. [Google Scholar] [CrossRef]
- Akalu, Y.; Molla, M.D.; Dessie, G.; Ayelign, B. Physiological Effect of Ghrelin on Body Systems. Int. J. Endocrinol. 2020, 2020, 1385138. [Google Scholar] [CrossRef]
- Nagaya, N.; Uematsu, M.; Kojima, M.; Ikeda, Y.; Yoshihara, F.; Shimizu, W.; Hosoda, H.; Hirota, Y.; Ishida, H.; Mori, H.; et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation 2001, 104, 1430–1435. [Google Scholar] [CrossRef]
- Nagaya, N.; Moriya, J.; Yasumura, Y.; Uematsu, M.; Ono, F.; Shimizu, W.; Ueno, K.; Kitakaze, M.; Miyatake, K.; Kangawa, K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation 2004, 110, 3674–3679. [Google Scholar] [CrossRef]
- Leite-Moreira, A.F.; Rocha-Sousa, A.; Henriques-Coelho, T. Cardiac, skeletal, and smooth muscle regulation by ghrelin. Vitam. Horm. 2008, 77, 207–238. [Google Scholar] [CrossRef]
- Soeki, T.; Koshiba, K.; Niki, T.; Kusunose, K.; Yamaguchi, K.; Yamada, H.; Wakatsuki, T.; Shimabukuro, M.; Minakuchi, K.; Kishimoto, I.; et al. Effect of ghrelin on autonomic activity in healthy volunteers. Peptides 2014, 62, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lilleness, B.M.; Frishman, W.H. Ghrelin and the Cardiovascular System. Cardiol. Rev. 2016, 24, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Okumura, H.; Nagaya, N.; Enomoto, M.; Nakagawa, E.; Oya, H.; Kangawa, K. Vasodilatory effect of ghrelin, an endogenous peptide from the stomach. J. Cardiovasc. Pharmacol. 2002, 39, 779–783. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, M.; Qu, X.; Yuan, F.; Yang, Y.; Xu, L.; Dai, J.; Wang, W.; Fei, J.; Hou, X. Ghrelin receptor deficiency aggravates atherosclerotic plaque instability and vascular inflammation. Front. Biosci. 2015, 20, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Ukkola, O. Ghrelin and atherosclerosis. Curr. Opin. Lipidol. 2015, 26, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Gavrila, D.; Liu, X.; Wang, L.; Gunnlaugsson, S.; Stoll, L.L.; McCormick, M.L.; Sigmund, C.D.; Tang, C.; Weintraub, N.L. Ghrelin inhibits proinflammatory responses and nuclear factor-kappaB activation in human endothelial cells. Circulation 2004, 109, 2221–2226. [Google Scholar] [CrossRef]
- Waseem, T.; Duxbury, M.; Ito, H.; Ashley, S.W.; Robinson, M.K. Exogenous ghrelin modulates release of pro-inflammatory and anti-inflammator cytokines in LPS-stimulated macrophages through distinct signaling pathways. Surgery 2008, 143, 334–342. [Google Scholar] [CrossRef]
- Takata, A.; Takiguchi, S.; Miyazaki, Y.; Miyata, H.; Takahashi, T.; Kurokawa, Y.; Yamasaki, M.; Nakajima, K.; Mori, M.; Kangawa, K.; et al. Randomized Phase II Study of the Anti-inflammatory Effect of Ghrelin During the Postoperative Period of Esophagectomy. Ann. Surg. 2015, 262, 230–236. [Google Scholar] [CrossRef]
- Farokhnia, M.; Portelli, J.; Lee, M.R.; McDiarmid, G.R.; Munjal, V.; Abshire, K.M.; Battista, J.T.; Browning, B.D.; Deschaine, S.L.; Akhlaghi, F.; et al. Effects of exogenous ghrelin administration and ghrelin receptor blockade, in combination with alcohol, on peripheral inflammatory markers in heavy-drinking individuals: Results from two human laboratory studies. Brain Res. 2020, 1740, 146851. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, H.; Guo, W.; Yu, L. Potential role of ghrelin in the regulation of inflammation. FASEB J. 2022, 36, e22508. [Google Scholar] [CrossRef]
- Lee, J.; Lim, E.; Kim, Y.; Li, E.; Park, S. Ghrelin attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. J. Endocrinol. 2010, 205, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cui, Z.; Luo, G.; Zhang, J.; Ma, T.; Hu, N.; Cui, T. Ghrelin attenuates intestinal ischemia/reperfusion injury in mice by activating the mTOR signaling pathway. Int. J. Mol. Med. 2013, 32, 851–859. [Google Scholar] [CrossRef]
- El-Shaer, N.O.; El Gazzar, W.B.; Allam, M.M.; Anwer, H.M. Ghrelin ameliorated inflammation and oxidative stress in isoproterenol induced myocardial infarction through the endothelial nitric oxide synthase (eNOS)/nuclear factor erythroid 2-related factor-2 (NRF2)/heme oxygenase-1 (HO-1) signaling pathway. J. Physiol. Pharmacol. 2021, 72, 273–282. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef]
- Dall, R.; Kanaley, J.; Hansen, T.K.; Møller, N.; Christiansen, J.S.; Hosoda, H.; Kangawa, K.; Jørgensen, J.O. Plasma ghrelin levels during exercise in healthy subjects and in growth hormone-deficient patients. Eur. J. Endocrinol. 2002, 147, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, R.R.; Durand, R.J.; Acevedo, E.O.; Johnson, L.G.; Kraemer, G.R.; Hebert, E.P.; Castracane, V.D. Rigorous running increases growth hormone and insulin-like growth factor-I without altering ghrelin. Exp. Biol. Med. 2004, 229, 240–246. [Google Scholar] [CrossRef]
- Schmidt, A.; Maier, C.; Schaller, G.; Nowotny, P.; Bayerle-Eder, M.; Buranyi, B.; Luger, A.; Wolzt, M. Acute exercise has no effect on ghrelin plasma concentrations. Horm. Metab. Res. 2004, 36, 174–177. [Google Scholar] [CrossRef]
- Zoladz, J.A.; Konturek, S.J.; Duda, K.; Majerczak, J.; Sliwowski, Z.; Grandys, M.; Bielanski, W. Effect of moderate incremental exercise, performed in fed and fasted state on cardio-respiratory variables and leptin and ghrelin concentrations in young healthy men. J. Physiol. Pharmacol. 2005, 56, 63–85. [Google Scholar]
- Erdmann, J.; Tahbaz, R.; Lippl, F.; Wagenpfeil, S.; Schusdziarra, V. Plasma ghrelin levels during exercise—Effects of intensity and duration. Regul. Pept. 2007, 143, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Jürimäe, J.; Hofmann, P.; Jürimäe, T.; Palm, R.; Mäestu, J.; Purge, P.; Sudi, K.; Rom, K.; von Duvillard, S.P. Plasma ghrelin responses to acute sculling exercises in elite male rowers. Eur. J. Appl. Physiol. 2007, 99, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Salvadori, A.; Brunani, A.; Verti, B.; Walker, G.E.; Fanari, P.; Tovaglieri, I.; De Medici, C.; Savia, G.; Liuzzi, A. Acylated ghrelin decreases during acute exercise in the lean and obese state. Clin. Endocrinol. 2008, 69, 970–971. [Google Scholar] [CrossRef]
- Thomas, G.A.; Kraemer, W.J.; Comstock, B.A.; Dunn-Lewis, C.; Volek, J.S.; Denegar, C.R.; Maresh, C.M. Effects of resistance exercise and obesity level on ghrelin and cortisol in men. Metabolism 2012, 61, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, D.R.; Blannin, A.K. Effects of exercise in the cold on Ghrelin, PYY, and food intake in overweight adults. Med. Sci. Sports Exerc. 2015, 47, 49–57. [Google Scholar] [CrossRef]
- Toshinai, K.; Kawagoe, T.; Shimbara, T.; Tobina, T.; Nishida, Y.; Mondal, M.S.; Yamaguchi, H.; Date, Y.; Tanaka, H.; Nakazato, M. Acute incremental exercise decreases plasma ghrelin level in healthy men. Horm. Metab. Res. 2007, 39, 849–851. [Google Scholar] [CrossRef]
- Malkova, D.; McLaughlin, R.; Manthou, E.; Wallace, A.M.; Nimmo, M.A. Effect of moderate-intensity exercise session on preprandial and postprandial responses of circulating ghrelin and appetite. Horm. Metab. Res. 2008, 40, 410–415. [Google Scholar] [CrossRef]
- Stokes, K.A.; Sykes, D.; Gilbert, K.L.; Chen, J.W.; Frystyk, J. Brief, high intensity exercise alters serum ghrelin and growth hormone concentrations but not IGF-I, IGF-II or IGF-I bioactivity. Growth Horm. IGF Res. 2010, 20, 289–294. [Google Scholar] [CrossRef]
- Kelly, P.J.; Guelfi, K.J.; Wallman, K.E.; Fairchild, T.J. Mild dehydration does not reduce postexercise appetite or energy intake. Med. Sci. Sports Exerc. 2012, 44, 516–524. [Google Scholar] [CrossRef]
- Halliday, T.M.; White, M.H.; Hild, A.K.; Conroy, M.B.; Melanson, E.L.; Cornier, M.A. Appetite and Energy Intake Regulation in Response to Acute Exercise. Med. Sci. Sports Exerc. 2021, 53, 2173–2181. [Google Scholar] [CrossRef]
- Jürimäe, J.; Jürimäe, T.; Purge, P. Plasma ghrelin is altered after maximal exercise in elite male rowers. Exp. Biol. Med. 2007, 232, 904–909. [Google Scholar]
- Burns, S.F.; Broom, D.R.; Miyashita, M.; Mundy, C.; Stensel, D.J. A single session of treadmill running has no effect on plasma total ghrelin concentrations. J. Sports Sci. 2007, 25, 635–642. [Google Scholar] [CrossRef]
- Martins, C.; Morgan, L.M.; Bloom, S.R.; Robertson, M.D. Effects of exercise on gut peptides, energy intake and appetite. J. Endocrinol. 2007, 193, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Sartorio, A.; Morpurgo, P.; Cappiello, V.; Agosti, F.; Marazzi, N.; Giordani, C.; Rigamonti, A.E.; Muller, E.E.; Spada, A. Exercise-induced effects on growth hormone levels are associated with ghrelin changes only in presence of prolonged exercise bouts in male athletes. J. Sports Med. Phys. Fit. 2008, 48, 97–101. [Google Scholar]
- Hagobian, T.A.; Sharoff, C.G.; Stephens, B.R.; Wade, G.N.; Silva, J.E.; Chipkin, S.R.; Braun, B. Effects of exercise on energy-regulating hormones and appetite in men and women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R233–R242. [Google Scholar] [CrossRef]
- Shiiya, T.; Ueno, H.; Toshinai, K.; Kawagoe, T.; Naito, S.; Tobina, T.; Nishida, Y.; Shindo, M.; Kangawa, K.; Tanaka, H.; et al. Significant lowering of plasma ghrelin but not des-acyl ghrelin in response to acute exercise in men. Endocr. J. 2011, 58, 335–342. [Google Scholar] [CrossRef]
- Plinta, R.; Olszanecka-Glinianowicz, M.; Drosdzol-Cop, A.; Chudek, J.; Skrzypulec-Plinta, V. The effect of three-month pre-season preparatory period and short-term exercise on plasma leptin, adiponectin, visfatin, and ghrelin levels in young female handball and basketball players. J. Endocrinol. Investig. 2012, 35, 595–601. [Google Scholar] [CrossRef]
- Ballard, T.P.; Melby, C.L.; Camus, H.; Cianciulli, M.; Pitts, J.; Schmidt, S.; Hickey, M.S. Effect of resistance exercise, with or without carbohydrate supplementation, on plasma ghrelin concentrations and postexercise hunger and food intake. Metabolism 2009, 58, 1191–1199. [Google Scholar] [CrossRef]
- Ghanbari-Niaki, A. Ghrelin and glucoregulatory hormone responses to a single circuit resistance exercise in male college students. Clin. Biochem. 2006, 39, 966–970. [Google Scholar] [CrossRef]
- Christ, E.R.; Zehnder, M.; Boesch, C.; Trepp, R.; Mullis, P.E.; Diem, P.; Décombaz, J. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. Eur. J. Endocrinol. 2006, 154, 397–403. [Google Scholar] [CrossRef]
- Rämson, R.; Jürimäe, J.; Jürimäe, T.; Mäestu, J. The influence of increased training volume on cytokines and ghrelin concentration in college level male rowers. Eur. J. Appl. Physiol. 2008, 104, 839–846. [Google Scholar] [CrossRef]
- Hedayati, M.; Saghebjoo, M.; Ghanbari-Niaki, A. Effects of circuit resistance training intensity on the plasma ghrelin to obestatin ratios in healthy young women. Int. J. Endocrinol. Metab. 2012, 10, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Rosenkilde, M.; Morville, T.; Andersen, P.R.; Kjær, K.; Rasmusen, H.; Holst, J.J.; Dela, F.; Westerterp, K.; Sjödin, A.; Helge, J.W. Inability to match energy intake with energy expenditure at sustained near-maximal rates of energy expenditure in older men during a 14-d cycling expedition. Am. J. Clin. Nutr. 2015, 102, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Rämson, R.; Jürimäe, J.; Jürimäe, T.; Mäestu, J. The effect of 4-week training period on plasma neuropeptide Y, leptin and ghrelin responses in male rowers. Eur. J. Appl. Physiol. 2012, 112, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M. Serum leptin and ghrelin changes-induced aerobic training in healthy young females. Int. J. Collab. Res. Intern. Med. Public Health 2012, 4, 1257–1264. [Google Scholar]
- Martins, C.; Kulseng, B.; King, N.A.; Holst, J.J.; Blundell, J.E. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J. Clin. Endocrinol. Metab. 2010, 95, 1609–1616. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet. Med. 2013, 30, e41–e50. [Google Scholar] [CrossRef]
- Rosenkilde, M.; Reichkendler, M.H.; Auerbach, P.; Toräng, S.; Gram, A.S.; Ploug, T.; Holst, J.J.; Sjödin, A.; Stallknecht, B. Appetite regulation in overweight, sedentary men after different amounts of endurance exercise: A randomized controlled trial. J. Appl. Physiol. 2013, 115, 1599–1609. [Google Scholar] [CrossRef]
- Gibbons, C.; Blundell, J.E.; Caudwell, P.; Webb, D.L.; Hellström, P.M.; Näslund, E.; Finlayson, G. The Role of Episodic Postprandial Peptides in Exercise-Induced Compensatory Eating. J. Clin. Endocrinol. Metab. 2017, 102, 4051–4059. [Google Scholar] [CrossRef]
- Elerian, A.E.; Abdeen, H.; Elmakaky, A.; Mostafa, M.S. Efficacy of gender, anaerobic exercise and low-calorie diet on leptin, ghrelin hormones and hunger perception: A comparative study. Obes. Med. 2020, 18, 100213. [Google Scholar] [CrossRef]
- Leidy, H.J.; Gardner, J.K.; Frye, B.R.; Snook, M.L.; Schuchert, M.K.; Richard, E.L.; Williams, N.I. Circulating ghrelin is sensitive to changes in body weight during a diet and exercise program in normal-weight young women. J. Clin. Endocrinol. Metab. 2004, 89, 2659–2664. [Google Scholar] [CrossRef] [PubMed]
- Foster-Schubert, K.E.; McTiernan, A.; Frayo, R.S.; Schwartz, R.S.; Rajan, K.B.; Yasui, Y.; Tworoger, S.S.; Cummings, D.E. Human plasma ghrelin levels increase during a one-year exercise program. J. Clin. Endocrinol. Metab. 2005, 90, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Mizia-Stec, K.; Zahorska-Markiewicz, B.; Olszanecka-Glinianowicz, M.; Janowska, J.; Mucha, Z.; Holecki, M.; Gasiora, Z. Ghrelin as a potential blood pressure reducing factor in obese women during weight loss treatment. Endokrynol. Pol. 2008, 59, 207–211. [Google Scholar] [PubMed]
- Kelishadi, R.; Hashemipour, M.; Mohammadifard, N.; Alikhassy, H.; Adeli, K. Short- and long-term relationships of serum ghrelin with changes in body composition and the metabolic syndrome in prepubescent obese children following two different weight loss programmes. Clin. Endocrinol. 2008, 69, 721–729. [Google Scholar] [CrossRef]
- Konopko-Zubrzycka, M.; Baniukiewicz, A.; Wróblewski, E.; Kowalska, I.; Zarzycki, W.; Górska, M.; Dabrowski, A. The effect of intragastric balloon on plasma ghrelin, leptin, and adiponectin levels in patients with morbid obesity. J. Clin. Endocrinol. Metab. 2009, 94, 1644–1649. [Google Scholar] [CrossRef]
- Gueugnon, C.; Mougin, F.; Nguyen, N.U.; Bouhaddi, M.; Nicolet-Guénat, M.; Dumoulin, G. Ghrelin and PYY levels in adolescents with severe obesity: Effects of weight loss induced by long-term exercise training and modified food habits. Eur. J. Appl. Physiol. 2012, 112, 1797–1805. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Vrabas, I.S.; Kapelouzou, A.; Lampropoulos, S.; Sailer, N.; Kostakis, A.; Liapis, C.D.; Angelopoulou, N. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med. Sci. Monit. 2012, 18, CR290–CR295. [Google Scholar] [CrossRef]
- Markofski, M.M.; Carrillo, A.E.; Timmerman, K.L.; Jennings, K.; Coen, P.M.; Pence, B.D.; Flynn, M.G. Exercise training modifies ghrelin and adiponectin concentrations and is related to inflammation in older adults. J. Gerontol. Ser. A 2014, 69, 675–681. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, Y.J.; Lee, S.Y.; Jeong, D.W.; Lee, J.G.; Yi, Y.H.; Cho, Y.H.; Choi, E.J.; Kim, H.J. Interactive effects of an isocaloric high-protein diet and resistance exercise on body composition, ghrelin, and metabolic and hormonal parameters in untrained young men: A randomized clinical trial. J. Diabetes Investig. 2014, 5, 242–247. [Google Scholar] [CrossRef]
- Kang, S.J.; Kim, J.H.; Gang, Z.; Yook, Y.S.; Yoon, J.R.; Ha, G.C.; Ko, K.J. Effects of 12-week circuit exercise program on obesity index, appetite regulating hormones, and insulin resistance in middleaged obese females. J. Phys. Ther. Sci. 2018, 30, 169–173. [Google Scholar] [CrossRef][Green Version]
- Ataeinosrat, A.; Haghighi, M.M.; Abednatanzi, H.; Soltani, M.; Ghanbari-Niaki, A.; Nouri-Habashi, A.; Amani-Shalamzari, S.; Mossayebi, A.; Khademosharie, M.; Johnson, K.E.; et al. Effects of Three Different Modes of Resistance Training on Appetite Hormones in Males with Obesity. Front. Physiol. 2022, 13, 827335. [Google Scholar] [CrossRef]
- Campos, R.M.; de Mello, M.T.; Tock, L.; Silva, P.L.; Masquio, D.C.; de Piano, A.; Sanches, P.L.; Carnier, J.; Corgosinho, F.C.; Foschini, D.; et al. Aerobic plus resistance training improves bone metabolism and inflammation in adolescents who are obese. J. Strength Cond. Res. 2014, 28, 758–766. [Google Scholar] [CrossRef]
- Mason, C.; Xiao, L.; Imayama, I.; Duggan, C.R.; Campbell, K.L.; Kong, A.; Wang, C.Y.; Alfano, C.M.; Blackburn, G.L.; Foster-Schubert, K.E.; et al. The effects of separate and combined dietary weight loss and exercise on fasting ghrelin concentrations in overweight and obese women: A randomized controlled trial. Clin. Endocrinol. 2015, 82, 369–376. [Google Scholar] [CrossRef]
- King, J.A.; Wasse, L.K.; Stensel, D.J.; Nimmo, M.A. Exercise and ghrelin. A narrative overview of research. Appetite 2013, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Pavlatou, M.; Diamanti-Kandarakis, E.; Chrousos, G.P. Exercise and the stress system. Hormones 2005, 4, 73–89. [Google Scholar] [PubMed]
- Broom, D.R.; Miyashita, M.; Wasse, L.K.; Pulsford, R.; King, J.A.; Thackray, A.E.; Stensel, D.J. Acute effect of exercise intensity and duration on acylated ghrelin and hunger in men. J. Endocrinol. 2017, 232, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Weigle, D.S.; Cummings, D.E.; Newby, P.D.; Breen, P.A.; Frayo, R.S.; Matthys, C.C.; Callahan, H.S.; Purnell, J.Q. Roles of leptin and ghrelin in the loss of body weight caused by a low fat, high carbohydrate diet. J. Clin. Endocrinol. Metab. 2003, 88, 1577–1586. [Google Scholar] [CrossRef]
- Klok, M.D.; Jakobsdottir, S.; Drent, M.L. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obes. Rev. 2007, 8, 21–34. [Google Scholar] [CrossRef]
- Marzullo, P.; Verti, B.; Savia, G.; Walker, G.E.; Guzzaloni, G.; Tagliaferri, M.; Di Blasio, A.; Liuzzi, A. The relationship between active ghrelin levels and human obesity involves alterations in resting energy expenditure. J. Clin. Endocrinol. Metab. 2004, 89, 936–939. [Google Scholar] [CrossRef]
- Broom, D.R.; Batterham, R.L.; King, J.A.; Stensel, D.J. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R29–R35. [Google Scholar] [CrossRef]
- Anderson, K.C.; Zieff, G.; Paterson, C.; Stoner, L.; Weltman, A.; Allen, J.D. The effect of acute exercise on pre-prandial ghrelin levels in healthy adults: A systematic review and meta-analysis. Peptides 2021, 145, 170625. [Google Scholar] [CrossRef] [PubMed]
- Vatansever-Ozen, S.; Tiryaki-Sonmez, G.; Bugdayci, G.; Ozen, G. The effects of exercise on food intake and hunger: Relationship with acylated ghrelin and leptin. J. Sports Sci. Med. 2011, 10, 283–291. [Google Scholar] [PubMed]
- Douglas, J.A.; Deighton, K.; Atkinson, J.M.; Sari-Sarraf, V.; Stensel, D.J.; Atkinson, G. Acute exercise and appetite-regulating hormones in overweight and obese individuals: A meta-analysis. J. Obes. 2016, 2016, 2643625. [Google Scholar] [CrossRef]
- Stensel, D. Exercise, appetite and appetite-regulating hormones: Implications for food intake and weight control. Ann. Nutr. Metab. 2010, 57, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Morgan, L.; Truby, H. A review of the effects of exercise on appetite regulation: An obesity perspective. Int. J. Obes. 2008, 32, 1337–1347. [Google Scholar] [CrossRef]
- Schubert, M.M.; Sabapathy, S.; Leveritt, M.; Desbrow, B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med. 2014, 44, 387–403. [Google Scholar] [CrossRef]
- Hazell, T.J.; Islam, H.; Townsend, L.K.; Schmale, M.S.; Copeland, J.L. Effects of exercise intensity on plasma concentrations of appetite-regulating hormones: Potential mechanisms. Appetite 2016, 98, 80–88. [Google Scholar] [CrossRef]
- Broom, D.R.; Stensel, D.J.; Bishop, N.C.; Burns, S.F.; Miyashita, M. Exercise-induced suppression of acylated ghrelin in humans. J. Appl. Physiol. 2007, 102, 2165–2171. [Google Scholar] [CrossRef]
- Leidy, H.J.; Dougherty, K.A.; Frye, B.R.; Duke, K.M.; Williams, N.I. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training in non-obese women. Obesity 2007, 15, 446–455. [Google Scholar] [CrossRef]
- Stojiljkovic-Drobnjak, S.; Fischer, S.; Arnold, M.; Langhans, W.; Ehlert, U. Menopause is associated with decreased postprandial ghrelin, whereas a history of anorexia nervosa is associated with increased total ghrelin. J. Neuroendocrinol. 2019, 31, e12661. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, M.; Goldsmith, R.; Bloomfield, D.; Magnano, A.; Weimer, L.; Heymsfield, S.; Gallagher, D.; Mayer, L.; Murphy, E.; Leibel, R.L. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Investig. 2005, 115, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Blom, W.A.; Lluch, A.; Stafleu, A.; Vinoy, S.; Holst, J.J.; Schaafsma, G.; Hendriks, H.F. Effect of a high-protein breakfast on the postprandial ghrelin response. Am. J. Clin. Nutr. 2006, 83, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Dhurandhar, E.J.; Allison, D.B.; van Groen, T.; Kadish, I. Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer’s disease pathology in a mouse model. PLoS ONE 2013, 8, e60437. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, F.G.; Biasinutto, C.; Mangogna, A.; Fiotti, N.; Vinci, P.; Pisot, R.; Mearelli, F.; Simunic, B.; Roni, C.; Biolo, G. Metabolic consequences of anabolic steroids, insulin, and growth hormone abuse in recreational bodybuilders: Implications for the World Anti-Doping Agency passport. Sports Med. Open 2024, 10, 28. [Google Scholar] [CrossRef]
- Lewiński, A.; Karbownik-Lewińska, M.; Wieczorek-Szukała, K.; Stasiak, M.; Stawerska, R. Contribution of ghrelin to the pathogenesis of growth hormone deficiency. Int. J. Mol. Sci. 2021, 22, 9066. [Google Scholar] [CrossRef]
- Holt, R.I.; Sönksen, P.H. Growth hormone, IGF-I and insulin and their abuse in sport. Br. J. Pharmacol. 2008, 154, 542–556. [Google Scholar] [CrossRef]
- Briggs, D.I.; Lockie, S.H.; Benzler, J.; Wu, Q.; Stark, R.; Reichenbach, A.; Hoy, A.J.; Lemus, M.B.; Coleman, H.A.; Parkington, H.C.; et al. Evidence that diet-induced hyperleptinemia, but not hypothalamic gliosis, causes ghrelin resistance in NPY/AgRP neurons of male mice. Endocrinology 2014, 155, 2411–2422. [Google Scholar] [CrossRef]
- Heidarianpour, A.; Shokri, E.; Baghian, T.; Shokri, B. Benefits of aerobic training in girls with precocious puberty: Involvement of CRP and cortisol. J. Pediatr. Endocrinol. Metab. 2019, 32, 1005–1011. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, L.; Dou, Z.; Hou, Q.; Wang, S.; Yuan, Z.; Li, B. Ghrelin in Focus: Dissecting Its Critical Roles in Gastrointestinal Pathologies and Therapies. Curr. Issues Mol. Biol. 2024, 46, 948–964. [Google Scholar] [CrossRef]
- Algul, S.; Ilcin, S.; Ozcelik, O. Effects of Excercise on Ghrelin. Prog. Nutr. 2021, 23, e2021114. [Google Scholar]
- Larsen, P.S.; Donges, C.E.; Guelfi, K.J.; Smith, G.C.; Adams, D.R.; Duffield, R. Effects of Aerobic, Strength or Combined Exercise on Perceived Appetite and Appetite-Related Hormones in Inactive Middle-Aged Men. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.P.; Ugwu, F.N.; Tam, B.T.; Lee, P.H.; Lai, C.W.; Wong, C.S.C.; Lam, W.W.; Sheridan, S.; Siu, P.M. One Year of Yoga Training Alters Ghrelin Axis in Centrally Obese Adults with Metabolic Syndrome. Front. Physiol. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Mitoiu, B.I.; Nartea, R.; Miclaus, R.S. Impact of Resistance and Endurance Training on Ghrelin and Plasma Leptin Levels in Overweight and Obese Subjects. Int. J. Mol. Sci. 2024, 25, 8067. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Billings, L.K.; Liebl, A.; Vilsbøll, T. Transitioning from basal-bolus or premix insulin therapy to a combination of basal insulin and glucagon-like peptide-1 receptor agonist in people with type 2 diabetes. Diabet. Med. 2022, 39, e14901. [Google Scholar] [CrossRef]

| Reference | Study Population | Intervention | Results (Ghrelin and Growth Hormone (GH)) |
|---|---|---|---|
| Short-term (<60 min) exercise with stable plasma ghrelin levels | |||
| Dall et al. [108] | Eight healthy males (age, 40.8 ± 2.9 years; BMI, 23.6 ± 0.5 kg/m2). Eight hypopituitary males with GH deficiency (age, 40.8 ± 4.7 years; BMI, 29.2 ± 0.8 kg/m2). | Submaximal aerobic exercise For 45 min At 65–70% of HRmax. | Ghrelin levels did not change significantly (p > 0.05). GH concentrations increased after 45 min of exercise (11.43 ± 3.61 µg/L). Infusion of GH in the patients resulted in a peak level after 45 min. GH did not increase after exercise without GH infusion (9.77 ± 2.40 with GH vs. 0.11 ± 0.07 µg/L without GH). |
| Kraemer et al. [109] | Six well-trained males (age, 27.7 ± 3.20 years; BM, 72.0 ± 4.6 kg). | Progressively intense exercise on a treadmill, including four exercise intensities: 60%, 75%, 90%, and 100% of VO2max. Blood samples were collected before and after 15 and 30 min. | Ghrelin levels did not change significantly. Intense running (75% to 100% of VO2max) increased IGF-1 and GH levels. |
| Schmidt et al. [110] | Eight healthy young males (age, 29.9 ± 1.9 years; BMI, 22.2 ± 0.5 kg/m2). | Treadmill exercise at 50%, 70%, and 90% of VO2max for 10–20 min on different days. | Ghrelin plasma concentrations remained unchanged (−9% at 50% VO2max, −10.65% at 70% VO2max, −2.64% at 70% VO2max, p > 0.05). GH increased significantly after 40 min at 50% VO2max and after 20 min at 70 and 90% VO2max. GH peak concentrations were 5.8 ± 2.3 ng/mL, 12.0 ± 3.2 ng/mL, and 9.8 ± 4.7 ng/mL, respectively. |
| Zoladz al. [111] | Eight healthy non-smoking men (age, 23.0 ± 0.5 years; BM, 71.9 ± 1.5 kg; BMI, 22.42 ± 0.49 kg/m2). | Two incremental cycling tests: one in the fed state until exhaustion, and one after overnight fasting until reaching 150 W, with 30 W increases every 3 min. | Pre-exercise leptin and ghrelin levels did not differ between fed and fasted states. Plasma GH was significantly higher at 90 W in the fed state (p = 0.016). At 150 W, GH reached 14.85 ± 4.67 ng/mL (fed) and 12.83 ± 3.62 ng/mL (fasted). |
| Erdmann et al. [112] | Seven healthy males and females (age, 24.4 ± 0.6 years; BMI, 21.4 ± 0.8 kg/m2). | Bicycle exercise for 30 min at 100 W, below the aerobic/anaerobic threshold. In a second group, 7 subjects cycled at 50 W for 30, 60, and 120 min, respectively. | Ghrelin levels increased during exercise at 50 W, rising by 50–70 pg/mL above baseline after 30, 60, and 120 min. However, during higher intensity cycling at 100 W, ghrelin levels remained unchanged, and hunger/satiety ratings, food intake, and postprandial ghrelin suppression were like the control group, showing no significant differences. |
| Diet (40–50% carbohydrate, 15–25% protein, and 30–40% fat). | |||
| Jürimäe et al. [113] | Nine elite male rowers (age, 20.1 ± 3.7 years; BMI, 20.1 ± 3.7 kg/m2; BM, 89.6 ± 4.6 kg; %body fat, 9.9 ± 2.5%). | Single-scull rowing was performed for 15 min below and above the individual anaerobic threshold. | Above anaerobic threshold |
| Plasma ghrelin concentration did not increase significantly (from 802 ± 181 to 808 ± 196 pg/mL, +0.74%, p > 0.05). GH significantly increased (from 2.6 ± 4.4 to 72.6 ± 19.2 ng/mL, +2692.3%, p < 0.05). | |||
| Below anaerobic threshold | |||
| Plasma ghrelin concentration did not increase significantly (from 791 ± 214 to 794 ± 170 pg/mL, +0.37%, p > 0.05). GH significantly increased (from 4.1 ± 4.8 to 41.9 ± 28.4 ng/mL, +922%, p < 0.05). | |||
| Marzullo et al. [114] | Eight obese males (BMI, 33.7 ± 1.5 kg/m2). | Cycling exercise, at 20 W, increased by 20 W every 4 min until exhaustion. | Plasma ghrelin concentration did not increase significantly (from 3053 ± 315 to 2929 ± 345 pg/mL, −4.06%, p > 0.05). GH increased significantly (from 1.3 ± 0.96 to 5.5 ± 1.6 µg/L, +323.1%, p < 0.01) |
| Thomas et al. [115] | Five class 1 obese men (age, 21.6 ± 2.5 years; BMI range, 30.00–34.99 kg/m2; BM, 97.8 ± 8.58 kg; %body fat, 34.7 ± 2.95%). Five class 2 obese men (age, 20.0 ± 1.4 years; BMI range, 35–39.99 kg/m2 BM, 120.8 ± 10.49 kg; %body fat, 40.5 ± 5.82%). Nine lean men (age, 20.1 ± 2.1 years; BMI range, 35–39.99 kg/m2; %body fat, 14.7 ± 3.54%). | Resistance exercise protocol: 6 exercises, 3 sets of 10 repetitions at 85–95%, 10 repetition maximum with 120- and 90-s rest periods. | The obese 2/3 class group had significantly greater ghrelin levels than the lean group (p = 0.009) and the obese class 1 group (p = 0.002). Higher GH was associated with lower ghrelin in lean individuals. |
| Crabtree and Blannin, [116] | Sixteen overweight participants: ten men and six women (age, 50.1 ± 11.6 years; BMI, 28.9 ± 4.2 kg/m2). | Treadmill walking (cold trial and neutral trial) for 45 min at 60% of VO2max in a randomized, counterbalanced design. | Total ghrelin concentration was significantly greater during walking in the cold versus those during walking in the neutral condition (p < 0.05). |
| Ouerghi et al. [19] | Seven inactive overweight men (age, 36.4 ± 4.35 years; BMI, 28.3 ± 1.66 kg/m2). | Two sessions of cycling exercise at 7-day intervals, each session lasting 20 min at 60% or 80% of peak aerobic power. | Ghrelin was unchanged in both groups. GH increased significantly at the end of exercise (from 0.12 ± 0.09 to 1.08 ± 0.73 ng/mL, +800%, p = 0.004) and 30 min later (from 0.12 ± 0.09 to 0.60 ± 0.36 ng/mL, +400%, p = 0.035) the session at 80% of peak aerobic power. |
| Tobin et al. [23] | Twenty-four overweight/obese subjects, twelve men and twelve women, matched for age: 32.3 ± 2 vs. 36.8 ± 2 years, and BMI: 28.1 ± 1.2 vs. 29.0 ± 1.5 kg/m2. |
| Total ghrelin showed no significant differences between men and women after acute exercise (all p > 0.05). |
| Short-term (<60 min) exercise with lower plasma ghrelin concentrations | |||
| Toshinai et al. [117] | Five inactive normal weight healthy men (age, 26 ± 0.5 years; BMI, 23.3 ± 0.2 kg/m2). | Incremental endurance exercise for 10 min, including 4 conditions:
| Ghrelin levels decreased significantly after incremental exercise. GH did not change after exercise at half the lactate threshold intensity incremental exercise (p > 0.05). GH increased after exercise at an intensity at the lactate threshold intensity (p < 0.05). GH increased after exercise at an intensity at the onset of blood lactate accumulation (p < 0.001). GH increased exercise at an intensity above the onset of blood lactate accumulation (p < 0.001). |
| Stokes et al. [119] | Seven healthy, active men (age, 26.0 ± 3.0 years; BMI, 24.9 ± 3.5 kg/m2). | In two exercise trials, participants performed a single 30-s sprint on a cycle ergometer against a resistance equivalent to 7% (FAST) or 9% (SLOW) of their body mass. A control group rested in the laboratory. | Total ghrelin concentrations decreased significantly after the sprint, with values lower at 30 min of recovery compared to pre-exercise (FAST: from 620 ± 190 to 490 ± 160 pg/mL, −21.0%, p < 0.001; SLOW: from 590 ± 150 to 470 ± 130 pg/mL, −20.3%, p < 0.001). GH concentrations increased in both exercise trials and were greater in the FAST than in the SLOW trial. |
| Kelly et al. [120] | Ten physically active men (age, 21.4 ± 1.3 years; BMI, 23.94 ± 2.1 kg/m2). | Treadmill running for 45 min at intensity of 70% of VO2peak. In a randomized, counterbalanced design, males completed three trials: exercise when hydrated (0–1% body mass), exercise when dehydrated (−1% to −2% body mass), and a hydrated resting control. | Exercise performed in a dehydrated state resulted in significantly lower concentrations of ghrelin compared with control (p = 0.045) and hydrated exercise conditions (p = 0.014). |
| Halliday et al. [121] | Twenty-four physically inactive and overweight/ obese adults: twelve men and twelve women (age, 35 ± 2 years; BMI, 28.5 ± 0.9 kg/m2; %body fat, 35 ± 2%). | Three conditions were initiated 35 min after breakfast: Aerobic exercise (walking at 65–70% HRmax for 45 min). Acute bout of resistance exercise (12–15 repetitions for 12 exercises). Sedentary control. | Ghrelin was lower after resistance exercise (131 ± 4 pg/mL) than after aerobic exercise (144 ± 7 pg/mL) (p = 0.006). |
| Li et al. [22] | Fourteen obese: seven men and seven females (age, 20.6 ± 1.5 years; BMI, 31.1 ± 2.0 kg/m2, BM; 88.7 ± 15.1 Kg; %body fat 38.8 ± 3.7%). | The participants were randomized into three groups performing:
| No significant differences in total ghrelin were detected before exercise. Immediately after exercise, total ghrelin in the (1) and (2) groups differed significantly from the (3) group (p < 0.05). One hour after exercise, total ghrelin in the (2) and (1) groups remained significantly different from the (3) group (p < 0.05). Total ghrelin in the (2) group was significantly lower than in the (1) group (p < 0.05). |
| Short-term (<60 min) exercise with higher plasma ghrelin levels | |||
| Erdmann et al. [112] | Seven normal-weight men and women (age, 24.4 ± 0.6 years; BMI, 21.4 ± 0.8 kg/m2). | Bicycle exercise on an ergometer for 30 min at 50 W, below the aerobic/anaerobic threshold. | In the control group, plasma ghrelin postprandial declined at 150 min (from 488.6 ± 80.8 pg/mL to 325.4 ± 54.1 pg/mL, −33.4%, p < 0.05). During 30 min of exercise at 50 W, ghrelin increased (from 520.4 ± 82.7 pg/mL to 566.6 ± 86.2 pg/mL, +8.9%, p < 0.05), then decreased to 386.8 ± 58.5 pg/mL, −25.7%, p < 0.05 at 135 min. At 100 W, ghrelin remained unchanged (485.7 ± 94.7 pg/mL to 473.4 ± 101.8 pg/mL, −2.47%, p > 0.05). |
| Diet (40–50% carbohydrate, 15–25% protein, and 30–40% fat). | |||
| Jürimäe et al. [122] | Eight male rowers (age, 21.3 ± 2.8 years; BM, 97.4 ± 67.4 kg). | Maximal rowing ergometer test for 20 min at 81% of VO2max. | Ghrelin significantly increased immediately after the exercise (+24.4%, p < 0.05) and was not significantly different than baseline after 30 min of recovery. GH increased from 0.9 ± 0.6 to 72.1 ± 9.5 μL/U/mL, +7902.2%, p < 0.05. |
| Long-term (≥60 min), exercise with stable plasma ghrelin levels | |||
| Burns et al. [123] | Eighteen healthy trained: nine men and nine women (age, ♂24.5 ± 1.3 years, ♀25.1 ± 1.2 years; BM, ♂74.03 ± 4.20 kg, ♀63.57 ± 2.55 kg; BMI, ♂23.4 ± 1.0 kg/m2 ♀22.5 ± 0.8 kg/m2; %body fat, ♀16.9 ± 1.7%, ♀28.3 ± 1.2%). | Treadmill run for 60 min at 73.5% of VO2max. | Ghrelin concentrations did not differ between trials (from 1387.6 ± 254.5 to 1380.6 ± 224.9 pg/mL, −0.5%, p > 0.05). |
| Martins et al. [124] | Twelve healthy, normal-weight volunteers (six males and six females) (age, 25.9 ± 4.6 years; BMI, 22.0 ± 3.2 kg m2). | Ergometer intermittent cycling for 60 min at 65% of HRmax. | No significant effect of exercise was observed on postprandial levels of ghrelin (p > 0.05). |
| Sartorio et al. [125] | Group 1: nineteen healthy males and eighteen females (age, 25 ± 6.7 years). Group 2: four healthy males (age, 28.2 ± 7.2 years). | Group 1: Aerobic exercise (treadmill running) for 60–90 min at 80% of VO2max. Group 2: Two consecutive 30-min cycling sessions at 80% VO2max with different time intervals (2 and 6 h) between bouts on two separate days. | Group 1: In males, ghrelin levels significantly decreased (from 1506.4 ± 859 to 1254.8 ± 661.7 pg/mL, −16.7%, p < 0.05), while no significant changes were observed in females. GH levels increased post-training (p < 0.0001), with no sex differences. Group 2: No significant changes in ghrelin levels were observed during or after the two bouts at different intervals. GH levels increased after the first exercise bout (peak: 26.8 ± 11.2 and 17.3 ± 3.5 ng/mL, p < 0.005). Peak GH after the second bout (4.3 ± 1.6 ng/mL) was lower (p < 0.01) after a 2-h interval, while GH responsiveness recovered after the 6-h interval (11.9 ± 3.3 ng/mL). GH responses to prolonged exercise (60–90 min) were linked to changes in ghrelin levels only in males, while repeated shorter bouts (30 min) with marked GH responses did not affect ghrelin concentrations. |
| Hagobian et al. [126] | Eighteen healthy overweight/obese individuals, nine men and nine women (age, ♂26.8 ± 11.8 years, ♀23.3 ± 8 years; BMI, ♂25.7 ± 2.3 kg/m2; ♀28.0 ± 3.5 kg/m2). | Treadmill running at 50–65% of VO2peak. Four bouts with energy added to the baseline diet to maintain energy balance and four bouts without added energy to induce energy deficit. | In men, no significant changes in ghrelin levels were observed. In women, ghrelin was higher after both the energy deficit (+32%) and energy balance (+25%) conditions, with the change from baseline being significantly higher in women compared to men (p < 0.05). |
| Shiiya et al. [127] | Nine healthy males (age, 25.2 ± 0.5 years; BMI, 22.6 ± 0.4 kg/m2). | Cycling exercise for 60 min at 50% of VO2max. | Plasma ghrelin levels decreased significantly during cycling (45–60 min) and tended to decline post-exercise (15–30 min, p ≈ 0.06). Deacyl and total ghrelin remained unchanged. GH significantly increased during exercise (p < 0.01) and decreased at 90 min. |
| Plinta et al. [128] | Fifty healthy young female professional basketball and handball players (age, 21 ± 2.4 years; BMI, 22.1 ± 1.8 kg/m2). | Three-month period of moderate aerobic training (pulse 140–160/min) or intensive aerobic training (pulse > 170/min). | Plasma ghrelin levels significantly decreased after long-term moderate aerobic exercise (921 ± 300 vs. 575 ± 572 pg/mL, −37.6%, p < 0.001), while they remained unchanged following short-term moderate aerobic exercise or intensive fitness and speed exercise. |
| Laursen et al. [24] | Eleven recreationally trained males (age, 25 ± 4 years; BM, 79.4 ± 13.5 kg). | Three 1-h cycling bouts at 60% Wmax in hot (33 °C), cold (7 °C), and room temperature (20 °C), followed by a 3-h recovery at room temperature. | Total and acylated ghrelin levels remained unchanged post- and 3 h post-exercise, regardless of ambient temperature (p > 0.05). |
| Long-term (≥60 min), exercise with lower plasma ghrelin levels | |||
| Ballard et al. [129] | Twenty-one healthy males (age, 20 ± 1.8 years; BMI, 24.8 ± 3.3 kg/m2). | Resistance exercise for 80 min at 55–70% of 1 RM and without carbohydrate supplementation. | Plasma ghrelin declined during and 110 min after exercise (p < 0.05). Plasma ghrelin increased in rest. |
| Liu et al. [11] | Eleven healthy young men (age, 23 ± 2 years; BMI, 22 ± 2 kg/m2). | Sixty minutes of moderate-load resistance exercise (4 sets of 8 repetitions at 85% 8 RM). Low-load resistance exercise (4 sets of 15 repetitions at 45% 8 RM). | Ghrelin concentrations were significantly lower immediately after both moderate and low-load exercise compared to control (p < 0.05). |
| Very long-term (≥90 min), exercise with lower plasma ghrelin levels | |||
| Ghanbari-Niaki, [130] | Fourteen volunteer male physical education students (age, 20.5 ± 0.5 years; BM, 78.25 ± 5.01 kg; BMI, 25.27 ± 1.18 kg/m2). | Circuit resistance training for 180 min (10 exercises, three circuits, with 8–12 repetitions, at 60% of 1 RM). | Plasma ghrelin showed a significant decrease immediately after the exercise (p < 0.05) and increased significantly 24 h following the exercise (p < 0.05). GH showed a significant increase immediately after exercise (p < 0.01) and returned to pre-exercise values. |
| Sartorio et al. [125] | Group 1: Nineteen elite male athletes and eighteen elite female athletes (age, 25 ± 6.7 years; BMI, 25 ± 6.7 kg/m2). Group 2: Four elite male athletes (age, 28.2 ± 0.2 years; BMI, 25 ± 6.7 kg/m2). | Group 1: One 60–90 min training session at approximately 80% of VO2max; Group 2: Two consecutive 30-min cycling sessions at 80% of VO2max at different intervals between workouts (2 and 6 h) on two different days. | Group 1: In males, ghrelin significantly decreased after the training session (from 1506.4 ± 859 to 1254.8 ± 661.7 pg/mL, −16.7%, p < 0.05), while no significant changes were found in females. GH levels increased after the training session (p < 0.0001), with no differences between males and females. Group 2: Ghrelin levels remained unchanged during or after the two exercise bouts at different time intervals. GH levels significantly increased after the first exercise bout (peak: 26.8 ± 11.2 and 17.3 ± 3.5 ng/mL). After the second bout, peak GH was lower (4.3 ± 1.6 ng/mL) at a 2-h interval (p < 0.01) but recovered after the 6-h interval (11.9 ± 3.3 ng/mL). |
| Very long-term (≥90 min), exercise with higher plasma ghrelin levels | |||
| Christ et al. [131] | Eleven healthy, endurance-trained male athletes (age, 31.4 ± 1.7 years; BMI, 22.6 ± 0.5 kg/m2). | Aerobic exercise test on a cycloergometer for 180 min at 50% of Wmax. High-fat (HF) or low-fat (LF) diet. | Ghrelin significantly increased after the LF diet compared to the HF diet (p < 0.03). No differences in ghrelin levels were observed during exercise, but post-exercise ghrelin was significantly higher after the LF diet. GH levels were not significantly different between the LF and HF diets during the exercise session. |
| Jürimäe et al. [21] | Nine national-level male rowers (age, 20.1 ± 1.5 years; BM, 81.0 ± 5.0 kg; %body fat, 10.8 ± 3.3%). | Rowing training session for 120 min (distance = 20.7 ± 1.4 km; HR = 133 ± 4 bpm at intensity of 80.2 ± 1.6% of the HR turn point) followed by a 30-min rest. | Ghrelin concentration increased (from 780.6 ± 207.4 to 876.2 ± 207.3 pg/mL, +12.2%, p < 0.05) 30 min after exercise. |
| Russel et al. [20] | Twenty-one endurance-trained runners: eleven men (age, 27 ± 9 years; BM, 69.8 ± 4.9 kg, BMI 21.9 ± 1.5 kg/m2, %body fat, 12.8 ± 2.4%) and ten women (age 29 ± 7 years; BM, 56.2 ± 4.9 kg, BMI, 21.0 ± 1.1 kg/m2, %body fat 20.6 ± 2.3%). | Intense endurance running by a 10-km time trial on a treadmill for 90 min at 62 ± 5% of VO2max. | Ghrelin was significantly higher after exercise (from 823 ± 94 to 976 ± 94 pg/mL, +18.6%, p < 0.0001). GH significantly increased after exercise (from 2.7 ± 6.0 to 23.8 ± 16.8 μg·mL−1, +781%, p < 0.001). |
| Reference | Study Population | Intervention | Results (Ghrelin and Body Mass/Body Fat /BMI) |
|---|---|---|---|
| Short-term chronic exercise programs (<12 weeks) with stable plasma ghrelin levels | |||
| Rämson et al. [132] | Eight trained male rowers (age, 20.2 ± 1.6 years; BM, 81.0 ± 5.4 kg). | Week 1: Maintain previous training volume (10 h/week). Week 2: Increase training load by 50%. Week 3: Increase training load by 10–15% (individualized). Week 4: Decrease training volume back to week 1 level. Training Breakdown: 80% low-intensity rowing; 10% low intensity running/cycling; 10% strength endurance training (40–50% 1 RM, 30–50 reps). Frequency: 6 training days/week, 1 recovery day. | Week 1: Ghrelin increased from 780.5 ± 221.7 to 842.1 ± 216.5 pg/mL, +7.9% (p > 0.05). Week 2–3: Ghrelin increased from 819.4 ± 194.6 to 822.9 ± 215.5 pg/mL, +0.43% (p < 0.05). Week 4: Ghrelin increased from 755.6 ± 140.9 to 851.3 ± 229.1 pg/mL, +12.7% (p > 0.05). |
| Hedayati et al. [133] | Twenty-seven female students (age, 22.2 ± 1.54 years; BM, 52.6 ± 3.2 Kg, BMI, 20.76 ± 1.86 kg/m2, %body fat, 21.1 ± 1.6%). | Subjects performed circuit resistance training with 40% and 80% of 1 RM for 4 weeks. | At 40% of 1 RM: Ghrelin increased from 414 ± 154 to 446 ± 186 pg/mL, +7.73% (p > 0.05). The %body fat decreased from 21.2 ± 2.4% to 20.4 ± 2% (p > 0.05). At 80% of 1 RM: Ghrelin increased from 397 ± 195 to 451 ± 142 pg/mL, +13.60% (p > 0.05). The %body fat did not change from 20.6 ± 2.3% to 20.6 ± 2.3% (p > 0.05). |
| Rosenkilde et al. [134] | Six older men cyclists (age, 61 ± 3 years; BM, 52.6 ± 3.2 Kg, BMI, 24.46 ± 0.9 kg/m2; %body fat, 21.1 ± 1.6%). | Cycling (2706 km) for 14 days. | Total ghrelin decreased from 867 ± 516 to 824 ± 497 pg/mL, −4.95% (p > 0.05). Body fat decrease from 14.0 ± 1.5 kg to 11.8 ± 1.1 kg (p = 0.02). |
| Ahmadi et al. [25] | Thirty elderly men (age range 60–70 years; BMI range, 25–30 kg/m2). | Eight weeks of aerobic training at 50–60% of HRmax, for 50–60 min/day. | Ghrelin increased from 640 ± 110 to 710 ± 20 pg/mL, +10.9% (p > 0.05). The %body fat decreased from 24.31 ± 3.39% to 21.43 ± 2.94% (p = 0.001). |
| Short-term chronic exercise programs (<12 weeks) with lower plasma ghrelin levels | |||
| Rämson et al. [135] | Twelve trained male rowers (age 22.2 ± 3.4 years; BMI, 23.95 ± 2.4 kg/m2). | At 50% resistance and 50% endurance rowing, cycling, or running training. Week 1: 10 h/week. Week 2–3: 15 h–20 h/week. Week 4: 10 h/week. | Week 1: Ghrelin increased from 973.46 ± 183.4 to 980 ± 300.2 pg/mL, +0.71% (p > 0.05). Week 2–3: Ghrelin decreased from 980 ± 300.2 to 873.35 ± 198.61 pg/mL, −10.88% (p < 0.05). Body fat decreased from 11.4 ± 6.2 to 9.3 ± 5.5 kg (p < 0.05). |
| Cho et al. [27] | Forty women cadets (age 22 ± 28 years; BMI 24.1 ± 2.7 kg/m2). | Training course for 8 weeks. | Ghrelin decreased from 1100 ± 100 to 1000 ± 100 pg/mL, −9.1% (p < 0.01). BMI decreased from 24.1 ± 2.7 to 22.4 ± 2.2 kg/m2 (p < 0.01). |
| Short-term chronic exercise programs (<12 weeks) with hyperplasma ghrelin levels | |||
| Azizi et al. [136] | Twenty-four inactive students (age 27.56 ± 0.48 years; BMI 32.68 ± 0.84 kg/m2). | Eight weeks of aerobic training for 60 min at 65–85% of HRmax. | Ghrelin increased from 321 ± 65 to 417 ± 72 pg/mL, +29.90% (p = 0.0001). BMI decreased from 32.94 to 31.65 kg/m2 (p < 0.05). |
| Tremblay et al. [29] | Seventy-one overweight adults/elderly females and males with metabolic syndrome (age range 50–70 years). Group 1: BMI 32.68 ± 0.84 kg/m2. Group 2: BMI, 34.4 ± 4.2 kg/m2. Group 3 BMI, 33.9 ± 4.0 kg/m2. | Resistance training for 90 min/week for 3 weeks. Group 1: High resistance/moderate endurance, 65–85% of 10 RM for resistance training and 30% VO2peak for endurance training. Group 2: Moderate resistance/high endurance intensity, 30% of 10 RM for resistance training and 70% VO2peak for endurance training. Group 3: moderate resistance/endurance, 30% of 10 RM for resistance training/30% VO2peak for endurance training. | Ghrelin was significantly increased after day 21 and month 3 (p < 0.001). Group 1: Body fat decreased from 27.7 ± 7.6 kg to 24.9 ± 7.1 kg (p < 0.05). Group 2: Body fat decreased from 32.2 ± 7.7 to 29.3 ± 7.3 kg (p < 0.05). Group 3: Body fat decreased from 32.3 ± 7.5 to 30.1 ± 7.3 kg (p < 0.05). |
| Liao et al. [28] | Sixteen obese children (age 12.74 ± 1.94 years; BMI, 27.74 ± 3.33 kg/m2). | Six weeks of moderate, high-intensity interval and resistance training at 50–60% of HRmax or 80–90% of HRmax; 12–15 RM. Diet. | Ghrelin was significantly enhanced after 6 weeks (p < 0.05). The %body fat decreased from 40.00 ± 4.99% to 32.95 ± 4.66% (p < 0.001). |
| Long-term training (≥12 weeks) with stable plasma ghrelin levels | |||
| Martins et al. [137] | Twenty-two overweight/ obese sedentary females and males (age range 56–70 years; BMI, 31.3 ± 2.3 kg/m2). | Twelve weeks of moderate, high-intensity interval treadmill walking or running at 74% of HRmax. | Ghrelin increased from 2074 ± 912.4 to 2371 ± 1022 pg/mL, +14.3% (p > 0.05). The %body fat decreased from 35.3 ± 5.6% to 33.5 ± 5.9% (p < 0.001). |
| Kadoglou et al. [138] | Ninety females and males with type 2 diabetes mellitus (age 36.9 ± 8.3 years; BMI, 31.3 ± 2.3 kg/m2). Group 1: BMI, 32.55 ± 3.11 kg/m2, resistance training. Group 2: BMI, 31.55 ± 3.11 kg/m2, aerobic training. Group 3: BMI, 31.91 ± 2.93 kg/m2, aerobic exercise + resistance training. Group 4: BMI, 32.1 ± 2.95 kg/m2, control. | Twenty-four weeks of aerobic, resistance, and combined training for 60 min at 60–75% of HRmax or 60–80% of 1 RM. | Subgroup analysis showed no significant effects on serum ghrelin levels (p = 0.228). Group 1: %Body fat decreased—0.12 ± 0.06%. Group 2: %Body fat decreased—0.66 ± 0.21%. Group 3: %Body fat decreased—1.97 ± 0.55% (p < 0.005). |
| Rosenkilde et al. [139] | Fifty-three sedentary, overweight males (age range 27–30 years). Group 1: BMI, 27.6 ± 1.4 kg/m2. Group 2: BMI, 28.6 ± 1.8 kg/m2. Group 3: Control BMI, 28.0 ± 2.3 kg/m2. | Twelve weeks of aerobic training at 20–60 %VO2max for 2984.6 ± 132.6 min. Group 1: High training dose, 60 min. Group 2: Moderate training dose, 30 min. | Group 1: Ghrelin increased from 648 ± 80 to 661 ± 84 pg/mL, +2% (p > 0.05). Body fat decreased from 27.4 ± 4.2 to 23.7 ± 3.7 kg (p < 0.001). Group 2: Ghrelin increased from 698 ± 209 to 714 ± 208 pg/mL, +2.29% (p > 0.05). Body fat decreased from 30.0 ± 4.6 to 25.8 ± 5.1 kg (p < 0.001). Group 3: Ghrelin increased from 674 ± 136 to 704 ± 122, +4.45% (p > 0.05). Body fat did not change from 29.0 ± 6.0 to 25.8 ± 5.1 kg (p > 0.05). |
| Gibbons et al. [140] | Thirty-two inactive males (age range 18–55 years). Group 1: BM, 83.5 ± 3.1 kg. Group 2: BM, 90.4 ± 2.7 kg. Group 3: BM, 93.1 ± 3.6 kg. | Twelve weeks of resistance training at 70% of HRmax. | Ghrelin does not change (p > 0.05). Group 1: %Body fat decreased from 33.1 ± 2.5 to 29.0 ± 3.1 kg (p < 0.01). Group 2: %Body fat did not change 35.5 ± 3.3 to 35.0 ± 3.5 kg (p > 0.05). Group 3: %Body fat increased from 35.1 ± 2.2 to 40 ± 7.2% (p < 0. 01). |
| Elerian et al. [141] | Twelve obese females and males (age 34.75 ± 4.18 years; BMI, 37.3 ± 2.6 kg/m2). | Fourteen weeks of 50 min/day, 80% of 1 RM. Diet (1200–1800 kcal/j). | Ghrelin did not change from 4.4 ± 0.53 to 4.2 ± 0.35 mg/mL, −4.6% (p > 0.05). %Body fat decreased from 45.4 ± 5.2% to 40 ± 7.2 % (p < 0.005). |
| Fico et al. [26] | Thirty-nine obese females and males with osteoarthritis (age 59 ± 1 years). Group Cycling: BMI, 32.5 ± 2.0 kg/m2. Group Swimming: BMI, 33.1 ± 1.6 kg/m2. | Twelve weeks of cycling or swimming training, 20–45 min at 40–70% of HRr. | Ghrelin did not change (p > 0.05). Group Cycling: BMI decreased from 33.1 ± 1.6 to 32.2 ± 1.5 kg/m2 (p < 0.05). Group Swimming: BMI decreased from 32.5 ± 2.0 to 32.0 ± 2.0 kg/m2 (p < 0.05). |
| Long-term training (≥12 weeks) with lower plasma ghrelin levels | |||
| Plinta et al. [128] | Fifty female professional basketball and handball players (age 21 ± 2.4 years); BMI, 22.1 ± 1.8 kg/m2). | Twelve weeks of moderate aerobic training or intensive aerobic training for 120 min at 140–170 bpm. | After 12 weeks of moderate aerobic training, ghrelin decreased from 921 ± 300 to 575 ± 572 pg/mL, −37.6% (p < 0.001). BM did not change (from 65.9 ± 7.1 kg to 65.5 ± 7.0 kg, p > 0.05). After 12 weeks of intensive aerobic training, ghrelin increased from 6.09 ± 5.62 to 6.89 ± 5.62 ng/mL, +13.1% (p > 0.05). BM did not change (p > 0.05). |
| Alyar et al. [10] | Sixty-two obese (BMI ≥ 30 kg/m2) and forty-eight healthy controls (BMI, range 18.50–29.99 kg/m2). | Twelve weeks of walking 5000 steps/day. Diet (1000–1500 kcal/day). | Ghrelin levels significantly decreased. |
| Long-term training (≥12 weeks) with higher plasma ghrelin levels | |||
| Leidy et al. [142] | Fifteen females (age 20.2 ± 1.4 years; BMI, range 18–25 kg/m2). | Aerobic exercise at 70–80% of HRmax for 12 weeks. Diet. | Ghrelin increased from 2593 ± 995 to 4449 ± 2234 pg/mL, +71.6% (p < 0.05). The %body fat decreased from 30.5 ± 3.5% (p < 0.05). |
| FosterSchubert et al. [143] | Eighty-seven postmenopausal women (age 60.7 ± 6.75 years; BMI, 30.4 ± 4.1 kg/m2). | Aerobic training, cycling at 60–75% of HRmax, 45 min/day for 12 weeks. | Ghrelin increased by +24.1% (p < 0.05). BM decreased (p < 0.05). |
| Mizia-Stec et al. [144] | Thirty-seven obese premenopausal females (age 29 ± 52 years; BMI, 36.5 ± 5 kg/m2). | Twelve weeks of aerobic exercise x 60 min at 65% of HRmax. Diet (1000 kcal/day). | Ghrelin increased from 66.9 ± 13.7 to 73.9 ± 15.4 pg/mL, +10.5% (p = 0.005). BMI decreased from 36.5 ± 5.4 to 33.4 ± 5.2 kg/m2 (p = 0.001). |
| Kelishadi et al. [145] | Ninety-two female and male obese children (age 7.7 ± 1.2 years; BMI, 21.1 ± 2.5 kg/m2). | Twenty-four weeks, 40 min training. Diet. | Ghrelin decreased (p < 0.05). BMI decreased (p < 0.05). |
| Konopko-Zubrzycka et al. [146] | Twenty-one obese females and males (age 41 ± 11.9 years; BMI, 47.3 ± 5.7 kg/m2). | Twenty-four weeks of walking for 45 min. Bioenterics intragastric balloon diet (1500 kcal/d). | Ghrelin increased from 621.9 ± 182.4 to 903.9 ± 237 pg/mL, +43.3% (p < 0.01), and gradually returned to baseline 3 months post-balloon removal. BM decreased. |
| Gueugnon et al. [147] | Thirty-two female and male obese adolescents (age 14.3 ± 0.3 years; BMI, 35.6 ± 0.7 kg/m2). | Twenty-eight weeks of aerobic training, 45–60 min at 50–85% of MAP. Diet (2300–2500 kcal/day). | Ghrelin increased (p < 0.05). BMI decreased (p < 0.001). |
| Kadoglou et al. [148] | Fifty-four overweight females and males with type 2 diabetes mellitus diagnosis (age range, 50–70 years; BMI, 32.1 ± 3.77 kg/m2). | Twelve weeks of aerobic training for 45–60 min/day at 60–75% of HRmax. | Ghrelin increased from 2140 ± 710 to 3870 ± 1070 pg/mL, +80.8% (p > 0.05). BMI did not change from 32.1 ± 3.77 to 31.98 ± 3.03 kg/m2 (p > 0.05). |
| Markofski et al. [149] | Twenty-nine older, healthy females and males (age 71.2 ± 5 years). | Twelve weeks of aerobic and resistance training for 20 min/day at 60–70% of HRr and 80% of 1 RM. | Ghrelin increased from 32.9 ± 4.0 to 48.2 ± 6.0 pg/mL, +47% (p < 0.01). BMI did not change (p > 0.05). |
| Kim et al. [150] | Eighteen untrained healthy males (age, 23.6 ± 2.8 years). Group 1: BMI, 23.6 ± 2.8 kg/m2, isocaloric high protein diet. Group 2: 24.5 ± 2.8 kg/m2, standard diet. | Twelve weeks of resistance training for 50–80 min/day at 60–80% 1 RM. | Group 1: Ghrelin increased from 658.2 ± 73.8 to 817.2 ± 87.0 pg/mL, +25% (p = 0.001). The %body fat decreased from 22.4 ± 5.8% to 20.0 ± 4.9% (p < 0.05). Group 2: Ghrelin did not change from 707 ± 133 to 760 ± 91.2 pg/mL, +9.3% (p > 0.05). The %body fat did not change from 19.2 ± 7.9% to 18.3 ± 7.3% (p > 0.05). |
| Kang et al. [151] | Twenty-six obese females. Intervention group (age, 50.1 ± 3.8 years; BMI, 31.8 ± 3.2 kg/m2). Control group (age, 49.84 ± 2.96 years; BMI, 30.4 ± 2.3 kg/m2). | Twelve weeks of aerobic and resistance training for 50 min at 12–14 of RPE. | Ghrelin increased from 588.5 ± 139 pg/mL to 821.4 ± 197.0, +39.5% (p < 0.001). The %body fat decreased from 38.7 ± 3.2% to 36.9 ± 3.5% (p < 0.05). |
| Tremblay et al. [29] | One hundred overweight adults/elderly females and males with metabolic syndrome group (age range 50–70 years). Group 1: BM, 85.4 ± 12.4 kg. Group 2: BM, 94.0 ± 13.7 kg. Group 3: BM, 89.0 ± 12.7 kg. | Twenty-four weeks of 90 min/day of endurance plus 90 min/week of resistance training. Group 1: High resistance (at 65–85% of 10 RM), moderate endurance (30% of VO2peak) training. Group 2: Moderate resistance (at 30% of 10 RM), high endurance (70% of VO2peak training). Group 3: Moderate resistance (at 30% of 10 RM) endurance, for resistance (30% of VO2peak). | Ghrelin significantly increased after day 21 (p < 0.001), returning to baseline levels between months 6 and 12 as body weight and fat plateaued. Group 1: Body fat decreased from 27.7 ± 7.6 to 22.1 ± 6.9 kg (p < 0.05). Group 2: Body fat decreased from 29.6 ± 0.7 to 26.3 ± 6.8 kg (p < 0.05). Group 3: Body fat decreased from 32.3 ± 7.5 to 28.3 ± 6.8 kg (p < 0.05). |
| Ataeinosrat et al. [152] | Forty-four obese males (age 27.5 ± 9.4 years; BMI, 32.9 ± 1.2 kg/m2). | Twelve weeks of 70 min at 50% of 1 RM training. Group 1: Circuit resistance training. Group 2: Traditional resistance. Group 3: Interval resistance training. | Group 1: Ghrelin increased from 650 ± 14 to 870 ± 11 pg/mL, +33.8% (p < 0.05). Body fat decreased from 29.9 ± 1.1 to 27.2 ± 0.8 kg (p < 0.05). Group 2: Ghrelin increased from 659 ± 20 to 779 ± 17 pg/mL, +18.2% (p < 0.05). Body fat did not change from 29.9 ± 1.1 to 28.5 ± 0.8 kg (p > 0.05). Group 3: Ghrelin increased from 669 ± 31 to 866 ± 18 pg/mL, +29.4% (p < 0.05). Body fat decreased from 30.4 ± 1.0 to 26.8 ± 1.0 kg (p < 0.05). |
| Najafi et al. [12] | Forty-five females with precocious puberty (age range 6–8 years). Group 1: Medication plus training, BMI, 17.9 ± 0.6 kg/m2. Group 2: Medication, BMI, 17.8 ± 0.68 kg/m2. Group 3: Control, BMI, 16.5 ± 0.49 kg/m2. | Twelve weeks of aerobic training, 20–75 min/day at 45–75% of HRmax. | Group 1: Ghrelin increased from 8210 ± 1400 to 9110 ± 1350 pg/mL, +11% (p = 0.001). BMI decreased from 17.9 ± 0.6 to 17.1 ± 0.44 kg/m2 (p < 0.05). Group 2: Ghrelin did not change from 8340 ± 1210 to 8400 ± 1210 pg/mL, +0.7% (p > 0.05). BMI did not change from 17.8 ± 0.68 to 17.9 ± 0.72 kg/m2 (p > 0.05). Group 3: Ghrelin did not change from 9880 ± 1300 to 9900 ± 1800 pg/mL, +0.2% (p > 0.05). BMI did not change from 16.5 ± 0.49 to 16.6 ± 0.29 kg/m2 (p > 0.05). |
| Very long-term training (≥48 weeks) with stable plasma ghrelin levels | |||
| Campos et al. [153] | Forty-two female and male obese adolescents (age 16.22 ± 1.35 years). Group1: BMI, 35.82 ± 4.52 kg/m2. Group 2: BMI, 37.6 ± 5.44 kg/m2. | Forty-eight weeks of 60 min/day. Group 1: Aerobic training. Group 2: Aerobic plus resistance (6–12 of RM) training. | Group 1: Ghrelin did not change from 7660 ± 6230 to 8890 ± 6450 pg/mL, +16.1% (p > 0.05). BMI decreased from 35.82 ± 4.52 to 32.06 ± 4.92 kg/m2 (p < 0.05). The %body fat decreased from 43.97 ± 6.46 to 38.44 ± 8.97% (p < 0.05); Group 2: Ghrelin increased from 1240 ± 480 to 1470 ± 1700 pg/mL, +18.6% (p < 0.05). BMI decreased from 37.6 ± 5.44 to 32.6 ± 4.56 kg/m2 (p < 0.05). The %body fat decreased from 50.5 ± 6.46 to 45.2 ± 8.14% (p < 0.01). |
| Very long-term training (≥48 weeks) with higher plasma ghrelin levels | |||
| Foster-Schubert et al. [143] | Eighty-seven postmenopausal obese women (age 60.7 ± 6.75 years; BM, 81 ± 14.1 Kg; BMI, 30.4 ± 4.1 kg/m2). | Forty-eight weeks of aerobic training, for 45 min/day at 60–75% of HRmax. Diet (1200–2000 kcal/day). | Ghrelin increased by 32 ± 16 pg/mL (p < 0.05). BM decreased from 81.7 ± 1.4 kg to 80.5 ± 0.3 (p < 0.05). |
| Mason et al. [154] | Three hundred ninety-nine overweight/ obese postmenopausal women (age 57.9 ± 5 years; BMI, 30.9 ± 4.0 kg/m2). | Twelve months of moderate-to-vigorous intensity exercise for 45 min/day (5 days/week) at 70–85% of HRmax. Diet: 1200–2000 kcal/day. | Ghrelin significantly increased in the diet plus exercise (+7.4%, p = 0.008) group but not in either the diet (+6.5%, p = 0.07) or exercise (+1.0%, p = 0.53) groups. BM decreased by 2.4% (p = 0.03) in the exercise group, by 8.5% (p < 0.001) in the diet group, and by 10.8% (p < 0.001) in the diet plus exercise group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abassi, W.; Ouerghi, N.; Muscella, A.; Marsigliante, S.; Feki, M.; Bouassida, A. Systematic Review: Does Exercise Training Influence Ghrelin Levels? Int. J. Mol. Sci. 2025, 26, 4753. https://doi.org/10.3390/ijms26104753
Abassi W, Ouerghi N, Muscella A, Marsigliante S, Feki M, Bouassida A. Systematic Review: Does Exercise Training Influence Ghrelin Levels? International Journal of Molecular Sciences. 2025; 26(10):4753. https://doi.org/10.3390/ijms26104753
Chicago/Turabian StyleAbassi, Wissal, Nejmeddine Ouerghi, Antonella Muscella, Santo Marsigliante, Moncef Feki, and Anissa Bouassida. 2025. "Systematic Review: Does Exercise Training Influence Ghrelin Levels?" International Journal of Molecular Sciences 26, no. 10: 4753. https://doi.org/10.3390/ijms26104753
APA StyleAbassi, W., Ouerghi, N., Muscella, A., Marsigliante, S., Feki, M., & Bouassida, A. (2025). Systematic Review: Does Exercise Training Influence Ghrelin Levels? International Journal of Molecular Sciences, 26(10), 4753. https://doi.org/10.3390/ijms26104753







