In-Situ Vascular Regeneration by Host Cells of Acellular Human Saphenous Vein Implanted in Porcine Carotid Artery
Abstract
1. Introduction
2. Results
2.1. Microbiology and Haematology
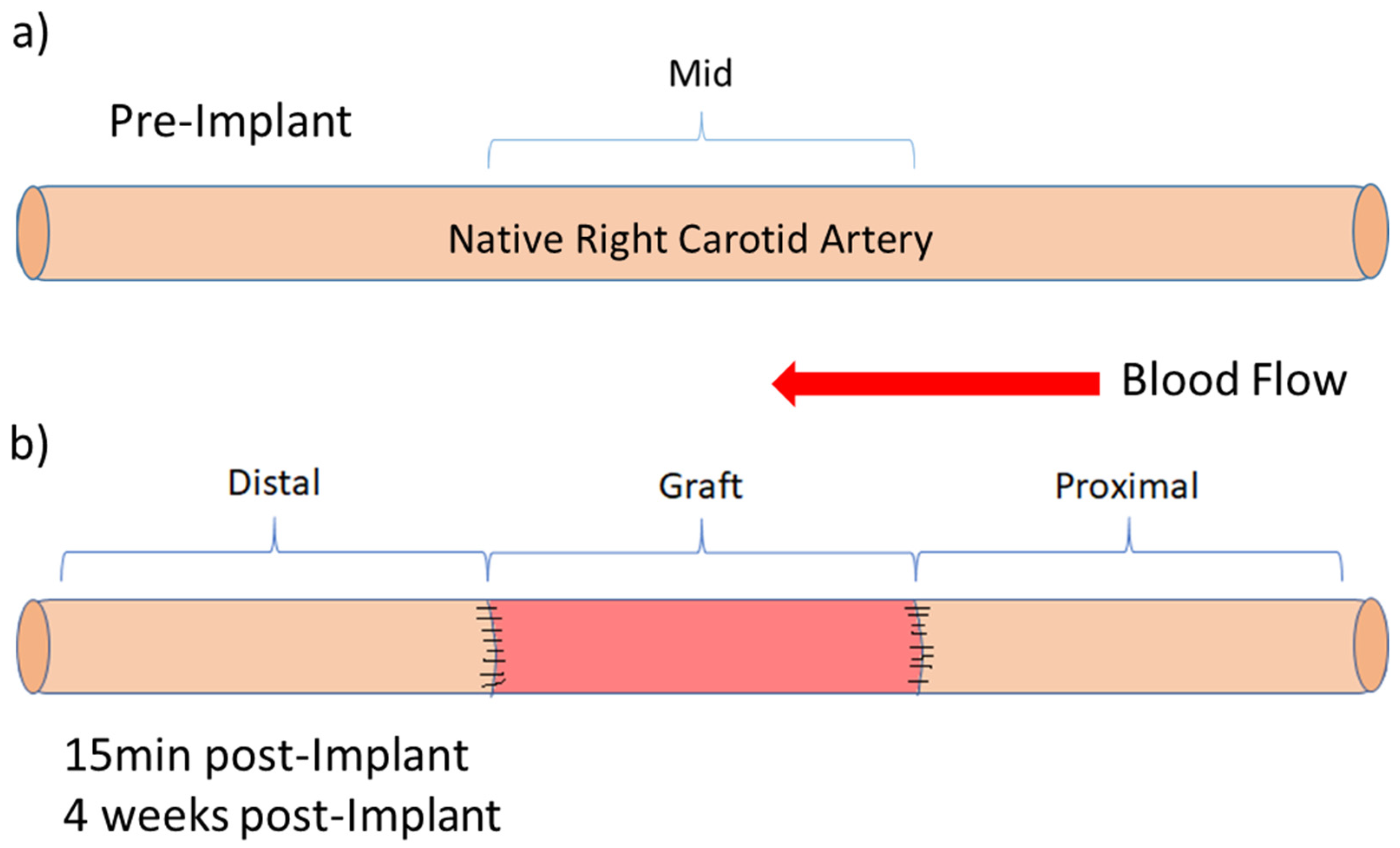
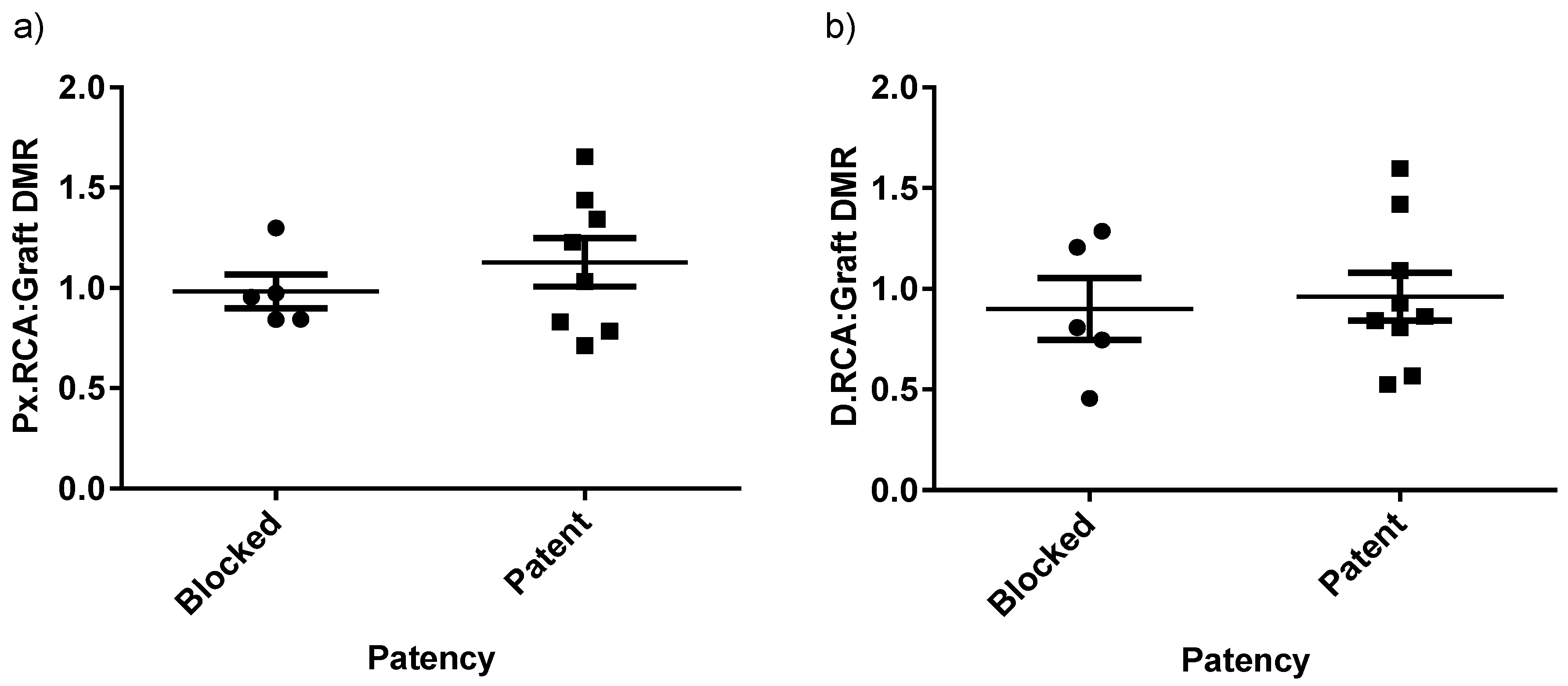
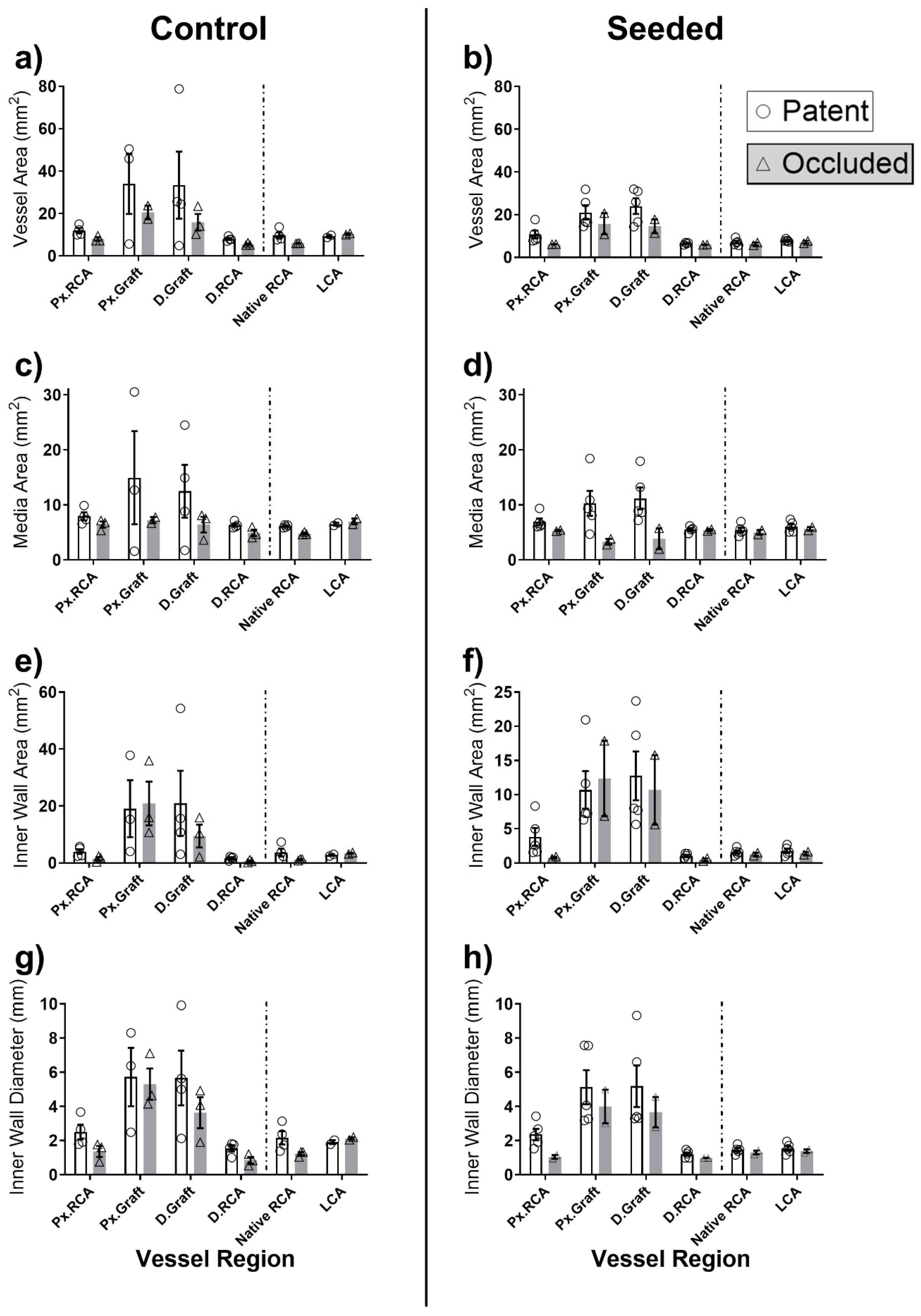
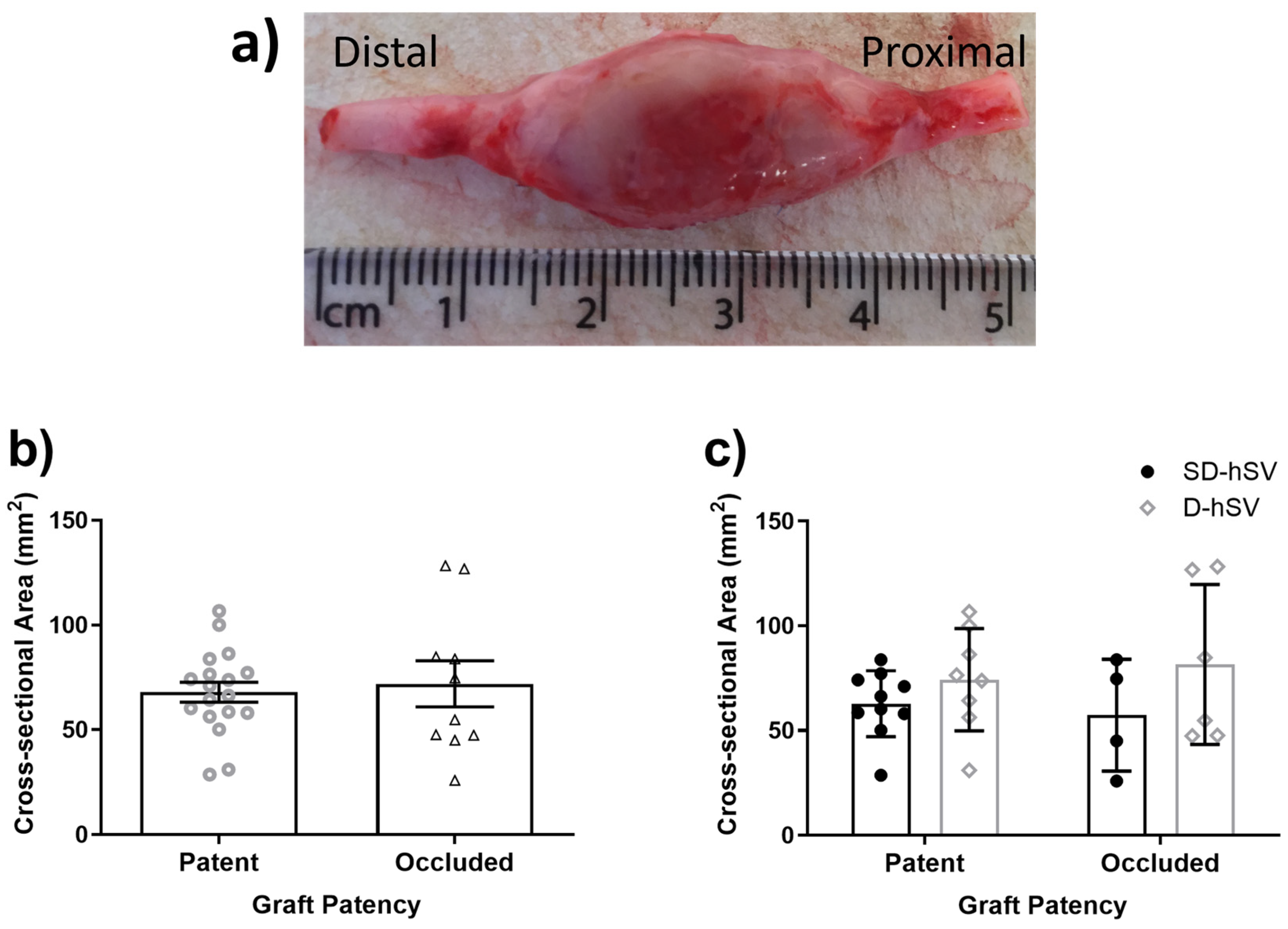
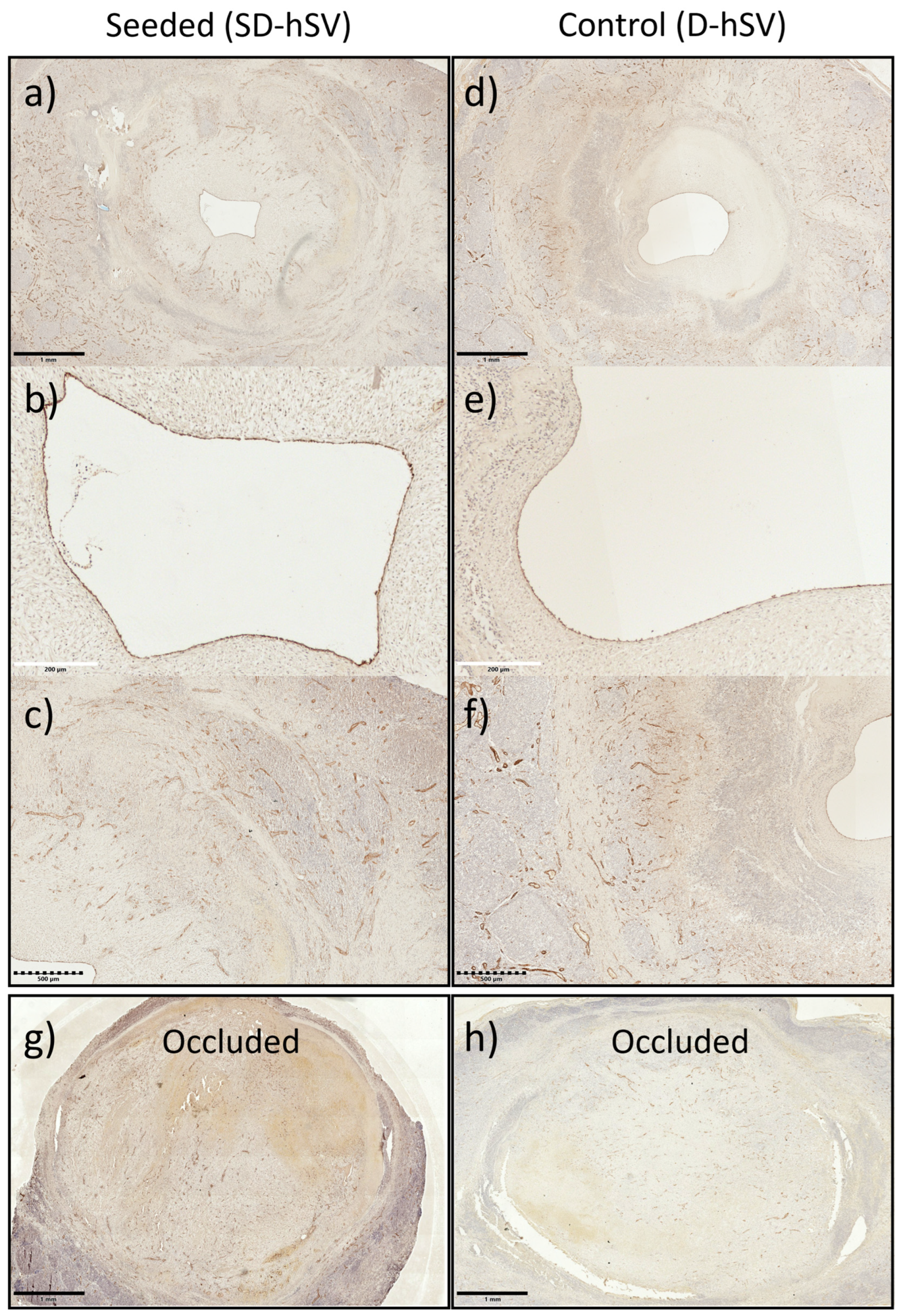
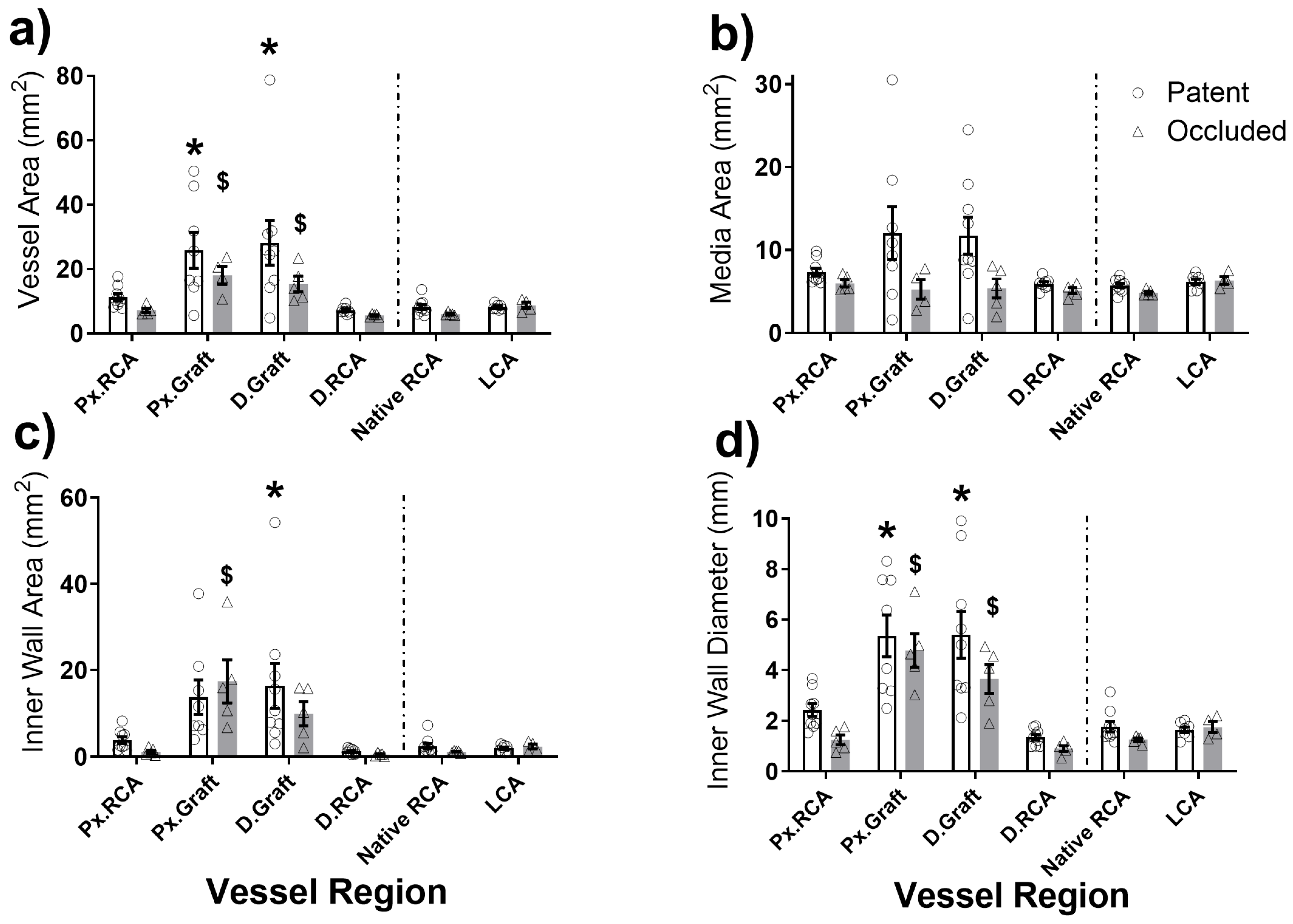
2.2. Graft Patency, Luminal Remodelling, and Intimal Thickening
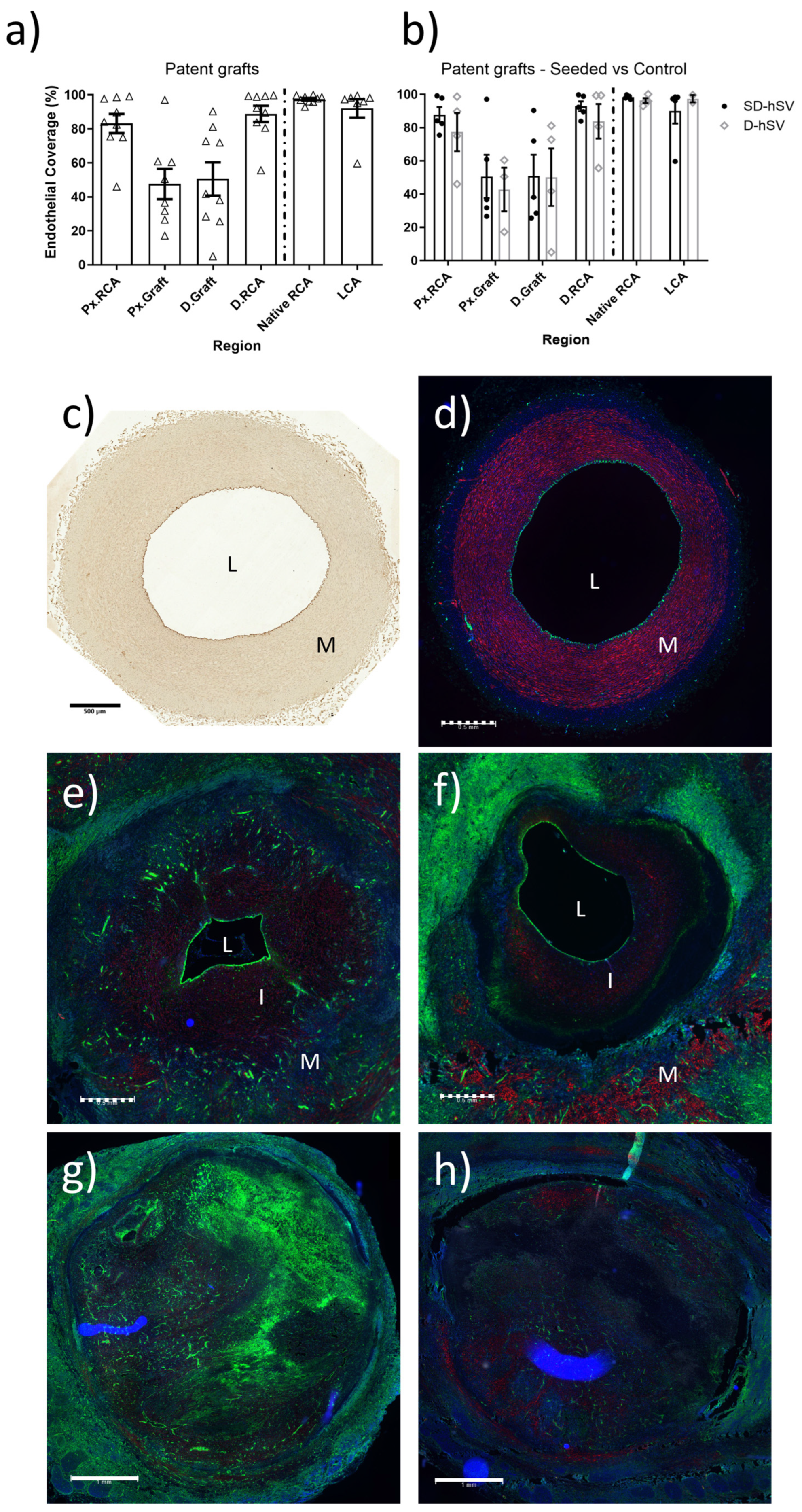
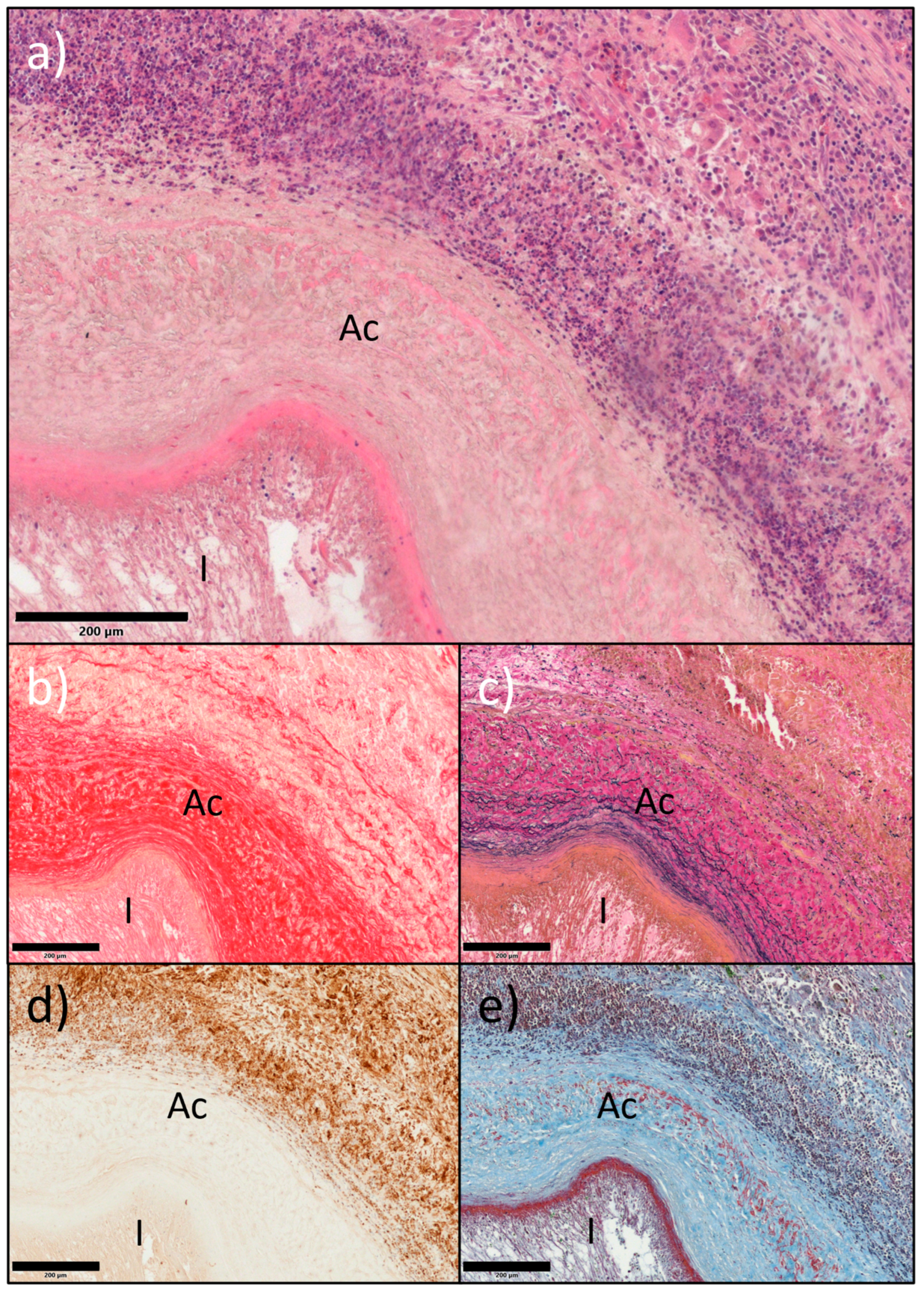
2.3. In Situ Vascular Regeneration by Host Cells
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Human Saphenous Vein Decellularisation
4.3. Porcine Endothelial-like Cell (ELC) Isolation and Seeding
4.4. In Vivo Surgical Grafting and Vascular Doppler Acquisition
4.5. Outcomes Measures
4.5.1. Microbiology
4.5.2. Haematology
4.5.3. Feasibility/Safety Measures
4.5.4. Patency, Intimal Thickening, and Vascular Regeneration
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | Carotid artery |
| CABG | Coronary artery bypass grafting |
| DBA | Dolichos Biflorus Agglutinin |
| DBFV | Diastolic blood flow velocity |
| D-hSV | Decellularised human saphenous vein |
| DMR | Diameter matching ratio |
| D.RCA | Distal right carotid artery |
| ECs | Endothelial cells |
| ECFCs | Endothelial colony-forming cells |
| ELCs | Endothelial-like cells |
| IT | Intimal thickening |
| LCA | Left carotid artery |
| LMR | Leukocyte/monocyte ratio |
| MSB | Martius scarlet blue |
| NLR | Neutrophil/lymphocyte ratio |
| PABG | Peripheral artery bypass grafting |
| PBFV | Peak blood flow velocity |
| pELCs | Porcine endothelial-like cells |
| PI | Pulsatility index |
| PWD | Pulse wave Doppler |
| Px.RCA | Proximal right carotid artery |
| RBC | Red blood cell |
| RCA | Right carotid artery |
| SD-hSV | D-hSV seeded with ELC |
| SMCs | Smooth muscle cells |
| SM-MHC | Smooth muscle myosin heavy chain |
| SV | Saphenous vein |
| SVGs | Saphenous vein grafts |
| USD | Ultrasound vascular Doppler |
Appendix A
Appendix A.1. Expanded Methods
Appendix A.1.1. Cell Seeding of Decellularised Human Saphenous Vein
Appendix A.1.2. Doppler Assessment of Grafts
Appendix A.1.3. Histological Assessment of Grafts
Appendix A.2. Results
Appendix A.2.1. Haematological Screening
Appendix A.2.2. Other Vascular Doppler Results



| Pig ID | Age at Implant (Months) | Weight at Implant (kg) | Weight at Termination (kg) | Weight Increase (%) | Graft Duration (Days) | Vein ID | Seeded vs. Control | Graft Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.7 | 57 | 71 | 19.7 | 28 | A | Control | Occluded |
| 2 | 4.0 | 64 | 84 | 23.8 | 28 | B | Control | Patent |
| 3 | 3.8 | 60 | 77 | 22.1 | 28 | B | Control | Patent |
| 4 | 3.9 | 61 | 80 | 23.8 | 29 | C | Seeded | Patent |
| 5 | 4.1 | 66 | 90 | 26.7 | 29 | C | Control | Patent |
| 6 | 3.9 | 61 | 85 | 28.2 | 30 | D | Control | Patent |
| 7 | 4.0 | 64 | 85 | 24.7 | 30 | D | Seeded | Patent |
| 8 | 4.1 | 67 | 84 | 20.2 | 27 | E | Seeded | Patent |
| 9 | 3.9 | 63 | 77 | 18.2 | 27 | F | Seeded | Occluded |
| 10 | 3.9 | 63 | 79 | 20.3 | 31 | G | Seeded | Occluded |
| 11 | 3.9 | 61 | 75 | 18.7 | 31 | H | Seeded | Patent |
| 12 | 3.7 | 55 | 80 | 31.3 | 31 | I | Seeded | Patent |
| 13 | 3.8 | 59 | 90 | 34.4 | 31 | J | Control | Occluded |
| 14 | 3.7 | 56 | 83 | 32.5 | 32 | I | Control | Occluded |
| Pre-Implant | Implant | Explant | |||||
|---|---|---|---|---|---|---|---|
| Control | Seeded | Control | Seeded | Control | Seeded | ||
| Inner Wall Diameter (cm) | Mid RCA | 0.45 ± 0.02 (n = 2) | 0.42 ± 0.04 (n = 2) | ||||
| Px.RCA | 0.38 ± 0.06 (n = 3) | 0.35 ± 0.00 (n = 2) | 0.34 ± 0.07 (n = 3) | 0.40 ± 0.03 (n = 2) | |||
| Mid RCA/Graft | 0.36 ± 0.02 (n = 3) | 0.39 ± 0.04 (n = 2) | 0.61 ± 0.26 (n = 3) | 0.54 ± 0.27 (n = 2) | |||
| D.RCA | 0.33 ± 0.04 (n = 3) | 0.32 ± 0.13 (n = 2) | 0.26 ± 0.05 (n = 3) | 0.37 ± 0.03 (n = 2) | |||
| LCA | 0.34 (n = 1) | 0.51 ± 0.04 (n = 2) | |||||
| Diameter Matching Index (DMI) | Proximal RCA/Graft Diameter | 1.03 ± 0.14 (n = 3) | 0.91 ± 0.07 (n = 2) | 0.67 ± 0.16 (n = 3) | 0.96 ± 0.42 (n = 2) | ||
| Distal RCA/Graft Diameter | 0.92 ± 0.14 (n = 3) | 0.88 ± 0.42 (n = 2) | 0.52 ± 0.13 (n = 3) | 0.88 ± 0.39 (n = 2) | |||
| PBFV (cm/s) | Mid RCA | 95.9 ± 31.2 (n = 2) | 154.2 ± 13.6 (n = 2) | ||||
| Px.RCA | 24.2 ± 0.2 (n = 2) | 38.1 ± 7.7 (n = 2) | 5.7 ± 5.7 (n = 2) | 22.9 ± 15.1 (n = 2) | |||
| Mid RCA/Graft | 164.0 ± 130.4 (n = 2) | 77.8 ± 31.0 (n = 2) | 0.0 ± 0.0 (n = 3) | 1.0 ± 1.0 (n = 2) | |||
| D.RCA | 80.5 ± 72.1 (n = 2) | 55.4 ± 22.2 (n = 2) | 0.0 ± 0.0 (n = 2) | 15.5 (n = 1) | |||
| Mid LCA | 193.0 (n = 1) | 154.4 ± 18.0 (n = 2) | |||||
| DBFV (cm/s) | Mid RCA | 34.0 ± 9.7 (n = 2) | 37.2 ± 3.1 (n = 2) | ||||
| Px.RCA | 7.2 ± 0.9 (n = 2) | 11.0 ± 2.4 (n = 2) | 1.2 ± 1.2 (n = 2) | 10.2 ± 7.1 (n = 2) | |||
| Mid RCA/Graft | 64.9 ± 59.0 (n = 2) | 14.0 ± 3.2 (n = 2) | 0.0 ± 0.0 (n = 3) | 0.7 ± 0.7 (n = 2) | |||
| D.RCA | 40.1 ± 37.1 (n = 2) | 7.7 ± 2.0 (n = 2) | 0.0 ± 0.0 (n = 2) | 3.7 (n = 1) | |||
| Mid LCA | 71.8 (n = 1) | 33.2 ± 9.9 (n = 2) | |||||
| Pulsatility Index | Mid RCA | 3.00 ± 1.34 (n = 2) | 6.78 ± 1.49 (n = 2) | ||||
| Px.RCA | 15.67 ± 11.53 (n = 2) | 10.00 ± 5.30 (n = 2) | nd | nd | |||
| Mid RCA/Graft | 6.31 ± 3.80 (n = 2) | 8.54 ± 6.26 (n = 2) | nd | 2.67 (n = 1) | |||
| D.RCA | 4.29 ± 2.70 (n = 2) | 2.40 ± 1.38 (n = 2) | nd | 4.27 (n = 1) | |||
| Mid LCA | 1.70 (n = 1) | 3.21 ± 0.52 (n = 2) | |||||
| Leukocytes (×109/L) | Neutrophils (×109/L) | Lymphocytes (×109/L) | NLR | Monocytes (×109/L) | LMR | Eosinophils (×109/L) | Basophils (×109/L) | ||
|---|---|---|---|---|---|---|---|---|---|
| Ref. Ranges [41] | 11.3–22.8 | 3.1–9.6 | 4.6–10 | - | 0.3–1.2 | - | 0–0.9 | 0–0.5 | |
| Baseline | Patent (n = 4) | 17.2 ± 0.9 | 5.3 ± 0.5 | 10.7 ± 0.9 | 0.5 ± 0.1 | 0.7 ± 0.1 | 20.2 ± 4.0 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| Occluded (n = 7) | 18.0 ± 3.1 | 6.9 ± 2.4 | 9.5 ± 0.6 | 0.7 ± 0.2 | 0.7 ± 0.1 | 14.6 ± 0.9 | 0.7 ± 0.3 | 0.1 ± 0.1 | |
| D-hSV (n = 4) | 15.5 ± 1.4 | 4.2 ± 0.4 | 10.3 ± 1.5 | 0.4 ± 0.1 | 0.4 ± 0.1 * | 25.0 ± 5.6 * | 0.5 ± 0.2 | 0.0 ± 0.0 * | |
| SD-hSV (n = 7) | 18.7 ± 1.5 | 6.9 ± 1.2 | 10.3 ± 0.6 | 0.7 ± 0.1 | 0.8 ± 0.1 | 14.3 ± 1.5 | 0.5 ± 0.2 | 0.1 ± 0.0 | |
| Explant | Patent (n = 4) | 17.3 ± 0.9 | 4.4 ± 0.5 | 11.4 ± 0.8 | 0.4 ± 0.1 | 0.8 ± 0.1 | 17.8 ± 3.1 | 0.5 ± 0.1 | 0.1 ± 0.0 |
| Occluded (n = 7) | 17.3 ± 1.8 | 6.0 ± 1.1 | 10.0 ± 0.4 | 0.6 ± 0.1 | 0.6 ± 0.2 | 19.1 ± 5.1 | 0.5 ± 0.3 | 0.1 ± 0.0 | |
| D-hSV (n = 4) | 16.4 ± 1.6 | 4.1 ± 0.1 | 11.1 ± 1.5 | 0.4 ± 0.0 | 0.5 ± 0.1 | 22.3 ± 4.4 | 0.5 ± 0.2 | 0.0 ± 0.0 | |
| SD-hSV (n = 7) | 17.8 ± 0.9 | 5.5 ± 0.8 | 10.8 ± 0.4 | 0.5 ± 0.1 | 0.8 ± 0.1 | 16.0 ± 3.0 | 0.5 ± 0.2 | 0.1 ± 0.0 | |
| Explant/Baseline Ratio | Patent (n = 4) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 1.3 ± 0.2 | 1.0 ± 0.1 | n/d | n/d |
| Occluded (n = 7) | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.1 ± 0.0 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.3 ± 0.3 | 0.5 ± 0.2 | 0.5 ± 0.3 | |
| D-hSV (n = 4) | 1.1 ± 0.0 | 1.0 ± 0.1 | 1.1 ± 0.0 | 0.9 ± 0.1 | 1.3 ± 0.3 | 1.1 ± 0.4 | n/d | n/d | |
| SD-hSV (n = 7) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.2 | 0.6 ± 0.2 | |
| Region | Pre-Implant | 15 min Post-Implant | 4 Weeks | ||||
|---|---|---|---|---|---|---|---|
| Patent | Occluded | Patent | Occluded | Patent | Occluded | ||
| Inner Wall Diameter (cm) | Mid RCA | 0.46 ± 0.03 (n = 9) | 0.43 ± 0.02 (n = 4) | ||||
| Px.RCA | 0.38 ± 0.03 (n = 8) | 0.37 ± 0.03 (n = 5) | 0.42 ± 0.03 (n = 9) | 0.36 ± 0.04 (n = 5) | |||
| Graft | 0.37 ± 0.03 (n = 9) | 0.37 ± 0.02 (n = 5) | 0.58 ± 0.11 (n = 9) | 0.58 ± 0.17 (n = 5) | |||
| D.RCA | 0.34 ± 0.04 a (n = 9) | 0.33 ± 0.05 (n = 5) | 0.46 ± 0.02 **,a (n = 9) | 0.30 ± 0.04 ** (n = 5) | |||
| Mid LCA | 0.52 ± 0.02 (n = 7) | 0.45 ± 0.06 (n = 3) | |||||
| Diameter Matching Ratio (DMR) | Px.RCA/Graft | 1.13 ± 0.12 (n = 8) | 0.98 ± 0.08 (n = 5) | 1.12 ± 0.31 (n = 9) | 0.78 ± 0.17 (n = 5) | ||
| D.RCA/Graft | 0.96 ± 0.12 (n = 9) | 0.90 ± 0.15 (n = 5) | 1.19 ± 0.31 (n = 9) | 0.67 ± 0.17 (n = 5) | |||
| Region | Pre-Implant | 15 min Post-Implant | 4 Weeks | ||||
|---|---|---|---|---|---|---|---|
| Patent | Occluded | Patent | Occluded | Patent | Occluded | ||
| Peak Blood Flow Velocity (cm/s) | Mid RCA | 164.6 ± 22.2 (n = 9) | 125.0 ± 21.8 (n = 4) | ||||
| Px.RCA | 63.2 ± 9.9 * (n = 9) | 31.1 ± 5.1 * (n = 4) | 50.0 ± 7.4 (n = 9) | 14.3 ± 8.3 (n = 4) | |||
| Graft | 162.1 ± 38.2 (n = 9) | 120.9 ± 60.1 (n = 4) | 226.0 ± 56.2 (n = 9) | 0.4 ± 0.4 (n = 5) | |||
| D.RCA | 185.2 ± 22.3 * (n = 9) | 67.9 ± 31.7 * (n = 4) | 114.1 ± 34.1 (n = 9) | 5.2 ± 5.2 (n = 3) | |||
| Mid LCA | 153.7 ± 16.2 (n = 7) | 167.2 ± 16.5 (n = 3) | |||||
| Diastolic Blood Flow Velocity (cm/s) | Mid RCA | 53.9 ± 15.9 (n = 9) | 35.6 ± 4.3 (n = 4) | ||||
| Px.RCA | 26.8 ± 4.4 ** (n = 9) | 9.1 ± 1.5 ** (n = 4) | 18.9 ± 2.4 (n = 9) | 5.7 ± 3.9 (n = 4) | |||
| Graft | 54.5 ± 12.3 (n = 9) | 39.5 ± 28.2 (n = 4) | 76.6 ± 20.9 (n = 9) | 0.3 ± 0.3 (n = 5) | |||
| D.RCA | 70.2 ± 8.6 *,b (n = 9) | 23.9 ± 17.8 * (n = 4) | 31.9 ± 9.5 b (n = 9) | 1.2 ± 1.2 (n = 3) | |||
| Mid LCA | 36.9 ± 5.3 (n = 7) | 46.1 ± 14.1 (n = 3) | |||||
| Pulsatility Index | Mid RCA | 4.32 ± 0.66 (n = 8) | 4.89 ± 1.36 (n = 4) | ||||
| Px.RCA | 1.82 ± 0.33 (n = 8) | 12.84 ± 5.43 (n = 4) | 5.42 ± 2.63 (n = 9) | n/d | |||
| Graft | 2.24 ± 0.32 (n = 8) | 7.42 ± 3.06 (n = 4) | 3.13 ± 0.39 (n = 9) | n/d | |||
| D.RCA | 1.88 ± 0.21 (n = 8) | 3.34 ± 1.35 (n = 4) | 15.40 ± 12.84 (n = 9) | n/d | |||
| Mid LCA | 3.89 ± 0.59 (n = 7) | 2.70 ± 0.58 (n = 3) | |||||
| Pre-Implant | Implant | 4 Weeks | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Patency | Native RCA a | Graft b | Px. RCA c | Px. Graft d | D. Graft e | D. RCA f | LCA g |
| All | 57.9 ± 3.9 b,d,e (n = 14) | 22.7 ± 3.1 a,c,d,f,g (n = 11) | 69.4 ± 4.1 b,d,e,f,g (n = 13) | 3.4 ± 1.6 a,b,c,f,g (n = 12) | 12.2 ± 4.3 a,c,f,g (n = 14) | 51.2 ± 3.7 b,c,d,e (n = 14) | 51.3 ± 4.4 b,c,d,e (n = 10) | |
| Patent | 59.1 ± 5.7 b,d,e (n = 9) | 16.8 ± 2.1 a,c,f,g (n = 7) | 73.2 ± 5.4 b,d,e,f,g (n = 9) | 2.0 ± 1.4 a,c,f,g (n = 8) | 10.1 ± 5.6 a,c,f,g (n = 9) | 51.8 ± 4.9 b,c,d,e (n = 9) | 48.1 ± 4.3 b,c,d,e (n = 7) | |
| Occluded | 55.7 ± 4.8 d,e (n = 5) | 33.0 ± 4.2 c (n = 4) | 61.0 ± 2.7 b,d,e (n = 4) | 6.2 ± 3.7 a,c,f,g (n = 4) | 16.1 ± 7.3 a,c,f,g (n = 5) | 50.1 ± 6.0 d,e (n = 5) | 58.7 ± 10.8 d,e (n = 3) | |
| Patent | D-hSV | 60.5 ± 9.8 b,d,e (n = 4) | 13.3 ± 1.0 a,c (n = 2) | 70.0 ± 8.8 b,d,e (n = 4) | 0.9 ± 0.8 a,c,f,g (n = 3) | 3.9 ± 3.6 a,c,f,g (n = 4) | 43.9 ± 6.2 d,e (n = 4) | 50.4 ± 3.0 d,e (n = 2) |
| SD-hSV | 58.0 ± 7.6 b,d,e (n = 5) | 18.2 ± 2.7 a,c,f (n = 5) | 75.7 ± 7.5 b,d,e (n = 5) | 2.7 ± 2.2 a,c,f,g (n = 5) | 15.1 ± 9.5 a,c,f,g (n = 5) | 58.2 ± 6.5 b,d,e (n = 5) | 47.1 ± 6.1 d,e (n = 5) | |
| Occluded | D-hSV | 51.0 ± 6.9 (n = 3) | 36.1 ± 7.6 (n = 2) | 58.5 ± 5.7 (n = 2) | 3.9 ± 3.5 (n = 2) | 17.0 ± 11.7 (n = 3) | 42.3 ± 5.3 (n = 3) | 42.8 (n = 1) |
| SD-hSV | 62.7 ± 1.6 (n = 2) | 30.0 ± 5.2 (n = 2) | 63.5 ± 0.7 (n = 2) | 8.5 ± 7.7 (n = 2) | 14.7 ± 11.2 (n = 2) | 61.8 ± 6.9 (n = 2) | 66.7 ± 12.7 (n = 2) | |
| Primary Antibody | Cat. No.; Supplier | Working Concentration | Secondary Antibody | Cat. No.; Supplier | Working Concentration |
|---|---|---|---|---|---|
| Anti-PECAM-1/CD31 | Ab28364; Abcam | 0.45 μg/mL | Goat anti-rabbit IgG, biotinylated | B7389; Sigma-Aldrich | 3.5 μg/mL |
| Anti-calponin 1 | NBP1-87029; Novus Biologicals | 0.2 μg/mL | |||
| Anti-smooth muscle myosin heavy chain 11 | AB53219; Abcam | 1.08 μg/mL | |||
| Rabbit IgG Isotype Control | Ab172730; Abcam | 0.45 μg/mL | |||
| Rabbit IgG Isotype Control | 10500C; Invitrogen | 0.45–1.08 μg/mL | |||
| Anti-vimentin | Ab8979; Abcam | 5 μg/mL | Goat anti-mouse IgG (H+L), biotinylated | BA-9200; Vector Labs | 7.5 μg/mL |
| Mouse IgG Isotype Control | 10400C; Invitrogen | 5 μg/mL | |||
| DBA-Lectin $$ | B-1035; Vector Laboratories | 25 μg/mL | Alexa-Fluor 488 Streptavidin | S32354; ThermoFisher Scientific | 10 μg/mL |
| Anti-SMA-Cy3 | C6198; Sigma | 2.5 μg/mL | n/a | n/a | n/a |
References
- Bauersachs, R.; Zeymer, U.; Briere, J.B.; Marre, C.; Bowrin, K.; Huelsebeck, M. Burden of Coronary Artery Disease and Peripheral Artery Disease: A Literature Review. Cardiovasc. Ther. 2019, 2019, 8295054. [Google Scholar] [CrossRef] [PubMed]
- Klinkert, P.; Post, P.N.; Breslau, P.J.; van Bockel, J.H. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. A review of the literature. Eur. J. Vasc. Endovasc. Surg. 2004, 27, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Bowdish, M.E.; D’Agostino, R.S.; Thourani, V.H.; Desai, N.; Shahian, D.M.; Fernandez, F.G.; Badhwar, V. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2020 Update on Outcomes and Research. Ann. Thorac. Surg. 2020, 109, 1646–1655. [Google Scholar] [CrossRef]
- Harskamp, R.E.; Lopes, R.D.; Baisden, C.E.; de Winter, R.J.; Alexander, J.H. Saphenous vein graft failure after coronary artery bypass surgery: Pathophysiology, management, and future directions. Ann. Surg. 2013, 257, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.M.; Darling, R.C., 3rd; Chang, B.B.; Fitzgerald, K.M.; Paty, P.S.; Leather, R.P. Long-term results of in situ saphenous vein bypass. Analysis of 2058 cases. Ann. Surg. 1995, 222, 438–446, discussion 446–438. [Google Scholar] [CrossRef]
- Thatte, H.S.; Biswas, K.S.; Najjar, S.F.; Birjiniuk, V.; Crittenden, M.D.; Michel, T.; Khuri, S.F. Multi-photon microscopic evaluation of saphenous vein endothelium and its preservation with a new solution, GALA. Ann. Thorac. Surg. 2003, 75, 1145–1152, discussion 1152. [Google Scholar] [CrossRef]
- Abendschein, D.R.; Recchia, D.; Meng, Y.Y.; Oltrona, L.; Wickline, S.A.; Eisenberg, P.R. Inhibition of thrombin attenuates stenosis after arterial injury in minipigs. J. Am. Coll. Cardiol. 1996, 28, 1849–1855. [Google Scholar] [CrossRef]
- Cosgrove, D.M.; Loop, F.D.; Lytle, B.W.; Gill, C.C.; Golding, L.A.; Gibson, C.; Stewart, R.W.; Taylor, P.C.; Goormastic, M. Predictors of reoperation after myocardial revascularization. J. Thorac. Cardiovasc. Surg. 1986, 92, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Loop, F.D.; Lytle, B.W.; Cosgrove, D.M.; Stewart, R.W.; Goormastic, M.; Williams, G.W.; Golding, L.A.; Gill, C.C.; Taylor, P.C.; Sheldon, W.C.; et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N. Engl. J. Med. 1986, 314, 1–6. [Google Scholar] [CrossRef]
- Lytle, B.W.; Blackstone, E.H.; Loop, F.D.; Houghtaling, P.L.; Arnold, J.H.; Akhrass, R.; McCarthy, P.M.; Cosgrove, D.M. Two internal thoracic artery grafts are better than one. J. Thorac. Cardiovasc. Surg. 1999, 117, 855–872. [Google Scholar] [CrossRef]
- Jouda, H.; Larrea Murillo, L.; Wang, T. Current Progress in Vascular Engineering and Its Clinical Applications. Cells 2022, 11, 493. [Google Scholar] [CrossRef]
- Pearson, J.D. Normal endothelial cell function. Lupus 2000, 9, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Verrier, E.D.; Boyle, E.M., Jr. Endothelial cell injury in cardiovascular surgery. Ann. Thorac. Surg. 1996, 62, 915–922. [Google Scholar] [CrossRef]
- Allaire, E.; Clowes, A.W. Endothelial cell injury in cardiovascular surgery: The intimal hyperplastic response. Ann. Thorac. Surg. 1997, 63, 582–591. [Google Scholar] [CrossRef]
- Paschalaki, K.E.; Randi, A.M. Recent Advances in Endothelial Colony Forming Cells Toward Their Use in Clinical Translation. Front. Med. 2018, 5, 295. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Bond, A.R.; Bruno, V.D.; Joseph, J.; Johnson, J.L.; Suleiman, M.S.; George, S.J.; Ascione, R. Effective decellularisation of human saphenous veins for biocompatible arterial tissue engineering applications: Bench optimisation and feasibility in vivo testing. J. Tissue Eng. 2021, 12, 2041731420987529. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Domenico Bruno, V.; Sulaiman, N.; Ward, A.; Johnson, T.W.; Baby, H.M.; Kerala Varma, P.; Jose, R.; Nair, S.V.; Menon, D.; et al. A novel small diameter nanotextile arterial graft is associated with surgical feasibility and safety and increased transmural endothelial ingrowth in pig. J. Nanobiotechnol. 2022, 20, 71. [Google Scholar] [CrossRef]
- Bond, A.; Bruno, V.; Johnson, J.; George, S.; Ascione, R. Development and Preliminary Testing of Porcine Blood-Derived Endothelial-like Cells for Vascular Tissue Engineering Applications: Protocol Optimisation and Seeding of Decellularised Human Saphenous Veins. Int. J. Mol. Sci. 2022, 23, 6633. [Google Scholar] [CrossRef]
- Solo, K.; Lavi, S.; Kabali, C.; Levine, G.N.; Kulik, A.; John-Baptiste, A.A.; Fremes, S.E.; Martin, J.; Eikelboom, J.W.; Ruel, M.; et al. Antithrombotic treatment after coronary artery bypass graft surgery: Systematic review and network meta-analysis. BMJ 2019, 367, l5476. [Google Scholar] [CrossRef]
- Xenogiannis, I.; Zenati, M.; Bhatt, D.L.; Rao, S.V.; Rodes-Cabau, J.; Goldman, S.; Shunk, K.A.; Mavromatis, K.; Banerjee, S.; Alaswad, K.; et al. Saphenous Vein Graft Failure: From Pathophysiology to Prevention and Treatment Strategies. Circulation 2021, 144, 728–745. [Google Scholar] [CrossRef] [PubMed]
- Hinokiyama, K.; Valen, G.; Tokuno, S.; Vedin, J.B.; Vaage, J. Vein graft harvesting induces inflammation and impairs vessel reactivity. Ann. Thorac. Surg. 2006, 82, 1458–1464. [Google Scholar] [CrossRef]
- Alrawi, S.J.; Raju, R.; Alshkaki, G.; Acinapura, A.J.; Cunningham, J.N., Jr. Saphenous vein endothelial cell viability: A comparative study of endoscopic and open saphenectomy for coronary artery bypass grafting. JSLS 2001, 5, 37–45. [Google Scholar]
- Samano, N.; Souza, D.; Pinheiro, B.B.; Kopjar, T.; Dashwood, M. Twenty-Five Years of No-Touch Saphenous Vein Harvesting for Coronary Artery Bypass Grafting: Structural Observations and Impact on Graft Performance. Braz. J. Cardiovasc. Surg. 2020, 35, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.C.; Souza, D.S.; Filbey, D.; Bomfim, V.; Dashwood, M.R. Preserved endothelial integrity and nitric oxide synthase in saphenous vein grafts harvested by a ’no-touch’ technique. Br. J. Surg. 2001, 88, 1209–1215. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Quint, C.; Kondo, Y.; Manson, R.J.; Lawson, J.H.; Dardik, A.; Niklason, L.E. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 2011, 108, 9214–9219. [Google Scholar] [CrossRef]
- Unger, E.R.; Sung, J.H.; Manivel, J.C.; Chenggis, M.L.; Blazar, B.R.; Krivit, W. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: A Y-chromosome specific in situ hybridization study. J. Neuropathol. Exp. Neurol. 1993, 52, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Harrington, J.K.; Chahboune, H.; Criscione, J.M.; Li, A.Y.; Hibino, N.; Yi, T.; Villalona, G.A.; Kobsa, S.; Meijas, D.; Duncan, D.R.; et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011, 25, 4150–4161. [Google Scholar] [CrossRef]
- Malek, A.M.; Alper, S.L.; Izumo, S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999, 282, 2035–2042. [Google Scholar] [CrossRef]
- Nerem, R.M.; Alexander, R.W.; Chappell, D.C.; Medford, R.M.; Varner, S.E.; Taylor, W.R. The study of the influence of flow on vascular endothelial biology. Am. J. Med. Sci. 1998, 316, 169–175. [Google Scholar] [CrossRef]
- Joddar, B.; Firstenberg, M.S.; Reen, R.K.; Varadharaj, S.; Khan, M.; Childers, R.C.; Zweier, J.L.; Gooch, K.J. Arterial levels of oxygen stimulate intimal hyperplasia in human saphenous veins via a ROS-dependent mechanism. PLoS ONE 2015, 10, e0120301. [Google Scholar] [CrossRef]
- Rafati, M.; Havaee, E.; Moladoust, H.; Sehhati, M. Appraisal of different ultrasonography indices in patients with carotid artery atherosclerosis. EXCLI J. 2017, 16, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.; Zadina, K.; Moritz, T.; Ovitt, T.; Sethi, G.; Copeland, J.G.; Thottapurathu, L.; Krasnicka, B.; Ellis, N.; Anderson, R.J.; et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: Results from a Department of Veterans Affairs Cooperative Study. J. Am. Coll. Cardiol. 2004, 44, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; Windecker, S.; Kolh, P.; Alfonso, F.; Collet, J.P.; Cremer, J.; Falk, V.; Filippatos, G.; Hamm, C.; Head, S.J.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur. Heart J. 2014, 35, 2541–2619. [Google Scholar] [CrossRef]
- Yamane, Y.; Uchida, N.; Okubo, S.; Morimoto, H.; Mukai, S. Impact of the size mismatch between saphenous vein graft and coronary artery on graft patency. Gen. Thorac. Cardiovasc. Surg. 2017, 65, 25–31. [Google Scholar] [CrossRef]
- Motwani, J.G.; Topol, E.J. Aortocoronary saphenous vein graft disease: Pathogenesis, predisposition, and prevention. Circulation 1998, 97, 916–931. [Google Scholar] [CrossRef]
- Rydhmer, L.; Zamaratskaia, G.; Andersson, H.K.; Algers, B.; Guillemet, R.; Lundström, K. Aggressive and sexual behaviour of growing and finishing pigs reared in groups, without castration. Acta Agric. Scand. Sect. A—Anim. Sci. 2006, 56, 109–119. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthi, I.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Vet. Clin. Pathol. 2012, 41, 27–31. [Google Scholar] [CrossRef]
- Turak, O.; Ozcan, F.; Isleyen, A.; Tok, D.; Sokmen, E.; Buyukkaya, E.; Aydogdu, S.; Akpek, M.; Kaya, M.G. Usefulness of the neutrophil-to-lymphocyte ratio to predict bare-metal stent restenosis. Am. J. Cardiol. 2012, 110, 1405–1410. [Google Scholar] [CrossRef]
- Fielder, S.E. Hematologic Reference Ranges. In The Merck Veterinary Manual; Merck & Co., Inc.: Rahway, NJ, USA, 2015. [Google Scholar]


| Region | Pre-Implant | 15 min Post-Implant | 4 Weeks Post-Implant | ||||
|---|---|---|---|---|---|---|---|
| D-hSV (n = 4) | SD-hSV (n = 5) | D-hSV | SD-hSV | D-hSV | SD-hSV | ||
| Inner Wall Diameter (cm) | Mid RCA | 0.48 ± 0.05 | 0.44 ± 0.03 | ||||
| Px.RCA | 0.37 ± 0.03 (n = 4) | 0.39 ± 0.04 (n = 4) | 0.44 ± 0.05 (n = 4) | 0.40 ± 0.03 (n = 5) | |||
| Graft | 0.36 ± 0.04 (n = 4) | 0.38 ± 0.04 (n = 5) | 0.45 ± 0.16 (n = 4) | 0.68 ± 0.16 (n = 5) | |||
| D.RCA | 0.29 ± 0.05 (n = 4) | 0.38 ± 0.05 (n = 5) | 0.45 ± 0.05 (n = 4) | 0.47 ± 0.02 (n = 5) | |||
| Mid LCA | 0.53 ± 0.02 (n = 2) | 0.51 ± 0.02 (n = 5) | |||||
| Diameter Matching Ratio (DMR) | Px.RCA/Graft | 1.08 ± 0.14 (n = 4) | 1.18 ± 0.22 (n = 4) | 1.52 ± 0.62 (n = 4) | 0.81 ± 0.25 (n = 5) | ||
| D.RCA/Graft | 0.79 ± 0.08 (n = 4) | 1.10 ± 0.19 (n = 5) | 1.51 ± 0.60 (n = 4) | 0.92 ± 0.29 (n = 5) | |||
| Region | Pre-Implant | 15 min Post-Implant | 4 Weeks Post-Implant | ||||
|---|---|---|---|---|---|---|---|
| D-hSV | SD-hSV | D-hSV | SD-hSV | D-hSV | SD-hSV | ||
| Peak Blood Flow Velocity (cm/s) | Mid RCA | 151.0 ± 16.9 (n = 4) | 175.5 ± 39.2 (n = 5) | ||||
| Px.RCA | 81.1 ± 11.7 a (n = 4) | 48.9 ± 12.6 (n = 5) | 43.3 ± 7.1 a (n = 4) | 55.4 ± 12.3 (n = 5) | |||
| Graft | 184.9 ± 66.5 (n = 4) | 143.8 ± 49.2 (n = 5) | 225.7 ± 70.2 (n = 4) | 226.3 ± 91.7 (n = 5) | |||
| D.RCA | 186.6 ± 33.8 (n = 4) | 184.1 ± 33.2 a (n = 5) | 151.6 ± 75.9 (n = 4) | 84.1 ± 15.2 a (n = 5) | |||
| Mid LCA | 163.6 ± 47.8 (n = 2) | 149.7 ± 17.5 (n = 5) | |||||
| Diastolic Blood Flow Velocity (cm/s) | Mid RCA | 35.0 ± 5.9 (n = 4) | 69.1 ± 27.6 (n = 5) | ||||
| Px.RCA | 33.9 ± 6.6 (n = 4) | 21.0 ± 5.0 (n = 5) | 18.1 ± 2.1 (n = 4) | 19.6 ± 4.2 (n = 5) | |||
| Graft | 59.9 ± 23.0 (n = 4) | 50.2 ± 14.6 (n = 5) | 79.6 ± 27.0 (n = 4) | 74.1 ± 33.6 (n = 5) | |||
| D.RCA | 77.9 ± 14.2 (n = 4) | 64.0 ± 11.2 a (n = 5) | 42.9 ± 20.7 (n = 4) | 23.1 ± 5.1 a (n = 5) | |||
| Mid LCA | 37.5 ± 12.4 (n = 2) | 36.6 ± 6.6 (n = 5) | |||||
| Pulsatility Index | Mid RCA | 3.04 ± 0.70 * (n = 4) | 5.61 ± 0.66 * (n = 4) | ||||
| Px.RCA | 1.59 ± 0.53 (n = 4) | 2.04 ± 0.43 (n = 4) | 8.28 ± 5.99 (n = 4) | 3.12 ± 0.61 (n = 5) | |||
| Graft | 2.25 ± 0.28 (n = 4) | 2.23 ± 0.64 (n = 4) | 2.75 ± 0.39 (n = 4) | 3.43 ± 0.64 (n = 5) | |||
| D.RCA | 1.55 ± 0.25 (n = 4) | 2.22 ± 0.26 (n = 4) | 2.30 ± 0.36 (n = 4) | 25.88 ± 23.06 (n = 5) | |||
| Mid LCA | 3.72 ± 0.05 (n = 2) | 3.96 ± 0.85 (n = 5) | |||||
| D-hSV | Proximal Graft | Distal Graft | ||
|---|---|---|---|---|
| All (n = 14) | 15.8 ± 3.5% | 71.4 ± 9.7% | 67.1 ± 9.2% | |
| Patent (n = 9) | 17.1 ± 4.7% | 54.1 ± 12.2% | 49.2 ± 10.1% | |
| Occluded (n = 5) | 12.8 ± 4.7% a | 99.0 ± 0.8% | 99.4 ± 0.6% | |
| Control n = 7 | All | 63.4 ± 17.6% | 60.2 ± 15.7% | |
| Patent | 28.5 ± 18.4% | 31.2 ± 14.2% | ||
| Occluded | 98.3 ± 1.1% | 98.9 ± 1.1% | ||
| Seeded n = 7 | All | 78.2 ± 10.4% | 74.1 ± 10.3% | |
| Patent | 69.5 ± 12.6% | 63.7 ± 11.3% | ||
| Occluded | 100.0 ± 0.0% | 100.0 ± 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bond, A.; Bruno, V.; Sulaiman, N.; Johnson, J.; George, S.; Ascione, R. In-Situ Vascular Regeneration by Host Cells of Acellular Human Saphenous Vein Implanted in Porcine Carotid Artery. Int. J. Mol. Sci. 2025, 26, 4718. https://doi.org/10.3390/ijms26104718
Bond A, Bruno V, Sulaiman N, Johnson J, George S, Ascione R. In-Situ Vascular Regeneration by Host Cells of Acellular Human Saphenous Vein Implanted in Porcine Carotid Artery. International Journal of Molecular Sciences. 2025; 26(10):4718. https://doi.org/10.3390/ijms26104718
Chicago/Turabian StyleBond, Andrew, Vito Bruno, Nadiah Sulaiman, Jason Johnson, Sarah George, and Raimondo Ascione. 2025. "In-Situ Vascular Regeneration by Host Cells of Acellular Human Saphenous Vein Implanted in Porcine Carotid Artery" International Journal of Molecular Sciences 26, no. 10: 4718. https://doi.org/10.3390/ijms26104718
APA StyleBond, A., Bruno, V., Sulaiman, N., Johnson, J., George, S., & Ascione, R. (2025). In-Situ Vascular Regeneration by Host Cells of Acellular Human Saphenous Vein Implanted in Porcine Carotid Artery. International Journal of Molecular Sciences, 26(10), 4718. https://doi.org/10.3390/ijms26104718








