Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Results
2.1. Plant Extract Phytochemical Characterization
2.2. Assessment of the In Vitro OS Markers
2.3. Evaluation of In Vivo Antioxidant Effects of P. ovata Ethanol Extract
2.4. In Vivo Inflammatory Markers Analysis
2.5. Assessment of In Vivo Metabolic and Hepatoprotective Activities
2.6. Effect of P. ovata Ethanolic Extract on Sex Hormones, Serum Insulin, and HOMA-IR in Rat LET-Induced PCOS
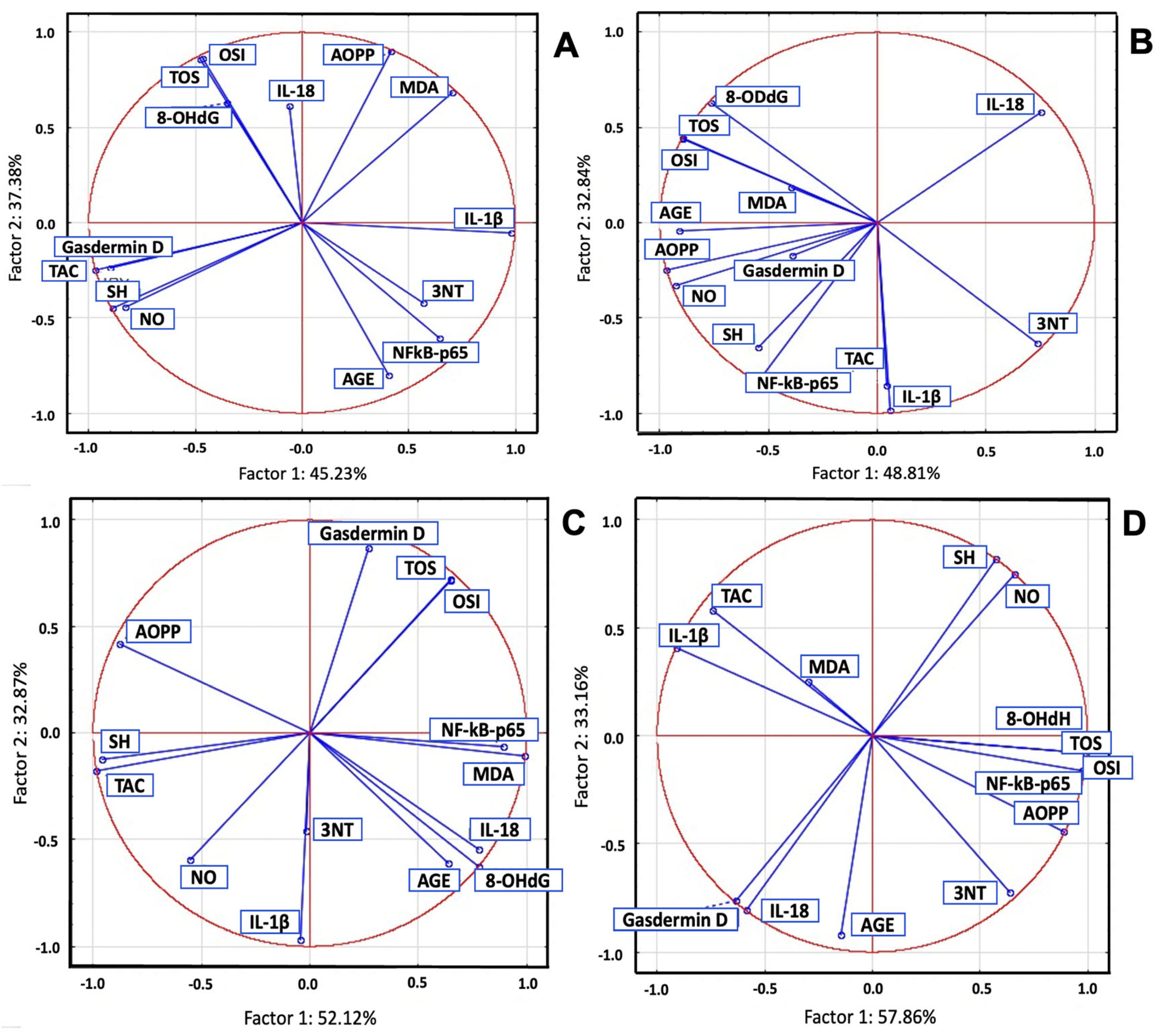
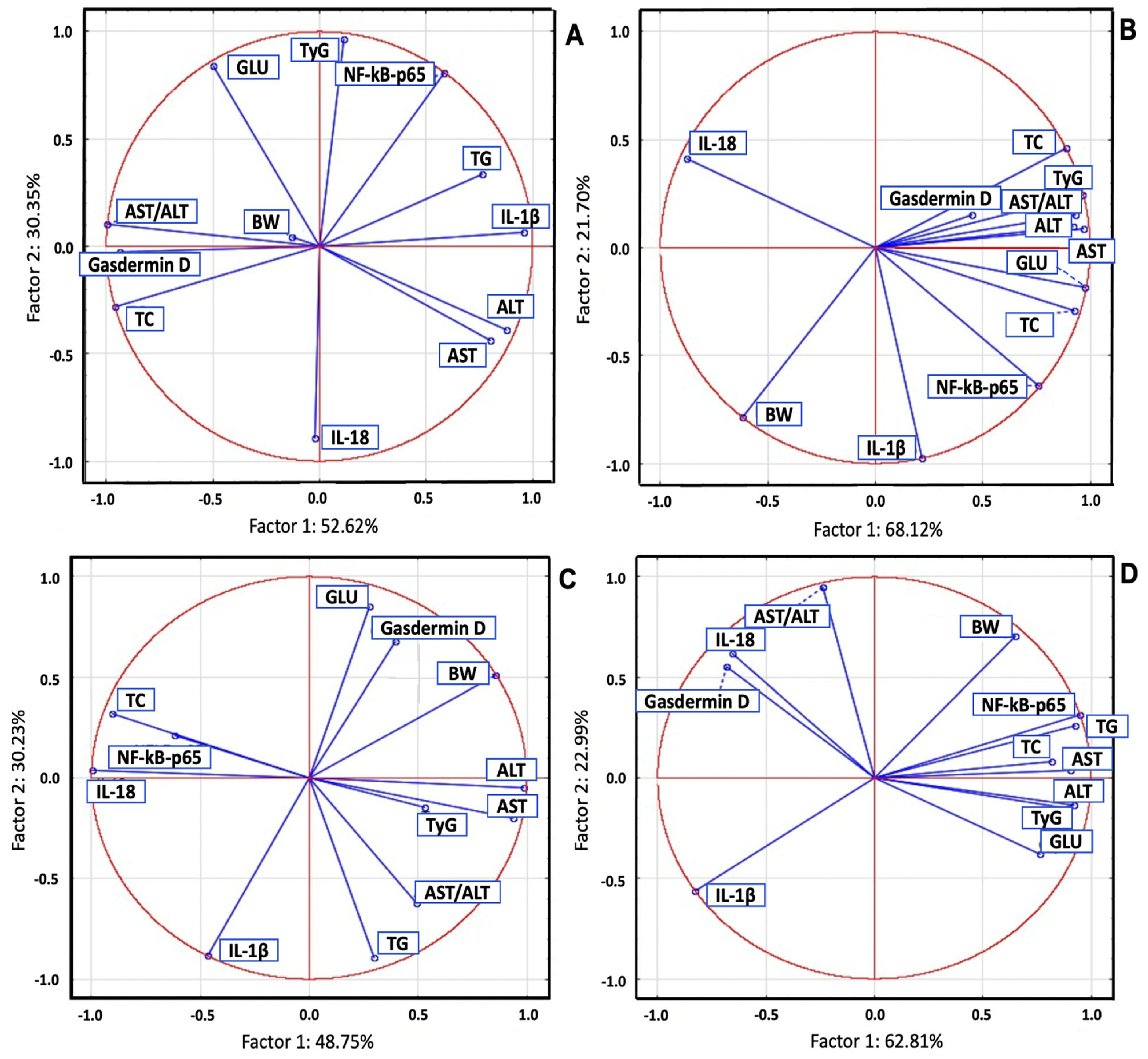
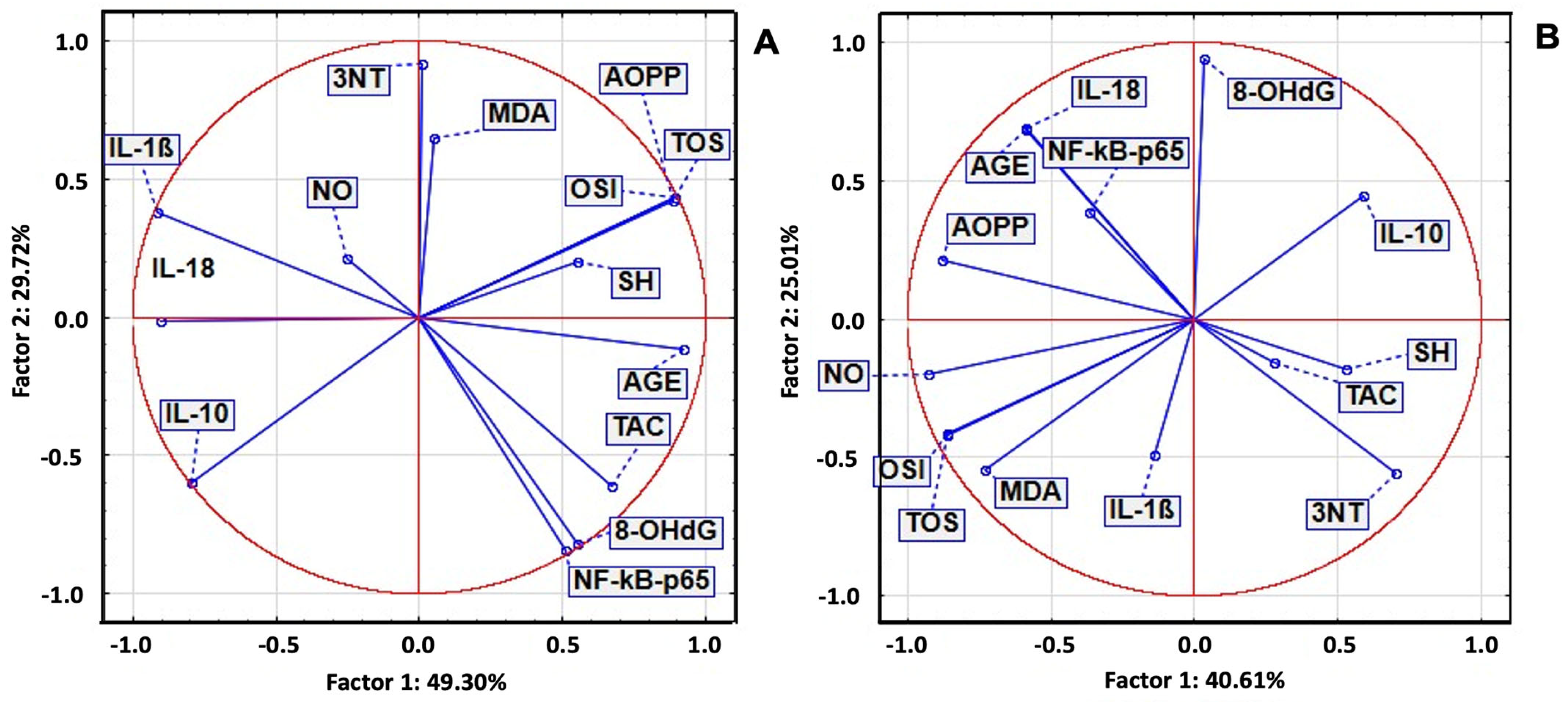
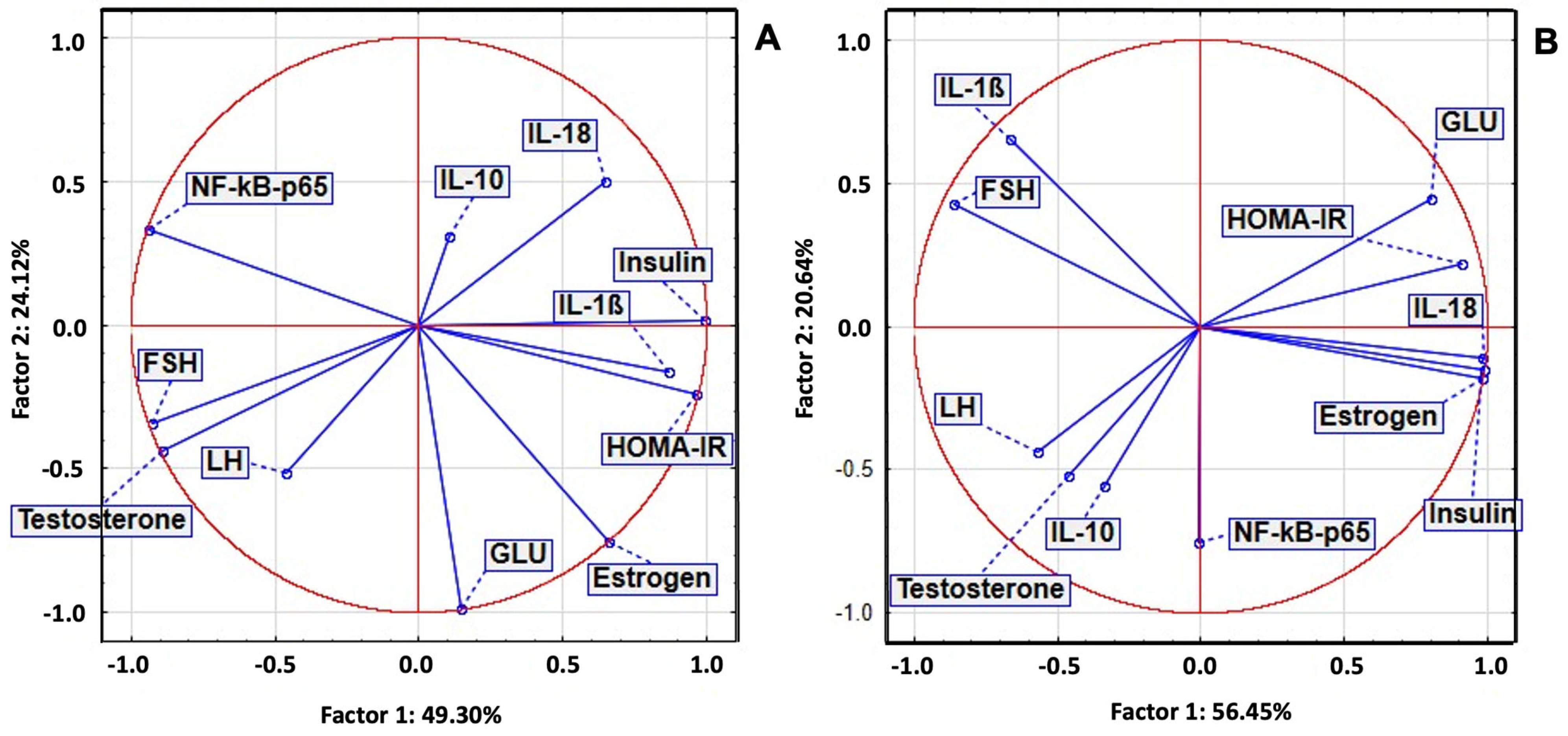
2.7. Principal Component Analysis of Serum Biomarkers
2.8. Histological and Follicular Changes in Letrozole-Induced Hyperandrogenized Rat Ovaries Following Treatment with P. ovata Ethanolic Extract
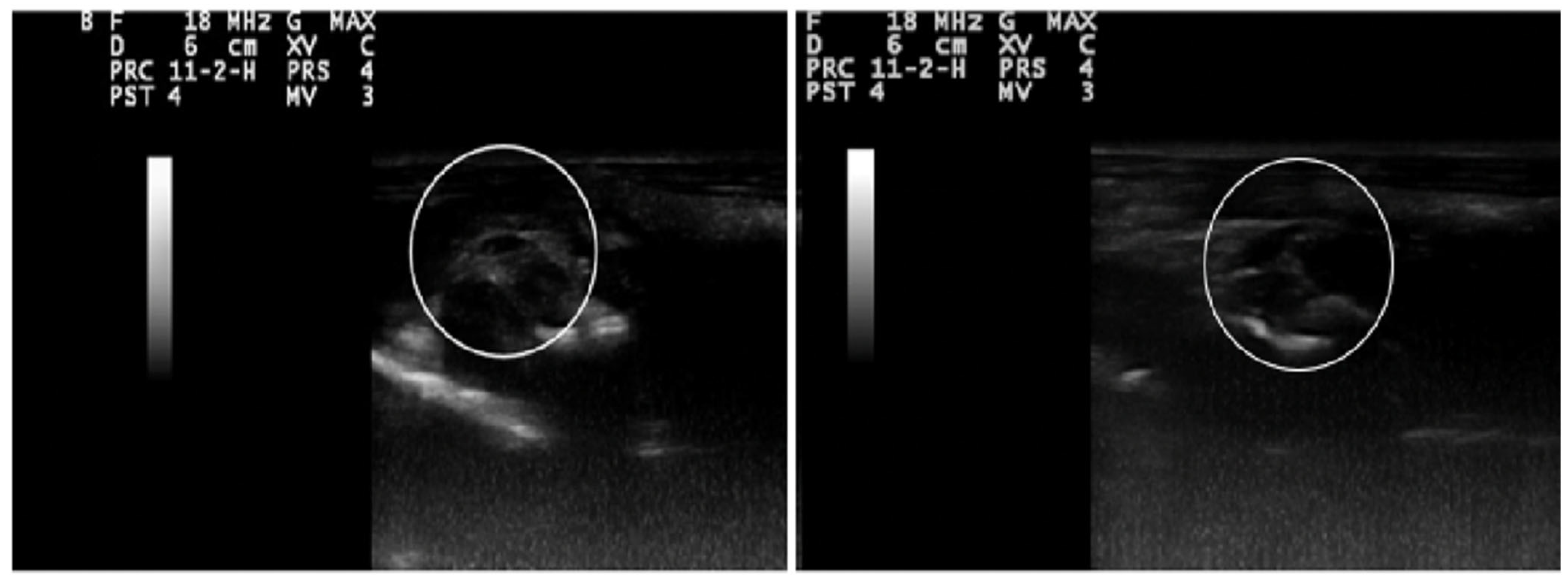
2.9. Ultrasonography Findings
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Extraction and Preparation of P. ovata Ethanol Extract
4.3. Quantification of Total Phenolic and Flavonoid Content
4.4. Chromatographic and Mass Spectrometric Profiling
4.5. Evaluation of In Vitro Antioxidant Activity
4.6. Experimental Design
- CONTROL: non-diabetic, untreated.
- STZ: diabetic control group (STZ 55 mg/kg, i.p.) [92].
- STZ + TX: diabetic rats treated with TX (20 mg/kg b.w.) [34].
- STZ + M: diabetic rats treated with metformin (100 mg/100 g b.w.) [91].
- POEE100%, POEE50%, and POEE25%: diabetic rats receiving 100%, 50%, and 25% dilutions of POEE, respectively.
- CONTROL: untreated, healthy control.
- LET: PCOS group receiving LET (1 mg/kg b.w., orally) [93].
- LET + TX: PCOS rats treated with TX (20 mg/kg b.w., orally).
- LET + M: PCOS rats treated with metformin (100 mg/100 g b.w., orally).
- LET + POEE100%: PCOS rats treated with 100% POEE dilution.
4.7. Pharmacological Studies
4.7.1. Evaluation of the Serum OS Markers
4.7.2. Assessment of Serum Inflammatory Biomarkers
4.7.3. Evaluation of the Blood Glucose, Lipid Profile, Triglyceride–Glucose Index, Liver Injury, and Anthropometric Parameters
4.7.4. Hormonal Assays
4.8. Histopathological Assessment of Reproductive Organs
4.9. Ultrasonographic Evaluation
4.10. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Diabetes Mellitus |
| PCOS | Polycystic ovary syndrome |
| POEE | Plantago ovata ethanol extract |
| STZ | Streptozotocin |
| LET | Letrozole |
| OS | Oxidative stress |
| ROS | Reactive oxygen species |
| TX | Trolox |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| TOS | Total oxidative status |
| TAC | Total antioxidant capacity |
| OSI | Oxidative stress index |
| MDA | Malondialdehyde |
| AOPPs | Advanced oxidation protein products |
| 8-OHdG | 8-Hydroxydeoxyguanosine |
| NO | Nitric oxide |
| 3-NT | 3-Nitrotyrosine |
| AGEs | Advanced glycation end products |
| SH | Total thiols |
| NF-kB-p65 | Nuclear Factor Kappa B-p65 |
| IL-1B | Interleukin-1 Beta |
| IL-18 | Interleukin-18. |
| IL-10 | Interleukin-10 |
| GLU | Blood glucose |
| LH | Luteinizing hormone |
| FSH | Follicle-stimulating hormone |
| TyG | Triglyceride–Glucose index |
| IR | Insulin resistance |
| HOMA-IR | Homeostatic Model Assessment for Insulin Resistance |
References
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Role of nitrosative stress in the pathogenesis of diabetic vascular dysfunction. Br. J. Pharmacol. 2009, 156, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Codner, E. Type 1 diabetes, obesity and PCOS: Is type 1 stepping into the shoes of type 2 diabetes? Clin. Endocrinol. 2021, 95, 265–266. [Google Scholar] [CrossRef]
- Kayama, Y.; Raaz, U.; Jagger, A.; Adam, M.; Schellinger, I.N.; Sakamoto, M.; Suzuki, H.; Toyama, K.; Spin, J.M.; Tsao, P.S. Diabetic Cardiovascular Disease Induced by Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 25234–25263. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A brief insight into the etiology, genetics, and immunology of polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef]
- Rudnicka, E.; Suchta, K.; Grymowicz, M.; Calik-Ksepka, A.; Smolarczyk, K.; Duszewska, A.M.; Smolarczyk, R.; Meczekalski, B. Chronic Low Grade Inflammation in Pathogenesis of PCOS. Int. J. Mol. Sci. 2021, 22, 3789. [Google Scholar] [CrossRef]
- Gholami, Z.; Clark, C.C.T.; Paknahad, Z. The effect of psyllium on fasting blood sugar, HbA1c, HOMA IR, and insulin control: A GRADE-assessed systematic review and meta-analysis of randomized controlled trials. BMC Endocr. Disord. 2024, 24, 82. [Google Scholar] [CrossRef]
- Dey, S.; Raychaudhuri, S.S. Selenium biofortification improves bioactive composition and antioxidant status in Plantago ovata Forsk., a medicinal plant. Genes Environ. 2023, 45, 38. [Google Scholar] [CrossRef]
- Patel, M.K.; Mishra, A.; Jaiswar, S.; Jha, B. Metabolic profiling and scavenging activities of developing circumscissile fruit of psyllium (Plantago ovata Forssk.) reveal variation in primary and secondary metabolites. BMC Plant Biol. 2020, 20, 116. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S.; Prodpran, T.; Nuthong, P. Effect of Psyllium (Plantago ovata Forks) Husk on Characteristics, Rheological and Textural Properties of Threadfin Bream Surimi Gel. Foods 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.R.; Gour, V.S.; Kothari, S.; Sharma, P.; Dar, K.B.; Ganie, S.A. Antioxidant, nutritional, structural, thermal and physico-chemical properties of psyllium (Plantago ovata) seeds. Curr. Res. Nutr. Food Sci. J. 2020, 8, 727–743. [Google Scholar] [CrossRef]
- Hawash, M.; Jaradat, N.; Elaraj, J.; Hamdan, A.; Lebdeh, S.A.; Halawa, T. Evaluation of the hypoglycemic effect of seven wild folkloric edible plants from Palestine. J. Complement. Integr. Med. 2019, 17, 20190032. [Google Scholar] [CrossRef] [PubMed]
- Banaszak, M.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. The Impact of Curcumin, Resveratrol, and Cinnamon on Modulating Oxidative Stress and Antioxidant Activity in Type 2 Diabetes: Moving beyond an Anti-Hyperglycaemic Evaluation. Antioxidants 2024, 13, 510. [Google Scholar] [CrossRef]
- Khedher, A.; Dhibi, S.; Bouzenna, H.; Akermi, S.; El Feki, A.; Teles, P.H.V.; Almeida, J.; Hfaiedh, N. Antiulcerogenic and antioxidant activities of Plantago ovata ethanolic extract in rats. Braz. J. Biol. 2022, 84, e255120. [Google Scholar] [CrossRef]
- Grubesić, R.J.; Vuković, J.; Kremer, D.; Vladimir-Knezević, S. Spectrophotometric method for polyphenols analysis: Prevalidation and application on Plantago L. species. J. Pharm. Biomed. Anal. 2005, 39, 837–842. [Google Scholar] [CrossRef]
- Iqbal, Y.; Ponnampalam, E.N.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Extraction and characterization of polyphenols from non-conventional edible plants and their antioxidant activities. Food Res. Int. 2022, 157, 111205. [Google Scholar] [CrossRef]
- Verhulst, E.P.; Brunton, N.P.; Rai, D.K. Polyphenols in Agricultural Grassland Crops and Their Health-Promoting Activities-A Review. Foods 2023, 12, 4122. [Google Scholar] [CrossRef]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Xu, D.; Hu, M.J.; Wang, Y.Q.; Cui, Y.L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-inflammatory effects of quercetin in a mouse model of MC903-induced atopic dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Haddad, P.S. The Antidiabetic Potential of Quercetin: Underlying Mechanisms. Curr. Med. Chem. 2017, 24, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, K.; Zhang, Y.; Li, H.; Li, A.; Xu, Y.; Wei, B. Quercetin liposomes ameliorate streptozotocin-induced diabetic nephropathy in diabetic rats. Sci. Rep. 2020, 10, 2440. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Song, W.; Liang, X.; Zhang, Q.; Shi, Y.; Liu, W.; Shi, X. Protective effect of quercetin on streptozotocin-induced diabetic peripheral neuropathy rats through modulating gut microbiota and reactive oxygen species level. Biomed. Pharmacother. 2020, 127, 110147. [Google Scholar] [CrossRef]
- Mihanfar, A.; Nouri, M.; Roshangar, L.; Khadem-Ansari, M.H. Therapeutic potential of quercetin in an animal model of PCOS: Possible involvement of AMPK/SIRT-1 axis. Eur. J. Pharmacol. 2021, 900, 174062. [Google Scholar] [CrossRef]
- Hua, Z.; Zhang, W.; Han, L.; Zhang, Y.; Jiang, X.; Ding, C. Kaempferol exerts antioxidant effects in age-related diminished ovarian reserve by regulating the HSP90/NRF2 pathway. Chem. Biol. Drug Des. 2024, 103, e14385. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Ali, M.; Hassan, M.; Ansari, S.A.; Alkahtani, H.M.; Al-Rasheed, L.S.; Ansari, S.A. Quercetin and Kaempferol as Multi-Targeting Antidiabetic Agents against Mouse Model of Chemically Induced Type 2 Diabetes. Pharmaceuticals 2024, 17, 757. [Google Scholar] [CrossRef]
- Alkhalidy, H.; Moore, W.; Wang, Y.; Luo, J.; McMillan, R.P.; Zhen, W.; Zhou, K.; Liu, D. The Flavonoid Kaempferol Ameliorates Streptozotocin-Induced Diabetes by Suppressing Hepatic Glucose Production. Molecules 2018, 23, 2338. [Google Scholar] [CrossRef]
- Kishore, L.; Kaur, N.; Singh, R. Effect of Kaempferol isolated from seeds of Eruca sativa on changes of pain sensitivity in Streptozotocin-induced diabetic neuropathy. Inflammopharmacology 2018, 26, 993–1003. [Google Scholar] [CrossRef]
- Zhou, Y.; Lan, H.; Dong, Z.; Li, W.; Qian, B.; Zeng, Z.; He, W.; Song, J.L. Rhamnocitrin Attenuates Ovarian Fibrosis in Rats with Letrozole-Induced Experimental Polycystic Ovary Syndrome. Oxid Med. Cell. Longev. 2022, 2022, 5558599. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Zou, H.; Hao, J.Y.; Huang, Y.; Zhang, J.N.; Xu, X.H.; Li, J. Metformin inhibits ovarian granular cell pyroptosis through the miR-670-3p/NOX2/ROS pathway. Aging 2023, 15, 4429–4443. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- But, A.E.; Pop, R.M.; Binsfeld, G.F.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Morar, I.I.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; et al. The Phytochemical Composition and Antioxidant Activity of Matricaria recutita Blossoms and Zingiber officinale Rhizome Ethanol Extracts. Nutrients 2024, 17, 5. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Overview of oxidative stress and inflammation in diabetes. J. Diabetes 2024, 16, e70014. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Al-Awar, A.; Kupai, K.; Veszelka, M.; Szűcs, G.; Attieh, Z.; Murlasits, Z.; Török, S.; Pósa, A.; Varga, C. Experimental Diabetes Mellitus in Different Animal Models. J. Diabetes Res. 2016, 2016, 9051426. [Google Scholar] [CrossRef]
- Usatiuc, L.O.; Pârvu, M.; Pop, R.M.; Uifălean, A.; Vălean, D.; Szabo, C.E.; Țicolea, M.; Cătoi, F.A.; Ranga, F.; Pârvu, A.E. Phytochemical Profile and Antidiabetic, Antioxidant, and Anti-Inflammatory Activities of Gypsophila paniculata Ethanol Extract in Rat Streptozotocin-Induced Diabetes Mellitus. Antioxidants 2024, 13, 1029. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Lin, H.; Ao, H.; Guo, G.; Liu, M. The Role and Mechanism of Metformin in Inflammatory Diseases. J. Inflamm. Res. 2023, 16, 5545–5564. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.Y.; Bolatai, A.; Wu, N. Progress in Research on Biomarkers of Gestational Diabetes Mellitus and Preeclampsia. Diabetes Metab. Syndr. Obes. 2023, 16, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Al-Aubaidy, H.A.; Dayan, A.; Deseo, M.A.; Itsiopoulos, C.; Jamil, D.; Hadi, N.R.; Thomas, C.J. Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus. Nutrients 2021, 13, 1133. [Google Scholar] [CrossRef] [PubMed]
- Alu, S.N.; Los, E.A.; Ford, G.A.; Stone, W.L. Oxidative Stress in Type 2 Diabetes: The Case for Future Pediatric Redoxomics Studies. Antioxidants 2022, 11, 1336. [Google Scholar] [CrossRef]
- Król, M.; Kepinska, M. Human Nitric Oxide Synthase-Its Functions, Polymorphisms, and Inhibitors in the Context of Inflammation, Diabetes and Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 22, 56. [Google Scholar] [CrossRef]
- Adebayo, S.A.; Ondua, M.; Shai, L.J.; Lebelo, S.L. Inhibition of nitric oxide production and free radical scavenging activities of four South African medicinal plants. J. Inflamm. Res. 2019, 12, 195–203. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Panda, A.; Raj, N.; Shrivastava, A.; Prathani, R. The evaluation of nitric oxide scavenging activity of Acalypha indica Linn root. Asian J. Res. Chem. 2009, 2, 148–150. [Google Scholar]
- Murri, M.; Luque-Ramírez, M.; Insenser, M.; Ojeda-Ojeda, M.; Escobar-Morreale, H.F. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Hum. Reprod. Update 2013, 19, 268–288. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M.; Schmidt, A.M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Gelen, V.; Kükürt, A.; Deveci, H.A.; Faruk Başer, Ö.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. In Accenting Lipid Peroxidation; Atukeren, P., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef]
- Al Mamun, A.; Shao, C.; Geng, P.; Wang, S.; Xiao, J. The Mechanism of Pyroptosis and Its Application Prospect in Diabetic Wound Healing. J. Inflamm. Res. 2024, 17, 1481–1501. [Google Scholar] [CrossRef]
- Wu, J.; Lv, T.; Liu, Y.; Liu, Y.; Han, Y.; Liu, X.; Peng, X.; Tang, F.; Cai, J. The role of quercetin in NLRP3-associated inflammation. Inflammopharmacology 2024, 32, 3585–3610. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Liu, L.; Gong, Y.; Peng, S.; Yan, X.; Bai, M.; Xu, E.; Li, Y. Kaempferol improves depression-like behaviors through shifting microglia polarization and suppressing NLRP3 via tilting the balance of PPARγ and STAT1 signaling. Int. Immunopharmacol. 2024, 143 Pt 3, 113538. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.Y.; Zhao, Z.C.; Liu, X.L.; Peng, Y.G.; Zhu, H.M.; Yan, S.F.; Liu, Y.J.; Xie, Q.; Jiang, Y.; Zeng, S.Z. Metformin Mitigates Sepsis-Induced Acute Lung Injury and Inflammation in Young Mice by Suppressing the S100A8/A9-NLRP3-IL-1β Signaling Pathway. J. Inflamm. Res. 2024, 17, 3785–3799. [Google Scholar] [CrossRef] [PubMed]
- Hannan, J.M.; Ali, L.; Khaleque, J.; Akhter, M.; Flatt, P.R.; Abdel-Wahab, Y.H. Aqueous extracts of husks of Plantago ovata reduce hyperglycaemia in type 1 and type 2 diabetes by inhibition of intestinal glucose absorption. Br. J. Nutr. 2006, 96, 131–137. [Google Scholar] [CrossRef]
- Rivers, C.R.; Kantor, M.A. Psyllium husk intake and risk of type 2 diabetes: An evidence-based scientific and regulatory review of a qualified health claim conducted by the US Food and Drug Administration. Nutr. Rev. 2020, 78, 787–797. [Google Scholar] [CrossRef]
- Gibb, R.D.; McRorie, J.W., Jr.; Russell, D.A.; Hasselblad, V.; D’Alessio, D.A. Psyllium fiber improves glycemic control proportional to loss of glycemic control: A meta-analysis of data in euglycemic subjects, patients at risk of type 2 diabetes mellitus, and patients being treated for type 2 diabetes mellitus. Am. J. Clin. Nutr. 2015, 102, 1604–1614. [Google Scholar] [CrossRef]
- McRae, M.P. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef]
- Sun, C.; Wang, L.; Sun, J.; Wang, Z.; Tang, Z. Hypoglycemic and hypolipidemic effects of rutin on hyperglycemic rats. J. Tradit. Chin. Med. 2020, 40, 640–645. [Google Scholar] [CrossRef]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.M.A.; Abdel-Wahab, Y.H.A. Therapeutic Potential of Quercetin in the Management of Type-2 Diabetes Mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, W.; Yun, J.M. Luteolin inhibits hyperglycemia-induced proinflammatory cytokine production and its epigenetic mechanism in human monocytes. Phytother. Res. 2014, 28, 1383–1391. [Google Scholar] [CrossRef]
- Jafari Azad, B.; Yaseri, M.; Daneshzad, E.; Koohdani, F. Interaction between Apo A-II -265T>C polymorphism and dietary total antioxidant capacity on some anthropometric indices and serum lipid profile in patients with type 2 diabetes mellitus. J. Nutr. Sci. 2021, 10, e9. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ji, H.; Sun, W.; An, X.; Lian, F. Triglyceride glucose (TyG) index: A promising biomarker for diagnosis and treatment of different diseases. Eur. J. Intern. Med. 2025, 131, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Teck, J. Diabetes-Associated Comorbidities. Prim. Care Clin. Off. Pract. 2022, 49, 275–286. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F.; Roldán-Martín, M.B. Type 1 Diabetes and Polycystic Ovary Syndrome: Systematic Review and Meta-analysis. Diabetes Care 2016, 39, 639–648. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Cacciapuoti, N.; Lonardo, M.S.; Nasti, G.; Gautiero, C.; Belfiore, A.; Guida, B.; Chiurazzi, M. Pathophysiology and Nutritional Approaches in Polycystic Ovary Syndrome (PCOS): A Comprehensive Review. Curr. Nutr. Rep. 2023, 12, 527–544. [Google Scholar] [CrossRef]
- Almeida Balieiro, C.C.; Hespanhol, L.C.; Mendes Fonseca, L.; Wantowski, S.; Freitas, M.A.A.; Dias, Y.J.M.; Burlá, M.M.; Maria Lima de Oliveira, L. Effects of polyphenol in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol Reprod. Biol. 2024, 294, 84–91. [Google Scholar] [CrossRef]
- Marouf, B.H.; Ismaeel, D.O.; Hassan, A.H.; Ali, O.J. Therapeutic Effects of Silibinin Against Polycystic Ovary Syndrome Induced by Letrozole in Rats via Its Potential Anti-Inflammatory and Anti-Oxidant Activities. J. Inflamm. Res. 2022, 15, 5185–5199. [Google Scholar] [CrossRef]
- Joshi, A. PCOS stratification for precision diagnostics and treatment. Front. Cell Dev. Biol. 2024, 12, 1358755. [Google Scholar] [CrossRef]
- Real, M.; Molina-Molina, J.M.; Jimenez, J.; Diéguez, H.R.; Fernández, M.F.; Olea, N. Assessment of hormone-like activities in Ginkgo biloba, Elettaria cardamomum and Plantago ovata extracts using in vitro receptor-specific bioassays. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 1531–1541. [Google Scholar] [CrossRef]
- Galisteo, M.; Morón, R.; Rivera, L.; Romero, R.; Anguera, A.; Zarzuelo, A. Plantago ovata husks-supplemented diet ameliorates metabolic alterations in obese Zucker rats through activation of AMP-activated protein kinase. Comparative study with other dietary fibers. Clin. Nutr. 2010, 29, 261–267. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-Enriched Green Rooibos Extract Reduces Hepatic Insulin Resistance by Modulating PI3K/AKT and AMPK Pathways. Int. J. Mol. Sci. 2019, 20, 663. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wang, C.; Jin, Y.; Meng, Q.; Wu, J.; Sun, H. Kaempferol-induced GPER upregulation attenuates atherosclerosis via the PI3K/AKT/Nrf2 pathway. Pharm. Biol. 2021, 59, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, X.; Wang, Y.; Wang, Y.; Zhou, Y.; Li, F.; Hou, X.; Xia, D. Dietary kaempferol exerts anti-obesity effects by inducing the browing of white adipocytes via the AMPK/SIRT1/PGC-1α signaling pathway. Curr. Res. Food Sci. 2024, 8, 100728. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Ishfaq, M.; Han, Y.; Zhang, X.; Li, X.; Wang, B.; Lu, X.; Gao, B. Quercetin and AMPK: A Dynamic Duo in Alleviating MG-Induced Inflammation via the AMPK/SIRT1/NF-κB Pathway. Molecules 2023, 28, 7388. [Google Scholar] [CrossRef]
- Zheng, S.; Ma, M.; Chen, Y.; Li, M. Effects of quercetin on ovarian function and regulation of the ovarian PI3K/Akt/FoxO3a signalling pathway and oxidative stress in a rat model of cyclophosphamide-induced premature ovarian failure. Basic Clin. Pharmacol. Toxicol. 2022, 130, 240–253. [Google Scholar] [CrossRef]
- Huang, M.; Liu, J.; Lin, X.; Goto, A.; Song, Y.; Tinker, L.F.; Chan, K.K.; Liu, S. Relationship between dietary carbohydrates intake and circulating sex hormone-binding globulin levels in postmenopausal women. J. Diabetes 2018, 10, 467–477. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.; Cao, J.; Chen, Y.; Dong, Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018, 16, 80. [Google Scholar] [CrossRef]
- Dong, X.C.; Liu, C.; Zhuo, G.C.; Ding, Y. Potential Roles of mtDNA Mutations in PCOS-IR: A Review. Diabetes Metab. Syndr. Obes. 2023, 16, 139–149. [Google Scholar] [CrossRef]
- Gleicher, N.; Darmon, S.; Patrizio, P.; Barad, D.H. Reconsidering the Polycystic Ovary Syndrome (PCOS). Biomedicines 2022, 10, 1505. [Google Scholar] [CrossRef]
- Wang, J.; Yin, T.; Liu, S. Dysregulation of immune response in PCOS organ system. Front. Immunol. 2023, 14, 1169232. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Cooney, L.G.; Stanic, A.K. Immune Dysfunction in Polycystic Ovary Syndrome. Immunohorizons 2023, 7, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qu, Y.; Jiang, H.; Wang, H.; Pan, Y.; Zhang, Y.; Wu, X.; Han, Y.; Zhang, Y. Therapeutic effect and safety of curcumin in women with PCOS: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 1051111. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini, E.S.L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; Cincotto Dos Santos Bueno, P. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef]
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.C.; Li, S.; Zhan, J.; Ho, C.T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Vodnar, D.C.; Bischin, C.; Hanganu, D.; Gheldiu, A.M.; Oprean, R.; Silaghi-Dumitrescu, R.; Crișan, G. Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense Mill. leaves. Molecules 2014, 19, 10056–10073. [Google Scholar] [CrossRef]
- Babotă, M.; Mocan, A.; Vlase, L.; Crișan, O.; Ielciu, I.; Gheldiu, A.M.; Vodnar, D.C.; Crișan, G.; Păltinean, R. Phytochemical Analysis, Antioxidant and Antimicrobial Activities of Helichrysum arenarium (L.) Moench. and Antennaria dioica (L.) Gaertn. Flowers. Molecules 2018, 23, 409. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Patel, A.; Patel, N.M. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea linn leaves (Leguminosae). Pharmacogn. Res. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Al-Majedy, Y.K.; Kadhum, A.A.; Mohamad, A.B. Hydrogen Peroxide Scavenging Activity of Novel Coumarins Synthesized Using Different Approaches. PLoS ONE 2015, 10, e0132175. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: A practical guide. Excli J. 2023, 22, 274–294. [Google Scholar] [CrossRef] [PubMed]
- Ragy, M.M.; Abdel-Hamid, H.A.; Toni, N.D.M. Pathophysiological changes in experimental polycystic ovary syndrome in female albino rats: Using either hemin or L-arginine. J. Cell. Physiol. 2019, 234, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Morar, I.I.; Pop, R.M.; Peitzner, E.; Ranga, F.; Orăsan, M.S.; Cecan, A.D.; Chera, E.I.; Bonci, T.I.; Usatiuc, L.O.; Țicolea, M.; et al. Phytochemical Composition and Antioxidant Activity of Manuka Honey and Ohia Lehua Honey. Nutrients 2025, 17, 276. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, M.A.; Mendoza-Núñez, V.M. Oxidative Stress Indexes for Diagnosis of Health or Disease in Humans. Oxid Med. Cell. Longev. 2019, 2019, 4128152. [Google Scholar] [CrossRef]
- Poimenova, I.A.; Sozarukova, M.M.; Ratova, D.-M.V.; Nikitina, V.N.; Khabibullin, V.R.; Mikheev, I.V.; Proskurnina, E.V.; Proskurnin, M.A. Analytical Methods for Assessing Thiol Antioxidants in Biological Fluids: A Review. Molecules 2024, 29, 4433. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Tsikas, D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: Appraisal of the Griess reaction in the l-arginine/nitric oxide area of research. J. Chromatogr. B 2007, 851, 51–70. [Google Scholar] [CrossRef]
- Xiao, L.-L.; Zhang, F.; Zhao, Y.-L.; Zhang, L.-J.; Xie, Z.-Y.; Huang, K.-Z.; Ouyang, X.-X.; Wu, X.-X.; Xu, X.-W.; Li, L.-J. Using advanced oxidation protein products and ischaemia-modified albumin to monitor oxidative stress levels in patients with drug-induced liver injury. Sci. Rep. 2020, 10, 18128. [Google Scholar] [CrossRef]
- Er, L.K.; Wu, S.; Chou, H.H.; Hsu, L.A.; Teng, M.S.; Sun, Y.C.; Ko, Y.L. Triglyceride Glucose-Body Mass Index Is a Simple and Clinically Useful Surrogate Marker for Insulin Resistance in Nondiabetic Individuals. PLoS ONE 2016, 11, e0149731. [Google Scholar] [CrossRef]
- Salgado, A.L.; Carvalho, L.; Oliveira, A.C.; Santos, V.N.; Vieira, J.G.; Parise, E.R. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq. Gastroenterol. 2010, 47, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, T.; Horváth, G.; Papp, N. Variability of total flavonoid, polyphenol and tannin contents in some Lythrum salicaria populations. Nat. Prod. Commun. 2011, 6, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Sumitra, M.; Manikandan, P.; Rao, K.V.; Nayeem, M.; Manohar, B.M.; Puvanakrishnan, R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar rats—A comparative analysis. Life Sci. 2004, 75, 1887–1896. [Google Scholar] [CrossRef]
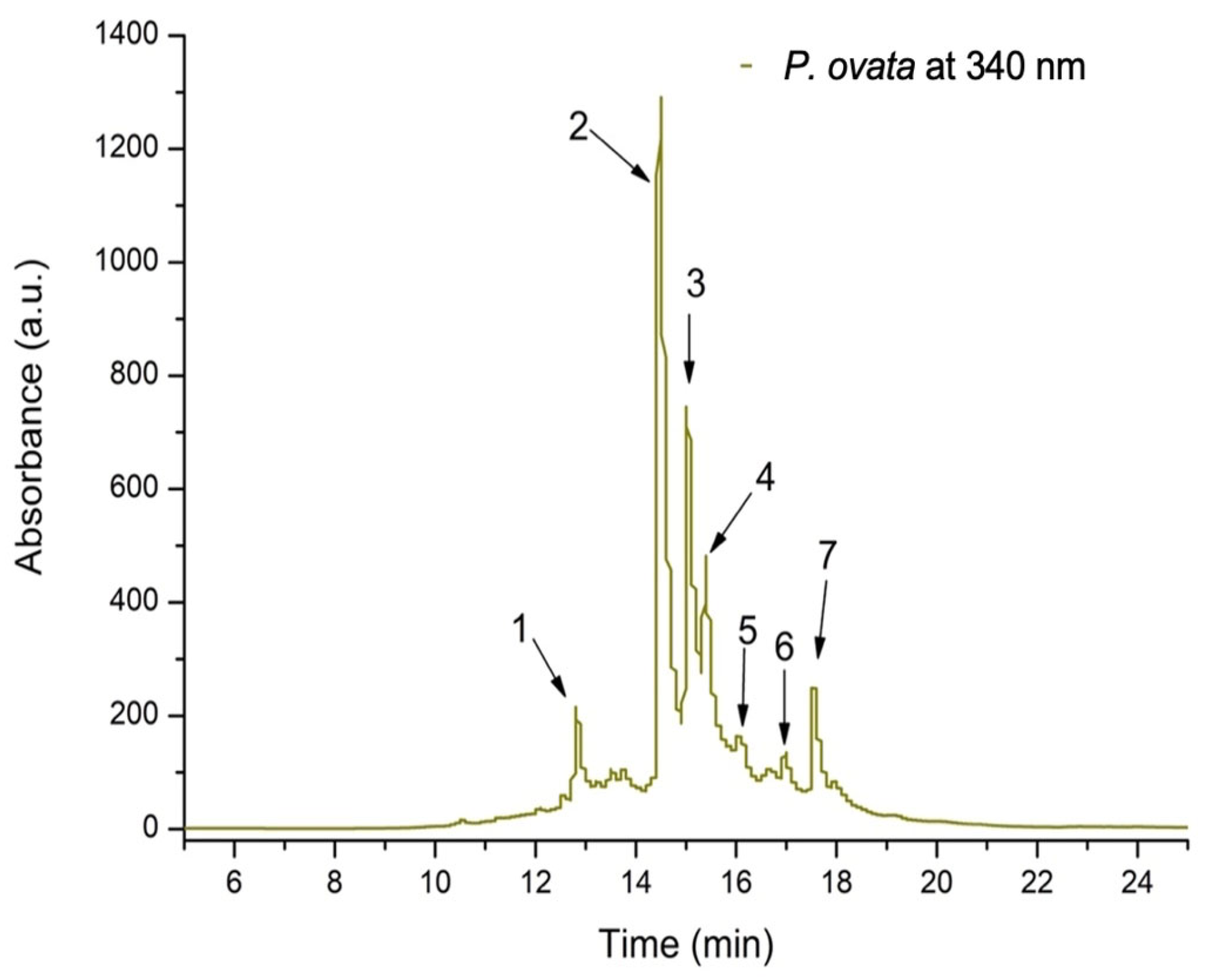
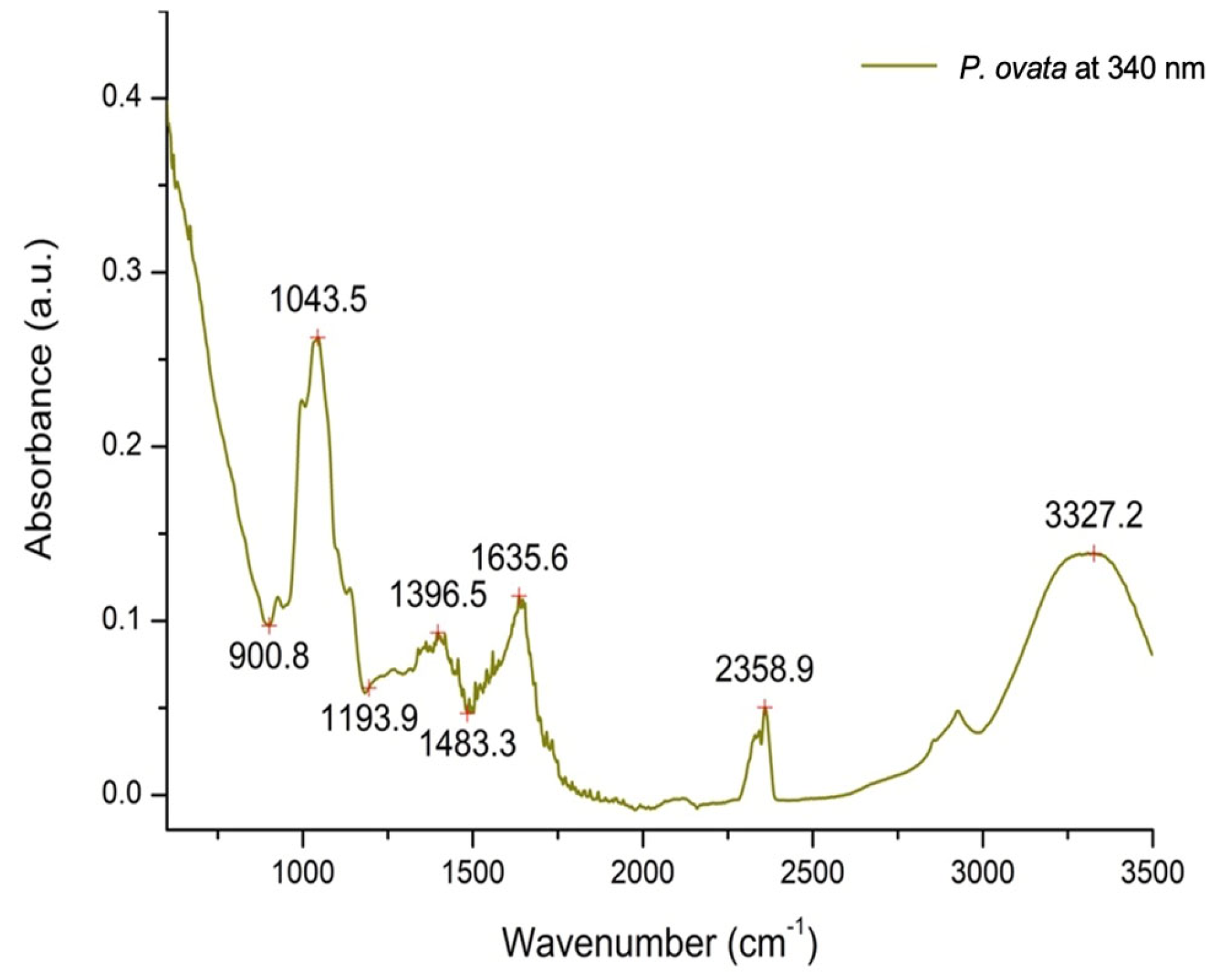

| Peak No. | Rt (min) | UV λmax (nm) | [M + H]+ (m/z) | Compound | Subclass | Quantity * (μg/mL) |
|---|---|---|---|---|---|---|
| 1 | 12.85 | 350,255 | 595,287 | Kaempferol-rutinoside | Flavonol | 98.695 ± 6.23 |
| 2 | 14.51 | 355,250 | 743,303 | Quercetin-xylosyl-rutinoside | Flavonol | 419.322 ± 25.98 |
| 3 | 15.04 | 355,250 | 611,303 | Quercetin-rutinoside (Rutin) | Flavonol | 259.952 ± 8.34 |
| 4 | 15.39 | 355,250 | 465,303 | Quercetin-glucoside | Flavonol | 225.034 ± 17.11 |
| 5 | 16.12 | 345,250 | 611,287 | Luteolin-diglucoside | Flavone | 36.901 ± 0.78 |
| 6 | 16.97 | 355,250 | 479,303 | Quercetin-glucuronide | Flavonol | 73.309 ± 4.65 |
| 7 | 17.76 | 350,255 | 611,287 | Kaempferol-diglucoside | Flavonol | 86.271 ± 7.43 |
| Sample | DPPH (μgTE/mL) | H2O2 Scavenging Activity (μgTE/mL) | NO Scavenging Activity (μgQE/mL) | FRAP (mgTE/mL) |
|---|---|---|---|---|
| P. ovata IC50 | 99.86 ± 4.32 | 87.06 ± 2.89 | 121.07 ± 16.22 | 92.58 ± 5.14 |
| TX IC50 | 11.2 ± 1.7 | 24.23 ± 3.12 | - | 12.07 ± 2.04 |
| Quercetin IC50 | - | - | 20.58 ± 3.67 | - |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 |
| Parameters | Control | STZ | STZ + POEE100% | STZ + POEE50% | STZ + POEE25% | STZ + M | STZ + TX |
|---|---|---|---|---|---|---|---|
| TOS (μM/L) | 18.11 ± 3.18 | 40.85 ± 3.18 aaa | 15.35 ± 3.18 bbb,ccc | 21.43 ± 3.18 bbb | 36.98 ± 3.18 b | 23.17 ± 3.18 bbb | 25.18 ± 11.55 bbb |
| TAC (mM/L) | 1.09 ± 0.001 | 1.084 ± 0.0014 a | 1.089 ± 0.001 bbb,c | 1.088 ± 0.001 bbb,c | 1.09 ± 0.0001 bbb | 1.091 ± 0.001 bb | 1.09 ± 0.001 b |
| OSI | 120.96 ± 2.53 | 37.67 ± 2.92 aaa | 14.08 ± 2.92 bbb | 19.47 ± 2.92 bbb,cc | 33.25 ± 2.92 b | 21.24 ± 2.92 bbb | 24.30 ± 12.54 b |
| MDA (nM/L) | 2.33 ± 0.04 | 3.53 ± 0.04 aaa | 2.31 ± 0.04 bbb | 2.58 ± 0.04 bbb | 2.86 ± 0.04 bbb | 2.13 ± 0.04 bbb | 2.20 ± 0.18 bbb |
| AOPP (μM/L) | 140.40 ± 7.77 | 166.66 ± 7.77 aaa | 118.93 ± 7.77 bbb,cc,dd | 121.67 ± 7.77 bbb,cc,dd | 139.11 ± 7.77 b,c,d | 161.59 ± 26.19 | 166.08 ± 36.79 |
| 8-OHdG (ng/mL) | 38.04 ± 12.36 | 45.45 ± 12.36 a | 23.68 ± 12.36 bb,c | 17.80 ± 12.36 bb,cc,dd | 30.20 ± 12.36 b | 44.63 ± 12.36 | 41.71 ± 31.66 |
| AGEs (ng/mL) | 11.18 ± 2.88 | 14.71 ± 2.88 a | 12.42 ± 2.88 b | 14.07 ± 2.88 cc | 13.42 ± 2.88 c | 11.01 ± 2.88 bb | 14.76 ± 2.12 |
| NOx (μM/L) | 31.38 ± 5.33 | 60.58 ± 5.33 aaa | 44.48 ± 5.33 bb,cc | 42.44 ± 5.33 bb,cc | 49.49 ± 5.33 | 57.83 ± 5.33 | 58.19 ± 5.45 |
| 3NT (ng/mL) | 36.86 ± 12.28 | 58.82 ± 12.28 a | 21.00 ± 12.28 bbb,cc,ddd | 19.27 ± 12.28 bbb,cc,ddd | 27.80 ± 12.28 bbb,cc,dd | 44.54 ± 12.28 b | 64.71 ± 46.28 |
| SH (μM/L) | 322.84 ± 14.47 | 253.13 ± 14.47 a | 387.77 ± 14.47 bb,cc,d | 414.63 ± 14.47 bb | 254.83 ± 14.47 cc,dd | 507.52 ± 150.89 bbb | 426.53 ± 109.40 bb |
| Parameters | Control | LET | LET + POEE100% | LET + M | LET + TX |
|---|---|---|---|---|---|
| TOS (μM/L) | 4.80 ± 0.46 | 14.40 ± 0.88 aaa | 12.29 ± 2.93 b,ddd | 13.15 ± 4.41 | 4.55 ± 0.36 bbb |
| TAC (mM/L) | 1.09 ± 0.0001 | 1.079 ± 0.0001 aaa | 1.083 ± 0.0001 bbb | 1.09 ± 0.0001 bbb | 1.08 ± 0.0001 bbb |
| OSI | 4.44 ± 0.34 | 12.45 ± 1.42 aaa | 11.18 ± 1.12 b,ddd | 12.43 ± 3.74 | 4.24 ± 0.28 bbb |
| MDA (nM/L) | 2.12 ± 0.31 | 3.84 ± 0.20 aaa | 2.78 ± 0.35 bb | 2.96 ± 0.27 bbb | 2.46 ± 0.44 bbb |
| AOPP (μM/L) | 57.54 ± 4.32 | 97.64 ± 8.05 aaa | 80.64 ± 8.20 bb | 77.88 ± 12.16 bb | 84.10 ± 12.25 b |
| 8-OHdG (ng/mL) | 109.05 ± 2.30 | 148.34 ± 48.09 aa | 48.97 ± 14.61 bbb,c | 75.53 ± 24.30 bb | 37.67 ± 8.75 bbb |
| AGEs (ng/mL) | 20.54 ± 10.79 | 30.69 ± 7.69 a | 18.81 ± 6.09 | 18.39 ± 1.12 | 15.64 ± 6.20 |
| NOx (μM/L) | 35.38 ± 5.48 | 63.89 ± 2.55 aaa | 60.00 ± 11.90 bb,c | 64.68 ± 7.33 | 64.73 ± 10.23 |
| 3NT (ng/mL) | 28.24 ± 7.12 | 59.07 ± 32.99 aa | 59.60 ± 31.33 | 65.70 ± 37.60 | 51.46 ± 34.43 |
| SH (μM/L) | 363.83 ± 27.24 | 304.70 ± 31.11 aaa | 319.04 ± 6.70 | 351.83 ± 41.71 b | 355.20 ± 62.51 b |
| Parameters | Control | STZ | STZ + POEE100% | STZ + POEE50% | STZ + POEE25% | STZ + M | STZ + TX |
|---|---|---|---|---|---|---|---|
| NFkB-p65 (pg/mL) | 165.37 ± 32.29 | 314.29 ± 59.94 aaa | 196.73 ± 55.44 bb,c,d | 231.17 ± 42.75 b | 256.67 ± 43.79 b | 230.48 ± 18.46 b | 223.32 ± 34.26 b |
| IL-1B (pg/mL) | 24.38 ± 3.92 | 132.33 ± 20.18 aaa | 19.62 ± 2.66 bbb,ddd | 20.85 ± 5.54 bbb,d | 26.49 ± 18.77 bbb | 19.61 ± 3.93 bbb | 27.91 ± 3.15 bbb |
| IL-18 (pg/mL) | 17.28 ± 8.12 | 60.12 ± 10.88 aaa | 0.01 ± 0.0002 bbb,ccc,ddd | 0.012 ± 0.0001 bbb,ccc,ddd | 0.0109 ± 0.0003 bbb,ccc,ddd | 11.50 ± 1.28 bbb | 12.182 ± 0.55 bbb |
| Gasdermin (ng/mL) | 6.002 ± 1.05 | 9.84 ± 1.27 aa | 5.25 ± 0.43 bb,cc | 5.77 ± 0.97 bb,cc | 6.13 ± 1.04 | 9.29 ± 1.05 | 6.51 ± 1.35 |
| Parameters | Control | LET | LET + POEE100% | LET + M | LET + TX |
|---|---|---|---|---|---|
| NFkB-p65 (pg/mL) | 258.35 ± 48.76 | 520.89 ± 73.55 aa | 281.30 ± 71.59 bb,cc | 541.52 ± 93.95 | 268.39 ± 90.45 bb |
| IL-1B (pg/mL) | 24.99 ± 1.60 | 44.06 ± 10.21 aa | 24.43 ± 3.66 bb | 22.17 ± 3.35 bb | 27.15 ± 0.89 bb |
| IL-18 (pg/mL) | 19.47 ± 2.48 | 33.20 ± 7.30 aa | 17.76 ± 3.67 bb | 16.03 ± 1.08 bbb | 20.92 ± 6.16 b |
| IL-10 (ng/mL) | 15.79 ± 2.19 | 29.66 ± 5.81 aaa | 16.01 ± 2.25 bbb,dd | 14.44 ± 2.77 bbb | 23.00 ± 1.46 b |
| Parameters | Control | STZ | STZ + POEE100% | STZ + POEE50% | STZ + POEE25% | STZ + M | STZ + TX |
|---|---|---|---|---|---|---|---|
| GLU (mg/dL) | 102.91 ± 8.39 | 426.76 ± 26.71 aaa | 360.51 ± 38.69 bb | 411.98 ± 36.79 | 422.25 ± 56.36 | 370.72 ± 49.08 bb | 412.5 ± 139.36 |
| TG (mg/dL) | 66.10 ± 23.86 | 129.26 ± 6.15 aaa | 92.96 ± 19.59 b,ccc,dd | 102.99 ± 12.25 b,cc,d | 113.00 ± 12.41 c,d | 133.09 ± 5.33 | 116.62 ± 7.53 b |
| TyG index | 4.38 ± 0.12 | 5.46 ± 0.11 aa | 5.20 ± 0.11 b | 5.32 ± 0.65 bb | 5.38 ± 0.13 | 5.40 ± 0.06 | 5.38 ± 0.11 |
| TC (mg/dL) | 72.97 ± 12.33 | 123.51 ± 8.21 aaa | 72.95 ± 12.09 bbb,ccc | 98.03 ± 12.12 bbb | 105.83 ± 6.99 bbb,c | 98.06 ± 12.27 bbb,dd | 112.36 ± 12.75 |
| AST (U/L) | 67.51 ± 15.04 | 156.8 ± 16.04 aaa | 119.06 ± 34.73 bb | 114.78 ± 20.13 bb | 125.61 ± 25.35 | 125.42 ± 24.38 | 131.07 ± 28.54 |
| ALT (U/L) | 62.87 ± 14.38 | 159.92 ± 18.24 aaa | 104.18 ± 32.003 bb | 117.48 ± 22.41 bb | 130.55 ± 24.53 | 126.76 ± 16.03 b | 137.79 ± 42.05 |
| AST/ALT | 1.08 ± 0.10 | 0.98 ± 0.03 aa | 1.04 ± 0.09 | 0.98 ± 0.18 | 0.96 ± 0.09 | 0.98 ± 0.18 | 0.94 ± 0.102 |
| BW (g) change | 15.14 ± 3.30 | 104.16 ± 26.62 aaa | 33.54 ± 13.46 bb | 64.63 ± 20.03 b,cc | 67.39 ± 22.46 b,cc | 27.39 ± 15.01 bb | 56.25 ± 18.55 bb |
| Parameters | Control | LET | LET + POEE100% | LET + M | LET + TX |
|---|---|---|---|---|---|
| Insulin (pg/mL) | 30.15 ± 6.63 | 99.53 ± 12.59 aaa | 24.08 ± 4.30 bbb | 25.81 ± 4.85 bbb | 27.89 ± 8.44 bbb |
| HOMA-IR | 7.91 ± 3.34 | 32.59 ± 8.86 aaa | 5.74 ± 4.38 bbb | 7.11 ± 4.43 bbb | 7.14 ± 2.41 bbb |
| GLU (mg/dL) | 100.97 ± 19.57 | 129.45 ± 12.59 a | 77.69 ± 14.20 bbb,c,d | 104.49 ± 5.94 b | 108.92 ± 18.02 b |
| FSH (mIU/ml) | 39.19 ± 4.48 | 99.86 ± 33.16 aa | 80.39 ± 18.30 c | 99.33 ± 3.70 | 101.13 ± 34.50 |
| LH (mIU/ml) | 9.97 ± 4.66 | 26.05 ± 10.10 aa | 14.60 ± 5.87 b,c | 20.69 ± 14.05 | 13.12 ± 2.97 b |
| Estrogen (pg/mL) | 596.34 ± 67.74 | 329.88 ± 55.80 aaa | 728.07 ± 166.13 bbb | 774.90 ± 55.74 bbb | 621.89 ± 166.06 bbb |
| Testosterone (ng/mL) | 0.76 ± 0.24 | 1.17 ± 0.83 a | 1.31 ± 0.17 b | 1.55 ± 1.05 | 2.44 ± 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usatiuc, L.-O.; Pop, R.M.; Adrian, S.; Pârvu, M.; Țicolea, M.; Uifălean, A.; Vălean, D.; Gavrilaș, L.-I.; Csilla-Enikő, S.; Leopold, L.F.; et al. Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2025, 26, 4712. https://doi.org/10.3390/ijms26104712
Usatiuc L-O, Pop RM, Adrian S, Pârvu M, Țicolea M, Uifălean A, Vălean D, Gavrilaș L-I, Csilla-Enikő S, Leopold LF, et al. Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. International Journal of Molecular Sciences. 2025; 26(10):4712. https://doi.org/10.3390/ijms26104712
Chicago/Turabian StyleUsatiuc, Lia-Oxana, Raluca Maria Pop, Surd Adrian, Marcel Pârvu, Mădălina Țicolea, Ana Uifălean, Dan Vălean, Laura-Ioana Gavrilaș, Szabo Csilla-Enikő, Loredana Florina Leopold, and et al. 2025. "Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome" International Journal of Molecular Sciences 26, no. 10: 4712. https://doi.org/10.3390/ijms26104712
APA StyleUsatiuc, L.-O., Pop, R. M., Adrian, S., Pârvu, M., Țicolea, M., Uifălean, A., Vălean, D., Gavrilaș, L.-I., Csilla-Enikő, S., Leopold, L. F., Ranga, F., Cătoi, F. A., & Pârvu, A. E. (2025). Multitargeted Effects of Plantago ovata Ethanol Extract in Experimental Rat Streptozotocin-Induced Diabetes Mellitus and Letrozole-Induced Polycystic Ovary Syndrome. International Journal of Molecular Sciences, 26(10), 4712. https://doi.org/10.3390/ijms26104712










