Structural Implications of H233L and H398P Mutations in Phospholipase Cζ: A Full-Atom Molecular Dynamics Study on Infertility-Associated Dysfunctions
Abstract
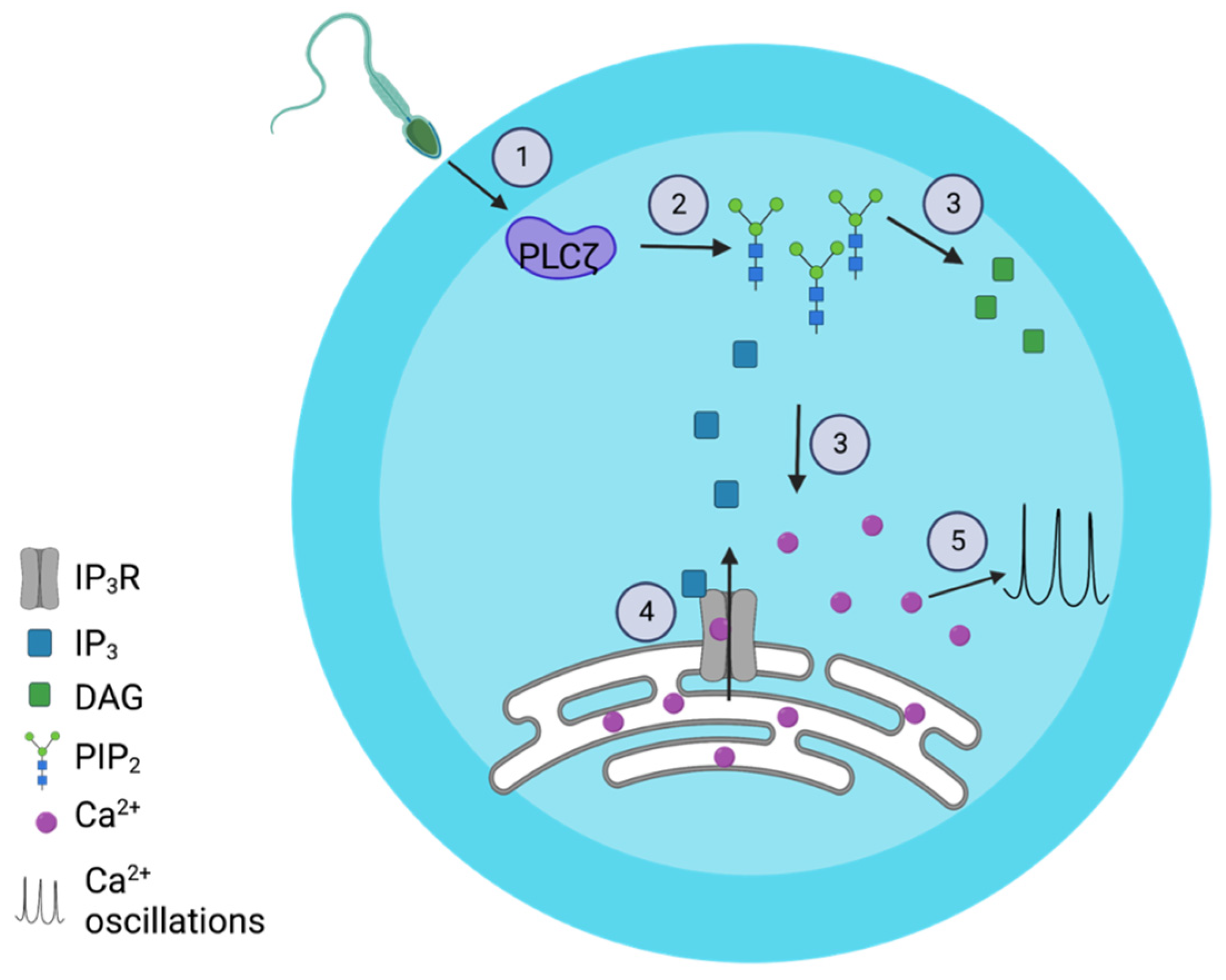
1. Introduction
2. Results
2.1. H233L and H398P Mutations Altered PIP2 Binding
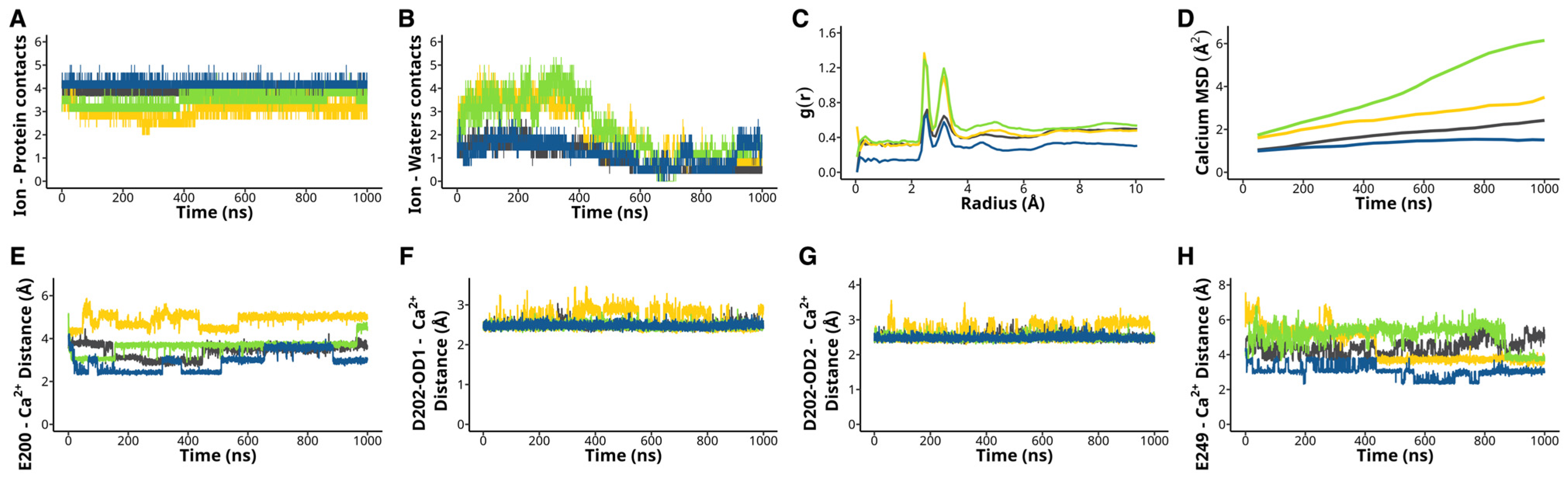
2.2. H233L and H398P Mutations Impair Ca2+ Binding
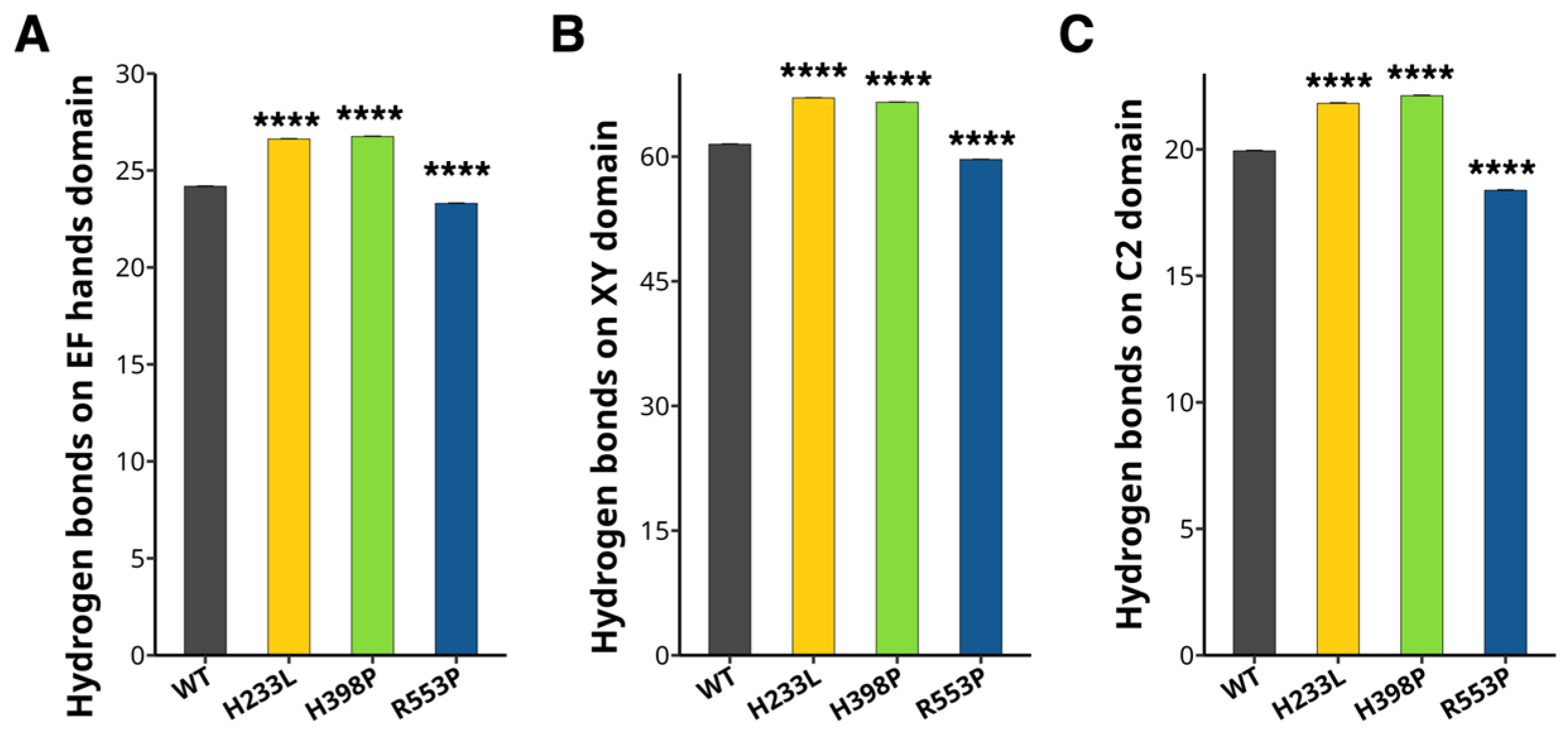
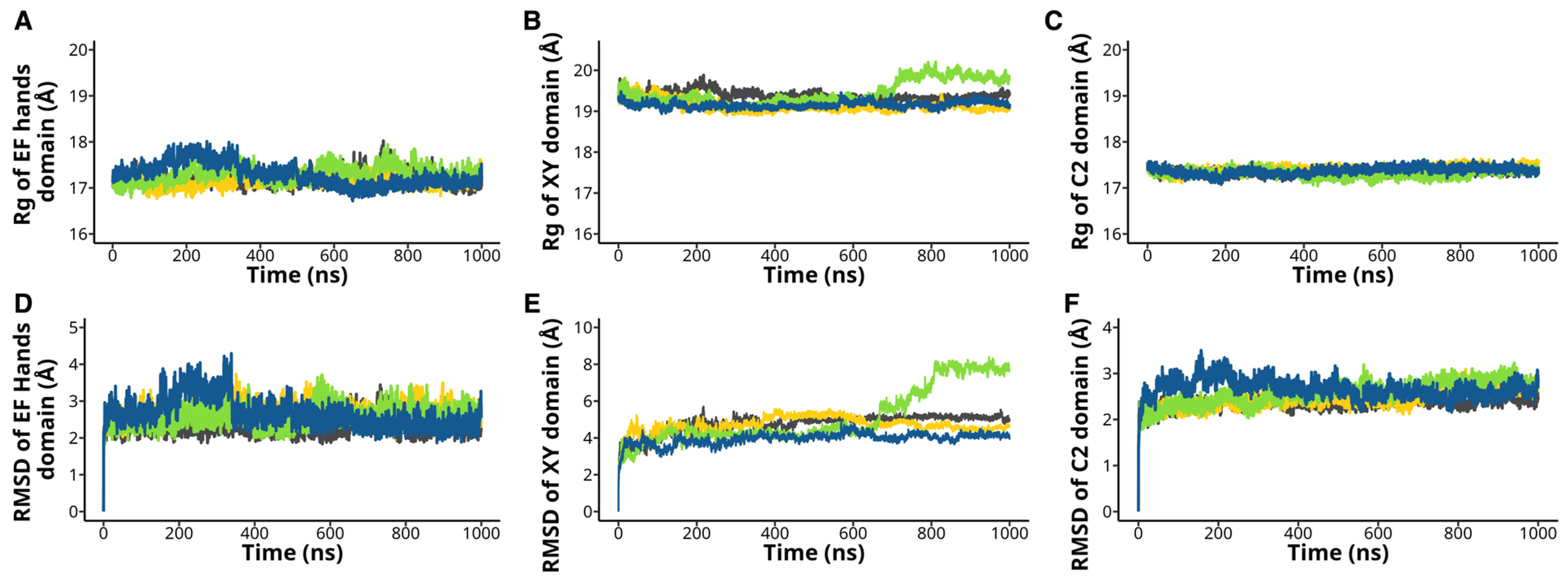
2.3. The H233L, H398P, and R553P Mutations Changes the PLCζ Intramolecular Interactions
3. Discussion
4. Materials and Methods
4.1. Full-Atom Molecular Dynamics Simulations and Docking
4.2. PIP2 Mean Square Displacement and Diffusion Coefficient
4.3. Binding Free Energy
4.4. Protein-Ligand Contacts
4.5. Root Mean Square Fluctuations
4.6. Ca2+ Ion Mean Square Displacement and Diffusion Coefficient
4.7. Radial Distribution Function
4.8. Root Mean Square Deviation
4.9. Intramolecular Hydrogen Bonds per Domain
4.10. Ion Coordination
4.11. Radius of Gyration
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ART | Assisted reproductive technology |
| DAG | Diacylglycerol |
| GAFF | The General Amber force field |
| IP3 | Inositol 1,4,5-triphosphate |
| IP3R | Inositol 1,4,5-triphosphate receptor |
| MD | Molecular dynamics simulations |
| MM-GBSA | Molecular Mechanics-Generalized Born Surface Area |
| MSD | Mean square deviation |
| NPT | Isobaric-isothermal ensemble |
| PDB | Protein Data Bank |
| PIP2 | Phosphatidylinositol 4,5-biphosphate |
| PLCζ | Phospholipase Cζ |
| PLC∂ | Phospholipase C∂ |
| PM | Plasma membrane |
| RDF | Radial distribution function |
| Rg | Radius of gyration |
| RMSD | Root mean square deviation |
| RMSF | Root mean square fluctuations |
| vdw | Van der Waals |
| VMD | Visual molecular dynamics |
| WT | Wild-type |
References
- Kashir, J.; Jones, C.; Lee, H.C.; Rietdorf, K.; Nikiforaki, D.; Durrans, C.; Ruas, M.; Tee, S.T.; Heindryckx, B.; Galione, A.; et al. Loss of Activity Mutations in Phospholipase C Zeta (PLCζ) Abolishes Calcium Oscillatory Ability of Human Recombinant Protein in Mouse Oocytes. Hum. Reprod. 2011, 26, 3372–3387. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Heindryckx, B.; De Sutter, P.; Parrington, J.; Wells, D.; Coward, K. Characterization of Two Heterozygous Mutations of the Oocyte Activation Factor Phospholipase C Zeta (PLCζ) from an Infertile Man by Use of Minisequencing of Individual Sperm and Expression in Somatic Cells. Fertil. Steril. 2012, 98, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Konstantinidis, M.; Jones, C.; Lemmon, B.; Lee, H.C.; Hamer, R.; Heindryckx, B.; Deane, C.M.; De Sutter, P.; Fissore, R.A.; et al. A Maternally Inherited Autosomal Point Mutation in Human Phospholipase C Zeta (PLCζ) Leads to Male Infertility. Hum. Reprod. 2012, 27, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.M.; Larman, M.G.; Parrington, J.; Cox, L.J.; Royse, J.; Blayney, L.M.; Swann, K.; Lai, F.A. PLC Zeta: A Sperm-Specific Trigger of Ca(2+) Oscillations in Eggs and Embryo Development. Development 2002, 129, 3533–3544. [Google Scholar] [CrossRef]
- Thanassoulas, A.; Swann, K.; Lai, F.A.; Nomikos, M. Sperm factors and egg activation: The Structure and Function Relationship of Sperm PLCZ1. Reproduction 2022, 164, F1–F8. [Google Scholar] [CrossRef]
- Kurokawa, M.; Sato, K.; Fissore, R.A. Mammalian Fertilization: From Sperm Factor to Phospholipase Czeta. Biol. Cell 2004, 96, 37–45. [Google Scholar] [CrossRef]
- Yu, Y.; Nomikos, M.; Theodoridou, M.; Nounesis, G.; Lai, F.A.; Swann, K. PLCζ Causes Ca(2+) Oscillations in Mouse Eggs by Targeting Intracellular and Not Plasma Membrane PI(4,5)P(2). Mol. Biol. Cell 2012, 23, 371–380. [Google Scholar] [CrossRef]
- Miyazaki, S.; Shirakawa, H.; Nakada, K.; Honda, Y. Essential Role of the Inositol 1,4,5-Trisphosphate Receptor/Ca2+ Release Channel in Ca2+ Waves and Ca2+ Oscillations at Fertilization of Mammalian Eggs. Dev. Biol. 1993, 158, 62–78. [Google Scholar] [CrossRef]
- Wakai, T.; Mehregan, A.; Fissore, R.A. Ca2+ Signaling and Homeostasis in Mammalian Oocytes and Eggs. Cold Spring Harb. Perspect. Biol. 2019, 11, a035162. [Google Scholar] [CrossRef]
- Hachem, A.; Godwin, J.; Ruas, M.; Lee, H.C.; Ferrer Buitrago, M.; Ardestani, G.; Bassett, A.; Fox, S.; Navarrete, F.; de Sutter, P.; et al. PLCζ Is the Physiological Trigger of the Ca2+ Oscillations That Induce Embryogenesis in Mammals but Conception Can Occur in Its Absence. Development 2017, 144, 2914–2924. [Google Scholar] [CrossRef]
- Nozawa, K.; Satouh, Y.; Fujimoto, T.; Oji, A.; Ikawa, M. Sperm-Borne Phospholipase C Zeta-1 Ensures Monospermic Fertilization in Mice. Sci. Rep. 2018, 8, 1315. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Mistry, B.V.; Rajab, M.A.; BuSaleh, L.; Abu-Dawud, R.; Ahmed, H.A.; Alharbi, S.; Nomikos, M.; AlHassan, S.; Coskun, S.; et al. The Mammalian Sperm Factor Phospholipase C Zeta Is Critical for Early Embryo Division and Pregnancy in Humans and Mice. Hum. Reprod. 2024, 39, 1256–1274. [Google Scholar] [CrossRef]
- Heytens, E.; Parrington, J.; Coward, K.; Young, C.; Lambrecht, S.; Yoon, S.-Y.; Fissore, R.A.; Hamer, R.; Deane, C.M.; Ruas, M.; et al. Reduced Amounts and Abnormal Forms of Phospholipase C Zeta (PLCzeta) in Spermatozoa from Infertile Men. Hum. Reprod. 2009, 24, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-Y.; Jellerette, T.; Salicioni, A.M.; Lee, H.C.; Yoo, M.-S.; Coward, K.; Parrington, J.; Grow, D.; Cibelli, J.B.; Visconti, P.E.; et al. Human Sperm Devoid of PLC, Zeta 1 Fail to Induce Ca(2+) Release and Are Unable to Initiate the First Step of Embryo Development. J. Clin. Investig. 2008, 118, 3671–3681. [Google Scholar] [CrossRef]
- Essen, L.O.; Perisic, O.; Katan, M.; Wu, Y.; Roberts, M.F.; Williams, R.L. Structural Mapping of the Catalytic Mechanism for a Mammalian Phosphoinositide-Specific Phospholipase C. Biochemistry 1997, 36, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Kouchi, Z.; Shikano, T.; Nakamura, Y.; Shirakawa, H.; Fukami, K.; Miyazaki, S. The Role of EF-Hand Domains and C2 Domain in Regulation of Enzymatic Activity of Phospholipase Czeta. J. Biol. Chem. 2005, 280, 21015–21021. [Google Scholar] [CrossRef]
- Nomikos, M.; Elgmati, K.; Theodoridou, M.; Calver, B.L.; Nounesis, G.; Swann, K.; Lai, F.A. Phospholipase Cζ Binding to PtdIns(4,5)P2 Requires the XY-Linker Region. J. Cell Sci. 2011, 124, 2582–2590. [Google Scholar] [CrossRef]
- Nomikos, M.; Mulgrew-Nesbitt, A.; Pallavi, P.; Mihalyne, G.; Zaitseva, I.; Swann, K.; Lai, F.A.; Murray, D.; McLaughlin, S. Binding of Phosphoinositide-Specific Phospholipase C-Zeta (PLC-Zeta) to Phospholipid Membranes: Potential Role of an Unstructured Cluster of Basic Residues. J. Biol. Chem. 2007, 282, 16644–16653. [Google Scholar] [CrossRef]
- Escoffier, J.; Lee, H.C.; Yassine, S.; Zouari, R.; Martinez, G.; Karaouzène, T.; Coutton, C.; Kherraf, Z.-E.; Halouani, L.; Triki, C.; et al. Homozygous Mutation of PLCZ1 Leads to Defective Human Oocyte Activation and Infertility That Is Not Rescued by the WW-Binding Protein PAWP. Hum. Mol. Genet. 2016, 25, 878–891. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Cornet-Bartolomé, D.; Barragán, M.; Durban, M.; Ferrer-Vaquer, A.; Zambelli, F.; Rodriguez, A.; Oliva, R.; Vassena, R. Novel Phospholipase C Zeta 1 Mutations Associated with Fertilization Failures after ICSI. Hum. Reprod. 2019, 34, 1494–1504. [Google Scholar] [CrossRef]
- Nomikos, M.; Stamatiadis, P.; Sanders, J.R.; Beck, K.; Calver, B.L.; Buntwal, L.; Lofty, M.; Sideratou, Z.; Swann, K.; Lai, F.A. Male Infertility-Linked Point Mutation Reveals a Vital Binding Role for the C2 Domain of Sperm PLCζ. Biochem. J. 2017, 474, 1003–1016. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, C.; Ren, Y.; Yan, J.; Nie, Y.; Yan, L.; Qiao, J. A Novel Homozygous Mutation of Phospholipase C Zeta Leading to Defective Human Oocyte Activation and Fertilization Failure. Hum. Reprod. 2020, 35, 977–985. [Google Scholar] [CrossRef]
- Torra-Massana, M.; Rodríguez, A.; Vassena, R. Exonic Genetic Variants Associated with Unexpected Fertilization Failure and Zygotic Arrest after ICSI: A Systematic Review. Zygote 2023, 31, 316–341. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Lee Fryar, K.; Landua, J.; Trevino, S.R.; Schell, D.; Thurlkill, R.L.; Imura, S.; Scholtz, J.M.; Gajiwala, K.; et al. Contribution of Hydrogen Bonds to Protein Stability. Protein Sci. 2014, 23, 652–661. [Google Scholar] [CrossRef]
- World Health Organization. Infertility Prevalence Estimates, 1990–2021; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Nomikos, M.; Blayney, L.M.; Larman, M.G.; Campbell, K.; Rossbach, A.; Saunders, C.M.; Swann, K.; Lai, F.A. Role of Phospholipase C-Zeta Domains in Ca2+-Dependent Phosphatidylinositol 4,5-Bisphosphate Hydrolysis and Cytoplasmic Ca2+ Oscillations. J. Biol. Chem. 2005, 280, 31011–31018. [Google Scholar] [CrossRef]
- Kouchi, Z.; Fukami, K.; Shikano, T.; Oda, S.; Nakamura, Y.; Takenawa, T.; Miyazaki, S. Recombinant Phospholipase Czeta Has High Ca2+ Sensitivity and Induces Ca2+ Oscillations in Mouse Eggs. J. Biol. Chem. 2004, 279, 10408–10412. [Google Scholar] [CrossRef]
- Ellis, M.V.; Sally, U.; Katan, M. Mutations within a Highly Conserved Sequence Present in the X Region of Phosphoinositide-Specific Phospholipase C-Delta 1. Biochem. J. 1995, 307 Pt 1, 69–75. [Google Scholar] [CrossRef]
- Ellis, M.V.; James, S.R.; Perisic, O.; Downes, C.P.; Williams, R.L.; Katan, M. Catalytic Domain of Phosphoinositide-Specific Phospholipase C (PLC). Mutational Analysis of Residues within the Active Site and Hydrophobic Ridge of Plcdelta1. J. Biol. Chem. 1998, 273, 11650–11659. [Google Scholar] [CrossRef] [PubMed]
- Hinostroza, F.; Neely, A.; Araya-Duran, I.; Marabolí, V.; Canan, J.; Rojas, M.; Aguayo, D.; Latorre, R.; González-Nilo, F.D.; Cárdenas, A.M. Dynamin-2 R465W Mutation Induces Long Range Perturbation in Highly Ordered Oligomeric Structures. Sci. Rep. 2020, 10, 18151. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Thakuri, B.; MohanKumar, K.; Roy, S.; Sljoka, A.; Sun, G.-Q.; Chakraborty, A. Mutation-Induced Long-Range Allosteric Interactions in the Spike Protein Determine the Infectivity of SARS-CoV-2 Emerging Variants. ACS Omega 2021, 6, 31305–31320. [Google Scholar] [CrossRef] [PubMed]
- Nomikos, M. Novel Signalling Mechanism and Clinical Applications of Sperm-Specific PLCζ. Biochem. Soc. Trans. 2015, 43, 371–376. [Google Scholar] [CrossRef]
- Theodoridou, M.; Nomikos, M.; Parthimos, D.; Gonzalez-Garcia, J.R.; Elgmati, K.; Calver, B.L.; Sideratou, Z.; Nounesis, G.; Swann, K.; Lai, F.A. Chimeras of Sperm PLCζ Reveal Disparate Protein Domain Functions in the Generation of Intracellular Ca2+ Oscillations in Mammalian Eggs at Fertilization. Mol. Hum. Reprod. 2013, 19, 852–864. [Google Scholar] [CrossRef]
- Igarashi, H.; Knott, J.G.; Schultz, R.M.; Williams, C.J. Alterations of PLCβ1 in Mouse Eggs Change Calcium Oscillatory Behavior Following Fertilization. Dev. Biol. 2007, 312, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Rajpert-De Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.-M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; Sapra, K.J.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef]
- Nachtigall, R.D.; Becker, G.; Wozny, M. The Effects of Gender-Specific Diagnosis on Men’s and Women’s Response to Infertility. Fertil. Steril. 1992, 57, 113–121. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, Z.; Wu, L.; Fu, J.; Chen, B.; Yan, Z.; Li, B.; Zhou, Z.; Wang, W.; Zhao, L.; et al. The Identification of Novel Mutations in PLCZ1 Responsible for Human Fertilization Failure and a Therapeutic Intervention by Artificial Oocyte Activation. Mol. Hum. Reprod. 2020, 26, 80–87. [Google Scholar] [CrossRef]
- Yan, Z.; Fan, Y.; Wang, F.; Yan, Z.; Li, M.; Ouyang, J.; Wu, L.; Yin, M.; Zhao, J.; Kuang, Y.; et al. Novel Mutations in PLCZ1 Cause Male Infertility Due to Fertilization Failure or Poor Fertilization. Hum. Reprod. 2020, 35, 472–481. [Google Scholar] [CrossRef]
- Nomikos, M.; Yu, Y.; Elgmati, K.; Theodoridou, M.; Campbell, K.; Vassilakopoulou, V.; Zikos, C.; Livaniou, E.; Amso, N.; Nounesis, G.; et al. Phospholipase Cζ Rescues Failed Oocyte Activation in a Prototype of Male Factor Infertility. Fertil. Steril. 2013, 99, 76–85. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A Web-Based Graphical User Interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks, C.L.; Mackerell, A.D.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Gao, Y.; Lee, J.; Smith, I.P.S.; Lee, H.; Kim, S.; Qi, Y.; Klauda, J.B.; Widmalm, G.; Khalid, S.; Im, W. CHARMM-GUI Supports Hydrogen Mass Repartitioning and Different Protonation States of Phosphates in Lipopolysaccharides. J. Chem. Inf. Model. 2021, 61, 831–839. [Google Scholar] [CrossRef]
- Lee, J.; Hitzenberger, M.; Rieger, M.; Kern, N.R.; Zacharias, M.; Im, W. CHARMM-GUI Supports the Amber Force Fields. J. Chem. Phys. 2020, 153, 035103. [Google Scholar] [CrossRef]
- Giorgino, T. Computing Diffusion Coefficients in Macromolecular Simulations: The Diffusion Coefficient Tool for VMD. J. Open Source Softw. 2019, 4, 1698. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Case, D.A.; Aktulga, H.M.; Belfon, K.; Ben-Shalom, I.Y.; Berryman, J.T.; Brozell, S.R.; Cerutti, D.S.; Cheatham, T.E.; Cisneros, G.A.; Cruzeiro, V.W.D.; et al. Amber 2022; University of California: San Francisco, CA, USA, 2022. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinostroza, F.; Albornoz-Muñoz, S.; Vergara, S.; Urra, G.; Araya-Durán, I.; Fissore, R.A.; González-Nilo, F.D.; Bustos, D.; Carvacho, I. Structural Implications of H233L and H398P Mutations in Phospholipase Cζ: A Full-Atom Molecular Dynamics Study on Infertility-Associated Dysfunctions. Int. J. Mol. Sci. 2025, 26, 4706. https://doi.org/10.3390/ijms26104706
Hinostroza F, Albornoz-Muñoz S, Vergara S, Urra G, Araya-Durán I, Fissore RA, González-Nilo FD, Bustos D, Carvacho I. Structural Implications of H233L and H398P Mutations in Phospholipase Cζ: A Full-Atom Molecular Dynamics Study on Infertility-Associated Dysfunctions. International Journal of Molecular Sciences. 2025; 26(10):4706. https://doi.org/10.3390/ijms26104706
Chicago/Turabian StyleHinostroza, Fernando, Sofía Albornoz-Muñoz, Sebastián Vergara, Gabriela Urra, Ingrid Araya-Durán, Rafael A. Fissore, Fernando Danilo González-Nilo, Daniel Bustos, and Ingrid Carvacho. 2025. "Structural Implications of H233L and H398P Mutations in Phospholipase Cζ: A Full-Atom Molecular Dynamics Study on Infertility-Associated Dysfunctions" International Journal of Molecular Sciences 26, no. 10: 4706. https://doi.org/10.3390/ijms26104706
APA StyleHinostroza, F., Albornoz-Muñoz, S., Vergara, S., Urra, G., Araya-Durán, I., Fissore, R. A., González-Nilo, F. D., Bustos, D., & Carvacho, I. (2025). Structural Implications of H233L and H398P Mutations in Phospholipase Cζ: A Full-Atom Molecular Dynamics Study on Infertility-Associated Dysfunctions. International Journal of Molecular Sciences, 26(10), 4706. https://doi.org/10.3390/ijms26104706





