Long-Term Real-World Outcomes and Insights of Biologic Therapies in Chronic Rhinosinusitis with Nasal Polyps
Abstract
1. Introduction
2. Results
2.1. Patient’s Characteristics
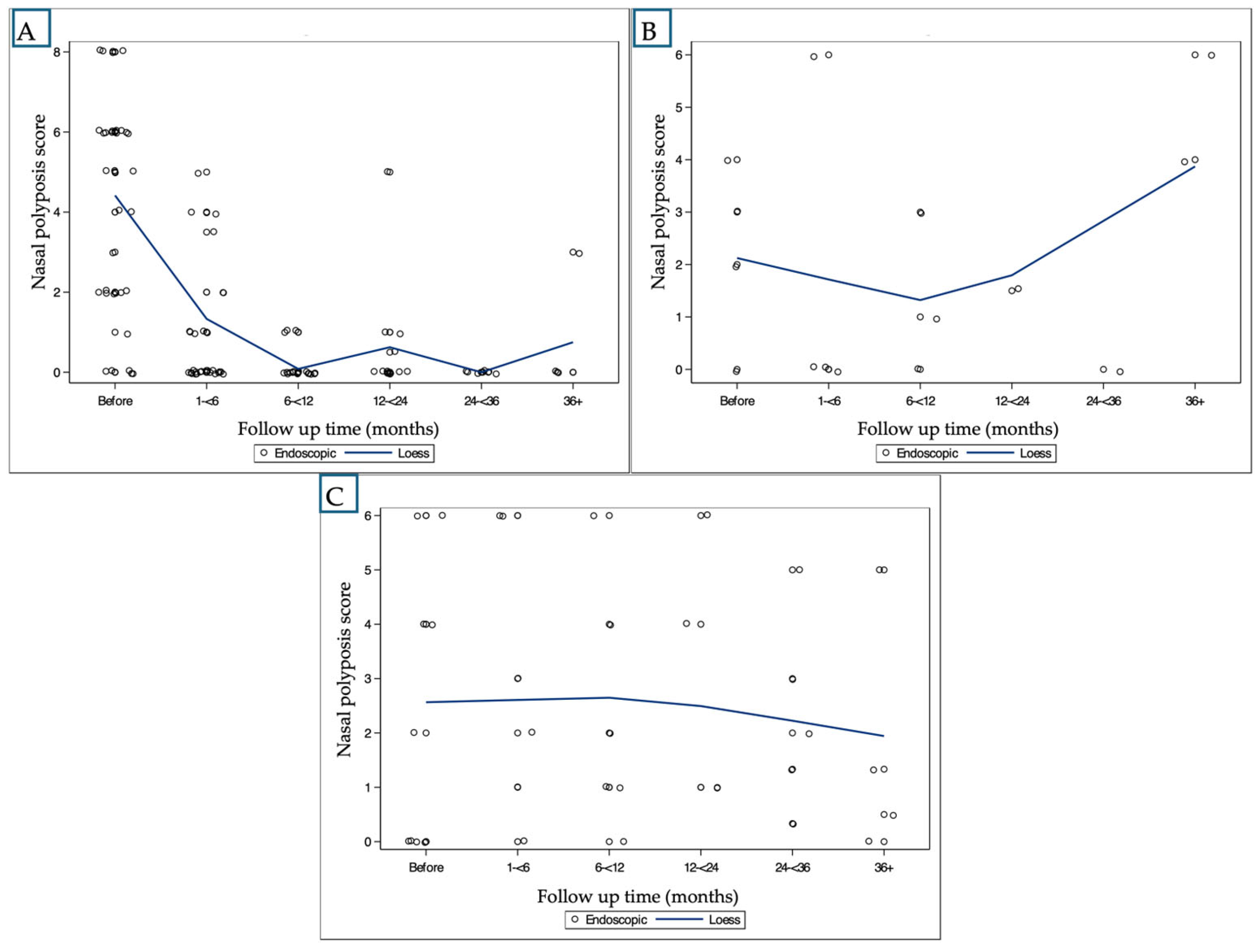
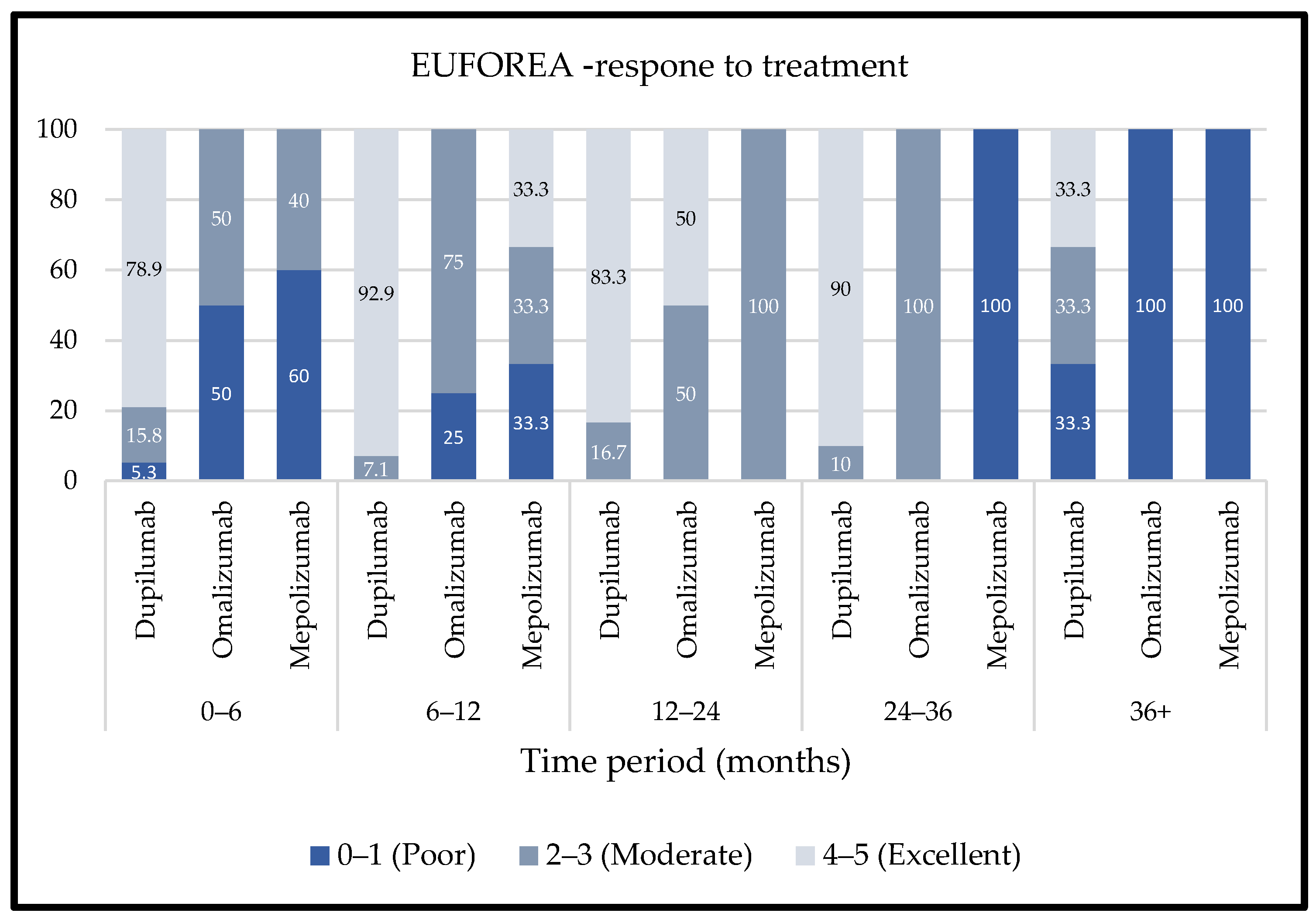
2.2. Treatment Outcomes and Responses
- Nasal Polyp Score (NPS):
- SNOT-22 Scores:
- Smell Scores:
2.3. Increasing Injection Intervals for Anti-IL-4 Treatment
2.4. Change in Treatment and Need for Additional Treatment
2.5. Discontinuation of Treatment and Side Effects
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRS | Chronic Rhinosinusitis |
| CRSwNP | Chronic Rhinosinusitis with Nasal Polyps |
| CRSsNP | Chronic Rhinosinusitis without Nasal Polyps |
| ESS | Endoscopic Sinus Surgery |
| INCS | Intranasal Corticosteroids |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| IL | Interleukin |
| IgE | Immunoglobulin E |
| NPS | Nasal Polyp Score |
| QALY | Quality-Adjusted Life Year |
| RCTs | Randomized Controlled Trials |
| EPOS | European Position Paper on Rhinosinusitis and Nasal Polyps |
| EUFOREA | European Forum for Research and Education in Allergy and Airway Diseases |
| SNOT-22 | 22-item Sino-Nasal Outcome Test |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, A.; Emmanuel, B.; Thomas, K.; Guiang, H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr. Med. Res. Opin. 2020, 36, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef]
- Adnane, C.; Adouly, T.; Khallouk, A.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. European Archives Oto-Rhino-Laryngol. 2017, 274, 879–885. [Google Scholar] [CrossRef]
- DeConde, A.S.; Mace, J.C.; Levy, J.M.; Rudmik, L.; Alt, J.A.; Smith, T.L. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 2017, 127, 550–555. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Kong, W.; Wang, X.; Yuan, L.; Zheng, R.; Qiu, H.; Huang, X.; Yang, Q. Which Is the Best Biologic for Nasal Polyps: Dupilumab, Omalizumab, or Mepolizumab? A Network Meta-Analysis. Int. Arch. Allergy Immunol. 2022, 183, 279–288. [Google Scholar] [CrossRef]
- Gerstacker, K.; Ketterer, M.C.; Jakob, T.F.; Hildenbrand, T. Real Life Observational Study of Treatment Success of Monoclonal Antibodies for Refractory Chronic Rhinosinusitis with Nasal Polyps. J. Clin. Med. 2023, 12, 4374. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Wagenmann, M.; Hosemann, W.; Lee, S.E.; Backer, V.; Mullol, J.; Gevaert, P.; Klimek, L.; Prokopakis, E.; et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J. Allergy Clin. Immunol. 2021, 147, 29–36. [Google Scholar] [CrossRef]
- Book, R.; Eligal, S.; Tal, Y.; Eliashar, R. Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. J. Clin. Med. 2023, 12, 3671. [Google Scholar] [CrossRef]
- Patel, G.B.; Peters, A.T. The Role of Biologics in Chronic Rhinosinusitis With Nasal Polyps. Ear. Nose Throat J. 2021, 100, 44–47. [Google Scholar] [CrossRef] [PubMed]
- van der Lans, R.J.L.; Fokkens, W.J.; Adriaensen, G.F.J.P.M.; Hoven, D.R.; Drubbel, J.J.; Reitsma, S. Real-life observational cohort verifies high efficacy of dupilumab for chronic rhinosinusitis with nasal polyps. Allergy 2022, 77, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Viskens, A.-S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Reaney, M.; Guillemin, I.; Nelson, L.; Qin, S.; Kamat, S.; Mannent, L.; Amin, N.; Whalley, D.; Hopkins, C. Development of Sinonasal Outcome Test (SNOT-22) Domains in Chronic Rhinosinusitis with Nasal Polyps. Laryngoscope 2022, 132, 933–941. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Ko, I.; Kim, M.S.; Yu, M.S.; Cho, B.-J.; Kim, D.-K. Association of Chronic Rhinosinusitis with Depression and Anxiety in a Nationwide Insurance Population. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 313–319. [Google Scholar] [CrossRef]
- Frankenberger, H.; Wiebringhaus, R.; Paul, B.; Huber, P.; Haubner, F.; Gröger, M.; Stihl, C. The use of biologics in patients suffering from chronic rhinosinusitis with nasal polyps—A 4-year real life observation. Eur. Arch. Oto-Rhino-Laryngol. 2024, 281, 5773–5782. [Google Scholar] [CrossRef]
- Russo, P.; Bassano, E.; Menichetti, M.; Lucidi, D.; Minniti, R.M.; Cigarini, E.; Menabue, S.; Marchioni, D.; Perano, D.; Ghidini, A. Long-Term Effectiveness of Dupilumab in Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: A Multicenter Retrospective Study. Am. J. Rhinol. Allergy 2025, 39, 175–180. [Google Scholar] [CrossRef]
- Qureshi, H.A.; Lane, A.P. Olfaction Now and in the Future in CRSwNP. Am. J. Rhinol. Allergy 2023, 37, 168–174. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.M.; Bertolini, F.; Carriero, V. The Role of Dupilumab in Severe Asthma. Biomedicines 2021, 9, 1096. [Google Scholar] [CrossRef]
- van der Lans, R.J.; Hopkins, C.; Senior, B.A.; Lund, V.J.; Reitsma, S. Biologicals and Endoscopic Sinus Surgery for Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: An Economic Perspective. J. Allergy Clin. Immunol. Pract. 2022, 10, 1454–1461. [Google Scholar] [CrossRef]
- Scangas, G.A.; Wu, A.W.; Ting, J.Y.; Metson, R.; Walgama, E.; Shrime, M.G.; Higgins, T.S. Cost Utility Analysis of Dupilumab Versus Endoscopic Sinus Surgery for Chronic Rhinosinusitis with Nasal Polyps. Laryngoscope 2021, 131, E26–E33. [Google Scholar] [CrossRef] [PubMed]
- Chapurin, N.; Khan, S.; Gutierrez, J.; Soler, Z.M. Economics of Medical and Surgical Management of Chronic Rhinosinusitis with Nasal Polyps: A Contemporary Review. Am. J. Rhinol. Allergy 2023, 37, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Little, D.H.W.; Tabatabavakili, S.; Shaffer, S.R.; Nguyen, G.C.; Weizman, A.V.; Targownik, L.E. Effectiveness of Dose De-escalation of Biologic Therapy in Inflammatory Bowel Disease: A Systematic Review. Am. J. Gastroenterol. 2020, 115, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Andersen, M.; Ring, T.; Andersen, G.; Ehlers, L.; Rasmussen, C.; Stensballe, A. Personalized rheumatic medicine through dose reduction reduces the cost of biological treatment—A retrospective intervention analysis. Scand. J. Rheumatol. 2019, 48, 398–407. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; E Lee, S.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- van der Lans, R.J.L.; Otten, J.J.; Adriaensen, G.F.J.P.M.; Hoven, D.R.; Benoist, L.B.; Fokkens, W.J.; Reitsma, S. Two-year results of tapered dupilumab for CRSwNP demonstrates enduring efficacy established in the first 6 months. Allergy 2023, 78, 2684–2697. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Cha, H.; Hong, S.-N.; Yang, M.-S.; Kim, D.W. Therapeutic Effectiveness of SNOT 22-Based Interdose Interval Adjustment of Dupilumab for Chronic Rhinosinusitis with Nasal Polyps. Clin. Exp. Otorhinolaryngol. 2024, 17, 317–325. [Google Scholar] [CrossRef]
- Cleveland, W.S.; Grosse, E. Computational methods for local regression. Stat. Comput. 1991, 1, 47–62. [Google Scholar] [CrossRef]


| Patient Characteristics | Baseline | |||
|---|---|---|---|---|
| All | Anti-IL-4 | Anti-IgE | Anti-IL-5 | |
| Number of patients | 52 | 38 | 4 | 10 |
| Age * | 54.14 (43.58–65.49, 50) | 57.68 (43.58–66.65, 37) | 42.21 (36.47–51.39, 4) | 52.10 (43.69–60.95, 9) |
| Gender (female) ** | 19 (36.54) | 13 (34.21) | 1 (25) | 5 (50) |
| Samter’s triad ** | 15 (28.85) | 11 (28.95) | 1 (25) | 3 (30) |
| Asthma ** | 46 (88.46) | 33 (86.84) | 3 (75) | 10 (100) |
| Allergic status ** | 35 (67.31) | 26 (68.42) | 3 (75) | 6 (60) |
| Blood Eosinophils% * | 7.4 (4.2–11.2, 33) | 6.65 (3.1–9.15, 24) | 20 (4.2–23, 3) | 9.85 (7.2–12.4, 6) |
| Total IgE * | 130 (40.6–274.5, 24) | 191.5 (40.6–335.5, 16) | 177 (114–274, 4) | 51.65 (18.8–150.5, 4) |
| Number of prior ESS * | 2 (1–2, 51) | 2 (1–2, 37) | 1.5 (1–2, 4) | 1.5 (1–3, 10) |
| Time from ESS * (months) | 20 (5–27, 46) | 21 (5–32, 34) | 13 (5.5–22.5, 4) | 19.5 (9–29, 8) |
| Nasal polyposis score * | 4 (2–6, 49) | 5 (2–6, 36) | 2.5 (1–3.5, 4) | 2 (0–4, 9) |
| SNOT-22 score * | 43 (19–60, 33) | 33 (16–58, 27) | 62 (53–71, 2) | 59.5 (28.5–85.5, 4) |
| Follow-up time *** | 19.13 (20.45) | 14.18 (13.89) | 43.00 (47.69) | 28.40 (19.13) |
| Treatment | Time (Months) | NPS * | SNOT-22 ** | Smell * |
|---|---|---|---|---|
| Anti-IL-4 | Baseline | 4.22, 36 | 38.52, 27 | 2.96, 27 |
| <6 | 1.08, 36 *** | +2.00, 20 | 2.44, 27 | |
| 6–12 | 0.19, 21 *** | −4.00, 16 | 2.05, 20 | |
| 12–24 | 0.44, 18 *** | −7.57, 14 *** | 1.13, 16 *** | |
| 24–36 | 0.0, 15 *** | −5.57, 14 *** | 1.29, 14 *** | |
| 36+ | 1.20, 5 *** | −5.00, 2 | 1.50, 4 | |
| Anti-IgE | Baseline | 2.25, 4 | 62.0, 2 | 5.00, 2 |
| <6 | 1.50, 4 | −3.50, 2 | - | |
| 6–12 | 1.75, 4 | +4.00, 1 | 5.00, 1 | |
| 12–24 | 1.50, 4 | – | - | |
| 24–36 | 0.0, 1 | – | - | |
| 36+ | 4.46, 13 | – | 4.70, 10 | |
| Anti-IL-5 | Baseline | 2.44, 9 | 57.00, 4 | 3.25, 4 |
| <6 | 3.00, 6 | 11.33, 3 | 3.40, 5 | |
| 6–12 | 1.88, 8 | −2.67, 3 | 4.00, 7 | |
| 12–24 | 2.57, 7 | +3.50, 2 | 3.00, 6 | |
| 24–36 | 1.67, 9 | +10.20, 5 | 3.50, 8 | |
| 36+ | 1.43, 7 | +24.00, 1 | 4.73, 11 |
| Domain | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Baseline | 14.78, 27 | 5.74, 27 | 4.07, 27 | 8.81, 27 | 7.04, 27 |
| <6 | 12.70, 33 | 5.39, 33 | 4.52, 33 | 8.82, 33 | 7.24, 33 |
| 6–12 | 11.23, 22 | 5.41, 22 | 3.36, 22 | 7.86, 221 | 4.95, 22 |
| 12–24 | 8.69, 16 *** | 4.38, 16 | 2.69, 16 | 5.44, 16 *** | 2.88, 16 *** |
| 24–36 | 7.29, 14 *** | 2.50, 14 *** | 1.64, 144 | 4.07, 14 | 2.64, 14 |
| 36+ | 11.25, 4 | 5.75, 46 | 9, 4 | 9.25, 4 | 9.75, 4 |
| Patient Characteristics | Baseline | |
|---|---|---|
| Regular Interval | Extended Intervals | |
| Number of patients | 25 | 13 |
| Age * | 56.93 (43.92–64.34) | 62.80 (40.18–71.05) |
| Gender (female) ** | 32.0% | 38.5% |
| Samter’s triad ** | 36.0% | 15.4% |
| Asthma ** | 88.0% | 84.6% |
| Allergic status ** | 56.0% | 92.3% |
| Blood Eosinophils% * | 6.90 (1.50–9.15) | 6.35 (4.15–9.30) |
| Total IgE * | 250.0 (52.0–760.0) | 51.60 (29.60–243.00) |
| Number of prior ESS * | 2.00 (1.00–2.00) | 2.00 (1.00–3.00) |
| Time from ESS * (months) | 18.00 (4.00–26.00) | 23.00 (19.00–54.00) |
| Nasal polyposis score * | 4.00 (2.00–6.00) | 6.00 (4.00–6.00) |
| SNOT-22 score * | 43.50 (31.50–69.50) | 43.00 (29.00–62.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Book, R.; Lazutkin, A.; Eliashar, R. Long-Term Real-World Outcomes and Insights of Biologic Therapies in Chronic Rhinosinusitis with Nasal Polyps. Int. J. Mol. Sci. 2025, 26, 4694. https://doi.org/10.3390/ijms26104694
Book R, Lazutkin A, Eliashar R. Long-Term Real-World Outcomes and Insights of Biologic Therapies in Chronic Rhinosinusitis with Nasal Polyps. International Journal of Molecular Sciences. 2025; 26(10):4694. https://doi.org/10.3390/ijms26104694
Chicago/Turabian StyleBook, Reut, Anna Lazutkin, and Ron Eliashar. 2025. "Long-Term Real-World Outcomes and Insights of Biologic Therapies in Chronic Rhinosinusitis with Nasal Polyps" International Journal of Molecular Sciences 26, no. 10: 4694. https://doi.org/10.3390/ijms26104694
APA StyleBook, R., Lazutkin, A., & Eliashar, R. (2025). Long-Term Real-World Outcomes and Insights of Biologic Therapies in Chronic Rhinosinusitis with Nasal Polyps. International Journal of Molecular Sciences, 26(10), 4694. https://doi.org/10.3390/ijms26104694







