Tissue- and Condition-Specific Biosynthesis of Ascorbic Acid in Glycine max L.: Insights from Genome-Wide Analyses of Pathway-Encoding Genes, Expression Profiling, and Mass Fraction Determination
Abstract
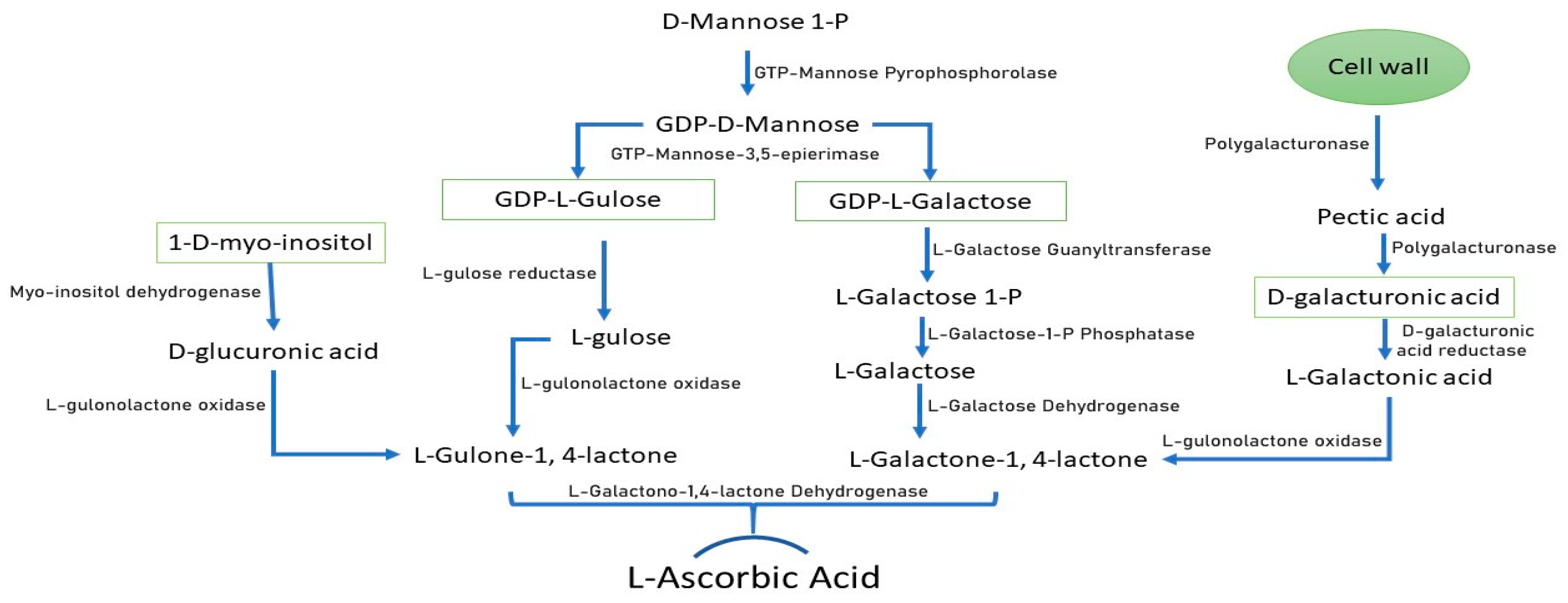
1. Introduction
2. Results
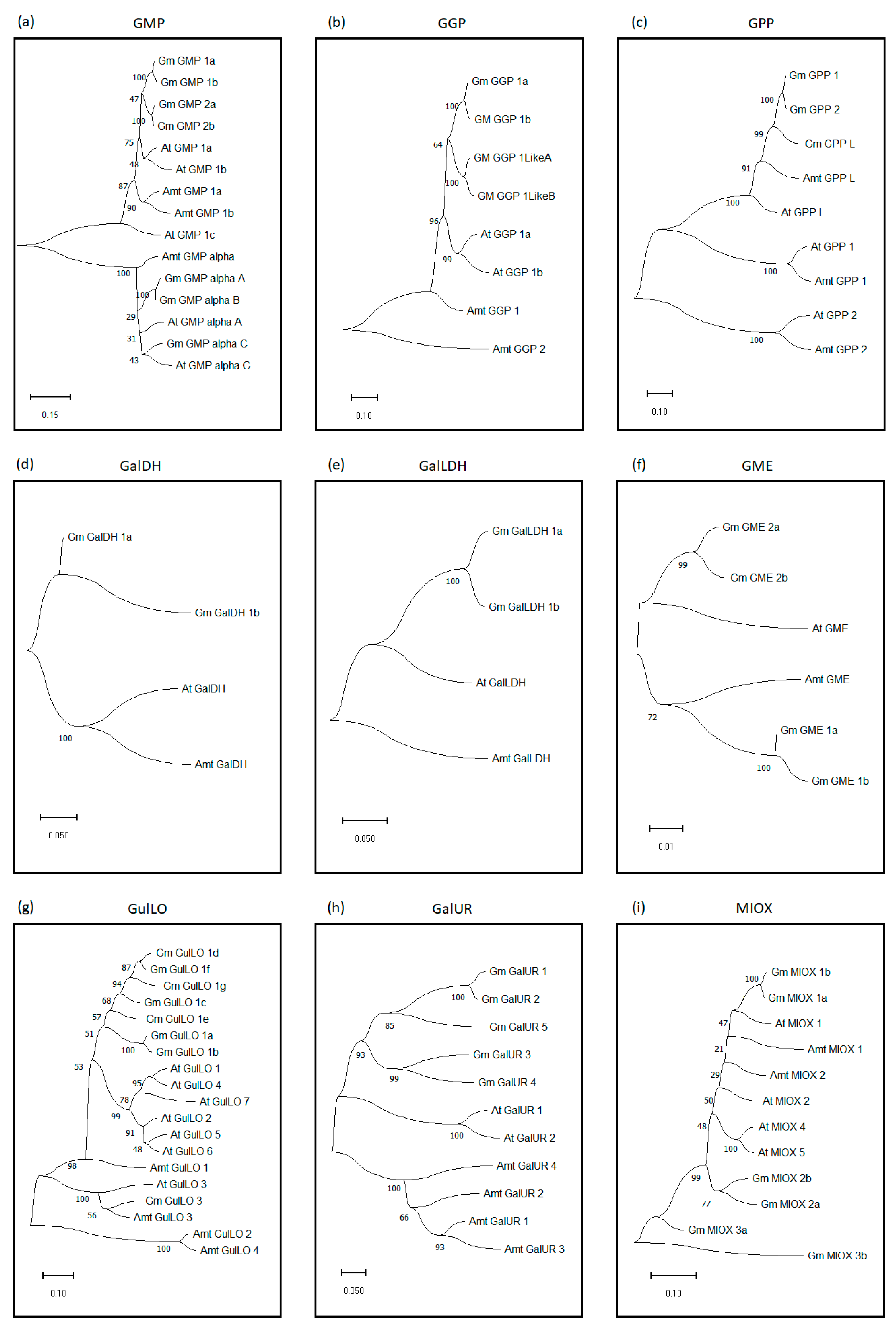
2.1. Identification and Phylogenetic Relationship of Genes Encoding AsA Biosynthesis Pathways in Soybean
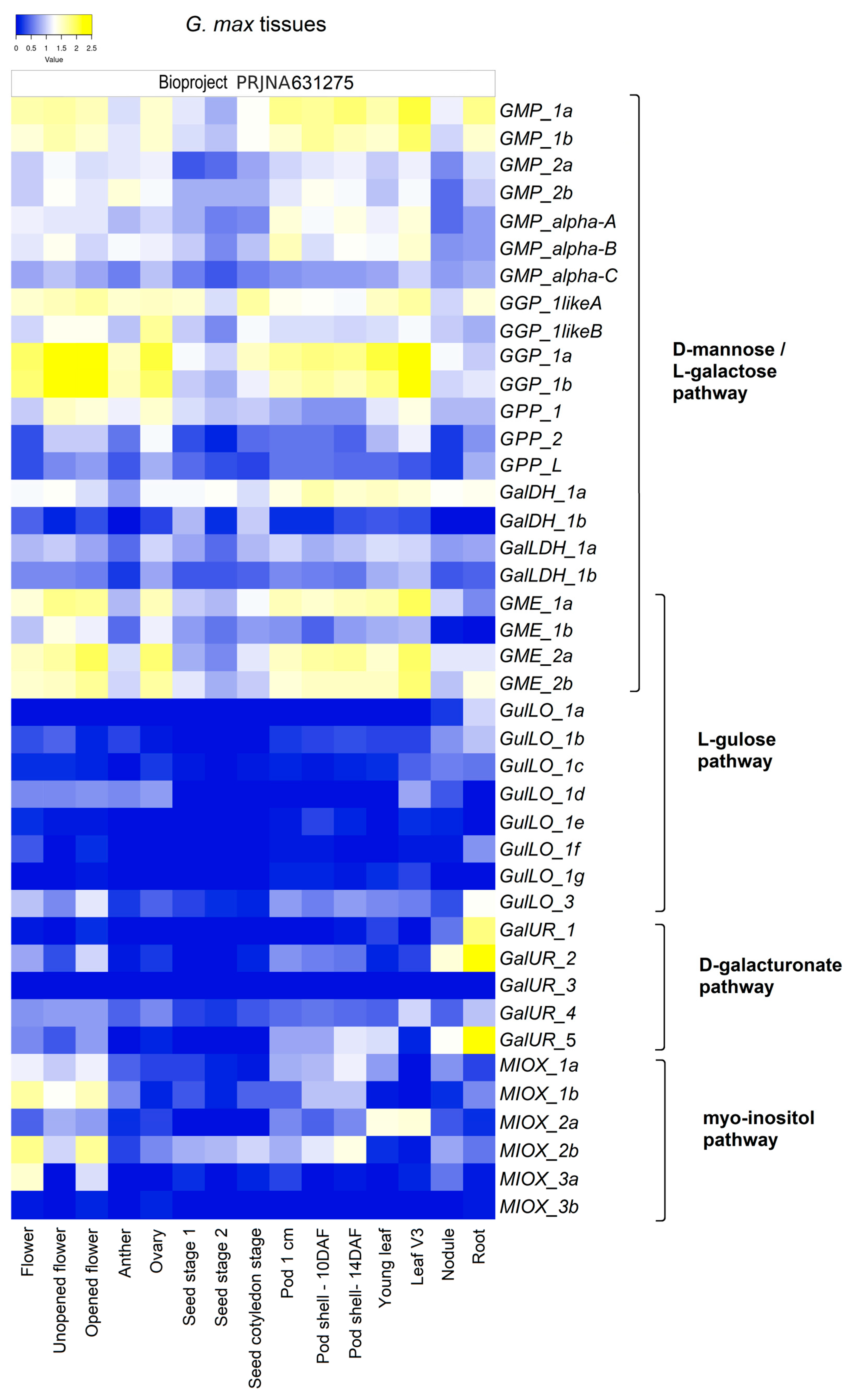
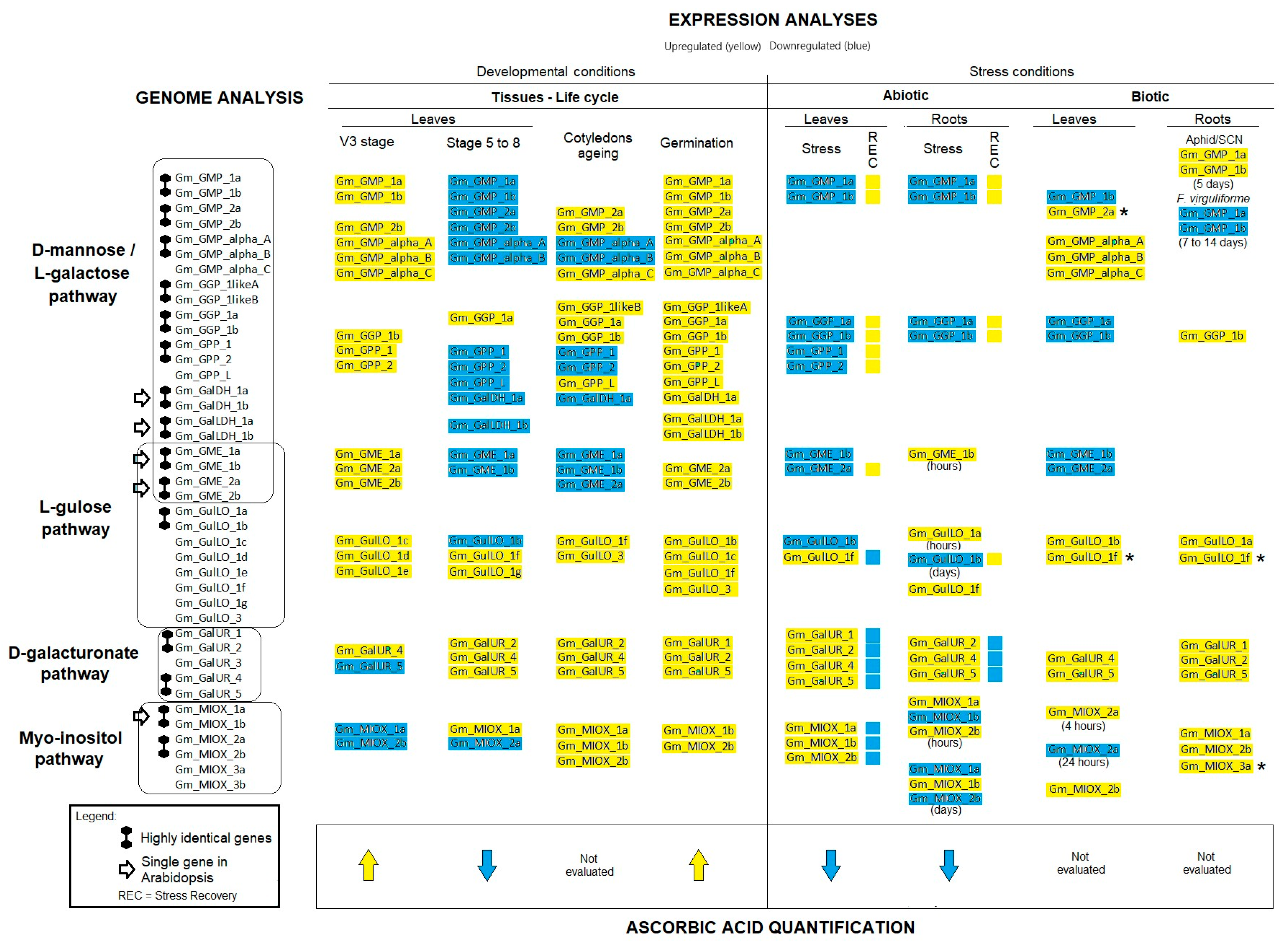
2.2. Gene Expression Analyses Indicate That All Proposed AsA Biosynthesis Pathways Play an Active Regulatory Role in G. max Species
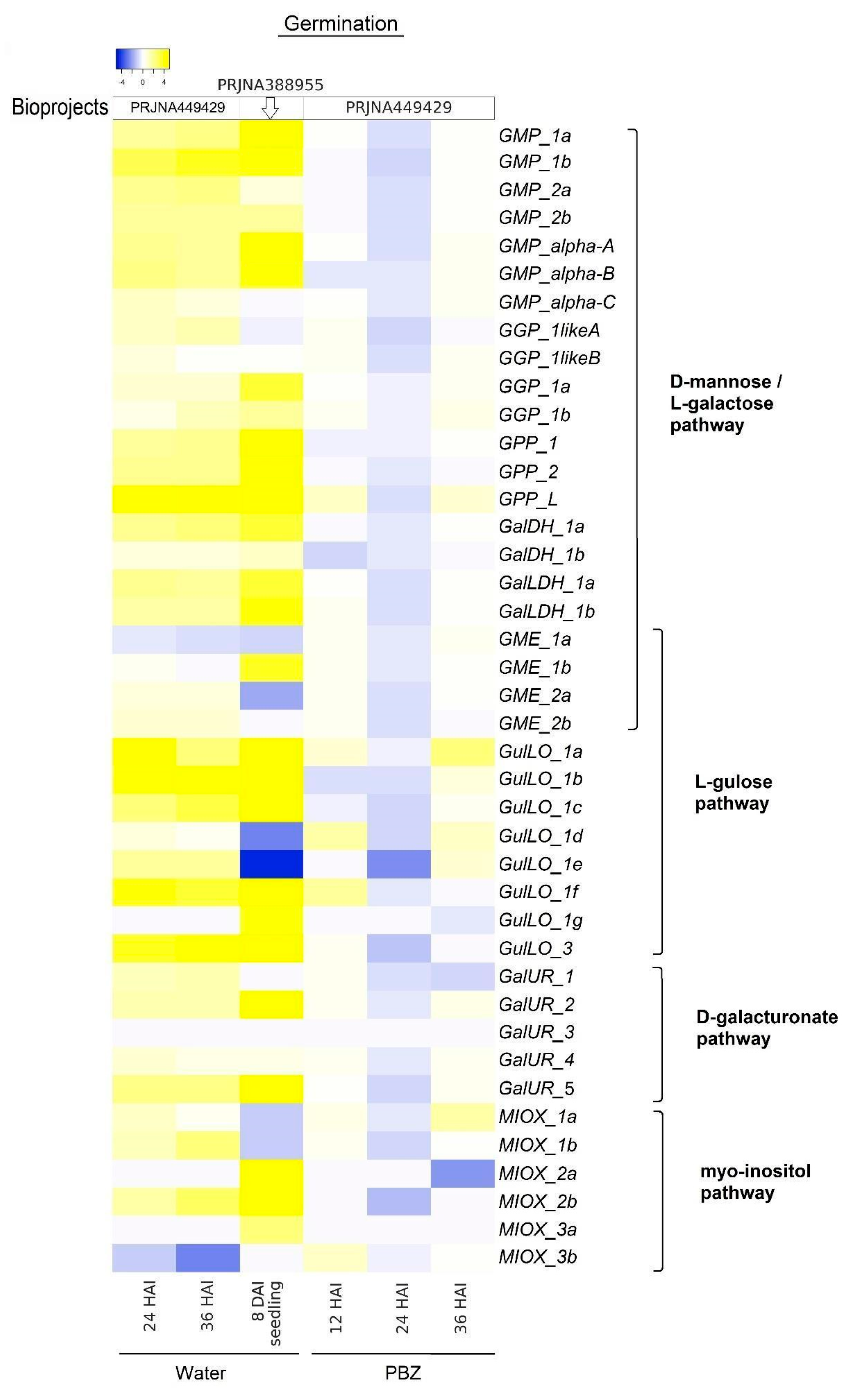
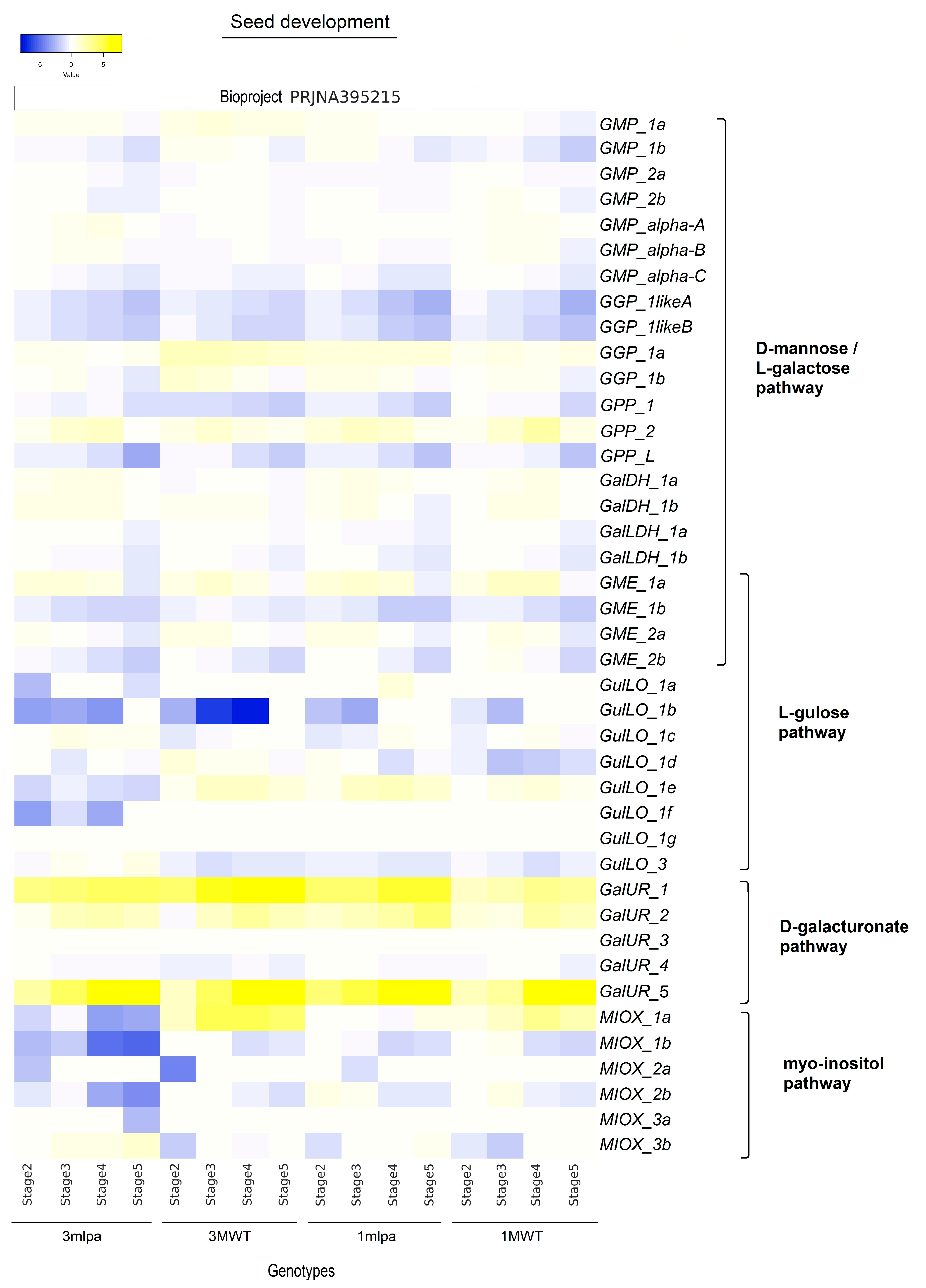
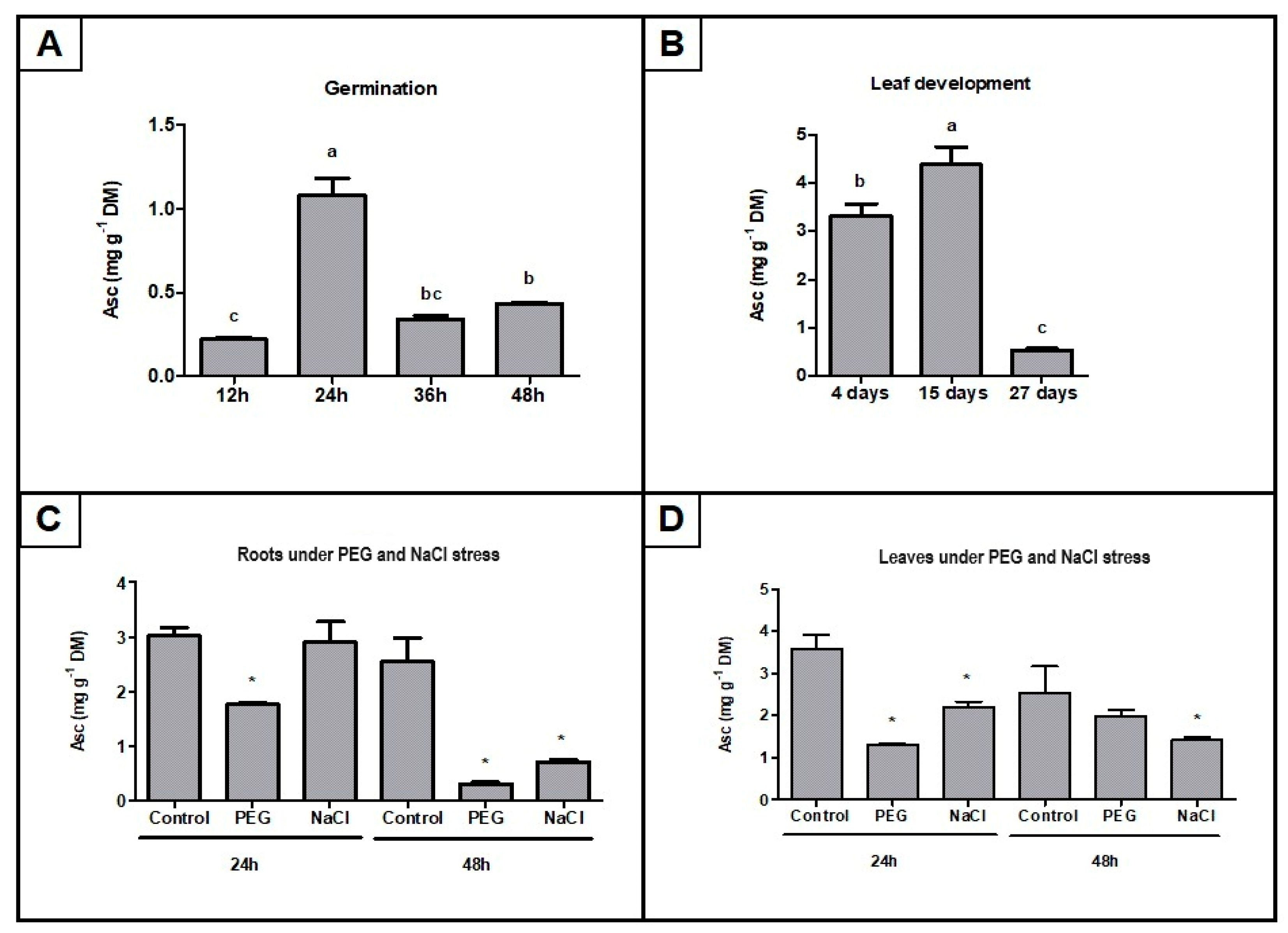
2.3. Expression Profile of Genes Linked to AsA Biosynthesis Pathways During Germination
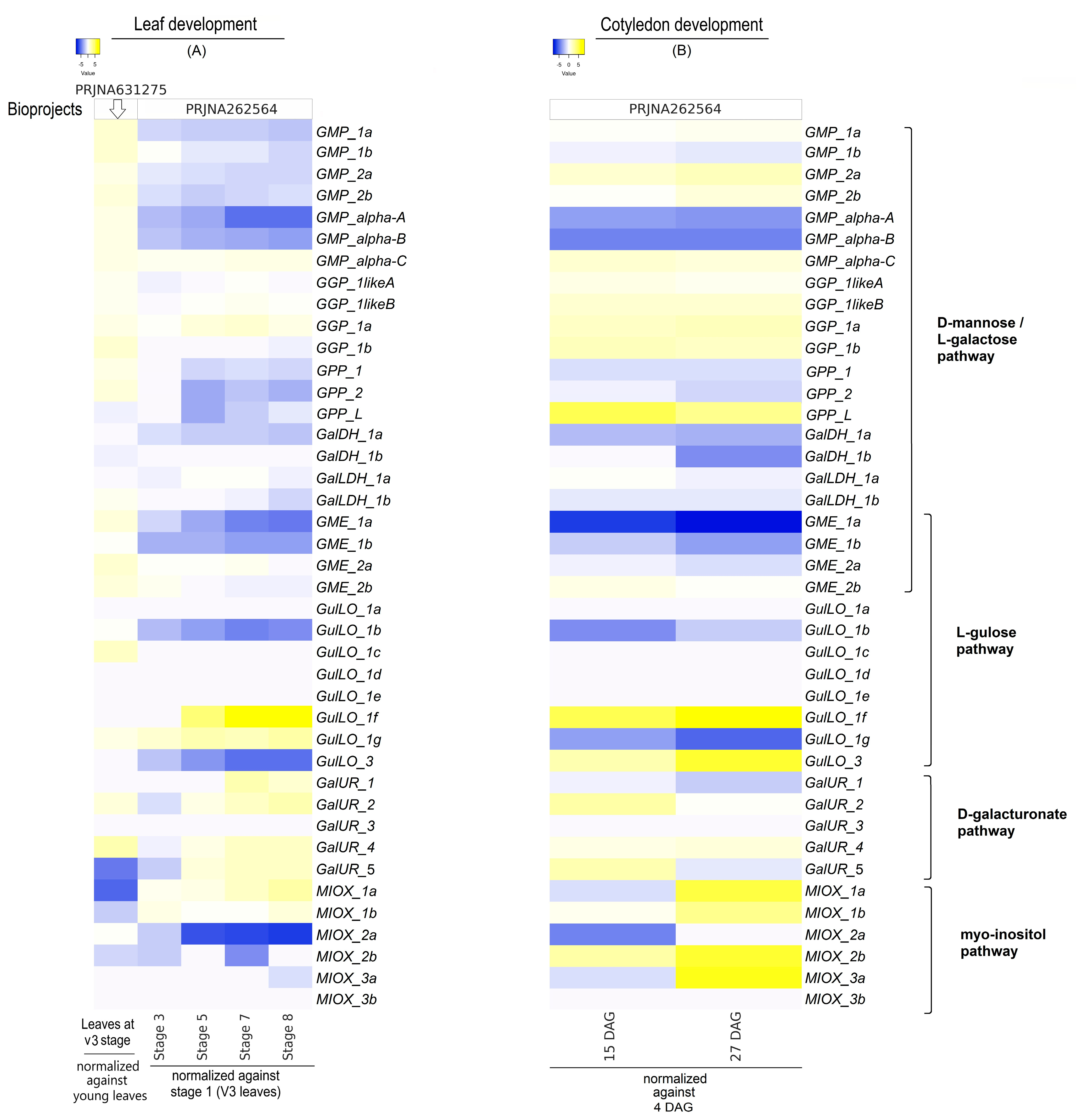
2.4. Gene Expression Levels of AsA Biosynthetic Pathways Concerning Tissue Development
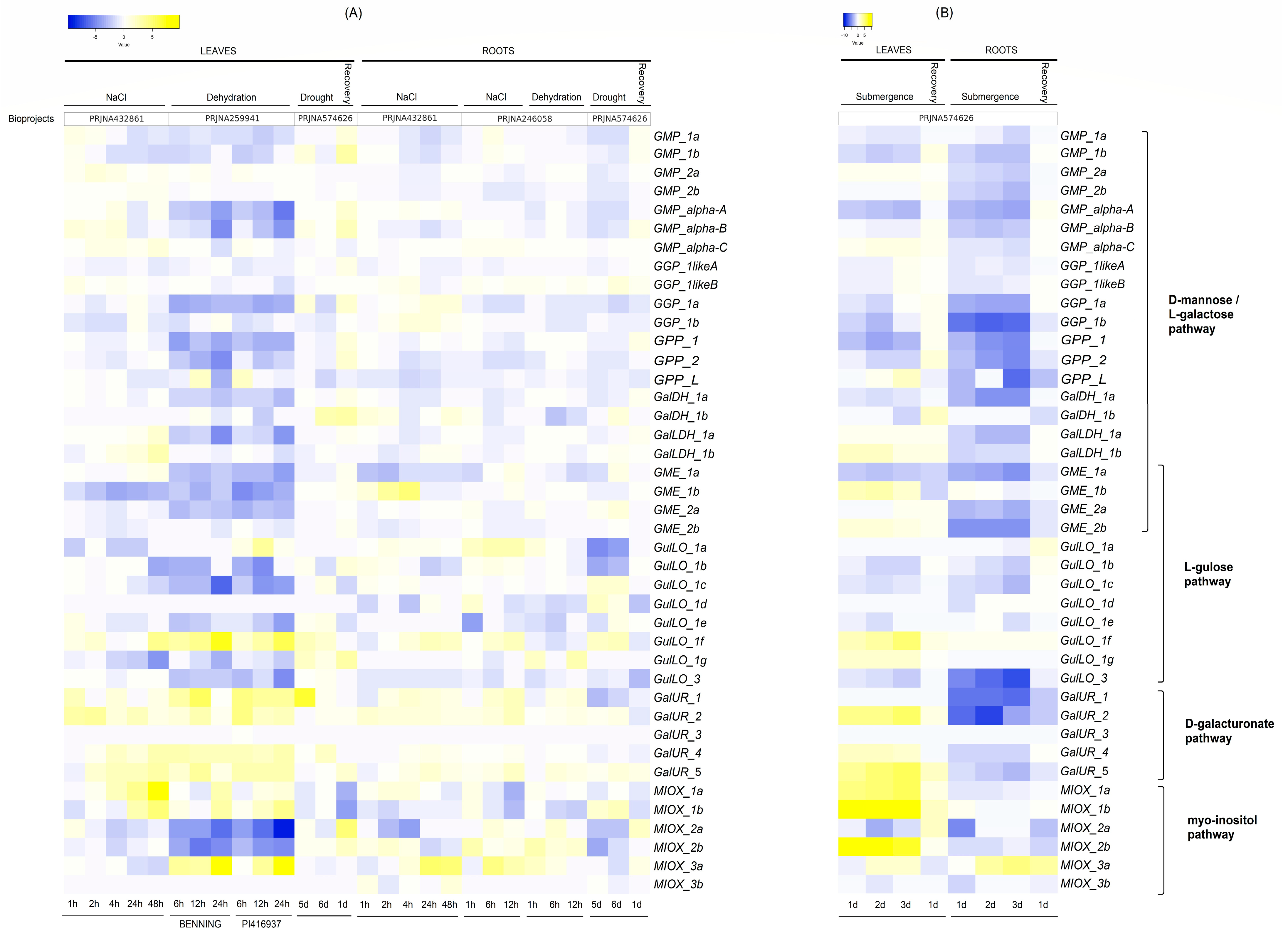
2.5. Expression Levels of Genes Involved in AsA Biosynthesis in G. max Roots and Leaves Under Abiotic Stresses
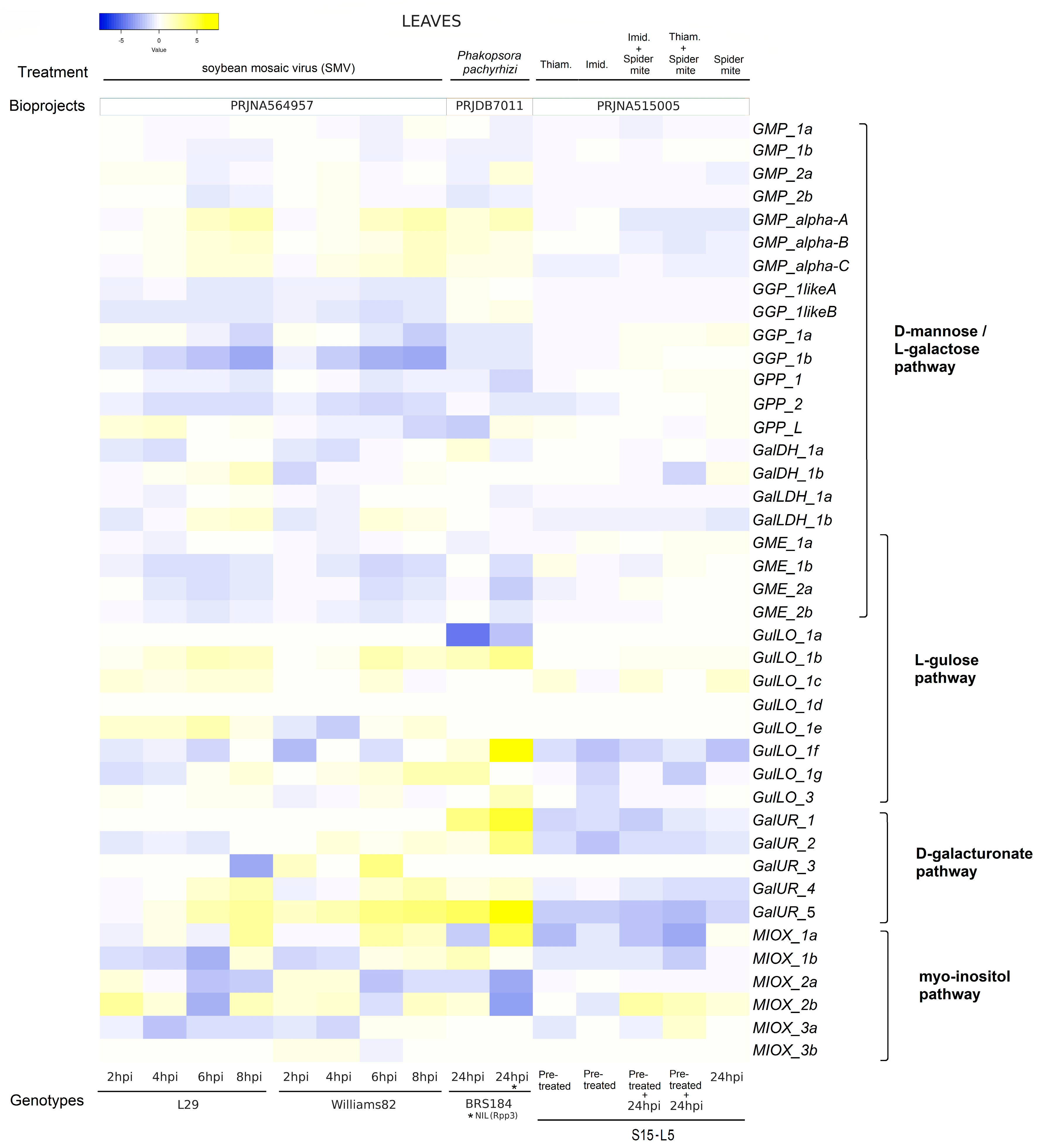
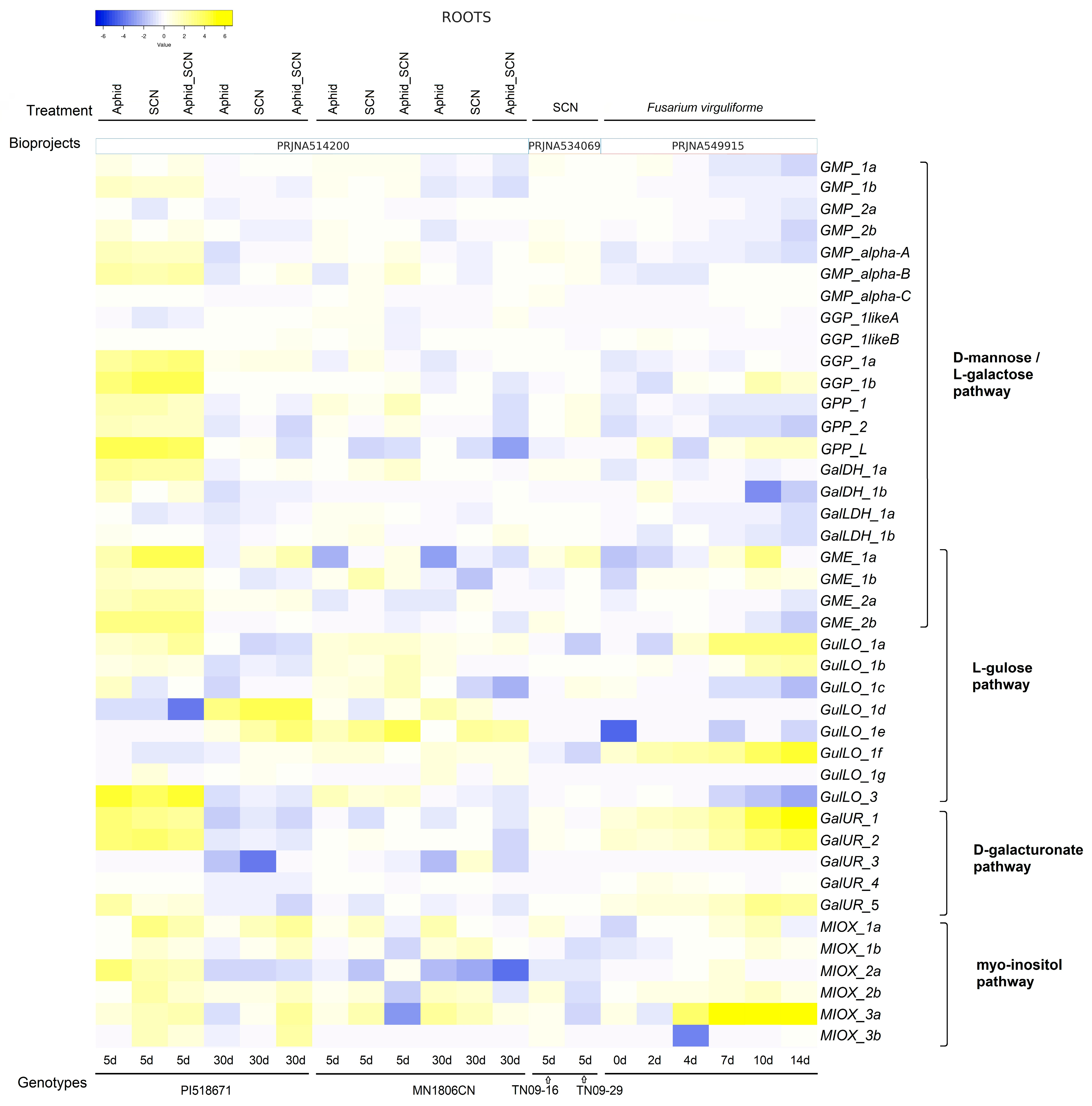
2.6. Expression Level of Genes Associated with AsA Biosynthesis in Leaves and Roots Under Biotic Stresses
2.7. AsA Mass Fraction Determination in Selected Soybean Tissues Under Development and Stress Conditions
3. Discussion
4. Material and Methods
4.1. Plant Materials and Growth Conditions
4.2. Abiotic Stress Induction
4.3. Quantification of AsA
4.4. Identification and Retrieval of Genes Associated with AsA Biosynthesis
4.5. Phylogenetic Analyses
4.6. In Silico Expression Analyses of AsA Pathway-Linked Genes in G. max RNA-seq Data
4.7. Statistical Analyses
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; De Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef] [PubMed]
- Tóth, S.Z.; Lőrincz, T.; Szarka, A. Concentration does matter: The beneficial and potentially harmful effects of ascorbate in humans and plants. Antioxid. Redox Signal. 2018, 29, 1516–1533. [Google Scholar] [CrossRef]
- Choudhary, A.; Kumar, A.; Kaur, N. ROS and oxidative burst: Roots in plant development. Plant Divers. 2020, 42, 33–43. [Google Scholar] [CrossRef]
- Steelheart, C.; Alegre, M.L.; Baldet, P.; Rothan, C.; Bres, C.; Just, D.; Okabe, Y.; Ezura, H.; Ganganelli, I.; Gergoff Grozeff, G.E.; et al. The effect of low ascorbic acid content on tomato fruit ripening. Planta 2020, 252, 36. [Google Scholar] [CrossRef]
- Barth, C.; De Tullio, M.; Conklin, P.L. The role of ascorbic acid in the control of flowering time and the onset of senescence. J. Exp. Bot. 2006, 57, 1657–1665. [Google Scholar] [CrossRef]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef]
- Khazaei, Z.; Esmaielpour, B.; Estaji, A. Ameliorative effects of ascorbic acid on tolerance to drought stress on pepper (Capsicum annuum L.) plants. Physiol. Mol. Biol. Plants 2020, 26, 1649–1662. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Costa, J.H.; Roque, A.L.M.; Aziz, S.; Dos Santos, C.P.; Germano, T.A.; Batista, M.C.; Thiers, K.L.L.; da Cruz Saraiva, K.D.; Arnholdt-Schmitt, B. Genome-wide identification of ascorbate-glutathione cycle gene families in soybean (Glycine max) reveals gene duplication events and specificity of gene members linked to development and stress conditions. Int. J. Biol. Macromol. 2021, 187, 528–543. [Google Scholar] [CrossRef]
- Gaafar, A.A.; Ali, S.I.; El-Shawadfy, M.A.; Salama, Z.A.; Sękara, A.; Ulrichs, C.; Abdelhamid, M.T. Ascorbic acid induces the increase of secondary metabolites, antioxidant activity, growth, and productivity of the common bean under water stress conditions. Plants 2020, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Cheng, H.; Chen, L.; Zhu, H.; Mao, P.; Wang, M. Influence of exogenous ascorbic acid and glutathione priming on mitochondrial structural and functional systems to alleviate aging damage in oat seeds. BMC Plant Biol. 2020, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, C.O.; Gesto-Borroto, R.; Wilson, R.V.; Hernández-Madrigal, S.V.; Lorence, A. Alternative pathways leading to ascorbate biosynthesis in plants: Lessons from the last 25 years. J. Exp. Bot. 2024, 75, 2644–2663. [Google Scholar] [CrossRef]
- Wolucka, B.A.; Van Montagu, M. GDP-Mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef]
- Castro, J.C.; Castro, C.G.; Cobos, M. Genetic and biochemical strategies for regulation of L-ascorbic acid biosynthesis in plants through the L-galactose pathway. Front. Plant Sci. 2023, 14, 1099829. [Google Scholar] [CrossRef]
- Liu, X.; Wu, R.; Bulley, S.M.; Zhong, C.; Li, D. Kiwifruit MYBS1-like and GBF3 transcription factors influence l-ascorbic acid biosynthesis by activating transcription of GDP-l-galactose phosphorylase 3. New Phytol. 2022, 234, 1782–1800. [Google Scholar] [CrossRef]
- Laing, W.A.; Martínez-Sánchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Bulley, S.M. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Radzio, J.A.; Lorence, A.; Chevone, B.I.; Nessler, C.L. L-Gulono-1, 4-lactone oxidase expression rescues vitamin C-deficient Arabidopsis (vtc) mutants. Plant Mol. Biol. 2003, 53, 837–844. [Google Scholar] [CrossRef]
- Aboobucker, S.; Suza, W.; Lorence, A. Characterization of two Arabidopsis l-gulono-1,4-lactone oxidases, AtGulLO3 and AtGulLO5, involved in ascorbate biosynthesis. React. Oxyg. Species 2017, 4, 389–417. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Y.; Pulla, R.K.; Park, J.M.; Harn, C.H.; Jeong, B.R. Over-expression of L-gulono-γ-lactone oxidase (GLOase) gene leads to ascorbate accumulation with enhanced abiotic stress tolerance in tomato. In Vitro Cell. Dev. Biol. Plant 2012, 48, 453–461. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, Y.; Cai, X.; Gong, P.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Overexpression of SlGMEs leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011, 30, 389–398. [Google Scholar] [CrossRef]
- Upadhyaya, C.P.; Young, K.E.; Akula, N.; Soon Kim, H.; Heung, J.J.; Oh, O.M.; Aswath, C.R.; Chun, S.C.; Kim, D.H.; Park, S.W. Over-expression of strawberry D-galacturonic acid reductase in potato leads to accumulation of vitamin C with enhanced abiotic stress tolerance. Plant Sci. 2009, 177, 659–667. [Google Scholar]
- Lisko, K.A.; Torres, R.; Harris, R.S.; Belisle, M.; Vaughan, M.M.; Jullian, B.; Chevone, B.I.; Mendes, P.; Nessler, C.L.; Lorence, A. Elevating vitamin C content via overexpression of Myo-inositol oxygenase and L-gulono-1,4-lactone oxidase in Arabidopsis leads to enhanced biomass and tolerance to abiotic stresses. In Vitro Cell. Dev. Biol. Plant 2013, 49, 643–655. [Google Scholar] [CrossRef]
- Maruta, T.; Ichikawa, Y.; Mieda, T.; Takeda, T.; Tamoi, M.; Yabuta, Y.; Ishikawa, T.; Shigeoka, S. The contribution of Arabidopsis homologs of L-gulono-1,4-lactone oxidase to the biosynthesis of ascorbic acid. Biosci. Biotechnol. Biochem. 2010, 74, 1494–1497. [Google Scholar] [CrossRef]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef]
- Kanter, U.; Usadel, B.; Guerineau, F.; Li, Y.; Pauly, M.; Tenhaken, R. The inositol oxygenase gene family of Arabidopsis is involved in the biosynthesis of nucleotide sugar precursors for cell-wall matrix polysaccharides. Planta 2005, 221, 243–254. [Google Scholar] [CrossRef]
- Adak, S.; Agarwal, T.; Das, P.; Ray, S.; Lahiri Majumder, A. Characterization of Myo-inositol oxygenase from rice (OsMIOX): Influence of salinity stress in different indica rice cultivars. Physiol. Mol. Biol. Plants 2023, 29, 927–945. [Google Scholar] [CrossRef]
- Zheng, X.; Gong, M.; Zhang, Q.; Tan, H.; Li, L.; Tang, Y.; Li, Z.; Peng, M.; Deng, W. Metabolism and regulation of ascorbic acid in fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Zhong, Y.; Zheng, J.; Ali, M.; Liu, G.D.; Zheng, X.L. L-ascorbic acid metabolism in an ascorbate-rich kiwifruit (Actinidia. Eriantha Benth.) cv. ‘White’ during postharvest. Plant Physiol. Biochem. 2018, 124, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kakan, X.; Yu, Y.; Li, S.; Li, X.; Huang, R.; Wang, J. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R. L-ascorbic acid: A multifunctional molecule supporting plant growth and development. Scientifica 2013, 2013, 795964. [Google Scholar] [CrossRef]
- Williams, D.J.; Edwards, D.; Pun, S.; Chaliha, M.; Burren, B.; Tinggi, U.; Sultanbawa, Y. Organic acids in kakadu plum (Terminalia ferdinandiana): The good (ellagic), the bad (oxalic) and the uncertain (ascorbic). Food Res. Int. 2016, 89, 237–244. [Google Scholar] [CrossRef]
- Castro, J.C.; Maddox, J.D.; Imán, S.A. Camu-camu–Myrciaria dubia (Kunth) McVaugh. In Exotic Fruits Reference Guide; Academic Press: Cambridge, MA, USA, 2018; pp. 97–105. [Google Scholar]
- Aniceto, A.; Montenegro, J.; da Cadena, R.S.; Teodoro, A.J. Physicochemical characterization, antioxidant capacity, and sensory properties of murici (Byrsonima crassifolia (L.) Kunth) and taperebá (Spondias mombin L.) beverages. Molecules 2021, 26, E332. [Google Scholar] [CrossRef]
- Carneiro Ferreira, I.; Pereira da Silva, V.; Vilvert, J.C.; de França Souza, F.; de Freitas, S.T.; Dos Santos Lima, M. Brazilian varieties of acerola (Malpighia emarginata DC.) produced under tropical semi-arid conditions: Bioactive phenolic compounds, sugars, organic acids, and antioxidant capacity. J. Food Biochem. 2021, 45, e13829. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Milić-Komić, S.; Vuleta, A.; Zechmann, B.; Prokić, L. Characterisation of antioxidants in photosynthetic and non-photosynthetic leaf tissues of variegated pelargonium zonale plants. Plant Biol. 2016, 18, 669–680. [Google Scholar] [CrossRef]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum-evolutionary trees. Mol. Biol. Evol. 1992, 9, 945. [Google Scholar] [CrossRef]
- Boratyn, G.M.; Thierry-Mieg, J.; Thierry-Mieg, D.; Busby, B.; Madden, T.L. Magic-BLAST, an accurate RNA-seq aligner for long and short reads. BMC Bioinform. 2019, 20, 405. [Google Scholar] [CrossRef]
- Putri, G.; Anders, S.; Pyl, P.T.; Pimanda, J.E.; Zanini, F. Analysing high-throughput sequencing data in Python with HTSeq 2.0. Bioinformatics 2022, 38, 2943–2945. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.F.; Morales, L.M.M.; Santana, D.B.; Silva, G.M.C.; Gomes, M.M.D.A.; Ayub, R.A.; Costa, J.H.; de Oliveira, J.G. Ascorbate synthesis as an alternative electron source for mitochondrial respiration: Possible implications for plant performance. Front. Plant Sci. 2022, 13, 987077. [Google Scholar] [CrossRef]
- Saraiva, K.D.D.S.; Oliveira, A.E.R.; dos Santos, C.P.; Lima, K.T.L.; de Sousa, J.M.; de Melo, D.F.; Costa, J.H. Phylogenetic analysis and differential expression of EF1 genes in soybean during development, stress, and phytohormone treatments. Mol. Genet. Genom. 2015, 291, 1505–1522. [Google Scholar] [CrossRef]
- Maia, R.A.; da Cruz Saraiva, K.D.; Roque, A.L.M.; Thiers, K.L.L.; Dos Santos, C.P.; da Silva, J.H.M.; Feijó, D.F.; Arnholdt-Schmitt, B.; Costa, J.H. Differential expression of recently duplicated PTOX genes in Glycine max during plant development and stress conditions. J. Bioenerg. Biomembr. 2019, 51, 355–370. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene duplication as a major force in evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef]
- Rensing, S.A. Gene duplication as a driver of plant morphogenetic evolution. Curr. Opin. Plant Biol. 2014, 17, 43–48. [Google Scholar] [CrossRef]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar] [CrossRef]
- Ishikawa, T.; Shigeoka, S. Recent advances in ascorbate biosynthesis and the physiological significance of ascorbate peroxidase in photosynthesizing organisms. Biosci. Biotechnol. Biochem. 2008, 72, 1143–1154. [Google Scholar] [CrossRef]
- Ishikawa, T.; Dowdle, J.; Smirnoff, N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol. Plant. 2006, 126, 343–355. [Google Scholar] [CrossRef]
- Smirnoff, N. Vitamin C: The metabolism and functions of ascorbic acid in plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2011; Volume 59, pp. 107–177. [Google Scholar]
- Sodeyama, T.; Nishikawa, H.; Harai, K.; Takeshima, D.; Sawa, Y.; Maruta, T.; Ishikawa, T. The d-mannose/l-galactose pathway is the dominant ascorbate biosynthetic route in the moss Physcomitrium patens. Plant J. 2021, 107, 1724–1738. [Google Scholar] [CrossRef]
- Jeevan Kumar, S.P.; Rajendra Prasad, S.; Banerjee, R.; Thammineni, C. Seed birth to death: Dual functions of reactive oxygen species in seed physiology. Ann. Bot. 2015, 116, 663–668. [Google Scholar] [CrossRef]
- Chen, Z.; Cao, X.L.; Niu, J.P. Effects of exogenous ascorbic acid on seed germination and seedling salt-tolerance of alfalfa. PLoS ONE 2021, 16, e0250926. [Google Scholar] [CrossRef]
- Nunes, L.R.D.L.; Pinheiro, P.R.; Silva, J.B.D.; Dutra, A.S. Effects of ascorbic acid on the germination and vigour of cowpea seeds under water stress. Rev. Ciência Agronômica 2020, 51, e20196629. [Google Scholar]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Wang, C.; García-Caparros, P.; Li, Z.; Chen, F. A comprehensive review on plant ascorbic acid. Trop. Plants 2024, 3, e042. [Google Scholar] [CrossRef]
- Moallemi, N.; Khaleghi, E. The effect of ascorbic acid on vegetative growth of olive plants cv. ‘Baghmalek’ under water deficit conditions. J. Hortic. Sci. Technol. 2021, 34, 621–632. [Google Scholar] [CrossRef]
- Rehman, S.; Wei, X. To study the effect of ascorbic acid as a foliar spray on the growth of Triticum aestivum L. Eur. J. Bot. Plant Sci. Phytol. 2022, 7, 62–72. [Google Scholar]
- Hu, L.; Zhou, K.; Ren, G.; Yang, S.; Liu, Y.; Zhang, Z.; Li, Y.; Gong, X.; Ma, F. Myo-inositol mediates reactive oxygen species-induced programmed cell death via salicylic acid-dependent and ethylene-dependent pathways in apple. Hortic. Res. 2020, 7, 138. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Q.; Wu, W.; Wang, J.; Li, G.; Xu, S.; Shao, S.; Liu, M.; Zhong, C.; He, Z.; et al. Genomic evidence for rediploidization and adaptive evolution following the whole-genome triplication. Nat. Commun. 2024, 15, 1635. [Google Scholar] [CrossRef]
- Aguiar, É.S.D.; Dias, A.N.; Sousa, R.M.; Germano, T.A.; de Sousa, R.O.; Miranda, R.D.S.; Costa, J.H.; Dos Santos, C.P. Genome and Transcriptome Analyses of Genes Involved in Ascorbate Biosynthesis in Pepper Indicate Key Genes Related to Fruit Development, Stresses, and Phytohormone Exposures. Plants 2023, 12, 3367. [Google Scholar] [CrossRef]
- Ioannidi, E.; Kalamaki, M.S.; Engineer, C.; Pateraki, I.; Alexandrou, D.; Mellidou, I. Expression profiling of ascorbic acid-related genes during tomato fruit development and ripening and in response to stress conditions. J. Exp. Bot. 2009, 60, 663–678. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Souza, I.C.; Ramos, M.V.; Costa, J.H.; Freitas, C.D.; Oliveira, R.S.; Moreno, F.B.; Moreira, R.A.; Carvalho, C.P. The osmotin of Calotropis procera latex is not expressed in laticifer-free cultivated callus and under salt stress. Plant Physiol. Biochem. 2017, 119, 312–318. [Google Scholar] [CrossRef]
- Freitas, N.M.D.; Silva, V.F.V.; Teixeira, C.A.S.; Ferreira, L.A.I.; Padovese, L.M.; Oliveira, R.S.D., Jr. Light, temperature, and sowing depth on germination of garden spurge. Ciência Rural 2021, 51, e20200764. [Google Scholar] [CrossRef]
- Hoagland, D.; Arnon, D.I. The water culture method for growing plants without soil. Calif. Agric. Exp. Stn. Circ. 1950, 347, 39. [Google Scholar]
- Chen, J.X.; Wang, X.F. Experimental Instruction of Plant Physiology; South China University of Technology Press: Guangzhou, China, 2002; pp. 125–127. [Google Scholar]
- Nogales, A.; Nobre, T.; Valadas, V.; Ragonezi, C.; Döring, M.; Polidoros, A.; Arnholdt-Schmitt, B. Can functional hologenomics aid tackling current challenges in plant breeding? Brief. Funct. Genom. 2016, 15, 288–297. [Google Scholar] [CrossRef]
- Gazara, R.K.; de Oliveira, E.A.G.; Rodrigues, B.C.; da Fonseca, R.N.; Oliveira, A.E.A.; Venancio, T.M. Transcriptional landscape of soybean (Glycine max) embryonic axes during germination in the presence of paclobutrazol, a gibberellin biosynthesis inhibitor. Sci. Rep. 2019, 9, 9601. [Google Scholar] [CrossRef]
- Brown, A.V.; Hudson, K.A. Developmental profiling of gene expression in soybean trifoliate leaves and cotyledons. BMC Plant Biol. 2015, 15, 169. [Google Scholar] [CrossRef]
- Redekar, N.; Pilot, G.; Raboy, V.; Li, S.; Maroof, M.A.S. Inference of Transcription Regulatory Network in Low Phytic Acid Soybean Seeds. Front. Plant Sci. 2017, 8, 2029. [Google Scholar] [CrossRef]
- Belamkar, V.; Weeks, N.T.; Bharti, A.K.; Farmer, A.D.; Graham, M.A.; Cannon, S.B. Comprehensive characterization and RNA-Seq profiling of the HD-Zip transcription factor family in soybean (Glycine max) during dehydration and salt stress. BMC Genom. 2014, 15, 950. [Google Scholar] [CrossRef]
- DeMers, L.C.; Redekar, N.R.; Kachroo, A.; Tolin, S.A.; Li, S.; Maroof, M.A.S. A transcriptional regulatory network of Rsv3-mediated extreme resistance against Soybean mosaic virus. PLoS ONE 2020, 15, e0231658. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ishiga, Y.; Yamanaka, N.; Ogiso-Tanaka, E.; Yamaoka, Y. Soybean leaves transcriptomic data dissects the phenylpropanoid pathway genes as a defence response against Phakopsora pachyrhizi. Plant Physiol. Biochem. 2018, 132, 424–433. [Google Scholar] [CrossRef]
- Wulff, J.A.; Kiani, M.; Regan, K.; Eubanks, M.D.; Szczepaniec, A. Neonicotinoid Insecticides Alter the Transcriptome of Soybean and Decrease Plant Resistance. Int. J. Mol. Sci. 2019, 20, 783. [Google Scholar] [CrossRef]
- Neupane, S.; Mathew, F.M.; Varenhorst, A.J.; Nepal, M.P. Transcriptome profiling of interaction effects of soybean cyst nematodes and soybean aphids on soybean. Sci. Data 2019, 6, 133. [Google Scholar] [CrossRef]
- Rambani, A.; Pantalone, V.; Yang, S.; Rice, J.H.; Song, Q.; Mazarei, M.; Arelli, P.R.; Meksem, K.; Stewart, C.N.; Hewezi, T. Identification of introduced and stably inherited DNA methylation variants in soybean associated with soybean cyst nematode parasitism. New Phytol. 2020, 227, 168–184. [Google Scholar] [CrossRef]
- Baetsen-Young, A.; Wai, C.M.; VanBuren, R.; Day, B. Fusarium virguliforme Transcriptional Plasticity Is Revealed by Host Colonization of Maize versus Soybean. Plant Cell 2019, 32, 336–351. [Google Scholar] [CrossRef]
- Arikit, S.; Xia, R.; Kakrana, A.; Huang, K.; Zhai, J.; Yan, Z.; Valdés-López, O.; Prince, S.; Musket, T.A.; Nguyen, H.T.; et al. An Atlas of Soybean Small RNAs Identifies Phased siRNAs from Hundreds of Coding Genes. Plant Cell 2014, 26, 4584–4601. [Google Scholar] [CrossRef]
- Bellieny-Rabelo, D.; De Oliveira, E.A.G.; Ribeiro, E.D.S.; Costa, E.P.; Oliveira, A.E.A.; Venancio, T.M. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Sci. Rep. 2016, 6, 36009. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, S.; Germano, T.A.; Sousa Do Nascimento, M.A.d.S.d.; dos Santos, C.P.; Arnholdt-Schmitt, B.; de Miranda, M.R.A.; Gomes, M.M.d.A.; Mazorra Morales, L.M.; Ayub, R.A.; Oliveira, J.G.d.; et al. Tissue- and Condition-Specific Biosynthesis of Ascorbic Acid in Glycine max L.: Insights from Genome-Wide Analyses of Pathway-Encoding Genes, Expression Profiling, and Mass Fraction Determination. Int. J. Mol. Sci. 2025, 26, 4678. https://doi.org/10.3390/ijms26104678
Aziz S, Germano TA, Sousa Do Nascimento MAdSd, dos Santos CP, Arnholdt-Schmitt B, de Miranda MRA, Gomes MMdA, Mazorra Morales LM, Ayub RA, Oliveira JGd, et al. Tissue- and Condition-Specific Biosynthesis of Ascorbic Acid in Glycine max L.: Insights from Genome-Wide Analyses of Pathway-Encoding Genes, Expression Profiling, and Mass Fraction Determination. International Journal of Molecular Sciences. 2025; 26(10):4678. https://doi.org/10.3390/ijms26104678
Chicago/Turabian StyleAziz, Shahid, Thais Andrade Germano, Maria Adriele dos Santos de Sousa Do Nascimento, Clesivan Pereira dos Santos, Birgit Arnholdt-Schmitt, Maria Raquel Alcântara de Miranda, Mara Menezes de Assis Gomes, Luis Miguel Mazorra Morales, Ricardo Antônio Ayub, Jurandi Gonçalves de Oliveira, and et al. 2025. "Tissue- and Condition-Specific Biosynthesis of Ascorbic Acid in Glycine max L.: Insights from Genome-Wide Analyses of Pathway-Encoding Genes, Expression Profiling, and Mass Fraction Determination" International Journal of Molecular Sciences 26, no. 10: 4678. https://doi.org/10.3390/ijms26104678
APA StyleAziz, S., Germano, T. A., Sousa Do Nascimento, M. A. d. S. d., dos Santos, C. P., Arnholdt-Schmitt, B., de Miranda, M. R. A., Gomes, M. M. d. A., Mazorra Morales, L. M., Ayub, R. A., Oliveira, J. G. d., & Costa, J. H. (2025). Tissue- and Condition-Specific Biosynthesis of Ascorbic Acid in Glycine max L.: Insights from Genome-Wide Analyses of Pathway-Encoding Genes, Expression Profiling, and Mass Fraction Determination. International Journal of Molecular Sciences, 26(10), 4678. https://doi.org/10.3390/ijms26104678







