A Novel Sample Preparation Method for GC-MS Analysis of Volatile Organic Compounds in Whole Blood for Veterinary Use
Abstract
1. Introduction
2. Results
2.1. Sensitivity Comparison Across Sample Preparation Reagent Combinations
2.2. Sensitivity Alterations According to Sample Volume
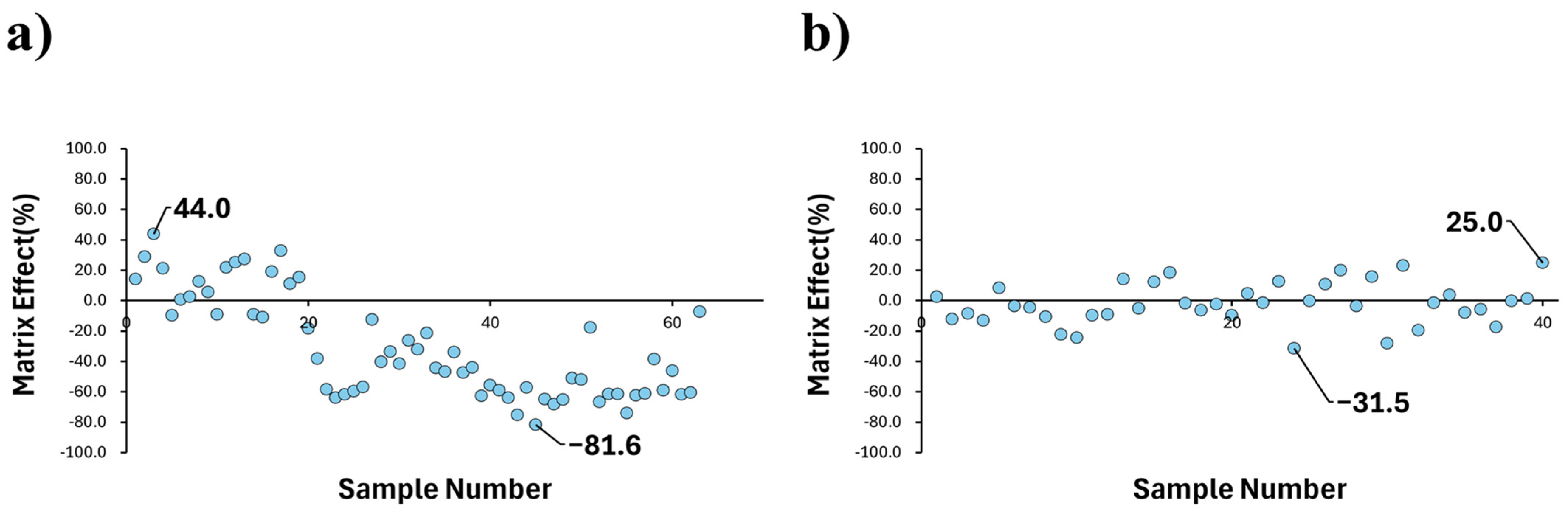
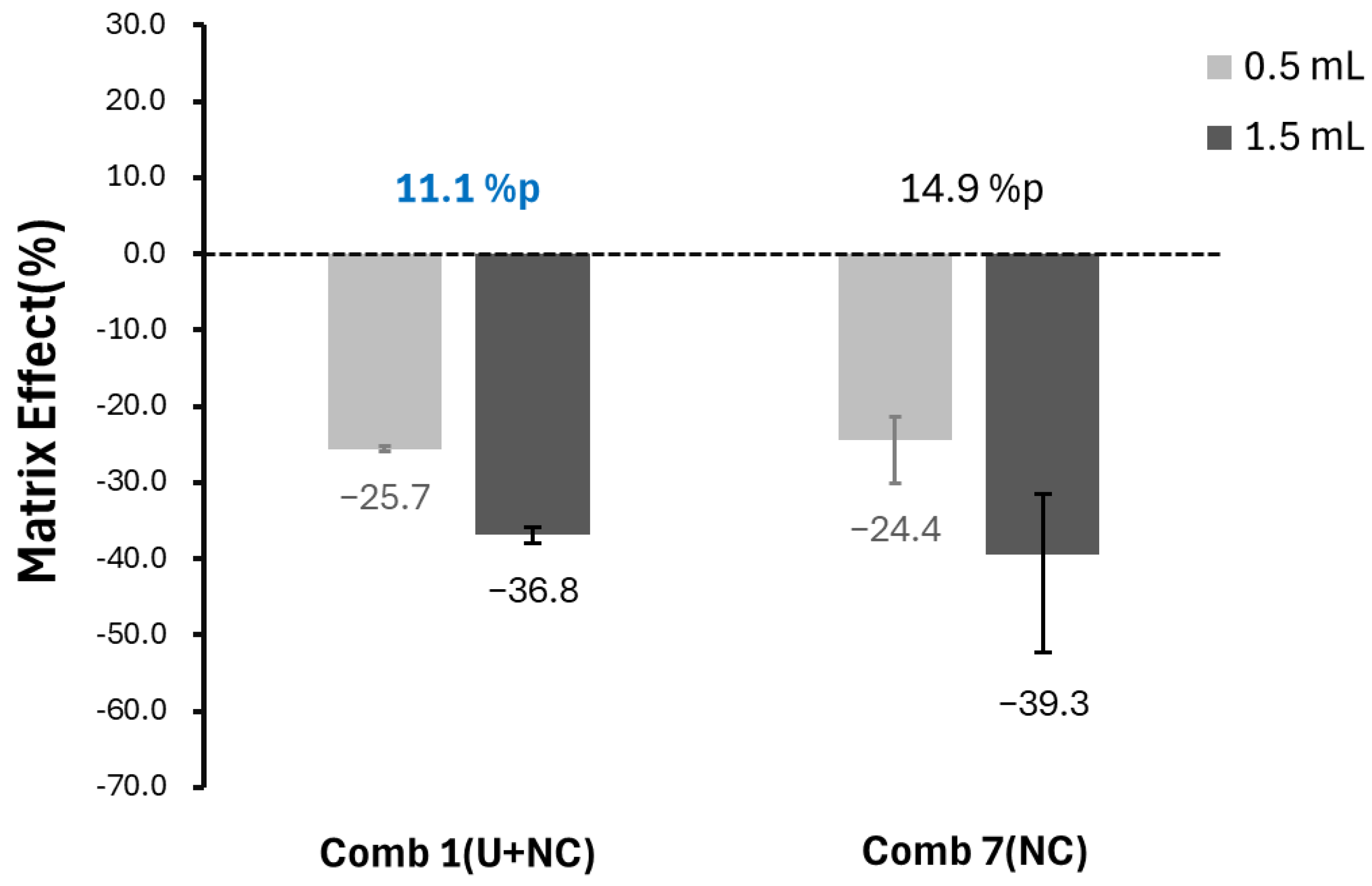
2.3. Comparison of Matrix Effect Uniformity Across Combinations
3. Discussion
4. Materials and Methods
4.1. Standards and Chemicals
4.2. Sample Collection
4.3. Sample Preparation
4.4. Gas Chromatography-Mass Spectrometry Analysis
4.5. Selection of Quantification and Qualification Ions for GC-MS Analysis
4.6. GC-MS Analysis
4.7. Comparison of Sensitivity and Matrix Effects Among Combinations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPDA | Citrate−phosphate−dextrose−adenine |
| SDS | Sodium dodecyl sulfate |
| VOC | Volatile organic compound |
| NaCl | Sodium chloride |
| K2SO4 | Potassium sulfate |
| Na2SO4 | Sodium sulfate |
| GC-MS | Gas chromatography−mass spectrometry |
| H/S | Headspace |
| SIM | Selective ion monitoring |
References
- Tan, T.; Xu, X.; Gu, H.; Cao, L.; Liu, T.; Zhang, Y.; Wang, J.; Chen, M.; Li, H.; Ge, X. The Characteristics, Sources, and Health Risks of Volatile Organic Compounds in an Industrial Area of Nanjing. Toxics 2024, 12, 868. [Google Scholar] [CrossRef]
- Chen, J.J.; Wang, T.B.; Chang, L.-T.; Chuang, K.-J.; Chuang, H.-C.; Chang, T.-Y. Exposure and health risk assessment of volatile organic compounds among drivers and passengers in long-distance buses. Environ. Res. 2024, 252 Pt 3, 118959. [Google Scholar] [CrossRef]
- Alsaadi, D.; Clements, N.; Gabuniya, N.; Francis, N.; Chand, M. Exhaled volatile organic compounds in the detection of colorectal cancer: A systematic review and meta-analysis. Excli. J. 2024, 23, 795–810. [Google Scholar] [PubMed]
- Dawson, J.; Green, K.; Lazarowicz, H.; Cornford, P.; Probert, C. Analysis of urinary volatile organic compounds for prostate cancer diagnosis: A systematic review. BJUI Compass 2024, 5, 822–833. [Google Scholar] [CrossRef]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Alkateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef] [PubMed]
- Perera, T.R.W.; Skerrett-Byrne, D.A.; Gibb, Z.; Nixon, B.; Swegen, A. The Future of Biomarkers in Veterinary Medicine: Emerging Approaches and Associated Challenges. Animals 2022, 12, 2194. [Google Scholar] [CrossRef] [PubMed]
- Einoch Amor, R.; Levy, J.; Broza, Y.Y.; Vangravs, R.; Rapoport, S.; Zhang, M.; Wu, W.; Leja, M.; Behar, J.A.; Haick, H. Liquid Biopsy-Based Volatile Organic Compounds from Blood and Urine and Their Combined Data Sets for Highly Accurate Detection of Cancer. ACS Sens. 2023, 8, 1450–1461. [Google Scholar] [CrossRef]
- Williams, M.L.; Olomukoro, A.A.; Emmons, R.V.; Godage, N.H.; Gionfriddo, E. Matrix effects demystified: Strategies for resolving challenges in analytical separations of complex samples. J. Sep. Sci. 2023, 46, e2300571. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.; Marcillo, A.; Davis, C.; Milkovska-Stamenova, S.; Hutschenreuther, A.; Birkemeyer, C. Matrix Effects in GC-MS Profiling of Common Metabolites after Trimethylsilyl Derivatization. Molecules 2023, 28, 2653. [Google Scholar] [CrossRef]
- Gosetti, F.; Mazzucco, E.; Zampieri, D.; Gennaro, M.C. Signal suppression/enhancement in high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2010, 1217, 3929–3937. [Google Scholar] [CrossRef]
- Kandaswamy, C.; Presley, D.; Ahammed, T.P.S.; Anadaram, S. Improved chromatographic performance in red chilli powder pesticide residue quantitation by retention gap introduction in gas chromatography tandem mass spectrometry. J. Food Sci. Technol. 2022, 59, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Perez, J.F.; Baslé, Q.; Dubois, M.; Theurillat, X. Multi-residue pesticides determination in complex food matrices by gas chromatography tandem mass spectrometry. Food Chem. 2024, 436, 137687. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kwon, S.-Y.; Seo, Y.-S.; Lee, Y.-B.; Mok, J.H. Application of static headspace GC-MS for detection of residual trichloroethylene and toluene solvents in beta-cyclodextrin. Food Res. Int. 2024, 197 Pt 2, 115292. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, M.; Liu, H.; Niu, Y.; Wang, S.; Zhang, F.; Liu, H. Simultaneous determination of seven endogenous aldehydes in human blood by headspace gas chromatography-mass spectrometry. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2019, 1118–1119, 85–92. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Moosavi-Movahedi, A.A. Human hemoglobin structural and functional alterations and heme degradation upon interaction with benzene: A spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 157, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Kuiper, B.P.; Thunnissen, A.-M.W.H.; Cool, R.H.; Zhou, L.; Huang, C.; Dijkstra, B.W.; Broos, J. The Role of Tryptophan in pi Interactions in Proteins: An Experimental Approach. J. Am. Chem. Soc. 2022, 144, 13815–13822. [Google Scholar] [CrossRef]
- Crowley, P.B.; Golovin, A. Cation-pi interactions in protein-protein interfaces. Proteins 2005, 59, 231–239. [Google Scholar] [CrossRef]
- Poulson, S.; Harrington, R.; Drever, J. The solubility of toluene in aqueous salt solutions. Talanta 1999, 48, 633–641. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Shao, H.; Zheng, Q. Non-targeted screening and analysis of volatile organic compounds in drinking water by DLLME with GC-MS. Sci. Total Environ. 2019, 694, 133494. [Google Scholar] [CrossRef]
- Cervera, M.I.; Beltran, J.; Lopez, F.; Hernandez, F. Determination of volatile organic compounds in water by headspace solid-phase microextraction gas chromatography coupled to tandem mass spectrometry with triple quadrupole analyzer. Anal. Chim. Acta 2011, 704, 87–97. [Google Scholar] [CrossRef]
- Dupree, E.J.; Jayathirtha, M.; Yorkey, H.; Mihasan, M.; Petre, B.A.; Darie, C.C. A Critical Review of Bottom-Up Proteomics: The Good, the Bad, and the Future of this Field. Proteomes 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
| Combination(s) | PDR | Salt |
|---|---|---|
| Cont. | H2O | H2O |
| Comb 1 | Urea | NaCl |
| Comb 2 | Urea | K2SO4 |
| Comb 3 | Urea | Na2SO4 |
| Comb 4 | SDS | NaCl |
| Comb 5 | SDS | K2SO4 |
| Comb 6 | SDS | Na2SO4 |
| Comb 7 | H2O | NaCl |
| Comb 8 | H2O | K2SO4 |
| Comb 9 | H2O | Na2SO4 |
| Comb 10 | Urea | H2O |
| Comb 11 | SDS | H2O |
| Combination | Benzene | Toluene | Ethylbenzene | m-/p-Xylene | o-Xylene | Styrene | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (1) | CV (2) | Mean | CV | Mean | CV | Mean | CV | Mean | CV | Mean | CV | |
| Cont. | 100.0 | 3.4 | 100.0 | 3.8 | 100.0 | 4.1 | 100.0 | 4.1 | 100.0 | 4.0 | 100.0 | 3.7 |
| Comb 1(U+NC) | 143.4 | 0.8 | 147.3 | 1.0 | 136.8 | 1.0 | 140.1 | 1.1 | 147.1 | 0.9 | 151.3 | 1.0 |
| Comb 2(U+KS) | 97.3 | 1.0 | 102.8 | 0.9 | 103.8 | 1.4 | 105.3 | 1.3 | 104.2 | 1.3 | 105.4 | 1.0 |
| Comb 3(U+NS) | 137.5 | 2.2 | 140.8 | 1.3 | 116.5 | 0.8 | 117.1 | 1.1 | 130.9 | 0.8 | 150.5 | 0.6 |
| Comb 4(S+NC) | 138.3 | 6.4 | 115.5 | 8.3 | 72.9 | 8.8 | 69.9 | 8.7 | 79.2 | 7.8 | 82.4 | 6.8 |
| Comb 5(S+KS) | 130.2 | 3.9 | 123.3 | 2.3 | 102.3 | 2.3 | 99.7 | 2.5 | 101.3 | 2.8 | 119.0 | 2.6 |
| Comb 6(S+NS) | 207.1 | 10.1 | 148.2 | 7.3 | 90.0 | 10.2 | 86.8 | 10.8 | 101.0 | 9.1 | 111.2 | 10.9 |
| Comb 7(NC) | 222.3 | 5.3 | 235.9 | 6.0 | 196.8 | 6.2 | 197.3 | 6.6 | 215.0 | 6.2 | 241.2 | 5.2 |
| Comb 8(KS) | 126.6 | 2.0 | 124.5 | 1.7 | 122.9 | 1.9 | 123.4 | 2.0 | 126.4 | 1.7 | 119.8 | 4.6 |
| Comb 9(NS) | 169.9 | 0.8 | 155.2 | 1.0 | 126.4 | 0.6 | 128.7 | 0.8 | 145.5 | 1.0 | 154.5 | 0.5 |
| Comb 10(U) | 82.2 | 2.3 | 84.5 | 2.8 | 85.3 | 3.0 | 86.1 | 2.9 | 84.0 | 3.2 | 89.6 | 2.9 |
| Comb 11(S) | 93.6 | 9.1 | 94.9 | 11.0 | 85.6 | 11.0 | 84.4 | 10.7 | 84.5 | 10.8 | 53.0 | 12.8 |
| Combination | Mean (%) ± SD (1) | |||||
|---|---|---|---|---|---|---|
| Benzene | Toluene | Ethylbenzene | m,p-Xylene | o-Xylene | Styrene | |
| Cont. | 12.8 ± 0.7 | 19.5 ± 0.9 | 22.8 ± 0.5 | 23.2 ± 0.5 | 23.3 ± 0.6 | 35.0 ± 0.3 |
| Comb 1(U+NC) | 16.7 ± 2.2 | 15.6 ± 3.0 | 26.6 ± 2.7 | 28.2 ± 2.6 | 28.5 ± 2.8 | 31.7 ± 1.8 |
| Comb 2(U+KS) | 10.3 ± 0.9 | 26.5 ± 0.9 | 14.6 ± 1.0 | 15.9 ± 0.8 | 16.6 ± 0.4 | 24.6 ± 1.6 |
| Comb 3(U+NS) | 16.6 ± 5.2 | 4.3 ± 7.0 | 43.5 ± 4.7 | 45.5 ± 4.7 | 47.7 ± 4.8 | 44.9 ± 4.7 |
| Comb 4(S+NC) | 31.8 ± 6.3 | 8.4 ± 7.4 | 39.9 ± 2.8 | 39.6 ± 2.8 | 41.3 ± 3.1 | 39.1 ± 3.9 |
| Comb 5(S+KS) | 6.3 ± 1.1 | 31.2 ± 7.6 | 13.3 ± 5.0 | 13.9 ± 5.3 | 14.4 ± 5.3 | 23.9 ± 3.8 |
| Comb 6(S+NS) | 39.1 ± 3.0 | 28.1 ± 3.8 | 48.7 ± 2.3 | 49.1 ± 2.1 | 51.2 ± 3.1 | 47.1 ± 3.0 |
| Comb 7(NC) | 19.2 ± 0.8 | 2.7 ± 2.3 | 30.8 ± 1.0 | 31.5 ± 1.0 | 32.6 ± 0.8 | 35.5 ± 1.8 |
| Comb 8(KS) | 18.9 ± 6.7 | 14.2 ± 4.9 | 26.2 ± 4.3 | 27.1 ± 4.4 | 27.3 ± 3.6 | 37.8 ± 1.7 |
| Comb 9(NS) | 25.4 ± 16.5 | 17.9 ± 16.3 | 42.8 ± 20.6 | 44.0 ± 21.1 | 42.9 ± 19.1 | 50.9 ± 18.5 |
| Comb 10(U) | 4.9 ± 0.3 | 34.5 ± 0.7 | 8.2 ± 0.4 | 9.4 ± 0.3 | 10.4 ± 0.2 | 29.2 ± 0.4 |
| Comb 11(S) | 12.8 ± 7.3 | 54.7 ± 6.0 | 3.5 ± 1.1 | 3.9 ± 1.5 | 4.7 ± 3.1 | 42.0 ± 5.6 |
| Compound | Structure | Molecular Formula | Molecular Weight | Retention Times | Quantification Ion (m/z) | Qualification Ion (m/z) |
|---|---|---|---|---|---|---|
| Benzene | 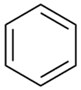 | C6H6 | 78.11 | 4.78 | 78 | 77 |
| Toluene | 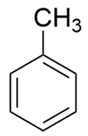 | C5H5CH3 | 92.14 | 8.08 | 91 | 92 |
| Ethylbenzene |  | C6H5CH2CH3 | 106.17 | 10.48 | 91 | 106 |
| m-xylene | 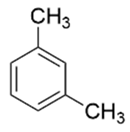 | C6H4(CH3)2 | 106.16 | 10.68 | 106 | 105 |
| p-xylene |  | C6H4(CH3)2 | 106.16 | 10.68 | 106 | 105 |
| o-xylene | 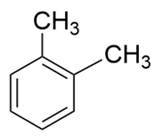 | C6H4(CH3)2 | 106.16 | 11.16 | 91 | 106 |
| Styrene |  | C6H5CH=CH2 | 104.15 | 11.24 | 104 | 78 |
| Fluorobenzene | 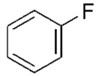 | C6H5F | 96.10 | 5.47 | 96 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, K.-G.; Choi, R.; Gwak, S.; Choi, I.; Jang, G.; Kim, J.-W.; Kim, G.A. A Novel Sample Preparation Method for GC-MS Analysis of Volatile Organic Compounds in Whole Blood for Veterinary Use. Int. J. Mol. Sci. 2025, 26, 4667. https://doi.org/10.3390/ijms26104667
Ahn K-G, Choi R, Gwak S, Choi I, Jang G, Kim J-W, Kim GA. A Novel Sample Preparation Method for GC-MS Analysis of Volatile Organic Compounds in Whole Blood for Veterinary Use. International Journal of Molecular Sciences. 2025; 26(10):4667. https://doi.org/10.3390/ijms26104667
Chicago/Turabian StyleAhn, Kyung-Geun, Ryuho Choi, Soonchul Gwak, Inyoung Choi, Giup Jang, Jin-Wook Kim, and Geon A Kim. 2025. "A Novel Sample Preparation Method for GC-MS Analysis of Volatile Organic Compounds in Whole Blood for Veterinary Use" International Journal of Molecular Sciences 26, no. 10: 4667. https://doi.org/10.3390/ijms26104667
APA StyleAhn, K.-G., Choi, R., Gwak, S., Choi, I., Jang, G., Kim, J.-W., & Kim, G. A. (2025). A Novel Sample Preparation Method for GC-MS Analysis of Volatile Organic Compounds in Whole Blood for Veterinary Use. International Journal of Molecular Sciences, 26(10), 4667. https://doi.org/10.3390/ijms26104667






