Pilot Investigation on Markers of Bone Metabolism, Angiogenesis, and Neuroendocrine Activity as Potential Predictors of Survival of Metastatic Prostate Cancer Patients with Bone Metastases
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of Patients
2.2. Identification of Molecular Clusters and Key Biomarkers
2.3. Influence of Clinical Factors and Prior Treatments on Biomarker Levels
2.4. Biomarker Dynamics
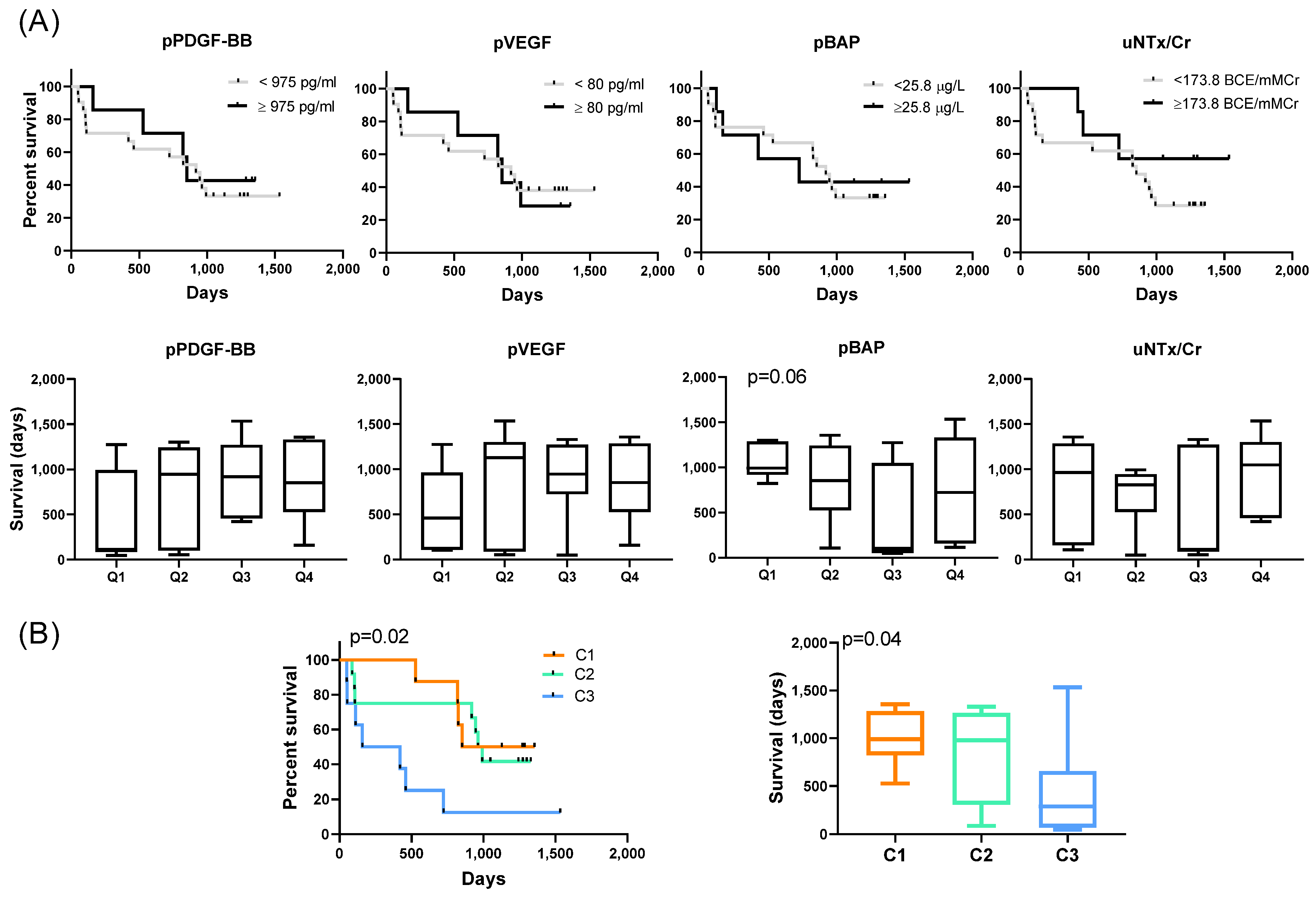
2.5. Prognostic Implications
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Determination of Plasma, Serum, and Urine Markers
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Roghmann, F.; Antczak, C.; McKay, R.R.; Choueiri, T.; Hu, J.C.; Kibel, A.S.; Kim, S.P.; Kowalczyk, K.J.; Menon, M.; Nguyen, P.L.; et al. The burden of skeletal-related events in patients with prostate cancer and bone metastasis. Urol. Oncol. 2015, 33, 17.e9–17.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, J.; Zeng, H. Our Current Understanding of the Heterogeneity in Prostate Cancer and Renal Cell Carcinoma. J. Clin. Med. 2023, 12, 1526. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Mao, J.; Sheng, L.; Wu, H.; Bai, G.; Zhong, Z.; Pan, Z. Biomarkers for Prostate Cancer Bone Metastasis Detection and Prediction. J. Pers. Med. 2023, 13, 705. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.N., Jr.; Mayerson, E.; Gertz, E.; Tangen, C.; Goldkorn, A.; van Loan, M.; Hussain, M.; Gupta, S.; Zhang, J.; Parikh, M.; et al. Bone Biomarkers and Subsequent Survival in Men with Hormone-sensitive Prostate Cancer: Results from the SWOG S1216 Phase 3 Trial of Androgen Deprivation Therapy with or Without Orteronel. Eur. Urol. 2024, 85, 171–176. [Google Scholar] [CrossRef]
- Brown, J.E.; Sim, S. Evolving role of bone biomarkers in castration-resistant prostate cancer. Neoplasia 2010, 12, 685–696. [Google Scholar] [CrossRef]
- Corey, E.; Brown, L.G.; Kiefer, J.A.; Quinn, J.E.; Pitts, T.E.; Blair, J.M.; Vessella, R.L. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005, 65, 1710–1718. [Google Scholar] [CrossRef]
- Ramaswamy, G.; Rao, V.R.; Krishnamoorthy, L.; Ramesh, G.; Gomathy, R.; Renukadevi, D. Serum levels of bone alkaline phosphatase in breast and prostate cancers with bone metastasis. Indian J. Clin. Biochem. 2000, 15, 110–113. [Google Scholar] [CrossRef]
- Thoms, J.W.; Dal Pra, A.; Anborgh, P.H.; Christensen, E.; Fleshner, N.; Menard, C.; Chadwick, K.; Milosevic, M.; Catton, C.; Pintilie, M.; et al. Plasma osteopontin as a biomarker of prostate cancer aggression: Relationship to risk category and treatment response. Br. J. Cancer 2012, 107, 840–846. [Google Scholar] [CrossRef]
- Garnero, P.; Buchs, N.; Zekri, J.; Rizzoli, R.; Coleman, R.E.; Delmas, P.D. Markers of bone turnover for the management of patients with bone metastases from prostate cancer. Br. J. Cancer 2000, 82, 858–864. [Google Scholar] [CrossRef]
- Rosar, F.; Ribbat, K.; Ries, M.; Linxweiler, J.; Bartholoma, M.; Maus, S.; Schreckenberger, M.; Ezziddin, S.; Khreish, F. Neuron-specific enolase has potential value as a biomarker for [(18)F]FDG/[(68)Ga]Ga-PSMA-11 PET mismatch findings in advanced mCRPC patients. EJNMMI Res. 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Ploussard, G.; Rozet, F.; Roubaud, G.; Stanbury, T.; Sargos, P.; Roupret, M. Chromogranin A: A useful biomarker in castration-resistant prostate cancer. World J. Urol. 2023, 41, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, L.; Hu, J.; Feng, D.; Xu, L. Diagnostic performance of choline PET/CT for the detection of bone metastasis in prostate cancer: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0203400. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Massard, C.; Smith, M.; Rader, M.; Brown, J.; Milecki, P.; Shore, N.; Oudard, S.; Karsh, L.; Carducci, M.; et al. Bone-related Parameters are the Main Prognostic Factors for Overall Survival in Men with Bone Metastases from Castration-resistant Prostate Cancer. Eur. Urol. 2015, 68, 42–50. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Hennenlotter, J.; Rausch, S.; Bock, C.; Erne, E.; Schwentner, C.; Stenzl, A. Oncological validation of bone turnover markers c-terminal telopeptide of type I collagen (1CTP) and peptides n-terminal propeptide of type I procollagen (P1NP) in patients with prostate cancer and bone metastases. Transl. Androl. Urol. 2021, 10, 4000–4008. [Google Scholar] [CrossRef]
- Goldkorn, A.; Tangen, C.; Plets, M.; Bsteh, D.; Xu, T.; Pinski, J.K.; Ingles, S.; Triche, T.J.; MacVicar, G.R.; Vaena, D.A.; et al. Circulating Tumor Cell Count and Overall Survival in Patients With Metastatic Hormone-Sensitive Prostate Cancer. JAMA Netw. Open 2024, 7, e2437871. [Google Scholar] [CrossRef]
- Enroth, S.; Johansson, A.; Enroth, S.B.; Gyllensten, U. Strong effects of genetic and lifestyle factors on biomarker variation and use of personalized cutoffs. Nat. Commun. 2014, 5, 4684. [Google Scholar] [CrossRef]
- Prensner, J.R.; Rubin, M.A.; Wei, J.T.; Chinnaiyan, A.M. Beyond PSA: The next generation of prostate cancer biomarkers. Sci. Transl. Med. 2012, 4, 127rv123. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Gaballah, M.S.A.; Gaballa, R.; Mahgoub, S.; Hassan, Z.A.; Toraih, E.A.; Drake, B.F.; Abd Elmageed, Z.Y. Small Extracellular Vesicle-Derived microRNAs Stratify Prostate Cancer Patients According to Gleason Score, Race and Associate with Survival of African American and Caucasian Men. Cancers 2021, 13, 5236. [Google Scholar] [CrossRef]
- Matei, D.; Kelich, S.; Cao, L.; Menning, N.; Emerson, R.E.; Rao, J.; Jeng, M.H.; Sledge, G.W. PDGF BB induces VEGF secretion in ovarian cancer. Cancer Biol. Ther. 2007, 6, 1951–1959. [Google Scholar] [CrossRef]
- Zhang, D.; Babayan, L.; Ho, H.; Heaney, A.P. Chromogranin A regulates neuroblastoma proliferation and phenotype. Biol. Open 2019, 8, bio036566. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Kim, K.B.; Kim, D.L.; Kim, S.G.; Choi, K.M.; Baik, S.H.; Choi, D.S.; Kang, Y.S.; Han, S.Y.; Han, K.H.; et al. Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in Type 2 diabetes mellitus. Diabet. Med. 2004, 21, 545–551. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Kim, H.; Chae, Y.J.; Jung, J.H.; Kim, W. Serum VEGF-D level is correlated with renal dysfunction and proteinuria in patients with diabetic chronic kidney disease. Medicine 2022, 101, e28804. [Google Scholar] [CrossRef]
- Cook, R.J.; Coleman, R.; Brown, J.; Lipton, A.; Major, P.; Hei, Y.J.; Saad, F.; Smith, M.R. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 2006, 12 Pt 1, 3361–3367. [Google Scholar] [CrossRef]
- Carano, A.; Teitelbaum, S.L.; Konsek, J.D.; Schlesinger, P.H.; Blair, H.C. Bisphosphonates directly inhibit the bone resorption activity of isolated avian osteoclasts in vitro. J. Clin. Investig. 1990, 85, 456–461. [Google Scholar] [CrossRef]
- Hughes, D.E.; Wright, K.R.; Uy, H.L.; Sasaki, A.; Yoneda, T.; Roodman, G.D.; Mundy, G.R.; Boyce, B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995, 10, 1478–1487. [Google Scholar] [CrossRef]
- Flanagan, A.M.; Chambers, T.J. Inhibition of bone resorption by bisphosphonates: Interactions between bisphosphonates, osteoclasts, and bone. Calcif. Tissue Int. 1991, 49, 407–415. [Google Scholar] [CrossRef]
- McClung, M.; Recker, R.; Miller, P.; Fiske, D.; Minkoff, J.; Kriegman, A.; Zhou, W.; Adera, M.; Davis, J. Intravenous zoledronic acid 5 mg in the treatment of postmenopausal women with low bone density previously treated with alendronate. Bone 2007, 41, 122–128. [Google Scholar] [CrossRef]
- Carter, S.; Braem, K.; Lories, R.J. The role of bone morphogenetic proteins in ankylosing spondylitis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 293–299. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Papadopetraki, A.; Karatza, E.; Vardakostas, D.; Philippou, A.; Kouraklis, G.; Mantas, D. Insulin-like growth factor-I and wound healing, a potential answer to non-healing wounds: A systematic review of the literature and future perspectives. Biomed. Rep. 2021, 15, 66. [Google Scholar] [CrossRef]
- Shanmugalingam, T.; Bosco, C.; Ridley, A.J.; Van Hemelrijck, M. Is there a role for IGF-1 in the development of second primary cancers? Cancer Med. 2016, 5, 3353–3367. [Google Scholar] [CrossRef] [PubMed]
- Francini, E.; Petrioli, R.; Fiaschi, A.I.; Laera, L.; Roviello, G. Effects of abiraterone acetate on chronic kidney disease in 2 patients with metastatic castration-resistant prostate cancer. Medicine 2014, 93, e163. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Merseburger, A.S.; Arlt, W.; Sternberg, C.N.; Feyerabend, S.; Berruti, A.; Joniau, S.; Géczi, L.; Lefresne, F.; Lahaye, M.; et al. Assessment of the Safety of Glucocorticoid Regimens in Combination With Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer: A Randomized, Open-label Phase 2 Study. JAMA Oncol. 2019, 5, 1232. [Google Scholar] [CrossRef]
- Parimi, V.; Goyal, R.; Poropatich, K.; Yang, X.J. Neuroendocrine differentiation of prostate cancer: A review. Am. J. Clin. Exp. Urol. 2014, 2, 273–285. [Google Scholar]
- Kim, S.; Ramakrishnan, R.; Lavilla-Alonso, S.; Chinnaiyan, P.; Rao, N.; Fowler, E.; Heine, J.; Gabrilovich, D.I. Radiation-induced autophagy potentiates immunotherapy of cancer via up-regulation of mannose 6-phosphate receptor on tumor cells in mice. Cancer Immunol. Immunother. 2014, 63, 1009–1021. [Google Scholar] [CrossRef]
- Costa, S.; Reagan, M.R. Therapeutic Irradiation: Consequences for Bone and Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 587. [Google Scholar] [CrossRef]
- Eisermann, K.; Broderick, C.J.; Bazarov, A.; Moazam, M.M.; Fraizer, G.C. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol. Cancer 2013, 12, 7. [Google Scholar] [CrossRef]
- Roberts, E.; Cossigny, D.A.; Quan, G.M. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer 2013, 2013, 418340. [Google Scholar] [CrossRef]
- Lange, S.; Heger, J.; Euler, G.; Wartenberg, M.; Piper, H.M.; Sauer, H. Platelet-derived growth factor BB stimulates vasculogenesis of embryonic stem cell-derived endothelial cells by calcium-mediated generation of reactive oxygen species. Cardiovasc. Res. 2009, 81, 159–168. [Google Scholar] [CrossRef]
- Brown, J.E.; Cook, R.J.; Major, P.; Lipton, A.; Saad, F.; Smith, M.; Lee, K.A.; Zheng, M.; Hei, Y.J.; Coleman, R.E. Bone turnover markers as predictors of skeletal complications in prostate cancer, lung cancer, and other solid tumors. J. Natl. Cancer Inst. 2005, 97, 59–69. [Google Scholar] [CrossRef]
- Saad, F.; Eastham, J.A.; Smith, M.R. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol. Oncol. 2012, 30, 369–378. [Google Scholar] [CrossRef]
- Brown, J.E.; Thomson, C.S.; Ellis, S.P.; Gutcher, S.A.; Purohit, O.P.; Coleman, R.E. Bone resorption predicts for skeletal complications in metastatic bone disease. Br. J. Cancer 2003, 89, 2031–2037. [Google Scholar] [CrossRef]
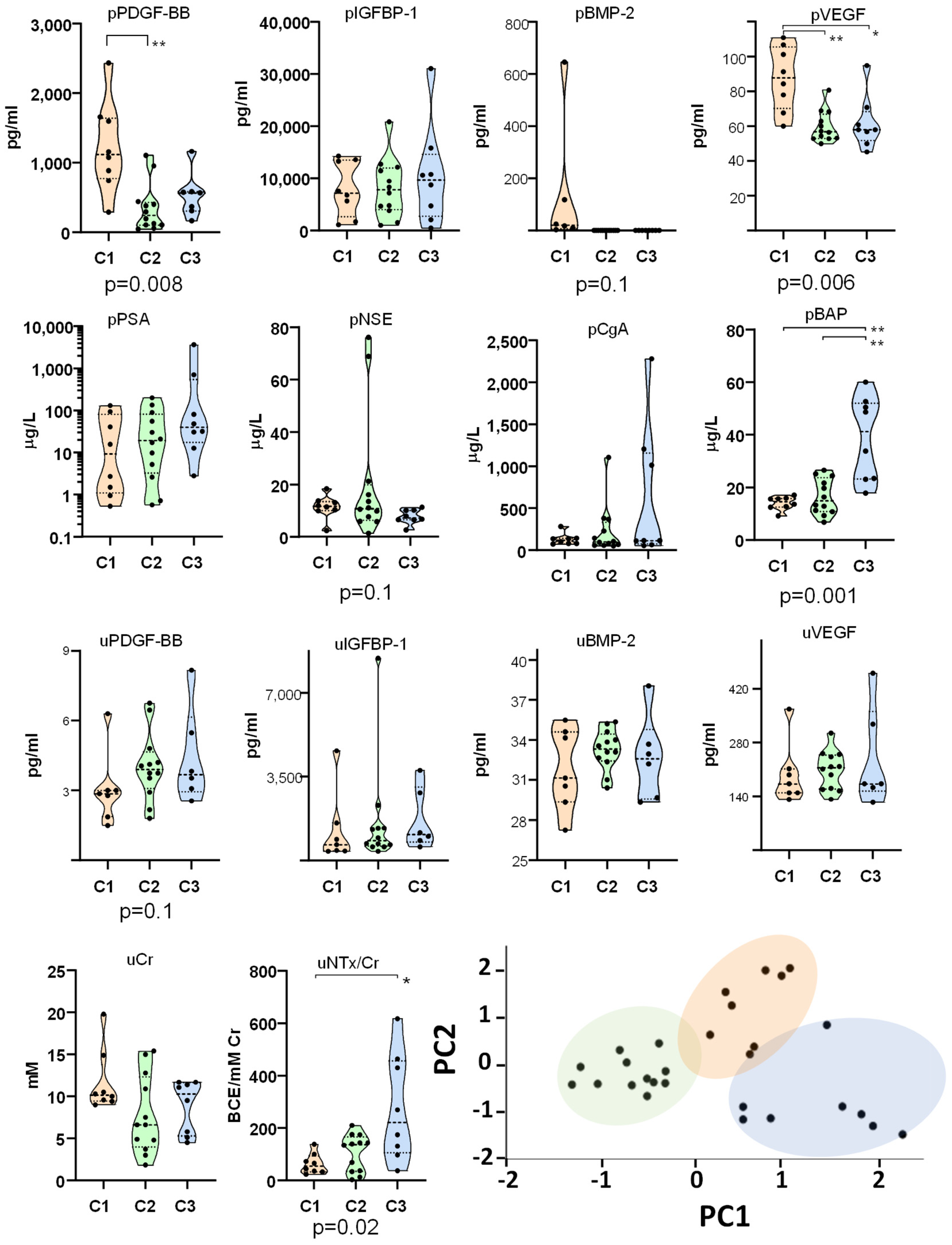


| Cluster | C1 | C2 | C3 | p |
|---|---|---|---|---|
| Number of patients | 8 | 12 | 8 | |
| Age at Dx, years (mean ± SD) | 68.63 ± 5.42 | 72.75 ± 8.47 | 74.88 ± 8.14 | ns |
| Gleason score at Dx: <8 n (%) | 2 (25) | 5 (41.66) | 1 (12.5) | ns |
| ≥8 n (%) | 6 (75) | 6 (50) | 4 (50) | ns |
| Metastasis at t0 n (%) | ||||
| Bone | 6 (75.00) | 10 (83.33) | 7 (87.50) | ns |
| Visceral | 2 (25.00) | 3 (25.00) | 4 (50.00) | ns |
| Adenopathic | 4 (50.00) | 9 (75.00) | 2 (25.00) | ns |
| NLR (mean ± SD) | 3.48 ± 1.54 | 5.81 ± 8.61 | 3.27 ± 1.34 | ns |
| PLTs × 109/L (mean ± SD) | 248 ± 53.10 | 199 ± 74.01 | 218 ± 36.61 | ns |
| Previous local therapy for prostate cancer at t0 n (%) | 6 (75.00) | 8 (66.66) | 4 (50.00) | ns |
| Years from Dx at t0 (mean ± SD) | 11.25 ± 6.92 | 8.00 ± 6.53 | 8.87 ± 6.89 | ns |
| Treated with Abi at t0 n (%) | 5 (62.5) | 7 (58.33) | 3 (37.50) | ns |
| Treated with BPs at t0 n (%) | 0 (0.00) | 2 (16.66) | 1 (12.50) | ns |
| Years from Dx at exitus (median (range)) | 16 (14–20) | 8 (1–19) | 8.42 (2–20) | ns |
| Abi | BPs | Sx | RT | Chemo | Gleason ≥ 8 | M1 n | ||
|---|---|---|---|---|---|---|---|---|
| n | Yes No | 17 12 | 4 25 | 11 18 | 12 17 | 7 22 | 17 8 | >4 15 <4 9 No 5 |
| # pPDGF-BB (pg/mL) | Yes No | 134.5 ± 660.5 522.5 ± 436.7 | 442.3 ± 652.7 667.8 ± 575.9 | 669.2 ± 604.5 607.7 ± 572.2 | 620.5 ± 650.8 661.0 ± 534.1 | 524.5 ± 506.7 676.1 ± 599.9 | 634.4 ± 530.9 668.6 ± 766.4 | 487.4 ± 404.1 896.9 ± 737.8 625.2 ± 622.6 |
| # pIGFBP-1 (pg/mL) | Yes No | 6948 ± 5760 & 11,294 ± 7418 | 2786 ± 1658 * 9534 ± 6785 | 8246 ± 5827 9176 ± 7446 | 8209 ± 4291 9262 ± 8255 | 6453 ± 4576 9454 ± 7189 | 9853 ± 7625 7711 ± 5409 | 8595 ± 5878 6929 ± 4653 12,800 ± 11,158 |
| # pBMP-2 (pg/mL) | Yes No | 18.65 ± 29.89 71.04 ± 183.8 | 26.69 ± 26.05 42.84 ± 129.4 | 72.69 ± 190.8 20.67 ± 36.87 | 13.16 ± 16.72 62.06 ± 160.1 | 16.83 ± 20.59 47.73 ± 137.6 | 20.09 ± 36.71 99.67 ± 221.9 | 17.86 ± 32.88 83.87 ± 211.3 29.21 ± 44.24 |
| # pVEGF (pg/mL) | Yes No | 30.98 ± 35.4 & 14.01 ± 28.69 | 25.62 ± 41.09 23.48 ± 33.19 | 32.92 ± 43.36 17.75 ± 24.35 | 16.80 ± 26.35 28.89 ± 37.58 | 28.26 ± 40.37 22.47 ± 32.01 | 29.40 ± 38.49 14.34 ± 18.95 | 10.45 ± 19.20 & 38.69 ± 38.89 33.87 ± 44.54 |
| pPSA (µg/L) | Yes No | 287 ± 877 93.9 ± 195 | 202 ± 268 207 ± 729 | 48.3 ± 66.05 304 ± 857 | 324 ± 1045 124 ± 206 | 118 ± 216 235 ± 776 | 294 ± 875 23.7 ± 45.0 | 368.4 ± 932.5 37.62 ± 43.99 28.37 ± 38.65 |
| pNSE (µg/L) | Yes No | 12.7 ± 14.8 16.0 ± 19.6 | 41.3 ± 36.0 & 9.77 ± 4.73 | 21.6 ± 25.5 & 9.52 ± 4.49 | 13.7 ± 17.8 14.3 ± 16.5 | 27.2 ± 30.9 & 9.95 ± 4.94 | 9.79 ± 3.26 19.3 ± 23.8 | 13.75 ± 17.92 17.63 ± 19.68 9.01 ± 2.89 |
| pCgA (µg/L) | Yes No | 167 ± 231 & 490 ± 691 | 297 ± 475 302 ± 507 | 236 ± 336 340 ± 576 | 386 ± 678 241 ± 319 | 370 ± 506 279 ± 500 | 263 ± 528 378 ± 484 | 393 ± 624.2 250.8 ± 363.6 117.6 ± 64.47 |
| pBAP (µg/L) | Yes No | 27.2 ± 23.0 21.4 ± 15.3 | 24.5 ± 17.4 21.5 ± 13.7 | 18.7 ± 12.2 & 28.5 ± 23.2 | 21.2 ± 12.7 27.4 ± 24.0 | 25.0 ± 18.2 24.7 ± 21.0 | 24.7 ± 22.8 20.0 ± 14.6 | 24.88 ± 12.88 & 18.85 ± 13.40 13.5 ± 3.38 |
| uPDGF-BB (pg/mL) | Yes No | 22.6 ± 11.48 21.49 ± 7.79 | 17.94 ± 4.77 22.78 ± 0.02 | 19.34 ± 6.85 24.45 ± 11.89 | 18.20 ± 5.95 & 24.28 ± 11.6 | 17.63 ± 3.20 23.64 ± 11.19 | 25.12 ± 11.66 20.13 ± 8.76 | 24.72 ± 10.99 19.91 ± 9.95 18.14 ± 4.84 |
| uIGFBP-1 (pg/mL) | Yes No | 1808 ± 4918 839.6 ± 1282 | 143.2 ± 44.9 * 844.0 ± 1534 | 898.5 ± 1876 1894 ± 51.01 | 3308 ± 6227 & 325.9 ± 366.9 | 1316 ± 2297 1531 ± 4557 | 748.1 ± 1634 2923 ± 6934 | 557.7 ± 1005 3292 ± 6583 287.0 ± 264.6 |
| uBMP-2 (pg/mL) | Yes No | 18.85 ± 4.73 15.71 ± 10.78 | 20.50 ± 5.22 17.34 ± 7.68 | 16.88 ± 7.96 18.37 ± 7.20 | 16.84 ± 6.66 18.3 ± 8.06 | 17.07 ± 6.58 17.92 ± 7.82 | 16.23 ± 6.28 18.23 ± 6.51 | 19.29 ± 7.26 14.23 ± 7.53 21.33 ± 5.24 |
| uVEGF (pg/mL) | Yes No | 90.92 ± 61.41 115.8 ± 147.6 | 119.7 ± 87.73 95.86 ± 100.8 | 87.08 ± 80.29 108.7 ± 110.5 | 101.1 ± 121.6 98.57 ± 83.73 | 101.9 ± 67.46 98.67 ± 108.2 | 94.87 ± 76.43 & 56.61 ± 34.33 | 108.2 ± 115.5 90.0 ± 84.23 87.52 ± 53.15 |
| uCr (mM) | Yes No | 10.5 ± 4.09 * 7.20 ± 3.57 | 6.37 ± 2.99 9.60 ± 4.20 | 8.15 ± 2.99 9.76 ± 4.72 | 8.31 ± 4.22 9.74 ± 4.15 | 7.37 ± 2.88 & 9.72 ± 4.40 | 9.73 ± 4.20 8.61 ± 4.67 | 8.43 ± 4.92 & 10.22 ± 3.58 9.40 ± 2.58 |
| uNTx/Cr (BCE/mM Cr) | Yes No | 95.2 ± 72.8 201 ± 195 | 30.4 ± 11.6 ** 156 ± 148 | 124 ± 125 148 ± 158 | 152 ± 154 130 ± 141 | 111 ± 164 147 ± 140 | 94.8 ± 69.3 163 ± 138 | 163.1 ± 167.3 126.0 ± 140.2 91.71 ± 59.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, M.A.; Anguera, G.; Cantó, E.; Alejandre, J.; Mora, J.; Osuna-Gómez, R.; Mulet, M.; Mora, P.; Antonijuan, A.; Sánchez, S.; et al. Pilot Investigation on Markers of Bone Metabolism, Angiogenesis, and Neuroendocrine Activity as Potential Predictors of Survival of Metastatic Prostate Cancer Patients with Bone Metastases. Int. J. Mol. Sci. 2025, 26, 4669. https://doi.org/10.3390/ijms26104669
Ortiz MA, Anguera G, Cantó E, Alejandre J, Mora J, Osuna-Gómez R, Mulet M, Mora P, Antonijuan A, Sánchez S, et al. Pilot Investigation on Markers of Bone Metabolism, Angiogenesis, and Neuroendocrine Activity as Potential Predictors of Survival of Metastatic Prostate Cancer Patients with Bone Metastases. International Journal of Molecular Sciences. 2025; 26(10):4669. https://doi.org/10.3390/ijms26104669
Chicago/Turabian StyleOrtiz, Maria Angels, Georgia Anguera, Elisabet Cantó, Jose Alejandre, Josefina Mora, Ruben Osuna-Gómez, Maria Mulet, Pradip Mora, Assumpta Antonijuan, Sofia Sánchez, and et al. 2025. "Pilot Investigation on Markers of Bone Metabolism, Angiogenesis, and Neuroendocrine Activity as Potential Predictors of Survival of Metastatic Prostate Cancer Patients with Bone Metastases" International Journal of Molecular Sciences 26, no. 10: 4669. https://doi.org/10.3390/ijms26104669
APA StyleOrtiz, M. A., Anguera, G., Cantó, E., Alejandre, J., Mora, J., Osuna-Gómez, R., Mulet, M., Mora, P., Antonijuan, A., Sánchez, S., Ramírez, O., Orantes, V., Maroto, P., & Vidal, S. (2025). Pilot Investigation on Markers of Bone Metabolism, Angiogenesis, and Neuroendocrine Activity as Potential Predictors of Survival of Metastatic Prostate Cancer Patients with Bone Metastases. International Journal of Molecular Sciences, 26(10), 4669. https://doi.org/10.3390/ijms26104669







