Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications
Abstract
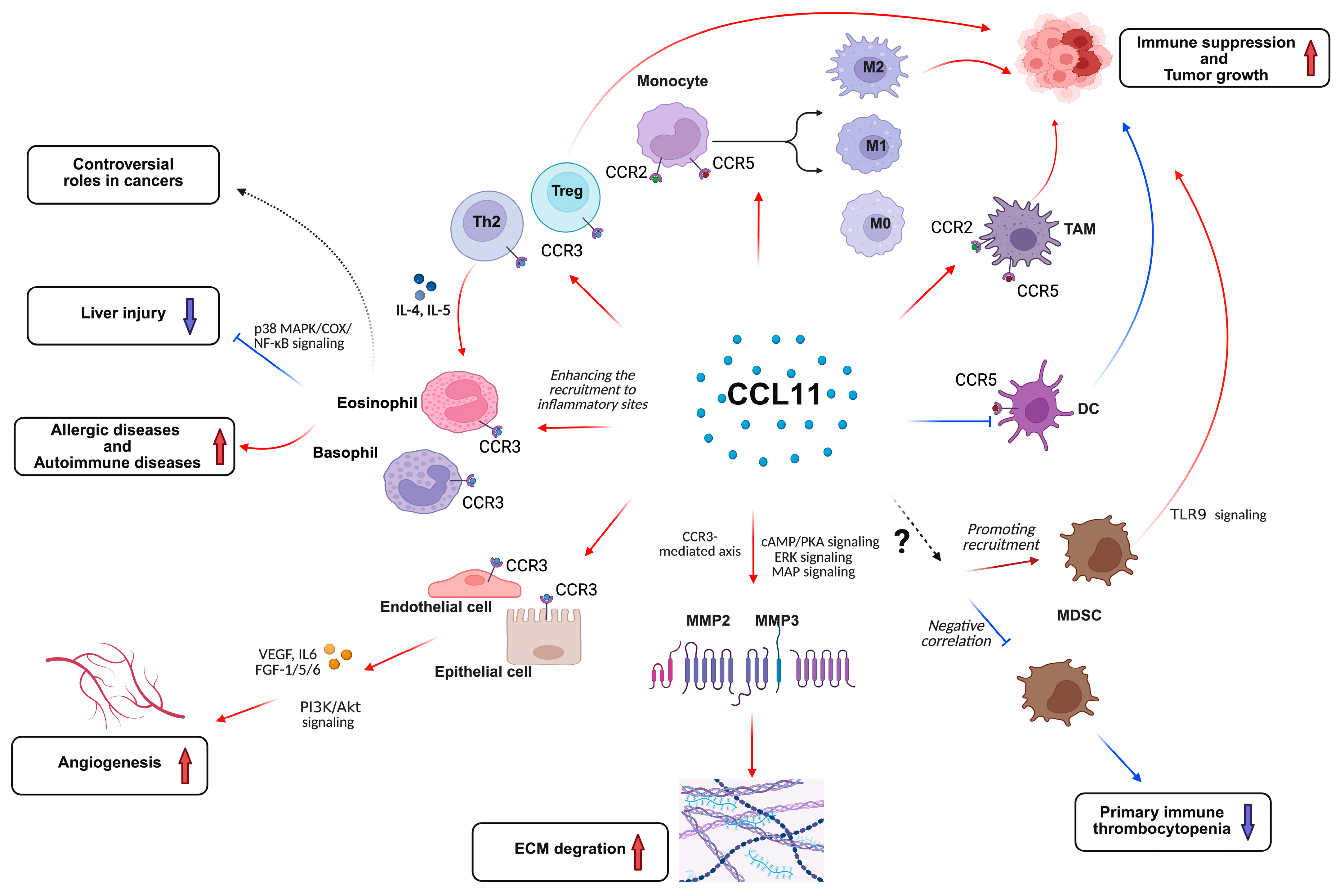
1. Introduction
1.1. Genetic, Molecular, and Protein Structure
1.2. Secretion Resources
1.2.1. Eosinophils
1.2.2. Fibroblasts
1.2.3. Smooth Muscle Cells
1.2.4. Endothelial Cells
1.2.5. Epithelial Cells
1.2.6. Hepatocytes
1.2.7. Tumor Cells
1.3. CCL11-Binding Receptors
1.3.1. Chemokine Receptor CCR3
1.3.2. Chemokine Receptor CCR5 and CCR2
1.3.3. Chemokine Receptor CXCR3
2. Mechanistic Functions of CCL11 Signaling
2.1. The Roles of CCL11 in Relation to Immune Response
2.1.1. Eosinophils/Basophils
2.1.2. T Cells
2.1.3. Monocytes/Macrophages
2.1.4. DCs
2.2. The Roles of CCL11 on Angiogenesis
2.3. Matrix Metalloproteinase (MMP) Expression and Extracellular Matrix (ECM) Degradation
3. Roles of CCL11 Signaling in Cancers
3.1. Pro-Tumor Functions
3.1.1. Renal Cell Carcinoma (RCC)
3.1.2. Ovarian Carcinoma
3.1.3. Breast Cancer
3.1.4. Pancreatic Ductal Adenocarcinoma
3.1.5. Lymphoma
3.2. Anti-Tumor Functions
Fibrosarcoma
3.3. Dual or Controversial Roles in Cancers
3.3.1. Non-Small-Cell Lung Cancer (NSCLC)
3.3.2. Colorectal Cancer (CRC)
3.3.3. Prostate Cancer
3.3.4. Esophageal Cancer
3.3.5. Melanoma
3.3.6. Hepatocellular Carcinoma
4. Roles of CCL11 Signaling in Liver Diseases
4.1. Liver Injury
4.2. Liver Cirrhosis
4.3. Hepatitis and Cholangitis
4.4. Alcoholic Liver Disease (ALD) and Non-Alcoholic Fatty Liver Disease (NAFLD)
5. Potential Treatments Targeting CCL11 and Its Receptors
5.1. CCL11
5.2. CCR3
5.3. CCR5
5.4. CCR2
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CCL11 | C-C motif ligand 11 |
| MCP | Macrophage chemoattractant protein |
| HSC | Hepatic stellate cell |
| HCC | Hepatocellular carcinoma |
| CAFs | Cancer-associated fibroblasts |
| SMCs | Smooth muscle cells |
| ConA | Concanavalin A |
| MDSCs | Myeloid-derived suppressor cells |
| Treg | Regulatory T cell |
| MS-K | Murine sarcoma cell line |
| TAM | Tumor-associated macrophage |
| IL-6 | Interleukin-6 |
| FGF | Fibroblast growth factors |
| VEGF | Vascular endothelial growth factor |
| MMP | Matrix Metalloproteinase |
| ECM | Extracellular matrix |
| PKA | Protein kinase A |
| ERK | Extracellular signal-regulated kinase |
| MAP | Mitogen-activated protein |
| PI3K | Phosphatidylinositol 3-kinase |
| JNK | c-Jun N-terminal kinase |
| RCC | Renal cell carcinoma |
| NSCLC | Non-small-cell lung cancer |
| EMT | Epithelial–mesenchymal transition |
| CRC | Colorectal cancer |
| HSCs | Hepatic stellate cells |
| PSC | Primary sclerosing cholangitis |
| PBC | Primary biliary cirrhosis |
| AIH | Autoimmune hepatitis |
| ALD | Alcoholic liver disease |
| NAFLD | Nonalcoholic fatty liver disease |
References
- Williams, T.J. Eotaxin-1 (CCL11). Front. Immunol. 2015, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Greenman, R.; Weston, C.J. CCL24 and Fibrosis: A Narrative Review of Existing Evidence and Mechanisms. Cells 2025, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Roy-O’Reilly, M.; Ritzel, R.M.; Conway, S.E.; Staff, I.; Fortunato, G.; McCullough, L.D. CCL11 (Eotaxin-1) Levels Predict Long-Term Functional Outcomes in Patients Following Ischemic Stroke. Transl. Stroke Res. 2017, 8, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, J.; Zhu, Z.; Hu, J.; Lin, Y. Spotlight on pro-inflammatory chemokines: Regulators of cellular communication in cognitive impairment. Front. Immunol. 2024, 15, 1421076. [Google Scholar] [CrossRef]
- Levina, V.; Nolen, B.M.; Marrangoni, A.M.; Cheng, P.; Marks, J.R.; Szczepanski, M.J.; Szajnik, M.E.; Gorelik, E.; Lokshin, A.E. Role of eotaxin-1 signaling in ovarian cancer. Clin. Cancer Res. 2009, 15, 2647–2656. [Google Scholar] [CrossRef]
- Garcia-Zepeda, E.A.; Rothenberg, M.E.; Ownbey, R.T.; Celestin, J.; Leder, P.; Luster, A.D. Human eotaxin is a specific chemoattractant for eosinophil cells and provides a new mechanism to explain tissue eosinophilia. Nat. Med. 1996, 2, 449–456. [Google Scholar] [CrossRef]
- Proctor, W.R.; Chakraborty, M.; Chea, L.S.; Morrison, J.C.; Berkson, J.D.; Semple, K.; Bourdi, M.; Pohl, L.R. Eosinophils mediate the pathogenesis of halothane-induced liver injury in mice. Hepatology 2013, 57, 2026–2036. [Google Scholar] [CrossRef]
- Landi, A.; Weismuller, T.J.; Lankisch, T.O.; Santer, D.M.; Tyrrell, D.L.J.; Manns, M.P.; Houghton, M. Differential serum levels of eosinophilic eotaxins in primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis. J. Interferon & Cytokine Res. 2014, 34, 204–214. [Google Scholar] [CrossRef]
- Kong, M.; Dong, W.; Kang, A.; Kuai, Y.; Xu, T.; Fan, Z.; Shi, L.; Sun, D.; Lu, Y.; Li, Z.; et al. Regulatory role and translational potential of CCL11 in liver fibrosis. Hepatology 2023, 78, 120–135. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Li, J.; Zhu, Z.; Cui, Z.; Liu, R.; Lu, R.; Yao, Z.; Xu, Q. Cancer associated fibroblast-derived CCL5 promotes hepatocellular carcinoma metastasis through activating HIF1α/ZEB1 axis. Cell Death & Dis. 2022, 13, 478. [Google Scholar] [CrossRef]
- Miyamasu, M.; Misaki, Y.; Yamaguchi, M.; Yamamoto, K.; Morita, Y.; Matsushima, K.; Nakajima, T.; Hirai, K. Regulation of human eotaxin generation by Th1-/Th2-derived cytokines. Int. Arch. Allergy Immunol. 2000, 122 (Suppl. S1), 54–58. [Google Scholar] [CrossRef]
- Watanabe, K.; Jose, P.J.; Rankin, S.M. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and IL-4 in monocytes and macrophages. J. Immunol. 2002, 168, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Yamasaki, A.; Yang, J.; Shan, L.; Halayko, A.J.; Gounni, A.S. IL-17A induces eotaxin-1/CC chemokine ligand 11 expression in human airway smooth muscle cells: Role of MAPK (Erk1/2, JNK, and p38) pathways. J. Immunol. 2006, 177, 4064–4071. [Google Scholar] [CrossRef] [PubMed]
- Kodali, R.B.; Kim, W.J.H.; Galaria, I.I.; Miller, C.; Schecter, A.D.; Lira, S.A.; Taubman, M.B. CCL11 (Eotaxin) induces CCR3-dependent smooth muscle cell migration. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1211–1216. [Google Scholar] [CrossRef]
- Jaruga, B.; Hong, F.; Sun, R.; Radaeva, S.; Gao, B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: Up-regulating eotaxins and IL-5 and recruiting leukocytes. J. Immunol. 2003, 171, 3233–3244. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.-T.; Chang, T.-T.; Jong, Y.-J.; Kuo, P.-L.; Lee, H.-M.; Lee, M.-S.; Chang, H.-W.; Hung, C.-H. Suppressive effects of formoterol and salmeterol on eotaxin-1 in bronchial epithelial cells. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2010, 21, 345–352. [Google Scholar] [CrossRef]
- Ma, J.; Chan, C.-C.; Huang, W.-C.; Kuo, M.-L. Berberine Inhibits Pro-inflammatory Cytokine-induced IL-6 and CCL11 Production via Modulation of STAT6 Pathway in Human Bronchial Epithelial Cells. Int. J. Med. Sci. 2020, 17, 1464–1473. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, X.; Chen, X.; Liu, H.; Miao, X.; Guo, Y.; Xu, Y.; Li, J.; Zou, X.; Li, Z. C–C motif chemokine CCL11 is a novel regulator and a potential therapeutic target in non-alcoholic fatty liver disease. JHEP Rep. 2023, 5, 100805. [Google Scholar] [CrossRef]
- Kataoka, S.; Konishi, Y.; Nishio, Y.; Fujikawa-Adachi, K.; Tominaga, A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004, 23, 549–560. [Google Scholar] [CrossRef]
- Cho, H.; Lim, S.-J.; Won, K.Y.; Bae, G.E.; Kim, G.Y.; Min, J.W.; Noh, B.-J. Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24. J. Pathol. Transl. Med. 2016, 50, 45–51. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. Eotaxins and Their Receptor in Colorectal Cancer-A Literature Review. Cancers 2020, 12, 1318. [Google Scholar] [CrossRef] [PubMed]
- Adar, T.; Shteingart, S.; Ben Ya’acov, A.; Bar-Gil Shitrit, A.; Goldin, E. From airway inflammation to inflammatory bowel disease: Eotaxin-1, a key regulator of intestinal inflammation. Clin. Immunol. 2014, 153, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Robinson, D.S.; Meng, Q.; Rottman, J.; Kennedy, R.; Ringler, D.J.; Mackay, C.R.; Daugherty, B.L.; Springer, M.S.; Durham, S.R.; et al. Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma. Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur. J. Immunol. 1997, 27, 3507–3516. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, S.; Robbiani, D.F.; Du, X.; Rodrigues, E.; Ignatius, R.; Wei, Y.; Ponath, P.; Young, J.W.; Pope, M.; Steinman, R.M.; et al. Expression of a functional eotaxin (CC chemokine ligand 11) receptor CCR3 by human dendritic cells. J. Immunol. 2002, 169, 2925–2936. [Google Scholar] [CrossRef]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Tagliamonte, M.; Gauzzi, M.C.; Lopalco, L. Dual CCR5/CCR2 targeting: Opportunities for the cure of complex disorders. Cell. Mol. Life Sci. 2019, 76, 4869–4886. [Google Scholar] [CrossRef]
- Martinelli, R.; Sabroe, I.; LaRosa, G.; Williams, T.J.; Pease, J.E. The CC chemokine eotaxin (CCL11) is a partial agonist of CC chemokine receptor 2b. J. Biol. Chem. 2001, 276, 42957–42964. [Google Scholar] [CrossRef]
- Ogilvie, P.; Bardi, G.; Clark-Lewis, I.; Baggiolini, M.; Uguccioni, M. Eotaxin is a natural antagonist for CCR2 and an agonist for CCR5. Blood 2001, 97, 1920–1924. [Google Scholar] [CrossRef]
- Weng, Y.; Siciliano, S.J.; Waldburger, K.E.; Sirotina-Meisher, A.; Staruch, M.J.; Daugherty, B.L.; Gould, S.L.; Springer, M.S.; DeMartino, J.A. Binding and functional properties of recombinant and endogenous CXCR3 chemokine receptors. J. Biol. Chem. 1998, 273, 18288–18291. [Google Scholar] [CrossRef]
- Clark-Lewis, I.; Mattioli, I.; Gong, J.-H.; Loetscher, P. Structure-function relationship between the human chemokine receptor CXCR3 and its ligands. J. Biol. Chem. 2003, 278, 289–295. [Google Scholar] [CrossRef]
- Combadiere, C.; Ahuja, S.K.; Murphy, P.M. Cloning and functional expression of a human eosinophil CC chemokine receptor. J. Biol. Chem. 1995, 270, 16491–16494. [Google Scholar] [CrossRef] [PubMed]
- Kitaura, M.; Nakajima, T.; Imai, T.; Harada, S.; Combadiere, C.; Tiffany, H.L.; Murphy, P.M.; Yoshie, O. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J. Biol. Chem. 1996, 271, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Neote, K.; DiGregorio, D.; Mak, J.Y.; Horuk, R.; Schall, T.J. Molecular cloning, functional expression, and signaling characteristics of a C-C chemokine receptor. Cell 1993, 72, 415–425. [Google Scholar] [CrossRef]
- Berridge, M.J. Inositol trisphosphate and calcium signalling. Nature 1993, 361, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Bober, A.; Mika, J.; Piotrowska, A. A Missing Puzzle in Preclinical Studies-Are CCR2, CCR5, and Their Ligands Roles Similar in Obesity-Induced Hypersensitivity and Diabetic Neuropathy?-Evidence from Rodent Models and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 11323. [Google Scholar] [CrossRef]
- Nolen, B.M.; Lokshin, A.E. Targeting CCL11 in the treatment of ovarian cancer. Expert Opin. Ther. Targets 2010, 14, 157–167. [Google Scholar] [CrossRef]
- Rigoni, A.; Colombo, M.P.; Pucillo, C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin. Immunol. 2018, 35, 29–34. [Google Scholar] [CrossRef]
- Zajkowska, M.; Mroczko, B. From Allergy to Cancer-Clinical Usefulness of Eotaxins. Cancers 2021, 13, 128. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; MacLean, J.A.; Pearlman, E.; Luster, A.D.; Leder, P. Targeted disruption of the chemokine eotaxin partially reduces antigen-induced tissue eosinophilia. J. Exp. Med. 1997, 185, 785–790. [Google Scholar] [CrossRef]
- Ezekwe, E.A.D., Jr.; Weskamp, A.L.; Rahim, R.; Makiya, M.A.; Wetzler, L.; Ware, J.M.; Nelson, C.; Castillo, P.A.; Riley, C.A.; Brown, T.; et al. Dupilumab Use in Patients With Hypereosinophilic Syndromes: A Multicenter Case Series and Review of the Literature. J. Allergy Clin. Immunol. Pract. 2025, 13, 167–175 e166. [Google Scholar] [CrossRef]
- Tomkinson, A.; Duez, C.; Cieslewicz, G.; Gelfand, E.W. Eotaxin-1-deficient mice develop airway eosinophilia and airway hyperresponsiveness. Int. Arch. Allergy Immunol. 2001, 126, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Wang, M.; Wang, S.; Jeong, J.-M.; Xu, L.; Wen, Y.; Emontzpohl, C.; Atkins, C.L.; Duong, K.; et al. Eosinophils attenuate hepatic ischemia-reperfusion injury in mice through ST2-dependent IL-13 production. Sci. Transl. Med. 2021, 13, eabb6576. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Wen, Y.; Jeong, J.-M.; Emontzpohl, C.; Atkins, C.L.; Sun, Z.; Poulsen, K.L.; Hall, D.R.; Steve Bynon, J.; et al. Hepatic recruitment of eosinophils and their protective function during acute liver injury. J. Hepatol. 2022, 77, 344–352. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Jiang, J.; Wen, Y.; Jeong, J.-M.; Emontzpohl, C.; Atkins, C.L.; Kim, K.; Jacobsen, E.A.; Wang, H.; et al. Eosinophils protect against acetaminophen-induced liver injury through cyclooxygenase-mediated IL-4/IL-13 production. Hepatology 2022, 77, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, R.; Lee, J.J.; Lotze, M.T. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): Role in the inflammatory response within tumors. J. Immunother. 2007, 30, 16–28. [Google Scholar] [CrossRef]
- Lotfi, R.; Herzog, G.I.; DeMarco, R.A.; Beer-Stolz, D.; Lee, J.J.; Rubartelli, A.; Schrezenmeier, H.; Lotze, M.T. Eosinophils oxidize damage-associated molecular pattern molecules derived from stressed cells. J. Immunol. 2009, 183, 5023–5031. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Mackay, C.R.; Lanzavecchia, A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science 1997, 277, 2005–2007. [Google Scholar] [CrossRef]
- Wang, R.; Huang, K. CCL11 increases the proportion of CD4+CD25+Foxp3+ Treg cells and the production of IL-2 and TGF-β by CD4+ T cells via the STAT5 signaling pathway. Mol. Med. Rep. 2020, 21, 2522–2532. [Google Scholar] [CrossRef]
- Xing, Y.; Tian, Y.; Kurosawa, T.; Matsui, S.; Touma, M.; Yanai, T.; Wu, Q.; Sugimoto, K. CCL11-induced eosinophils inhibit the formation of blood vessels and cause tumor necrosis. Genes Cells Devoted Mol. Cell. Mech. 2016, 21, 624–638. [Google Scholar] [CrossRef]
- Yang, J.; Hawkins, O.E.; Barham, W.; Gilchuk, P.; Boothby, M.; Ayers, G.D.; Joyce, S.; Karin, M.; Yull, F.E.; Richmond, A. Myeloid IKKβ promotes antitumor immunity by modulating CCL11 and the innate immune response. Cancer Res. 2014, 74, 7274–7284. [Google Scholar] [CrossRef]
- Zambirinis, C.P.; Levie, E.; Nguy, S.; Avanzi, A.; Barilla, R.; Xu, Y.; Seifert, L.; Daley, D.; Greco, S.H.; Deutsch, M.; et al. TLR9 ligation in pancreatic stellate cells promotes tumorigenesis. J. Exp. Med. 2015, 212, 2077–2094. [Google Scholar] [CrossRef]
- Shao, X.; Wu, B.; Cheng, L.; Li, F.; Zhan, Y.; Liu, C.; Ji, L.; Min, Z.; Ke, Y.; Sun, L.; et al. Distinct alterations of CD68+CD163+ M2-like macrophages and myeloid-derived suppressor cells in newly diagnosed primary immune thrombocytopenia with or without CR after high-dose dexamethasone treatment. J. Transl. Med. 2018, 16, 48. [Google Scholar] [CrossRef]
- Salcedo, R.; Young, H.A.; Ponce, M.L.; Ward, J.M.; Kleinman, H.K.; Murphy, W.J.; Oppenheim, J.J. Eotaxin (CCL11) induces in vivo angiogenic responses by human CCR3+ endothelial cells. J. Immunol. 2001, 166, 7571–7578. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kang, Y.W.; Choi, B.Y.; Yang, Y.C.; Cho, B.P.; Cho, W.G. CCL11 promotes angiogenic activity by activating the PI3K/Akt pathway in HUVECs. J. Recept. Signal Transduct. Res. 2017, 37, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Beck, L.A.; Tancowny, B.; Brummet, M.E.; Asaki, S.Y.; Curry, S.L.; Penno, M.B.; Foster, M.; Bahl, A.; Stellato, C. Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J. Immunol. 2006, 177, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Nave, O.; Sigron, M. A mathematical model for cancer treatment based on combination of anti-angiogenic and immune cell therapies. Results Appl. Math. 2022, 16, 100330. [Google Scholar] [CrossRef]
- Kodali, R.; Hajjou, M.; Berman, A.B.; Bansal, M.B.; Zhang, S.; Pan, J.J.; Schecter, A.D. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc. Res. 2006, 69, 706–715. [Google Scholar] [CrossRef]
- Chao, P.-Z.; Hsieh, M.-S.; Cheng, C.-W.; Lin, Y.-F.; Chen, C.-H. Regulation of MMP-3 expression and secretion by the chemokine eotaxin-1 in human chondrocytes. J. Biomed. Sci. 2011, 18, 86. [Google Scholar] [CrossRef]
- Koç, Ü.; Çetinkaya, E.; Bostanci, E.B.; Kemık, A.S.; Tez, M.; Gömceli, İ.; Akoğlu, M. Diagnostic significance of serum eotaxin-1 level in gastric cancer patients. Dis. Markers 2013, 35, 363–367. [Google Scholar] [CrossRef]
- Jöhrer, K.; Zelle-Rieser, C.; Perathoner, A.; Moser, P.; Hager, M.; Ramoner, R.; Gander, H.; Höltl, L.; Bartsch, G.; Greil, R.; et al. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 2459–2465. [Google Scholar] [CrossRef]
- Bekaert, S.; Rocks, N.; Vanwinge, C.; Noel, A.; Cataldo, D. Asthma-related inflammation promotes lung metastasis of breast cancer cells through CCL11-CCR3 pathway. Respir. Res. 2021, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Miyagaki, T.; Sugaya, M.; Murakami, T.; Asano, Y.; Tada, Y.; Kadono, T.; Okochi, H.; Tamaki, K.; Sato, S. CCL11-CCR3 interactions promote survival of anaplastic large cell lymphoma cells via ERK1/2 activation. Cancer Res. 2011, 71, 2056–2065. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Huang, G.; Cheng, L.; Lv, J.; Zheng, D.; Lin, S.; Wang, S.; Wu, Q.; Long, Y.; et al. Myeloid-derived suppressor cells promote lung cancer metastasis by CCL11 to activate ERK and AKT signaling and induce epithelial-mesenchymal transition in tumor cells. Oncogene 2021, 40, 1476–1489. [Google Scholar] [CrossRef]
- Siva, S.; MacManus, M.; Kron, T.; Best, N.; Smith, J.; Lobachevsky, P.; Ball, D.; Martin, O. A pattern of early radiation-induced inflammatory cytokine expression is associated with lung toxicity in patients with non-small cell lung cancer. PLoS ONE 2014, 9, e109560. [Google Scholar] [CrossRef]
- Tsao, A.S.; Liu, S.; Lee, J.J.; Alden, C.M.; Blumenschein, G.R.; Herbst, R.; Davis, S.E.; Kim, E.; Lippman, S.; Heymach, J.; et al. Clinical and biomarker outcomes of the phase II vandetanib study from the BATTLE trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2013, 8, 658–661. [Google Scholar] [CrossRef]
- Wågsäter, D.; Löfgren, S.; Hugander, A.; Dienus, O.; Dimberg, J. Analysis of single nucleotide polymorphism in the promoter and protein expression of the chemokine eotaxin-1 in colorectal cancer patients. World J. Surg. Oncol. 2007, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Polosukhina, D.; Singh, K.; Asim, M.; Barry, D.P.; Allaman, M.M.; Hardbower, D.M.; Piazuelo, M.B.; Washington, M.K.; Gobert, A.P.; Wilson, K.T.; et al. CCL11 exacerbates colitis and inflammation-associated colon tumorigenesis. Oncogene 2021, 40, 6540–6546. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Liu, P.; Li, J.; Zhang, Y. Eotaxin-1 promotes prostate cancer cell invasion via activation of the CCR3-ERK pathway and upregulation of MMP-3 expression. Oncol. Rep. 2014, 31, 2049–2054. [Google Scholar] [CrossRef]
- Heidegger, I.; Höfer, J.; Luger, M.; Pichler, R.; Klocker, H.; Horninger, W.; Steiner, E.; Jochberger, S.; Culig, Z. Is Eotaxin-1 a serum and urinary biomarker for prostate cancer detection and recurrence? Prostate 2015, 75, 1904–1909. [Google Scholar] [CrossRef]
- Blank, S.; Nienhüser, H.; Dreikhausen, L.; Sisic, L.; Heger, U.; Ott, K.; Schmidt, T. Inflammatory cytokines are associated with response and prognosis in patients with esophageal cancer. Oncotarget 2017, 8, 47518–47532. [Google Scholar] [CrossRef]
- Simson, L.; Ellyard, J.I.; Dent, L.A.; Matthaei, K.I.; Rothenberg, M.E.; Foster, P.S.; Smyth, M.J.; Parish, C.R. Regulation of carcinogenesis by IL-5 and CCL11: A potential role for eosinophils in tumor immune surveillance. J. Immunol. 2007, 178, 4222–4229. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.T.; Liu, J.; Yeung, O.W.; Pang, L.; Shiu, H.C.; Liu, H.; Yang, X.X.; Chan, A.C.; Wong, T.C.; Lo, C.M.; et al. Post-transplant inflammatory cytokine signature adds value for predicting tumor recurrence after liver transplantation for hepatocellular carcinoma. Hepatol. Int. 2023, 17, 1596–1609. [Google Scholar] [CrossRef]
- Tacke, F.; Trautwein, C.; Yagmur, E.; Hellerbrand, C.; Wiest, R.; Brenner, D.A.; Schnabl, B. Up-regulated eotaxin plasma levels in chronic liver disease patients indicate hepatic inflammation, advanced fibrosis and adverse clinical course. J. Gastroenterol. Hepatol. 2007, 22, 1256–1264. [Google Scholar] [CrossRef]
- Pham, B.N.; Bemuau, J.; Durand, F.; Sauvanet, A.; Degott, C.; Prin, L.; Janin, A. Eotaxin expression and eosinophil infiltrate in the liver of patients with drug-induced liver disease. J. Hepatol. 2001, 34, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Monga, S.P. Wnt/-Catenin Signaling and Liver Regeneration: Circuit, Biology, and Opportunities. Gene Expr. 2021, 20, 189–199. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yang, H.Y. Mechanism of hepatocytes differentiation and dedifferentiation in liver regeneration: Process and exploration. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 331–332. [Google Scholar] [CrossRef]
- Fan, Z.; Kong, M.; Dong, W.; Dong, C.; Miao, X.; Guo, Y.; Liu, X.; Miao, S.; Li, L.; Chen, T.; et al. Trans-activation of eotaxin-1 by Brg1 contributes to liver regeneration. Cell Death Dis. 2022, 13, 495. [Google Scholar] [CrossRef]
- Wong, S.-W.; Ting, Y.-W.; Yong, Y.-K.; Tan, H.-Y.; Barathan, M.; Riazalhosseini, B.; Bee, C.J.; Tee, K.-K.; Larsson, M.; Velu, V.; et al. Chronic inflammation involves CCL11 and IL-13 to facilitate the development of liver cirrhosis and fibrosis in chronic hepatitis B virus infection. Scand. J. Clin. Lab. Investig. 2021, 81, 147–159. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Wang, S.; Wang, J.; Zhou, A.; Gong, S.; Wang, Y.; Miao, X.; Guo, Y.; Wang, H.; et al. The CC motif chemokine ligand 11 contributes to alcoholic liver disease. Life Sci. 2025, 363, 123409. [Google Scholar] [CrossRef]
- Tarantino, G.; Costantini, S.; Finelli, C.; Capone, F.; Guerriero, E.; La Sala, N.; Gioia, S.; Castello, G. Carotid intima-media thickness is predicted by combined eotaxin levels and severity of hepatic steatosis at ultrasonography in obese patients with Nonalcoholic Fatty Liver Disease. PLoS ONE 2014, 9, e105610. [Google Scholar] [CrossRef]
- Zimmermann, N.; Hershey, G.K.; Foster, P.S.; Rothenberg, M.E. Chemokines in asthma: Cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 2003, 111, 227–242; quiz 243. [Google Scholar] [CrossRef] [PubMed]
- Main, S.; Handy, R.; Wilton, J.; Smith, S.; Williams, L.; Du Fou, L.; Andrews, J.; Conroy, L.A.; May, R.; Anderson, I.; et al. A potent human anti-eotaxin1 antibody, CAT-213: Isolation by phage display and in vitro and in vivo efficacy. J. Pharmacol. Exp. Ther. 2006, 319, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, A.; Tamura, N.; Yatabe, Y.; Onodera, S.; Hiruma, T.; Inaba, N.; Kusunoki, J.; Tomioka, H. Suppressive effects of F-1322 on the antigen-induced late asthmatic response and pulmonary eosinophilia in guinea pigs. Eur. J. Pharmacol. 2001, 430, 123–133. [Google Scholar] [CrossRef]
- Heath, H.; Qin, S.; Rao, P.; Wu, L.; LaRosa, G.; Kassam, N.; Ponath, P.D.; Mackay, C.R. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J. Clin. Investig. 1997, 99, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef]
- Emmelkamp, J.M.; Rockstroh, J.K. CCR5 antagonists: Comparison of efficacy, side effects, pharmacokinetics and interactions--review of the literature. Eur. J. Med. Res. 2007, 12, 409–417. [Google Scholar]
- Emmelkamp, J.M.; Rockstroh, J.K. Maraviroc, risks and benefits: A review of the clinical literature. Expert Opin. Drug Saf. 2008, 7, 559–569. [Google Scholar] [CrossRef]
- Korbecki, J.; Grochans, S.; Gutowska, I.; Barczak, K.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of Receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 Ligands. Int. J. Mol. Sci. 2020, 21, 7619. [Google Scholar] [CrossRef]
- Gonzalez, E.O.; Boix, V.; Deltoro, M.G.; Aldeguer, J.L.; Portilla, J.; Montero, M.; Belda, E.B.; Abril, V.; Gutierrez, F.; Minguez, C.; et al. The effects of Maraviroc on liver fibrosis in HIV/HCV co-infected patients. J. Int. AIDS Soc. 2014, 17, 19643. [Google Scholar] [CrossRef]
- Vergunst, C.E.; Gerlag, D.M.; Lopatinskaya, L.; Klareskog, L.; Smith, M.D.; van den Bosch, F.; Dinant, H.J.; Lee, Y.; Wyant, T.; Jacobson, E.W.; et al. Modulation of CCR2 in rheumatoid arthritis: A double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2008, 58, 1931–1939. [Google Scholar] [CrossRef]
- Witherington, J.; Bordas, V.; Cooper, D.G.; Forbes, I.T.; Gribble, A.D.; Ife, R.J.; Berkhout, T.; Gohil, J.; Groot, P.H. Conformationally restricted indolopiperidine derivatives as potent CCR2B receptor antagonists. Bioorganic & Med. Chem. Lett. 2001, 11, 2177–2180. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Xu, M.; Wang, L.; Chen, X.; Feng, Y.; Li, Y.; Zhang, X.; Cui, W.; Jia, X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer 2020, 19, 41. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Weston, C.J.; Miao, Z.; Corbett, C.; Armstrong, M.J.; Ertl, L.; Ebsworth, K.; Walters, M.J.; Baumart, T.; Newland, D.; et al. CC chemokine receptor 2 promotes recruitment of myeloid cells associated with insulin resistance in nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G483–G493. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.; Walters, M.; Ertl, L.; Ebsworth, K.; Tan, J.; McMahon, J.; Powers, J.; Adams, D.; Jaen, J.; Schall, T. Therapeutic use of a clinical stage CCR2 inhibitor, CCX872, in obesity-associated steatohepatitis. Lancet 2014, 383, S78. [Google Scholar] [CrossRef]
- Humbles, A.A.; Conroy, D.M.; Marleau, S.; Rankin, S.M.; Palframan, R.T.; Proudfoot, A.E.; Wells, T.N.; Li, D.; Jeffery, P.K.; Griffiths-Johnson, D.A.; et al. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: Analysis in a guinea pig model in vivo. J. Exp. Med. 1997, 186, 601–612. [Google Scholar] [CrossRef]
| Cell Types | Diseases | Stimulating Factors | Ref. | |
|---|---|---|---|---|
| Eosinophils | Asthma, ulcerative colitis, Crohn’s disease, drug-induced liver injury | Th2 cytokines, like IL4 | [6,7] | |
| Fibroblasts | HSCs | Liver fibrosis | (1) Pro-fibrogenic growth factors: TGF-β, PDHF (2) Promoter: zinc finger factor 281 | [8,9] |
| CAFs | Metastasis and invasion of HCC | Under stimulations of inflammatory cytokines | [10] | |
| Dermal fibroblasts | Cutaneous inflammation | (1) Promoted by IL4/13 (2) Inhibited by IFN-γ | [11,12] | |
| Smooth muscle cell | Airway smooth muscle cell | Allergic asthma | (1) Under IL-17A stimulation (2) Signaling: MAPK, STAT-3 pathway | [13] |
| Vascular smooth muscle cells | Atherosclerosis | Ischemic situation | [14] | |
| Endothelial cells | ConA-induced liver injury | IL-4/STAT6 signaling pathway | [15] | |
| Epithelial cells | Allergic asthma | (1) IL-4/STAT6 signaling pathway (2) With the stimulations of TNF-a, IL-1, or IFN-a | [16,17] | |
| Hepatocytes | NASH, ConA-induced liver injury | (1) Activated by free fatty acids (2) IL-4/STAT6 signaling pathway | [15,18] | |
| Tumor cells | Ovarian cancer, HCC, colorectal cancer | Under stimulations of inflammatory cytokines | [5,19,20] | |
| CCL11 Receptor | Key Roles | Major Expressing Cells | Other Binding Chemokines | Ref. |
|---|---|---|---|---|
| CCR3 | Recruits eosinophils and basophils | Eosinophils, basophils, TH2 lymphocytes, mast cells, and dendritic cells | (1) Agonist: CCL 3, 4, 5, 7, 13, 15, 23, 24, 26, 28 (2) Antagonist: CCL 9, 10, 18 | [21,22,23,24] |
| CCR5 | Induces the internalization of CCR5 in monocytes and macrophages | T lymphocytes, macrophages, dendritic cells (DCs), granulocytes, fibroblasts, epithelium, endothelium, and vascular smooth muscle | (1) Agonist: CCL3, CCL4, CCL5, CCL8, CCL13, CCL14, CCL16, CCL3L1 (2) Antagonist: CCL7 | [5,25,26] |
| CCR2 | Dual activity of CCL11/CCR2 on inflammatory effects by partial agonists or natural antagonists in different studies | Monocytes, NK, and T lymphocytes | (1) Agonist: CCL2, CCL7, CCL8, CCL12, CCL13 | [26,27,28] |
| CXCR3 | (1) Low affinity for binding CXCR3 (2) Impaired Th-1 response | T cells, NK cells, and some epithelial cells | (1) Agonist: CXCL4, CXCL9, CXCL10, CXCL11 | [29,30] |
| Functions | Cancer Types | Mechanisms of CCL11 Signaling | Ref. |
|---|---|---|---|
| Pro-tumor | Renal cell carcinoma | (1) Stimulate progression and invasiveness of CCR3+ RCC. (2) Correlation: overexpression of CCR3 with the grade of malignancy. | [60] |
| Ovarian carcinoma | (1) Promote the proliferation and dissemination of ovarian carcinoma cells by CCR2/3/5. (2) The growth-stimulatory effects of CCL11 on activation of ERK1/2, MEK1, and pSTAT3, as well as increased levels of CXCL8, G-CSF, GM-CSF, M-CSF, IL6R, IL8, VEGF, SDF-1a, and ICAM-1. (3) Biomarker: decreased circulating levels of CCL11 in cancer. (4) Prognostic: postoperative levels of CCL11 were negatively correlated with relapse-free survival. (5) Therapy: inhibition of CCL11 signaling increased sensitivity to cisplatin. | [5] | |
| Breast cancer | (1) Overexpression of serum CCL11 increased the proportion of Tregs and the production of TGF-β1 and IL-2 through the STAT5 signaling pathway. (2) Promote the lung metastasis of breast cancer via CCL11. | [48,61] | |
| Pancreatic ductal adenocarcinoma | Protumorigenic effects on TLR9/CCL11/MDSC recruitment. | [51] | |
| Lymphoma | Enhance tumor growth via CCL11/CCR3/ERK1/2 signaling. | [62] | |
| Anti-tumor | Fibrosarcoma | Reduce the formation of blood vessels and induce tumor necrosis by CCL11-induced eosinophils. | [49] |
| Dual/ controversial roles | Non-small-cell lung cancer | Pro-tumor: Promote tumor metastasis via MDSC-CCL11-ERK/AKT-EMT axis. Anti-tumor: (1) High ability to destroy cancer cells. (2) Low serum concentrations of CCL11 were associated with poor prognosis after vandetanib treatment. | [63,64,65] |
| Colorectal cancer | Pro-tumor: (1) Higher levels of tissue CCL11 were found in CRC patients. (2) Epithelial-cell-derived CCL11 was associated with carcinogenesis. Anti-tumor: (1) CCL11 serum levels were significantly below that of controls. (2) Decreased expression of CCL11 in tumor glandular cells induced a decrease in eosinophilia and achieved immune evasion. | [20,66,67] | |
| Prostate cancer | Pro-tumor: Promote cancer cell migration and invasion by CCR3/ERK1/2/MMP3. Anti-tumor: Prognostic: decreased serum levels of CCL11 in cancer and hyperplasia compared to normal. | [68,69] | |
| Esophageal cancer | Pro-tumor: Lower levels of serum CCL11 were associated with an adverse prognosis. Anti-tumor: Lower levels of CCL11 in tissue and serum were correlated with better survival. | [70] | |
| Hepatocellular carcinoma | (1) A combination of IL5 and CCL11 could recruit eosinophils into the tumors and cause immune evasion. (2) The single roles of CCL11 on HCC are unclear. | [19] | |
| Melanoma | (1) A combination of IL5 and CCL11 could recruit eosinophils into the tumors and cause immune evasion. (2) The single roles of CCL11 on melanoma are unclear. | [71] |
| Target | Drug Names | Targeting Diseases | Ref. | ||
|---|---|---|---|---|---|
| Cancers | Liver Diseases | Other Diseases | |||
| CCL11 | Bertilimumab | Ovarian carcinoma, NSCLC | Liver injury, liver fibrosis, ALD, NAFLD | Allergic disorders, asthma, dermal eosinophilia, nasal congestion, ulcerative colitis | [5,9,15,18,36,63,79,81,82,95] |
| CCR3 | SB-328437, F-1322, 7B11 | Ovarian carcinoma, melanoma | Liver fibrosis, ALD, NAFLD | Allergic diseases, chronic asthma | [9,18,79,83,84,85] |
| CCR5 | Maraviroc, GSK706769, INCB009471, vicriviroc, GW873140 | Breast cancer, prostate cancer, colorectal cancer | Liver fibrosis | HIV-1 | [86,87,88,89] |
| CCR2 | MLN1202, BMS-741672 indolopiperidine derivative | Esophageal cancer, pancreatic cancer, HCC | NAFLD, steatohepatitis | Allergic diseases, atherosclerosis, rheumatoid arthritis, type II diabetes | [85,90,92,93,94] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Man, K.; Ng, K.T.-P. Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2025, 26, 4662. https://doi.org/10.3390/ijms26104662
Wang J, Man K, Ng KT-P. Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications. International Journal of Molecular Sciences. 2025; 26(10):4662. https://doi.org/10.3390/ijms26104662
Chicago/Turabian StyleWang, Jiaqi, Kwan Man, and Kevin Tak-Pan Ng. 2025. "Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications" International Journal of Molecular Sciences 26, no. 10: 4662. https://doi.org/10.3390/ijms26104662
APA StyleWang, J., Man, K., & Ng, K. T.-P. (2025). Emerging Roles of C-C Motif Ligand 11 (CCL11) in Cancers and Liver Diseases: Mechanisms and Therapeutic Implications. International Journal of Molecular Sciences, 26(10), 4662. https://doi.org/10.3390/ijms26104662







