Regulation of MdANR in Anti-Burning Process of Apple Peel
Abstract
1. Introduction
2. Results
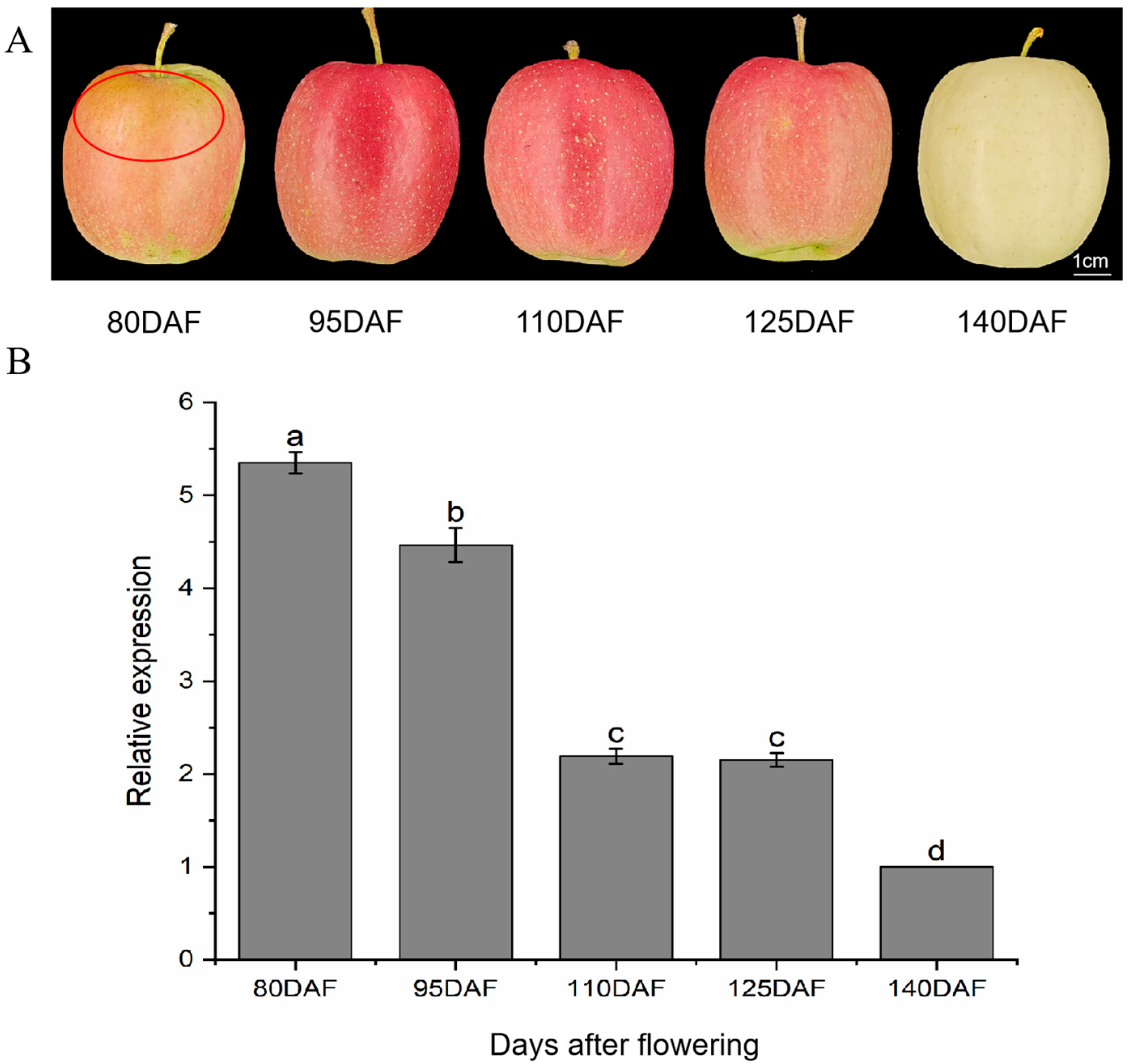
2.1. Effects of Different Bagging Removal Times on MdANR Expression in Apple Peel
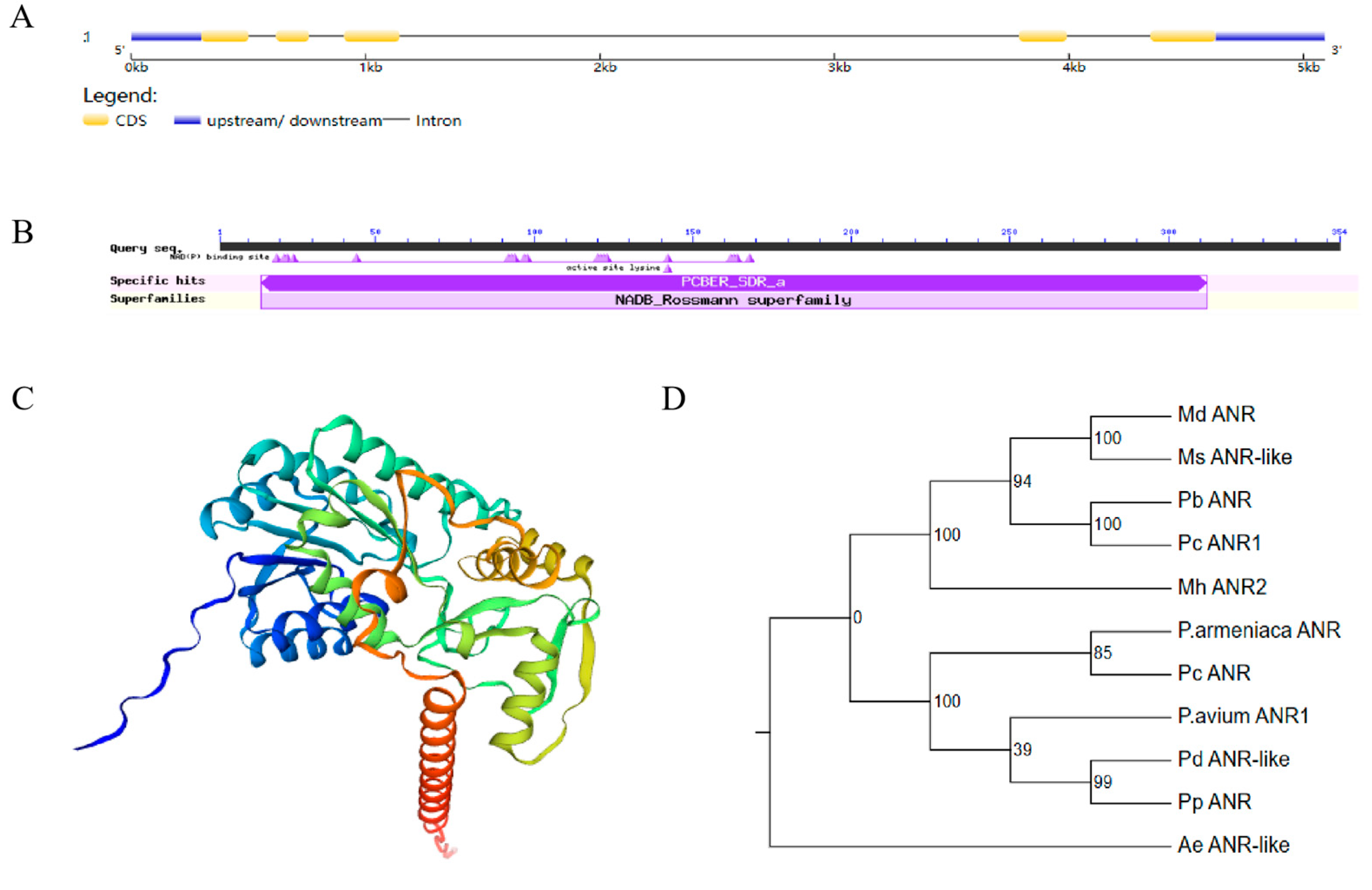
2.2. Gene Structure and Phylogenetic Analysis of MdANR
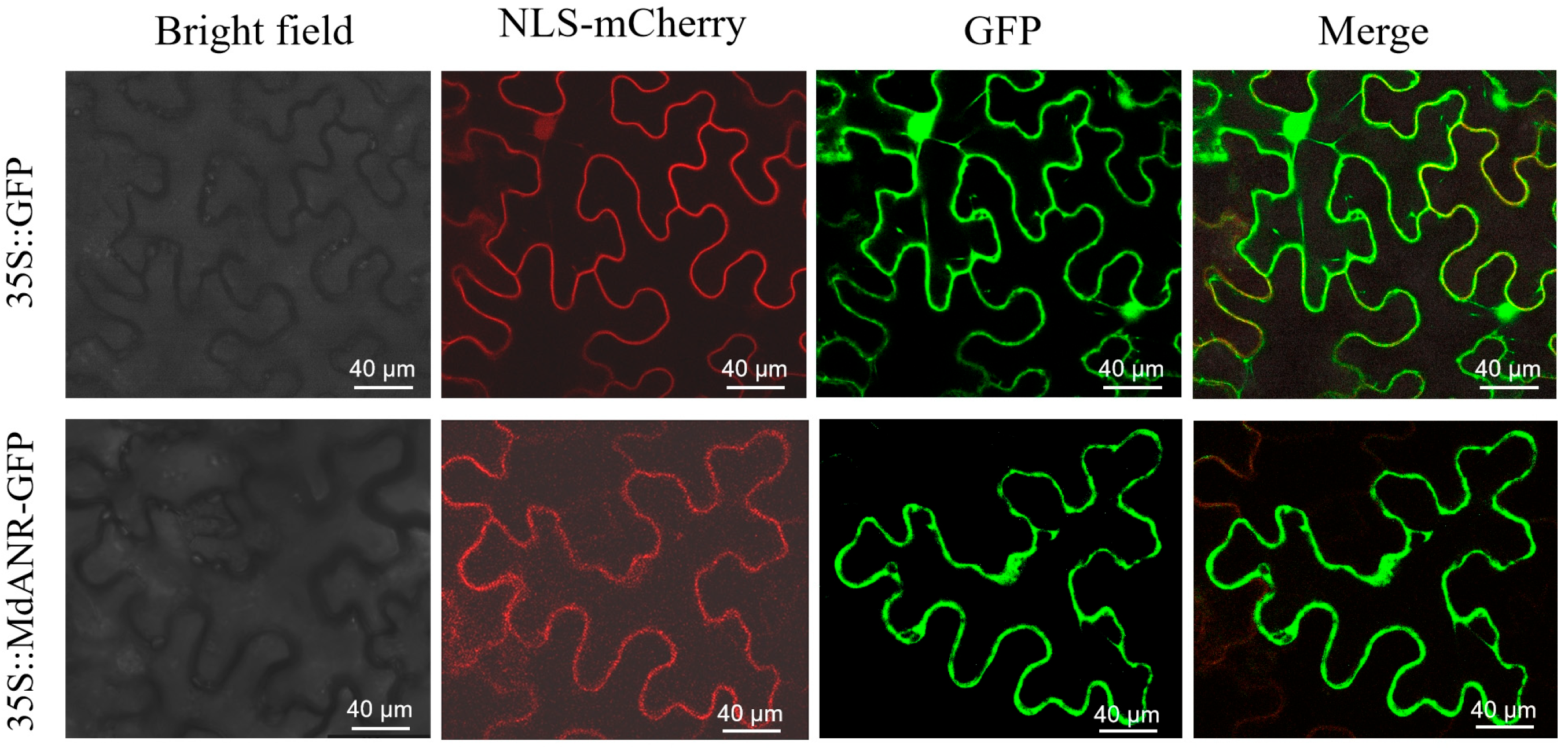
2.3. Subcellular Localization of MdANR
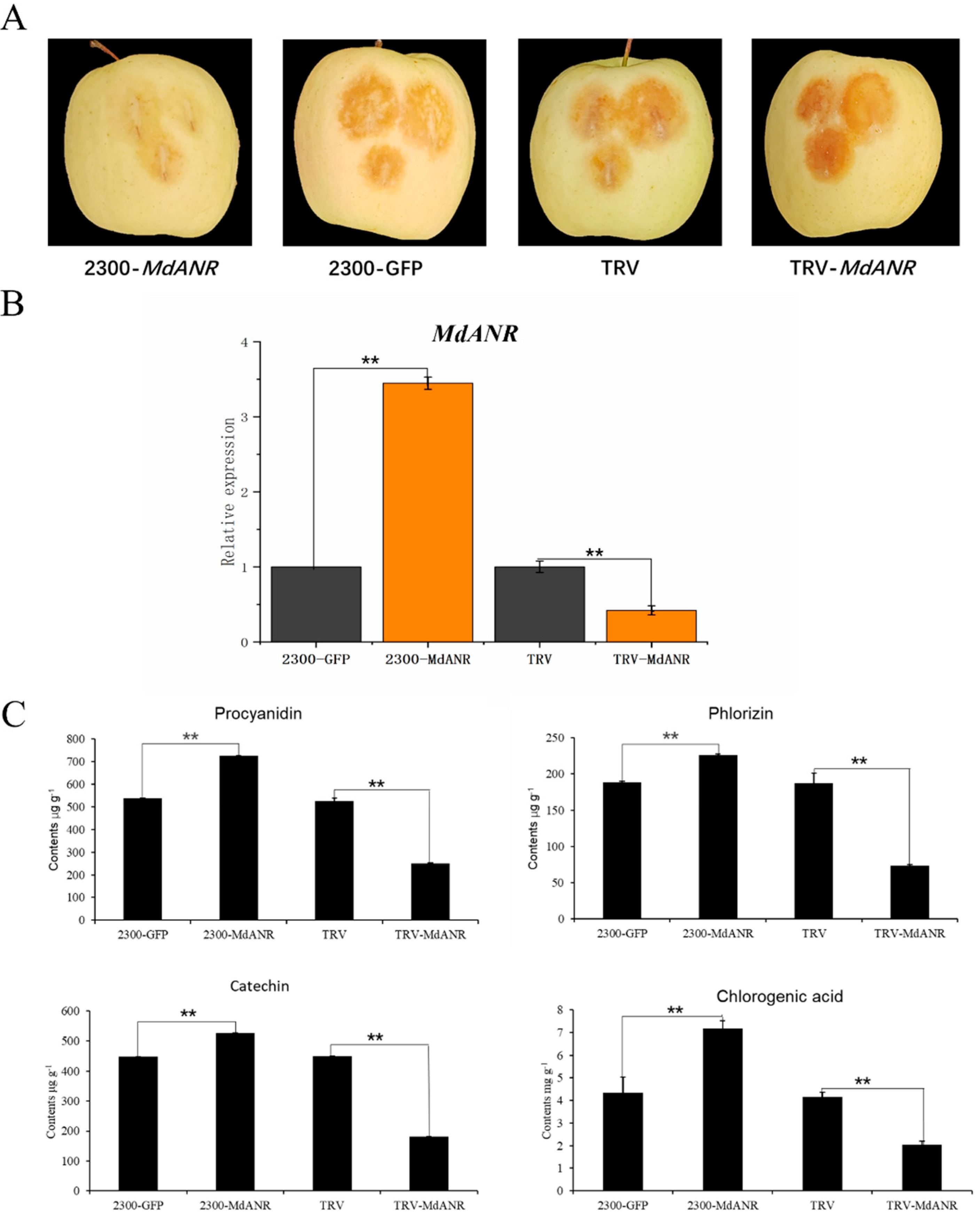
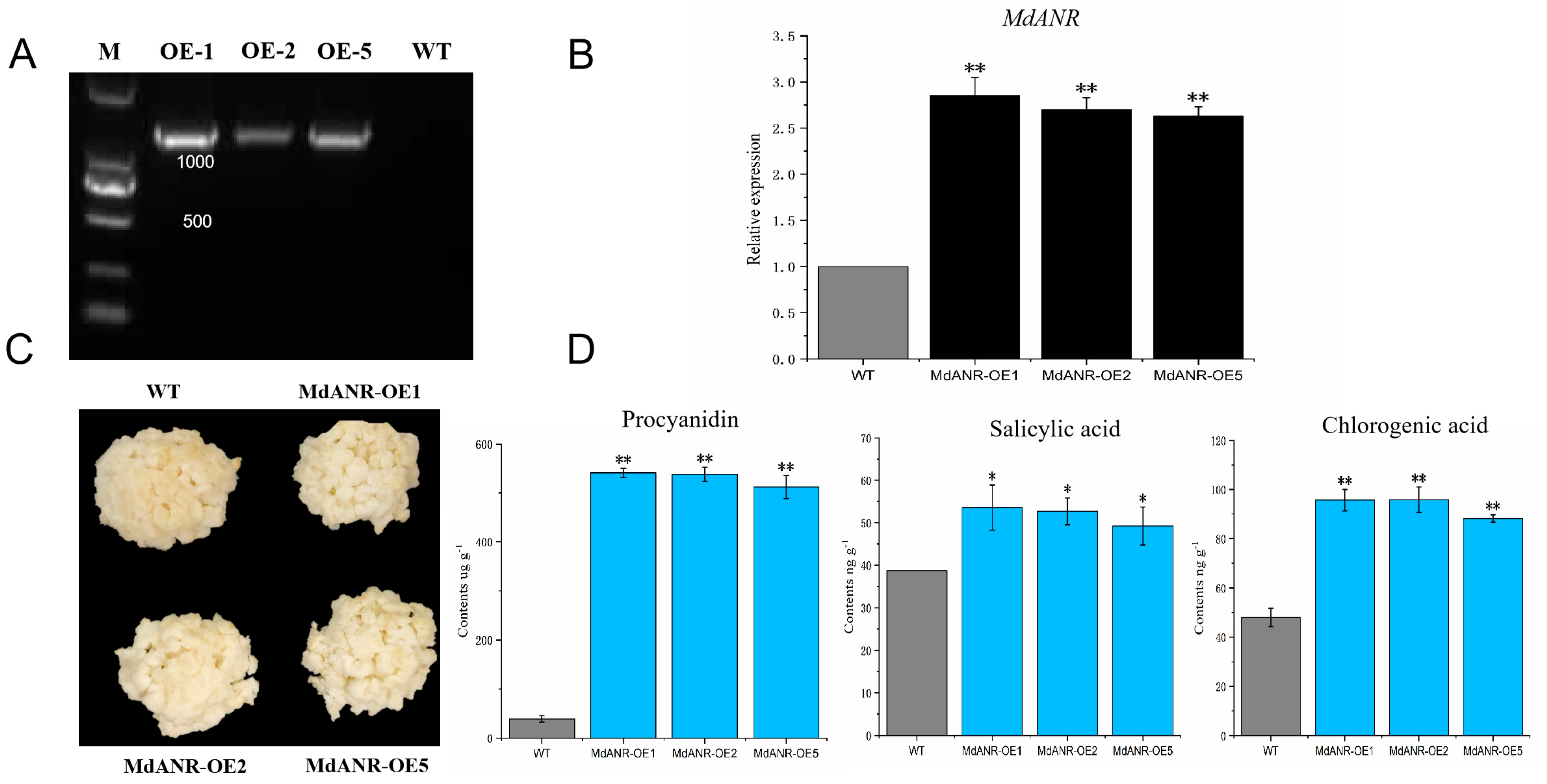
2.4. Functional Verification of MdANR in Apple Peel
2.5. Functional Verification of MdANR in Apple Callus
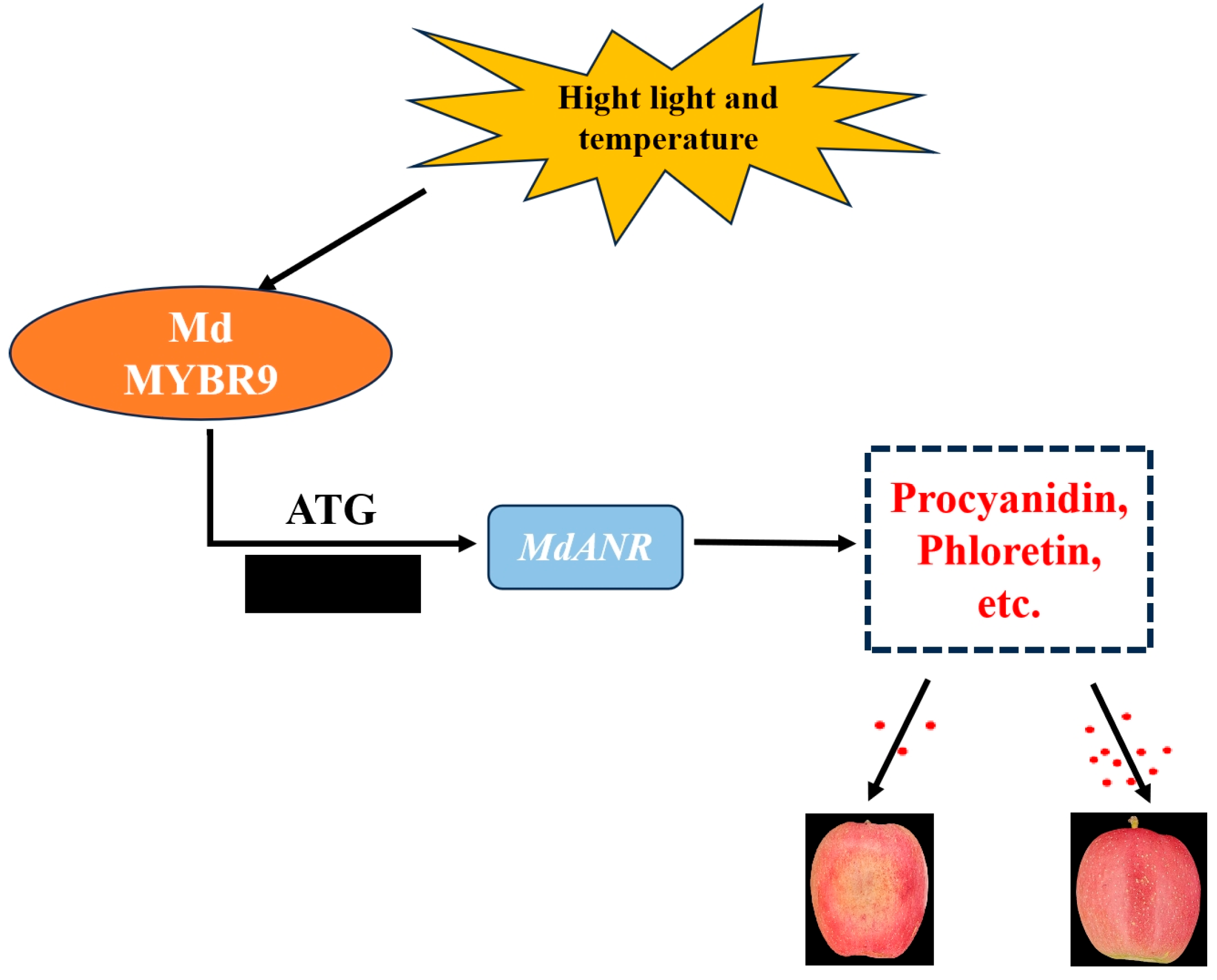
2.6. MdMYBR9 Binds to the MdANR Promoter and Induces Its Activity
3. Discussion
4. Materials and Methods
4.1. Experimental Design of Apple Picking Bag Treatment
4.2. Selection of Transgenic Materials
4.3. Ligation of Gene Clones and Vectors for Genetic Transformation
4.4. Transient Transformation of Apple Fruit
4.5. Stable Genetic Transformation of Apple Callus
4.6. RT-qPCR Expression Analysis
4.7. Metabolite Content Determination and Analysis
4.8. Gene Structure Analysis
4.9. Subcellular Localization Analysis
4.10. Dual-Luciferase Reporter Assay
4.11. Yeast One-Hybrid Assay
4.12. Electrophoretic Mobility Shift Assay
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Racsko, J.; Schrader, L.E. Sunburn of Apple Fruit: Historical Background, Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- Kalcsits, L.; Musacchi, S.; Layne, D.R.; Schmidt, T.; Mupambi, G.; Serra, S.; Mendoza, M.; Asteggiano, L.; Jarolmasjed, S.; Sankaran, S. Above and below-ground environmental changes associated with the use of photoselective protective netting to reduce sunburn in apple. Agric. For. Meteorol. 2017, 237–238, 9–17. [Google Scholar] [CrossRef]
- Xu, H.; Blatt, S.; Ediger, D. Tools for climate resilience in tree fruit I: Large-dwarfing rootstocks can alleviate sunburn damage in “Buckeye Gala” apple. Can. J. Plant Sci. 2022, 108, 128–132. [Google Scholar] [CrossRef]
- Yoo, J.; Sepulveda, G.; Rudell, D.; Torres, C.A. Comparative analysis of metabolic differences between sunburn and sunscald disorder on ’Packham’s triumph’ pear. Postharvest Biol. Technol. 2022, 195, 112153. [Google Scholar] [CrossRef]
- Domanda, C.; Paradiso, V.M.; Migliaro, D.; Pappaccogli, G.; Failla, O.; Rustioni, L. Epicuticular waxes: A natural packaging to deal with sunburn browning in white grapes. Sci. Hortic. 2024, 328, 112153. [Google Scholar] [CrossRef]
- Blasco, J.; Cubero, S.; Gómez-Sanchís, J.; Mira, P. Development of a machine for the automatic sorting of pomegranate (Punica granatum) arils based on computer vision. J. Food Eng. 2009, 90, 27–34. [Google Scholar] [CrossRef]
- Jiang, J.-M.; Lin, Y.-X.; Chen, Y.-Y.; Deng, C.-J.; Gong, H.-W.; Xu, Q.-Z.; Zheng, S.-Q.; Chen, W. Proteomics approach reveals mechanism underlying susceptibility of loquat fruit to sunburn during color changing period. Food Chem. 2015, 176, 388–395. [Google Scholar] [CrossRef]
- Morales-Quintana, L.; Waite, J.M.; Kalcsits, L.; Torres, C.A.; Ramos, P. Sun injury on apple fruit: Physiological, biochemical and molecular advances, and future challenges. Sci. Hortic. 2020, 260, 108866. [Google Scholar] [CrossRef]
- Felicetti, D.A.; Schrader, L.E. Photooxidative sunburn of apples: Characterization of a third type of apple sunburn. Int. J. Fruit Sci. 2008, 8, 160–172. [Google Scholar] [CrossRef]
- Weerakkody, P.; Jobling, J.; Infante, M.M.V.; Rogers, G. The effect of maturity, sunburn and the application of sunscreens on the internal and external qualities of pomegranate fruit grown in Australia. Sci. Hortic. 2010, 124, 57–61. [Google Scholar] [CrossRef]
- Li, M.; Hu, G.; Chen, Y. Evaluation of the antioxidant capacity of natural polyphenolic compounds using a macrocyclic Ni-(II) complex-catalysed Briggs–Rauscher reaction. Food Chem. 2016, 197 Pt A, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Shao, T.; Zeng, S.; Guo, Y.; Wu, Y.; Wei, W.; Pan, Q.; Xia, X.-L. Longan seed tannins as a quality tyrosinase inhibitor and browning resistance agent: Activity and mechanism. Ind. Crop. Prod. 2024, 222, 119918. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Wu, J.-Y.; Liu, X. Enhancement of chitosan-based film physicochemical and storage properties by interaction with proanthocyanidin and natural deep eutectic solvent. Int. J. Biol. Macromol. 2024, 278 Pt 1, 134611. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Feng, Y.; Zheng, Y.; Zhong, Y.; Li, X.; Liu, L.; Zhao, Z. MdGATA15 regulates MdANR to promote the synthesis of procyanidin and enhance the sunburn resistance of apple fruit. Sci. Hortic. 2025, 339, 113854. [Google Scholar] [CrossRef]
- Wang, W.Q.; Pu, Y.F.; Wen, H.; Lu, D.Y.; Yan, M.; Liu, M.Z.; Bai, H.J.; Shen, L.R.; Wu, C.Y. Transcriptome and weighted gene co-expression network analysis of jujube (Ziziphus jujub Mill.) fruit reveal putative genes involved in proanthocyanin biosynthesis and regulation. Food Sci Hum Well. 2023, 12, 1557–1570. [Google Scholar] [CrossRef]
- Bogs, J.; Downey, M.O.; Harvey, J.S.; Ashton, A.R.; Tanner, G.J.; Robinson, S.P. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 2005, 139, 652–663. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Zhang, J.; Song, T.-T.; Li, K.-T.; Yao, Y.-C. Identification of leucoanthocyanidin reductase and anthocyanidin reductase genes involved in proanthocyanidin biosynthesis in Malus crabapple plants. Plant Physiol. Biochem. 2019, 139, 141–151. [Google Scholar] [CrossRef]
- Su, Z.; Jiao, Y.; Jiang, Z.; Liu, P.; Chen, Q.; Qu, Y.; Deng, X. Gb_ANR-47 Enhances the Resistance of Gossypium barbadense to Fusarium oxysporum f. sp. vasinfectum (FOV) by Regulating the Content of Proanthocyanidins. Plants 2022, 11, 3529. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Z.; Wang, X.; Han, C.; Dong, Y.; Wang, Y. Functional identification of ANR genes in apple (Malus halliana) that reduce saline–alkali stress tolerance. Plant Biol. 2023, 25, 892–901. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, L.; Yu, J.; Chen, R.; Hu, C.; Wang, H.; Li, D.; Wang, Z.; Zhao, Z. Combined transcriptomic and metabolomic analyses reveal the mechanism of debagged ‘Fuji’ apple sunburn. Food Sci. Technol. 2023, 181, 114680. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, G.; Yue, W.; Zhang, S.; Wu, J. Transcriptome profiling reveals differential gene expression in proanthocyanidin biosynthesis associated with red/green skin color mutant of pear (Pyrus communis L.). Front. Plant Sci. 2015, 6, 795. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.Y.; Sharma, S.B.; Dixon, R.A. Anthocyanidin reductases from Medicago truncatula and Arabidopsis thaliana. Arch Biochem. Biophys. 2004, 422, 91–102. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, R.; Qu, F.; You, C.; Wang, X.; Hao, Y. R2R3-MYB transcription factor MdMYB23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018, 96, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Vimolmangkang, S.; Soria-Guerra, R.E.; Korban, S.S. Introduction of apple ANR genes into tobacco inhibits expression of both CHI and DFR genes in flowers, leading to loss of anthocyanin. J. Exp. Bot. 2012, 63, 2437–2447. [Google Scholar] [CrossRef]
- Xin, Y.; Meng, S.; Ma, B.; He, W.; He, N. Mulberry genes MnANR and MnLAR confer transgenic plants with resistance to Botrytis cinerea. Plant Sci. 2020, 296, 110473. [Google Scholar] [CrossRef]
- Wang, X.-L.; Hu, Z.-Y.; You, C.-X.; Kong, X.-Z.; Shi, X.-P. Subcellular localization and vacuolar targeting of sorbitol dehydrogenase in apple seed. Plant Sci. 2013, 210, 36–45. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Chang, Q.; Zhou, Z.; Han, R.; Liang, Z. Antioxidant and Antidiabetic Activity of Proanthocyanidins from Fagopyrum dibotrys. Molecules 2021, 26, 2417. [Google Scholar] [CrossRef]
- Liao, L.; Vimolmangkang, S.; Wei, G.; Zhou, H.; Korban, S.S.; Han, Y. Molecular characterization of genes encoding leucoanthocyanidin reductase involved in proanthocyanidin biosynthesis in apple. Front. Plant Sci. 2015, 6, 243. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, W.; Peng, X.; Sun, B.; Wang, X.; Tang, H. Characterization of anthocyanin and proanthocyanidin biosynthesis in two straw-berry genotypes during fruit development in response to different light qualities. J. Photochem. Photobiol. B 2018, 186, 225–231. [Google Scholar] [CrossRef]
- Verma, P.; Sen, R.; Bamanna, A.; Elhindawy, M.; Nagpal, K.; Krishnan, V. Structural chemistry to therapeutic functionality: A comprehensive review on proanthocyanidins. Biocatal. Agric. Biotechnol. 2024, 55, 102963. [Google Scholar] [CrossRef]
- Wang, N.; Qu, C.; Jiang, S.; Chen, Z.; Xu, H.; Fang, H.; Su, M.; Zhang, J.; Wang, Y.; Liu, W.; et al. The proanthocyanidin-specific transcription factor MdMYBPA1 initiates anthocyanin synthesis under low-temperature conditions in red-fleshed apples. Plant J. 2018, 96, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Junior, W.D.O.C.; Guimarães, M.L.R.; Freitas, K.M.; Pereira, R.S.; de Pádua, R.M.; Campana, P.R.V.; Braga, F.C. Structural characterization of a proanthocyanidin-rich fraction from Hancornia speciosa leaves and its effect on the release of pro-inflammatory cytokines and oxidative stress in THP-1 cells. J. Ethnopharmacol. 2024, 333, 118471. [Google Scholar]
- Simiyu, D.C.; Bayaraa, U.; Jang, J.H.; Lee, O.R. The R2R3-MYB transcription factor PgTT2 from Panax ginseng interacts with the WD40-repeat protein PgTTG1 during the regulation of proanthocyanidin biosynthesis and the response to salt stress. Plant Physiol. Biochem. 2024, 214, 108877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ran, L.; Hou, Y.; Tian, Q.; Li, C.; Liu, R.; Fan, D.; Luo, K. The transcription factor MYB115 contributes to the regulation ofproanthocya-nidin biosynthesis and enhances fungal resistance inpoplar. New Phytol. 2017, 215, 351–367. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Shen, Y.; Xing, W.; Cui, Y.; Luo, P. RhMYB1 and RhMYB123 form a positive feedback loop to regulate the proanthocyanidin biosynthesis in rose. Ind. Crop. Prod. 2023, 196, 116492. [Google Scholar] [CrossRef]
- Zhao, W.; Wu, W.; Liu, Y.; Fan, L.; Meng, H.; Wang, L.; Wu, G. The TT2-type MYB transcription factor JrMYB12 positively regulates proanthocyanidin biosynthesis in red walnut. Sci. Hortic. 2023, 307, 111515. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, S.; Zhang, T.; Xu, H.; Fang, H.; Zhang, J.; Su, M.; Wang, Y.; Zhang, Z.; Wang, N.; et al. Apple NAC transcription factor MdNAC52 regulates biosynthesis of anthocyanin and proanthocyanidin through MdMYB9 and MdMYB11. Plant Sci. 2019, 289, 110286. [Google Scholar] [CrossRef]
- An, X.-H.; Tian, Y.; Chen, K.-Q.; Liu, X.-J.; Liu, D.-D.; Xie, X.-B.; Cheng, C.-G.; Cong, P.-H.; Hao, Y.-J. MdMYB9 and MdMYB11 are Involved in the Regulation of the JA-Induced Biosynthesis of Anthocyanin and Proanthocyanidin in Apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Wang, Z.; Li, D.; Yan, L.; Feng, Y.; Liu, X.; Chen, R.; Fan, W.; Sun, L.; et al. Resistance index and browning mechanism of apple peel under high temperature stress. Hortic. Plant J. 2024, 10, 305–317. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Feng, Y.; Li, S.; Jia, R.; Yang, J.; Su, Q.; Zhao, Z. Physiological Characteristics of Sunburn Peel after Apple Debagged. Molecules 2022, 27, 3775. [Google Scholar] [CrossRef] [PubMed]
- Saha, G.; Park, J.-I.; Ahmed, N.U.; Kayum, A.; Kang, K.-K.; Nou, I.-S. Characterization and expression profiling of MYB transcription factors against stresses and during male organ development in Chinese cabbage (Brassica rapa ssp pekinensis). Plant Physiol. Biochem. 2016, 104, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, L.; Yu, J.; Li, S.; Li, D.; Feng, Y.; Zhao, Z. Role of the transcription factor MdABF2 in abscisic acid-induced volatile compound biosynthesis in ‘Ruixue’ (Malus × domestica) apple during storage. Postharvest Biol. Technol. 2023, 205, 112524. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.; Fu, Q.; Wang, Z.; Liu, X.; Sun, L.; Zhao, Z. Transcriptomic and metabolomic analysis reveals a protein module involved in preharvest apple peel browning. Plant Physiol. 2023, 192, 2102–2122. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, H.J.; Qu, D.; Zhu, Z.Z.; Yang, Y.Z.; Zhao, Z.Y. The MdBBX22-miR858-MdMYB9/11/12 module regulates proanthocyanidin biosynthesis in apple peel. Plant Biotechnol. J. 2022, 20, 1683–1700. [Google Scholar] [CrossRef]

| Primer Name | Sequence |
|---|---|
| MdANR-F | ATGACCGTTTCATCTTCTCTTTCTG |
| MdANR-R | AGCACAAGTGGCAGTGACAGTC |
| 35S-F | TCCTTCGCAAGACCCTTCCTCTAT |
| Seq-2300-F | CACTTTATGCTTCCGGCTCGTATG |
| SeqGFP-R | CAGGGTCAGCTTGCCGTAG |
| 2300-XbaI-R | CCATGGTGTCGACTCTAGA |
| 2300-KpnI-F | CGGGGGACGAGCTCGGTACC |
| MdANR-2300-F | ACGGGGGACGAGCTCGGTACCATGACCGTTTCATCTTCTCTTTCTG |
| MdANR-2300-R | GGTGTCGACTCTAGAGGATCCAGCACAAGTGGCAGTGACAGTC |
| MdANR-TRV2-F | GTGAGTAAGGTTACCACATCAATGGGCTTGCATCC |
| MdANR-TRV2-R | GAGACGCGTGAGCTCAGCACAAGTGGCAGTGACAGTC |
| MdMYBR9-LUC-F | AGAGGACAGCCCAAGCTGAGCTCATGTCGTCGGGCACGTGC |
| MdMYBR9-LUC-R | TTTCAGCGTACCGAATTGGTACCGGTGACGCTGATCATGCTATCC |
| MdANR-LUC-F | CACTATAGGGCGAATTGGGTACCATGGACTTGTTTTTGACCCTCAA |
| MdANR-LUC-R | TATGTTTTTGGCGTCTTCCATGGGGCTGCTGCTGCTCTTCTTTC |
| pAbi-F | TCTGTGCTCCTTCCTTCGT |
| pAbi-R | TGTATTTGTGTTTGCGTGTCTAT |
| pGADT7-F | AATACCACTACAATGGATGAT |
| pGADT7-R | ACTGTGCATCGTGCACCATCTC |
| MdMYBR9- pGADT7-F | GCCATGGAGGCCAGTTGAATTCATGTCGTCGGGCACGTGC |
| MdMYBR9- pGADT7-R | CAGCTCGAGCTCGATGGATCCTTAGGTGACGCTGATCATGCTATC |
| MdANR-pAbAi-1-F | ATGAATTGAAAAGCTTATGCCACATCTACCTATGAGACAGAT |
| MdANR- pAbAi-1-R | GTCGACAGATCCCCGGGTACCCTCCGTTCTCTTGCAAGATAGATCC |
| MdANR-pAbAi-2-F | ATGAATTGAAAAGCTTAATTAGATGGAGATCTCAACCATAGATATA |
| MdANR- pAbAi-2-R | GTCGACAGATCCCCGGGTACCCCGGAGCGTCAGTCGGCC |
| MdANR-pAbAi-3-F | ATGAATTGAAAAGCTTCTAGGAAAACTTTGGAAGAGACTCTAAG |
| MdANR- pAbAi-3-R | GTCGACAGATCCCCGGGTACCGGCTGCTGCTGCTCTTCTTTC |
| MdActin-F | TGACCGAATGAGCAAGGAAATTACT |
| MdActin-R | TACTCAGCTTTGGCAATCCACATC |
| MdANR-F | GACGGTACGGATATCGGGAAG |
| MdANR-R | GCAGCAAGGGCTAGTAGGTGA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Tian, W.; Guo, J.; Fu, J.; Wang, J.; Wang, Y.; Zhao, Z. Regulation of MdANR in Anti-Burning Process of Apple Peel. Int. J. Mol. Sci. 2025, 26, 4656. https://doi.org/10.3390/ijms26104656
Feng Y, Tian W, Guo J, Fu J, Wang J, Wang Y, Zhao Z. Regulation of MdANR in Anti-Burning Process of Apple Peel. International Journal of Molecular Sciences. 2025; 26(10):4656. https://doi.org/10.3390/ijms26104656
Chicago/Turabian StyleFeng, Yifeng, Wenya Tian, Junjiao Guo, Jianghong Fu, Jiangbo Wang, Yan Wang, and Zhengyang Zhao. 2025. "Regulation of MdANR in Anti-Burning Process of Apple Peel" International Journal of Molecular Sciences 26, no. 10: 4656. https://doi.org/10.3390/ijms26104656
APA StyleFeng, Y., Tian, W., Guo, J., Fu, J., Wang, J., Wang, Y., & Zhao, Z. (2025). Regulation of MdANR in Anti-Burning Process of Apple Peel. International Journal of Molecular Sciences, 26(10), 4656. https://doi.org/10.3390/ijms26104656







